Abstract
Nerve conduits have become an established option for repair of sensory deficits of up to 2 cm. More recently, decellularized nerve allograft has also been advocated as an option for nerve repair; however, no clinical studies have examined its efficacy for the treatment of sensory nerve defects. The aim of this study was to examine our early experience with the use of decellularized nerve allograft for repair of segmental nerve defects within the hand and fingers. From July 2007 to March 2008, seven patients who had ten nerve gaps were treated surgically using decellularized nerve allograft. Eight digital and two dorsal sensory nerves were repaired. The etiologies of the nerve defects were traumatic nerve transection in eight defects and neuroma resection and reconstruction in two defects. All of the affected nerves were pure sensory fibers. Functional recovery was evaluated by blinded hand therapist using moving and static two point discrimination tests. Implantation sites were also evaluated for any signs of infection, rejection, or graft extrusion. There were five men and two women with a mean age of 44 years (range 23–65). Mean nerve graft length was 2.23 cm with a range of 0.5–3 cm. Mean follow up time was 9 months (range 5–12). Average two point discrimination was 4.4 mm moving and 5.5 mm static at last recorded follow-up. There were no wound infections observed around the graft material and sensory improvement was observed in all of the patients despite this short-term follow-up. Re-exploration of two fingers was required for flexor tendon rupture in one and flexor tendon tenolysis in the other. In both cases, the nerve allograft was visualized and appeared well incorporated in the repair site. Decellularized nerve allografts were capable of returning adequate sensation in nerve defects ranging from 0.5 to 3 cm. There were no cases of infection or rejection. Decellularized nerve allograft may provide an option for segmental nerve gaps beyond 2 cm. Randomized comparative studies will be required to determine efficacy in comparison to collagen conduits or nerve autograft.
Keywords: Nerve repair, Allograft, Nerve graft, Trauma
Introduction
The reconstruction of injured peripheral nerves remains as a formidable challenge in reconstructive surgery. Nerve grafting is indicated for nerve repair when tension-free direct repair is not possible or when there is segmental nerve loss [15, 23]. The most common material used to bridging segmental nerve defects is autogenous nerve grafts [16]. Disadvantages of autogenous nerve graft include the requirements for graft harvest, increased operating times, limited sites for harvest, and donor site morbidity which can include pain, scarring, neuroma formation, and sensory loss with the area of harvest [15, 26, 28]. The morbidity associated with autogenous nerve graft harvest has motivated researchers to engage in finding alternatives to autogenous nerve graft and to improve the process of peripheral nerve regeneration in the patients with multiple nerve injuries.
An alternative to nerve autografts is nerve allograft. Nerve allografts have the advantage of being readily available and can provided an unlimited source of graft material; however, they require systemic immunosuppression in the patient for approximately 18 months [19]. Systemic immunosuppression allows host axons and Schwann cells to regenerate across the allograft scaffold, but leaves the patient vulnerable to infection and tumor formation [19, 29]. For these reasons, predegenerated decellularized human nerve allograft has become an attractive alternative to nerve allograft as they are capable of acting as a temporary scaffold for regenerating axons but circumvent the need for immunosuppression. Chemical decellularized nerve allograft models have been developed experimentally but no clinical data have been presented [11, 27].
The aim of this preliminary study was to report our early clinical experience with the use of decellularized nerve allograft for the repair of the segmental sensory nerve defects within the hand. This is the first study to examine the short-term clinical recovery of nerve function following nerve repair with acellular allogenous nerve graft material.
Method
From July 2007 to March 2008, seven patients who had ten nerve gaps were treated surgically using AxoGen® nerve allograft (AxoGen Inc, Alachua, FL, USA; Fig. 1). The material is a decellularized cadaveric nerve which is prepared through a process of detergent decellularization, enzyme degradation, and gamma irradiation sterilization which allows for the preservation of the nerve extracellular matrix (i.e., basal lamina, endoneural tubes, and laminin) and renders the graft nonimmunogenic [11, 12, 14, 25]. The material is stored frozen and thawed within the operating room prior to implantation.
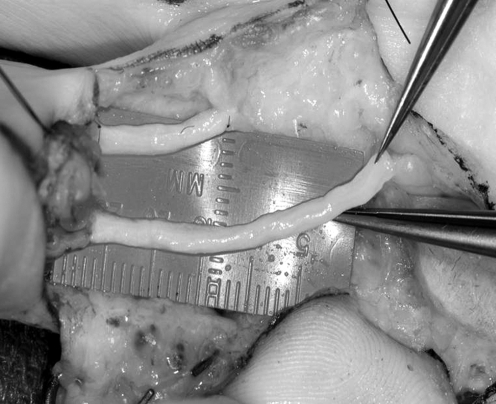
Figure 1.
An intraoperative image of the Axogen nerve graft being used to repair a bilateral digital nerve injury. The material handling properties are similar to autograft and standard microsurgical technique is employed during the nerve grafting procedure.
Nerve repair was performed by first preparing the defect site, with nerve endings being resected back until healthy fascicles were visualized under ×3.5 loop magnification. The diameter of the injured nerve was noted and a corresponding nerve graft diameter was chosen. The nerve graft material was then thawed in warm sterile saline and transferred to the defect site. Nerve grafts were cut to fill the defect site, allowing for a tension-free nerve repair using 8–0 nylon suture. All nerve repairs were performed under ×3.5 loop magnification.
After obtaining institutional research approval, moving and static two-point discrimination (2PD) tests were performed on all patients by certified hand therapist blinded to the treatment modality [5, 6]. Implantation sites were also evaluated for any signs of pain, infection, rejection, or graft extrusion. All postoperative complications were noted.
Final evaluation of sensibility was categorized according to the Mackinnon classification of excellent, good or poor results. An excellent functional result was noted if static two-point discrimination was less than or equal to 6 mm or moving two-point discrimination was less than or equal to 3 mm. A good functional result was noted if static two-point discrimination was between 7 and 15 mm and moving two-point discrimination was between 4 and 7 mm. Absence of either static or moving two-point discrimination was graded as a poor result [17, 18].
Results
There were eight digital nerves and two dorsal sensorial branches of ulnar nerve defects. The etiologies of the nerve defects were traumatic nerve transection in eight defects, neuroma resection, and reconstruction in two defects. Both cases of neuroma reconstruction involved the dorsal sensory branch of the ulnar nerve. All repairs were performed in pure sensory nerves.
There were five men and two women within the study, with a mean age of 44 years (range 23–65). Mean nerve graft length was 2.23 cm with a range of 0.5–3 cm. Mean follow-up time was 9 months (range 5–12 months). Two patients were smokers. None of the patients were diabetic and there were no cases of preexisting peripheral neuropathies.
Sensory Tests
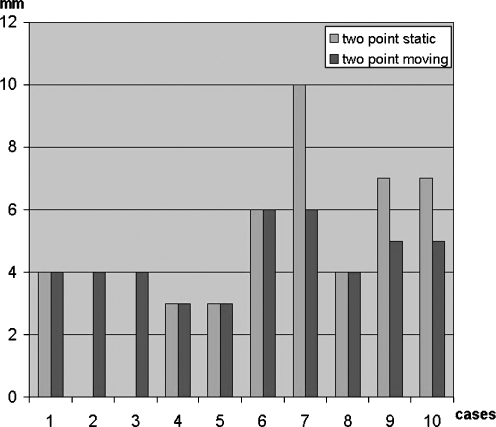
Average 2PD results were 4.4 mm moving and 5.5 mm static at the last recorded follow-up (Fig. 2; Table 1). Five patients were graded as an excellent result and five were graded as a good result. All patients recovered at least 10 mm or better static 2PD and there were no poor results. The two patients who underwent neuroma resection and repair were both graded as good results according to the Mackinnon scale. Pain relief was judged by each patient to be excellent at the time of final follow-up.
Figure 2.
At 9-month mean follow-up time, average 2PD results were 4.4 mm moving and 5.5 mm static.
Table 1.
A summary of all ten nerve reconstructions with defect length, follow-up, and values obtained on final evaluation of static and moving two-point discrimination (2PDs and 2PDM).
| No | Age | Sex | Side | Injured nerve | Etiology | Length | Diameter | Follow-up | 2PDS | 2PDM |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | f | L | Digital nerve | Laceration | 0.5 | 2 | 5.5 | 4 | 4 |
| 2 | 37 | f | R | Dorsal sensory branch of the ulna | Neuroma excision | 3 | 1 | 8.5 | * | 4 |
| 3 | 37 | f | R | Dorsal sensory branch of the ulna | Neuroma excision | 3 | 2 | 8.5 | * | 4 |
| 4 | 57 | m | R | r ring finger radial digital nerve | Laceration | 1 | 2 | 12 | 3 | 3 |
| 5 | 57 | m | R | r ring finger ulnar digital nerve | Laceration | 3 | 2 | 12 | 3 | 3 |
| 6 | 31 | m | R | Long finger radial digital nerve | Laceration | 3 | 2 | 9 | 6 | 6 |
| 7 | 48 | m | L | Digital nerve | Laceration | 3 | 2 | 5 | 10 | 6 |
| 8 | 65 | m | L | Digital nerve | Laceration | 3 | 2 | 5 | 4 | 4 |
| 9 | 23 | m | L | Thumb radial digital nerve | Laceration | 1 | 2 | 12 | 7 | 5 |
| 10 | 23 | m | L | Thumb ulnar digital nerve | Laceration | 1 | 2 | 12 | 7 | 5 |
| Average | 43 | 2.15 | 1.90 | 8.95 | 5.50 | 4.40 |
*Two point static discrimination was not recorded in this patient
m male, f female, L left, R right
No rejection or infection sign were seen around the graft material and sensory improvement was observed in all of the patients. Re-exploration of two fingers was required for flexor tendon rupture in one and flexor tendon tenolysis in the other (Fig. 3). In both cases, the nerve allografts were visualized and appeared well incorporated in the repair site.
Figure 3.
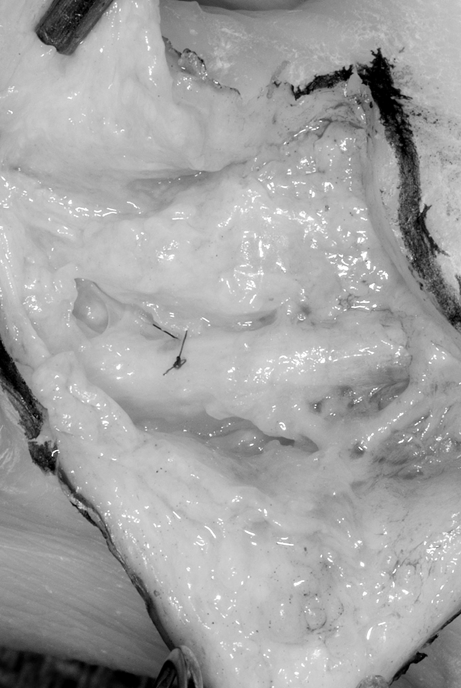
An intraoperative image of a decellularized nerve conduit at 8 weeks following implantation. The microsuture marks the junction between the decellularized nerve graft material and the native nerve. Re-exploration in this case was required for repair of a flexor tendon rupture. The nerve graft was visualized and appeared well incorporated in the repair site.
Discussion
The treatment of segmental nerve loss in upper extremity trauma remains a challenge. Nerve autograft remains the most common strategy for repairing segmental nerve defects; however, the use of nerve autograft has several disadvantages, including donor site morbidity, increased operative time, and limited harvest sites [23, 24, 26, 28]. The use of decellularized nerve allograft may provide an effective means of repairing segmental nerve defects while sparing the patient the secondary morbidity of nerve autograft harvest. This is the first study to show clinical efficacy for the use of decellularized nerve autografts in the treatment of sensory defects in the hand of up to 3 cm.
Various artificial materials have been used in place of nerve autograft in an attempt to minimize donor morbidity and improve neural regeneration. The majority of these devices have been variations of hollow conduits, designed to guide regenerating axons toward the distal nerve stump [2, 17, 30]. Most clinical studies have examined the use of these nerve conduits for sensory nerve gaps of 20 mm or less [9, 22]. Weber et al. have reported their results after using a polyglycolic acid nerve conduits for the treatment of nerve injuries distal to the wrist crease. They showed an average recovery of 6.8 mm of moving 2PD for patients undergoing repairs of 8 mm or greater [30]. Bushnell and colleagues have also reported on the efficacy of collagen conduits for the repair of nerve gaps within the finger. In their study of 12 patients followed for 15 months, they found a mean recovery of 6.9 mm of moving 2PD [3]. The results of both of these studies are comparable to the results obtained with decellularized nerve graft.
Decellularized nerve may offer some advantages over hollow conduits. The first advantage is that the material preserves the three-dimensional collagen scaffolding present within native nerve [10–12]. Cell migration and nerve fiber elongation require internal structure and extracellular matrix components to lead nerve regeneration in a nerve graft [13, 20]. This three-dimensional structure is absent within hollow conduits.
Hudson and colleagues have shown that macrophages are capable of migrating into this decellularized scaffolding where they are capable of producing growth factors to accelerate nerve regeneration [11]. Hudson et al. have shown that chemically decellularized nerve graft produces higher axon densities when compared to fresh nerve isografts in the cross section [11].
The second advantage of decellularized allograft is that it maintains laminin within its collagen matrix. Laminin and collagen are components of the basil lamina and are know to play an important role in axonal outgrowth [8]. The addition of laminin to collagen conduits has been shown to improve results of hollow conduit tubes in animal studies [21].
Finally, the graft material has been treated to remove chondroitin sulfate proteoglycans (CSPG). It has been shown that CSPG can inhibit axonal growth and its production is initially upregulated following peripheral nerve injury. The removal of CSPG from nerve graft material may allow for the acceleration of nerve recovery. Degradation of CSPG has been shown to increase the ability of axonal growth cones to cross the nerve–graft interface in animal models [14, 25].
The weaknesses of the present study are its retrospective nature, brief follow-up, and small numbers. Future studies should focus on prospective randomized trials comparing decellularized allograft to autograft and nerve conduits. This study serves as an initial case series suggesting efficacy for this form of nerve grafting. The results of this study have shown nerve recovery which is comparable to previous reports of primary nerve repair as well as nerve repair with conduits [1, 3, 4, 7, 17, 30].
In conclusion, decellularized nerve allografts are capable of returning adequate sensation in nerve defects ranging from 0.5 to 3 cm without infection or rejection. Decellularized nerve allograft may provide another option for segmental nerve gaps up to 3 cm. Randomized comparative studies will be required to determine efficacy in comparison to collagen conduits or nerve autograft.
Footnotes
A portion of this paper was presented at the AAHS meeting 2009.
References
- 1.Berger A, Mailander P. Advances in peripheral nerve repair in emergency surgery of the hand. World J Surg. 1991;15(4):493–500. doi:10.1007/BF01675646. [DOI] [PubMed]
- 2.Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg [Am]. 2005;30(3):513–8. doi:10.1016/j.jhsa.2004.12.009. [DOI] [PubMed]
- 3.Bushnell BD, McWilliams AD, Whitener GB, Messer TM. Early clinical experience with collagen nerve tubes in digital nerve repair. J Hand Surg [Am]. 2008;33(7):1081–7. doi:10.1016/j.jhsa.2008.03.015. [DOI] [PubMed]
- 4.Cheng AS, Hung L, Wong JM, Lau H, Chan J. A prospective study of early tactile stimulation after digital nerve repair. Clin Orthop Relat Res. 2001;384:169–75. doi:10.1097/00003086-200103000-00020. [DOI] [PubMed]
- 5.Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber receptor system. J Hand Surg. 1978;3:474–81. [DOI] [PubMed]
- 6.Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg. 1987;8:693–6. [DOI] [PubMed]
- 7.Glickman LT, Mackinnon SE. Sensory recovery following digital replantation. Microsurgery. 1990;11(3):236–42. doi:10.1002/micr.1920110311. [DOI] [PubMed]
- 8.Hall SM. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol Appl Neurobiol. 1986;12:27–46. doi:10.1111/j.1365-2990.1986.tb00679.x. [DOI] [PubMed]
- 9.Heath CA, Rutkowski GE. The development of bioartificial nerve grafts for peripheral nerve regeneration. Trends Biotechnol. 1998;16:163–8. doi:10.1016/S0167-7799(97)01165-7. [DOI] [PubMed]
- 10.Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Orthop Clin North Am. 2000;31(3):485–98. doi:10.1016/S0030-5898(05)70166-8. [DOI] [PubMed]
- 11.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10(11–12):1641–51. doi:10.1089/ten.2004.10.1641. [DOI] [PubMed]
- 12.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9–10):1346–58. doi:10.1089/ten.2004.10.1346. [DOI] [PubMed]
- 13.Ide C, Osawa T, Tohyama K. Nerve regeneration through allogeneic nerve grafts, with special reference to the role of the Schwann cell basal lamina. Prog Neurobiol. 1990;34(1):1–38. doi:10.1016/0301-0082(90)90024-B. [DOI] [PubMed]
- 14.Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21(16):6206–13. [DOI] [PMC free article] [PubMed]
- 15.Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuro scientific concepts and clinical significance. J Hand Surg [Am]. 2000;25(3):391–414. doi:10.1053/jhsu.2000.4165. [DOI] [PubMed]
- 16.Mackinnon SE. Surgical management of the peripheral nerve gap. Clin Plast Surg. 1989;16(3):587–603. [PubMed]
- 17.Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85:419–24. doi:10.1097/00006534-199003000-00015. [DOI] [PubMed]
- 18.Mackinnon SE, Dellon AL (eds) Surgery of the peripheral nerve. Thieme Medical, New York; 1988
- 19.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107(6):1419–29. doi:10.1097/00006534-200105000-00016. [DOI] [PubMed]
- 20.Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol. 1994;23(1):1–28. doi:10.1007/BF01189813. [DOI] [PubMed]
- 21.Matsumoto K, Ohnishi K, Sekine T, Ueda H, Yamamoto Y, Kiyotani T, et al. Use of a newly developed artificial nerve conduit to assist peripheral nerve regeneration across a long gaps in dogs. ASAIO. 2000;46:415–20. doi:10.1097/00002480-200007000-00009. [DOI] [PubMed]
- 22.Meek MF, Coert JH. Clinical use of nerve conduits in peripheral nerve repair: review of the literature. J Reconstr Microsurg. 2002;18:97–109. doi:10.1055/s-2002-19889. [DOI] [PubMed]
- 23.Millesi H. Nerve grafting. Clin Plast Surg. 1984;11(1):105–13. [PubMed]
- 24.Millesi H, Meissl G, Berger A. Further experience with interfascicular grafting of the median, ulnar, and radial nerves. J Bone Jnt Surg Am. 1976;58(2):209–18. [PubMed]
- 25.Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007;207(1):163–70. doi:10.1016/j.expneurol.2007.06.006. [DOI] [PMC free article] [PubMed]
- 26.Rappaport WD, Valente J, Hunter GC, Rance NE, Lick S, Lewis T, et al. Clinical utilization and complications of sural nerve biopsy. Am J Surg. 1993;166(3):252–6. doi:10.1016/S0002-9610(05)80968-7. [DOI] [PubMed]
- 27.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795(1–2):44–54. doi:10.1016/S0006-8993(98)00251-0. [DOI] [PubMed]
- 28.Staniforth P, Fisher TR. The effects of sural nerve excision in autogenous nerve grafting. Hand. 1978;10(2):187–90. doi:10.1016/S0072-968X(78)80012-6. [DOI] [PubMed]
- 29.Udina E, Gold BG, Navarro X. Comparison of continuous and discontinuous FK506 administration on autograft or allograft repair of sciatic nerve resection. Muscle Nerve. 2004;29(6):812–22. doi:10.1002/mus.20029. [DOI] [PubMed]
- 30.Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106(5):1036–45. doi:10.1097/00006534-200010000-00013. discussion 1046-8. [DOI] [PubMed]