Abstract
A rare previously unreported cause of flexor tendon rupture is described. A 66-year-old man presented with a fully extended left middle finger, accompanied by swelling and purulent drainage. Prior to presentation, he had received a steroid injection for left middle finger stenosing tenosynovitis and subsequently developed culture-proven phaeohyphomycosis fungal infection and secondary enterococcal bacterial infection, requiring pharmacotherapy and incision, drainage, and debridement for abscess formation. Clinical and magnetic resonance imaging findings were consistent with the diagnosis of closed flexor tendon rupture of the left middle finger. Antifungal and antibiotic therapy followed by two-stage flexor tendon reconstruction was performed. Six months postoperatively, full passive range of motion was achieved and the proximal interphalangeal and distal interphalangeal joints of the left middle finger actively flexed to 125° and 90°, respectively.
Keywords: Flexor tendon rupture, Phaeohyphomycosis, Fungal infection, Steroid injection, Stenosing tenosynovitis, Trigger finger, Two-stage flexor tendon reconstruction
Introduction
Closed flexor tendon rupture is most commonly caused by forceful hyperextension against resistance of the distal interphalangeal (DIP) joint, leading to avulsion at the tendon bone insertion [1, 3, 13]. Other sites of closed rupture are unique and are often due to underlying pathology such as rheumatoid arthritis, infectious tenosynovitis, gout, fractures, nonunion, or congenital abnormalities [2, 5, 6, 8–11, 18]. We present a case of a patient who had an unusual fungal infection involving the flexor tendons of his left middle finger leading to a zone two flexor tendon rupture and the ensuing surgical management. Patient consent for publication of this case report was obtained prior to the collection of data concerning this report.
Case Report
A 66-year-old right-hand dominant East Indian male presented to the emergency room (ER) at a community hospital with a 1-week history of pain and swelling (3 cm × 4 cm) in the region of his left middle finger and palm. Six months prior to presentation, the patient was treated with a single steroid injection in the region of the A1 pulley of the left middle finger for presumed stenosing tenosynovitis. Subsequent follow-up visits since the injection demonstrated a moderate improvement of his symptoms, with occasional triggering only and no functional limitations.
The patient was an immunocompetent individual with a past medical history significant for coronary artery disease, hypercholesterolemia, and hypertension. Prior surgical history was unremarkable; he had no known allergies and was a nondrinker and a nonsmoker.
Following assessment in the ER, the patient was admitted to the hospital for an incision, drainage, and debridement of the left palm for a presumed abscess. Postoperatively, the patient had a penrose drain in place and was initially managed with cephazolin. Intraoperative cultures returned positive for coagulase-negative staphylococci, as well as unidentified fungal species, which was sent for further laboratory analysis. Due to ongoing discharge (yellowish) from the wound and residual swelling, a repeat irrigation and debridement of the left palm was undertaken 2 weeks later. Repeat wound cultures showed heavy growth of both coagulase-negative staphylococci and enterococcus faecalis, and the patient was treated with i.v. ampicillin and gentamicin for a period of 2 weeks. Fungus cultures returned positive for Phaeoacremonium species and antifungal therapy (itraconazole 400 mg po od) was commenced for a period of 3 months after a consultation with the infectious diseases team.
Six weeks later, the patient was referred to our hand service with an inability to flex the middle finger that was discovered shortly after the repeat irrigation and debridement procedure. He reported that the swelling was diminishing slowly. There were no systemic signs of infection including fever, chills, or night sweats, although locally there was ongoing yellowish drainage.
Physical examination revealed a healthy man who appeared his stated age and did not look septic. There was a fullness of the left volar hand over the middle finger A1 pulley. In a relaxed posture, the middle finger was fully extended. There was no tenodesis effect. The patient was unable to actively flex the left middle finger proximal interphalangeal (PIP) or DIP joints; however, passive range of motion measured 105° and 80°, respectively. Dynamic two-point discrimination in the middle finger measured 4 mm along both radial and ulnar aspects.
An ultrasound of the left hand was performed to assess the level of flexor tendon rupture and to rule out any occult abscess. The ultrasound demonstrated thickened and heterogeneous middle flexor tendons forming a complex fusiform mass that measured 1.3 cm in diameter. No detectable tendon rupture or tear was detected and there was no focal abscess or fluid collection.
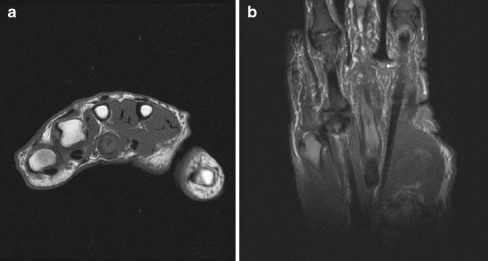
Magnetic resonance imaging (MRI) demonstrated a 9 × 9-mm non-specific mass approximately 3.2 cm in length involving the flexor digitorum profundus (FDP) tendon of the left middle finger. Within the abnormal profundus tendon near the metacarpophalangeal (MCP) joint, there was a small focus of fluid within the substance of the tendon, likely delineating a cleft filled with fluid. In addition, there was a longitudinal split tear of the flexor digitorum superficialis (FDS) tendon that extended as far distally as the level of the MCP joint (Fig. 1a, b). There was no significant marrow edema of the imaged third metacarpal or joint effusion at the MCP joint.
Figure 1.
a Preoperative axial T1-weighted MRI demonstrating a non-specific mass involving the FDP tendon of the left middle finger. b Preoperative coronal T2-weighted MRI demonstrating a longitudinal split tear involving the FDS tendon of the left middle finger.
The white blood cell count was normal as were the eosinophil and neutrophil counts. The patient was rheumatoid factor-negative, had normal circulating levels of IgG/IgM/IgA, normal urate values as well as normal hepatic, renal, and thyroid function. The erythrocyte sedimentation rate was not elevated.
The overall picture was consistent with a flexor tendon rupture involving the left middle finger following phaeohyphomycosis and a secondary bacterial infection. The yellowish discharge from the wound continued even after the antibiotic therapy for the enterococcal infection. Given the possibility of ongoing indolent or latent fungal infection, we elected to continue antifungal treatment with itraconazole until the 3-month course was completed. Once the patient was cleared by infectious diseases, a two-stage flexor tendon reconstruction was performed. During stage one of the reconstruction, it was discovered that the left middle finger flexors were ruptured in zone two. A yellowish solid mass measuring 1 × 1 cm was observed to be closely adherent to the flexor tendons. Excision of the FDS, FDP, and most of the flexor sheath was performed (Fig. 2). A 5-mm Hunter rod was inserted and the FDS was used to reconstruct the A2 pulley. Postoperatively, there were no wound complications. A hand therapy program was implemented to ensure passive mobility. At the 6-month follow-up, passive range of motion of the MCP joint showed flexion to 90° while the PIP and the DIP joints passively flexed to 90° and 65°, respectively. In subsequent follow-up visits, there was complete resolution of pain, swelling, and discharge.
Figure 2.
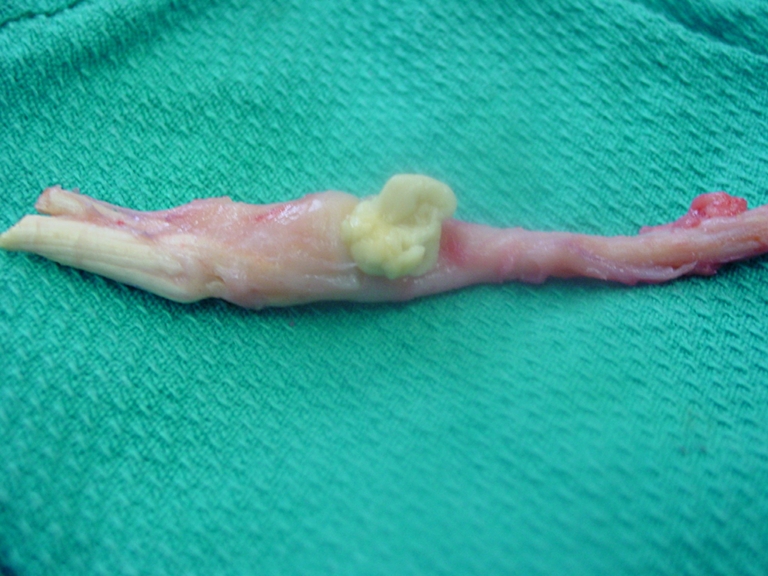
Excision of the FDS, FDP, and flexor sheath during stage one flexor tendon reconstruction.
Approximately 8 months later, the patient was taken back to the operating room for the second stage of the proposed reconstruction. Intraoperatively, there were no signs of infection. A left palmaris longus tendon graft was harvested from the ipsilateral side and used to reconstruct the FDP of the left middle finger following removal of the Hunter rod. The FDP tendon of the middle finger was reconstructed with the palmaris tendon graft and then transferred to the FDP of the ring finger at the level of the forearm.
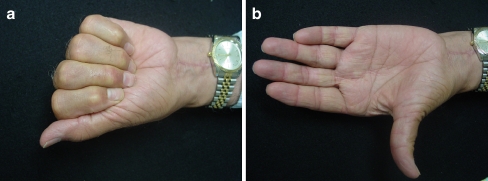
At the 6-month follow-up, excellent passive range was achieved. The PIP and the DIP joints of the left middle finger flexed to 125° and 90°, respectively (Fig. 3a, b). Grip strength (right/left = 30:8 lbs) and key pinch strength (right/left = 8:4 lbs) were markedly diminished on the operated side. A 2-year postoperative MRI of the left hand identified an attenuated but intact FDP tendon.
Figure 3.
a Active flexion of the left hand, post second stage flexor tendon reconstruction. b Active extension of the left hand, post second stage flexor tendon reconstruction.
Discussion
Our literature search revealed no previous reports of tenosynovitis or tendon rupture associated with Phaeoacremonium species.
In the case presented, the patient was not at risk for environmental exposure, nor did he suffer known inoculating trauma at home, the workplace, or during leisure activities. The presumed source of the fungus was the direct inoculation that occurred with the steroid injection in the region of the A1 pulley. Reyes et al. reported a case where the patient had a 3-month history of a mass over the dorsum of the middle phalanx of the right index finger following a steroid injection for painful extensor tendonitis [16]. There was no recurrence of symptoms but a swelling that persisted after the injection. At surgery, there was white viscous material surrounded by scar tissue. Biopsy confirmed the presence of Phaeoacremonium species. There was no tendon or joint involvement. Direct inoculation was thought to be the route of infection, in this case, presumably due to contamination at the time of steroid injection [16].
In terms of the delayed presentation of symptoms following the injection, it is possible that the injection introduced an infective nidus that over time went on to become symptomatic. There are other cases in the literature where a similar sequence of events has occurred. Torstrick et al. reported a case of a slowly enlarging olecranon bursal mass due to Phialophora richardsiae after a thorn puncture wound sustained over 30 years before [17]. Kaell et al. reported a case where a patient had a glass injury to the knee in childhood which may have served a portal of entry for an infective nidus [7]. Several years later, this nidus was likely the source of the acute fungal arthritis observed in this case (Phaeloacremonium parasiticum).
Phaeohyphomycosis is the general term used to describe infections caused by dematiaceous, or darkly pigmented fungi [12, 15]. These fungi, which are present in the soil and are distributed worldwide, can be responsible for human infections in immunocompromised and immunocompetent individuals. More than 100 species and 60 genera of dematiaceous fungi have been implicated in human disease. The common characteristic is the presence of melanin in their cell walls, which imparts a dark color to their conidia or spores or hyphae. The melanin may act as a virulence factor by scavenging free radicals and hypochlorite produced by phagocytic cells in their oxidative burst. It also binds to hydrolytic enzymes. This may explain the effect of these bacteria even in immunocompetent hosts [15].
Phaeoacremonium spp. are among the several fungi that can lead to the development of phaeohyphomycosis. Woody plants appear to be the main environmental source of these fungi [12]. Some species of Phaeoacremonium produce a yellowish discharge as was observed in this case. P. parasiticum (original name was Phialophora parasitica) was the first species of Phaeoacremonium reported to cause phaeohyphomycosis in humans. Subsequently, Phaeoacremonium rubrigenum and Phaeoacremonium inflatipes have also been reported from phaeohyphomycosis cases [14]. Reported cases involve subcutaneous abscesses, cysts, or chronic or acute arthritis in immunocompetent or immunocompromised patients, most often initiated by traumatic inoculation [4]. There was also a case that implicated these pathogens in olecranon bursitis and osteomyelitis. Disseminated infections, fungemia, and endocarditis are possible in immunocompromised patients [12].
The case presented highlights the importance of maintaining a high index of suspicion for unusual causes of infective tenosynovitis when symptoms do not resolve following irrigation and debridement and antibiotic therapy (i.e., ongoing swelling/yellowish discharge). It also emphasizes that seemingly routine procedures such as a steroid injection for trigger finger can be associated with rare complications. Appropriate antifungal and antibiotic therapy followed by a delayed two-stage flexor tendon reconstruction provided for an excellent clinical outcome in the management of this complex and challenging case.
References
- 1.Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Jt Surg 1960;42:637–46. [PubMed]
- 2.Cognet JM, Dujardin C, Popescu A, et al. Rupture of the flexor tendons on an anterior plate for distal radial fracture: four cases and a review of the literature. Rev Chir Orthop Repar Appar Mot 2005;91:476–81. doi:10.1016/S0035-1040(05)84366-1. [DOI] [PubMed]
- 3.Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Jt Surg 1972;54:579–84. [PubMed]
- 4.Guarro J, Alves SH, Gene J, et al. Two cases of subcutaneous infection due to phaeoacremonium spp. J Clin Microbiol 2003;41(3):1332–6. doi:10.1128/JCM.41.3.1332-1336.2003. [DOI] [PMC free article] [PubMed]
- 5.Hashizume H, Nishida K, Fujiwara K, et al. Spontaneous “spaghetti” flexor tendon ruptures in the rheumatoid wrist. Mod Rheumatol 2004;14:257–9. doi:10.1007/s10165-004-0303-8. [DOI] [PubMed]
- 6.Hay EL, Collawn SS, Middleton FG, et al. Sporothrix schenckii tenosynovitis: a case report. J Hand Surg [Am] 1986;11(3):431–4. [DOI] [PubMed]
- 7.Kaell AT, Weitzman I. Acute monoarticular arthritis due to Phialophora parasitica. Am J Med 1983;74:519–22. doi:10.1016/0002-9343(83)91001-X. [DOI] [PubMed]
- 8.Kato N, Nemoto K, Arino H, et al. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg 2002;36:245–8. doi:10.1080/02844310260259950. [DOI] [PubMed]
- 9.Koizumi M, Kanda T, Satoh S, et al. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg 2005;30:142–6. doi:10.1016/j.jhsa.2004.08.007. [DOI] [PubMed]
- 10.Lillmars SA, Bush DC. Flexor tendon rupture associated with an anomalous muscle. J Hand Surg 1988;13:115–9. doi:10.1016/0363-5023(88)90213-4. [DOI] [PubMed]
- 11.McLain RF, Stevers CM. Tendon ruptures with scaphoid nonunion: a case report. Clin Orthop Relat Res 1990;255:117–20. [PubMed]
- 12.Mostert L, Groenewald JZ, Summerbell RC, et al. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J Clin Microbiol 2005;43(4):1752–67. doi:10.1128/JCM.43.4.1752-1767.2005. [DOI] [PMC free article] [PubMed]
- 13.Murphy BA, Mass DP. Zone I flexor tendon injuries. Hand Clin 2005;21:167–71. doi:10.1016/j.hcl.2004.12.004. [DOI] [PubMed]
- 14.Padhye AA, Davis MS, Baer D, et al. Phaeohyphomycosis caused by phaeoacremonium inflatipes. J Clin Microbiol 1998;36(9):2763–5. [DOI] [PMC free article] [PubMed]
- 15.Revankar SG. Phaeophyphomycosis. Infect Dis Clin North Am 2006;20(3):609–20. doi:10.1016/j.idc.2006.06.004. [DOI] [PubMed]
- 16.Reyes FA, Buchman MT. Phialophora richardsiae infection mimicking a soft tissue mass of a finger. J Hand Surg [Br] 1986;11(2):274. doi:10.1016/0266-7681(86)90282-2. [DOI] [PubMed]
- 17.Torstrick RF, Harrison K, Heckman JD, et al. Chronic bursitis caused by Phialophora richardsiae. J Bone Jt Surg 1979;61:772–4. [PubMed]
- 18.Wurapa RK, Zelof DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg 2002;27:591–3. doi:10.1053/jhsu.2002.34312. [DOI] [PubMed]