Abstract
Background:
Bleeding on probing (BOP) is an indicator of tissue inflammatory response to bacterial pathogens. Due to anatomical limitations, the entity and physical state of microbial aggregations located under the gingival margin and their relations to BOP have been hardly investigated till now. The recent introduction of the endoscopy has allowed clinicians to observe the subgingival environment in a non-traumatic way. The aim of this study is to evaluate the correlation between BOP and subgingival deposits by using this new technology.
Methods:
107 teeth (642 individual sites) from 16 periodontal patients, treated with scaling and root planing, were evaluated for plaque index (PI), gingival index (GI), probing pocket depth (PPD), bleeding on probing (BOP), endoscopic biofilm index (EBI), and endoscopic calculus index (ECI) at one-month revaluation.
Results:
A linear association between BOP and PD, EBI, and ECI was detected. The BOP provided a high level of specificity but quite low sensitivity values both for ECI (sensitivity 40%, specificity 86%) and EBI (sensitivity 37%, specificity 89%). The BOP sensitivity was directly linked to the amount of subgingival deposits.
Conclusions:
This study demonstrates a direct relationship between BOP and presence/amount of subgingival deposits. More investigations on larger samples are, however, needed.
Keywords: Endoscopy, bleeding on probing, pocket depth, root planing, biofilm, calculus.
INTRODUCTION
Customary diagnosis of periodontal disease features the gathering of certain clinical and radiographical parameters. By using these observations, the clinician attempts to determine the patient’s periodontal status, and identify clinical signs that allow predicting the disease course.
The bleeding on probing (BOP) is a widely used clinical sign as indicator of the periodontal condition and disease progression [1].
Its clinical relevance has been shown and supported by several studies. A study on this topic, published in 1990 by Lang and colleagues, demonstrates how the absence of BOP represents a reliable indicator of periodontal stability [2]. Some years later Albandar and colleagues reported that, in individuals with early-onset periodontitis, existing lesions show more disease progression in sites with overt gingival inflammation than in sites where it was absent [3]. Recently, it has been demonstrated how a persistent presence of gingivitis in a periodontal site all over a long period of observation (26 years), is responsible for future periodontal breakdown [4]. Besides, the value of BOP as predictor of future periodontal deterioration seems to significantly increase when associated with periodontal pocket depth greater than or equal to 6 mm [5].
Even though all these findings point out the important role of BOP on present and future periodontal conditions, many are the variables that can play a confounding role on it. For instance it has been clearly shown how the smoke habit reduces the bleeding response to the periodontal probing [6]. As well, the fact that BOP could appear either from a deep periodontal tissue inflammation or to a superficial one could have important implications that shouldn’t be underestimated. Additionally, in spite of a quite clear connection between gingival bleeding and inflammation state, very few are known about the entity and physical state of subgingival bacterial deposits and BOP.
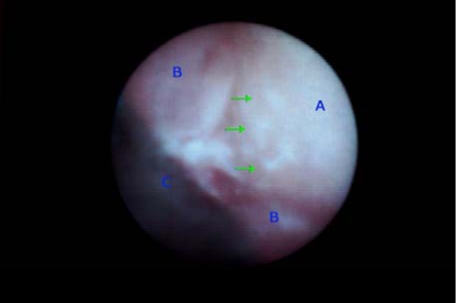
To date the anatomical peculiarities of the area have undeniably limited the investigation on this topic. The recent introduction of the endoscopy in periodontology (DV2, Dental View, Irvine, CA) has given for the first time a non-traumatic visual access to the subgingival area, consequently, opening new clinical research possibilities [7] (Fig. 1).
Fig. (1).
Subgingival visualization of the root surface (A). It is possible to distinguish: some calcified deposits (green arrows), the pocket wall (B) and the “shield” (C), which is a portion of the instrument developed to convey the endoscopical fiber beneath the gingival margin.
The aim of this study is to verify, by using this innovative tool, the presence of subgingival deposits and evaluate their correlation with BOP and other periodontal clinical parameters.
MATERIALS AND METHODS
From the beginning of February 2008, sixteen consecutive subjects attending the Periodontal Department of the University of Bologna were selected for the study. The inclusion criteria were: presence of generalized moderate to severe periodontitis [8], non-smoker, no drug consumption for at least one month, no systemic pathologies, and no prosthetic dental treatments. Informed consents were obtained and the tenets of the Declaration of Helsinki were followed.
Patients were treated with closed subgingival scaling and root-planing under local anesthesia. Oral hygiene instruction and motivation were included. After a healing period of 1 month, re-evaluation was carried out and the endoscopic data collected. The endoscopical observation was performed on a randomly selected quadrant for each patient. The random allocation was obtained drowning from a box containing 16 tickets reporting the four oral quadrants all equally represented. The chosen ticket was automatically thrown away, consequently, at the final observation, an automatic assignation was resulted.
The same general dentist with special postgraduate training in periodontics performed the initial preparations. The operator was unacquainted with the aim of the clinical trial.
Two examiners (A and B) collected the data; both were experienced dentists with graduate training in periodontics. Both the examiners were blinded to the findings obtained by the other and both had been calibrated for reproducibility prior to the study. Six sites for each tooth were evaluated: mesiofacial, midfacial, distofacial, distolingual, midlingual, and mesiolingual. All sites of third molars were disregarded.
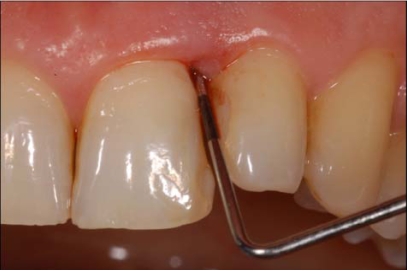
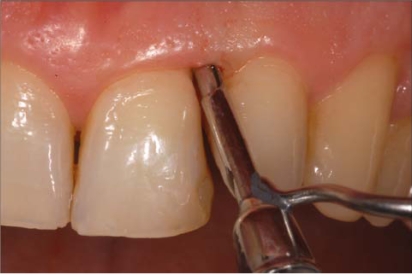
Examiner A using a standardized periodontal probe (CP11 Hu Friedy, Europe) detected: Plaque Index (PI) [9], Gingival Index (GI) [10], Probing Pocket Depth (PPD), and BOP (Fig. 2). The BOP was considered positive if bleeding occurred between 30 seconds after probing. Examiner B under endoscopic vision recorded the Endoscopic Biofilm Index (EBI) and the Endoscopic Calculus Index (ECI) at least 15 minutes later the preceding examination (Fig. 3) [11]. A time interval of 15 minutes was used in order to limit the possibility that the first exam, especially when bleeding was present, could affect the subsequent evaluation. The two endoscopic indices, reported on Table 1, have been recently purposed with the aim to distinguish different degrees and physical state of subgingival deposits.
Fig. (2).
Probing depth (PD) and bleeding on probing (BOP) recording.
Fig. (3).
Endoscopical visualsization of the subgingival area. The fiber is delivered to the gingival margin coupled into an instrument called “explorer”.
Table 1.
Endoscopic Biofilm Index and Endoscopic Calculus Index: Characteristics and Definitions [11]
| Endoscopic Biofilm Index | |
|---|---|
| Degree | Definition |
| 0 | No observable biofilm on root surface |
| 1 | Separate flecks of biofilm on less than 1/3 of the surface |
| 2 | A thin continuous band of biofilm on 2/3 of the surface |
| 3 | A continuous band of biofilm on the entire surface |
| Endoscopic Calculus Index | |
| 0 | No observable calculus on root surface |
| 1 | Separate flecks of calculus |
| 2 | A coalition of calculus deposits covering < 50% of the visual field |
| 3 | A thick,, diffuse accumulation of calculus covering >50% of the visual field |
The statistical analysis, performed on tooth sites, was made using the simple and multiple Chi Square tests (χ2), the Mantel-Haenszel test for linear association and the Relative Risk with a 95% confidence interval. The Kruskal-Wallis test was used to study the correlations between both tooth type or tooth site and the three variables BOP, EBI, ECI. The SPSS program was utilized for our data analysis.
Relative risk analysis for EBI and ECI was carried out using all values greater than or equal to 1 as a common variable.
RESULTS
None of the selected patients dropped the study or was disqualified. The 16 subjects examined were 9 females and 7 males with an average age of 50 years (45-54). PI percentage was between 22% and 46%. PPD at the time of the analysis was between 1 and 9 mm with a median value of 3 mm. The study sample consisted of 107 teeth corresponding to 642 sites.
33% of the sites had GI=0, 55% had GI =1, and the remaining 12 % had GI=2.
The BOP resulted correlated to the tooth type (χ²=114.9 p<0.0001), while either EBI or ECI correlated to tooth site too (EBI-tooth type χ²=81.9 p<0.0001, EBI-tooth site χ²=124.7 p<0.0001; ECI-tooth type χ²=60.1 p<0.0001, ECI-tooth site χ²=110.1 p<0.0001). The BOP as the subgingival deposits was more frequently observed on molars and premolars than on anterior teeth.
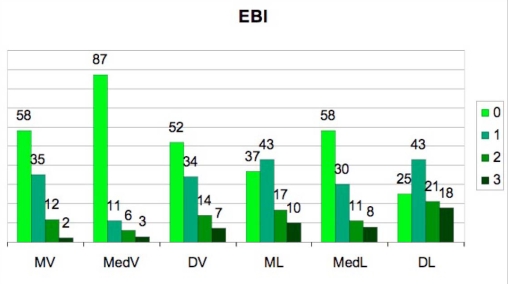
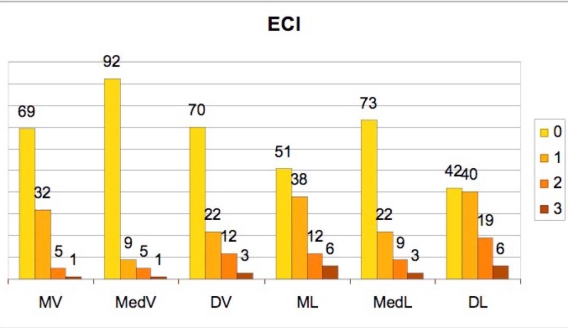
Figs. (4) and (5) show the distributions of EBI and ECI, respectively. The presence of subgingival residual deposits tended to be higher in the lingual area relative to the vestibular surfaces. This difference is statisticaly significant (EBI χ²=36.95 p=0.0001; ECI χ²=27.89 p=0.0001).
Fig. (4).
Incidence of the Endoscopic Biofilm Index (EBI) degrees in the 6 studied sites.
(MV=mesio-vestibular, MedV=medio-vestibular, DV=disto-vestibular, ML=mesio-lingual, MedL=medio-lingual DL=disto-lingual).
Fig. (5).
Incidence of the Endoscopic Calculus Index (ECI) degrees in the 6 studied sites.
(MV=mesio-vestibular, MedV=medio-vestibular, DV=disto-vestibolar, ML=mesio-lingual, MedL=medio-lingual, DL=disto-lingual).
The analysis of linear association between BOP and GI, PI, PD, EBI, and ECI is reported in Table 2.
Table 2.
Linear Association Analysis Between Bleeding on Probing and respectively Gingeval Index, Probing Depth, Plaque Index, Endoscopic Biofilm Index, and Endoscopic Calculus Index
| BOP-GI | BOP-PI | BOP-PD | BOP- EBI | BOP-ECI | |
|---|---|---|---|---|---|
| P χ2 | P χ2 | P χ2 | P χ2 | P χ2 | |
| Mesio-Vestibular | 0.009 6.72 | 0.007 7.24 | 0.0001 27.3 | 0.0001 24.06 | 0.0001 21.3 |
| Medio-Vestibular | 0.009 6.80 | 0.026 4.96 | 0.0001 21.8 | 0.0004 12.50 | 0.0002 18.3 |
| Disto-Vestibular | 0.006 7.51 | NS | 0.001 10.1 | 0.0001 15.3 | 0.0002 18.2 |
| Mesio-Lingual | 0.025 5.04 | NS | 0.0001 20.2 | 0.002 9.2 | 0.0001 14.5 |
| Medio-Lingual | 0.005 7.87 | NS | 0.0001 16.4 | 0.0001 22.2 | 0.0037 8.4 |
| Disto-Lingual | NS | NS | 0.0004 12.5 | 0.0002 18.2 | 0.0006 11.8 |
BOP: bleeding on probing; GI: gingival index; PD: probing depth; PI: plaque index; EBI: endoscopic biofilm index; ECI: endoscopic calculus index; NS: statistically not significant.
The analysis of the data shows a strong significance of the linear association to PD, EBI, and ECI in every site examined, but not the same for GI and PI. In particular, PI has a weak significant correlation with BOP only in 2 of the 6 areas analyzed.
Table 3 shows the analysis of the linear association between PD and EBI, and between PD and ECI. A significant correlation was found in all sites with the exception of the disto-vestibular one.
Table 3.
. Linear Association Analysis Between Probing Depth and Respectively Endoscopic Biofilm Index and Endoscopic Calculus Index
| PD - EBI | PD - ECI | |
|---|---|---|
| P χ2 | P χ2 | |
| Mesio-Vestibular | 0.0001 15.9 | 0.0007 11.5 |
| Medio-Vestibular | 0.0001 38.4 | 0.0001 50.1 |
| Distal-Vestibular | NS | NS |
| Mesio-Lingual | 0.0001 15.4 | 0.002 9.6 |
| Medio-Lingual | 0.0001 19.3 | 0.024 5.06 |
| Distal-Lingual | 0.0005 11.9 | 0.047 3.9 |
PD: probing depth; EBI: endoscopic biofilm index; ECI: endoscopic calculus Index; NS: statistically not significant.
The analysis of the relative risk between the presence of BOP and the presence of subgingival deposits (ECI and EBI) is seen in Table 4. From such analysis, there is a significant relative risk in almost all examined sites. Only one site resulted not significant for both ECI and EBI, however, the obtained values were very close to the limits.
Table 4.
. Relative Risk Analysis between Bleeding on Probing and, Respectively, Endoscopic Biofilm Index and Endoscopic Calculus Index
| EBI-BOP | ECI-BOP | |||
|---|---|---|---|---|
| RR | Confidence Limits 95 % | RR | Confidence Limits 95 % | |
| Mesio-Vestibular | 4.34 | 1.91 - 9.84 | 3.27 | 1.68 - 6.35 |
| Medio-Vestibular | 5.44 | 2.46 - 12.01 | 6.13 | 2.91 - 12.93 |
| Disto-Vestibular | 4.35 | 1.79 - 10.60 | 3.41 | 1.76 - 6.61 |
| Mesio-Lingual | 1.59 NS | 0.74 - 3.38 | 2.28 | 1.10 - 4.71 |
| Medio-Lingual | 2.71 | 1.21 - 6.04 | 1.97 NS | 0.97 - 4.00 |
| Disto-Lingual | 8.23 | 1.18 - 57.60 | 2.37 | 1.05 - 5.35 |
BOP: bleeding on probing; EBI: endoscopic biofilm index; ECI: endoscopic calculus index; RR: relative risk; NS: statistically not significant.
The diagnostic sensitivity and specificity of BOP in sites exhibiting subgingival residual deposits under endoscopic observation are, respectively, 37% and 89% for EBI [sensitivity: 119/(119+206); specificity: 283/(34+283)] and 40% and 86% for ECI [sensitivity: 98/(98+147); specificity: 342/(55+342)]. The BOP provided a very high level of specificity but low sensitivity values both for ECI and EBI.
In order to better understand the sensitivity of BOP, a detailed analysis on the different classes of ECI and EBI was performed. As a result, the BOP sensitivity was directly linked to the dimension of the subgingival deposits, as reported on Table 5.
Table 5.
Diagnostic Sensitivity of Bleeding on Probing to the Degree of Subgingival Deposits
| EBI & ECI Degree | EBI N | BOP N | Sensitivity % | ECI N | BOP N | Sensitivity % |
|---|---|---|---|---|---|---|
| 1 | 224 | 54 | 24 | 163 | 170 | 29 |
| 2 | 81 | 36 | 44 | 62 | 45 | 56 |
| 3 | 48 | 32 | 67 | 20 | 16 | 75 |
EBI: endoscopic biofilm index; ECI: endoscopic calculus index; BOP: bleeding on probing.
DISCUSSION
Nowadays it is commonly accepted that bacterial deposits are the key factor in the onset of periodontal disease [12]. As a consequence the primary aim of periodontal treatment is to recreate a healthy periodontal state by the removal of microorganism and their derivations from the tooth surfaces [13]. The evaluation of the efficacy of removal of bacterial deposits from the subgingival root surface has been, therefore, an important topic of many scientific studies on the periodontal treatments efficiency [14-19]. Unfortunately, all of these studies evaluated the presence of residual deposits on extracted teeth, condition that undoubtedly limits clinical considerations.
The association between BOP, important sign of clinical inflammation, and periodontal destruction has been studied [2,4,5]. Though a direct association between them has been demonstrated, it has also been shown that a high percentage of sites with gingival inflammation and/or calculus could not develop attachment loss [3].
These observations derive the consciousness that a deeper knowledge on this topic is still needed.
The recent introduction of the endoscopy in periodontology has open new research possibilities in this field. The use of this new high tech instrument seems to give additional and interesting information, rather than just using traditional clinical parameters. Actually, it is the first time that it is given the possibility to atraumatically observe the root deposits beneath the gingival margin and their effect on the surrounding periodontal soft tissues [7].
The present study is based on this innovation.
Our endoscopical observations of subgingival residues after scaling and root-planing gave results in line with those found by previous studies on extracted teeth [15,16,18]. The plaque and calculus residues were detected, respectively, in 55% and 38% of the examined sites. The residues, both calcified and not, were mostly found on the lingual side (Fig. 4, Fig. 5) emphasizing the difficulty of access to this area, both for the clinician and for personal oral hygiene.
The low correlation detected between GI and BOP is probably due to the study design (Table 2). The scaling and root planing together with the patients’ oral hygiene have undoubtedly contributed to a general state of health of the periodontal soft tissue as measured by the GI. This result demonstrates how the clinical parameters of the gingival tissues do not always reflect the state of the deeper portion of the periodontal soft tissues.
The absence of correlation between PI and BOP can be explained by variations in personal oral hygiene (Table 2). We know, from the work by Löe et al. (1965), that the onset of gingivitis requires a presence of bacterial plaque for some length of time [20].
Another explanation of the absence of correlation detected could be the plaque index used in this study [9]. It does not allow a distinction in degree of plaque deposits quantity, therefore, a small deposit was counted equal to a much larger one. Thus, it is difficult to show the specific influence of supragingival soft deposits on periodontal tissues.
ECI and EBI resulted correlated either to tooth type or site. Higher values of these indices were reported for molar teeth and inter-proximal areas. This result confirms the challenge these areas represent for the clinician to obtain, during scaling and root planing, an exhaustive debridement of the root surfaces.
Regarding the relationship between subgingival deposits and the surrounding soft tissues, a recent endoscopical publication has interestingly described a pathological connection. In that study, both calculus and biofilm were in fact directly related to pocket wall inflammation as measured by increased redness of the pocket epithelium [11].
Our findings disclose that a linear association between BOP and the presence of subgingival deposits is not particularly strong for each examined sites, anyway the data show how the amount of subgingival deposits rise with the incidence of BOP (Table 2). Relative Risk analysis further demonstrates that the incidence of BOP could be up to 6 times with calculus and up to 8 times with plaque (Table 4).
The present research shows a linear association between the increase in quantity of subgingival deposits and increase of PPD (Table 3), a result in accordance with previous findings [14-16]. The disto-vestibular site was the only area where it was not possible to observe a linear association between subgingival deposits and PPD. The anatomical features of this area, that undeniably limit the endoscope handling, could be the reason of this finding. In spite of its flexibility, the fiber of the endoscope finds few limitations in anatomical conditions like fairly small mouths and distal portion of the molar teeth. This hypothesis is supported by the observation that among the other sites studied is the disto-lingual, the one with the lower significance (Table 3).
Overall, it is possible to conclude that, when BOP is present especially in deep periodontal pockets after an adequate non-surgical therapy, a higher probability of residual deposits can be assumed.
Bleeding on probing was found to have high levels of specificity of residual deposits but low sensitivity values. These findings are in accordance with those reported by Sherman et al in 1990 on subgingival calculus [21].
The BOP’s sensitivity interestingly changes if the classes either of EBI or ECI are considered singularly. As reported on Table 5, the sensitivity values increase with the index classes. This observation confirms the previous statements of a possible level of periodontal soft tissues tolerance of subgingival calculus [22, 23]. At the same time, it is possible to deduce that the presence of bleeding upon probing could be a quite good indicator of important amounts of residual deposits in the subgingival area.
These results show that either soft or hard deposits are associated to the presence of BOP underlining their insight virulence.
However, further studies are recommended to determine if the present observations are true for a larger number of periodontal patients, since the sites within a mouth behave more alike than surfaces from different mouths [24, 25].
Finally, the results of this study suggest that the measurements obtained using the endoscope should be considered reliable in better predicting the disease establishment and progression, therefore, future studies investigating this hypothesis are required.
CONCLUSION
In conclusion, the obtained results confirm and corroborate the importance of BOP as indicator of subgingival deposits. However, though a correlation between BOP and amount of deposits has been observed in the present study, an exact level of the assumed tolerance of the periodontal tissues is still not clearly defined.
Until this level will not be identified, the total removal of tooth-borne subgingival deposits remain a reasonable goal to reach. According to this consideration the BOP can be judged as a concrete, suitable, and useful aid for the clinician interested in this task.
CONFLICTS OF INTEREST
The authors report no financial relationships related to any products involved in this study and declare that no funding has been available other than that of the author's institution.
REFERENCES
- 1.Newbrun E. Indices to measure gingival bleeding. J Periodontol. 1996;67:555–61. doi: 10.1902/jop.1996.67.6.555. [DOI] [PubMed] [Google Scholar]
- 2.Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990;17:714–21. doi: 10.1111/j.1600-051x.1990.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 3.Albandar JM, Kingman A, Brown LJ, Löe H. Gingival inflammation and subgingival calculus as determinants of disease progression in early-onset periodontitis. J Clin Periodontol. 1998;25:231–7. doi: 10.1111/j.1600-051x.1998.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 4.Schätzle M, Löe H, Bürgin W, Ånerud , Boysen H, Lang NP. Clinical course of chronic periodontitis. J Clin Periodontol. 2003;30:887–901. doi: 10.1034/j.1600-051x.2003.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Claffey N, Egelberg J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J Clin Periodontol. 1995;22:690–6. doi: 10.1111/j.1600-051x.1995.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 6.Shimazaki Y, Saito T, Kiyohara Y, et al. The influence of current and former smoking on gingival bleeding: the hisayama study. J Periodontol. 2006;77:1430–5. doi: 10.1902/jop.2006.050422. [DOI] [PubMed] [Google Scholar]
- 7.Stambaugh RV, Myers G, Ebling W, Beckman B, Stambaugh K. Endoscopic visualization of the submarginal gingiva dental sulcus and tooth root surfaces. J Periodontol. 2002;73:374–82. doi: 10.1902/jop.2002.73.4.374. [DOI] [PubMed] [Google Scholar]
- 8.Hirschfeld LI, Wasserman B. A long term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225–37. doi: 10.1902/jop.1978.49.5.225. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary T, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 10.Löe H, Silness J. Periodontal disease in pregnancy. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 11.Wilson TG, Harrel SK, Nunn ME, Francis B, Webb K. The relationship between the presence of tooth-borne subgingival deposits and inflammation found with a dental endoscope. J Periodontol. 2008;79:2029–35. doi: 10.1902/jop.2008.080189. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GB, Palmer JA, Bye FL, Smith BA, Caffesse RG. Effectiveness of subgingival scaling and root planing: single versus multiple episodes of instrumentation. J Periodontol. 1996;67:367–73. doi: 10.1902/jop.1996.67.4.367. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein G. Periodontal responce to mechanical and non-surgical therapy: a review. J Periodontol. 1992;63:118–30. doi: 10.1902/jop.1992.63.2.118. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan SA, Robertson PB. Calculus removal by scaling/root planing with and without surgical access. J Periodontol. 1987;58:159–63. doi: 10.1902/jop.1987.58.3.159. [DOI] [PubMed] [Google Scholar]
- 15.Caffesse RG, Sweeney PL, Smith BA. Scaling and root planing with and without periodontal flap surgery. J Clin Periodontol. 1986;13:205–10. doi: 10.1111/j.1600-051x.1986.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 16.Rabbani GM, Ash MM, Caffesse RG. The effectiveness of subgingival scaling and root planning in calculus removal. J Periodontol. 1981;52:119–23. doi: 10.1902/jop.1981.52.3.119. [DOI] [PubMed] [Google Scholar]
- 17.Sherman PR, Hutchens LH, Jewson LG, Moriarty JM, Greco GW, McFall WT Jr. The effectiveness of subgingival scaling and root planing I. Clinical detection of residual calculus. J Periodontol. 1990;61:3–8. doi: 10.1902/jop.1990.61.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Wylam JM, Mealey BL, Mills MP, Waldrop TC, Moskowicz DC. The clinical effectiveness of open versus closet scaling and root planing on multi-rooted teeth. J Periodontol. 1993;64:1023–8. doi: 10.1902/jop.1993.64.11.1023. [DOI] [PubMed] [Google Scholar]
- 19.Geisinger ML, Mealey BL, Schoolfield J, Mellonig JT. The effectiveness of subgingival scaling and root planing: an evaluation of therapy with and without the use of the periodontal endoscope. J Periodontol. 2007;78:22–8. doi: 10.1902/jop.2007.060186. [DOI] [PubMed] [Google Scholar]
- 20.Löe H, Theilade E, Jensen SB. Experimental gingivitis in men. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 21.Sherman PR, Hutchens LH Jr, Jewson LG. The effectiveness of subgingival scaling and root planing II. Clinical responses related to residual calculus. J Periodontol. 1990;61:9–15. doi: 10.1902/jop.1990.61.1.9. [DOI] [PubMed] [Google Scholar]
- 22.Robertson PB. The residual calculus paradox. J Periodontol. 1990;61:65–6. doi: 10.1902/jop.1990.61.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Fujikawa K, O'Leary TJ, Kafrawy AH. The effect of retained subgingival calculus on healing after flap surgery. J Periodontol. 1988;59:170–5. doi: 10.1902/jop.1988.59.3.170. [DOI] [PubMed] [Google Scholar]
- 24.Fleiss JL, Park MH, Chilton NW. Within-mouth correlations and reliabilities for probing depth and attachment level. J Periodontol. 1987;58:460–3. doi: 10.1902/jop.1987.58.7.460. [DOI] [PubMed] [Google Scholar]
- 25.Fleiss JL, Wallenstein S, Chilton NW, Goodson JM. A re-examination of within-mouth correlations of attachment level and of change in attachment level. J Clin Periodontol. 1988;15:411–4. doi: 10.1111/j.1600-051x.1988.tb01594.x. [DOI] [PubMed] [Google Scholar]