Abstract
Background and Purpose
White matter (WM) hyperintensities upon magnetic resonance imaging (MRI) or leukoaraiosis is characteristic of stroke syndromes. Increased MRI signals in the anterior temporal pole are suggested to be diagnostic for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), with 90% sensitivity and 100% specificity. The structural correlates of these specific WM hyperintensities seen on T2-weighted and FLAIR sequences in the temporal pole of CADASIL are unclear. We assessed pathological changes in post-mortem tissue from the temporal pole to reveal the cause of CADASIL specific WM hyperintensities.
Materials & Methods
A combination of tinctorial and immunostaining approaches and in vitro imaging methods were used to quantify the extent of perivascular space (PVS), arteriosclerosis determined as the sclerotic index (SI), WM myelination as the myelin index (MI) and damage within the WM as accumulated degraded myelin basic protein (dMBP) in samples of the anterior temporal pole from 9 CADASIL and 8 sporadic subcortical ischaemic vascular dementia (SIVD) cases, and 5 similar age (young) and 5 older controls. Luxol fast blue (LFB) stained serial sections from a CADASIL case were also used to reconstruct the temporal pole, which was then compared to the MR images.
Results
LFB sections used to reconstruct the temporal pole revealed an abundance of enlarged PVS in the WM that topographically appeared as indistinct opaque regions. The mean and total areas of the PVS per WM area (%PVS) were significantly greater in CADASIL compared to the controls. The MI was severely reduced in CADASIL in relation to the SIVD and control sample that was consistent with increased immunoreactivity of dMBP, indicating myelin degeneration. Cerebral microvessels associated with the PVS exhibited a 4.5 fold greater number of basophilic (hyalinised) vessels and a 57% increase in the SI values in CADASIL subjects compared to young controls. A significant correlation between the quantity of hyalinised vessels and SI values was also apparent (P<0.05).
Conclusions
Our findings suggest that MRI hyperintensities in the temporal pole of CADASIL patients are explained by enlarged PVS and degeneration of myelin accompanied by lack of drainage of the interstitial fluid rather than lacunar infarcts. Consistent with the lack of MR hypersignals in the temporal pole of older SIVD subjects, our observations imply greater progression of pathological changes in CADASIL patients.
Keywords: CADASIL, dementia, cognitive impairment, stroke, subcortical ischaemic vascular dementia, vascular dementia
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is likely the most common form of all hereditary small vessel diseases of the brain, which leads to cognitive decline and dementia.1 CADASIL is caused by mutations within the epidermal growth factor-like repeat domain of the NOTCH3 gene located on chromosome 19p13. Over 150 mutations have been described, nearly all involving either a gain or loss of cysteine residue(s). Although the pathological mechanisms remain unclear, the gene defects are linked to degeneration of vascular smooth muscle cells (VSMC) both in peripheral organs and the brain leading to multiple small infarcts in the white matter (WM), deep grey matter and the pons.2 The distinct arteriopathy in CADASIL, which involves thickening of vessel wall, loss of VSMC and endothelial cell abonormalities3 ultimately affects cerebral perfusion4 as demonstrated by neuroimaging tracer studies5, 6
Magnetic resonance imaging (MRI) has enabled characterisation of the burden of leukoaraiosis and lacunar infarction in stroke syndromes. In CADASIL,7, 8 T2-weighted hyperintensities in the deep WM, internal, and external capsules, and especially in the temporal pole are almost selective, with a suggested 90% sensitivity and 100% specificity.9–11 Curiously, patients often present the temporal pole hyperintensities seen on T2-weighted (T2-W) and FLAIR MR sequences even in early twenties while major ischaemic events typically begin around the fifties.2, 11 Chabriat and colleagues12 have reported that dilated perivascular spaces (PVS) in CADASIL patients were located in the lentiform nuclei (94%) and subcortical WM of the temporal lobes (66%). PVS around small perforating arteries are pial-lined, interstitial fluid-filled spaces13 and are readily seen to be enlarged in the WM of elderly subjects. PVS play an important role in lymphatic drainage from the brain.14, 15 Similar to the small infarcts or lacunes, they may be detected as hypointense (T1-weighted) and hyperintense (T2-W) areas in MR images, and thus clustered PVS could be mistaken as a lacune or cavitated infarct. The pathological correlates of these radiological findings are unknown. We performed a post-mortem study to quantify the degrees and extent of perivascular space and arteriopathic changes within the temporal pole WM of CADASIL subjects compared to young and older controls, and those with subcortical ischaemic vascular dementia (SIVD).
Materials and Methods
Subjects
Samples of temporal pole were collected from 9 CADASIL cases, 5 controls of similar age (young) and 5 older controls. To prove disease and age specific changes, the study also included samples from 8 SIVD subjects (Table). Formalin fixed blocks of the right or left temporal pole representing Brodmann areas 20–22 were sampled coronally approximately 2 cm from the tip of the pole and anterior to the accumbens nucleus. The samples were acquired from the Newcastle Brain Tissue Resource Centre, Newcastle General Hospital and two other sources: the Neuropathology Department, Frenchay Hospital, Bristol and the Institute of Psychiatry, London (courtesy of Drs Claire Troakes and Safa Al Sarraj). Available case notes and radiological reports indicated that CADASIL subjects showed extensive WM changes consistent with SIVD of the brain and met the minimum criteria for cognitive impairment used in our post-stroke study.16 None of the controls, young or older group, had neurological or pathological evidence for cerebrovascular disease or neurodegenerative disorder. Clinical and pathological features were defined according to established criteria.17, 18 CADASIL diagnosis was confirmed by direct DNA sequencing for NOTCH3 gene mutations.19 The Table shows the demographics, duration of disease, relevant risk factors and the genetic disposition of the CADASIL cases and data summaries of the SIVD group, and controls.
Table 1.
Details of CADASIL cases, SIVD and control groups and measures of vascular pathology
| Subjects | n | Mean age at death* (years) | Age range (years) | Gender | Mutation site† | Mean duration of Disease (range in years)^ | Dint (µm) | Dext (µm) | Dpvs (µm) | Area of PVS (mm2) | %PVS | SI | Notable clinical features and risk factors†† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAD1 | 1 | 44 | - | F | p.Arg153Cys | 8.0 | 42.4 | 80.7 | 221.0 | 0.041 | 5.71 | 0.52 | Cardiac arrhythmias |
| CAD2 | 1 | 52 | - | M | p.Arg141Cys | 10.0 | 41.2 | 81.5 | 148.0 | 0.012 | 2.69 | 0.51 | No vascular risk |
| CAD3 | 1 | 53 | - | F | p.Arg133Cys | 6.0 | 41.3 | 77.1 | 237.7 | 0.042 | 7.10 | 0.5 | No vascular risk |
| CAD4 | 1 | 55 | - | M | p.Arg558Cys | 11.0 | 69.6 | 106.0 | 171.2 | 0.015 | 2.15 | 0.38 | Brief history of gout |
| CAD5 | 1 | 59 | - | M | p.Arg169Cys | 12.0 | 41.3 | 76.1 | 272.4 | 0.062 | 5.84 | 0.48 | No other risk |
| CAD6 | 1 | 61 | - | M | p.Arg169Cys | 10.0 | 43.7 | 79.7 | 295.1 | 0.075 | 4.15 | 0.47 | Obesity 55 years of age |
| CAD7 | 1 | 63 | - | M | p.Arg141Cys | 10.0 | 47.8 | 86.3 | 155.8 | 0.013 | 6.83 | 0.46 | Enlarged thyroid |
| CAD8 | 1 | 65 | - | M | p.Arg141Cys | 13.0 | 50.0 | 85.7 | 163.8 | 0.017 | 6.06 | 0.44 | Parenchymatous goitre |
| CAD9 | 1 | 68 | - | F | p.Arg133Cys | 18.0 | 42.1 | 71.3 | 145.2 | 0.014 | 5.87 | 0.41 | Smoking history |
| CADASIL | 9 | 58 (8) | 44–68 | 6M/3F | 11 (6–18) | 46.3 (28.0) |
82.6 (35.0) |
196.5 (91.3) |
0.030 (0.04) |
5.16 (3.8) |
0.47 (0.12) |
As above | |
| Similar Age (Young) controls | 5 | 60 (7) | 52–69 | 3F/2M | - | - | 53.2 (21.8) |
75.1 (25.1) |
156.0 (67.6) |
0.018 (0.02) |
1.65 (0.95) |
0.30 (9.16) |
None |
| SIVD | 8 | 83 (10)* | 67–96 | 4F/4M | - | 8 (6–9) | 49.5 (19.0) |
76.8 (24.1) |
140.2 (51.9) |
0.012 (0.01) |
2.25 (1.41) |
0.36 (0.09) |
Mild- moderate hypertension in 3 subjects |
| Old controls | 5 | 83 (6)* | 75–90 | 4F/1M | - | - | 51.8 (16.7) |
74.4 (19.0) |
141.9 (51.6) |
0.013 (0.01) |
2.54 (2.15) |
0.31 (0.07) |
Mild hypertension in 2 subjects |
Numbers represent mean (± standard deviation) unless otherwise stated.
There were significant differences in age between young and old controls (P<0.001) and between CADASIL and SIVD subjects (P<0.001).
Mutation site of protein change in the NOTCH3 gene.
Duration of disease was considered from the first stroke or cerebrovascular event. +In CADASIL cases, there were multiple lacunar infarcts predominantly in subcortical structures, WM hyperintensities and moderate to severe arteriopathy. However, there was an absence of large or lacunar infarcts in the temporal pole.
Life time risk factors related to cardiovascular or systemic disease. Available medication records indicated that non-steriodal anti-inflammatory compounds, e.g. aspirin were most commonly used. Radiological reports were not available for the SIVD cases or the controls. No significant pathology diagnostic of a disease including Alzheimer type of changes (mostly hyperphosphorylated tau positive neurones) was evident either the cases or controls. All except CAD4 exhibited high degree of gliosis in WM. Abbreviations: CAD, CADASIL; SIVD, subcortical ischaemic vascular dementia.
Histopathology and immunohistochemistry
Paraffin embedded temporal pole blocks were serially cut into 10µm-thick sections. Sections were stained with luxol fast blue (LFB), cresyl fast violet (CFV) and haematoxylin and eosin (H&E). For immunohistochemistry, tissue sections were processed essentially as described previously.19 To reveal changes in vascular components19 within microvessels, adjacent sections were immunostained with antibodies to α-smooth muscle actin (Dako) and medin (courtesy of Dr P. Naslund, Karolinska Institute, Sweden) for VSMC, collagen IV (Sigma, UK) for the basement membrane and the glucose transporter 1 (GLUT1; Chemicon) for the endothelium. There were no apparent relationships between the density of immunostaining and length of fixation or post-mortem interval from death to fixation of tissue between the groups. Variation in the LFB-CFV, LFB only and H&E staining, and immunohistochemistry was minimised by processing sets of sections on the same occasion including the reference specimens, by the same technician. All of the histopathological analyses were performed on coded specimens.
Temporal pole reconstruction
To simulate MR images in vitro, serial sections of 10µm thickness were cut from a ~1mm coronal block of the temporal pole from one of the CADASIL cases (CAD6, Table). One section at every 50µm, total of 20 sections were stained with LFB and then their digital images were precisely overlaid as a stacking column using Adobe Photoshop. The resultant composite 2D image was compared to the MR scan of the temporal pole. In addition, a sample of the temporal pole from another CADASIL case (CAD3, Table) was cut in the sagittal plane of the cerebral hemispheres and stained with H&E and anti-α-smooth muscle actin to follow the longitudinal course of the distorted perforator arterioles.
Arteriosclerotic changes, perivascular space and in vitro digital imaging
To determine the extent of small vessel arteriopathic and PVS changes, serial sections from each sample of similar size (Table) were stained with H&E and LFB-CFV for the SI and PVS analysis, respectively. H&E stained sections were viewed at 6.3x magnification and images of arterial/arteriolar vessels (>50µm) and PVS were randomly taken. We measured the outer and inner diameters (Dext and Dint) of at least 30 arterial vessels per sample, of which Dext were 50–200 µm in diameter (Figure 1). The sclerotic index (SI)19–21 and the area of each PVS were calculated by incorporating the diameters into the devised formulae (Figure 1). Total PVS, which included all of the PVS with >100µm diameter in WM, were divided by whole WM area evaluated with Image Pro Plus 4.0 (Media Cybernetics, Bethesda, MD) to obtain the total area of PVS per WM area (% PVS).
Figure 1.
Measures of arteriosclerosis (SI) and PVS in WM of the temporal pole. Values of the internal diameter (Dint) and external diameter (Dext) of a vessel in cross-section were used to calculate the SI and area of vessel (V). Area of PVS was calculated from the Dpvs (hatched area and diameter of PVS). Tissue section stained with H&E from CAD3. Formulae below indicate how the variables were derived. All computer assisted measures were undertaken using Image Pro-Plus 4. Scale bar = 100µm.
Assessment of myelin and WM abnormalities
Degrees of myelin and axonal degeneration were determined by calculating the myelin index (MI). Serial coronal sections from the disease groups and controls were stained with LFB alone to select the whole WM region(s) of interest (ROI). Standardised images of the LFB stained slides were then captured, white balanced, converted to monochrome and analyzed using Image J software. The detected range of grey levels within the outlined WM, corresponding to the staining intensity, from point 0 to 127 (0, white; 255, black) was divided into four quartiles (the first quartile, 0–29; the second, 30–62; the third, 63–94; and the fourth, 95–127). The median grey level of each quartile was then multiplied by %area/100 for each quartile and totalled to yield the MI. For example, in the case of a 74 year old with SIVD, the MI was calculated to be 31.52 derived from 14.5 × 0.4648 + 46.0 × 0.5303 + 78.5 × 0.0045 + 111.0 × 0.0003 where values represent the median grey level×%area for each quartile.
Antibodies to epileptogenic peptide or degraded myelin basic protein22 (dMBP, Chemicon) and amyloid precursor protein (APP; 22C11, Chemicon) were used to quantify19 the damaged area in WM. Images of 15–20 randomly selected WM ROI were taken and the percentages of immunostained area were measured, which were averaged to yield mean percentage area containing dMBP (%dMBP) and APP (%APP) for each sample.
Statistical analysis
Statistical analysis was performed using SPSS v.15. The statistical tests include Mann-Whitney U test, one-way analysis of variance (ANOVA) and post hoc (Tukey) comparisons and non-parametric Spearman’s (rho) correlation.
Results
Gross pathological changes in WM of temporal pole
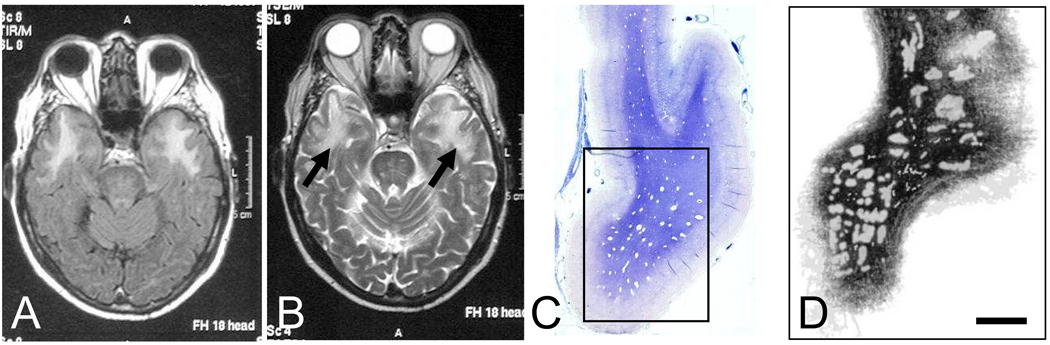
Gross examination of the temporal pole sections showed clearly visible PVS (Figure 1 and Figure 2) in the WM of the majority of the CADASIL cases. Macroimages of sections stained with LFB showed that in 8 of 9 CADASIL cases PVS were visible accompanied by high degree of WM pallor or rarefaction (Figure 2). However, similar PVS within the WM were not evident in the SIVD cases or the controls (data not shown). Reconstructed two-dimensional image simulated as a MR scan of temporal pole (Figure 2) revealed variable regions of low intensity created by the PVS when sections were stacked together; these collectively represented the hyperintensities seen in MRI (Figure 2 A, B and D). The temporal pole sections cut in the sagittal plane of the brain along the axis of the vessel length also confirmed the existence of PVS along distorted penetrating vessels (not shown). One of the cases with the p.Arg558Cys mutation, apparently did not reveal bilateral WM hyperintensities upon MRI in the anterior temporal lobe (CAD4, Table) but showed that T2-W signal changes in subcortical structures including the internal and external capsules. This case was, however, retained in the morphological analyses
Figure 2.
MR images and low power views of serial sections of the temporal pole (simulating MR image) cut in the coronal plane. A and B, show FLAIR and T2W MR sequences from CAD6 case. The arrows (B) show hyperintense signal in the WM of the anterior temporal pole. C, coronal section of the temporal pole stained with luxol fast blue (LFB) and CFV showing multiple “white spaces” in the WM with a preponderance in the border regions. Similar large PVS were not evident in temporal poles of SIVD cases or controls (n=5–8) indicating these do not arise from fixation or staining artefacts. The rectangle denotes the area shown in image D. D, a two-dimensional macroscopic image of twenty stacked 10 µm thick sections of temporal pole, cut 50µm apart, from a ~1mm block of tissue. Note the opaque fuzzy patches or PVS covering a large area of the WM. Scale bar= 4 mm (C) and 2 mm (D).
Enlarged PVS in WM
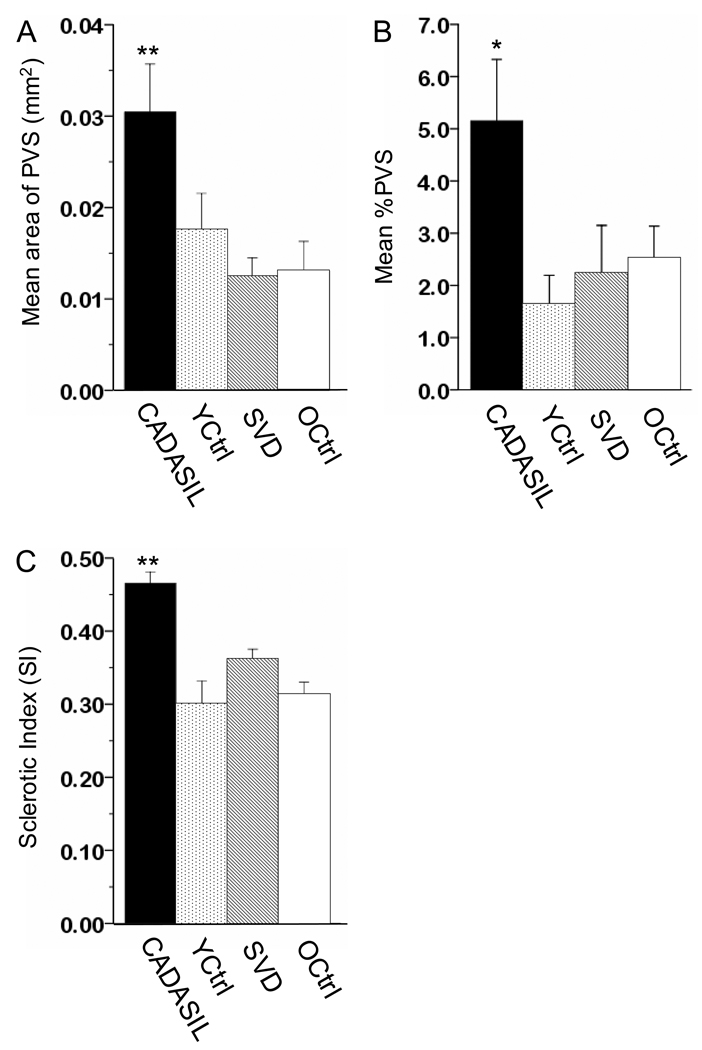
The total area (mm2) of PVS was significantly greater in CADASIL samples compared to the other groups vis a vis CADASIL (0.030) > young controls (0.018) > older controls (0.013) > SIVD (0.012) (F=15.64; P=0.0001, ANOVA) (Figure 3A; Table). There were no significant differences between SIVD and young (P=0.512) and older controls (P=0.999). Ratios of the area of PVS to the area of vessel (V, Figure 1) revealed very similar results: ratio of the mean area (and SD) of PVS to V in CADASIL (8.83, 10.4) was significantly higher compared to the SIVD group (3.95, 4.0), and young (4.89, 3.2) and older (4.36, 3.7) controls (F=18.5; P=0.0001 by ANOVA). The mean %PVS in CADASIL (5.16) was determined to be 50% greater in comparison to the young controls (1.65), SIVD (2.25) and older control (2.54) groups (Figure 3B). The differences in %PVS between CADASIL and the other groups were significant (P=0.036, ANOVA).
Figure 3.
Histograms showing quantification of the mean area of PVS (A), %PVS (B) and SI (C) in CADASIL, SIVD and similar age (YCtrl) and older controls. Columns show mean area (± 2SE for n=5–9) PVS (A) and total area of PVS per WM area mm2 (%PVS) (B). Mean area of PVS and %PVS in CADASIL were significantly increased compared to young controls (P=0.0001 and P=0.036) suggesting the abnormal enlargement of PVS and decreased WM mass in CADASIL. SI values were also significantly increased by 57% in CADASIL compared to young controls (0.47, SD 0.12 versus 0.30, 0.16; P= 0.0001), which indicates the thickening of vessel wall (C). * P<0.05 and **P<0.0001 versus young controls (Mann-Whitney U test).
None of the changes was related to any of the risk factors noted in the CADASIL and SIVD cases (Table). The analyses thus revealed that increases in both the mean area of PVS and number of PVS (>100µm) represented the WM fuzzy patches in CADASIL compared to SIVD cases or the controls (Table, Figure 3). Consistent with the lack of WM hyperintensities in the temporal pole, CAD4 case exhibited the lowest value of %PVS (2.15) that was comparable to values in SIVD and older controls but still higher than young controls (1.65) (Table).
Microvascular changes in WM
Prior to the SI analysis, it was confirmed that there were no significant differences in the sizes of vessels between the cases. H&E revealed numerous basophilic (hyalinised) vessels in the CADASIL cases (Table). Cerebral vessels in CADASIL showed characteristic arteriopathic changes and abnormalities in vascular wall components19 that included disruption of VSMC markers, basement membrane thickening and endothelial abnormalities. The percentage of hyalinised vessels in CADASIL cases (n=290 vessels) was significantly increased compared to similar age (young) controls (n=120): 60.0 % (SD=21.2) in CADASIL and 13.3 % (5.5) in the controls (P<0.001; Mann-Whitney U test). As expected, SI was significantly increased, by 57%, in the CADASIL patients compared to young controls (0.47 versus 0.30, P<0.001, Mann-Whitney U test) (Figure 3C and Table). We also observed that there was a significant correlation between the number of hyalinised vessels and SI values (P<0.05). Again, CAD4 case had the lower SI (0.38) compared to the other CADASIL cases. There was no apparent relationship between duration of disease and the number of hyalinised vessels or SI values.
Myelin and axonal damage in WM of temporal pole
MI analysis revealed that the myelin was severely affected in CADASIL, with the mean MI value as 24.3 in comparison to the other samples (SIVD, 34.6; young controls, 45.0; and older controls, 43.6) (Figure 4A). A significant difference was detected between CADASIL and young controls (P=0.028) while the difference between CADASIL versus SIVD and older controls failed to reach significance (P=0.093 and P=0.072, respectively). Low MI values were consistent with increased dMBP immunoreactivity in CADASIL (Figure 4 and Figure 5). We noted dMBP immunoreactivity in the myelinated nerve fibres and the soma of oligodendrocytes in patients with CADASIL and SIVD (Figure 5A–C) that was also consistent with the regions of pallor seen with LFB (Figure 2). However, in CADASIL cases (Figure 5A and B), the immunoreactivity for dMBP was more intense in the soma of oligodendrocytes than in the myelin sheaths surrounding the axon (Figure 5B, inset), and appeared to be different from that in SIVD, a pattern previously seen in acute phase of cerebral hemorrhage.22 The young or older controls generally lacked dMBP-positive cell bodies and fibres (Figure 5D). Quantitative analysis confirmed that the %dMBP was significantly greater in CADASIL compared to young controls (P=0.026), and in SIVD it was greater than in older controls (P=0.0038) (Figure 4B). In addition to myelin degradation, we also noted abundant deformed axons as well as punctate deposits of APP immunoreactivity in CADASIL cases (Figure 5E). Compared to the young controls, the mean %APP was 60-fold greater in CADASIL samples (P<0.05).
Figure 4.
Histograms showing mean Myelin Index (MI) and % immunoreactivities of dMBP in CADASIL and SIVD cases, and young (YCtrl) and older controls. (A) MI (± 2SE) was the lowest of all the groups and showed significant difference compared to young controls (P=0.028). (B) %dMBP (± 2SE) was significantly greater in CADASIL compared to the other groups. Significant differences were noted in CADASIL versus young controls (P = 0.026) and in SIVD versus older controls (P = 0.0038).
Figure 5.
Myelin damage in WM in the temporal pole in CADASIL as assessed by dMBP immunocytochemistry. Representative images of dMBP accumulation in CADASIL (A, B) and SIVD (C) cases and in an older control (D). Similar to the older control, there was lack of dMBP immunoreactivity in young controls (not shown). Inset in B (higher power) shows magnified image of a dMBP reactive oligodendrocyte cell body with myelin sheaths. (E) APP immunoreactivity showing axon fibre disruption in WM adjacent to a hyalinised vessel with small PVS (*) in a CADASIL subject (CAD3). Scale bar =100µm (A,C,D,E), = 50µm (B) and =10 µm (inset in B).
Discussion
Our main findings suggest that WM hyperintensities in the temporal pole best relate to the numerous fluid-filled PVS13 or increased number of PVS rather than the presence of lacunar infarcts. A previous report had indicated ageing-related subcortical MRI changes are associated with arteriosclerosis and enlarged PVS.23 Our data are consistent with this report but suggest that dilated PVS with the accompanying vascular changes are more profound in CADASIL. We suggest enlarged PVS are evident more so in the temporal pole because of its unique convolutional structure and vascularisation by the branches of the anterior temporal artery.24 Since the resolution of MRI is currently not sufficiently high18 to distinguish single large fluid-filled space (~ 2mm) from clustered smaller spaces, we suggest increased PVS collectively reflect WM hypeintensities upon MRI.
The increased PVS may only partially specify the almost confluent hypersignals upon T2-W and FLAIR MRI in CADASIL. We also demonstrated quantitative WM changes as indicated by decreased MI and increased accumulation of dMBP in CADASIL cases compared to young controls or older SIVD cases. Our data are consistent with the previous findings from diffusion tensor imaging studies25, 27, 28 showing increased diffusivity and decreased anisotropy indicating WM disintegration or rarefaction may progress rapidly over relatively short periods of time and affect executive function. Other factors that may contribute to the integrity of the WM include breakdown or structural changes within the WM25 caused by changes in the microvasculature including breach of the blood-brain barrier (BBB). It is likely that the increased burden and insufficiency in the drainage of the interstitial fluid15 and degraded protein products26 out to the lymphatic systems also play a role in PVS enlargement not only in the temporal pole but several subcortical structures. Thus the evident myelin depletion, oedema between myelin tracts and subsequent axonal disruption,25, 28 together with increased PVS likely intensify the strength of the hyperintensities in the temporal poles. Although there were greater WM changes in SIVD we did not observe significant differences in PVS size between SIVD and older controls. This lends support to the notion that PVS enlargement in CADASIL results from an unique mechanism or accelerated degenerative WM pathology not clearly apparent in SIVD.
Various studies have reported progressive vascular abnormalities in CADASIL.9, 29 that are apparent in skin even at early age (20–30 years old).3, 29–32 When present in brain such vascular abnormalities, also indicated by the SI, no doubt contribute to the reduced cerebral blood flow (CBF) and cerebral blood volume (CBV) to induce WM hypoperfusion and progressively affect cognitive function in CADASIL.33–36 Endothelial cell abnormalities and BBB dysfunction may further contribute to WM damage. Studies by Adler et al.37 indicated that BBB disruption can cause osmotic demyelination, and result in increased permeability of the vessel wall and mobilisation of inflammatory factors, such as macrophages, lymphocytes and complement, which may also cause myelin damage.38 Although incontrovertible evidence for an inflammatory response has not yet been reported,39 involvement of these factors in CADASIL pathology requires further assessment.
Is there functional significance of the dilated or enlarged PVS in CADASIL? While we found no clear relationship between the %PVS and disease duration in CADASIL subjects, previous studies have indicated that size of dilated PVS in other regions of the brain correlates with cognitive impairment.12, 40–42 MacLullich et al.42 showed that increased enlarged PVS in the basal ganglia and centrum semiovale were correlated with worsening cognitive function, particularly verbal memory in healthy elderly men.
One of the potential limitations of our study was that we focused on one region relative to the WM hyperintensities and PVS, which have been described in other subcortical structures in CADASIL.21 Despite this caveat, we believe to have adequately evaluated the temporal pole of the cases and similar age controls by assessing large numbers of cerebral microvessels and PVS. We observed that the CADASIL case with the p.Arg558Cys (in exon11) change lacked enlarged PVS consistent with the apparent absence of WM hyperintensities in temporal pole. Other mutations in exon 11 reported so far did reveal hyperintensities in the temporal pole, suggesting either this mutation is distinct, or more likely, NOTCH3 mutation alone does not lead to the WM changes. While genotype-phenotype relationships in CADASIL are not widely established43, 44 meta-analysis involving a large number of cases may tease out such outlier cases. Our individual case analysis revealed wide variation in PVS pathology similar to MR hyperintense signals between CADASIL cases. This supports the concept that other factors like lifestyle may be involved in the cause of temporal pole hyperintensities upon MRI in CADASIL.
In summary, our findings showed that the area, and by extension volume, of PVS in WM were significantly increased in temporal pole of CADASIL subjects. We suggest that PVS resulting collectively from abnormalities within arterial walls and WM demyelination account for the morphological correlates of the temporal pole WM hyperintensities evident upon MRI. Our observations are consistent with the previous findings of lack of WM hyperintense signals in the temporal pole of ageing controls or sporadic SIVD cases, and accord with the rapid progression of vascular pathology in CADASIL.
Acknowledgements
We are grateful to the patients and families for their co-operation in the investigation of this study. We acknowledge the MRC London Brain Bank for Neurodegenerative Diseases (Dr Claire Troakes) for providing one of the CADASIL cases. We thank Professor Andy Blamire (MR Centre, Newcastle General Hospital) for discussions on the image reconstruction of the temporal pole in earlier phases of this project. We also thank Dr Veronica Miller and Ros Hall for technical assistance in some of the experiments. Mr Jack Shields of the CADASIL Trust is gratefully acknowledged for supporting the fellowship to RWCL. Our work was enabled by grants from the Medical Research Council (UK), the CADASIL Trust (UK), the Alzheimer’s Research Trust (UK) and the National Institutes of Health (NINDS).
Footnotes
Conflicts on Interest Disclosures
We declare no conflicts of interest.
References
- 1.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis E-A, Ruchoux MM, Weissanbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 2.Chabriat H, Vahedi K, Iba-Zizen M, Joutel A, Nibbio A, Nagy TG, Krebs MO, Julien J, Dubois B, Ducrocq X, Levasseur M, Homeyer P, Mas JL, Lyon-Caen O, Tournier-Lasserve E, Bousser M-G. Clinical spectrum of CADASIL: a study of 7 families. Lancet. 1995;346:934–939. doi: 10.1016/s0140-6736(95)91557-5. [DOI] [PubMed] [Google Scholar]
- 3.Ruchoux M-M, Maurage C-A. Endothelial changes in muscle and skin biopsies in patients with CADASIL. Neuropathology and Applied Neurobiology. 1998;24:60–65. doi: 10.1046/j.1365-2990.1998.00087.x. [DOI] [PubMed] [Google Scholar]
- 4.Pfefferkorn T, von Stuckrad-Barre S, Herzog J, Grasser T, Hamann GF, Dichgans M. A transcranial Doppler sonography study. Stroke. 2001;32:17–21. doi: 10.1161/01.str.32.1.17. [DOI] [PubMed] [Google Scholar]
- 5.Chabriat H, Bousser MG, Pappata S. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a positron emission tomography study in two affected family members. Stroke. 1995;26:1729–1730. [PubMed] [Google Scholar]
- 6.Tuominen S, Miao Q, Kurki T, Tuisku S, Poyhonen M, Kalimo H, Viitanen M, Sipila HT, Bergman J, Rinne JO. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke. 2004;35:1063–1067. doi: 10.1161/01.STR.0000124124.69842.2d. [DOI] [PubMed] [Google Scholar]
- 7.Chabriat H, Levy C, Taillia H, Iba-Zizen MT, Vahedi K, Joutel A, Tournier-Lasserve E, Bousser MG. Patterns of MRI lesions in CADASIL. Neurology. 1998;51:452–457. doi: 10.1212/wnl.51.2.452. [DOI] [PubMed] [Google Scholar]
- 8.O'Sullivan M, Jarosz JM, Martin RJ, Deasy N, J.F. P, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56:628–634. doi: 10.1212/wnl.56.5.628. [DOI] [PubMed] [Google Scholar]
- 9.Kalimo H, Ruchoux M-M, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts. Brain Pathology. 2002;12:350–359. doi: 10.1111/j.1750-3639.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markus HS, Martin RJ, Simpson MS, Dong YB, Ali N, Crosby AH, Powell JF. Diagnositic strategies in CADASIL. Neurology. 2002;59:1134–1138. doi: 10.1212/wnl.59.8.1134. [DOI] [PubMed] [Google Scholar]
- 11.Singhal S, Rich P, Markus HS. The spatial distribution of MR imaging abnormalities in cerebral autosomal leukoencephalopathy and their relationships to age and clinical features. AJNR Am J Neuroradiol. 2005;26:2481–2487. [PMC free article] [PubMed] [Google Scholar]
- 12.Cumurciuc R, Guichard J-P, Reizine D, Gray F, Bousser MG, Chabriat H. Dilation of Virchow-Robin spaces in CADASIL. European Journal of Neurology. 2006;13:187–190. doi: 10.1111/j.1468-1331.2006.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Salzman KL, Osborn AG, House P, Jinkins JR, Ditchfield A, Cooper JA, Weller RO. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol. 2005;26:298–305. [PMC free article] [PubMed] [Google Scholar]
- 14.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Research. 2005;2:6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997;191(Pt 3):337–346. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballard C, Rowan E, Stephens S, Kalaria R, Kenny RA. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among demetia-free stroke survivoes >75 years of age. Stroke. 2003;34:2440–2444. doi: 10.1161/01.STR.0000089923.29724.CE. [DOI] [PubMed] [Google Scholar]
- 17.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo J-M, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E. Vascular dementia: Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 19.Low WC, Junna M, Börjesson-Hanson A, Morris CM, Moss TH, Stevens DL, St. Clair D, Mizuno T, Zhang WW, Mykkänen K, Wahlstrom J, Andersen O, Kalimo H, Viitanen M, Kalaria RN. Hereditary multi-infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain. 2007;130:357–367. doi: 10.1093/brain/awl360. [DOI] [PubMed] [Google Scholar]
- 20.Lammie GA, Brannan F, Slattery J, Warlow C. Nonhypertensive cerebral small-vessel disease. Stroke. 1997;28:2222–2229. doi: 10.1161/01.str.28.11.2222. [DOI] [PubMed] [Google Scholar]
- 21.Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, Kalimo H. Arterioles of the lenticular nucleus in CADASIL. Stroke. 2006;37:2242–2247. doi: 10.1161/01.STR.0000236838.84150.c2. [DOI] [PubMed] [Google Scholar]
- 22.Tomimoto H, Akiguchi I, Matsuo A, Terai K, Wakita H, Kimura J, McGeer PL, Budka H. Encephalitogenic peptide (EP) in human cerebrovascular white matter lesions. Neuroreport. 1997;8:3727–3730. doi: 10.1097/00001756-199712010-00014. [DOI] [PubMed] [Google Scholar]
- 23.Awad IA, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- 24.Salamon G. Atlas de la vascularisation arterielle du cerveau chez l'homme. Sandoz Editions 1971. [Google Scholar]
- 25.O'Sullivan M, Barrick TR, Morris RG, Clark CA, Markus HS. Damage within a network of white matter regions underlies executive dysfunction in CADASIL. Neurology. 2005;65:1584–1590. doi: 10.1212/01.wnl.0000184480.07394.fb. [DOI] [PubMed] [Google Scholar]
- 26.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chabriat H, Pappata S, Poupon C, Clark CA, Vahedi K, Poupon F, Mangin JF, Pachot-Clouard M, Jobert A, Le Bihan D, Bousser MG. Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke. 1999;30:2637–2643. doi: 10.1161/01.str.30.12.2637. [DOI] [PubMed] [Google Scholar]
- 28.Molko N, Pappata S, Mangin JF, Poupon F, LeBihan D, Bousser MG, Chabriat H. Monitoring disease progression in CADASIL with diffusion magnetic resonance imaging: a study with whole brain histogram analysis. Stroke. 2002;33:2902–2908. doi: 10.1161/01.str.0000041681.25514.22. [DOI] [PubMed] [Google Scholar]
- 29.Ruchoux M-M, Guerouaou D, Vandenhaute B, Pruvo J-P, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathologica. 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- 30.Ruchoux MM, Maurage CA. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56 947-064. [PubMed] [Google Scholar]
- 31.Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, Kalimo H. Fibrosis and stenosis of the long penetrating cerebral arteries: The cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathology. 2004;14:358–364. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brulin P, Godfraind C, Leteurtre E, Ruchoux M-M. Morphometric analysis of ultrastructural vascular changes in CADASIL: Analysis of 50 skin biopsy specimens and pathogenic implications. Acta Neuropathologica. 2002;104:241–248. doi: 10.1007/s00401-002-0530-z. [DOI] [PubMed] [Google Scholar]
- 33.Bruening R, Dichgans M, Berchtenbreiter C, Yousry T, Seelos KC, Wu RH, Mayer M, Brix G, Reiser M. Cerebral Autosomal dominant arteriopathy with subcortical Infarcts and leukoencephalopathy: decrease in regional cerebral blood volume in hyperintense subcortical lesions inversely correlates with disability and cognitive performance. American Journal of Neuroradiology. 2001;22:1268–1274. [PMC free article] [PubMed] [Google Scholar]
- 34.Chabriat H, Pappata S, Ostergaard L, Clark CA, Pachot-Clouard M, Vahedi K, Jobert A, Le Bihan D, Bousser MG. Cerebral homodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. 2000;31:1904–1912. doi: 10.1161/01.str.31.8.1904. [DOI] [PubMed] [Google Scholar]
- 35.Spilt A, Weverling-Rijinsburger AW, Middelkoop HA, van Der Flier WM, Gussekloo J, de Craen AJ, Bollen EL, Blauw GJ, van Buchem MA, Westendorp RG. Late-onset dementia: structural brain damage and total cerebral blood flow. Radiology. 2005;236:990–995. doi: 10.1148/radiol.2363041454. [DOI] [PubMed] [Google Scholar]
- 36.Osawa A, Maeshima S, Shimamoto Y, Maeshima E, Sekiguchi E, Kakishita K, Ozaki F, Moriwaki H. Relationship between cognitive function and regional cerebral blood flow in different types of dementia. Disability and Rehabilitation. 2004;26:739–745. doi: 10.1080/09638280410001704331. [DOI] [PubMed] [Google Scholar]
- 37.Adler S, Martinez J, Williams DS, Verbalis JG. Positive association between blood brain barrier disruption and osmotically-induced demyelination. Multiple Sclerosis. 2000;6:24–31. doi: 10.1177/135245850000600106. [DOI] [PubMed] [Google Scholar]
- 38.Baker EA, Tian Y, Adler S, Verbalis JG. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Experimental Neurology. 2000;165:221–230. doi: 10.1006/exnr.2000.7474. [DOI] [PubMed] [Google Scholar]
- 39.Unlu M, de Langa RP, de Silva R, Kalaria R, St Clair D. Detection of complement factor B in the cerebrospinal fluid of patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy disease using two-dimensional gel electrophoresis and mass spectrometry. Neurosci Lett. 2000;282:149–152. doi: 10.1016/s0304-3940(00)00875-2. [DOI] [PubMed] [Google Scholar]
- 40.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 41.Uggetti C, Egitto MG, Pichiecchio A, Sinforiani E, Bevilacqua MS, Cavallini A, Micieli G. Subcortical dementia associated with striking enlargement of the Virchow-Robin spaces and transneural degeneration of the left mammillo-thalamic tract. Cerebrobasc Dis. 2001;12:287–290. doi: 10.1159/000047722. [DOI] [PubMed] [Google Scholar]
- 42.MacLullich AMJ, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1519–1523. doi: 10.1136/jnnp.2003.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters N, Opherk C, Bergmann T, Castro M, Herzog J, Dichgans M. Spectrum of mutations in biopsy-proven CADASIL: Implications for diagnostic strategies. Archives of Neurology. 2005;62:1091–1094. doi: 10.1001/archneur.62.7.1091. [DOI] [PubMed] [Google Scholar]
- 44.Opherk C, Peters N, Herzog J, Dichgans M, Luedtke R. Long-term prognosis and causes of death in CADASIL: A retrospective study in 411 patients. Brain. 2004;127:2533–2539. doi: 10.1093/brain/awh282. [DOI] [PubMed] [Google Scholar]