Abstract
Dupuytren’s Disease (DD) is a common, fibroproliferative disorder affecting the palmar surface of the hands which is often irreversible and progressive. Understanding the epidemiology of DD is important in order to provide clues to its etiopathogenesis. This review aims to evaluate the epidemiological studies carried out in DD since 1951. Studies evaluating the epidemiology of DD were searched using Medline, Pubmed, and Scopus which dated back from 1951 to current date. Inclusion criteria were any studies investigating the prevalence or incidence of DD in any population group. A total of 620 articles were cited. Forty-nine studies were subsequently identified as relevant to evaluating the epidemiology of DD. The prevalence of DD in all studies increased with age with a male to female ratio of approximately 5.9:1. Prevalence rates ranged from 0.2% to 56% in varying age, population groups, and methods of data collection. The highest prevalence rate was reported in a study group of epileptic patients. Although, only one study calculated the incidence (as opposed to prevalence) of DD to be equal to 34.3 per 100,000 men (0.03%). In conclusion, the prevalence of DD in different geographical locations is extremely variable, and it is not clear whether this is genetic, environmental, or a combination of both. The majority of the prevalence studies have been conducted in Scandinavia or the UK, and the vast changes in population structure, the changes in prevalence of associated diseases, and the change in diagnostic criteria of DD makes understanding the epidemiology of this condition difficult.
Keywords: Dupuytren’s disease, Epidemiology, Etiology, Prevalence
Introduction
Dupuytren’s Disease (DD) is a common, fibroproliferative disorder affecting the palmar surface of the hands which can present itself as a clinically challenging disorder for the patient and the surgeon alike. The disease is often progressive, irreversible, and commonly bilateral. DD can be a psychosocially and physically disabling condition which can also have a significant impact on healthcare economy [65]. It is, therefore, considered important to time surgical operative intervention appropriately [45, 59]. The disease is thought to involve abnormal tissue contraction, shown to be mediated by the myofibroblast in the palmar fascia causing a digital flexion deformity [101]. Therefore, knowledge of the exact causation of DD may provide clues to mechanism of DD as well as other fibrotic disorders. In addition, understanding the epidemiology of DD is important in order to provide clues to its etiopathogensis.
By looking at the medical literature, it soon becomes apparent that the epidemiology of DD has been previously studied extensively albeit only in limited geographical areas [23]. There has been a much quoted concept of DD being labeled as the “Vikings” or “Nordic” disease. Other than the common prevalence of DD in Scandinavia, no objective scientific evidence has been found to date to support and substantiate the “Nordic” origin of the disease.
In addition, with the exception of a high prevalence rate in Northern European communities, no actual genetic factors have implicated DD as having arisen in the Scandinavian population [23, 29]. The global presence of DD in most countries suggests that the origin and spread of the disease was much earlier than previously speculated and patterns of migration have led to a hypothesis that DD is a genetic disease [69].
DD is found to be most prevalent in Northern European Caucasians [8] where it is one of the most commonly inherited connective tissue disorders with a prevalence reaching 30% in the Norwegian population aged over 60 years [15]. In contrast the prevalence rate of DD is reported to be just over 4% in the male population in England [26]. These particular prevalence rates have been quoted in many previous epidemiological studies.
DD is a condition which has been linked to many risk factors including a history of smoking [14], alcohol consumption [77], frozen shoulder [95], epilepsy [71], diabetes mellitus [6], carpal tunnel syndrome [11], history of manual labor [21], and hand injury [47, 68]. All of these reports are controversial and frequently delivered based on selective data. Factors associated with increased severity include male gender and a young age at onset, which are often reported although the evidence appears to be weak [45]. With an evolving population these prevalence values may or may not be accurate in relation to the present day. It is likely that the population structure will be quite different today than that of 50 years ago. Increasing changes in the environment, working patterns and social structure may also have a greater impact on the observed changes in the epidemiology of DD.
This review aims to assess in detail the epidemiology of DD since by evaluating all published literature with reference to DD epidemiology. The knowledge gained from this review will provide better clues to the reported epidemiological trends, relevance of genetic and environmental factors as well as associated factors implicated in the evolution of this enigmatic disorder.
Identification of Epidemiological Studies
Studies evaluating the epidemiology of DD were searched using Medline and subsequent cross referencing to earlier articles. The following keywords were used to cite relevant articles: Dupuytren’s, disease, contracture, history, population, prevalence, incidence, and epidemiology. The search included all case reports, letters, communications, prospective, and retrospective studies. Inclusion criteria were based on incorporating any study investigating the prevalence or incidence of DD in any population group. Studies were excluded if they limited their data to a known DD cohort.
A total of 620 articles were cited and reviewed. Forty-nine studies since 1951 were identified as relevant studies investigating the epidemiology of DD. A relevant study was one that calculated a prevalence or incidence rate of DD. These studies were categorized as cross-sectional, longitudinal, observational, review, or cohort, and the results tabulated (Table 1). The study setting was noted, e.g., to see if patients were examined as inpatients or outpatients. In addition to this, the data collector and the person who examined subjects were tabulated to enable evaluation of accurate disease diagnosis.
Table 1.
Population studies.
| Author | Date | Geographical Location | Study Design | Data Collector | Prevalence or incidence rate | Age group | Presence of heritability | Significant risk factors | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Male | Female | |||||||
| Herzog EG [43] | 1951 | North England | Cross-sectional study of 1,434 manual laborers and clerks | Author | 4.25% | 4.25% | – | >40 years | – | No mention | No significant difference in prevalence of DD in manual workers (4.5%) and clerks (3.8%). |
| Gordon S [38] | 1954 | Canada | Cross-sectional study of 2,705 hospital inpatients | Author | – | 26% | 33% | 66–75 years | 66–75 years | No mention | No mention |
| Yost J [105] | 1955 | New York, USA | Cross-sectional study of 5,062 hospital inpatients | Author | 3.4% | 4.3% | 2.3% | 37–100 years | 37–100 years | One case with definite family history—early age at onset in offspring. | No significant correlation with hand trauma. |
| Hueston JT [46] | 1960 | Australia | Cross-sectional study of 3,700 people in community | Author | – | 23% | 22% | >60 years | >60 years | No mention | No mention |
| Early PF [26] | 1962 | Lancashire, UK | Cross- sectional study of 6,979 people in community. | Author | 3.5% | 3.8% | 2.3% | 15 to >75 years | 15 to >75 years | No mention | Strong association between epilepsy. No relationship between DD and manual work. |
| Mikkelson OA [74] | 1972 | Haugesund, Norway | Cross- sectional study of 16,000 inpatients | Author/Surgeon | – | 9.4% | 2.8% | >16 years 70 to 74 years = 36.8% (max prev’) | >16 years 85 to 89 years = 25% (max prev’) | No mention | No mention |
| Critchley EM [21] | 1976 | Preston, UK | Cross-sectional study of chronic epileptics | Author | 56% | 57% | 56% | 35 to >75 years | 35 to >75 years | No mention | Link to anti-convulsants to DD due to peripheral stimulation of growth factors |
| Stuhler T [24] | 1977 | Germany | Cross-sectional study of 524 epileptics | Author | 21.6% | – | – | – | – | Genetic aspect must be taken into consideration | Idiopathic epilepsy is most associated with DD |
| Ravid M [43] | 1977 | Israel | Cross-sectional study of 959 diabetics | Author | 17.6% | – | – | – | – | No mention | Increased risk of DD in older diabetic patients |
| Mackenney RP [61] | 1983 | Cotswolds and Chilterns, England | Cross- sectional study of 919 patients attending the orthopedic clinic | Surgeon | – | 5% | 3.5% | – | – | No mention | Positive correlation between stretching of palm and DD |
| Arafa M [4] | 1984 | Manchester, UK | Cross-sectional study of 392 patients with Rheumatoid Arthritis (RA) | Rheumatologist | 6.4% with RA. 16% without RA | 7.6% with RA. 16.1% without RA | 5.7% with RA. 15.9% without RA | 30 to >70 years | 30 to >70 years | Genetic factor in RA as a protective factor against DD? | No mention |
| Noble J [78] | 1984 | UK | Cross-sectional study of 273diabetic patients | Authors (physician and hand surgeon) | 18% when examined by physician, 42% when examined by hand surgeon | 25% when examined by physician, 41% when examined by hand surgeon | 13% when examined by physician, 42% when examined by hand surgeon | >16 years | >16 years | No mention | Increased risk of DD in diabetic patients |
| Egawa T [28] | 1985 | Japan | Cross sectional study of 3,383 patients attending orthopedic clinic | Author | 1.8% | – | – | 40–59 years | 40–59 years | No mention | No mention |
| Laplane D [51] | 1985 | France | Cross-sectional study of 197 epileptic patients | Author | 8% | – | – | – | – | No mention | No evidence to suggest increased risk of DD in epileptics. |
| Stewart HD [98] | 1985 | UK | Cross-sectional study of 235 patients with Colles’ fracture | Author | 6.8% | – | – | 18–86 years | 18–86 years | No mention | Mild disease in patients who developed DD after fracture. No DD progression after 20 months. |
| Patri B [82] | 1986 | France | Cross-sectional study of 155 patients | Author | 9% | - | - | 20-90 years | 20-90 years | No mention | Increased prevalence of DD with increasing age |
| Guitian AQ [42] | 1988 | Spain | Cross sectional study of 1,455 undergoing general medical examinations | Author | 9.9% | – | – | 45–54 years | 45–54 years | No mention | No mention |
| 25.5% | >75 years | >75 years | |||||||||
| Eadington DW [24] | 1989 | Edinburgh, UK | Cross-sectional study of 266 type 1 diabetic patients | Author | 24% smokers | – | – | 36–84 years | 36–84 years | No mention | Increased risk of DD in diabetics and smokers |
| Egawa T [93] | 1990 | Japan | Cross sectional study of 1,154 nursing home patients | Author | – | 19.7% | 9% | Over 60 years | Over 60 years | No mention | No mention |
| French PD [33] | 1990 | London, UK | Cross-sectional study of 50 male AIDS patients | Genito-Urinary Physician | – | 6% | – | 23–56 years | – | No mention | No significance of HIV on DD. |
| Bower M [13] | 1990 | London, UK | Cross-sectional study of 50 male HIV infected patients | Physician | – | 36% | – | 19–54 years | – | No mention | Significant impact of HIV on DD. |
| Eadington DW [25] | 1991 | Edinburgh, UK | Cross-sectional study of 200 type 2 diabetic patients | Experienced observer | 24% | – | – | 36–84 years | 36–84 years | No mention | Increased risk of DD in diabetes and in smokers |
| Thomas PR [100] | 1992 | Middlesbrough, UK | Cross-sectional study of 500 claimants with vibration white finger. | Author | 13.6% | – | – | >45 years | >45 years | No mention | Positive correlation between DD and vibration white finger. |
| Arafa M [3] | 1992 | Manchester, UK | Cross-sectional study of 484 patients in 2 epileptic centers | Author | 12% and 38% | 13% and 44% | 11% and 28% | <30 to >70 years | <30 to >70 years | No mention | Increased prevalence of DD with increasing age. No evidence to link DD and epilepsy |
| Noble J [77] | 1992 | Manchester UK | Cross-sectional study of 50 patients with liver disease | Author | 28% | – | – | <30 to 70 years | <30 to 70 years | No mention | Increased risk of DD in patients with liver disease |
| Kelly SA [47] | 1992 | Derby, UK | Cross-sectional study of 235 patients with Colles’ fracture | Author | 8.5% | – | – | 47–79 years | 47–79 years | 15% had a positive family history | 25% consumed large amounts of alcohol; 5% taking anticonvulsant |
| Lennox IAC [52] | 1993 | Aberdeen, Scotland | Cross-sectional study of 200 nursing home patients over 60 years. | Author | 30% | 39% | 21% | >60 years | >60 years | No mention | No mention |
| Bergenudd H [10] | 1993 | Malmo, Sweden | Longitudinal study of 574 people in community | Author | 6% | 10% | 2% | 55 years | 55 years | No mention | Non except poor peripheral pulses. |
| Bovenzi M [12] | 1994 | Italy | Cross-sectional study of 570 quarry drillers and stone carvers | Author | 10% | – | – | Mean age—39 years | – | No mention | Increased risk in vibration exposed manual workers |
| Renard E [88] | 1994 | France | Cross-sectional study of 120 diabetic patients | Author | 35% type 1, 30% type 2 diabetes | – | – | – | – | No mention | Increased prevalence of DD in diabetics |
| Chammas M [18] | 1995 | France | Cross-sectional study of 120 diabetic patients | Author | 35% type 1 diabetics, 28% type 2 | – | – | – | – | No mention | Increased prevalence of DD in diabetics, risk increases with longer duration of diabetes. |
| Arkilla PE [7] | 1996 | Finland | Longitudinal study of 207 type 1 diabetic patients | Author | 4% | – | – | 21–39 years | 21–39 years | No mention | DD risk increases with patients age and duration of DM. |
| Dasgupta AK [20] | 1996 | India | Cross-sectional study of manual workers | Author | 4% | – | – | >21 years | – | No mention | Vibration exposure increases risk of DD. |
| Arkilla PE [6] | 1997 | Finland | Cross-sectional study of 139 type 2 diabetic patients | Author | 14% | – | – | 49–73 years | 49–73 years | No mention | DD risk greater in diabetic patients. Prevalence equal in males and females. |
| Gudmundsson KG [41] | 1999 | Reykjavik, Iceland | Cohort study of 2,165 inpatients | Physician | 13.3% | 19.2% | 4.4% | 45–74 years | 45–74 years | No mention | Significant association between DD and manual work, smoking, fasting glucose level. |
| Gudmundsson [40] | 2000 | Reykjavik, Iceland | Cohort study of 1,297 males in the community. | Physician | – | 19.2% | – | 45–74 years | – | No mention | Negative association between DD and joint complaints. |
| Kilian O [49] | 2001 | Germany | Cross-sectional study of 172 patients with shoulder pathology | Author | 2.9% | – | – | – | – | No mention | Similar histology in DD and frozen shoulder |
| Smith SP [95] | 2001 | UK | Cross-sectional study of 58 patients with frozen shoulder | 3 surgeons | 52% | – | – | Mean 54.9 years | Mean 54.9 years | 7% of people with DD had first degree relative with DD. | Increased prevalence of DD in frozen shoulder patients |
| Omari A [79] | 2001 | UK | Cross-sectional study of 75 patients with frozen shoulder | Author | 1.3% | – | – | – | – | No mention | No mention for DD |
| Finsen V [31] | 2002 | Finnmark, Norway | Cross-sectional study 456 people in community. | Surgeon | 7.5% | 11.9% | 1.5% | >50 years | >50 years | Higher prevalence in family members in same niche | No mention |
| White HA [103] | 2003 | Wales | Observational study of 197 inpatients. | Physician | 6.6% | – | – | 19–101 years | 19–101 years | No mention | No mention |
| Ardic F [5] | 2003 | Turkey | Cross-sectional study of 78 type II diabetics | Author | 21.8% | – | – | 46–70 years | 46–70 years | No mention | Diabetes is risk factor for DD |
| Zerajic D [108] | 2004 | Bosnia | Cross-sectional study of 1,207 people in community appearing to look over 50 years. | Medical student | 25% | 31% | 16% | >50 years | >50 years | No mention | Significant higher prevalence in diabetics. |
| Geohagan JM [35] | 2004 | West Midland, UK | Cross-sectional study of 383,000 GP patients. | GP | 0.2% | 0.15% | 0.05% | 24–97 years | 24–97 years | No mention | Diabetes is a strong risk factor for DD |
| Khan AA [48] | 2004 | Oxford, UK | Review of National Morbidity Survey of 502,493 men | GP | Incidence rate 34.3 per 100,000 men | – | – | 40–84 years | – | No mention | No mention |
| Godtfredsen NS [36] | 2004 | Denmark | Cohort study of 7,254 subjects | Trained nurse/med student | 11% | 16% | 7% | 20–93 years | 20–93 years | No mention | Alcohol and smoking risk factors |
| Logan AJ [56] | 2005 | UK | Questionnaire survey of 1,100 climbers | Patient | 19.5% | 19.5% | – | 23–93 years | – | No mention | No mention |
| Burke FD [16] | 2007 | UK | Cross-sectional study of 97,537 miners | Trained doctors | 8.1% | 8.1% | – | 25–99 years | – | No mention | Increased risk of DD in diabetics and increased age |
| Lucas G [58] | 2008 | France | Cross-sectional study of 2,406 male civil servants. | Physician | – | 8.8% | – | Mean age 50.7 years | – | 20% had family history of DD | Manual work exposure appears to be associated with DD |
Prevalence or incidence rates for each study were tabulated and, where possible, compared between males and females with reference to age groups within each population. Familial aggregation which has been confirmed in DD [69] is considered to be important in relation to the epidemiology of DD; therefore, heritability data was explored in each study. Risk factors (e.g. diabetes and smoking) for DD investigated in each study were also examined and tabulated.
Review of Significant Studies
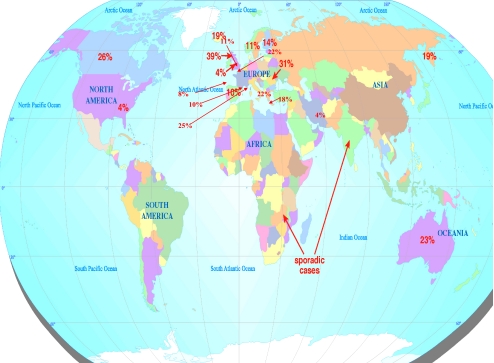
The 49 studies identified and tabulated (Table 1) in this study dates back from 1951 to 2008 covering a range of countries from North America to East Asia and Australasia (Fig. 1). Forty-one studies were cross-sectional studies with three cohort, two longitudinal, one observational study, one questionnaire survey, and one review. The prevalence rate was calculated in 48 studies with only one study presenting an incidence rate, where numbers of new cases of DD were calculated over a period of time. The incidence rate of DD for the British population in 2004 was calculated as 34.4 per 100,000 men between the ages of 40 and 84 years with a gradual increase in incidence with increasing age [48].
Figure 1.
Prevalence rates of DD across the globe.
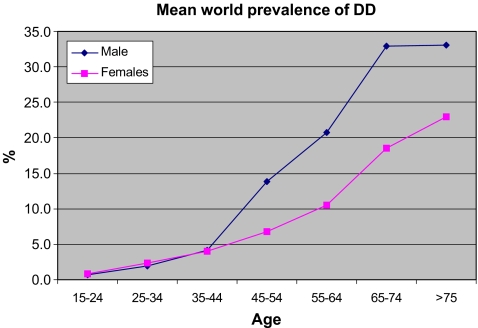
In order to better understand the distribution of the disease, our findings were further subclassified into age and gender. Prevalence rates of DD ranged from 0.2% to 56% in varying age groups and depended on methods of data collection. The prevalence of DD increased with increasing age, a similar finding in many studies (Table 1, Figs. 1 and 2). The highest prevalence rate (56%) was seen in a study group of epileptic [19] patients.
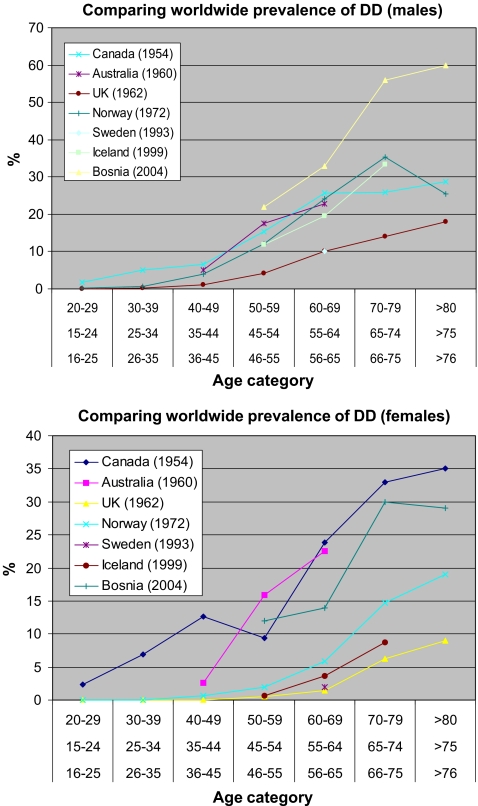
Figure 2.
Comparing worldwide prevalence rates in i males and ii females. The diagnosis of DD in each study was standard with authors having the criteria of identifying nodules, cords, and digit contracture.
Geohagan et al. in 2004 calculated the prevalence of DD for patients between the ages of 24 and 97 years of age in the West Midlands, UK [35]. The prevalence rate for a population of 383,000 was 0.2%. This, however, was not based on clinical examination of every subject but was dependent on the coding for DD within general practice databases. Although it included both sexes and all ages, it provided little information on the distribution of the disease. This may have been due to the fact that the coding was not done accurately as the coding term and coding numbers were not specific enough. We know that in the UK, there are a number of different generic codes for DD including the umbrella term musculoskeletal disorders.
Six studies [16, 26, 45, 58, 73, 99] related the epidemiological findings to the heritable nature of the disease. Finsen in 2002 noted that there was a higher prevalence of DD in family members living within the same geographical area as their diseased relative. A further genetic component of relevance to the epidemiology of DD was noted in the cross-sectional study from Manchester, UK carried out in 1984 which examined the hands of 392 patients with rheumatoid arthritis [4]. This study noted that there was a significantly reduced prevalence rate of DD in those with a diagnosis of rheumatoid arthritis, suggesting a genetic protective factor against the disease [4]. Yost in 1955 identified one family of DD with a comment on the development of early age at onset of DD in that familial case [105].
Various studies have examined the epidemiology of DD and also attempted to solve its etiological mystery. Herzog [43] in 1951 and Early [26] in 1962 concluded that there was no significant difference in the prevalence of DD in manual and non-manual workers. A similar result was concluded by Yost [105] in 1955 with a negative correlation between DD and hand trauma. Contrary to these findings, Gudmundsson [40] in 2000 concluded a significant association between manual work and DD while Thomas [100] in 1992 found a positive correlation between vibration white finger and DD. A more recent study to determine whether DD is more prevalent from repetitive trauma found that rock climbing increases the risk of disease development [56].
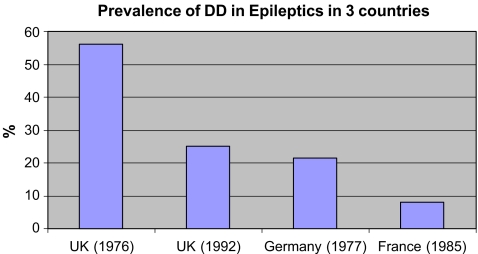
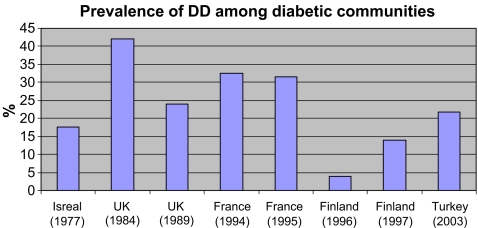
A similar scenario exists in the prevalence of DD and HIV infected patients, with conflicting data on the significance of HIV in the etiology of DD [13, 32]. There was found to be a high prevalence rate of DD among HIV infected patients. There is however more consistent data in the findings for a link between DD, diabetes mellitus and epilepsy, with many studies finding a higher prevalence of DD in the diabetic population [5, 18, 24, 25, 78, 87, 88, 103, 108]. We compared the prevalence of DD in global epileptic (Fig. 5) and diabetic cohorts (Fig. 6) and found all studies showed a positive correlation between DD, epilepsy and diabetes.
Figure 5.
Comparison of prevalence of DD in epileptic communities in three different countries.
Figure 6.
Prevalence rates of DD in diabetic communities. Note that prevalence of DD in study conducted in Finland (1996) examined patients between the ages of 21 and 39 years. Other studies conducted included a wide age range between 30 and >70 years.
We compared the prevalence rates of DD in different parts of the world (Fig. 2). Studies compared, were ones which calculated prevalence rates for various age categories. Of the seven studies compared, the highest prevalence in males was seen in Bosnia and in Canada for females. The high numbers of affected individuals in both of these studies suggest the diagnostic criteria may be different. Generally the prevalence of DD increases with age (Figs. 2 and 3). From all the reported studies the prevalence in males and females is similar up to 45 years of age after which the rate is significantly greater in males (Fig. 3).
Figure 3.
Comparison of prevalence of DD between males and females. Values calculated are mean values from Fig. 2.
Prevalence Rates Calculated in a Clinical and Community Setting
Of the 49 studies evaluated, 12 were conducted in a hospital setting, two examining the prevalence rates of DD in a nursing home, and 37 studies carried out in the community. Studies carried out in the nursing home identified a higher prevalence rate of DD compared to those in a hospital or community setting. Prevalence from the community studies on the whole identified a higher prevalence rate than those conducted in a hospital setting (Table 1). The exception to this was the study conducted by Geohagan [35] in 2004 where a very low prevalence rate was calculated.
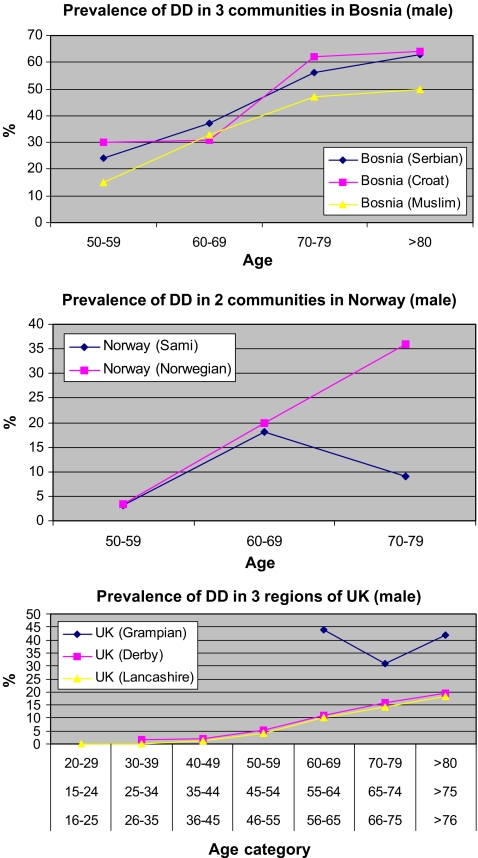
The differences in the findings can be potentially based upon the diagnostic criteria used by the various groups. The prevalence of DD in one country among different communities or regions may differ considerably (Fig. 4). We have already seen a high prevalence of DD in Bosnia, there is a lower prevalence in the Bosnian Muslim community compared to the Serbian and Croat cohort. Similarly in Norway, after 60 years of age the prevalence of the disease in the Norwegian community is significantly greater when compared to that of the Sami population. In the UK it appears that a higher reported prevalence is much higher in Scotland when compared to England. Although, again, this may reflect on the use of different diagnostic criteria in different regions (Fig. 4).
Figure 4.
Comparison of prevalence of DD in different communities/regions in i Bosnia, ii Norway, and iii UK. The results in Norway and Bosnia are likely to be real differences in rates of DD as the patients have been examined by one author. However, in the UK, the differences may be as a result of different diagnostic criteria.
Prevalence Rates and its Relation to Assessor of Disease
Of the 49 studies represented in Table 1, not all cases of DD were identified by experienced clinicians. The study by Logan et al. [56] in 2005 identified a high rate of DD in rock climbers; however, diagnosis of DD was made by patients via a questionnaire survey. A high rate of DD identified in the Bosnian community by Zerajic [108] in 2004 may have resulted from the diagnosis of DD made by a junior clinician or non hand surgical specialist. On the contrary, diagnosis made by a medical student or a nurse in a Danish study [36] identified a prevalence rate of 11%. However, this finding may be an underestimate. Diagnosis is likely to be underestimated when it is not made by a specialist hand surgeon. This is highlighted in a study where the prevalence rate of DD was 18% when patients were examined by a physician and 42% when examined by a hand surgeon [78].
Cases of DD in Other Atypical Geographical Locations
As we have seen from Table 1 and Fig. 1, DD appears to be most prevalent in Caucasian males, but not necessarily of Northern European extraction. There are several other reports of cases of DD around the world where one would not expect to find the disease [27]. Sladicka, Plasse, and Zaworski identified DD in an African–American patient [85, 94, 106]; these cases were completely sporadic with no evidence of familial clustering or interracial marital relationships. The patient reported by Zaworski in 1979 did, however, have epilepsy and worked as a manual laborer [106].
Similar sporadic cases were identified by Furnas in East Africa in 1979 [34], by Mennen, Richard-Kadio, and Aladin in 1990 and 2001, respectively, in an African patient [2, 70, 90] and by Maes and Pai in 1979 and 1994 in Taiwan [62, 80]. It appears that disease progression and intraoperative findings are similar in African–American and Northern European patients [37]. Muguti in 1993 identified DD in four indigenous Zimbabwean patients [76]. Three cases were male and one female. There was a history of manual trauma in the male patients and a history of epilepsy in the female patient. None of the cases had a family history of DD [76].
Liu in 1991 identified 41 cases of DD in the Oriental patient between 1970 and 1988 with 35 cases undergoing fasciectomy. There was no mention of familial clustering in this case series [55]. A similar case series has been reported by Vathana [102]. Srivasta in 1989 reported a series of ten cases of DD operated on in a UK hospital, with all ten cases being male (age range 45–68 years) originating from the Indian subcontinent [96]. None of the cases had a positive family history, three had recurrent disease, and eight out of ten had a history of repetitive hand trauma. This important publication raises questions of a possible long term environmental factor. Mitra reviewed 23 patients with DD of non-Caucasian origin and commented on these patients having a less extensive degree of DD diathesis [75]; again, in this series of patients, there was no mention of family history.
The majority of the studies reviewed have been those within the English literature. Although this review provides an exhaustive geographical representation of the epidemiology of DD, it may not be complete.
Epidemiology of DD and its Relationship to Age and Gender
We have seen from the population studies conducted over the last half century that DD increases with age (Table 1, Figs. 1 and 2). Interestingly, DD has also been frequently recognized in the younger population [86]. The diagnosis of DD is not a common finding in pediatric practice. Differential diagnosis made in a child who presents with a nodule in the hand, contracture of a digit, or a joint knuckle pad on the dorsum of the hand [64, 81] would include sarcoma of the upper limb [89]. A non-familial, sporadic case of DD has been identified in a child as young as 6 months old [9] which may suggest a new genetic mutation(s) responsible for disease development.
The majority of studies have found that DD is more prevalent in the male population (Table 1, Fig. 2), with a male to female ratio of approximately 5.9:1 [104]. There appears to be a significantly stronger genetic element of DD in women with familial cases of the disease predominantly in women [44, 66].
Incidence and Prevalence of DD
Two key aspects of DD epidemiology in terms of its incidence and prevalence rates are of interest. Incidence rate is defined as the number of new cases of a disease over a specified time period as a percentage of the population as opposed to the prevalence rate which is the current number of cases of disease at a single point time. This review has shown that most studies that have quoted “incidence” rates were in fact referring to “prevalence” rates. Interestingly, only one study to date has calculated an actual incidence rate of DD [48] which identified an increasing incidence with increasing age.
Comparing Epidemiological Data for DD from Different Sources
Each study has provided evidence for contributing etiological risk factors. However, studies are not standardized making epidemiological comparisons rather difficult. Each study assessed population groups of different age ranges. A problem with the calculation of prevalence rates for DD is the inconsistency in which these studies have been carried out. For example, the low prevalence rate calculated by Geohagan et al. in 2004 [35] may be due to the fact that DD is inappropriately coded within the general practice community resulting in a gross underestimate of the actual prevalence of DD.
One complication of several studies evaluating the epidemiology of DD was the experience of the investigator/s involved in diagnosing the disease in patients. Diagnosis of advanced disease is relatively straightforward with extensive signs of digit contracture and prominent nodules, cords, and palmar pits. However, in patients with minor disease, diagnostic criteria can be difficult to interpret [30]. Diagnosis of DD can be presumed to be relatively straightforward; however, this can lead to misdiagnosis or even over diagnosis of the condition. The highest rate of misdiagnosis of DD is most likely when asking a patient to self-diagnose. Therefore, studies attempting to find the prevalence of DD by questionnaires have to be interpreted with caution, e.g., a study in rock climbers via a patient questionnaire survey [56] had as mild DD (presence of palmar nodules only) is likely to be missed, leading to a potentially inaccurate prevalence rate.
Epidemiology Linked to Etiology of DD
Two elements in the etiology of DD clearly continue to stand out. One is the familial nature of the disease and the other is that DD appears to be an extremely common disorder affecting Caucasians of Northern European ancestry [8, 97], although this may reflect the geography of the actual studies. The heritable nature of DD has been of great interest, with reports of the disease present in as many as three generations [84] and studies suggesting a possible autosomal dominant inheritance pattern [67, 84]. There is a possibility that the multi-factorial etiology of DD has a strong environmental factor, based on the results produced by Finsen in 2002 [73] who found that family members were more likely to develop DD if they were residing in the same geographical area as their diseased relative.
The wide variation in prevalence (6–36%) in HIV-infected patients [13, 32] may not be secondary to HIV infection but secondary to the familial nature of DD. Neither study looking at the association between HIV and DD mentioned familial cases in their series of patients. Further studies are required to assess the exact association between DD and HIV.
The origin and spread of DD is presumed to be from Northern Europe [69]. If this is the case, we can see that the DD gene(s) may have spread with its migrating population (Figs. 1, 2). It is surprising that the genetic nature of DD has been discussed for over 50 years; yet, epidemiological studies conducted have not stringently analyzed this aspect of the disease. The presence of sporadic cases of DD around the globe suggests there may be spontaneous genetic mutations causing the disease. The report of ten cases of DD from the Indian subcontinent [96] suggests that environmental factors may also play a role in the development of the disease as all the patients in that series had been living in the UK for several years. DD may not only be a disease of Northern European Caucasians, the disease in other populations is either not recognized as frequently or there is a lack of initial presentation [63]. This is confirmed with the highest prevalence rates being calculated in Bosnia (males) and Canada (females; Fig. 2).
The presence of DD in children may be secondary to a genetic or environmental influence. The report of DD present in only one identical twin [60] may be the result of sole environmental factors or that the disease may have developed at a later date in the other sibling. Cases of DD in the younger population are likely to have a genetic predisposition, and this should be sought in these patients [50].
Early literature had stated that DD did not occur in women [93]; however, the male to female ratio of DD varies between 7:1 and 15:1 and women tend to suffer more postoperative complications [107]. Females are older at the time of their first operation and have a higher recurrence rate and heritability [44] compared to males [104].
Significance of Associated Risk Factors and Prevalence of DD
Etiological risk factors and their significance have been evaluated by several authors (Table 1, Figs. 5 and 6). The relevance of some of the many implicated etiological factors such as alcohol abuse, cirrhosis of the liver, smoking, epilepsy, diabetes mellitus, and hypercholesterolemia on the prevalence of DD is questioned [17, 57, 92].
Alcohol consumption has doubled over the last 40 years [53]. The prevalence of DD appears to have increased, but estimated rates after 50 years have not doubled, suggesting that alcohol consumption is unlikely to be the direct cause of the disease. The prevalence of smoking is decreasing [83]; there is an unlikely increased risk associated with smoking habits and the development of DD. In spite of this, cigarette smoking and increased alcohol consumption are more likely to result in surgical, rather than conservative management of DD [81]. In relation to smoking, epidemiological studies have attempted to identify the risk of mortality, in particular cancer mortality in patients with DD. Gudmundsson concluded that mortality in DD patients is higher [91]. This may be an observational finding and not have any actual significance as DD severity increases with advancing age, as does the incidence of cancer.
Hypercholesterolemia is known to increase with age as does the prevalence of DD, it is also known that patients with DD are more likely to have raised serum triglyceride concentrations [22]; with an aging population both hypercholesterolemia and DD are likely to become more prevalent and hence an increased prevalence of hypercholesterolemia may not be directly related to an increased prevalence of DD.
The prevalence of diabetes mellitus is increasing [46], and there is a positive association with diabetes mellitus and DD (Table 1, Fig. 6). A large retrospective study evaluating the experience of treating DD in one center concluded that there was no significant correlation between DD and diabetes mellitus, alcohol consumption, heavy smoking, or epilepsy; however, the prevalence of these risk factors is greater in DD patients than in the general population [61]. All these factors associated with DD can be debated as to their role in the pathogenesis of the disease; therefore, the change in prevalence rate of DD cannot be confidently attributed to the change in prevalence of these associated factors.
Hand trauma and a history of manual labor are an integral part of establishing whether they are causes of disease development with suggestions that DD is, in certain cases, precipitated or aggravated by hand injury, hand infection, elective hand surgery [1, 58, 72], and vibration exposure [54]. It has gone so far to associate other musculoskeletal conditions with DD which have an increased prevalence in manual workers; a recent study has concluded that patients with a history of frozen shoulder are eight times more likely to develop DD [95]. Since this finding, a further study has concluded that occupational history and social class has no bearing on DD development or progression [48].
A history of rheumatoid arthritis has a negative correlation with DD [4, 39]. There may be a common genetic pathway in the development of carpal tunnel syndrome and DD with chromosomal instability present in the palmar fascia in both of these conditions [11]. It would be important to establish an epidemiological link between carpal tunnel syndrome and DD in order to gain more of an insight into the etiology of both these conditions.
The changes in estimated prevalence of DD may be attributed by an increase in awareness of disease. There have been many reports of sporadic cases of DD in populations we would never expect; again, a full epidemiological study in these populations to calculate the actual prevalence or incidence in these communities has not been carried out. For accurate comparison in different population groups more standardized epidemiological studies need to be conducted.
Conclusion
This review has provided a detailed and thorough account of the epidemiology of DD to date. It gives a concise yet insightful summary of all the studies conducted to date with an attempt to accurately calculate the prevalence and incidence of DD in addition to known etiological factors. The prevalence of DD in different geographical locations is extremely variable, and this maybe due to a genetic element, environmental, or a combination of the two. There have been many prevalence studies which have been informative however the number of true incidence studies is limited. The majority of the prevalence studies have been conducted in Scandinavia or the UK and the vast changes in population structure, changes in prevalence of associated diseases and the change in diagnostic criteria of DD makes further elucidation of the epidemiology of this condition an intriguing challenge.
References
- 1.Abe Y, Rokkaku T, Ebata T, et al. Dupuytren’s disease following acute injury in Japanese patients: Dupuytren’s disease or not? J Hand Surg [Br] 2007. 32(5):569–72. doi:10.1016/j.jhse.2007.06.005. [DOI] [PubMed]
- 2.Aladin A, Oni JA. Bilateral Dupuytren’s contracture in a black patient. Int J Clin Pract 2001;55(9):641–2. [PubMed]
- 3.Arafa M, Noble J, Royle SG, Trail IA, Allen J. Dupuytren’s and epilepsy revisited. J Hand Surg [Br] 1992. 17(2):221–4. doi:10.1016/0266-7681(92)90095-J. [DOI] [PubMed]
- 4.Arafa M, Steingold RF, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg [Br] 1984. 9(2):165–6. doi:10.1016/S0266-7681(84)80020-0. [PubMed]
- 5.Ardic F, Soyupek F, Kahraman Y, Yorgancioglu R. The musculoskeletal complications seen in type II diabetics: predominance of hand involvement. Clin Rheumatol 2003. 22:229–33. doi:10.1007/s10067-003-0704-7. [DOI] [PubMed]
- 6.Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: Association with chronic diabetic complications. J Rheumatol 1997;24:153–9. [PubMed]
- 7.Arkkila PE, Kantola IM, Viikari JSA, Ronnemaa T, Vahatalo MA. Dupuytren’s disease in type 1 diabetic patients: A 5-year prospective study. Clin Exp Rheumatol 1996;14:59–65. [PubMed]
- 8.Bayat A, McGrouther DA. Management of Dupuytren's disease–clear advice for an elusive condition. Ann R Coll Surg Engl 2006. 88(1):3–8. doi:10.1308/003588406X83104. [DOI] [PMC free article] [PubMed]
- 9.Bebbington A, Savage R. Dupuytren’s disease in an infant. J Bone Joint Surg Br 2005;87B(1):111–3. [PubMed]
- 10.Bergenudd H, Lindgarde F, Nilsson BE. Prevalence of Dupuytren’s contracture and its correlation with degenerative changes of the hands and feet and with criteria of general health. J Hand Surg [Br] 1993. 18B:254–7. doi:10.1016/0266-7681(93)90123-W. [DOI] [PubMed]
- 11.Bonnici AV, Birjandi F, Spencer JD, Fox SP, Berry AC. Chromosomal abnormalities in Dupuytren’s contracture and carpal tunnel syndrome. J Hand Surg [Br] 1992. 17(3):349–55. doi:10.1016/0266-7681(92)90128-O. [DOI] [PubMed]
- 12.Bovenzi M. Hand-arm vibration syndrome and dose-response relation for vibration induced white finger among quarry drillers and stonecarvers. Italian Study Group on Physical Hazards in the Stone Industry. Occup Environ Med 1994;51(9):603–11. [DOI] [PMC free article] [PubMed]
- 13.Bower M, Nelson M, Gazzard BG. Dupuytren’s contractures in patients infected with HIV. BMJ 1990;300:164–5. [DOI] [PMC free article] [PubMed]
- 14.Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren’s contracture. J Bone Joint Surg 1997;79(B):206–10. [DOI] [PubMed]
- 15.Burge P. Genetics of Dupuytren’s disease. Hand Clin 1999;15(1):63–71. [PubMed]
- 16.Burke FD, Proud G, Lawson IJ, McGeoch KL, Miles NV. An assessment of the effects of exposure to vibration, smoking, alcohol and diabetes on the prevalence of Dupuytren’s disease in 97537 miners. J Hand Surg [Br] 2007. 32E(4):400–6. doi:10.1016/j.jhse.2005.02.002. [DOI] [PubMed]
- 17.Carson J, Clarke C. Dupuytren’s contracture in pensioners at the Royal Hospital Chelsea. J R Coll Physicians Lond 1993;27(1):25–7. [PMC free article] [PubMed]
- 18.Chammas M, Bousquet P, Renard E, et al. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg [Am] 1995. 20(1):109–14. doi:10.1016/S0363-5023(05)80068-1. [DOI] [PubMed]
- 19.Critchley EM, Vakil SD, Hayward HW, Owen VM. Dupuytren’s disease in epilepsy: result of prolonged administration of anticonvulsants. J Neurol Neurosurg Psychiatry 1976. 39(5):498–503. doi:10.1136/jnnp.39.5.498. [DOI] [PMC free article] [PubMed]
- 20.Dasgupta AK, Harrison J. Effects of vibration on the hand-arm system of miners in India. Occup Med 1996;46(1):71–8. [DOI] [PubMed]
- 21.de la Caffiniere JY, Wagner R, Etscheid J, Metzger F. Manual labour and Dupuytrens disease. The results of a computerized survey in the field of iron metallurgy. Ann Chir Main 1983. 2(1):66–72. doi:10.1016/S0753-9053(83)80084-2. [DOI] [PubMed]
- 22.Dunstan DW, Zimmet PZ, Welborn TA, et al. The rising prevalence of diabetes and impaired glucose tolerance: The Australian Diabetes, Obesity and Lifestyle study. Diabetes Care 2002. 25(5):829–34. doi:10.2337/diacare.25.5.829. [DOI] [PubMed]
- 23.Dupuytren BG. Permanent retraction of the fingers, produced by an affection of the palmar fascia. Lancet 1833;34(2):222.
- 24.Eadington DW, Patrick AW, Collier A, Frier BM. Limited joint mobility, Dupuytren’s contracture and retinopathy in type 1 diabetes: association with cigarette smoking. Diabet Med 1989;6(2):152–7. [DOI] [PubMed]
- 25.Eadington DW, Patrick AW, Frier BM. Association between connective tissue changes and smoking habit in type 2 diabetes and in non-diabetic humans. Diabetes Res Clin Pract 1991. 11(2):121–5. doi:10.1016/0168-8227(91)90101-I. [DOI] [PubMed]
- 26.Early PF. Population studies in Dupuytren’s contracture. J Bone Joint Surg 1962;44B(3):602–13.
- 27.Egawa T, Senrui H, Horiki A, et al. Epidemiology of the Oriental patient. In: Mcfarlane RM, McGrouther DA, Flint MH, editors. Dupuytren’s Disease. Edinburgh: Churchill Livingstone; 1990. p. 239–45.
- 28.Egawa T. Dupuytren’s contracture in Japan: Incidental study on outpatients in a private practice of general orthopaedics. J Jpn Soc Surg Hand 1985;2:204–7.
- 29.Elliot D. The early history of Dupuytren’s disease. Hand Clin 1999;15:1–19. [PubMed]
- 30.Erdmann MW, Quaba AA, Sommerlad BC. Epithelioid sarcoma masquerading as Dupuytren’s disease. Br J Plast Surg 1995. 48(1):39–42. doi:10.1016/0007-1226(95)90029-2. [DOI] [PubMed]
- 31.Finsen V, Dalen H, Nesheim J. The prevalence of Dupuytren’s disease among 2 different ethnic groups in Northern Norway. J Hand Surg [Am] 2002;27(1):115–7. [DOI] [PubMed]
- 32.French PD, Kitchen VS, Harris JRW. Prevalence of Dupuytren’s contracture in patients infected with HIV. BMJ 1990;301:967. [DOI] [PMC free article] [PubMed]
- 33.Friedman GD Primer of epidemiology. 5th ed. McGraw-Hill; 2004. pp. 1-5.
- 34.Furnas DW. Dupuytren’s contracture in a black patient in East Africa. Plast Reconstr Surg 1979. 64:250–1. doi:10.1097/00006534-197908000-00021. [DOI] [PubMed]
- 35.Geohegan JM, Forbes J, Clark DI, Smith C, Hubbard R. Dupuytren’s disease risk factors. J Hand Surg [Br] 2004. 29B(5):423–6. doi:10.1016/j.jhsb.2004.06.006. [DOI] [PubMed]
- 36.Godtfredsen NS, Lucht H, Prescott E, Sorensen TI, Gronbaek M. A prospective study linked both alcohol and tobacco to Dupuytren’s disease. J Clin Epidemiol 2004. 57(8):858–63. doi:10.1016/j.jclinepi.2003.11.015. [DOI] [PubMed]
- 37.Gonzalez MH, Sobeski J, Grindel S, Chunprapah B, Weinzweig N. Dupuytren’s disease in African-Americans. J Hand Surg [Br] 1998. 23(3):306–7. doi:10.1016/S0266-7681(98)80046-6. [DOI] [PubMed]
- 38.Gordon S. Dupuytren’s contracture. The significance of various factors in its etiology. Ann Surg 1954;5:683–6. [DOI] [PMC free article] [PubMed]
- 39.Gudmundsson KG, Arngrimsson F, Sigfusson N, Jonsson T. Increased total mortality and cancer mortality in men with Dupuytren’s disease: a 15 year follow up study. J Clin Epidemiol 2002. 55(1):5–10. doi:10.1016/S0895-4356(01)00413-9. [DOI] [PubMed]
- 40.Gudmundsson KG, Arngrimsson R, Sigfusson N, Bjornsson A, Jonsson T. Epidemiology of Dupuytren’s disease. Clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol 2000. 53(3):291–6. doi:10.1016/S0895-4356(99)00145-6. [DOI] [PubMed]
- 41.Gudmundsson KG, Arngrmsson R, Sigfusson N, Jonsson T. Prevalence of joint complaints amongst individuals with Dupuytren’s disease – from the Reykjavik study. Scand J Rheumatol 1999. 28:300–4. doi:10.1080/03009749950155481. [DOI] [PubMed]
- 42.Guitian AQ. Quelques aspects epidemiologiques de la maladie de Dupuytren. Ann Chir Main 1988. 7:256–62. doi:10.1016/S0753-9053(88)80013-9. [DOI] [PubMed]
- 43.Herzog EG. The etiology of Dupuytren’s contracture. Lancet 1951. 257:1305–6. doi:10.1016/S0140-6736(51)91775-8. [DOI] [PubMed]
- 44.Hindocha S, John S, Stanley JK, Watson SJ, Bayat A. The Heritability of Dupuytren's Disease: Familial Aggregation and Its Clinical Significance. J Hand Surg [Am] 2006. 31(2):204–10. doi:10.1016/j.jhsa.2005.09.018. [DOI] [PubMed]
- 45.Hindocha S, Stanley JK, Watson S, Bayat A. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg [Am] 2006. 31(10):1626–34. doi:10.1016/j.jhsa.2006.09.006. [DOI] [PubMed]
- 46.Hueston JT. The incidence of Dupuytren’s contracture. Med J Aust 1960;2:999–1002.
- 47.Kelly SA, Burke FD, Elliot D. Injury to the distal radius as a trigger to the onset of Dupuytren’s disease. J Hand Surg [Br] 1992. 17B:225–9. doi:10.1016/0266-7681(92)90096-K. [DOI] [PubMed]
- 48.Khan AA, Rider OJ, Jayadev CU, Heras-Palou C, Giele H, Goldacre M. The role of manual occupation in the etiology of Dupuytren’s disease in men in England and Wales. J Hand Surg [Br] 20004;29(1):12–4. [DOI] [PubMed]
- 49.Kilian O, Kriegsmann J, Berghauser K, et al. The frozen shoulder. Arthroscopy, histological findings and transmission electron microscopy. Chirurg 2001. 72(11):1303–8. doi:10.1007/s001040170036. [DOI] [PubMed]
- 50.Lane JG, Hankin FM. Dupuytren’s contracture in an adolescent. Am Fam Physician 1988;37(4):133–6. [PubMed]
- 51.Laplane D, Carydakis C. Side effects of antiepileptic therapy. Study of 197 cases. Rev Neurol 1985;141(6–7):447–55. [PubMed]
- 52.Lennox IAC, Murali SR, Porter R. A study of the repeatability of the diagnosis of Dupuytren’s contracture and its prevalence in the Grampian region. J Hand Surg [Br & Eur] 1993;18B:258–61. [DOI] [PubMed]
- 53.Leon DA, McCambridge J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: an analysis of routine data. Lancet 2006. 367:52–6. doi:10.1016/S0140-6736(06)67924-5. [DOI] [PubMed]
- 54.Liss GM, Stock SR. Can Dupuytren’s contracture be work related?: review of the evidence. Am J Ind Med 1996. 29(5):521–32. doi:10.1002/(SICI)1097-0274(199605)29:5<521::AID-AJIM12>3.0.CO;2-2.. [DOI] [PubMed]
- 55.Liu Y, Chen WY. Dupuytren’s disease amongst the Chinese in Taiwan. J Hand Surg [Am] 1991;16(5):779–86. [DOI] [PubMed]
- 56.Logan AJ, Mason G, Dias J, Makwana N. Can rock climbing lead to Dupuytren’s disease? Br J Sports Med 2005. 39(9):639–44. doi:10.1136/bjsm.2004.015792. [DOI] [PMC free article] [PubMed]
- 57.Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord 2007. 8:60. doi:10.1186/1471-2474-8-60. [DOI] [PMC free article] [PubMed]
- 58.Lucas G, Brichet A, Roquelaure Y, Leclerc A, Descatha A. Dupuytren’s disease: Personal factors and occupational exposure. Am J Ind Med 2008. 51:9–15. doi:10.1002/ajim.20542. [DOI] [PubMed]
- 59.Luck JV. Dupuytren’s contracture: a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg 1959;41A:635–64. [PubMed]
- 60.Lyall HA. Dupuytren’s disease in identical twins. J Hand Surg [Br] 1993. 18B:368–70. doi:10.1016/0266-7681(93)90066-O. [DOI] [PubMed]
- 61.Mackenney RP. A population study of Dupuytren’s contracture. Hand 1983. 15(2):155–61. doi:10.1016/S0072-968X(83)80007-2. [DOI] [PubMed]
- 62.Maes J. Dupuytren’s contracture in an Oriental patient. Plast Reconstr Surg 1979. 64:251. doi:10.1097/00006534-197908000-00022. [DOI] [PubMed]
- 63.Makhlouf MV, Cabbabe EB, Shively RE. Dupuytren’s disease in blacks. Ann Plast Surg 1987. 19(4):334–6. doi:10.1097/00000637-198710000-00008. [DOI] [PubMed]
- 64.Mandalia VI, Lowdon IM. Dupuytren’s disease in a child: a case report. J Pediatr Orthop 2003. 12(3):198–9. doi:10.1097/00009957-200305000-00005. [DOI] [PubMed]
- 65.Maravic M, Landais P. Dupuytren’s disease in France – 1831 to 2001 – from description to economic burden. J Hand Surg [Br] 2005. 30B(5):484–7. doi:10.1016/j.jhsb.2005.05.004. [DOI] [PubMed]
- 66.Matthews P. Familial Dupuytren’s contracture with predominantly female expression. Br J Plast Surg 1979. 32(2):120–3. doi:10.1016/0007-1226(79)90010-9. [DOI] [PubMed]
- 67.Maza RK, Goodman RM. A family with Dupuytren’s contracture. J Hered 1968;59(2):155–6. [DOI] [PubMed]
- 68.McFarlane RM. Dupuytren’s disease: relation to work and injury. J Hand Surg [Am] 1991;16:775–9. [DOI] [PubMed]
- 69.McFarlane RM. On the origin and spread of Dupuytren’s disease. J Hand Surg [Am] 2002. 27(3):385–90. doi:10.1053/jhsu.2002.32334. [DOI] [PubMed]
- 70.Mennen U. Dupuytren’s contracture in the Negro. J Hand Surg [Br] 1986. 11(1):61–4. doi:10.1016/0266-7681(86)90015-X. [DOI] [PubMed]
- 71.Mikkelsen OA. Dupuytren’s disease-initial symptoms, age of onset and spontaneous course. Hand 1977. 9(1):11–5. doi:10.1016/S0072-968X(77)80023-5. [DOI] [PubMed]
- 72.Mikkelsen OA. Dupuytren’s disease—the influence of occupation and previous hand injuries. Hand 1978. 10(1):1–8. doi:10.1016/S0072-968X(78)80019-9. [DOI] [PubMed]
- 73.Mikkelsen OA. The prevalence of Dupuytren’s disease in Norway. A study in a representative population sample of the municipality of Haugesund. Acta Chir Scand 1972;138(7):695–700. [PubMed]
- 74.Mikkelson OA. The prevalence of Dupuytren’s disease in Norway. A study in a representative population sample in the municipality of Haugesund. Acta Chir Scand 1972;138(7):695–700. [PubMed]
- 75.Mitra A, Goldstein RY. Dupuytren’s contracture in the black population: a review. Ann Plast Surg 1994. 32(6):619–22. doi:10.1097/00000637-199406000-00010. [DOI] [PubMed]
- 76.Muguti GI, Appelt B. Dupuytren’s contracture in black Zimbabweans. Cent Afr J Med 1993;39(6):129–32. [PubMed]
- 77.Noble J, Arafa M, Royle SG, McGeorge G, Crank S. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg [Br].1992;17(1):71–4. [DOI] [PubMed]
- 78.Noble J, Heathcote JG, Cohen H. Diabetes mellitus in the aeitiology of Dupuytren’s disease. J Bone Joint Surg Br 1984;66(3):322–5. [DOI] [PubMed]
- 79.Omari A, Bunker TD. Open surgical release for frozen shoulder: surgical findings and results of the release. J Shoulder Elbow Surg 2001. 10(4):353–7. doi:10.1067/mse.2001.115986. [DOI] [PubMed]
- 80.Pai CH, Tseng CH. Dupuytren’s contracture: report of a Taiwanese case. J Formos Med Assoc 1994;93(8):724–6. [PubMed]
- 81.Paller AS, Hebert AA. Knuckle pads in children. Am J Dis Child 1986;140(9):915–7. [DOI] [PubMed]
- 82.Patri B, Gatto A. Raynaud’s syndrome, Dupuytren’s disease, force of prehension and sensitivity of the hand. Study of age-related changes. Ann Chir Main 1986. 5(2):144–7. doi:10.1016/S0753-9053(86)80029-1. [DOI] [PubMed]
- 83.Peto R, Darby s, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case control studies. BMJ 2000. 321:323–9. doi:10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed]
- 84.Pierce ERF. —dupuytren’s contracture in three successive generations. Birth Defects Orig Artic Ser 1974;10(5):206–7. [PubMed]
- 85.Plasse JS. Dupuytren’s contracture in a black patient. Plast Reconstr Surg 1979. 64:250. doi:10.1097/00006534-197908000-00020. [DOI] [PubMed]
- 86.Rao GS, Luthra PK. Dupuytren’s disease of the foot in children; a report of three cases. Br J Plast Surg 1988. 41(3):313–5. doi:10.1016/0007-1226(88)90117-8. [DOI] [PubMed]
- 87.Ravid M, Dinai Y, Sohar E. Dupuytren’s disease in diabetes mellitus. Acta Diabetol Lat 1977. 14(3–4):170–4. doi:10.1007/BF02581405. [DOI] [PubMed]
- 88.Renard E, Jacques D, Chammas M, et al. Increased prevalence of soft tissue hand lesions in type 1 and type 2 diabetes mellitus: various entities and associated significance. Diabete Metab 1994;20(6):513–21. [PubMed]
- 89.Rhomberg M, Rainer C, Gardetto A, Piza-Katzer H. Dupuytren’s disease in children-differential diagnosis. J Pediatr Surg 2002. 37(4):E7. doi:10.1053/jpsu.2002.31646. [DOI] [PubMed]
- 90.Richard-Kadio M, Guedegbe F, Dick R, et al. Dupuytren’s contracture: review of the literature. Case report of a black African. Med Trop 1990;50(3):311–3. [PubMed]
- 91.Sanderson PL, Morris MA, Stanley JK, Fahmy NRM. Lipids and Dupuytren’s disease. J Bone Joint Surg Br 1992;74B:923–7. [DOI] [PubMed]
- 92.Schiavon F, Circhetta C, Dani L. The diabetic hand. Rheumatismo 2004;56(3):139–42. [DOI] [PubMed]
- 93.Skoog T. Dupuytren’s contracture with special reference to etiology and improved surgical treatment, its occurance in epileptics, note on knuckle pads. Acta Chir Scand 1948;96(suppl 39):25–175.
- 94.Sladicka MS, Benfanti P, Raab M, Becton J. Dupuytren’s contracture in the black population: a case report and review of the literature. J Hand Surg [Am] 1996. 21(5):898–9. doi:10.1016/S0363-5023(96)80211-5. [DOI] [PubMed]
- 95.Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg 2001. 10(2):149–51. doi:10.1067/mse.2001.112883. [DOI] [PubMed]
- 96.Srivastava S, Nancarrow JD, Cort DF. Dupuytren’s disease in patients from the Indian sub-continent. Report of ten cases. J Hand Surg [Br] 1989. 14(1):32–4. doi:10.1016/0266-7681(89)90009-0. [DOI] [PubMed]
- 97.Stadner F, Ulreich A, Pfeiffer KP. Dupuytren's contracture as a concomitant disease in diabetes mellitus. Wien Med Wochenschr 1987;137(4):89–92. [PubMed]
- 98.Stewart HD, Innes AR, Burke FD. The hand complications of Colles’ fractures. J Hand Surg [Br] 1985. 10(1):103–6. doi:10.1016/S0266-7681(85)80032-2. [DOI] [PubMed]
- 99.Stuhler T, Stankovic P, Ritter G, Schmulder E. Epilepsy and Dupuytren’s contracture—asyntropy of 2 diseases? Handchirurgie 1977;9(4):219–23. [PubMed]
- 100.Thomas PR, Clarke D. Vibration white finger and Dupuytren’s contracture: are they related? Occup Med 1992. 42:155–8. doi:10.1093/occmed/42.3.155. [DOI] [PubMed]
- 101.Tomasek JJ, Vaughan MB, Haaksma CJ. Cellular structure and biology of Dupuytren’s disease. Hand Clin 1999;15(1):21–33. [PubMed]
- 102.Vathana P, Setpakdi A, Srimongkol T. Dupuytren’s contracture in Thailand. Bull Hosp Jt Dis Orthop Inst 1990;50(1):41–7. [PubMed]
- 103.White HA, Alcolado R, Alcolado JC. Examination of the hands: An insight in to the Welsh population. Postgrad Med J 2003. 79:588–9. doi:10.1136/pmj.79.936.588. [DOI] [PMC free article] [PubMed]
- 104.Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of re-operation and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg [Br] 1999. 24(4):456–9. doi:10.1054/jhsb.1999.0154. [DOI] [PubMed]
- 105.Yost J, Winters T, Fett HC. Dupuytren’s contracture. A statistical study. J Hand Surg [Am] 1955;90(4):568–72. [DOI] [PubMed]
- 106.Zaworski RE, Mann RJ. Dupuytren’s contracture in a black patient. Plast Reconstr Surg 1979. 63(1):122–4. doi:10.1097/00006534-197901000-00030. [DOI] [PubMed]
- 107.Zemel NP. Dupuytren’s contracture in women. Hand Clin 1991;7(4):707–11. [PubMed]
- 108.Zerajic D, Finsen V. Dupuytren’s disease in Bosnia and Herzegovina. An epidemiological study. BMC Musculoskelet Disord 2004. 5:10. doi:10.1186/1471-2474-5-10. [DOI] [PMC free article] [PubMed]