Abstract
We examined the incidence of first primary central nervous system tumors (PCNST) in California from 2001–2005. This study period represents the first five years of data collection of benign PCNST by the California Cancer Registry. California’s age-adjusted incidence rates (AAIR) for malignant and benign PCNST (5.5 and 8.5 per 100,000, respectively). Malignant PCNST were highest among non-Hispanic white males (7.8 per 100,000). Benign PCNST were highest among African American females (10.5 per 100,000). Hispanics, those with the lowest socioeconomic status, and those who lived in rural California were found to be significantly younger at diagnosis. Glioblastoma was the most frequent malignant histology, while meningioma had the highest incidence among benign histologies (2.6 and 4.5 per 100,000, respectively). This study is the first in the US to compare malignant to benign PCNST using a population-based data source. It illustrates the importance of PCNST surveillance in California and in diverse communities.
Keywords: Brain and other central nervous system neoplasms, Epidemiology, Cancer incidence, Ethnic groups, Health disparities
Introduction
Central nervous system (CNS) cancers are neoplasms of the neuroepithelial tissue and membranous coverings of the brain and spinal cord, tumors of the pituitary gland, and cancers arising from the cranial nerves and CNS hematopoietic cells. Compared to other forms of cancer, PCNST are rare. In California, these cancers generally represent only 1.5% of incident cancer cases and 2.6% of cancer deaths [1]. Despite these statistics, PCNST are an important source of cancer morbidity and mortality and generate intense interest from clinicians, researchers, the public health community, and the general public.
Many population-based epidemiologic studies of PCNST are methodologically inconsistent and potentially unreliable. CNS cancers are a heterogeneous group of diseases, comprising many histopathological forms and encompassing numerous gross anatomic sites. Their histopathological progression may be benign, malignant, or of uncertain tumor behavior. Although CNS tumor classification was standardized by the World Health Organization (WHO) in 1993, there remains variation in the organization of histologic codes for presentation and in the creation of histology sub-groups [2]. Coding trends by neuropathologists change with time, resulting in erroneous incidence fluctuations [3]. Furthermore, many epidemiologic studies are retrospective, have small sample sizes, or lack broad ethnic, cultural, and socioeconomic demographics.
With the enactment of federal Public Law 107-260 in 2004, the Benign Brain Tumor Cancer Registries Amendment Act, all state and metropolitan cancer registries are required to collect data on benign PCNST and those of uncertain tumor behavior [4]. Prior to 2004, many state and metropolitan cancer registries voluntarily collected these data, including the California Cancer Registry (CCR), which has been collecting data on benign and uncertain tumor behavior CNS tumors since 2001. In this study, we examined the incidence of first primary PCNST collected by the CCR from 2001–2005. For benign PCNST, this study period represents the first five years of data collection in California and the first population-based study of benign PCNST in the country. With a population of 36.5 million, California contains over 12% of the U.S. population, with broad ethnic, cultural, and socioeconomic representation [5]. Delineating the epidemiology of PCNST in California will create a backdrop for future basic, translational, clinical, and public health research and can be used as a baseline for monitoring incidence trends over time.
Materials and methods
Case identification
Cases used in these analyses were identified using the California Cancer Registry (CCR), a population-based registry composed of eight regional registries collecting cancer incidence and mortality data for the entire population of California. In 1985, California state law mandated the reporting of all newly diagnosed cancers in California, and statewide implementation began January 1, 1988. This state law was amended to require the collection of benign and uncertain behavior brain and other nervous system tumors beginning January 1, 2001. Cases are reported to the Chronic Disease Surveillance and Research Branch of the California Department of Public Health from all hospitals and any other facilities providing care or therapy to cancer patients residing in California (approximately 2,500 facilities) [6]. Cases diagnosed outside of California, at autopsy, or from death certificates were excluded.
For this study, first primary cases of malignant, benign and uncertain tumor behavior brain and other nervous system tumors diagnosed between January 1, 2001 and December 31, 2005 and reported to the CCR as of October 2007 were used [7]. Only cases diagnosed or treated at the reporting facility were included in these analyses. Diagnoses of 98% of the 24,944 cases were confirmed by histology (79.0%) and radiography (19.3%); the remaining 1.7% was confirmed by a variety of methods, including but not limited to cytology and clinical determination. Less than 0.5% of cases included in these analyses were confirmed by an unknown method. In this manuscript, the term “uncertain behavior” is used and synonymous with “borderline behavior”. These terms are defined similarly but their use is specific to certain classification systems. Uncertain behavior is used in the International Classification of Diseases (ICD) systems [7], while borderline behavior is the term used by the Centers for Disease Control and Prevention’s (CDC), National Programs for Central Cancer Registries (NPCR) [8], Surveillance, Epidemiology and End Results (SEER) [9] program of the National Cancer Institute, and thus the CCR. Additionally, primitive neuroectodermal tumors are abbreviated throughout the text and tables as PNET.
Only cases with anatomical sites, histology codes and tumor behavior defined as reportable in Cancer Reporting in California: Abstracting and Coding Procedures for Hospitals, Volume 1, Section II.1.9.1 and Appendix V were included in these analyses. Anatomical sites included were the meninges (C70.0–C70.9); brain (C71.0–C71.9); cerebrum (C71.0); brain lobes (C71.1–C71.4); ventricle, NOS (C71.5); cerebellum, NOS (C71.6); brain stem (C71.7); spinal cord (C72.0); cauda equina (C72.1); cranial nerves (C72.2); pituitary gland (C75.1); craniopharyngeal duct (C75.2); and pineal gland (C75.3) [10]. For other selected analyses, overlapping lesions of brain (C71.8); brain, NOS (C71.9); and/or nervous system, NOS (C72.9) were included. Although the use of some anatomical sites and histology codes differed, the 2007–2008 report of the Central Brain Tumor Registry of the United States (CBTRUS), 2000–2004, was used as a guide for the organization of histology codes in Table 2 [11]. In this study, pilocytic astrocytoma (ICD-O-3, 9421) is classified as having benign tumor behavior as is directed in ICD-O-3 [7]. This is contrary to the classification stipulated in Cancer Reporting in California: Abstracting and Coding Procedures for Hospitals, Volume 1, Section II.1.9.1 and Appendix V, in which it is classified as malignant, in accordance with SEER reporting requirements [10].
Table 2.
Number of cases and percent of first primary central nervous system tumors by behavior and histology, California, 2001–2005
| Histology | ICD-O3 histology codes | Malignant | Benign | Uncertain | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Tumors of the neuroepithelial tissue | ||||||||||
| Pilocytic astrocytoma | 9421 | 0 | 0.0 | 488 | 3.5 | 0 | 0.0 | 488 | 2.0 | |
| Diffuse astrocytoma (protoplasmic, fibrillary) | 9410, 9420 | 87 | 0.9 | 0 | 0.0 | 0 | 0.0 | 87 | 0.3 | |
| Anaplastic astrocytoma | 9401, 9411 | 644 | 7.0 | 0 | 0.0 | 0 | 0.0 | 644 | 2.6 | |
| Unique astrocytoma variants | 9383, 9384, 9424 | 36 | 0.4 | 0 | 0.0 | 82 | 5.0 | 118 | 0.5 | |
| Astrocytoma, NOS | 9400, 9412 | 587 | 6.4 | 0 | 0.0 | 3 | 0.2 | 590 | 2.4 | |
| Glioblastoma | 9440, 9441, 9442 | 4,180 | 45.3 | 0 | 0.0 | 0 | 0.0 | 4,180 | 16.8 | |
| Oligodendroglioma | 9450 | 448 | 4.9 | 0 | 0.0 | 0 | 0.0 | 448 | 1.8 | |
| Anaplastic oligodendroglioma | 9451, 9460 | 207 | 2.2 | 0 | 0.0 | 0 | 0.0 | 207 | 0.8 | |
| Ependymoma/Anaplastic ependymoma | 9391, 9392, 9393 | 418 | 4.5 | 0 | 0.0 | 0 | 0.0 | 418 | 1.7 | |
| Ependymoma variant, Myxopapillary | 9394 | 0 | 0.0 | 0 | 0.0 | 146 | 9.0 | 146 | 0.6 | |
| Mixed glioma | 9382 | 313 | 3.4 | 0 | 0.0 | 0 | 0.0 | 313 | 1.3 | |
| Glioma malignant, NOS | 9380 | 497 | 5.4 | 0 | 0.0 | 0 | 0.0 | 497 | 2.0 | |
| Choroid plexus | 9390 | 20 | 0.2 | 68 | 0.5 | 5 | 0.3 | 93 | 0.4 | |
| Neuroepithelial | 9381, 9423, 9430, 9444 | 25 | 0.3 | 0 | 0.0 | <5 | 0.2 | 27 | 0.1 | |
| Neuronal/glial, neuronal and mixed | 8680, 9413, 9490, 9492, 9493, 9500, 9501, 9505, 9506, 9508 | 59 | 0.6 | 91 | 0.6 | 200 | 12.3 | 350 | 1.4 | |
| Pineal parenchymal | 9360, 9361, 9362 | 34 | 0.4 | 0 | 0.0 | 26 | 1.6 | 60 | 0.2 | |
| PNET/medulloblastoma | 9470, 9471, 9472, 9473, 9474 | 399 | 4.3 | 0 | 0.0 | 0 | 0.0 | 399 | 1.6 | |
| Tumors of cranial & spinal nerves | ||||||||||
| Nerve sheath tumors | 9540, 9550, 9560, 9561, 9570 | 17 | 0.2 | 2,537 | 18.0 | 23 | 1.4 | 2,577 | 10.3 | |
| Tumors of meninges | ||||||||||
| Meningioma | 9530, 9531, 9532, 9533, 9534, 9537, 9538, 9539 | 138 | 1.5 | 7,257 | 51.6 | 424 | 26.0 | 7,819 | 31.4 | |
| Other mesenchymal | 8324, 8728, 8801, 8806, 8810, 8815, 8850, 8861, 8890, 8900, 8920, 9150, 9260 | 25 | 0.3 | 21 | 0.1 | 49 | 3.0 | 95 | 0.4 | |
| Hemangioblastoma | 9131, 9161, 9535 | 0 | 0.0 | 17 | 0.1 | 217 | 13.3 | 234 | 0.9 | |
| Lymphomas | ||||||||||
| 9590, 9591, 9650, 9670, 9671, 9675, 9680, 9684, 9687, 9690, 9691, 9695, 9698, 9699, 9702, 9705, 9714, 9719, 9727, 9728, 9729, 9731, 9733, 9734, 9740, 9741, 9750, 9755, 9930 | 694 | 7.5 | 0 | 0.0 | 0 | 0.0 | 694 | 2.8 | ||
| Germ cell tumors | ||||||||||
| 9060, 9064, 9065, 9070, 9071, 9080, 9081, 9084, 9085 | 157 | 1.7 | 25 | 0.2 | 0 | 0.0 | 182 | 0.7 | ||
| Tumors of sellar region | ||||||||||
| Pituitary tumors | 8270, 8271, 8272, 8280, 8290, 8300,9580 | 15 | 0.2 | 3,384 | 24.1 | 0 | 0.0 | 3,399 | 13.6 | |
| Craniopharyngioma | 9350, 9351, 9352 | 0 | 0.0 | 0 | 0.0 | 271 | 16.6 | 271 | 1.1 | |
| Local extensions from regional tumors | ||||||||||
| Chordoma | 9370, 9371, 9372 | 23 | 0.2 | 0 | 0.0 | 0 | 0.0 | 23 | 0.1 | |
| Unclassifed tumors | ||||||||||
| Hemangioma | 9120, 9121, 9122, 9130, 9133 | 0 | 0.0 | 93 | 0.7 | 3 | 0.2 | 96 | 0.4 | |
| Neoplasm, unspecified | 8000, 8005, 8010 | 208 | 2.3 | 76 | 0.5 | 179 | 11.0 | 463 | 1.9 | |
| All other | 8720, 8728, 9580, 9751 | 5 | 0.1 | 0 | 0.0 | 0 | 0.0 | 5 | 0.0 | |
| Total | – | 9,236 | – | 14,057 | – | 1,630 | – | 24,923 | – | |
PNET primitive neuroectodermal tumors
The collection of benign and borderline brain and other nervous system tumors became a statewide effort beginning in 2001; however, the nationwide effort did not begin until 2004. Inconsistencies in data collection may have occurred as a result of coding rule changes for cases diagnosed in 2004 and 2005. To utilize all eligible cases, we identified inconsistencies by cross tabulations of cases by histology, tumor behavior, and anatomical site; these were reviewed by the authors, a regional registry quality control coordinator, and a neurosurgeon to determine the accuracy of coding and the appropriate categorization for presentation. Based on their assessment, approximately 400 cases were reassigned histology, tumor behavior, and/or anatomical site codes, and approximately 100 cases were deleted from the research database entirely for this study. Specifically, all craniopharyngiomas were recoded to uncertain behavior of the craniopharyngeal duct. All cases coded as benign adenomas (ICD-O-3 8140) of the pituitary gland were recoded to pituitary adenomas (ICD-O-3 8272). Some cases (n = 65), were recoded based on visual review of the individual case abstract for various reasons. Cases with ICD-O-3 codes 9450, 9391-9393, 9380, 9370-9372, were recoded to malignant behavior. Myxopapillary ependymomas were recoded to uncertain behavior. The cases coded as hemangioblastic meningioma were recoded to hemangioblastoma.
Variables
The age, sex, race/ethnicity, and residential address of the patients that were used in these analyses were collected by the CCR from each patient’s medical record. Race/ethnicity was derived from patient self-identification, assumptions based on personal appearance, or inferences based on the race/ethnicity of the parents, birthplace, surname, or maiden name. Race/ethnicity was classified into four mutually exclusive categories of non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander. Hispanic ethnicity identification was enhanced by the use of computerized comparisons to the 1980 U.S. census list of Hispanic surnames. Patients identified as Hispanic on the medical record, or patients identified as white, black, or of unknown race with a Hispanic surname were classified as Hispanic. Use of this method can misclassify some persons as Hispanic when they are not [12].
Residential census tracts of cases, including those who used post office boxes as residential addresses and the denominator population were classified by the 2000 Rural Urban Commuting Areas (RUCA) codes. The RUCAs were developed by the University of Washington’s Rural Health Research Center and the Economic Research Service [13]. The RUCAs are a census tract-based taxonomy that utilizes the standard Census Bureau Urbanized Area and Urban Cluster definitions in combination with other work that commuted data to characterize the nation’s census tracts regarding their urban and rural status and functional relationships [14]. For these analyses, urban and rural census tracks in California were dichotomized based on Categorization C as recommended in “Using RUCA Data,” published by the Rural Health Research Center [14].
Socioeconomic status (SES) was assigned based on the patient’s census block group (2000 U.S. census) derived from their address at time of initial diagnosis as reported in the medical record. This SES variable is an index that utilizes education, employment characteristics, median household income, proportion of the population living 200% below the Federal Poverty Level, median rent, and median housing value of census tract of residence for case and denominator population. A principal components analysis was used to identify quintiles of SES ranging from one, the lowest, to five, the highest [15].
Statistical analysis
Counts and proportions were calculated using SAS 9.0 (SAS Institute, Cary, NC). Age-adjusted incidence rates (AAIR) were adjusted by the direct method and standardized to the 2000 U.S. population [16]. Age-specific incidence rates (ASIR) were calculated specific to each 5 year age group. Denominators were based on the 2000 U.S. census. All rates were calculated using SEER*Stat 6.3.6 (National Cancer Institute, Silver Spring, MD). Statistical comparisons of incidence rates were assessed using their respective confidence intervals [16]. If two comparative AAIR or ASIR confidence intervals do not overlap, the difference in the rates is considered statistically significantly.
Results
We identified 24,923 cases of first PCNST in the CCR from 2001 to 2005. A total of 9,236 (37.1%) cases were malignant, 14,057 (56.4%) cases were benign, and 1,630 (6.5%) cases were of uncertain tumor behavior. As shown in Table 1, our study population was 59.5% adult, 20–64 years old; 54.5% female; 60.8% non-Hispanic white; 46.5% of high SES; and 93.5% urban residents at time of diagnosis. The proportion of cases by age groups, SES, and location of residence at diagnosis was similar across tumor behavior, although tumors of uncertain tumor behavior appeared to occur more in the 0–19 year old age group than malignant and benign. There were statistically significant differences in the median age at diagnosis of patients with PCNST by tumor behavior (P < 0.05). For malignant PCNST, males, ethnic minorities, those of low SES, and those who lived in urban areas were significantly younger at diagnosis than females, those of high SES, non-Hispanic whites, and those who lived in rural areas. For benign PCNST, Hispanics and Asian/Pacific Islanders, those of low SES, and those who lived in urban areas were significantly younger at diagnosis (P < 0.05). The proportional incidence of PCNST, regardless of tumor behavior, was highest among those at the highest SES.
Table 1.
Number of cases and percent of first primary central nervous system tumors, population demographic characteristics and median age at diagnosis by behavior, California, 2001–2005
| Demographic characteristics | Malignant | Benign | Uncertain | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Median age | n | % | Median age | n | % | Median age | n | % | ||
| Age group, years | ||||||||||||
| 0–19 | 1,114 | 12.1 | 698 | 5.0 | 284 | 17.4 | 2,096 | 8.4 | ||||
| 20–64 | 5,202 | 56.3 | 8,676 | 61.7 | 941 | 57.7 | 14,819 | 59.5 | ||||
| 65+ | 2,920 | 31.6 | 4,683 | 33.3 | 405 | 24.8 | 8,008 | 32.1 | ||||
| Sex | ||||||||||||
| Male | 5,232 | 56.6 | 53* | 5,277 | 37.5 | 55 | 830 | 50.9 | 44* | 11,339 | 45.5 | |
| Female | 4,004 | 43.4 | 57 | 8,780 | 62.5 | 56 | 800 | 49.1 | 50 | 13,584 | 54.5 | |
| Race/ethnicity | ||||||||||||
| Non-Hispanic White | 5,965 | 64.6 | 58 | 8,266 | 58.8 | 58 | 919 | 56.4 | 53 | 15,150 | 60.8 | |
| Non-Hispanic Black | 378 | 4.1 | 51* | 902 | 6.4 | 57 | 116 | 7.1 | 47* | 1,396 | 5.6 | |
| Hispanic | 2,122 | 23.0 | 43* | 3,092 | 22.0 | 47* | 424 | 26.0 | 34* | 5,638 | 22.6 | |
| Asian-Pacific Islander | 726 | 7.9 | 51* | 1,605 | 11.4 | 57* | 157 | 9.6 | 48* | 2,488 | 10.0 | |
| Other/unknown | 45 | 0.5 | 40* | 192 | 1.4 | 52* | 14 | 0.9 | 49 | 251 | 1.0 | |
| Socioeconomic status | ||||||||||||
| Low | 3,020 | 32.7 | 51* | 4,573 | 32.5 | 54* | 585 | 35.9 | 42* | 8,180 | 32.8 | |
| Medium | 1,907 | 20.6 | 56 | 2,909 | 20.7 | 56 | 352 | 21.6 | 50 | 5,169 | 20.7 | |
| High | 4,309 | 46.7 | 56 | 6,575 | 46.8 | 56 | 693 | 42.5 | 49 | 11,579 | 46.5 | |
| Level of urbanization | ||||||||||||
| Rural | 631 | 6.8 | 58 | 859 | 6.1 | 58 | 127 | 7.8 | 54 | 1,617 | 6.5 | |
| Urban | 8,605 | 93.2 | 54* | 13,198 | 93.9 | 55* | 1,503 | 92.2 | 47 | 23,306 | 93.5 | |
| Total |
9,236 | 14,057 | 1,630 | 24,923 | ||||||||
* P-value < 0.05; female; Non-Hispanic white; SES high and rural were used as the referent catagories
The overall sex-specific AAIR for malignant PCNST was highest among males, at 6.6 per 100,000 (C.I., 6.4, 6.8). For benign PCNST, the AAIR was highest for females, at 10.0 per 100,000 (C.I., 9.8, 10.2). The sex-specific AAIR for PCNST of uncertain tumor behavior were nearly equal for males and females at 1.0 and 0.9 per 100,000.
Age-specific incidence of malignant tumors was lowest, for both males and females, in their early 20s. The ASIR for males was higher than the ASIR for females, with the gap increasing between ages 40 and 79. Incidence of malignant tumors peaked for both males and females in their late 70s and decreased thereafter. In contrast to malignant tumors, benign tumors were lowest in childhood and adolescence and increased with increasing age. ASIRs for benign tumors were consistently higher among females for all age groups except ages 10–14 (Fig. 1).
Fig. 1.
Age-specific incidence rates (ASIR) of first primary benign and malignant brain and other nervous system tumors by sex, California, 2001–2005
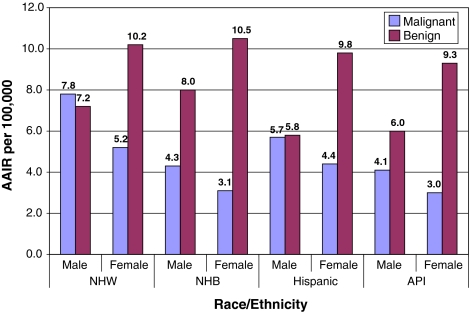
In Fig. 2, incidence of malignant tumors was highest for non-Hispanic white males, followed by Hispanic males (7.8 and 5.7 per 100,000, respectively), and lowest for Asian/Pacific Islander and non-Hispanic black females (3.0 and 3.1 per 100,000, respectively). Incidence of benign tumors was highest for all females, with rates for non-Hispanic black, non-Hispanic white, and Hispanic females being very close (10.5, 10.2 and 9.8 per 100,000, respectively).
Fig. 2.
Age-adjusted incidence rates (AAIR) of first primary central nervous system tumors by race/ethnicity, behavior and sex, California, 2001–2005
In Table 2 we see that malignant PCNST were primarily glioblastoma (45.3%), followed distantly by lymphoma (7.5%) and anaplastic astrocytoma (7.0%). The vast majority of benign tumors consisted of meningiomas (51.6%), pituitary tumors (24.1%), and nerve sheath tumors (18.0%). Tumors of uncertain behavior were distributed similarly to benign tumors, with the largest proportion being meningiomas (26.0%), followed by craniopharyngiomas (16.6%), which are tumors of the sellar region like those of the pituitary gland. These two groups were followed by hemangioblastoma (13.3%), which are considered tumors of the meninges.
As shown in Table 3, the highest incident histologies were glioblastoma (2.6 per 100,000) for malignant and meningioma (4.5 per 100,000) for benign PCNST. The AAIR for glioblastomas was 1.7 times greater in males compared to females, while the rate of meningiomas was 2.3 times greater in females compared to males. Non-Hispanic white males had the highest AAIR of glioblastoma (3.9 per 100,000), which was significantly higher than all other race/ethnic groups by sex. Non-Hispanic white males had significantly higher AAIRs than other race/ethnic groups; a higher incidence of anaplastic astrocytoma, except for Hispanic males; and a higher incidence of nerve sheath tumors, except for Asian/Pacific Islander males. The AAIRs for astrocytoma, NOS, and pilocytic astrocytoma were significantly higher for non-Hispanic white males compared to Asian/Pacific Islander males. Among females, non-Hispanic white females had the highest rate of glioblastoma (2.4 per 100,000), regardless of race/ethnicity. Non-Hispanic white females had a significantly higher AAIR compared to other race/ethnic groups for nerve sheath tumors, except for Asian/Pacific Islander females.
Table 3.
Age-Adjusted Incidence Rates (AAIR)* of selected first primary malignant and benign central nervous system tumors by histology and sex, California, 2001–2005
| Behavior | Histology | Sex | Total | NH White | NH Black | Hispanic | API | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAIR | 95% CI | AAIR | 95% CI | AAIR | 95% CI | AAIR | 95% CI | AAIR | 95% CI | |||
| Malignant | Astrocytoma, anaplastic | Total | 0.4 | 0.3, 0.4 | 0.5 | 0.4, 0.5 | 0.1 | 0.1, 0.2 | 0.3 | 0.3, 0.4 | 0.2 | 0.2, 0.3 |
| Male | 0.4 | 0.4, 0.5 | 0.6 | 0.5, 0.6 | 0.1 | 0.0, 0.2 | 0.4 | 0.3, 0.5 | 0.3 | 0.2, 0.4 | ||
| Female | 0.3 | 0.3, 0.4 | 0.4 | 0.3, 0.5 | 0.2 | 0.1, 0.3 | 0.3 | 0.2, 0.4 | 0.2 | 0.1, 0.3 | ||
| Astrocytoma, NOS | Total | 0.3 | 0.3, 0.4 | 0.4 | 0.4, 0.5 | 0.2 | 0.2, 0.4 | 0.3 | 0.3, 0.4 | 0.2 | 0.1, 0.2 | |
| Male | 0.4 | 0.3, 0.4 | 0.5 | 0.4, 0.5 | 0.4 | 0.2, 0.6 | 0.4 | 0.3, 0.5 | 0.2 | 0.1, 0.3 | ||
| Female | 0.3 | 0.3, 0.3 | 0.3 | 0.3, 0.4 | 0.1 | 0.1, 0.3 | 0.3 | 0.2, 0.4 | 0.2 | 0.1, 0.3 | ||
| Glioblastoma | Total | 2.6 | 2.5, 2.7 | 3.1 | 3.0, 3.2 | 1.5 | 1.3, 1.8 | 2.3 | 2.1, 2.5 | 1.2 | 1.1, 1.4 | |
| Male | 3.3 | 3.1, 3.4 | 3.9 | 3.7, 4.1 | 2.0 | 1.6, 2.5 | 2.8 | 2.5, 3.1 | 1.6 | 1.3, 1.9 | ||
| Female | 2.0 | 1.9, 2.1 | 2.4 | 2.3, 2.5 | 1.1 | 0.9, 1.5 | 1.9 | 1.7, 2.2 | 0.9 | 0.7, 1.1 | ||
| Oligodendroglioma | Total | 0.3 | 0.2, 0.3 | 0.3 | 0.3, 0.4 | 0.1 | 0.0, 0.2 | 0.2 | 0.2, 0.2 | 0.2 | 0.1, 0.2 | |
| Male | 0.3 | 0.3, 0.3 | 0.4 | 0.3, 0.5 | 0.1 | 0.0, 0.3 | 0.2 | 0.1, 0.3 | 0.2 | 0.1, 0.3 | ||
| Female | 0.2 | 0.2, 0.2 | 0.3 | 0.2, 0.3 | 0.1 | 0.0, 0.2 | 0.2 | 0.1, 0.3 | 0.1 | 0.1, 0.2 | ||
| Anaplastic oligodendroglioma | Total | 0.1 | 0.1, 0.1 | 0.2 | 0.1, 0.2 | 0.0 | 0.0, 0.1 | 0.1 | 0.1, 0.1 | 0.1 | 0.1, 0.1 | |
| Male | 0.1 | 0.1, 0.1 | 0.1 | 0.1, 0.2 | 0.1 | 0.0, 0.2 | 0.1 | 0.1, 0.2 | 0.1 | 0.1, 0.2 | ||
| Female | 0.1 | 0.1, 0.1 | 0.2 | 0.1, 0.2 | 0.0 | 0.0, 0.1 | 0.1 | 0.0, 0.1 | 0.1 | 0.0, 0.1 | ||
| Ependymomas, anaplastic | Total | 0.2 | 0.2, 0.3 | 0.3 | 0.2, 0.3 | 0.2 | 0.1, 0.3 | 0.2 | 0.2, 0.3 | 0.2 | 0.1, 0.2 | |
| Male | 0.2 | 0.2, 0.3 | 0.3 | 0.2, 0.3 | 0.2 | 0.1, 0.4 | 0.2 | 0.2, 0.3 | 0.1 | 0.1, 0.2 | ||
| Female | 0.2 | 0.2, 0.3 | 0.3 | 0.2, 0.3 | 0.2 | 0.1, 0.3 | 0.2 | 0.2, 0.3 | 0.2 | 0.1, 0.3 | ||
| Mixed glioma | Total | 0.2 | 0.2, 0.2 | 0.2 | 0.2, 0.3 | 0.1 | 0.0, 0.2 | 0.1 | 0.1, 0.2 | 0.2 | 0.1, 0.2 | |
| Male | 0.2 | 0.2, 0.2 | 0.3 | 0.2, 0.3 | 0.1 | 0.0, 0.2 | 0.1 | 0.1, 0.2 | 0.2 | 0.1, 0.3 | ||
| Female | 0.2 | 0.1, 0.2 | 0.2 | 0.1, 0.2 | 0.1 | 0.0, 0.3 | 0.1 | 0.1, 0.2 | 0.2 | 0.1, 0.2 | ||
| Glioma malignant, NOS | Total | 0.3 | 0.3, 0.3 | 0.3 | 0.3, 0.4 | 0.3 | 0.2, 0.4 | 0.3 | 0.2, 0.3 | 0.2 | 0.2, 0.3 | |
| Male | 0.3 | 0.3, 0.4 | 0.4 | 0.3, 0.4 | 0.3 | 0.1, 0.5 | 0.3 | 0.2, 0.4 | 0.3 | 0.2, 0.4 | ||
| Female | 0.3 | 0.2, 0.3 | 0.3 | 0.2, 0.4 | 0.3 | 0.2, 0.5 | 0.3 | 0.2, 0.4 | 0.2 | 0.1, 0.3 | ||
| PNET/Meduloblastoma | Total | 0.2 | 0.2, 0.2 | 0.2 | 0.2, 0.3 | 0.1 | 0.1, 0.2 | 0.2 | 0.2, 0.3 | 0.2 | 0.1, 0.2 | |
| Male | 0.3 | 0.2, 0.3 | 0.3 | 0.2, 0.4 | 0.1 | 0.0, 0.2 | 0.3 | 0.2, 0.4 | 0.1 | 0.1, 0.2 | ||
| Female | 0.2 | 0.1, 0.2 | 0.2 | 0.1, 0.2 | 0.1 | 0.0, 0.2 | 0.2 | 0.1, 0.2 | 0.2 | 0.1, 0.3 | ||
| Lymphomas | Total | 0.4 | 0.4, 0.5 | 0.4 | 0.4, 0.5 | 0.4 | 0.3, 0.5 | 0.4 | 0.3, 0.5 | 0.5 | 0.4, 0.6 | |
| Male | 0.5 | 0.5, 0.6 | 0.5 | 0.4, 0.6 | 0.4 | 0.3, 0.6 | 0.5 | 0.4, 0.6 | 0.6 | 0.4, 0.7 | ||
| Female | 0.3 | 0.3, 0.4 | 0.3 | 0.3, 0.4 | 0.3 | 0.2, 0.5 | 0.3 | 0.2, 0.4 | 0.4 | 0.3, 0.5 | ||
| Benign | Pilocytic astrocytoma | Total | 0.3 | 0.2, 0.3 | 0.4 | 0.3, 0.4 | 0.2 | 0.2, 0.3 | 0.2 | 0.2, 0.2 | 0.1 | 0.1, 0.2 |
| Male | 0.3 | 0.2, 0.3 | 0.4 | 0.3, 0.4 | 0.2 | 0.1, 0.4 | 0.2 | 0.2, 0.3 | 0.1 | 0.1, 0.2 | ||
| Female | 0.3 | 0.2, 0.3 | 0.4 | 0.3, 0.5 | 0.2 | 0.1, 0.4 | 0.2 | 0.2, 0.3 | 0.2 | 0.1, 0.3 | ||
| Nerve sheath | Total | 1.5 | 1.4, 1.6 | 1.8 | 1.7, 1.8 | 0.7 | 0.5, 0.9 | 1.0 | 0.9, 1.1 | 1.5 | 1.3, 1.7 | |
| Male | 1.6 | 1.5, 1.7 | 1.9 | 1.7, 2.0 | 0.8 | 0.5, 1.0 | 0.9 | 0.8, 1.1 | 1.6 | 1.4, 1.9 | ||
| Female | 1.4 | 1.4, 1.5 | 1.7 | 1.5, 1.8 | 0.6 | 0.4, 0.9 | 1.0 | 0.9, 1.2 | 1.4 | 1.2, 1.6 | ||
| Meningioma | Total | 4.5 | 4.4, 4.6 | 4.7 | 4.5, 4.8 | 5.0 | 4.5, 5.5 | 4.0 | 3.7, 4.2 | 4.2 | 3.9, 4.5 | |
| Male | 2.7 | 2.5, 2.8 | 2.9 | 2.7, 3.1 | 3.2 | 2.6, 3.8 | 2.0 | 1.7, 2.2 | 2.3 | 2.0, 2.7 | ||
| Female | 6.1 | 5.9, 6.3 | 6.3 | 6.1, 6.5 | 6.5 | 5.8, 7.2 | 5.7 | 5.3, 6.1 | 5.8 | 5.3, 6.2 | ||
| Pituitary tumors | Total | 2.0 | 1.9, 2.1 | 1.7 | 1.6, 1.8 | 3.2 | 2.9, 3.6 | 2.5 | 2.3, 2.7 | 1.8 | 1.6, 2.0 | |
| Male | 2.1 | 2.0. 2.2 | 1.8 | 1.7, 2.0 | 3.8 | 3.2, 4.5 | 2.5 | 2.3, 2.8 | 1.8 | 1.5, 2.1 | ||
| Female | 2.0 | 1.9, 2.1 | 1.6 | 1.5, 1.7 | 3.0 | 2.5, 3.5 | 2.6 | 2.4, 2.8 | 1.8 | 1.6, 2.1 | ||
* Age-adjusted incidence rates are per 100,000 population. Rates are standardized to the 2000 US population
PNET primitive neuroectodermal tumors
Non-Hispanic black males had significantly higher AAIR for pituitary tumors (3.8 per 100,000) compared to all other race/ethnic groups by sex and a significantly higher AAIR for meningiomas compared to Hispanic males. Non-Hispanic black females had a significantly higher AAIR for pituitary tumors compared to males and females in other race/ethnic groups, except non-Hispanic black males and Hispanic males and females.
Table 4 shows that malignant tumors occurred most often among adults, 20–64 years old. The exception was PNET/medulloblastoma (71.9%) which occurred mostly among children, adolescents and teens, 0–19 years old. Adults aged 65 years and older had higher proportions of glioblastomas (44.3%) and lymphomas (40.6%). Malignant tumors occurred chiefly among males with the exception of glioma and pilocytic astrocytoma, which occurred slightly more often among females. Hispanics had higher proportions of PNET/medulloblastoma (47.4%) and ependymoma/anaplastic ependymoma (33.5%). A higher proportion of lymphoma (13.5%) was seen among Asian/Pacific Islanders compared to other histologies. Most cases lived in areas of high SES, except for PNET/medulloblastoma, where 41.9% of the cases were of the lowest SES group.
Table 4.
Number of cases and percent of selected first primary malignant and benign central nervous system tumors by demographics and histology, California, 2001–2005
| Malignant | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | Anaplastic astrocytoma | Glioblastoma | Ependymoma/anaplastic ependymoma | Glioma, NOS | PNET/medullo-blastoma | Lymphoma | Other | Total | ||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Age group (years) | ||||||||||||||||
| 0–19 | 52 | 8.1 | 53 | 1.3 | 128 | 30.6 | 166 | 33.4 | 287 | 71.9 | 18 | 2.6 | 410 | 17.1 | 1,114 | 12.1 |
| 20–64 | 443 | 68.8 | 2,277 | 54.5 | 253 | 60.5 | 183 | 36.8 | 110 | 27.6 | 394 | 56.8 | 1,542 | 64.1 | 5,202 | 56.3 |
| 65+ | 149 | 23.1 | 1,850 | 44.3 | 37 | 8.9 | 148 | 29.8 | <5 | 0.5 | 282 | 40.6 | 452 | 18.8 | 2,920 | 31.6 |
| Sex | ||||||||||||||||
| Male | 363 | 56.4 | 2,441 | 58.4 | 212 | 50.7 | 247 | 49.7 | 248 | 62.2 | 398 | 57.3 | 1,323 | 55.0 | 5,232 | 56.6 |
| Female | 281 | 43.6 | 1,739 | 41.6 | 206 | 49.3 | 250 | 50.3 | 151 | 37.8 | 296 | 42.7 | 1,081 | 45.0 | 4,004 | 43.4 |
| Race/ethnicity | ||||||||||||||||
| Non-Hispanic White | 423 | 65.7 | 3,059 | 73.2 | 219 | 52.4 | 276 | 55.5 | 164 | 41.1 | 400 | 57.6 | 1,424 | 59.2 | 5,965 | 64.6 |
| Non-Hispanic Black | 16 | 2.5 | 143 | 3.4 | 23 | 5.5 | 29 | 5.8 | 13 | 3.3 | 38 | 5.5 | 116 | 4.8 | 378 | 4.1 |
| Hispanic | 152 | 23.6 | 724 | 17.3 | 140 | 33.5 | 140 | 28.2 | 189 | 47.4 | 156 | 22.5 | 621 | 25.8 | 2,122 | 23.0 |
| Asian-Pacific Islander | 50 | 7.8 | 242 | 5.8 | 34 | 8.1 | 47 | 9.5 | 31 | 7.8 | 94 | 13.5 | 228 | 9.5 | 726 | 7.9 |
| Other/unknown | <5 | 0.5 | 12 | 0.3 | <5 | 0.5 | 5 | 1.0 | <5 | 0.5 | 6 | 0.9 | 15 | 0.6 | 45 | 0.5 |
| Socioeconomic status | ||||||||||||||||
| Low | 218 | 33.9 | 1,218 | 29.1 | 157 | 37.6 | 184 | 37.0 | 167 | 41.9 | 245 | 35.3 | 831 | 34.6 | 3,020 | 32.7 |
| Medium | 131 | 20.3 | 875 | 20.9 | 69 | 16.5 | 105 | 21.1 | 77 | 19.3 | 141 | 20.3 | 509 | 21.2 | 1,907 | 20.6 |
| High | 295 | 45.8 | 2,087 | 49.9 | 192 | 45.9 | 208 | 41.9 | 155 | 38.8 | 308 | 44.4 | 1,064 | 44.3 | 4,309 | 46.7 |
| Level of urbanization | ||||||||||||||||
| Rural | 52 | 8.1 | 305 | 7.3 | 26 | 6.2 | 34 | 6.8 | 19 | 4.8 | 47 | 6.8 | 148 | 6.2 | 631 | 6.8 |
| Urban | 592 | 91.9 | 3,875 | 92.7 | 392 | 93.8 | 463 | 93.2 | 380 | 95.2 | 647 | 93.2 | 2,256 | 93.8 | 8,605 | 93.2 |
| Total | 644 | 4,180 | 418 | 497 | 399 | 694 | 2,404 | 9,236 | ||||||||
| Benign | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pilocytic astrocytoma | Nerve sheath tumors | Meningioma | Pituitary tumors | Other | Total | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||||
| Age group (years) | ||||||||||||||||
| 0–19 | 371 | 76.0 | 48 | 1.9 | 30 | 0.4 | 143 | 4.2 | 106 | 27.1 | 698 | 5.0 | ||||
| 20–64 | 111 | 22.7 | 2,037 | 80.3 | 3,863 | 53.2 | 2,444 | 72.2 | 221 | 56.5 | 8,676 | 61.7 | ||||
| 65+ | 6 | 1.2 | 452 | 17.8 | 3,364 | 46.4 | 797 | 23.6 | 64 | 16.4 | 4,683 | 33.3 | ||||
| Sex | ||||||||||||||||
| Male | 240 | 49.2 | 1,290 | 50.8 | 1,923 | 26.5 | 1,634 | 48.3 | 190 | 48.6 | 5,277 | 37.5 | ||||
| Female | 248 | 50.8 | 1,247 | 49.2 | 5,334 | 73.5 | 1,750 | 51.7 | 201 | 51.4 | 8,780 | 62.5 | ||||
| Race/ethnicity | ||||||||||||||||
| Non-Hispanic White | 250 | 51.2 | 1,639 | 64.6 | 4,626 | 63.7 | 1,528 | 45.2 | 223 | 57.0 | 8,266 | 58.8 | ||||
| Non-Hispanic Black | 31 | 6.4 | 74 | 2.9 | 460 | 6.3 | 322 | 9.5 | 15 | 3.8 | 902 | 6.4 | ||||
| Hispanic | 168 | 34.4 | 423 | 16.7 | 1,273 | 17.5 | 1,116 | 33.0 | 112 | 28.6 | 3,092 | 22.0 | ||||
| Asian-Pacific Islander | 30 | 6.1 | 326 | 12.8 | 829 | 11.4 | 385 | 11.4 | 35 | 9.0 | 1,605 | 11.4 | ||||
| Other/unknown | 9 | 1.8 | 75 | 3.0 | 69 | 1.0 | 33 | 1.0 | 6 | 1.5 | 192 | 1.4 | ||||
| Socioeconomic status | ||||||||||||||||
| Low | 191 | 39.1 | 630 | 24.8 | 2,323 | 32.0 | 1,299 | 38.4 | 130 | 33.2 | 4,573 | 32.5 | ||||
| Medium | 94 | 19.3 | 488 | 19.2 | 1,537 | 21.2 | 689 | 20.4 | 101 | 25.8 | 2,909 | 20.7 | ||||
| High | 203 | 41.6 | 1,419 | 55.9 | 3,397 | 46.8 | 1,396 | 41.3 | 160 | 40.9 | 6,575 | 46.8 | ||||
| Level of urbanization | ||||||||||||||||
| Rural | 35 | 7.2 | 165 | 6.5 | 469 | 6.5 | 159 | 4.7 | 31 | 7.9 | 859 | 6.1 | ||||
| Urban | 453 | 92.8 | 2,372 | 93.5 | 6,788 | 93.5 | 3,225 | 95.3 | 360 | 92.1 | 13,198 | 93.9 | ||||
| Total | 488 | 2,537 | 7,257 | 3,384 | 391 | 14,057 | ||||||||||
NOS not otherwise specified, PNET primitive neuroectodermal tumors
Patients with benign tumors were more similar demographically across histology groups than those with malignant tumors. Unlike malignant tumors, benign tumors occurred chiefly among females (62.5%). Benign PCNST occurred most often among adults, 20–64 years old (61.7%); non-Hispanic whites (58.8%) followed by Hispanics (22.0%); those in the higher SES groups (46.8%) followed by those in the lowest (32.5%); and urban residents (93.9%) at time of diagnosis. Meningiomas were highest among females (73.5%). Pilocytic astrocytoma (76.0%) occurred mostly among children, adolescents and teens, 0–19 years old. Among Hispanics and non-Hispanic blacks, tumors of the pituitary gland occurred more often than other anatomic sites (33% and 9.5%, respectively).
Discussion
Thirty-seven percent of California’s PCNST were found to be malignant and 56% benign. The AAIR of malignant PCNST in California was 5.8 cases per 100,000 persons, and benign PCNST was 8.5 cases per 100,000 persons.
Consistent with findings of other studies, the incidence of malignant tumors increased with increasing age except for the youngest (0–14 years) and oldest (>65 years) members of our study population [11, 17, 18]. A similar, but more monotonic age-related pattern was found for benign tumors. The highest incidence for both malignant and benign PCNST was among adults, 20–64 years old. Median age at diagnosis ranged widely by sex, race/ethnicity, SES, and the level of urbanization of patient residence. Significant differences were found in median age at diagnosis by sex for malignant but not for benign PCNST. Non-Hispanic whites were the oldest (57 years old) while Hispanics were the youngest (41 years old). Those in the lowest SES level and those who lived in rural California were significantly younger at the time of diagnosis. Disparities in median age at diagnosis could indicate etiologic differences in PCNST or disparities in healthcare utilization, access and diagnostics among these groups.
The incidence of malignant PCNST was highest among non-Hispanic white males, followed by Hispanic males [17, 19, 20]. Benign PCNST in California occurred more often among females. The AAIRs were similar across all race/ethnic groups, lead slightly by non-Hispanic black females (10.5 per 100,000). In our race/ethnicity analyses, we found significantly higher AAIRs for glioblastoma, pilocytic astrocytoma, anaplastic astrocytoma, oligodendroglioma, and nerve sheath tumors among non-Hispanic white males and females. In comparison, non-Hispanic black males and females had significantly higher rates of meningiomas and pituitary tumors [21–28]. We found higher incidence rates of nerve sheath tumors among Asian/Pacific Islanders. Unlike other studies, we found that the AAIR of pituitary tumors among Asian/Pacific Islanders was nearly the same rate as reported for non-Hispanic whites [29–32].
Overall PCNST proportional incidence, regardless of tumor behavior, was highest for the highest SES group, followed by the lowest SES group [28, 33]. Inskip et al. (2003) found similar results for gliomas and meningiomas, in which the incidence was highest among those with more education, greater income, and private insurance. The incidence among those with public insurance was also somewhat higher, possibly betraying a reporting bias linked to population healthcare coverage [33, 34]. Differential tumor detection and reporting between those with and without health insurance may account for some of the observed differences.
As in other studies, glioblastoma was the dominant histologic category for malignant PCNST (43%) in California [35, 36]. Although the proportion of cases we found in California was exceedingly higher than that found by others [11, 37], California’s AAIR (2.6 per 100,000 cases) was lower than those recorded elsewhere, which range from 3.1 to 4.8/100,000 [11, 38, 39]. The AAIR of lymphoma (0.4 per 100,000) was similar in California to that reported elsewhere [11, 38–40], despite changes in their histological classification and their effect over time [41].
The dominant histologic categories for benign PCNST were meningiomas (53.5%), tumors of the pituitary gland (24.9%), and tumors of the nerve sheath (18.7%), collectively representing 97% of California’s benign tumor cases. The AAIRs for meningiomas, tumors of the pituitary gland, and tumors of the nerve sheath were 4.5, 2.0, and 1.5 per 100,000, respectively. Californian rates for meningiomas were similar to those reported by some other studies [11, 35, 38, 39, 42] but not all [43]. The rate of pituitary tumors in California was higher than that reported by CBTRUS, 1.37 per 100,000 [11], while the incidence rate for tumors of the nerve sheath was the same as that reported for the nation.
Cancer incidence is difficult to compare across geographical areas, time-periods and information sources. Data used in national and international incidence studies can differ in diagnostic and neuropathological assessment, case ascertainment practices [44–47]. Some studies are single institution or if population-based may lack a sufficient case population to calculate incidence rates or if rates can be calculated, the use of the world standard population for incidence rates standardization rather than the U.S. standard population [48, 49].
Among U.S. CNS tumor incidence studies, counts and rates can vary by data collection sources and tumor classifications systems. All national CNS tumor incidence statistics derived cases from one of four centralized data collection sources: the NPCR from the CDC; the North American Association of Central Cancer Registries (NAACCR); SEER and CBTRUS. Both NPCR and NAACCR are population-based and cover more than 95% of the U.S. population [8, 50], while SEER, a non-random sample of central cancer registries, represents 26% of the population [9]. CBTRUS differs from these agencies since it is a voluntary repository for CNS tumor data. In 1999, CBTRUS was estimated to cover 15% of the U.S. population with contributions from 16 state registries (excluding California) [11, 51]. All of these cancer data collection agencies use the ICD-O tumor classification system [7]; whereas neuropathologists use the WHO grading system, which is specific to classifying CNS tumors [52]. Both of these tumor malignancy scales were created by the World Health Organization and can differ substantially, one essential difference is in tumor behavior categories. The ICD-O category of uncertain tumor behavior applies to CNS tumors, whereas the WHO grading scheme does not have an option for coding tumors of uncertain behavior. Coding discrepancies can occur at the individual pathology report level. This issue is further complicated by the application of data collection agency specific coding rules. Pilocytic astrocytoma is considered benign using the WHO grading system and ICD-O-3, but it is classified as malignant for reporting purposes by NAACCR and SEER and thus all North American central tumor registries. Given that pilocytic astrocytoma is a high-incidence histology, its inclusion or exclusion could significantly sway pediatric PCNST statistics. On the other hand, some statistical sources choose not to partition histologies by tumor behavior, thus the incidence of CNS tumors calculated by these sources can appear higher than from other sources [11].
Other differences in CNS tumor incidence statistics can arise from the inclusion of all CNS tumors from a single case, regardless of tumor sequence (first primary tumors versus all primary tumors) and the organization of tumor histology codes. The CCR as well as all member central cancer registries of NPCR and NAACCR, use the SEER program’s incidence site recode system that standardizes ICD-O histology subgroups [7, 9]. When comparing the incidence of pituitary gland tumors between sources, we found wide variation in the ICD-O-3 histology codes used by CBTRUS and SEER. These code subgroup differences may explain a nearly two-fold difference in reported incidence in California compared to the nation, as reported by CBTRUS [11].
This is the first study to examine both malignant and benign PCNST in California. California is a large, heavily populated state with a diverse ethnic, cultural, and socioeconomic population. This diversity is reflected in the CCR, and we were able to conduct robust analyses and make comparisons that few others can perform. The CCR’s epidemiologic value stems from the 1988 state-mandated comprehensive reporting of cancer cases from all physicians, hospitals, clinics, treatment facilities and pathology laboratories. Because a single standard is used for statewide data collection, quality assurance, and training and education for cancer registration, we acquire optimal case ascertainment and a high level of accuracy for many data items.
Our study and data source are not without limitations. This study was conducted solely using the CCR and was not supplemented with other data. The CCR data are collected for the purpose of surveillance and can be less detailed than those derived from medical records to support the design of a specific research study. Population-based cancer registry data are derived from many sources, thus, the quality of some variables may vary. Individual-level social indicators are not available to the CCR. Our SES measure is a composite of census-derived data and is more efficient for data analysis, and it avoids biases inherent in the use of individual component indicators. Another potential source of error is the misclassification of cases. Despite a rigorous data review and cleaning process, cases could have been misclassified based on tumor behavior, histology, and/or anatomical site.
Cancer surveillance identifies populations at greatest risk and which of their specific population attributes are associated with disease, providing valuable insights into disease etiology and prevention. This study of California PCNST establishes a foundation for future studies to examine age-group differences (i.e., children and seniors), specific histologies (i.e., glioblastoma and pituitary tumors), risk factors, and incidence trends over time.
Acknowledgement
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Sect. 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The authors would like to thank Mark Allen, Research Scientist II, Winny Roshala, CTR, Quality Control Specialist II and Jennifer Dodge, MS, Research Scientist I, of the California Cancer Registry for their technical support and Dr. Erica Whitney of the University of California, Davis and Cynthia Klutznick for their editorial guidance.
Disclaimer The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Monica Brown, Phone: +916-779-2687, FAX: +916-779-0264, Email: MBrown@ccr.ca.gov, Email: MonBrown@ucdavis.edu.
Rudolph Schrot, Email: Rudolph.Schrot@ucdmc.ucdavis.edu.
Katrina Bauer, Email: KBauer@ccr.ca.gov.
Deanna LeTendre, Email: DLeTendre@ccr.ca.gov.
References
- 1.California Cancer Registry (CCR): Brain and CNS Cancer Incidence, 2001–2005. SEER*Stat Database: Incidence - California, April 2008 (1988–2006), released April 2008. National Center for Health Statistics (NCHS) population estimates for 1990–2006; Benchmarked 1988–1989. California Department of Finance (DOF) population estimates July 2007. California Department of Public Health, Chronic Disease Surveillance an`d Research Section, generated by K. Bauer
- 2.Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumours. Brain Pathol 3:255–268. doi:10.1111/j.1750-3639.1993.tb00752.x [DOI] [PubMed]
- 3.McCarthy BJ, Propp JM, Davis FG, Burger PC (2008) Time trends in oligodendroglial and astrocytic tumor incidence. Neuroepidemiology 30:34–44. doi:10.1159/000115440 [DOI] [PubMed]
- 4.U.S. Congress: Public Law 107–260, Benign Brain Tumor Cancer Registries Amendment Act
- 5.U.S. Census Bureau: American Community Survey, 2003 Summary Tables. Generated by Monica Brown; using American FactFinder
- 6.Cancer Reporting in California: Abstracting and Coding Procedures for Hospitals. California Cancer Reporting System Standards, Volume 1. Section II19, Determining Reportability, Intracranial/CNS Tumors. California Department of Public Health, Chronic Disease Surveillance and Research Branch, Sacramento
- 7.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S (eds) (2000) International classification of diseases for oncology: ICD-O. World Health Organization, Geneva
- 8.National Program of Central Cancer Registries (NPCR). Centers for Disease Control and Prevention (CDC)
- 9.Surveillance Epidemiology and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch
- 10.Appendix V: Brain and CNS Site/Histology Listing. Cancer Reporting in California: Abstracting and Coding Procedures for Hospitals, California Cancer Reporting System Standards. California Department of Public Health, Chronic Disease Surveillance and Research Branch
- 11.CBTRUS Statistical Report: Primary Brain Tumors in the United States, 2000–2004. Central Brain Tumor Registry of the United States, 2008
- 12.Stewart SL, Swallen KC, Glaser SL, Horn-Ross PL, West DW (1999) Comparison of methods for classifying Hispanic ethnicity in a population-based cancer registry. Am J Epidemiol 149:1063–1071 [DOI] [PubMed]
- 13.Rural Urban Commuting Area Code US Department of Agriculture Economic Research Service, 2001, Washington, DC
- 14.Rural Health Research Center (RHRC): The Use of RUCAS (Version 2.0) in Health Care. The University of Washington, WWAMI, Center for Health Workforce Studies, Seattle
- 15.Yost K, Perkins C, Cohen R, Morris C, Wright W (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711. doi:10.1023/A:1011240019516 [DOI] [PubMed]
- 16.Fliess JL, Levin B, Paik MC (2003) Statistical methods for rates and proportions. Wiley, New York
- 17.Surveillance Epidemiology and End Results (SEER) Program Age-Adjusted Incidence Rates & 95% Confidence Intervals for Brain and Other Nervous System Cancer, All Ages SEER 17 Registries for 2000–2004. In: Surveillance E, and End Results Program (ed). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch
- 18.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC (2006) Trends in brain cancer incidence and survival in the United States: surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg Focus 20:E1. doi:10.3171/foc.2006.20.4.1 [DOI] [PubMed]
- 19.Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, Miller B, Williams M, Ward E, Wingo PA, Ramirez A, Edwards BK (2006) Annual report to the nation on the status of cancer, 1975–2003 featuring cancer among U.S. Hispanic/Latino populations. Cancer 107:1711–1742. doi:10.1002/cncr.22193 [DOI] [PubMed]
- 20.Canto MT, Chu KC (2000) Annual cancer incidence rates for Hispanics in the United States: surveillance, epidemiology, and end results, 1992–1996. Cancer 88:2642–2652. doi:10.1002/1097-0142(20000601)88:11<2642::AID-CNCR29>3.0.CO;2-S [DOI] [PubMed]
- 21.Robertson JT, Gunter BC, Somes GW (2002) Racial differences in the incidence of gliomas: a retrospective study from Memphis, Tennessee. Br J Neurosurg 16:562–566 [PubMed]
- 22.Davis FG, Malinski N, Haenszel W, Chang J, Flannery J, Gershman S, Dibble R, Bigner DD (1996) Primary brain tumor incidence rates in four United States regions, 1985–1989: a pilot study. Neuroepidemiology 15:103–112. doi:10.1159/000109895 [DOI] [PubMed]
- 23.Polednak AP, Flannery JT (1995) Brain, other central nervous system, and eye cancer. Cancer 75:330–337. doi:10.1002/1097-0142(19950101)75:1+<330::AID-CNCR2820751315>3.0.CO;2-5 [DOI] [PubMed]
- 24.Fan KJ, Pezeshkpour GH (1992) Ethnic distribution of primary central nervous system tumors in Washington, DC, 1971 to 1985. J Natl Med Assoc 84:858–863 [PMC free article] [PubMed]
- 25.Fan KJ, Kovi J, Earle KM (1977) The ethnic distribution of primary central nervous system tumors: AFIP, 1958 to 1970. J Neuropathol Exp Neurol 36:41–49. doi:10.1097/00005072-197701000-00005 [DOI] [PubMed]
- 26.Choi NW, Schuman LM, Gullen WH (1970) Epidemiology of primary central nervous system neoplasms. I. mortality from primary central nervous system neoplasms in Minnesota. Am J Epidemiol 91:238–259 [DOI] [PubMed]
- 27.Heshmat MY, Kovi J, Simpson C, Kennedy J, Fan KJ (1976) Neoplasms of the central nervous system. Incidence and population selectivity in the Washington DC, metropolitan area. Cancer 38:2135–2142. doi:10.1002/1097-0142(197611)38:5<2135::AID-CNCR2820380543>3.0.CO;2-T [DOI] [PubMed]
- 28.Preston-Martin S, Henderson BE, Peters JM (1982) Descriptive epidemiology of central nervous system neoplasms in Los Angeles County. Ann N Y Acad Sci 381:202–208. doi:10.1111/j.1749-6632.1982.tb50386.x [DOI] [PubMed]
- 29.Kuratsu J, Ushio Y (1996) Epidemiological study of primary intracranial tumors: a regional survey in Kumamoto prefecture in the southern part of Japan. J Neurosurg 84:946–950 [DOI] [PubMed]
- 30.Kepes JJ, Chen WY, Pang LC, Kepes M (1984) Tumors of the central nervous system in Taiwan, Republic of China. Surg Neurol 22:149–156. doi:10.1016/0090-3019(84)90043-0 [DOI] [PubMed]
- 31.Ho-cheong H (1979) Intracranial tumours among Chinese in Hong Kong. Surg Neurol 12:317–318 [PubMed]
- 32.Cheng MK (1982) Brain tumors in the People’s Republic of China: a statistical review. Neurosurgery 10:16–21 [PubMed]
- 33.Inskip PD, Tarone RE, Hatch EE, Wilcosky TC, Fine HA, Black PM, Loeffler JS, Shapiro WR, Selker RG, Linet MS (2003) Sociodemographic indicators and risk of brain tumours. Int J Epidemiol 32:225–233. doi:10.1093/ije/dyg051 [DOI] [PubMed]
- 34.Barker DJ, Weller RO, Garfield JS (1976) Epidemiology of primary tumours of the brain and spinal cord: a regional survey in southern England. J Neurol Neurosurg Psychiatry 39:290–296. doi:10.1136/jnnp.39.3.290 [DOI] [PMC free article] [PubMed]
- 35.Bauchet L, Rigau Vr, Mathieu-Daude′ Hln, Figarella-Branger D, Hugues D, Palusseau L, Bauchet F, Fabbro M, Campello C, Capelle L, Durand A, Tre′tarre B, Frappaz D, Henin D, Menei P, Honnorat Jr, Segnarbieux Fo (2007) French brain tumor data bank: methodology and first results on 10,000 cases. J Neurooncol [DOI] [PubMed]
- 36.Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG (1999) Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro-oncol 1:14–25 [DOI] [PMC free article] [PubMed]
- 37.Hoffman S, Propp JM, McCarthy BJ (2006) Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro-oncol 8:27–37. doi:10.1215/S1522851705000323 [DOI] [PMC free article] [PubMed]
- 38.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL (2007) Epidemiology of brain tumors. Neurol Clin 25:867–890 vii [DOI] [PubMed]
- 39.Dobec-Meic B, Pikija S, Cvetko D, Trkulja V, Pazanin L, Kudelic N, Rotim K, Pavlicek I, Kostanjevec AR (2006) Intracranial tumors in adult population of the Varazdin County (Croatia) 1996–2004: a population-based retrospective incidence study. J Neurooncol 78:303–310. doi:10.1007/s11060-005-9100-2 [DOI] [PubMed]
- 40.Cote TR, Manns A, Hardy CR, Yellin FJ, Hartge P (1996) Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. AIDS/Cancer Study Group. J Natl Cancer Inst 88:675–679. doi:10.1093/jnci/88.10.675 [DOI] [PubMed]
- 41.Clarke CA, Undurraga DM, Harasty PJ, Glaser SL, Morton LM, Holly EA (2006) Changes in cancer registry coding for lymphoma subtypes: reliability over time and relevance for surveillance and study. Cancer Epidemiol Biomarkers Prev 15:630–638. doi:10.1158/1055-9965.EPI-05-0549 [DOI] [PubMed]
- 42.Pobereskin LH, Chadduck JB (2000) Incidence of brain tumours in two English counties: a population based study. J Neurol Neurosurg Psychiatry 69:464–471. doi:10.1136/jnnp.69.4.464 [DOI] [PMC free article] [PubMed]
- 43.Christensen HC, Kosteljanetz M, Johansen C (2003) Incidences of gliomas, meningiomas in Denmark, 1943 to 1997. Neurosurgery 52:1327–1333 discussion 1333–1324 [DOI] [PubMed]
- 44.Castillo MS, Davis FG, Surawicz T, Bruner JM, Bigner S, Coons S, Bigner DD (2004) Consistency of primary brain tumor diagnoses and codes in cancer surveillance systems. Neuroepidemiology 23:85–93. doi:10.1159/000073980 [DOI] [PubMed]
- 45.Bruner JM, Inouye L, Fuller GN, Langford LA (1997) Diagnostic discrepancies and their clinical impact in a neuropathology referral practice. Cancer 79:796–803. doi:10.1002/(SICI)1097-0142(19970215)79:4<796::AID-CNCR17>3.0.CO;2-V [DOI] [PubMed]
- 46.(1989) Intraobserver reproducibility in assigning brain tumors to classes in the World Health Organization diagnostic scheme. The Childhood Brain Tumor Consortium J Neurooncol 7:211–224 [DOI] [PubMed]
- 47.Prayson RA, Agamanolis DP, Cohen ML, Estes ML, Kleinschmidt-DeMasters BK, Abdul-Karim F, McClure SP, Sebek BA, Vinay R (2000) Interobserver reproducibility among neuropathologists and surgical pathologists in fibrillary astrocytoma grading. J Neurol Sci 175:33–39. doi:10.1016/S0022-510X(00)00274-4 [DOI] [PubMed]
- 48.Bray F, Guilloux A, Sankila R, Parkin DM (2002) Practical implications of imposing a new world standard population. Cancer Causes Control 13:175–182. doi:10.1023/A:1014344519276 [DOI] [PubMed]
- 49.Anderson RN, Rosenberg HM (1998) Age standardization of death rates: implementation of the year standard 2000. Natl Vital Stat Rep 47:1–16 20 [PubMed]
- 50.North American Association of Central Cancer Registries (NAACCR). NAACCR
- 51.Davis FG, McCarthy BJ (2001) Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther 1:395–401. doi:10.1586/14737140.1.3.395 [DOI] [PubMed]
- 52.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO classification of tumours of the central nervous system. WHO Press, Geneva [DOI] [PMC free article] [PubMed]