Abstract
Pyramidal neurons in hippocampal CA1 regions are highly sensitive to cerebral ischemia. Alterations of excitatory and inhibitory synaptic transmission may contribute to the ischemia-induced neuronal degeneration. However, little is known about the changes of GABAergic synaptic transmission in the hippocampus following reperfusion. We examined the GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) in CA1 pyramidal neurons 12 hours and 24 hours after transient forebrain ischemia. The amplitudes of evoked IPSCs (eIPSCs) were increased significantly 12 hours after ischemia and returned to control levels 24 hours following reperfusion. The potentiation of eIPSCs was accompanied by an increase of miniature IPSCs (mIPSCs) amplitude, and an enhanced response to exogenous application of GABA, indicating the involvement of postsynaptic mechanisms. Furthermore, there was no obvious change of the paired-pulse ratio (PPR) of eIPSCs and the frequency of mIPSCs, suggesting that the potentiation of eIPSCs might not be due to the increased presynaptic release. Blockade of adenosine A1 receptors led to a decrease of eIPSCs amplitude in post-ischemic neurons but not in control neurons, without affecting the frequency of mIPSCs and the PPR of eIPSCs. Thus, tonic activation of adenosine A1 receptors might, at least in part, contribute to the enhancement of inhibitory synaptic transmission in CA1 neurons after forebrain ischemia. The transient enhancement of inhibitory neurotransmission might temporarily protect CA1 pyramidal neurons, and delay the process of neuronal death after cerebral ischemia.
Keywords: GABAA receptor, Cell death, Stroke, Excitotoxicity
INTRODUCTION
In the hippocampus, CA1 pyramidal neurons exhibit delayed neuronal death that occurs 2–4 days following transient cerebral ischemia (Kirino, 1982, Pulsinelli et al., 1982). The underlying mechanisms of this neuronal death are not completely understood. Numerous studies have shown that ischemic insults cause profound changes in neuronal excitability, including excitatory and inhibitory synaptic inputs, which may be crucial for ischemia-induced neuronal damage. Indeed, the excitatory synaptic inputs in ischemia-vulnerable CA1 pyramidal neurons are potentiated after ischemic insults (Urban et al., 1989, Gao et al., 1998), suggesting that enhancement of excitatory neurotransmission might be involved in ischemic cell death. However, other studies indicate that changes of inhibitory synaptic inputs may also contribute to the neuronal death in CA1 pyramidal neurons after transient cerebral ischemia. For example, the expression levels of GABAA receptor mRNAs in the CA1 region decreased within 4 hours, and continued to decline over 96 hours after ischemia, which was accompanied by a decrease in the binding activity of GABAA receptors (Mileson et al., 1992, Li et al., 1993). In contrast, the GABAA receptor mRNAs in ischemia-resistant CA3 pyramidal cells decreased only at early phase (4 hours) after ischemia, and then gradually recovered to control levels within 96 hours following reperfusion (Li et al., 1993). It remains to be elucidated whether these changes lead to corresponding alterations of inhibitory synaptic function. A recent study has demonstrated that the GABAA receptor-mediated responses in CA1 pyramidal neurons are suppressed 4 hours after transient cerebral ischemia (Zhan et al., 2006). However, changes of inhibitory synaptic inputs in hippocampal neurons at later phase following reperfusion are still unknown.
Adenosine is an important neuromodulator of synaptic transmission in the central nervous system (Dunwiddie and Masino, 2001). It has been shown that activation of adenosine A1 receptors may be responsible for the depression of excitatory neurotransmission in the hippocampus and the neostriatum after ischemia (Tanaka et al., 2001, Pang et al., 2002). In addition, endogenous adenosine, through A1 receptor activation, may contribute to the ischemic inhibition of IPSCs in striatal neurons (Centonze et al., 2001). Therefore, the present study was designed to examine whether the inhibitory synaptic inputs in CA1 pyramidal were changed 12–24 hours after transient forebrain ischemia, and to explore the roles of adenosine in ischemia-induced changes of inhibitory neurotransmission in the hippocampus.
EXPERIMENTAL PROCEDURES
Adult male Wistar rats (150–200g; Charles River Laboratories, Wilmington, MA) were used in all experiments. Experimental protocols were institutionally approved in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize both the suffering and number of animals used.
Transient forebrain ischemia model
Transient forebrain ischemia was induced using the four vessels occlusion method (Pulsinelli and Brierley, 1979) with modifications (Ren et al., 1997). The animals were fasted overnight to provide uniform blood glucose levels. For surgical preparation, the animals were anesthetized with a mixture of 1–2% halothane, 33% O2, and 66% N2 via a gas mask placed around the nose. A silicon tube loop was placed loosely around each common carotid artery to allow subsequent occlusion of these vessels. The animal was then placed on a sereotaxic frame, and the vertebral arteries were electrocauterized. A very small temperature probe (Pehysitemp, Clifton, NJ) was inserted beneath the skull in the extradural space, and the brain temperature was maintained at 37°C with a heating lamp using a temperature-control system (BAT-10, physitemp). Transient forebrain ischemia was induced by occluding both common carotid arteries to induced ischemic depolarization for ~12 min. The cerebral blood flow resumed immediately on release of the carotid arteries clasps.
Slice preparation and whole-cell voltage-clamp recording
Brain slices were prepared from animal before ischemia, and at 12–14 and 24 hours following reperfusion. The animals were anesthetized with ketamine-HCL (80mgh/kg, i.p.) and decapitated. The brains were quickly removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF), which was composed of the following (in mM): 130 NaCl, 3 KCl, 2 CaCl2, 2MgCl2, 1.25 NaH2PO4, 26NaHCO3, and 10 glucose, pH 7.4, 290–305 mosM/L. Coronal hippocampal slices of 400 μm thickness were cut using a vibratome (VT 1000S; Leica, Nussloch, Germany) and incubated in ACSF for 30 min at 34°C and for > 1 hour at room temperature before being transferred to the recording chamber. The slices were submerged beneath the fluid surface and superfused continuously with oxygenated ACSF. The flow rate was adjusted to 2–3ml/min. All recordings were performed at 32–34°C.
For whole-cell recording, patch electrodes were prepared from borosilicate glass (Warner Instruments, Hamden, CT) using a horizontal electrode puller (P-97; Faming/Brown; Sutter, Novato, CA) to produce tip opening of 1–2 μm (3–5 MΩ). Electrodes were filled with an intracellular solution containing (in mM): 43 CsCl, 92 CsMeSO4, 5 TEA, 2 EGTA, 1 MgCl2, 10 HEPES, and 4 Mg-ATP, pH 7.4, 290–300 mosM/L. Neurons were visualized with an infrared-differential interference contrast microscope (BX 50 WI; Olympus Optical, Tokyo, Japan) and a CCD camera. Voltage-clamped recording was performed with an Axopatch 200B amplifier (Molecular Devices, Forster City, CA.). Signals were filtered at 2k Hz and digitized at a sampling rate of 5k Hz using a data-acquisition program (Axograph 4.6; Molecular Devices). The series resistance of the pipette was ~15 MΩ. Stimulation was delivered every 10 s using a bipolar tungsten electrode (Microprobe, Potomac, MD) located in the stratum of radiatum, and 0.1 ms current pulses were used to evoke the inhibitory responses. One to five times of threshold stimulus intensity (1-5T) was used in the present experiments. The paired-pulse experiments were performed using 0.1 ms current pulses and the two consecutive stimuli with the same intensity were delivered at an interval of 50 ms. The amplitude of the second response was compared to that of the first one to calculate the paired-pulse ratio (PPR).
Drug application
(−)-2-amino-5-phosphonopentanoic acid (D-APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), bicuculline, tetrodotoxin (TTX), γ-aminobutyric acid (GABA) were purchased from Sigma (St. Louis, MO). Antagonists were applied via bath solution. Agonist (GABA, 100 and 300 μM) was applied through a “Y” tube system (Pang et al., 2002). The tip of the Y-tube had a diamter of 100–150 μm and was placed close to the recorded neuron. To isolate inhibitory synaptic currents, 50 μM D-APV was used to block NMDA receptors and 20 μM CNQX was used to block AMPA receptors. In addition, 1 μM TTX was used to block the sodium channels for recording miniature inhibitory postsynaptic currents (mIPSCs).
Data analysis
The miniature IPSCs were analyzed with Axograph 4.6 (Molecular Devices). Synaptic events with fast onset and exponential decay kinetics were captured with template detectors in Axograph 4.6 software. The detection parameters for analyzing synaptic events in each cell in the absence or presence of drugs were the same. All data were presented as mean ± SEM. Paired or unpaired student’s t-tests were used for two groups compare. Changes were considered significant if P < 0.05.
RESULTS
Transient enhancement eIPSCs after ischemia
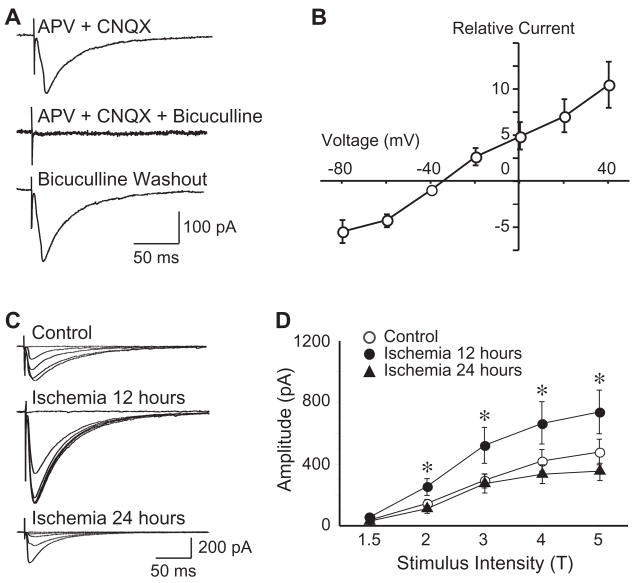
Whole-cell voltage-clamp recordings were performed on CA1 pyramidal neurons before and after ischemia. To isolate eIPSCs, 50 μM D-APV and 20 μM CNQX were used to block the NMDA receptors and AMPA receptors, respectively. At a holding potential of −60 mV, the evoked currents were completely blocked by application of bicuculline (30 μM), indicating that the evoked currents are mediated mainly by GABAA receptors (Fig. 1A). To examine the efficacy of inhibitory synaptic transmission in the pyramidal neurons, we first determined the threshold of stimulus intensity, which was defined as the stimulating current evoking the smallest detectable response from pyramidal neurons. Then a step increase in stimulating current, which was normalized as one to five times of the threshold intensity (1-5T), was performed to evoke postsynaptic response. The amplitude of eIPSCs increased with step increment of stimulus intensities accordingly. The current-voltage relationship of the eIPSCs was examined, and the reversal potential of eIPSCs was very close to the theoretical equilibrium potential (−29 mV, calculated with intracellular Cl− concentration of 45 mM and extracellular Cl− concentration of 141 mM; Fig. 1B).
Figure 1.
Transient increase of eIPSCs in CA1 pyramidal neurons after forebrain ischemia. A, Representative traces showing the isolation of eIPSCs (evoked at 3T). In the presence of APV (50 μM) and CNQX (20 μM), evoked postsynaptic currents could be reversibly blocked by selective GABAA receptor antagonist bicuculline (30 μM). B, Group data showing the current-voltage relationship of the eIPSCs, with a reversal potential close to the theoretical equilibrium potential (−29 mV). C, Representative traces of eIPSCs evoked in CA pyramidal neurons of control and after ischemia. D, Group data showing that the eIPSCs amplitude was significantly increased 12 hours after ischemia, and then returned to control levels 24 hours following reperfusion. All traces are average of 10 consecutive recordings. *, P < 0.01.
We compared the amplitude of eIPSCs between control and post-ischemic neurons, and found that the eIPSCs amplitude in CA1 pyramidal neurons was significantly increased 12 hours after ischemia, and then returned to control levels 24 hours following reperfusion (evoked at 2-5T, Fig. 1C & D). For example, when evoked at 3T, the amplitudes were 181.64 ± 16.81 pA (n=29) in control neurons, 574.13 ± 96.77 pA (n=31) in neurons 12 hours after ischemia (P < 0.01, compared with control), and 164.00 ± 33.29 pA (n=19) in neurons 24 following reperfusion (P > 0.05, compared with control). These results demonstrate a transient enhancement of GABAA receptor-mediated synaptic transmission in CA1 pyramidal neurons after forebrain ischemia. Thus, in the following experiments, we focused on CA1 pyramidal neurons of control and 12 hours after forebrain ischemia.
Involvement of postsynaptic mechanisms in eIPSCs enhancement after ischemia
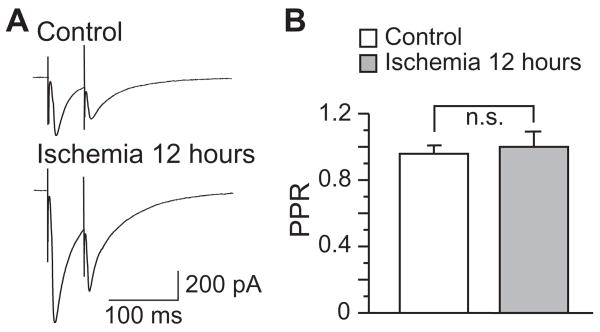
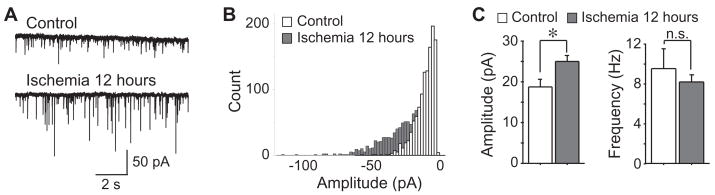
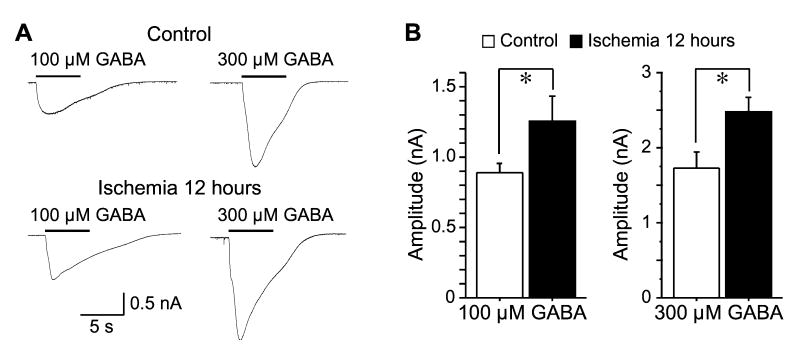
To investigate the involvement of pre- or postsynaptic mechanisms in the enhancement of inhibitory synaptic transmission after ischemia, we first analyzed the paired-pulse ratio (PPR) of eIPSCs. However, no significant difference of PPR was detected between neurons of control and 12 hours after ischemia (P > 0.05, n=9 in control and n=5 in ischemic group, Fig 2). Then, we analyzed the miniature inhibitory postsynaptic currents (mIPSCs) in the presence of 1 μM TTX, 50 μM D-APV and 20 μM CNQX. The mIPSCs of CA1 pyramidal neurons were recorded at a holding potential of −60 mV. As shown in Figure 3, mIPSCs in control neurons (n=8) had a mean amplitude of 18.85 ± 1.71 pA and a mean frequency of 9.41 ± 1.99 Hz. The mIPSCs amplitude was significantly increased 12 hours after ischemia (25.05 ± 1.32 pA, n=14, P < 0.01), whereas the frequency remained unchanged (8.16 ± 0.70 Hz, n=14, P > 0.05). Finally, we examined the postsynaptic responses of CA1 neurons to focal application of exogenous GABA, in the presence of 50 μM APV, 20 μM CNQX and 1 μM TTX. Application of exogenous GABA evoked an inward current at a holding voltage of −60 mV. Two different concentrations of GABA (100 or 300 μM) were tested. Application of 100 μM GABA induced currents of 886.26 ± 64.39 pA in control neurons (n=14), and currents of 1254.72 ± 174.58 pA in neurons 12hours after ischemia (n=8, P < 0.05), indicating an enhanced response of post-ischemic neurons to exogenous GABA. Consistently, application of 300 μM GABA induced currents of 1729.22 ±201.58 pA in control neurons (n=12), and currents of 2475.74 ± 197.77 pA in neurons 12 hours after ischemia (n=12, P < 0.05, Fig. 4). Therefore, our findings indicate that postsynaptic mechanisms might be responsible for the post-ischemic potentiation of inhibitory neurotransmission in CA1 pyramidal neurons.
Figure 2.
No change of PPR after forebrain ischemia. A, Representative traces of eIPSCs evoked with paired pulses (3T) in CA1 pyramidal neurons before and after ischemia. B, Group data showing that the PPR of eIPSCs remained unchanged after ischemia.
Figure 3.
Changes of mIPSCs after ischemia. A, Representative traces of mIPSCs recorded before and 12 hours after ischemia. B, Histogram showing the distribution of mIPSC amplitude. C, Group data showing that the mIPSC amplitude, but not the frequency, was increased after ischemia. *, P < 0.01.
Figure 4.
Enhanced responses to exogenous GABA after ischemia. A, Representative traces showing the responses to exogenous GABA (100 or 300 μM) in CA1 pyramidal neurons before and after ischemia. B, Group data showing that the amplitude of exogenous GABA-induced currents was increased 12 hours after ischemia. *, P < 0.05.
Involvement of adenosine A1 receptors in eIPSC enhancement after ischemia
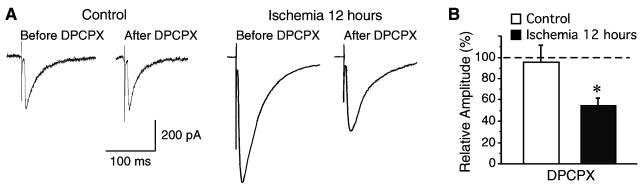
Adenosine has been shown to modulate neurotransmission and play neuroprotective roles after ischemia (Pang et al., 2002, Fredholm et al., 2005). To investigate whether adenosine regulates inhibitory neurotransmission in CA1 neurons after ischemia, the effects of selective adenosine A1 receptor antagonist DPCPX (500 nM) on eIPSCs were examined in both control and post-ischemic neurons. The amplitude of eIPSCs in control neurons showed no change in the presence of DPCPX (n=8, P > 0.05, Fig 5). However, application of DPCPX dramatically reduced the eIPSCs amplitude in neurons 12 hours after ischemia (n=11, P < 0.05, Fig 5). These data indicate that tonic modulation of inhibitory neurotransmission by adenosine A1 receptor activation might be involved in the post-ischemic enhancement of eIPSCs in CA1 neurons.
Figure 5.
Effects of DPCPX on eIPSCs. A, Representative traces of eIPSCs recorded with or without DPCPX application (500 nM) in CA pyramidal neurons before and after ischemia. B, Group data showing that application of DPCPX (500 nM) dramatically inhibited the eIPSC amplitude in post-ischemic neurons, but not in control neurons. *, P <0.05.
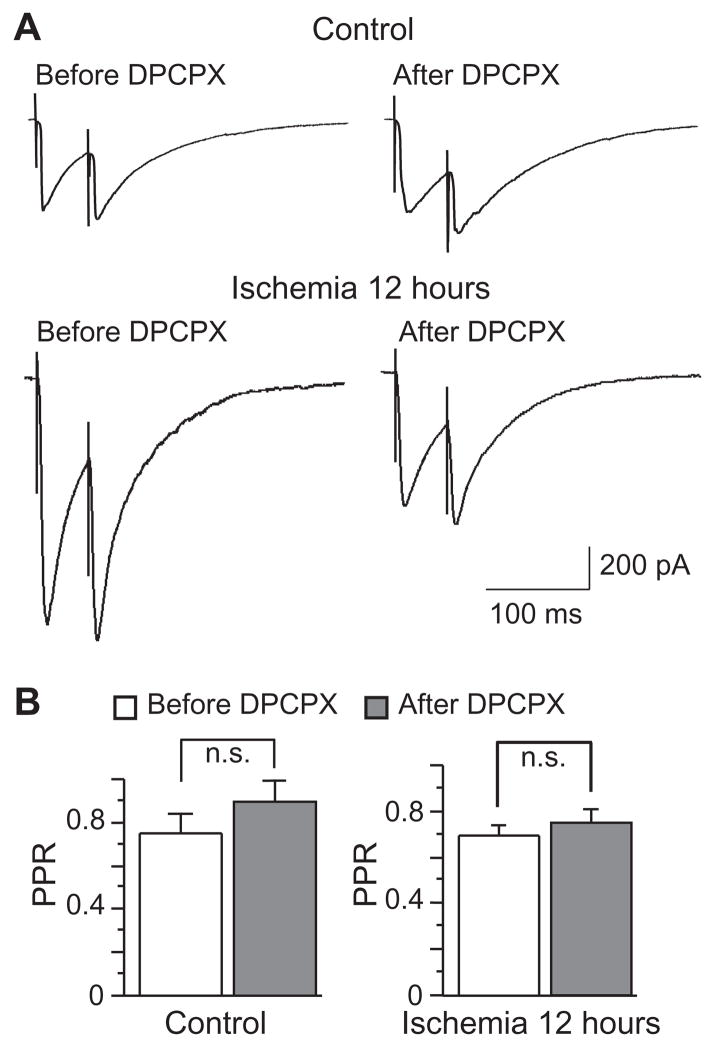
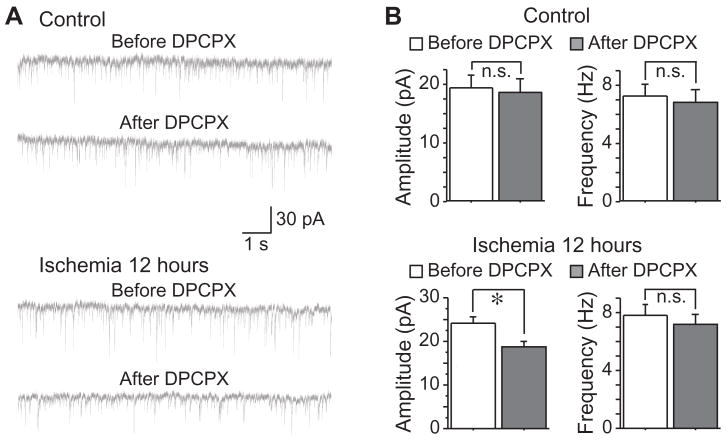
We further examined the effects of DPCPX on the PPR of eIPSCs. In both control and post-ischemic neurons, bath application of DPCPX (500 nM) had no effect on PPR (Fig. 6), suggesting that adenosine A1 receptor activation might modulate eIPSCs through postsynaptic mechanisms in post-ischemic neurons. In agreement with this notion, we found that application of DPCPX (500 nM) significantly reduced the mIPSCs amplitude in post-ischemic neurons (from 24.02 ± 1.73 pA to 19.10 ± 1.25 pA, n=13, P < 0.05), but had no effects on the mIPSCS frequency (from 7.85 ± 0.83 Hz to 7.18 ± 0.75 Hz, n=13, P > 0.05, Fig 7). Moreover, bath application of DPCPX (500 nM) had no effect on mIPSCS in control neurons (amplitude: from 19.44 ± 2.17 pA to 18.55 ± 2.33 pA, n=9, P > 0.05; frequency: from 7.28 ± 0.81 Hz to 6.82 ± 0.87 Hz, n=9, P > 0.05; Fig 7). These results strongly suggest that the transient enhancement of inhibitory neurotransmission in post-ischemic neurons might be due to a tonic activation of adenosine A1 receptor via postsynaptic mechanisms.
Figure 6.
No effect of DPCPX on PPR. A, Representative traces of eIPSCs evoked by paired pulses with or without DPCPX application (500 nM) in CA pyramidal neurons before and after ischemia. B, Group data showing that application of DPCPX (500 nM) had no effect on PPR in both control and post-ischemic neurons.
Figure 7.
Effects of DPCPX on mIPSCs. A, Representative traces of mIPSCs recorded with or without DPCPX application (500 nM) in CA pyramidal neurons before and after ischemia. B, Group data showing that application of DPCPX (500 nM) had no effect on mIPSCs in control neurons. However, DPCPX application significantly decreased the mIPSC amplitude, but not frequency, in neurons 12 hours after ischemia. *, P < 0.05.
DISCUSSION
The present study demonstrates that the eIPSCs in CA1 pyramidal neurons are transiently enhanced 12 hours after forebrain ischemia. Tonic activation of adenosine A1 receptors may contribute to the ischemic enhancement of inhibitory neurotransmission through postsynaptic mechanisms. Since an increase of GABAergic neurotransmission may protect neurons against ischemic insults (Schwartz-Bloom and Sah, 2001), the transient enhancement of inhibitory synaptic transmission might be associated with the delayed neuronal death in the hippocampus after cerebral ischemia.
Both morphological and electrophysiological studies have shown that GABAergic neurotransmission in ischemia-vulnerable neurons is highly sensitive to ischemic insults. In the CA1 region of the hippocampus, the mRNA expression level and binding activity of GABAA receptors are decreased early (4 hours) after ischemia, and continue to decline over 96 hours following reperfusion (Mileson et al., 1992, Li et al., 1993). Importantly, cerebral ischemia may cause a functional depression of inhibitory synaptic inputs in CA1 pyramidal neurons. The inhibitory postsynaptic potentials in CA1 neurons disappear during in vivo forebrain ischemia (Xu and Pulsinelli, 1994). In addition, GABAA receptor-mediated responses to either synaptic stimulus or exogenous GABA are significantly suppressed in CA1 pyramidal neurons 4 hours after cerebral ischemia (Zhan et al., 2006). The ischemia-induced depression of GABAergic neurotransmission has also been observed in other brain regions, such as the neostriatum. For example, oxygen and glucose deprivation in vitro causes a decrease of IPSCs in ischemia-sensitive striatal spiny neurons during ischemic insults (Centonze et al., 2001). Considering that CA1 pyramidal neurons exhibit delayed neuronal death that occurs 2–4 days after transient cerebral ischemia (Kirino, 1982, Pulsinelli et al., 1982), changes of synaptic function just before the occurrence of degeneration might play an important role in the delayed cell death. Interestingly, our results demonstrated that the GABAergic responses in CA1 pyramidal neurons were enhanced 12 hours after transient forebrain ischemia, and then returned to control levels 24 hours following reperfusion. It has been indicated that a dysfunction of GABAergic neurotransmission may contribute to ischemic cell death, whereas an increase of GABAergic neurotransmission may provide neuroprotective effects against cerebral ischemia (Schwartz-Bloom and Sah, 2001). Indeed, ischemia-resistant interneurons in the CA1 region exhibit no change of GABAA receptor-mediated responses after ischemia (Zhan et al., 2006). It is therefore possible that the transient enhancement of inhibitory neurotransmission might be associated with the delayed cell death in CA1 pyramidal neurons. This possibility is supported by the fact that striatal spiny neurons, most of which die in 24 hours after transient forebrain ischemia, display a persistent decrease of GABAergic neurotransmission within 9–12 hours following reperfusion, a time point at which spiny neurons start to degenerate (Gajendiran et al., 2001, Zhang et al., 2006).
Previous studies have suggested that ischemia-induced changes of inhibitory neurotransmission might be due to pre- or postsynaptic mechanisms. Presynaptic mechanisms might be involved in the inhibition of IPSCs in striatal spiny neurons during in vitro ischemic insults (Centonze et al., 2001). In contrast, the depression of GABAergic neurotransmission in CA1 pyramidal neurons early (4 hours) after ischemia may results from postsynaptic mechanisms, as indicated by the decrease of GABAA receptor mRNAs and binding activity (Li et al., 1993), and the reduce of GABAA receptor responses to exogenous GABA (Zhan et al., 2006). In the present study, we found that the enhancement of eIPSCs was accompanied by an increase of the amplitude of both mIPSCs and exogenous GABA-induced currents. Moreover, neither mIPSC frequency nor PPR showed significant changes. These findings indicate that the transient enhancement of GABAergic neurotransmission in CA1 pyramidal neurons might be mediated by postsynaptic mechanisms.
It is well established that adenosine is an important neuromodulator of synaptic transmission (Dunwiddie and Masino, 2001). In the hippocampus, activation of adenosine A1 receptors leads to a presynaptic inhibition of GABAergic neurotransmission in CA1 neurons in immature rat (Jeong et al., 2003). It has been shown that adenosine, by activating A1 receptors, may be responsible for the ischemic depression of excitatory and inhibitory neurotransmission in striatal neurons through presynaptic mechanisms (Centonze et al., 2001, Pang et al., 2002). Different from previous studies, our data demonstrated that application of DPCPX, a selective antagonist of adenosine A1 receptor, caused an inhibition of IPSCs in post-ischemic (12 hours) pyramidal neurons but not in control cells. Furthermore, application of DPCPX reduced the amplitudes of mIPSCs, without affecting the frequency of mIPSCs and the PPR of eIPSCs. It is therefore suggested that tonic activation of adenosine A1 receptors, through postsynaptic mechanisms, might be involved in the ischemia-induced enhancement of GABAergic neurotransmission in CA1 pyramidal neurons. However, the underlying pathways are still unclear. Several lines of evidence indicate that the function of GABAA receptors can be regulated by protein kinases. In particular, synaptic GABAA receptors in CA1 pyramidal neurons are tonically phosphorylated by cAMP-dependent protein kinase A (PKA), which reduces mIPSC amplitudes, and probably dephosphorylated by phosphatases (Poisbeau et al., 1999). It is possible that neuromodulators capable of regulating intracellular cAMP levels and PKA activities may influence the function of GABAA receptors. For example, activation of dopamine D3 receptors results in a suppression of inhibitory neurotransmission in the nucleus accumbens by enhancing the phospho-dependent endocytosis of GABAA receptors (Chen et al., 2006). It is therefore reasonable to speculate that, activation of adenosine A1 receptors in CA1 pyramidal neurons may causes a decrease of intracellular cAMP level (Burnstock, 2007), and a subsequent reduce of PKA activity, which in turn limits the phosphorylation of GABAA receptors, and thus contributes to the enhancement of GABAergic neurotransmission after cerebral ischemia.
Endogenous adenosine may protect neurons against cerebral ischemia (Fredholm et al., 2005), at least partially by suppressing neuronal excitability, such as depressing excitatory synaptic transmission (Pang et al., 2002, Pearson et al., 2006) and inhibiting hyperpolarization-activated cation currents (Deng et al., 2008). Adenosine A1 receptor-mediated enhancement of inhibitory neurotransmission may provide a novel mechanism to protect neurons against cerebral ischemia.
Acknowledgments
This work was supported by National Institutes of Health grant (NS38053), and American Heart Association grants (0120566Z, 0630172N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Centonze D, Saulle E, Pisani A, Bernardi G, Calabresi P. Adenosine-mediated inhibition of striatal GABAergic synaptic transmission during in vitro ischaemia. Brain. 2001;124:1855–1865. doi: 10.1093/brain/124.9.1855. [DOI] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci. 2006;26:2513–2521. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Inhibition of Ih in striatal cholinergic interneurons early after transient forebrain ischemia. J Cereb Blood Flow Metab. 2008;28:939–947. doi: 10.1038/sj.jcbfm.9600583. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Gajendiran M, Ling GY, Pang Z, Xu ZC. Differential changes of synaptic transmission in spiny neurons of rat neostriatum following transient forebrain ischemia. Neuroscience. 2001;105:139–152. doi: 10.1016/s0306-4522(01)00163-4. [DOI] [PubMed] [Google Scholar]
- Gao TM, Pulsinelli WA, Xu ZC. Prolonged enhancement and depression of synaptic transmission in CA1 pyramidal neurons induced by transient forebrain ischemia in vivo. Neuroscience. 1998;87:371–383. doi: 10.1016/s0306-4522(98)00150-x. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Nabekura J, Akaike N. Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol. 2003;89:1214–1222. doi: 10.1152/jn.00516.2002. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Li H, Siegel RE, Schwartz RD. Rapid decline of GABAA receptor subunit mRNA expression in hippocampus following transient cerebral ischemia in the gerbil. Hippocampus. 1993;3:527–537. doi: 10.1002/hipo.450030412. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Ehrmann ML, Schwartz RD. Alterations in the gamma-aminobutyric acid-gated chloride channel following transient forebrain ischemia in the gerbil. J Neurochem. 1992;58:600–607. doi: 10.1111/j.1471-4159.1992.tb09761.x. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Deng P, Ruan YW, Xu ZC. Depression of fast excitatory synaptic transmission in large aspiny neurons of the neostriatum after transient forebrain ischemia. J Neurosci. 2002;22:10948–10957. doi: 10.1523/JNEUROSCI.22-24-10948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Damian K, Lynas RE, Frenguelli BG. Sustained elevation of extracellular adenosine and activation of A1 receptors underlie the post-ischaemic inhibition of neuronal function in rat hippocampus in vitro. J Neurochem. 2006;97:1357–1368. doi: 10.1111/j.1471-4159.2006.03823.x. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Ren Y, Li X, Xu ZC. Asymmetrical protection of neostriatal neurons against transient forebrain ischemia by unilateral dopamine depletion. Exp Neurol. 1997;146:250–257. doi: 10.1006/exnr.1997.6525. [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Sah R. gamma-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J Neurochem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Yasumoto S, Hattori G, Niiyama S, Matsuyama S, Higashi H. Mechanisms underlying the depression of evoked fast EPSCs following in vitro ischemia in rat hippocampal CA1 neurons. J Neurophysiol. 2001;86:1095–1103. doi: 10.1152/jn.2001.86.3.1095. [DOI] [PubMed] [Google Scholar]
- Urban L, Neill KH, Crain BJ, Nadler JV, Somjen GG. Postischemic synaptic physiology in area CA1 of the gerbil hippocampus studied in vitro. J Neurosci. 1989;9:3966–3975. doi: 10.1523/JNEUROSCI.09-11-03966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZC, Pulsinelli WA. Responses of CA1 pyramidal neurons in rat hippocampus to transient forebrain ischemia: an in vivo intracellular recording study. Neurosci Lett. 1994;171:187–191. doi: 10.1016/0304-3940(94)90636-x. [DOI] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV, Schwartz-Bloom RD. Depressed responses to applied and synaptically-released GABA in CA1 pyramidal cells, but not in CA1 interneurons, after transient forebrain ischemia. J Cereb Blood Flow Metab. 2006;26:112–124. doi: 10.1038/sj.jcbfm.9600171. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Deng P, Li Y, Xu ZC. Enhancement of excitatory synaptic transmission in spiny neurons after transient forebrain ischemia. J Neurophysiol. 2006;95:1537–1544. doi: 10.1152/jn.01166.2005. [DOI] [PubMed] [Google Scholar]