Abstract
The fraudulent addition of hazelnut oil to more expensive olive oil not only causes economical loss but may also result in problems for allergic individuals as they may inadvertently be exposed to potentially allergenic hazelnut proteins. To improve consumer safety, a rapid and sensitive direct biosensor immunoassay, based on a highly specific monoclonal antibody, was developed to detect the presence of hazelnut proteins in olive oils. The sample preparation was easy (extraction with buffer); the assay time was fast (4.5 min only) and the limit of detection was low (0.08 μg/g of hazelnut proteins in olive oil). Recoveries obtained with an olive oil mixed with different amounts of a hazelnut protein containing hazelnut oil varied between 93% and 109%.
Electronic supplementary material
The online version of this article (doi:10.1007/s00216-009-2720-1) contains supplementary material, which is available to authorized users.
Keywords: Biosensor, Immunoassay, Monoclonal antibody, Olive oil, Hazelnut oil, Hidden allergens
Introduction
Undeclared traces of allergenic substances in food may cause problems for allergic individuals as they are inadvertently exposed to the offending food. In particular, traces of peanut and hazelnut are a risk as they can elicit severe reactions [1–3]. Traces of hazelnut proteins might unexpectedly be present in olive oils as, for unethical olive oil producers and importers, it may be economically attractive to adulterate extra virgin olive oils with less-expensive hazelnut oil. Within the European Union, this adulteration causes an estimated loss of four million euros per year [4]. Apart from economical loss, this adulteration may also cause problems for hazelnut-allergic individuals as Teuber et al. [5] showed that sera from allergic patients react with extract of hazelnut oil. It is now accepted that the allergenicity of edible oils is a function of the residual protein remaining after pressing and, if applicable, refining and other processing. Crude oils, having the highest protein content, provoke the majority of reactions [6].
To improve consumer safety, it is necessary to have rapid and sensitive detection methods for food allergens that can be routinely employed by food control authorities and food processors. At present, enzyme-linked immunosorbent assay (ELISA) is the most widely used method for allergen detection [7–12]. However, ELISAs are relatively labor-intensive and time-consuming, in particular for a small number of samples. These drawbacks can be overcome by the use of automated biosensors. The major advantages of these systems are their short assay time, i.e., minutes, their high degree of automation reducing labor time, the option of simultaneous detection of several analytes (in most machines), and the label-free detection. So far, only Mohammed et al. [13] and Malmheden Yman et al. [14] developed biosensor immunoassays (BIAs) intended for the detection of allergenic products in the low microgram per gram range. However, Mohammed et al. only measured standard solutions and Malmheden Yman et al. had extensive problems with nonspecific binding from food extracts using polyclonal antibodies (PAbs). These matrix problems could only partially be solved (i.e., for relatively high allergenic product concentrations) by affinity purification of the PAbs used and by applying a sandwich immunoassay format. Moreover, using polyclonal antibodies means that there is a finite amount of antibodies. Also, the use of a sandwich format doubles the analysis time.
In the present study, antihazelnut monoclonal antibodies (MAbs), having in principle an infinite supply, were developed and tested for specificity and sensitivity. We show that using a very specific MAb and a biosensor equipped with reference flow channels, in which the specific and the nonspecific binding for each sample can be observed simultaneously, a sensitive and specific direct BIA for quantitative analysis of proteins from allergenic products at trace levels in food can be developed. Furthermore, two single-step BIA formats (direct and inhibition) were compared. Hazelnut oils having different grades of refinement and olive oils contaminated with crude hazelnut oil were analyzed to evaluate this newly developed BIA.
Experimental
Instruments and reagents
1-Butanol, hydrochloric acid, phosphoric acid, sodium carbonate, sodium chloride, sodium hydrogen carbonate, and Tween 20 were purchased from VWR International (Amsterdam, The Netherlands). Antifoam A, bovine serum albumin (BSA), ovalbumin, and Tris(hydroxymethyl)aminomethane (Tris) were supplied by Sigma-Aldrich Chemie (Zwijndrecht, The Netherlands), liquid nitrogen by Linde Gas Benelux B.V. (Rotterdam, The Netherlands), and acetone by Biosolve (Valkenswaard, The Netherlands). The bicinchoninic protein assay reagents were purchased from Pierce (Rockford, IL, USA). Rabbit antimouse immunoglobulins horseradish peroxidase (RAM-HRP) was obtained from DAKO (Heverlee, Belgium), goat antirabbit HRP (GAR-HRP) was from Sigma-Aldrich Chemie (Zwijndrecht, The Netherlands), and goat antimouse immunoglobulins alkaline phosphatase (GAM-AP) from Southern Biotechnology (Birmingham, AL, USA). 5-Bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (BCIP/NBT) phosphatase substrate and solutions of tetramethylbenzidine (TMB) peroxidase substrate and peroxide were purchased from Kirkegaard and Perry Labs (Gaithersburg, MD, USA). Marvel dried skimmed milk powder was purchased from Premier International Foods Ltd. (Spalding, UK) and the RIDASCREEN® Allergen extraction buffer and RIDASCREEN® FAST Hazelnut were from R-Biopharm AG (Darmstadt, Germany). Colloidal gold total protein stain and Criterion Tris–HCl 10–20% linear gradient gels were purchased from Bio-Rad Laboratories, Inc. (Veenendaal, The Netherlands). Acrodisc filters were purchased from Pall Corporation (Ann Arbor, MI, USA) and glass filter funnels from Fisher Emergo B.V. (Landsmeer, The Netherlands). High-binding 96-well microtiter plates were purchased from Greiner Bio One (Alphen a/d Rijn, The Netherlands) and the microplate reader (Bio-tek Elx808 ultra) was from Bio-tek Instruments, Inc. (Winooski, VT, USA). Protran nitrocellulose transfer membrane of 0.2 μm was purchased from Schleicher & Schuell Bioscience Inc. (Dassel, Germany). The Akta purifier, HiTrap protein G columns (1 mL), the BIACORE 3000, sensor chips (CM5), HBS-EP buffer (pH 7.4, consisting of 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 150 mM sodium chloride, 3 mM ethylenediaminetetraacetic acid, 0.005% v/v surfactant polysorbate 20), and an amine coupling kit (containing 0.1 M N-hydroxysuccinimide (NHS), 0.4 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), and 1 M ethanolamine hydrochloride (pH 8.5)) were purchased from GE Healthcare (Uppsala, Sweden).
Immunogens, hazelnut protein extracts, and extracts for cross-reactivity studies
R-Biopharm AG kindly donated raw and roasted (140–160 °C for over 30 min) hazelnut mixtures containing different hazelnut varieties. For cross-reactivity studies, extracts were derived from legumes and fruits (soybean; chick pea; green pea; brown lentil; white bean; apple; mango; apricot; raisin), nuts and stone fruits (pecan; walnut; Brazil nut; hazelnut; coconut; almond; chestnut; cashew; pistachio nut; peanut), and various ingredients (dried egg white; egg yolk powder; cocoa butter; vanillin; butter fat; cinnamon; cocoa; milk; yeast; pumpkin seed; sunflower seed; sesame seed; poppy seed; wheat; rye; corn; rolled oats; barley; rice) as well as thickening and gelling agents (lecithin; carob; potato starch) and pollen (birch pollen; hazel pollen; walnut pollen). The pollen were obtained from Allergon AB (Ängelholm, Sweden); all other products were obtained from the local market. Extractions were performed as described elsewhere [10].
Monoclonal and polyclonal antibodies
Antihazelnut MAbs were developed according to a procedure previously described for the production of anticasein MAbs [15]. However, in this case, mice were immunized with 50 μg extracted hazelnut protein (obtained from roasted or mixtures of raw and roasted hazelnuts) and booster injections contained 25 μg extracted hazelnut protein. In total, 23 MAbs were obtained from four immunized mice. Eight of the MAbs selected in this study (see “Results and discussion” section) were isolated from the raw cell culture media (about 1 L) by ammonium sulfate precipitation followed by affinity chromatography using a HiTrap Protein G column in accordance with the manufacturer’s instruction manual. Thirty milligrams of purified MAb 50-5H9 (obtained from an immunization with roasted hazelnuts), the MAb used in the BIA, were obtained from 1 L of raw cell culture medium. Antihazelnut PAbs were raised in rabbit and sheep according to a similar immunization protocol as previously described for the development of antiflumequine PAbs [16]. However, in this case, extracted hazelnut proteins were used as the immunogen.
Antipeanut MAbs were obtained according to the same procedure as described above for the antihazelnut MAbs now using peanut proteins as immunogen. One of these MAbs, MAb 51–12D2 selected for its specificity toward peanut proteins, was used in this study as a reference MAb in the BIA.
Inhibition ELISA
All incubations were performed at a microplate shaker, for 1 h at room temperature (RT), unless stated otherwise. High-binding 96-well microtiter plates were coated with hazelnut protein (100 μL per well of a 1 μg/mL solution) in coating buffer (pH 9.6, 15 mM Na2CO3 and 35 mM NaHCO3). Subsequently, the wells were emptied and blocked with 200 μL of an ovalbumin solution (0.1% (w/v) in coating buffer). The plates were emptied, sealed, and could be stored at −20 °C for at least 4 weeks. Prior to use and after every step, as described in the following, the plate was washed three times with washing buffer (phosphate-buffered saline (PBS) containing 0.05% (v/v) Tween 20 and 0.004% (v/v) antifoam A). Wells of the microtiter plate were filled with 50 μL of standard or sample extract diluted in PBS and 50 μL in PBS containing 0.05% (v/v) Tween 20 (PBST) diluted MAbs or PAbs (e.g., in the final protocol, MAb 50-5H9 (1.5 mg/mL) was applied in a 1:375,000 dilution). After incubation and washing, the plate was incubated with 100 μL of GAR-HRP (in the case of rabbit PAbs) in a 1:5,000 dilution in PBST, RASH-HRP (in case of sheep PAbs) in a 1:8,000 dilution in PBST, or RAM-HRP (in the case of MAbs) in a 1:2,500 dilution in PBST. After washing the plate, the bound peroxidase was assessed by adding 100 μL of a freshly prepared mixture (1:1 (v/v)) of TMB peroxidase substrate and peroxidase. After incubation in the dark for 30 min at RT, the reaction was stopped by adding 100 μL of 1 M phosphoric acid and the colored product was measured at 450 nm using a microplate reader.
SDS-PAGE and immunoblotting
On an 18 comb Tris–HCl 10–20% linear gradient gel, 5 μg of protein per lane were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently semi-dry-blotted onto a nitrocellulose membrane as described elsewhere [17]. After blotting, the protein pattern was visualized using a colloidal gold total protein staining in accordance with the manufacturer’s manual. All the buffers described in the following were based on a Tris buffer (TB; pH 7.4, 50 mM TRIS, pH adjusted with 5 M HCl). The binding pattern of MAb 50-5H9 was visualized by incubation of the blotted and blocked (1 h with TB containing 1% w/v BSA) membrane with a dilution of MAb 50-5H9 in TB containing 0.5% w/v Tween 20 for 1 h at RT. After three washing steps with TB, the blot was incubated for 1 h at RT with GAM-AP in a 1:1,000 dilution in TB containing 0.33% w/v Marvel. After washing the blot, the bound alkaline phosphatase was assessed by incubating with BCIP/NBT phosphatase substrate until substantial color was obtained. Washing with water stopped the reaction.
Biosensor chip preparation
In the direct BIA format, Prot-G-purified MAbs were immobilized onto the biosensor chip (CM5) surface by the use of the amine coupling kit and the Surface Preparation Wizard as present in the BIACORE 3000 control software. The biosensor surface was activated by injecting (35 μL at a flow rate of 5 μL/min) a mixture of EDC and NHS (1:1; v/v) into one of the four flow channels (Fcs). Then the MAb, diluted (0.1 mg/mL) in coupling buffer (10 mM sodium acetate, pH 4.5), was injected and bound to the activated carboxymethylated dextran surface via its primary amine groups. After coupling, the remaining active groups were blocked with ethanolamine (1 M).
Hazelnut oils and olive oils
Two hazelnut oils and six olive oils were bought at local stores and two hazelnut oils and two olive oils were kindly donated by Minerva S.A. Edible Oils Enterprises (Athens, Greece). All oils were stored in the dark at room temperature. Using a commercially available ELISA, it was confirmed that the olive oils were blank.
Extraction procedure for oils
For extraction, 2.5 g of oil was rotated end over end with 2.5 mL of heated (60 °C) RIDASCREEN® Allergen extraction buffer for 20 min at 60 °C. Prior to use in the BIA, the samples were allowed to cool to room temperature. Upon cooling, a phase separation occurred. Samples were taken from the lower water phase. RIDASCREEN® Allergen extraction buffer is included in Allergen ELISA test kits from R-Biopharm AG and is developed for extraction of matrices with high fat content.
Direct biosensor immunoassay
In the final format, the selected antipeanut MAb (MAb 51–12D2) was immobilized in the reference Fc (Fc1) and the antihazelnut MAb 50-5H9 was immobilized in the measurement Fc (Fc2). The BIACORE 3000 operated at a temperature of 25 °C and the running buffer was HBS-EP. During all analyses, 15 μL injections at a flow rate of 20 μL/min were applied and for regeneration 20 μL of a 10 mM NaOH solution was injected at a flow rate of 50 μL/min. The total run time between two sample injections, including washing and regeneration steps in the biosensor, was 4.5 min. The relative responses measured 10 s before the regeneration started were used for calculations. For quantitative analysis, a calibration graph was prepared by spiking extract of pure extra virgin olive oil with aqueous hazelnut protein extracts [10] to give final hazelnut protein concentrations of 0–5 μg/mL. Extracts of pure hazelnut oils were diluted in extract of pure extra virgin olive oil when needed. All samples and spiked samples were analyzed in duplicate.
Results and discussion
Specificity and sensitivity of the antisera
Antihazelnut antibodies were tested in the inhibition ELISA for sensitivity with standard solutions of hazelnut protein (0 to 1,000 μg/mL) and for specificity with over 40 different foods and ingredients. The extracts of these foods and ingredients contained between 0.04 and 22 mg/mL of protein. Extracts with high protein content were tested at a protein concentration of 1 mg/mL. Extracts with protein content lower than 1 mg/mL were tested at the highest concentration possible. Practically all PAbs cross-reacted with various foods and/or ingredients. This was also observed by Holzhauser and Vieths [7] and by Malmheden Yman et al. [14], whose PAbs all displayed cross-reactivity with various food extracts even after affinity purification. A major part of the developed antihazelnut MAbs also displayed strong cross-reactions, in particular, with tree nuts. Eight out of the 23 obtained MAbs showed high specificity (no detectable cross-reactivity with any of the 40 extracts) and sensitivity (50% inhibition around 5 μg/mL) for hazelnut proteins. The antipeanut MAb applied in the reference Fc of the biosensor was selected in the inhibition ELISA (using peanut-protein-coated microplates) because of its specificity towards peanut proteins.
SDS-PAGE and immunoblotting of raw and roasted hazelnuts
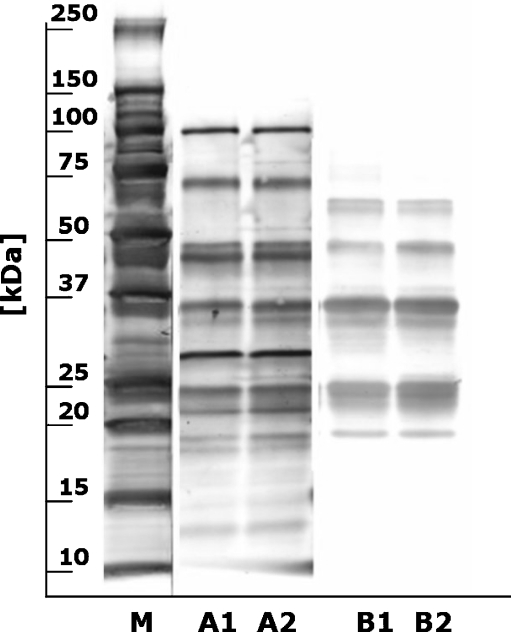
In food and oil production, raw and roasted hazelnuts are both used. To evaluate whether the protein binding profile of the MAbs was affected by roasting, a comparison was made between extracts from raw and roasted hazelnuts. The amount of extracted protein was about 10% higher for raw than for roasted hazelnuts. Holzhauser and Vieths [7] also observed that the amount of extractable protein from hazelnut and peanut varied, depending on the type of nut and the roasting conditions. This means that, depending on the nut extract used for calibration, there might be a (small) deviation between measured and exact amount of hazelnut protein present in food. Extracts of a raw and roasted mixture of different hazelnut varieties were characterized by SDS-PAGE followed by Western blotting and immunoblotting (Fig. 1). The protein profiles (Fig. 1, lanes A1 and A2) are in good agreement with hazelnut protein profiles described in literature [7, 9] and are almost identical for raw and roasted extracts. All 23 MAbs were tested in immunoblotting and the results for MAb 50-5H9 are shown in Fig. 1 (lanes B1 and B2). MAb 50-5H9 recognized proteins between 20 and 65 kDa and no difference in binding profile to raw and roasted extracts was observed. Out of all MAbs, MAb 50-5H9 was selected for application in the BIA as it demonstrated good sensitivity (50% inhibition around 5 μg/mL) and good specificity (no detectable cross-reactivity with the 40 tested foods and food ingredient extracts); it was obtained from a productive cell line and its detection of hazelnut proteins was not influenced by the roasting process. Thus, MAb 50-5H9 can be applied in immunoassays to detect traces of hazelnut in a range of processed food products with minimal risk of false-positive or false-negative test results.
Fig. 1.
Colloidal gold-stained Western blot (lanes M, A1, and A2) and MAb 50-5H9 incubated immunoblot (lanes B1 and B2) of raw (1) and roasted (2) hazelnut extracts (M = molecular mass marker)
Inhibition biosensor assay
In the biosensor, immunoassays can be developed in an inhibition and direct format (with extension to a sandwich format). In general, the inhibition format, with the antigen coated on the chip, is the more robust and stable assay format [15, 18]. On the other hand, the direct assay format, with the antibodies coated, has the advantages of a single reagent format, the use of only small amounts of antibodies, and a wide measurement range [15]. In this study, both assay formats were compared. For the inhibition assay, a hazelnut protein extract was coated to the chip and a high final immobilization response was observed (approximately 4,500 RU). The reference Fc was only activated with EDC/NHS and deactivated with ethanol amine; no reference protein was coated. MAb 50-5H9 was injected over the coated surface but only a very low response (approximately 60 RU) was observed. However, after injection of a PAb, a high response (approximately 2,500 RU) was observed in the hazelnut-coated Fc and a low response in the reference Fc, indicating specific binding of the PAb to the coated hazelnut proteins. This difference in binding of both antibodies to the coated hazelnut proteins must be a result of the higher specificity of MAb 50-5H9. This MAb only binds to a few specific proteins in the hazelnut extract (see Fig. 1, lanes B1 and B2) whereas PAbs can bind to a whole range of proteins. As a total protein extract is used for chip coating, the relative amount of the MAb-specific proteins on the chip is small, leading to low responses, whereas the amount of protein to which the PAbs can bind is much higher, leading to high responses. This problem might be overcome by affinity isolation of specific hazelnut proteins by MAb 50-5H9 and subsequent coating of these purified proteins on the chip. However, this is a labor-intensive protocol that requires high amounts of antibody. This renders the inhibition format less suitable for this specific application.
Direct biosensor assay
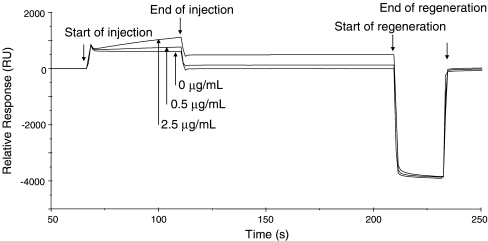
A direct BIA was developed to detect hazelnut proteins in hazelnut and olive oils. For this, prot-G-purified MAb 50-5H9 was immobilized onto the biosensor chip surface into Fc 2. A final response of 12,500 RU was obtained corresponding to 15 ng protein. In the reference Fc (Fc 1), the antipeanut MAb was immobilized to serve as blank and a final response similar to that obtained in Fc 2 was obtained. To assess the suitability of the BIA, extracts of pure extra virgin olive oil spiked with hazelnut proteins were injected through the two serially connected Fcs. For Fc 2 (the antihazelnut-coated Fc), this resulted in sensorgrams as shown in Fig. 2. The sharp change in signal upon switching between the olive oil extract and the HBS-EP buffer are caused by the difference in refractive index of both solutions. During sample injection, the signal in the antihazelnut channel increased steadily with time as a result of the binding of hazelnut proteins. Low responses (approximately 4 RU) were observed for all solutions in the antipeanut-coated reference Fc, which indicates the absence of nonspecific binding. This is in strong contrast to the findings of Malmheden Yman et al. [14], who had extensive problems with nonspecific binding from food extracts. These matrix problems were only partially solved by affinity purification of the PAbs used and by applying a sandwich immunoassay format. The use of a sandwich immunoassay format, however, doubled the analysis time and in the low concentration range nonspecific surface binding was still observed. The low nonspecific binding observed in our case may be the result of the use of a very specific MAb and of low concentrations of interfering components in oil. Moreover, the use of a biosensor equipped with a reference Fc would have allowed for subtraction of nonspecific binding when encountered. The hazelnut proteins were strongly bound by the antihazelnut MAb, as rinsing with HBS-EP buffer (after the end of the injection) did not cause any dissociation. To completely remove the bound hazelnut proteins, i.e., regeneration, 20 μL of a 10 mM NAOH solution was injected. The time needed for one complete analysis is about 4.5 min, whereas the analysis time of an (commercial) ELISA is much longer, approximately 1 or 3 h for fast or classical ELISAs, respectively. Although more samples can be analyzed in parallel using ELISAs, approximately 15 samples for fast ELISAs and 40 for classical ELISAs, the same analysis time is required for smaller number of samples. Hence, the developed BIA is very suitable for online detection and it produces results the fastest for approximately 15 samples or less.
Fig. 2.
Sensorgrams obtained with the direct BIA in the antihazelnut-coated flow channel after injecting olive oil extracts spiked with different hazelnut protein concentrations
Calibration curve and limit of detection
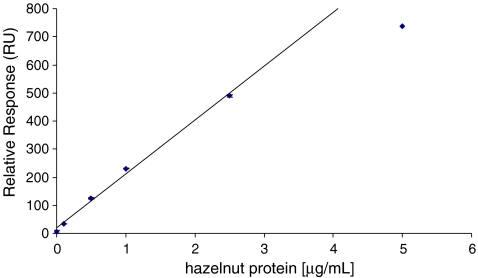
A calibration curve was constructed by plotting the concentrations of hazelnut protein (0–5 μg/mL) in extracts of pure extra virgin olive oil against the responses in the biosensor (Fig. 3). The response in the antihazelnut-coated Fc increased linearly with hazelnut protein concentration up to a concentration of 2.5 μg/mL. At a concentration of 5 μg/mL, the signal slightly leveled off. For reasons of accuracy, we used the linear calibration curve between 0 and 2.5 μg/mL. The limit of detection (LOD) of the assay was determined by analyzing eight different blank olive oils obtained from local markets in The Netherlands and Greece. Due to the applied extraction procedure (2.5 g of oil with 2.5 mL of extraction buffer), the concentrations of hazelnut proteins in the oil (μg/g) are similar to the concentrations in the extraction buffer (μg/mL). An LOD, defined as the concentration corresponding to the average signal from blank samples plus three standard deviations, of 0.08 μg/g was determined. This LOD is in the same range as commercially available ELISA kits and as the biosensor assay described previously [14]. To increase food safety for hazelnut-allergic individuals, analytical methods should be able to detect at least 1–2 μg/g of hazelnut protein in a foodstuff. In this way, only mild or no allergic reactions are to be expected [7]. Obviously, our BIA meets this requirement.
Fig. 3.
Calibration curve of hazelnut proteins in an extract of pure olive oil as obtained with the direct BIA. Samples were measured in duplicate and standard deviations are shown as error bars
Analysis of hazelnut oils
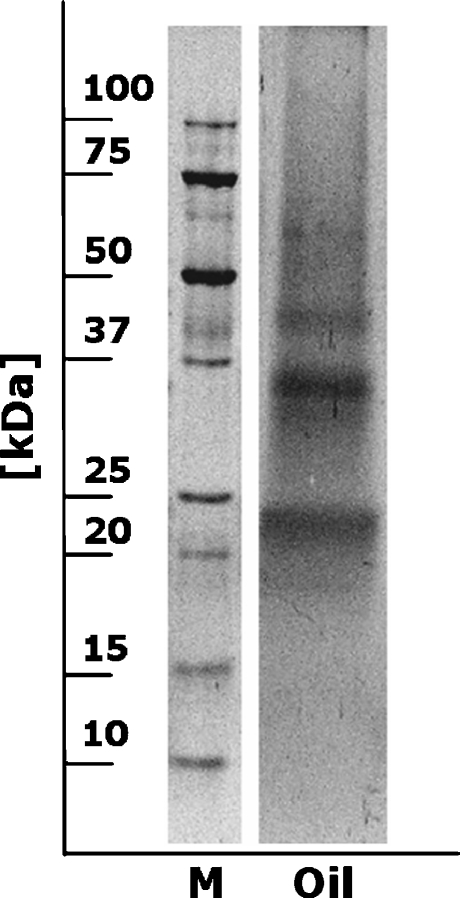
Four hazelnut oils were extracted and tested in the BIA. In one of the Greek virgin hazelnut oils, a high concentration of approximately 1,670 μg/g of hazelnut proteins was measured (after analyzing in and correcting for high diluted extracts). In the other Greek hazelnut oil, a concentration of only 0.35 μg/g hazelnut proteins was detected. In both hazelnut oils from the Dutch local market, the concentration of hazelnut proteins was below the LOD of 0.08 μg/g. A hazelnut oil analyzed by Teuber et al. [5], which was a blend of refined and unrefined oil in unknown proportion, contained 62 μg/mL of hazelnut protein. It is clear that the protein content of hazelnut oils depends on the refinement grade and that it can vary significantly. Similar observations were made for other vegetable and nut oils [5, 19–21]. In general, crude oils may contain up to a few hundred micrograms of protein per milliliter whereas highly refined oils may have a 100-fold lower protein content [6]. From the protein content, it is obvious that one Greek hazelnut oil was a crude oil and that the low-priced ones from the local market were highly refined. An important observation is that proteins present in oils can still be allergenic [5, 6]. Therefore, olive oils adulterated with crude hazelnut oils, even at a low percentages, may contain enough protein to pose a threat to allergenic individuals. A protein profile of the crude hazelnut oil extract is shown in Fig. 4. This protein profile and the profile of the hazelnut extract used for calibration are similar (Fig. 1). The protein bands from the hazelnut oil extract are less intense and some bands seem to be missing due the low protein concentration in the extract. However, all proteins detected by MAb 50-5H9 (Fig. 1) are present in the oil extract. This demonstrates that the extract used for calibration is representative for extracts obtained from (crude) hazelnut oil and can be used for calibration.
Fig. 4.
Colloidal gold-stained Western blot of crude hazelnut oil extract (M = molecular mass marker, Oil = hazelnut oil extract)
Analysis of olive oil adulterated with hazelnut oil
To assess the suitability of the assay for the detection of hazelnut proteins in olive oil, we mixed pure olive oil with different amounts (from 0.00625% up to 100%) of the crude Greek hazelnut oil that contained a high concentration of hazelnut proteins. By mixing these two oils, a more homogenous distribution of hazelnut proteins in the olive oil is obtained than by mixing olive oil with an aqueous solution of hazelnut proteins. The hazelnut protein concentrations were determined and results are presented in Table 1 which shows that even a low concentration as 0.00625% hazelnut oil, i.e., 0.10 μg/g hazelnut protein, can be detected. From the protein concentrations in these mixtures, the concentrations of hazelnut protein in pure hazelnut oil were calculated by multiplying for the applied dilutions in olive oil. Recoveries, calculated as percentages of the protein concentration found in the pure hazelnut oil, varied between 93% and 109% and, even at very low concentrations, there was a good recovery. The standard deviation of the recovery over all samples was about 5%.
Table 1.
Concentrations of hazelnut proteins found with the BIA in prepared mixtures of hazelnut oil and olive oil. Hazelnut protein concentrations in pure hazelnut oil and recoveries were calculated from the concentrations in the mixtures (correcting for the dilution)
| Prepared mixture of hazelnut oil in olive oil (%) | Hazelnut protein concentration (μg/g) | Recovery (%) | |
|---|---|---|---|
| Found in mixture | Calculated to pure hazelnut oil | ||
| 0.00625 | 0.10 | 1,559 | 93 |
| 0.0125 | 0.20 | 1,604 | 96 |
| 0.025 | 0.41 | 1,636 | 98 |
| 0.05 | 0.80 | 1,605 | 96 |
| 0.25 | 4.2 | 1,689 | 101 |
| 0.5 | 9.1 | 1,825 | 109 |
| 1 | 16.3 | 1,625 | 97 |
| 5 | 87.4 | 1,748 | 105 |
| 30 | 530 | 1,744 | 104 |
| 100 | 1,673 | 1,674 | 100 |
In literature, the protein content of only one partially unrefined hazelnut oil could be found [5]. This blend of refined and unrefined hazelnut oil contained 62 μg/mL of hazelnut protein which correlates to 68 μg/g (using a density of 0.91 g/cm3 at 20 °C, http://www.oilsbynature.com). The developed BIA should be able to detect hazelnut proteins in olive oils adulterated with only 0.12% of this hazelnut oil. From these results, it can be concluded that the developed BIA can indeed be used for the detection of hazelnut protein from (unrefined) hazelnut oil in olive oils at very low adulteration levels.
Conclusions
In conclusion, in this study, it is demonstrated that a biosensor immunoassay, based on a highly specific monoclonal antibody and developed in a biosensor equipped with a reference channel, can be used for specific and quantitative analysis of proteins from allergenic products at trace levels. With the developed assay, a concentration as low as 0.08 μg/g of hazelnut proteins can be detected in olive oils, rendering this assay sufficiently sensitive to detect hazelnut protein at levels where only mild or no allergic reactions are to be expected. With a limit of detection that is comparable to or even lower than the most sensitive ELISAs, it is obvious that the biosensor immunoassay, which is much faster and less labor consuming and due to its high degree of automation suitable for online analysis, can play an important role in increasing food safety.
Electronic Supplementary Material
Below is the link to the electronic Supplementary Material
(PDF 8.83 KB)
Acknowledgements
We are thankful for the financial support received from the European Commission within the EU project “Allergen Test” QLRT-2000-01151. We thank G. Siragakis from Minerva S.A. Edible Oils Enterprises (Athens, Greece) for supplying us with oils.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bousquet J, Bjorgsten B, Bruijnzeel-Koomen K, Huggett A, Ortolani C, Warner C, Smith M. Allergy. 1998;53:3. doi: 10.1111/j.1398-9995.1998.tb04987.x. [DOI] [PubMed] [Google Scholar]
- 2.Hefle SL, Nordlee JA, Taylor SL. Crit Rev Food Sci Nutr. 1996;36:69. doi: 10.1080/10408399609527760. [DOI] [PubMed] [Google Scholar]
- 3.Hourihane JOB. Allergy. 1998;53:84. doi: 10.1111/j.1398-9995.1998.tb04971.x. [DOI] [PubMed] [Google Scholar]
- 4.European Union Research Committee. (2001) Development and assessment of methods for the detection of adulteration of olive oil with hazelnut oil. Press Release, Brussels, Belgium
- 5.Teuber SS, Brown RL, Haapanen LAD. J. Allergy Clin Immunol. 1997;99:502. doi: 10.1016/S0091-6749(97)70077-0. [DOI] [PubMed] [Google Scholar]
- 6.Crevel RWR, Kerkhoff MAT, Koning MMG. Food Chem Tox. 2000;38:385. doi: 10.1016/S0278-6915(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 7.Holzhauser T, Vieths S. J Agric Food Chem. 1999;47:4209. doi: 10.1021/jf990478q. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SL, Nordlee JA. Food Technol. 1995;5:231. [Google Scholar]
- 9.Koppelman SA, Knulst AC, Koers WJ, et al. J Immunol Methods. 1999;229:107. doi: 10.1016/S0022-1759(99)00119-2. [DOI] [PubMed] [Google Scholar]
- 10.Drs E, Baumgartner S, Bremer M, et al. Anal Chim Acta. 2004;520:223. doi: 10.1016/j.aca.2004.04.054. [DOI] [Google Scholar]
- 11.Kiening M, Niessbner R, Drs E, et al. J Agric Food Chem. 2005;53:3321. doi: 10.1021/jf048394r. [DOI] [PubMed] [Google Scholar]
- 12.Poms RE, Klein CL, Anklam E. Food Addit Contam. 2004;21:1. doi: 10.1080/02652030310001620423. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed I, Mullett WM, Lai EP, et al. Anal Chim Acta. 2001;444:97. doi: 10.1016/S0003-2670(01)01166-7. [DOI] [Google Scholar]
- 14.Malmheden Yman I, Eriksson A, Johansson MA, et al. J AOAC Int. 2006;89:856. [PubMed] [Google Scholar]
- 15.Haasnoot W, Smits NGE, Kemmers-Voncken AEM, et al. J Dairy Res. 2004;71:322. doi: 10.1017/S0022029904000317. [DOI] [PubMed] [Google Scholar]
- 16.Haasnoot W, Gerçek H, Cazemier G, et al. Anal Chim Acta. 2007;586:312. doi: 10.1016/j.aca.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Verheijen R, Salden M, van Vernooij WJ. Manual of biological markers of disease. Dordrecht: Kluwer Academic; 1993. [Google Scholar]
- 18.Heutmekers THJ, Bremer MGEG, Haasnoot W, et al. Anal Chim Acta. 2007;586:239. doi: 10.1016/j.aca.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 19.Zitouni N, Errahali Y, Metche M, et al. J Allergy Clin Immunol. 2000;106:962. doi: 10.1067/mai.2000.110229. [DOI] [PubMed] [Google Scholar]
- 20.Olszewski A, Pons L, Moutété F, et al. Clin Exp Allergy. 1998;28:850. doi: 10.1046/j.1365-2222.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman DR, Collins-Williams C. J Allergy Clin Immunol. 1994;93:801. doi: 10.1016/0091-6749(94)90262-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 8.83 KB)