Abstract
Critical steps in coronary vascular formation include the epithelial–mesenchyme transition (EMT) that epicardial cells undergo to become sub-epicardial; the invasion of the myocardium; and the differentiation of coronary lineages. However, the factors controlling these processes are not completely understood. Epicardial and coronary vascular precursors migrate to the avascular heart tube during embryogenesis via the proepicardium (PE). Here, we show that in the quail embryo fibroblast growth factor receptor (FGFR)-1 is expressed in a spatially and temporally restricted manner in the PE and epicardium-derived cells, including vascular endothelial precursors, and is up-regulated in epicardial cells after EMT. We used replication-defective retroviral vectors to over-express or knock-down FGFR-1 in the PE. FGFR-1 over-expression resulted in increased epicardial EMT. Knock-down of FGFR-1, however, did not inhibit epicardial EMT but greatly compromised the ability of PE progeny to invade the myocardium. The latter could, however, contribute to endothelia and smooth muscle of sub-epicardial vessels. Correct FGFR-1 levels were also important for correct coronary lineage differentiation with, at E12, an increase in the proportion of endothelial cells amongst FGFR-1 over-expressing PE progeny and a decrease in the proportion of smooth muscle cells in antisense FGFR-1 virus-infected PE progeny. Finally, in a heart explant system, constitutive activation of FGFR-1 signaling in epicardial cells resulted in increased delamination from the epicardium, invasion of the sub-epicardium, and invasion of the myocardium. These data reveal novel roles for FGFR-1 signaling in epicardial biology and coronary vascular lineage differentiation, and point to potential new therapeutic avenues.
INTRODUCTION
The coronary vasculature is essential for heart function, yet the processes that govern its formation are incompletely understood. Endothelial and smooth muscle cells of the coronary vasculature are derived from the epicardium and its transient precursor, the proepicardium (PE; Dettman et al., 1998; Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996; Pérez-Pomares et al., 1998). Before formation of the epicardium, the primitive heart tube consists of two layers, the myocardium and endocardium (Manasek, 1969). The PE appears as a grape-like cluster of cells, comprising villus protrusions, that emanates from the pericardial serosa posterior to the sino-atrium (Hiruma and Hirakow, 1989; Ho and Shimada, 1978; Virágh and Challice, 1981; Virágh et al., 1993). The PE appears to be induced by the liver bud (Ishii et al., 2007) and during development extends to the double-walled heart tube, probably with the aid of an extracellular matrix bridge between it and the myocardardium (Nahirney et al., 2003). It then envelops the developing heart, thus giving rise to the epicardium, the outer, mesothelial layer of the heart (Hiruma and Hirakow, 1989; Ho and Shimada, 1978; Virágh and Challice, 1981). Epicardium-derived cells form a coronary capillary plexus by a vasculogenic process (Mikawa and Fischman, 1992) that is remodeled into a mature coronary vasculature (reviewed by Bernanke and Velkey, 2002). Recent studies have indicated that PE identity is reliant on correct bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) signaling (Kruithof et al., 2006; Schlueter et al., 2006).
A critical step in coronary vascular formation is the epithelial–mesenchyme transition (EMT) that epicardial cells undergo to invade the sub-epicardium (Virágh et al., 1993). Another is the decision to contribute to the sub-epicardial coronary vasculature or, alternatively, to invade the myocardium and contribute to intramural vessels. Fibroblast growth factor (FGF)s and transforming growth factor β (TGFβ)?s expressed in the myocardium have been implicated in epicardial EMT, delamination, and invasion of the sub-epicardium (Dettman et al., 2003; Dokic and Dettman, 2006; Morabito et al., 2001). However, it remains unclear why only a portion of the epicardial cells undergoes EMT whilst others remain a part of the epicardium. Furthermore, the intrinsic factors that determine whether epicardium-derived cells will invade the myocardium or remain sub-epicardial are unknown.
The high affinity receptors for FGFs, FGFR-1–4, have been implicated in coronary vascular development: FGFR-1 and -2 signaling in cardiomyocytes is required for activation of hedgehog-dependent pathways controlling coronary vasculogenesis (Lavine et al., 2006). It remains unclear, however, if FGFR-1 signaling in epicardial cells is required for EMT, myocardial invasion, and coronary vessel formation. Recent studies on zebrafish reveal an important role for myocardial expression of FGF ligand and FGFR signaling for epicardial EMT and subsequent invasion of the myocardium during regeneration after surgical resection, and in normal homeostasis and maintenance of the adult heart (Lepilina et al., 2006; Wills et al., 2008). In the former study, it was shown that FGFR-2 and -4 were up-regulated in the epicardium upon heart damage, but the contribution of FGFR-mediated signaling in the myocardium toward repair remains unclear as the transgenic approach inhibited FGFR-mediated signaling in all cardiac tissues (Lepilina et al., 2006).
Other key steps in the formation of the coronary vasculature include the determination and differentiation of vascular lineages, including endothelial and smooth muscle cells. Lineage tracing of coronary vessel precursors in the chick embryo using replication-defective retroviral vectors expressing a reporter gene demonstrated that the coronary endothelial, smooth muscle and adventitial cell lineages have already segregated at the PE stage (Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996). The extent of their commitment, however, has not been experimentally addressed. Numerous angiogenic and vasculogenic factors have been identified, including the FGF family. FGFs are capable of stimulating angioblast formation, the earliest stage of vascular induction (Poole et al., 2001; Vokes and Krieg, 2002), and have long been implicated in other aspects of vascular development, being able to act directly on vascular cells, affecting vessel induction, and formation (for reviews, see Flamme et al., 1997; Folkman and D'Amore, 1996; Presta et al., 2005; Slavin, 1995). FGF expression in the myocardium has an effect on coronary vessel formation through paracrine signaling (Fernandez et al., 2000; Tomanek et al., 1998), and has a role patterning the intramural capillary network (Pennisi and Mikawa, 2005). In addition, ectopic over-expression of FGFs in PE cells promotes the formation and branching of coronary blood vessels in an autocrine manner (Hyer et al., 1999).
Most FGF effects are mediated through the FGFRs in numerous cell types, including developing vascular cells (reviewed in Powers et al., 2000; Presta et al., 2005). FGFRs are expressed dynamically and can be up-regulated in response to FGF (Estival et al., 1996; Saito et al., 1991). Moreover, epicardium-derived cells up-regulate FGFR-1 expression in response to myocardial over-expression of FGF (Pennisi and Mikawa, 2005). Thus, there is evidence indicating a role for FGFR-mediated signaling in different stages of epicardial and coronary vascular development. We hypothesised that FGFR-1 signaling plays a key role in epicardial EMT and coronary vasculature development. This was tested by modulating FGFR-1 levels in epicardial and coronary precursors in the PE with replication-defective retroviral vectors to over-express or knock-down receptor levels. Over-expression of FGFR-1 in PE progeny resulted in increased epicardial EMT. Knock-down of FGFR-1, however, did not reduce epicardial EMT but greatly compromised the ability of PE progeny to invade the myocardium. PE progeny with knock-down of FGFR-1 were able to invade the sub-epicardium and express endothelial and smooth muscle markers and contribute to the sub-epicardial coronary vasculature. At E12, there was an increased proportion of coronary endothelial cells amongst FGFR-1 over-expressing PE progeny, while there was a decreased proportion of coronary smooth muscle in PE progeny with knock-down of FGFR-1. In cultured PE explants, FGF2 induced EMT whilst FGFR antagonism inhibited EMT. Furthermore, FGF2 modestly promoted endothelial differentiation whilst FGFR antagonism decreased smooth muscle differentiation. Finally, upon constitutive activation of FGFR-1, more epicardial cells invaded the sub-epicardium and myocardium in whole-heart explant cultures. Thus, FGFR-1 signaling can promote epicardial EMT; is essential for invasion of the myocardium by PE progeny; and influences coronary lineage differentiation.
MATERIALS AND METHODS
Embryos
Fertilized quail eggs were purchased from CBT Farms (Chestertown, Maryland) and incubated at 38°C under humidified conditions. Embryos were staged according to the number of days incubated or by the system of Hamburger and Hamilton (Hamburger and Hamilton, 1951).
Immunohistochemistry and Immunofluorescence
Whole embryos or embryonic hearts were dissected in phosphate-buffered saline (PBS), fixed in 2% paraformaldehyde (PFA) in PBS at 4°C for 2–4 hours, dehydrated in an ethanol series, cleared in CitriSolv (Fisher Scientific, USA), and embedded in paraffin. Serial, 7 µm, transverse sections were cut and mounted on Superfrost slides (VWR, USA). Immunohistochemistry was performed as described (Pennisi et al., 2003) with the following antibodies: anti-FGFR-1 (sc-15, Santa Cruz Biotechnology), QH1 (Developmental Studies Hybridoma Bank, The University of Iowa), anti-cytokeratin (BT-571, Biomedical Technologies), anti-SMαA (clone 1A4, Sigma). Some sections were counterstained with nuclear fast red to aid quantification. Indirect immunofluorescence using the QH1 and anti-FGFR-1 antibodies was performed on sections prepared as above. Sections were blocked in 2% BSA in PBS/0.1% Tween 20 and incubated with primary antibodies. Sections were then washed in PBS and incubated with Alexa Fluor 488-and Alexa Fluor 594-conjugated secondary antibodies (Molecular Probes). After washing in PBS, slides were mounted with Vectashield Mounting Medium (Vector Laboratories).
In ovo Injection of Retroviral Constructs and Sample Analyses
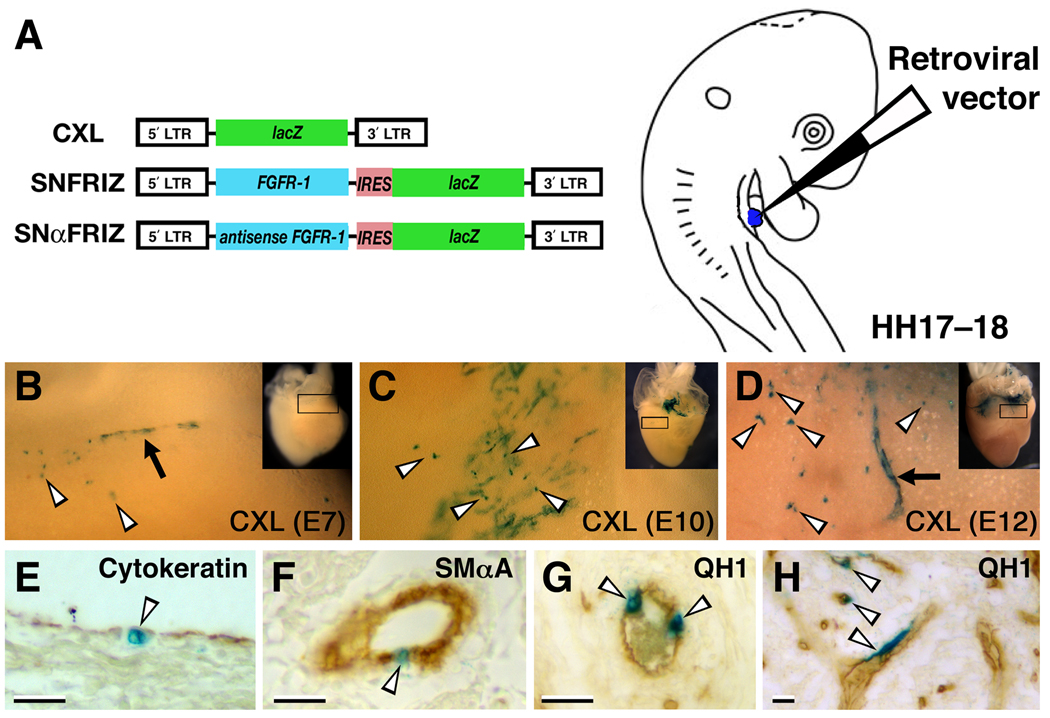
High titre preparations (107–108 plaque forming units/mL) of replication-defective retroviruses were prepared and pressure-injected in ovo into the PE of HH17–18 embryos to infect epicardial and epicardial derived cells as previously described (Figure 2; Hatcher et al., 2004; Hyer et al., 1999; Mikawa and Gourdie, 1996). The retroviral vectors employed in this study have been described previously: CXL (expressing β-galactosidase; Mikawa et al., 1992; Mikawa et al., 1991), SNFRIZ (expressing chick FGFR-1 and β-galactosidase from a di-cistronic mRNA; Itoh et al., 1996; Mima et al., 1995), and SNαFRIZ (expressing antisense FGFR-1 and β-galactosidase from a di-cistronic mRNA; Itoh et al., 1996; Mima et al., 1995). The effective expression and biological activity of FGFR-1 from SNFRIZ and knock-down of FGFR-1 from SNαFRIZ have been shown previously (Itoh et al., 1996; Mima et al., 1995). Hearts were dissected at embryonic days (E) 7, E10, and E12 in PBS, fixed in 2% PFA in PBS at 4°C for 2–4 hours, and stained with X-Gal at 37°C overnight. After numerous washes in PBS and whole-mount photography, samples displaying X-Gal staining were embedded in paraffin and processed for immunohistochemistry as described above or counterstained with nuclear fast red. For quantification of virus-infected PE progeny at E7 and E12, all X-Gal+ cells from sectioned hearts were scored as set out in Supplemental Table 1 (generally after immunohistochemistry with the QH1 or anti-SMαA antibodies). A minimum of three hearts displaying X-Gal staining for each viral vector, at each developmental stage, was used for quantification of cell fate. The data in Supplemental Table 1 are further summarized in Figure 5 to show the percentage of X-Gal+ PE progeny that (i) remained in the epicardium, (ii) occupied an intra-myocardial location (intra-atrial or intra-ventricular), (iii) were QH1-immunoreactive or (iv) were anti-SMαA-immunoreactive. When expressing the percentage of PE-derived cells were intra-myocardial or not, X-Gal+ cells from the atrial, atrio-ventricular and ventricular components of the heart were considered, not adventitial regions around the great vessels or truncus/OFT.
Figure 2. Experimental procedure for retroviral targeting epicardial and coronary vascular precursors in the quail embryo.
A, The retroviral vectors, CXL (β-galactosidase), SNFRIZ (FGFR-1 and β-galactosidase) and SNαFRIZ (antisense FGFR-1 and β-galactosidase) were injected into the PE (shown in blue) of quail embryos. B–D, Whole-mount views of control hearts X-Gal-stained at E7, E10, and E12, respectively. High magnification views are of the boxed areas in insets. Note X-Gal+ cells amongst the epicardium (arrowheads) and vascular structures (arrows). E, Cytokeratin immunostaining of an E10 heart injected with control virus showing labelling of the epicardium. F, SMαA immunostaining of an E12 heart injected with control virus showing labelling of smooth muscle cells of a coronary artery. G, QH1 immunostaining of an E12 heart injected with control virus showing labelling of coronary artery endothelia. H, QH1 immunostaining of an E12 heart injected with control virus showing labelling of intramural coronary endothelia. Scale bars, 20µm.
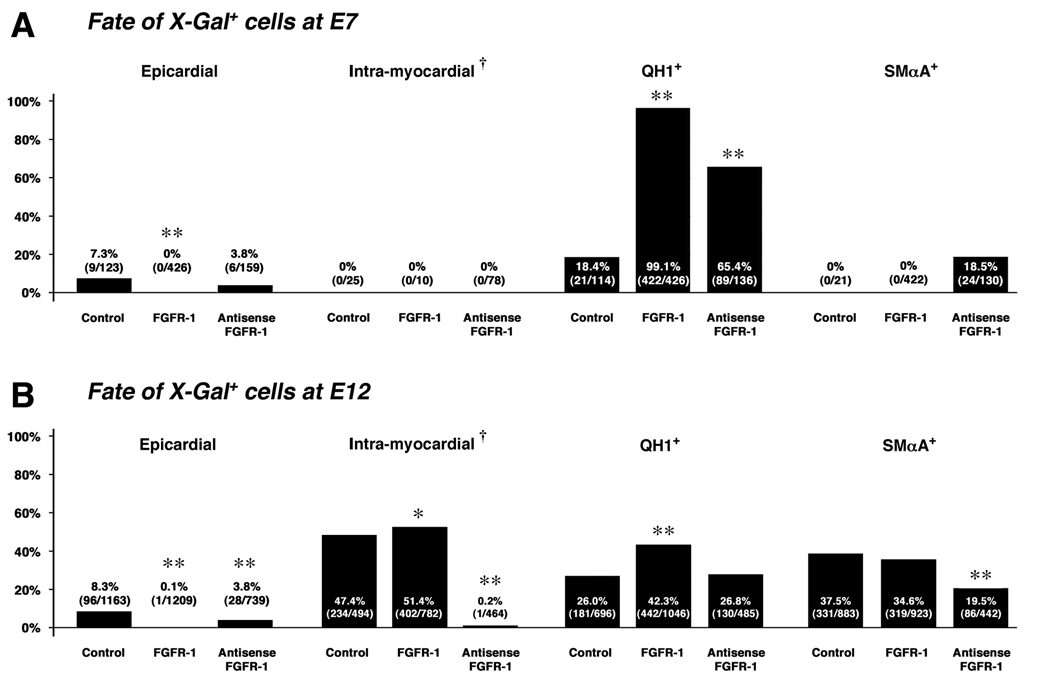
Figure 5. Summary of the fate of control, FGFR-1, and antisense FGFR-1 virus-infected proepicardial cells.
A, The fate of virus-infected cells at E7. The total number of X-Gal+ cells from all hearts in each category are in parentheses. Note the increase in the proportion of QH1+ cells in FGFR-1, and antisense FGFR-1 virus-infected hearts relative to controls, and the increase in the proportion of SMαA+ cells in antisense FGFR-1 virus-infected hearts. B, The fate of virus-infected cells at E12. Note the marked decrease in the number of intra-myocardial X-Gal+ cells in antisense FGFR-1 virus-infected hearts. As at E7, an increase in the proportion of QH1+ cells in FGFR-1 virus-infected hearts relative to controls was observed at E12, although the proportion for antisense FGFR-1 virus-infected hearts was similar to controls. Also note the decrease in proportion of X-Gal+/SMαA+ cells in antisense FGFR-1 virus-infected samples. At both E7 and E12, in FGFR-1 virus-infected hearts there was almost a complete reduction in X-Gal+ cells resident in the epicardium relative to control and antisense FGFR-1 virus-infected hearts. †, expressed as a percentage of all X-Gal+ cells from the atrial, atrio-ventricular and ventricular components of the heart (described in Materials and Methods). *, P value <0.05; **, P value <0.001.
Proepicardial Explant Cultures
PE of stage HH16–17 quail embryos were isolated using fine dissecting forceps in PBS/0.05% BSA to prevent tissue from adhering to the plastic dishes. They were then cultured in serum-free DMEM supplemented with penicillin and streptomycin for 16 hours and allowed to attach to fibronectin-coated coverslips. The media was then replaced with fresh serum-free DMEM (−FCS) or DMEM supplemented with 10% fetal calf serum (+FCS); FGFR inhibitor (SU5402; Merck); 0.1% DMSO; 10ng/mL or 50ng/mL FGF2 (1104616, Roche Diagnostics); or 1ng/mL TGFβ2 (302-B2-002, R&D Systems). Explants were cultured for a further 5 days, fixed in 4% PFA and processed for immunofluorescence (with anti-SMαA or QH1 antibodies) and DAPI staining. Low magnification images were captured such that entire explants were photographed. DAPI-stained nuclei and immunofluorescent cells were quantified with ImageJ software (NIH; http://rsb.info.nih.gov/ij/) or manually using Adobe Photoshop software (version 7.0, Adobe Systems Inc.). At least four explants for each culture condition and antibody staining were examined and the mean percentage of immunoreactivity calculated.
Construction of a Constitutively Activated FGFR-1
To constitutively activate FGFR-1-mediated signaling pathway in epicardial cells, we used a derivative of human FGFR-1, myrR1-TDII (Hart et al., 2000). This clone consists of the cytoplasmic domain of human FGFR-1 with a Lys656>Glu mutation in the activation loop and a myristylation signal to target the protein to the plasma membrane. This has been shown to phosphorylate STAT1, STAT3 and p44/42 MAPK, components of the FGFR-mediated signaling pathway (Hart et al., 2000). This modified FGFR-1 cDNA was cloned into the proviral vector, pCXIZ (Mikawa et al., 1991), upstream of an IRES and LacZ sequence, and termed pCAFR-1. To confirm the ability of the pCAFR-1 construct to activate components of the FGFR-mediated signaling pathway, the levels of STAT1, STAT3 and p44/42 phosphorylation were examined after transfection in cultured cells. To this end, D17.2G cells were transiently transfected with pCAFR-1 or pCXIZ. Cells were stained with X-Gal to gauge transfection efficiency or used for preparation of whole-cell lysate that was separated by SDS-PAGE and transferred to nitrocellulose membranes. Immunoblotting was performed as described (Hart et al., 2000), using the commercial antibodies described therein. Immunoblotting data are representative examples of three transfections.
Explant Invasion Assay
Freshly dissected E6 hearts were transferred to electroporation cuvettes (5mm gap) containing 40µg of pCXIZ (encoding β-galactosidase) or pCAFR-1 (encoding β-galactosidase and a constitutively activated FGFR-1) in 110µL PBS. Constructs were introduced into epicardial cells by electroporation (5 pulses of 30 msec at 60 volts). After incubation on ice for 5 minutes, hearts were cultured in serum-free DMEM supplemented with penicillin and streptomycin in BSA-coated tissue culture dishes to avoid adherence of the explanted tissue. After 48 hours, hearts were processed for X-Gal staining and paraffin embedding as above. In all cases, explanted hearts maintained a rhythmic heartbeat and remained unattached to the tissue culture dishes during incubation. 10µm coronal sections were cut on a microtome and processed for nuclear fast red counterstaining. For quantification of epicardial EMT and invasion, only X-Gal-positive cells in the ventricular component of the explant with a clearly stained nucleus were counted and scored for their location (epicardial, sub-epicardial, or intra-myocardial). Every third section was used for quantification to avoid counting the same cell more than once. The relative proportions of epicardial, sub-epicardial or intra-myocardial X-Gal+ cells were expressed as a percentage of total X-Gal+ cells counted from that explant. At least three explants of each type were examined and the mean and standard deviation of the mean calculated. In addition to nuclear fast red counterstaining, some sections were stained for immunoreactivity with a phospho-p44/42 MAPK-specific antibody (Cell Signaling Technology, #4376) according to the manufacturers specifications.
Data Documentation
Images were captured using either a Digital Photo Camera (DKC-5000, Sony) or a Spot RT Slider (2.3.1, Diagnostic Instruments Inc., USA) using Adobe Photoshop software (version 7.0, Adobe Systems Inc.) or Spot (version 3.2.6 Diagnostic Instruments Inc, USA) software, respectively. Images were adjusted for color levels, brightness and contrast, and figures compiled, using Adobe Photoshop software.
Statistical Analyses
Statistical significance of the difference in the fate of FGFR-1 and antisense FGFR-1 virus-infected proepicardial cells relative to control data was determined using the Chi-square test. For proepicardial explant cell phenotype and epicardial invasion in heart explants, statistical significance was determined using an unpaired two-tailed Student’s t test.
RESULTS
FGFR-1 is expressed in a subset of PE and epicardium-derived cells including endothelial precursors
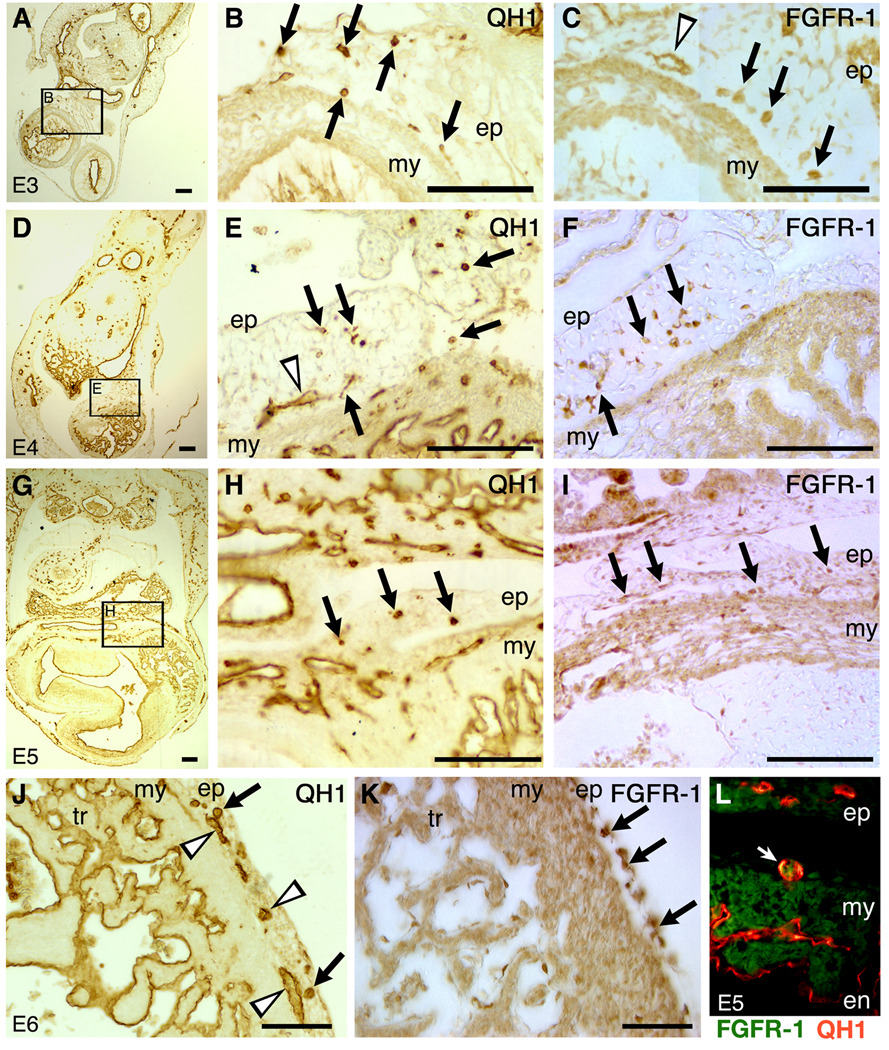
As FGFs have been implicated in numerous aspects of heart development, including formation of the coronary vasculature, we examined the expression of FGFR-1 in the population of cells that give rise to the coronary vasculature from the stage when the PE is forming and contacting the tubular heart (~E3 in the quail embryo). In parallel, we used the QH1 antibody, a marker for quail endothelial cells and their precursors (Pardanaud et al., 1987). In addition, non-immune serum controls were conducted. At no stage was non-specific signal detected (not shown). At E3, QH1+ cells are present in the PE that bridges the dorsal body wall and the myocardium of the tubular heart (Figure 1A, B). Likewise, FGFR-1+ cells were observed in the PE (Figure 1C). At E4 and E5 (Figure 1D–I), a similar distribution of QH1+ and FGFR-1+ cells was observed in the primitive epicardium and sub-epicardium. At E6, a stage when the epicardium has almost enveloped the entire heart (Virágh et al., 1993; Vrancken Peeters et al., 1995), QH1+ cells are evident in the sub-epicardium, appearing as isolated cells and in lumenised structures (Figure 1J). At this stage, sub-epicardial cells are more strongly FGFR-1-immunoreactive than epicardial cells (Figure 1K). At the stages examined, we observed comparatively large, round, QH1+ cells, presumably angioblasts (Pardanaud et al., 1987; Poole et al., 2001). The overlap of QH1 and FGFR-1 immunoreactivity, for at least a proportion of epicardium-derived cells, was demonstrated at E5 (Figure 1L). Consistent with previous reports, FGFR-1 expression was observed in cardiomyocytes (Patstone et al., 1993; Pennisi et al., 2003). It was, therefore, difficult to examine FGFR-1 expression amongst coronary progenitors that had invaded the myocardium. Examining expression in the cells of the PE and sub-epicardium, however, allowed us to assess the relative FGFR-1 immunoreactivity before such progenitors invaded the myocardium. Thus, at the different stages examined, FGFR-1 was expressed in a spatially restricted manner amongst primitive epicardial and sub-epicardial cells, the population of cells that include coronary vascular progenitors. Furthermore, sub-epicardial cells expressed FGFR-1 at levels higher than epicardial cells, suggestive of it having a role in EMT and invasion.
Figure 1. FGFR-1 expression is spatially and temporally restricted in the PE and epicardium-derived cells, and in some vascular endothelial precursors.
A–C, E3; D–F, E4; G–I, E5; J, K, E6; L, E5. QH1 immunohistochemical detection of vascular endothelia and their precursors (A, B, D, E, G, H and J). B, E and H are higher magnification views of the boxed areas in A, D and G, respectively. Comparable regions were stained for FGFR-1 immunoreactivity (C, F, I and K). Note the cells (arrows) and putative lumen structures (arrowheads) that are immunoreactive for QH1 or FGFR-1. Also note, at E6, sub-epicardial cells are more strongly FGFR-1+ than are epicardial cells (arrows, K). L, Double immunofluorescence for QH1 (red) and FGFR-1 (green) at E5 showing a sub-epicardial cell immunoreactive for both antibodies (arrow). en, endocardium; ep, epicardium; my, myocardium; tr, trabeculae. Scale bars, 100µm.
Modulation of FGFR-1 levels in PE cells affects epicardial EMT, myocardial invasion and coronary lineage differentiation
We tested the role of FGFR-1 in epicardial EMT and invasion, and for coronary cell differentiation, by modulating FGFR-1 levels in epicardial and coronary vessel precursors. Replication-defective retroviral vectors were injected into the PE of quail embryos in ovo at HH17–18 (~E3); CXL, expressing β-galactosidase; SNFRIZ, expressing chick FGFR-1 and β-galactosidase; and SNαFRIZ, expressing antisense FGFR-1 and β-galactosidase (Figure 2A; Itoh et al., 1996; Mikawa et al., 1991; Mima et al., 1995). The efficacy of PE injections in quail embryos was determined using control virus. Hearts dissected at E7, E10, and E12 regularly displayed X-Gal+ cell clusters reminiscent of epicardial and coronary vascular colonies (Figure 2B–D; Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996). Immunohistochemistry for cytokeratin (a marker for epicardial cells; Vrancken Peeters et al., 1995), smooth muscle α actin (SMαA) and QH1 were used to determine cellular identity. We found X-Gal+ cells amongst the epicardium, coronary artery smooth muscle, coronary artery endothelial cells and intramural, capillary endothelial cells (Figure 2E–H). This indicated epicardial and coronary precursors were successfully targeted by PE injection as described for the chick embryo (Hatcher et al., 2004; Hyer et al., 1999; Mikawa and Gourdie, 1996).
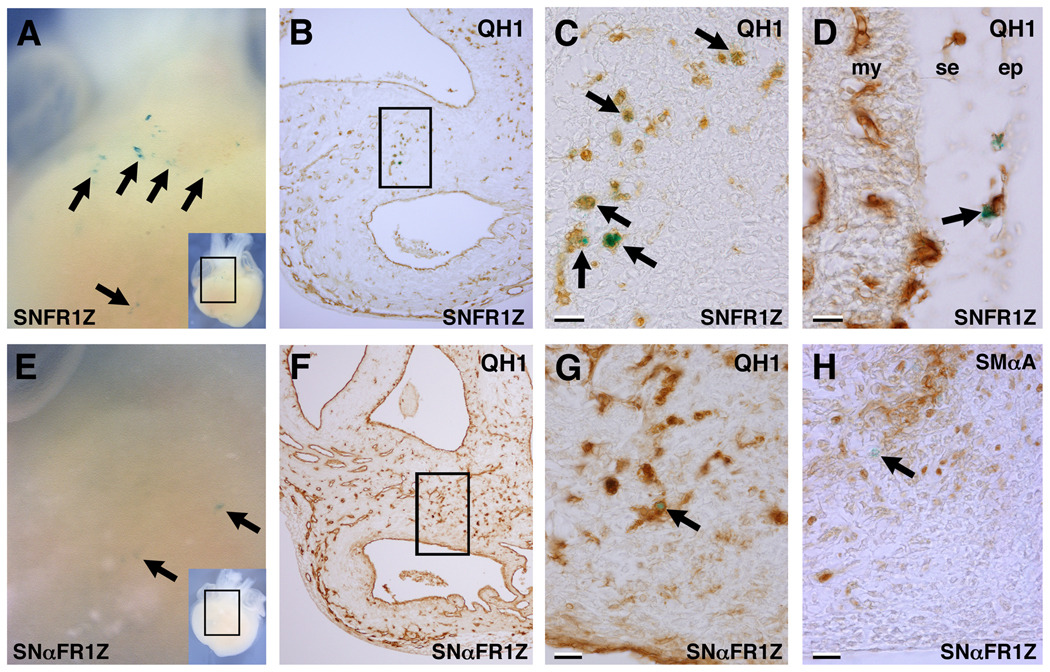
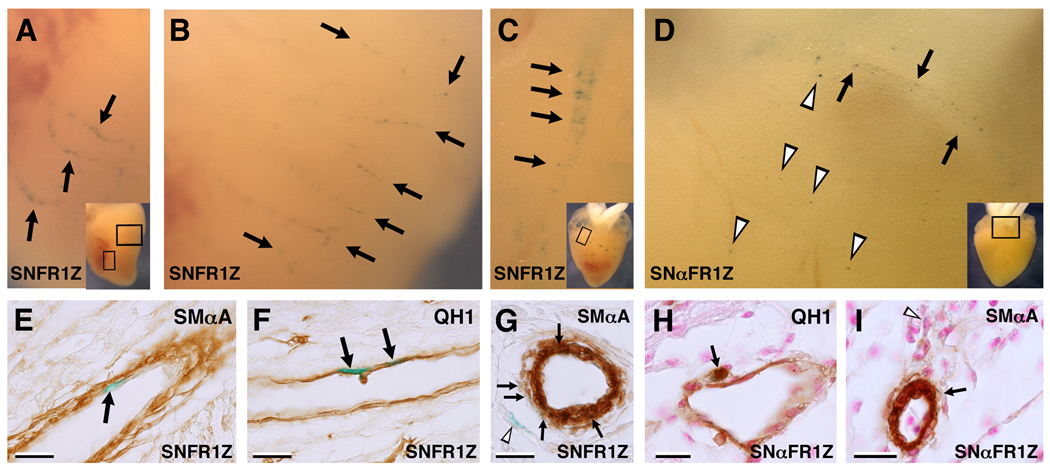
Next, FGFR-1 levels were modulated in PE cells and their progeny using either the FGFR-1 or the antisense FGFR-1 viruses. Embryos were allowed to develop until E7 or E12 before X-Gal staining and section immunohistochemistry. At E7, in FGFR-1 virus-infected hearts we observed numerous X-Gal+/QH1+ and X-Gal+/SMαA− cells around the outflow tract (OFT) and sub-epicardium at the level of the ventricles (Figure 3). Many of these, particularly around the OFT, appeared as large, round isolated cells, probably representing angioblasts. Likewise, in antisense FGFR-1 virus-infected hearts at E7, numerous QH1+/SMαA− cells were observed around the OFT. At E12, X-Gal+ structures were frequently observed in stained virus-infected hearts viewed in whole-mount similar to virally tagged epicardial and sub-epicardial cells, and portions of coronary vessels as described previously (Hatcher et al., 2004; Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996). An example where X-Gal+ cells are arranged along the axes of vessels, indicative of endothelia, is shown (Figure 4A, B; Mikawa and Fischman, 1992). Immunostaining showed these to be luminal, QH1+ and SMαA−, confirming their endothelial identity (Figure 4E, F). Also observed were X-Gal+ cells arranged spirally with their axis transverse to that of the vessel (Figure 4C). Section immunostaining showed these to be SMαA+. In addition, X-Gal+/SMαA− cells were observed in close proximity to the X-Gal+ smooth muscle cells (Figure 4G). In antisense FGFR-1 virus-infected hearts at E12, X-Gal+ vascular structures were also observed, but only superficially on the heart (Figure 4D). Section analysis revealed both X-Gal+/SMαA+ and X-Gal+/QH1+ cells associated with the coronary vasculature (Figure 4H, I). Importantly, these were rarely found in the ventricular or atrial myocardium. Thus, antisense FGFR-1 virus-infected PE progeny were able to express endothelial or smooth muscle markers and contribute to the coronary vasculature, but only in the sub-epicardium.
Figure 3. Test virus-infected hearts X-Gal-stained at E7.
An example of an FGFR-1 virus-infected heart (A–D) and an antisense FGFR-1 virus-infected heart (E–F) at E7. A, Whole-mount view showing X-Gal+ cells close to the surface of the heart (boxed area of inset). B, QH1 immunostaining on a transverse section at the level of the outflow tract. C, Higher magnification view of the boxed area in B showing X-Gal+/QH1+ cells (arrows). D, QH1 immunostaining at the level of the ventricle showing an X-Gal+/QH1+ cell in the sub-epicardium (arrow). E, Whole-mount view showing X-Gal+ cells close to the surface of the heart (boxed area of inset). F, QH1 immunostaining at the level of the outflow tract. G, Higher magnification view of the boxed area in F. Note the X-Gal+/QH1+ cell (arrow). H, SMαA immunostaining of an adjacent section to that shown in G showing an X-Gal+/SMαA− cell (arrow). Scale bars, 20µm.
Figure 4. Test virus-infected hearts X-Gal-stained at E12.
Examples of FGFR-1 virus-infected hearts (A–C, E–G) and an antisense FGFR-1 virus-infected heart (D, H, I) at E12. A, Whole-mount view showing X-Gal+ cells in coronary vessels (boxed area of inset). B, View of the left ventricle of the heart shown in A. X-Gal+ endothelial cells are present in coronary vessels (arrows). C, Whole-mount view of another FGFR-1 virus-infected heart showing X-Gal+ cells amongst a vascular structure (boxed area of inset). D, Whole-mount view of an antisense FGFR-1 virus-infected heart showing X-Gal+ cells in a vascular structure (arrows) and the epicardium or sub-epicardium (arrowheads; boxed area of inset). E, SMαA immunostaining of a section of the heart shown in A and B revealing an X-Gal+ cell luminal to the presumptive media (SMαA+) of a coronary vessel (arrow). F, QH1 immunostaining of an adjacent section to that shown in E, showing X-Gal+/QH1+ cells (arrows). G, SMαA immunostaining of a section of the heart shown in C at the level of the boxed area of inset revealing X-Gal+/SMαA+ cells in the media of a coronary artery (arrows). Note X-Gal+ cells that do not appear to be part of the media, but rather adventitia or fibroblasts (arrowhead). H, QH1 immunostaining on a section of the heart shown in D showing an X-Gal+/QH1+ cell associated with a sub-epicardial vessel. I, SMαA immunostaining of a nearby section, revealing X-Gal+ cells in the sub-epicardium (arrowheads) and amongst the smooth muscle of a sub-epicardial arteriole (arrow). Scale bars, 20µm.
The quantification of fate and location of all X-Gal+ cells in virus-infected hearts in this study are detailed in Supplemental Table 1. These data were summarized to highlight the percentage of X-Gal+ cells at E7 and E12 that were; (i) epicardial, (ii) intra-myocardial, (iii) QH1+ or (iv) SMαA+ (Figure 5). At E7 in controls, 7.3% of X-Gal+ cells were epicardial, 13.0% were sub-epicardial, and 79.7% were in the truncus/OFT region. No X-Gal+ cells were found in the adventitia around the OFT and atria. In FGFR-1 virus-infected hearts at E7, however, no X-Gal+ epicardial cells were observed. 2.3% of X-Gal+ cells were sub-epicardial, 89.7% were in the truncus/OFT region, and 8.0% were in the adventitia around the OFT and atria. In antisense FGFR-1 virus-infected hearts at E7, 3.8% of X-Gal+ cells were epicardial, 45.3% were sub-epicardial, 34.6% were in the truncus/OFT region, and 16.4% in the adventitia around the OFT and atria. In controls at E12, 8.3% of all X-Gal+ PE-derived cells were epicardial while 47.4% of X-Gal+ cells from the atrial, atrio-ventricular and ventricular components of the heart were in intra-myocardial locations. In FGFR-1 virus-infected hearts, however, a marked reduction in X-Gal+ epicardial cells was observed (0.1%), with an increase in intra-myocardial relative to controls (51.4%). In antisense FGFR-1 virus-infected hearts, a decrease in X-Gal+ epicardial cells was observed (3.8%), however, we found a dramatic reduction in the number of PE-derived X-Gal+ cells located in the myocardium (0.2%). Thus, there was a concomitant increase in the proportion of X-Gal+ cells that were sub-epicardial (ventricular and atrio-ventricular regions of the heart) by E12 in FGFR-1 virus-infected hearts (31.3% of all X-Gal+ cells) and antisense FGFR-1 virus-infected hearts (58.9%) relative to controls (14.1%). There was, however, a decrease in the proportion of X-Gal+ cells residing in the adventitia around the great vessels and atria in FGFR-1 virus-infected hearts (35.3% of all X-Gal+ cells) and antisense FGFR-1 virus-infected hearts (37.2%) relative to controls (57.6%) at E12.
In controls at E7, 18.4% of X-Gal+, PE-derived cells were QH1+. FGFR-1 and antisense FGFR-1 virus-infected hearts both displayed an increase in X-Gal+/QH1+, PE-derived cells (99.1% and 65.4%, respectively). At E12, the proportion of X-Gal+/QH1+, PE-derived cells in controls was 26.0%. A value similar to controls was observed in antisense FGFR-1 virus-infected hearts (26.8%), although these cells were in different locations in the heart. In FGFR-1 virus-infected hearts at E12, however, an increase in the proportion of X-Gal+/QH1+ cells was observed (42.3%). At E7, we did not detect any X-Gal+/SMαA+, PE-derived cells in control or FGFR-1 virus-infected hearts. In antisense FGFR-1 virus-infected hearts, however, 18.5% of X-Gal+, PE-derived cells were SMαA+. At E12, in control and FGFR-1 virus-infected hearts, 37.5% and 34.6% of X-Gal+, PE-derived cells were SMαA+, respectively. By contrast, 19.5% were SMαA+ in antisense FGFR-1 virus-infected hearts.
Thus, over-expression of FGFR-1 in PE cells resulted in an increase in the proportion of QH1+ cells. At E7, knock-down of FGFR-1 in PE cells also resulted in an increase in the proportion of QH1+ cells, although by E12 the proportion was similar to controls. Similarly, knock-down of FGFR-1 also resulted in an increase in the proportion of SMαA+ cells at E7, although by E12 there was a reduction relative to controls. Strikingly, over-expression of FGFR-1 in PE progeny resulted in a decreased ability to remain epicardial. Knock-down of FGFR-1, however, compromised the ability of epicardial cells to invade the myocardium and contribute to intramural vessels, although they were able to undergo EMT and invade the sub-epicardium. They were also able to express endothelial and smooth muscle markers and contribute to sub-epicardial vessels.
Modulating FGF signaling affects EMT and coronary lineage differentiation in cultured PE explants
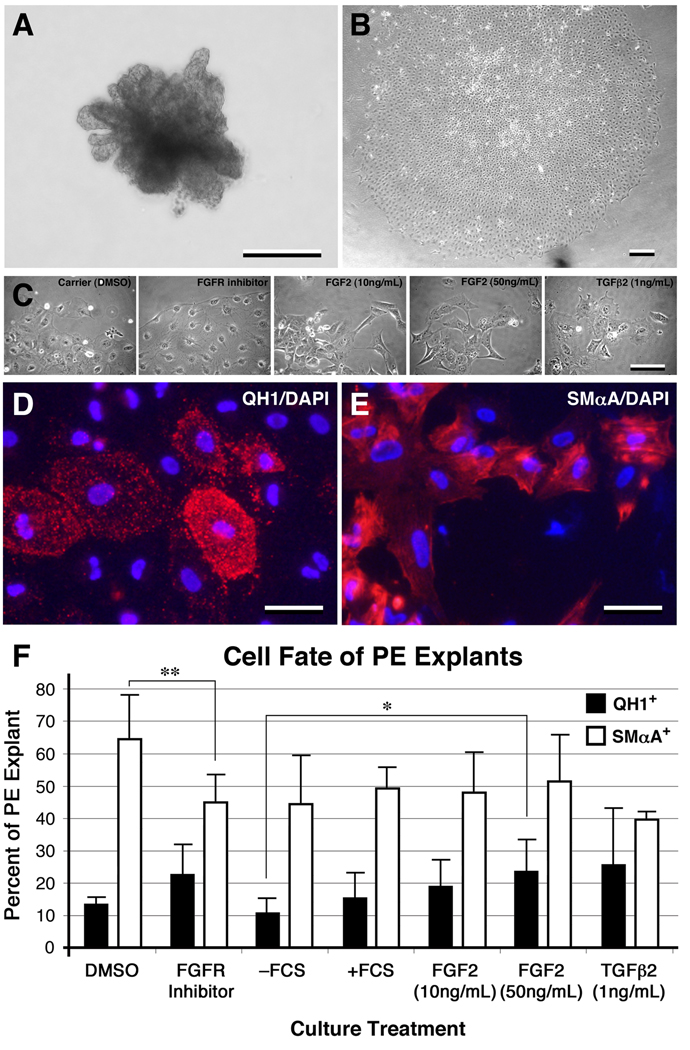
To further investigate the role of FGFR-mediated signaling in EMT of the epicardial mesothelium, as well as vascular phenotype differentiation, we utilized a PE explant culture system. Once explanted, isolated PE formed epithelial sheets in culture (Figure 6A, B) and co-expressed WT-1, cytokeratin, and vimentin, characteristic of the epicardial mesothelium (data not shown). To experimentally modulate cellular phenotype in cultured PE explants, media was supplemented with FGF2 or a pharmacological inhibitor of FGFR signaling, SU5402. TGFβ2 was used as a positive control for epicardial EMT (Compton et al., 2006; Dokic and Dettman, 2006). After 3 days of culture, cells at the leading edge of explants cultured with FGF2 (10ng/mL and 50ng/mL) or TGFβ2 were more likely to be separated from the main explant and fibroblast-like in appearance than in control explants. Cells at the leading edge of PE explants cultured with FGFR inhibitor, however, were less likely to adopt a fibroblast-like appearance and more likely to remain part of the main explant and retain an epithelial phenotype (Figure 6C). Similar to earlier time points, PE cultured in the presence of TGFβ2 or FGF2 for 5 days showed more isolated cells than those cultured in the presence of fetal calf serum or FGFR inhibitor (Supplemental Figure 1). After 5 days of culture, the number of fibroblast-like cells in explanted PE increased under all conditions.
Figure 6. Modulating FGF signaling affects EMT and endothelial and smooth muscle cell phenotype in PE explant culture.
A PE immediately after dissection (A) and one that has formed a monolayer after 36 hours of culture (B). C, Higher magnification of the leading edge of PE explant monolayers after 3 days of culture in the presence of factors as indicated. The cells at the leading edge of explants cultured in the presence of FGFR inhibitor maintained an epithelial phenotype. Examples of cultured PE explants displaying QH1 and DAPI staining (D) and SMαA and DAPI staining (E). F, Quantification of QH1 and SMαA immunoreactivity after 5 days of various culture conditions. There was a reduction in the percentage of SMαA+ cells in PE cultured with FGFR inhibitor relative controls. In addition, there was a modest increase in the percentage of QH1+ cells in PE treated with FGF2 (50ng/mL). Under all conditions except TGFβ2 treatment there was a significant difference between the numbers of QH1+ and SMαA+ cells (P value <0.02). Error bars, standard deviation of the mean. *, P value <0.1; **, P value <0.05. Scale bars; A and B, 200µm; C, 100µm; D and E, 50µm.
To further examine the effect of FGFR signaling on coronary lineage differentiation, PE explants were cultured with exogenous FGF2, FCS, TGFβ2 or FGFR inhibitor. After 5 days, explants were stained with QH1 or anti-SMαA antibodies and the number of immunoreactive cells quantified (Figure 6 D–F). The percentage of SMαA+ cells in PE explants were: 44.3% for – FCS, 49.3% for +FCS, 48% for FGF2 (10ng/mL), 51.4% for FGF2 (50ng/mL), 39.6% for TGFβ2 (1ng/mL), 64.4% for DMSO (carrier control), and 45% for FGFR inhibitor. QH1 immunoreactivity amongst PE explants were: 10.9% for –FCS, 15.8% for +FCS, 19% for FGF2 (10ng/mL), 23.7% for FGF2 (50ng/mL), 25.7% for TGFβ2 (1ng/mL), 13.6% for DMSO, and 22.7% for FGFR inhibitor (Figure 6F). We observed a significant reduction in the percentage of SMαA+ cells in PE cultured with FGFR inhibitor (45%) relative to controls (64.4%). In addition, there was a modest increase in the percentage of QH1+ cells in PE treated with FGF2 (50ng/mL; P value <0.1). Under all culture conditions except TGFβ2 treatment, there were significantly more SMαA+ cells (44.3–64.4%) than QH1+ cells (10.9–23.7%) amongst explants (P value <0.02). The data show that FGF and FGFR signaling promote EMT in PE explants. Moreover, exogenous FGF and an inhibitor of FGFR signaling can affect coronary lineage differentiation in cultured PE. Together with the changes in the proportion of QH1+ and SMαA+ cells amongst virus-infected PE progeny, the data demonstrate that FGFR-mediated signaling modulates coronary vascular lineage differentiation.
Constitutive activation of FGFR-1-mediated signaling pathway in epicardial cells increases invasiveness
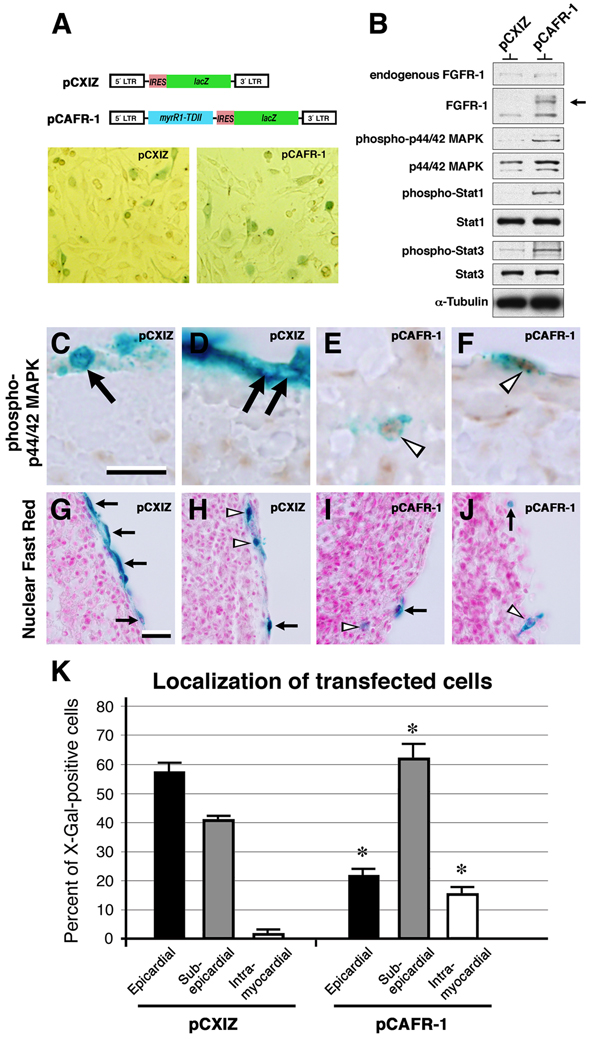
The lineage tracing data of virus-infected PE progeny indicated that FGFR-1 expression was sufficient but not necessary to induce epicardial EMT. FGFR-1 expression did, however, appear necessary for epicardium-derived cells to invade the myocardium. It remained unclear, however, whether activation of the FGFR-1 signaling pathway was sufficient for epicardial cells to invade the sub-epicardium and the myocardium. To test this, we used a construct encoding β-galactosidase and a constitutively activated FGFR-1, pCAFR-1, based on the pCXIZ proviral construct encoding β-galactosidase alone (Hart et al., 2000; Mikawa et al., 1991). We confirmed pCAFR-1 activated components of the FGFR-1 pathway in the D17.2G cell line: Compared to pCXIZ-transfected cell lysate, pCAFR-1-transfected cell lysate had elevated levels of phospho-p44/42 MAPK, phospho-STAT1, and phospho-STAT3, indicative of pathway activation (Figure 7A, B; Hart et al., 2000). These constructs were then applied to a heart explant system where they were introduced into epicardial cells by electroporation. We found that pCAFR-1-transfected cells were more likely to have anti-phospho-p44/42 MAPK-immunoreactive nuclei than pCXIZ-transfected cells, indicating that pCAFR-1 activated the FGFR-1 signaling pathway in epicardial cells (Figure 7C–F). Quantification of X-Gal+ cells in the ventricular component of pCXIZ-transfected hearts revealed that 57.4% were epicardial, 41.1% were sub-epicardial, and 1.5% were intra-myocardial (Figure 7K). This is consistent with previous reports using epicardial invasion assays showing that the majority of cells remained epicardial (Dettman et al., 2003; Dokic and Dettman, 2006). Transfecting epicardia of explants with pCAFR-1, however, resulted in 22.3% of X-Gal+ cells remaining epicardial, 62.2%, sub-epicardial, and 15.5% intra-myocardial (Figure 7K). Relative to controls, epicardial cells expressing pCAFR-1 were less likely to remain epicardial and more likely to occupy either the sub-epicardium or the myocardium (P value <0.005). Thus, constitutively activating the FGFR-1 signaling pathway in epicardial cells increased their propensity to undergo EMT and invade both the sub-epicardium and the myocardium.
Figure 7. Constitutive activation of the FGFR signaling pathway in epicardial cells increases their invasiveness.
A, The proviral constructs pCXIZ (β-galactosidase) and pCAFR-1 (constitutively activated FGFR-1 and β-galactosidase); examples of D17.2G cells X-Gal stained after transient transfection with pCXIZ or pCAFR-1, as indicated. B, Immunoblotting of D17.2G whole-cell lysate transiently transfected with either pCXIZ or pCAFR-1. In addition to endogenous FGFR-1, note the truncated, mutant form of FGFR-1 (arrow; Hart et al., 2000). Also note the relative increase in the levels of phospho-p44/42 MAPK, phospho-STAT1 and phospho-STAT3 in pCAFR-1-transfected cells. C–F, Transfected heart explants processed for X-Gal staining and anti phospho-p44/42 MAPK immunohistochemistry. C, D, Examples of pCXIZ-transfected explants. E, F, Examples of pCAFR-1-transfected explants. pCAFR-1-transfected cells were more likely to have anti-phospho-p44/42-immunoreactive nuclei (open arrowheads) than pCXIZ-transfected cells (arrows). G–J, Explants processed for X-Gal staining and histology. G, H, Examples of pCXIZ-transfected explants. I, J, Examples of pCAFR-1-transfected explants. Note the epicardial (arrows, G and H) and sub-epicardial (arrowheads, H) locations of X-Gal+ cells from pCXIZ-transfected heart explants. Note the epicardial (arrows, I and J), intra-myocardial (arrowhead, I), and sub-epicardial (arrowhead, J) locations of X-Gal+ cells from pCAFR-1-transfected heart explants. K, Quantification of the localization of X-Gal+ cells. Solid bars, values for epicardial localization of X-Gal+ cells; grey bars, sub-epicardial localization; open bars, intra-myocardial localization. Error bars, standard deviation of the mean. *, P value <0.005 for pCAFR-1-transfected values relative to pCXIZ-transfected values. Scale bars; C, 10µm; G, 5µm.
DISCUSSION
A number of factors have been identified that are involved in epicardial EMT, including FGFs and TGFβs that can promote EMT (Compton et al., 2006; Morabito et al., 2001) and integrin α4 and VCAM-1 that inhibit EMT (Dettman et al., 2003; Dokic and Dettman, 2006). Studies have also implicated FGFR signaling in epicardial EMT in adult zebrafish hearts (Lepilina et al., 2006; Wills et al., 2008). FGFR-1 and -2 signaling in coronary endothelial cells were reported to be dispensable for coronary development in mouse embryos based on conditional deletion driven by Tie1-Cre and Flk1-Cre (Lavine et al., 2006). It remains a possibility that FGFR-1 or -2 signaling may have had a role before Flk1-Cre and Tie1-Cre are expressed at levels necessary to complete the Cre-mediated recombination. Furthermore, it remains unclear whether FGF expression is affected upon disruption of FGFR-1 and -2 in cardiomyocytes. In this study, we utilized replication-defective retroviral vectors in the avian embryo to better understand the function of FGFR-1 in epicardial and coronary vascular development.
FGFs and FGFRs are involved in many aspects of cardiovascular development, including the specification and differentiation of various lineages through both paracrine and autocrine mechanisms (Poole et al., 2001; Presta et al., 2005; Slavin, 1995). Cardiomyocyte-expressed FGFs have an autocrine effect and a paracrine effect on coronary vascular cells (Fernandez et al., 2000; Mima et al., 1995; Pennisi and Mikawa, 2005; Tomanek et al., 1998). FGF signaling has also been implicated in cardiovascular pathology, as well as being the subject of research for therapeutic intervention (Molin and Post, 2007; Syed et al., 2004). We further defined the role of FGFR-1 in epicardial EMT and identified a requirement upon FGFR-1 for myocardial invasion by epicardium-derived cells. We show that over-expression of FGFR-1 promotes EMT from the epicardium, but is not necessary for EMT as antisense FGFR-1 virus-infected epicardial cells can occupy the sub-epicardium. Therefore, a receptor other than FGFR-1 may play a redundant role in epicardial EMT during development, although such roles for other FGFRs remain to be determined. What have not been identified previously are the factors that determine whether epicardium-derived cells will remain in the sub-epicardium or invade the myocardium. Here, we show that FGFR-1 expression in epicardium-derived cells is important for their ability to invade the myocardium and contribute to the intramural vasculature. In this particular process, it appears FGFR-1 plays a non-redundant role. Identification of a role for FGFR-1 provides a starting point for investigation into what other factors are required for epicardium-derived cells to invade the myocardium.
FGFR-1 displays a spatially restricted pattern of expression in the PE making it difficult to determine whether each of the PE-derived cells express FGFR-1 at some point. The retroviral injection technique employed here does not allow us to specifically infect a sub-population of PE cells, but rather infect a fraction of PE cells indiscriminately. Nevertheless, compared to controls, clear changes were observed in the proportion of test virus-infected PE progeny undergoing epicardial EMT, invading the myocardium, and adopting coronary vascular lineages. This allowed us to analyse the cell-autonomous role of FGFR-1 in PE-derived cells in mosaic embryos, thus avoiding potentially confounding problems associated with whole-tissue gene deletion and embryonic lethality.
Previous studies using quail-chick chimeræ have indicated that epicardium-derived cells contribute to atrio-ventricular cushions and valves (Gittenberger-de Groot et al., 1998; Männer, 1999). Although we focused our attention on the coronary vascular lineages in this study, we did not encounter X-Gal+ cells in the AV or OFT cushions. This apparent discrepancy could be due to the differences between the viral targeting of PE cells used in this study and the chimeric transplantation used in the previous studies. The chimeric technique were typified by i) the inclusion of part of the donor liver primordium, ii) on occasions, the analysis of later-stage embryos, or iii) in some instances, transplanting donor tissue to the inner curvature of recipient hearts rather than adjacent to the sinus venosis. In addition, the viral targeting used in this study will label a portion of PE cells and it is possible that a minor population of PE-derived cells (eg. AV or OFT cushion cells) could go undetected. It is also worth noting that FGFR signaling affects differentiation of other mesoderm-derived lineages, although this was not specifically tested in this study. As we focused on the coronary vascular lineages, the antibodies used will only identify vascular smooth muscle and endothelial cells (and their precursors). It remains unknown whether FGFR signaling occurs in other epicardium-derived cell types and, therefore, we can not rule out that differentiation of other mesoderm-derived lineages is affected. Another unanswered question is whether PE progeny with altered FGFR-1 expression are able to co-express endothelial and smooth muscle markers. Due to technical limitations associated with the antibodies used being mouse IgG molecules, co-staining was not performed. We were, therefore, unable to determine if any X-Gal+ cells co-expressed both markers.
Amongst PE progeny that had been infected with antisense FGFR-1 virus, we did not observe a reduction in the proportion that could undergo epicardial EMT. By contrast, PE explants cultured with SU5402, an inhibitor of all FGFRs, showed a marked inhibition of EMT. This difference may reflect the varied experimental systems or, alternatively, be suggestive of functional redundancy amongst FGFRs in epicardial EMT. As mentioned above, FGFR-1 and FGFR-2 have been shown to act redundantly in the myocardium (Lavine et al., 2006; Lavine et al., 2005). It remains to be determined whether other FGFRs play a redundant role with FGFR-1 in the epicardium and epicardium-derived cells. Retroviral over-expression of FGFR-1 in the embryonic PE and constitutive activation of the FGFR signaling pathway in established epicardial cells of explanted hearts both led to an increase in the number of epicardial cells invading the myocardium. These data are consistent with the idea that FGFR-mediated signaling promotes active EMT and invasion in epicardium-derived cells.
Consistent with previous studies on the timing of coronary vascular smooth muscle recruitment in the developing heart, X-Gal+/SMαA+ cells were not detected in control (and FGFR-1) virus-infected hearts at E7 (Hood and Rosenquist, 1992; Vrancken Peeters et al., 1997). Moreover, SMαA+ cells were never detected amongst the myocardium (the location of myocardial fibroblasts) at stages before overt recruitment of vascular smooth muscle cells to the coronary vasculature, indicating changes in X-Gal+/SMαA+ cells relate to the coronary vascular smooth muscle lineage. We did, however, observe an increase in the proportion of SMαA+ cells in antisense FGFR-1 virus-infected hearts at E7, although, ultimately, the proportion of SMαA+ cells was reduced after knock-down of FGFR-1 by E12. It is not clear why there was a temporary, and seemingly premature, increase in these populations in antisense FGFR-1 virus-infected hearts. One possibility is that proper FGFR-1 expression maintains a progenitor population in an undifferentiated state during coronary development at earlier embryonic stages and that the experimental down-regulation of FGFR-1 led to premature differentiation and exhaustion of the progenitor pool. Indeed, FGFR expression levels (and FGFR-mediated signaling) have been implicated in maintaining the undifferentiated state of cells of contractile lineages until appropriate differentiation. These include FGFR-1 in skeletal muscle (Itoh et al., 1996) and FGFR-2 in lung smooth muscle (De Langhe et al., 2006).
By contrast, we observed a marked increase in the proportion of QH1+ cells in FGFR-1 virus-infected PE progeny, indicating FGFR-1 expression ultimately favours the endothelial lineage over the smooth muscle lineage in PE cells. This notion is consistent with recent studies showing high FGF activity promotes endothelial cell fate (Nakazawa et al., 2006) and FGF2 favours the endothelial lineage over smooth muscle lineage in ES-derived cells (Ishisaki et al., 2003). Lineage tracing studies indicate that the coronary vascular lineages have segregated at the PE stage (Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996). Here we show that altering FGFR-1 signaling affects the differentiation of coronary endothelial and smooth muscle lineages. There are a number of possible mechanisms by which altering FGFR-1 levels in precursors led to a change in the proportion of endothelial and smooth muscle cells: i) by altering the lineage of already segregated coronary vascular precursors or ii) by positive or negative selection for a particular lineage, thus altering their final number. Possible mechanisms by which FGFR signaling may affect the relative numbers of cells of coronary vascular lineages are by differential effects on cell survival and/or proliferation, depending upon which cell type is acted on. Indeed, FGFs and FGFRs have been shown to promote proliferation and survival in numerous cell types (Powers et al., 2000; Slavin, 1995). Yet another possibility is that changing the ultimate destination of PE-derived cells (due to altered FGFR-1 levels) affected coronary lineage differentiation due to a different extracellular environment. It remains a possibility that altering FGFR-1 levels at the stages performed here was too late to alter determination of particular coronary progenitors although differentiation may have been altered. Currently it is not feasible to target the coronary progenitors at stages earlier than the PE stage.
Recently, thymosin β4 signaling in the myocardium has been shown to be necessary for coronary vessel formation in the embryo, which may be related to altered levels of myocardial VEGF (Smart et al., 2007). Adult epicardial cells in culture treated to undergo EMT with thymosin β4, TGFβ1, BMP-2 or myocardin can adapt smooth muscle, endothelial and fibroblastic phenotypes, highlighting the therapeutic potential of mobilised epicardial cells (Smart et al., 2007). The data presented here further our understanding of the molecular mechanisms that govern epicardial EMT, myocardial invasion, and coronary lineage differentiation. They show that FGFR-1 plays a redundant role in epicardial EMT and a non-redundant role in myocardial invasion. Furthermore, precise regulation of FGFR-1 levels in PE-derived cells is required for proper coronary vascular lineage differentiation. Moreover, they reveal an uncoupling of the molecular processes that govern epicardial EMT and myocardial invasion. This may open avenues for development of cellular therapies for coronary artery diseases based on the ability to mobilise epicardial cells and, potentially, control coronary lineage differentiation in vivo.
In summary, the key findings of this study are:
FGFR-1-mediated signaling is sufficient but not necessary for epicardial EMT
FGFR-1-mediated signaling is necessary for epicardium-derived cells to invade the myocardium
FGFR-mediated signaling can modulate coronary vascular cell fate/differentiation in the developing heart
Supplementary Material
ACKNOWLEDGMENTS
The myrR1-TDII plasmid was a kind gift from Dr Daniel Donoghue, University of California San Diego. The monoclonal antibody, QH1, developed by Dr F. Dieterlen-Lièvre, was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences. We are grateful to Dr Diane Maresco-Pennisi for critical reading of this manuscript. This work was supported by the NIH and the American Heart Foundation. DJP was a Charles H. Revson Foundation Postdoctoral Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bernanke DH, Velkey JM. Development of the coronary blood supply: changing concepts and current ideas. The Anatomical Record. 2002;269:198–208. doi: 10.1002/ar.10139. [DOI] [PubMed] [Google Scholar]
- Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-β induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Developmental Dynamics. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Warburton D, Hajihosseini MK, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Developmental Biology. 2006;299:52–62. doi: 10.1016/j.ydbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental Biology. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- Dettman RW, Pae SH, Morabito C, Bristow J. Inhibition of α4-integrin stimulates epicardial-mesenchymal transformation and alters migration and cell fate of epicardially derived mesenchyme. Developmental Biology. 2003;257:315–328. doi: 10.1016/s0012-1606(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Dokic D, Dettman RW. VCAM-1 inhibits TGFβ stimulated epithelial-mesenchymal transformation by modulating Rho activity and stabilizing intercellular adhesion in epicardial mesothelial cells. Developmental Biology. 2006;299:489–504. doi: 10.1016/j.ydbio.2006.08.054. [DOI] [PubMed] [Google Scholar]
- Estival A, Monzat V, Miquel K, Gaubert F, Hollande E, Korc M, Vaysse N, Clemente F. Differential regulation of fibroblast growth factor (FGF) receptor-1 mRNA and protein by two molecular forms of basic FGF. Modulation of FGFR-1 mRNA stability. Journal of Biological Chemistry. 1996;271:5663–5670. doi: 10.1074/jbc.271.10.5663. [DOI] [PubMed] [Google Scholar]
- Fernandez B, Buehler A, Wolfram S, Kostin S, Espanion G, Franz WM, Niemann H, Doevendans PA, Schaper W, Zimmermann R. Transgenic myocardial overexpression of fibroblast growth factor-1 increases coronary artery density and branching. Circulation Research. 2000;87:207–213. doi: 10.1161/01.res.87.3.207. [DOI] [PubMed] [Google Scholar]
- Flamme I, Frölich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. Journal of Cellular Physiology. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation Research. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hart KC, Robertson SC, Kanemitsu MY, Meyer AN, Tynan JA, Donoghue DJ. Transformation and Stat activation by derivatives of FGFR1, FGFR3, and FGFR4. Oncogene. 2000;19:3309–3320. doi: 10.1038/sj.onc.1203650. [DOI] [PubMed] [Google Scholar]
- Hatcher CJ, Diman NY, Kim MS, Pennisi D, Song Y, Goldstein MM, Mikawa T, Basson CT. A role for Tbx5 in proepicardial cell migration during cardiogenesis. Physiological Genomics. 2004;18:129–140. doi: 10.1152/physiolgenomics.00060.2004. [DOI] [PubMed] [Google Scholar]
- Hiruma T, Hirakow R. Epicardial formation in embryonic chick heart: computer-aided reconstruction, scanning, and transmission electron microscopic studies. American Journal of Anatomy. 1989;184:129–138. doi: 10.1002/aja.1001840204. [DOI] [PubMed] [Google Scholar]
- Ho E, Shimada Y. Formation of the epicardium studied with the scanning electron microscope. Developmental Biology. 1978;66:579–585. doi: 10.1016/0012-1606(78)90263-4. [DOI] [PubMed] [Google Scholar]
- Hood LC, Rosenquist TH. Coronary artery development in the chick: origin and deployment of smooth muscle cells, and the effects of neural crest ablation. The Anatomical Record. 1992;234:291–300. doi: 10.1002/ar.1092340215. [DOI] [PubMed] [Google Scholar]
- Hyer J, Johansen M, Prasad A, Wessels A, Kirby ML, Gourdie RG, Mikawa T. Induction of Purkinje fiber differentiation by coronary arterialization. Proceedings of the National Academy of Sciences USA. 1999;96:13214–13218. doi: 10.1073/pnas.96.23.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Langberg JD, Hurtado R, Lee S, Mikawa T. Induction of proepicardial marker gene expression by the liver bud. Development. 2007;134:3627–3637. doi: 10.1242/dev.005280. [DOI] [PubMed] [Google Scholar]
- Ishisaki A, Hayashi H, Li AJ, Imamura T. Human umbilical vein endothelium-derived cells retain potential to differentiate into smooth muscle-like cells. Journal of Biological Chemistry. 2003;278:1303–1309. doi: 10.1074/jbc.M207329200. [DOI] [PubMed] [Google Scholar]
- Itoh N, Mima T, Mikawa T. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development. 1996;122:291–300. doi: 10.1242/dev.122.1.291. [DOI] [PubMed] [Google Scholar]
- Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Pérez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Developmental Biology. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes and Development. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Developmental Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Manasek FJ. Embryonic development of the heart. II. Formation of the epicardium. Journal of Embryology and Experimental Morphology. 1969;22:333–348. [PubMed] [Google Scholar]
- Männer J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. The Anatomical Record. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Borisov A, Brown AM, Fischman DA. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus: I. Formation of the ventricular myocardium. Developmental Dynamics. 1992;193:11–23. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proceedings of the National Academy of Sciences USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA, Dougherty JP, Brown AM. In vivo analysis of a new lacZ retrovirus vector suitable for cell lineage marking in avian and other species. Experimental Cell Research. 1991;195:516–523. doi: 10.1016/0014-4827(91)90404-i. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental Biology. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- Mima T, Ueno H, Fischman DA, Williams LT, Mikawa T. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proceedings of the National Academy of Sciences USA. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin D, Post MJ. Therapeutic angiogenesis in the heart: protect and serve. Current Opinion in Pharmacology. 2007;7:158–163. doi: 10.1016/j.coph.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Developmental Biology. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Developmental Dynamics. 2003;227:511–523. doi: 10.1002/dvdy.10335. [DOI] [PubMed] [Google Scholar]
- Nakazawa F, Nagai H, Shin M, Sheng G. Negative regulation of primitive hematopoiesis by the FGF signaling pathway. Blood. 2006;108:3335–3343. doi: 10.1182/blood-2006-05-021386. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Altmann C, Kitos P, Dieterlen-Lièvre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Patstone G, Pasquale EB, Maher PA. Different members of the fibroblast growth factor receptor family are specific to distinct cell types in the developing chicken embryo. Developmental Biology. 1993;155:107–123. doi: 10.1006/dbio.1993.1011. [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, Ballard VL, Mikawa T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Developmental Dynamics. 2003;228:161–172. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, Mikawa T. Normal patterning of the coronary capillary plexus is dependent on the correct transmural gradient of FGF expression in the myocardium. Developmental Biology. 2005;279:378–390. doi: 10.1016/j.ydbio.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Pérez-Pomares JM, Macías D, García-Garrido L, Muñoz-Chápuli R. The origin of the subepicardial mesenchyme in the avian embryo: an immunohistochemical and quail-chick chimera study. Developmental Biology. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- Poole TJ, Finkelstein EB, Cox CM. The role of FGF and VEGF in angioblast induction and migration during vascular development. Developmental Dynamics. 2001;220:1–17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine and Growth Factor Reviews. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Saito H, Kasayama S, Kouhara H, Matsumoto K, Sato B. Up-regulation of fibroblast growth factor (FGF) receptor mRNA levels by basic FGF or testosterone in androgen-sensitive mouse mammary tumor cells. Biochemical and Biophysical Research Communications. 1991;174:136–141. doi: 10.1016/0006-291x(91)90496-t. [DOI] [PubMed] [Google Scholar]
- Schlueter J, Männer J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Developmental Biology. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Slavin J. Fibroblast growth factors: at the heart of angiogenesis. Cell Biology International. 1995;19:431–444. doi: 10.1006/cbir.1995.1087. [DOI] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Syed IS, Sanborn TA, Rosengart TK. Therapeutic angiogenesis: a biologic bypass. Cardiology. 2004;101:131–143. doi: 10.1159/000075994. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Lotun K, Clark EB, Suvarna PR, Hu N. VEGF and bFGF stimulate myocardial vascularization in embryonic chick. American Journal of Physiology. 1998;274:H1620–H1626. doi: 10.1152/ajpheart.1998.274.5.H1620. [DOI] [PubMed] [Google Scholar]
- Virágh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. The Anatomical Record. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- Virágh S, Gittenberger-de Groot AC, Poelmann RE, Kálmán F. Early development of quail heart epicardium and associated vascular and glandular structures. Anatomy and Embryology. 1993;188:381–393. doi: 10.1007/BF00185947. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Krieg PA. Endoderm is required for vascular endothelial tube formation, but not for angioblast specification. Development. 2002;129:775–785. doi: 10.1242/dev.129.3.775. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Hungerford JE, Little CD, Poelmann RE. Differences in development of coronary arteries and veins. Cardiovascular Research. 1997;36:101–110. doi: 10.1016/s0008-6363(97)00146-6. [DOI] [PubMed] [Google Scholar]
- Vrancken Peeters MP, Mentink MM, Poelmann RE, Gittenberger-de Groot AC. Cytokeratins as a marker for epicardial formation in the quail embryo. Anatomy and Embryology. 1995;191:503–508. doi: 10.1007/BF00186740. [DOI] [PubMed] [Google Scholar]
- Wills AA, Holdway JE, Major RJ, Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135:183–192. doi: 10.1242/dev.010363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.