Abstract
Acinetobacter baumannii is a Gram-negative opportunistic nosocomial pathogen. This microorganism survives in hospital environments despite unfavorable conditions such as desiccation, nutrient starvation and antimicrobial treatments. It is hypothesized that its ability to persist in these environments, as well as its virulence, is a result of its capacity to form biofilms. A. baumannii forms biofilms on abiotic surfaces such as polystyrene and glass as well as biotic surfaces such as epithelial cells and fungal filaments. Pili assembly and production of the Bap surface-adhesion protein play a role in biofilm initiation and maturation after initial attachment to abiotic surfaces. Furthermore, the adhesion and biofilm phenotypes of some clinical isolates seem to be related to the presence of broad-spectrum antibiotic resistance. The regulation of the formation and development of these biofilms is as diverse as the surfaces on which this bacterium persists and as the cellular components that participate in this programmed multistep process. The regulatory processes associated with biofilm formation include sensing of bacterial cell density, the presence of different nutrients and the concentration of free cations available to bacterial cells. Some of these extracellular signals may be sensed by two-component regulatory systems such as BfmRS. This transcriptional regulatory system activates the expression of the usher-chaperone assembly system responsible for the production of pili, needed for cell attachment and biofilm formation on polystyrene surfaces. However, such a system is not required for biofilm formation on abiotic surfaces when cells are cultured in chemically defined media. Interestingly, the BfmRS system also controls cell morphology under particular culture conditions.
Keywords: abiotic surface, Acinetobacter, biofilm, biotic surface, pili production, quorum sensing, signal transduction, transcriptional regulation
The genus Acinetobacter is a genetically diverse group of aerobic Gram-negative nonfermenting bacteria [1]. Although acinetobacters are commonly described as being ubiquitous in nature, those strains belonging to the Acinetobacter baumannii–Acinetobacter calcoaceticus cluster are emerging as problematic opportunistic pathogens due to the rapid increase in multidrug or pandrug resistance [2–4]. These infections manifest as serious diseases in compromised human hosts particularly in the case of ventilator-acquired pneumonia, urinary tract infections, septicemia, wound infections and more recently in severe cases of necrotizing fasciitis [5]. Furthermore, these infections have recently come under scrutiny because of their prevalence in wounded American military personnel returning from the Middle East [6]. Owing to the increase in mortality as well as the lack of treatment options for these infections, prevention of host colonization is absolutely necessary [7]. In addition, since a large proportion of these infections are acquired in healthcare environments, a better understanding of how this microorganism survives and persists in this environment is essential. It is hypothesized that the ability of clinical strains to survive desiccation, antimicrobial therapies and nutrient availability stress is mediated by the microorganism's ability to form biofilms on medically relevant surfaces.
Biofilm structure & function
A. baumannii clinical isolates have been observed to possess the ability to survive long stretches of time under highly desiccated conditions on abiotic surfaces [8,9]. For instance, Jawad and colleagues discovered that the mean survival time for a bank of 39 clinical isolates when subjected to desiccation on glass coverslips was 27 days, a response that is more common among Gram-positive than Gram-negative bacteria [8]. Accordingly, analysis of a collection of clinical isolates showed that the ability to form biofilms on abiotic surfaces is a common trait among different A. baumannii strains, particularly in those isolated from catheter-related urinary tract or bloodstream infections as well as a case of shunt-related meningitis [10]. In addition, A baumannii clinical isolates have shown a correlation between antibiotic resistance and the ability to adhere to clinically relevant surfaces including polystyrene and respiratory epithelial cells [11]. It was observed that cell adhesiveness and biofilm formation on plastic is higher in strains harboring the blaPER-1 gene than in those that do not harbor this genetic trait. Furthermore, the level of expression of this gene, as determined by reverse transcription-PCR, is positively correlated with the level of biofilm formed on plastic and the adhesiveness of bacteria to human epithelial cells [11]. Similar findings were reported by Rao et al., who found a significant association between multidrug resistance and biofilms, although they believe that the presence of blaPER-1 is more critical for cell adhesion than the formation of bacterial biofilms on abiotic surfaces [12]. Thus, the functions of the biofilm structures formed by this bacterial pathogen encompass its ability to resist antimicrobial therapies as well as other environmental stresses such as dehydration and limited nutrient availability.
A biofilm is a community of multiple bacterial cells associated with a surface (either biotic or abiotic), arranged in a tertiary structure in intimate contact with each other and encased in an extracellular matrix that can be comprised of carbohydrates, nucleic acids, proteins and other macromolecules [13]. Furthermore, this structure can confer resistance to antimicrobial therapies on the order of one thousand times greater than that of their planktonic counterparts [14]. Bacterial biofilm initiation and development is not simply a serendipitous adherence of bacterial cells to a surface. On the contrary, it is a highly regulated series of molecular events, which cells keep under tight regulation. The most common factors that can influence biofilm formation are: nutrient availability, bacterial appendages (pili and flagella), bacterial surface components (outer membrane proteins, adhesins), quorum sensing and macromolecular secretions (polysaccharides, nucleic acids and so on) [15]. In addition, complex regulatory networks including two-component regulatory systems and transcriptional regulators are known to be responsible for the expression of a variety of biofilm-associated gene products in response to a wide range of environmental signals [16].
Acinetobacter baumannii biofilm formation & regulation
The A. baumannii 19606-type strain forms biofilms on abiotic surfaces such as glass and polystyrene [17]. With respect to the medically relevant surface polystyrene, a polymer that is commonly used in the manufacturing of a variety of medical devices, the production of pili is essential for biofilm formation by this clinical strain. These pili are the product of the csuA/BABCDE six open-reading frame polycistronic operon. Comparative analysis showed that operons similar to those described in the 19606-type strain are also found in the genome of strains AB0056 (GenBank accession number NC_011586) [101], AYE [18] and 17978 [19]. Interestingly, the two latter strains also contain three additional loci that code for secretion functions potentially associated with pili assembly and adhesion [101]. A locus coding for a putative chaperon-usher secretion system was also found in the ACICU strain, although the number of genes and their order seems to be different from that described for the 19606 strain [20]. By contrast, no similar csu loci were located in the genome of A. baumannii SDF and Acinetobacter baylyi ADP1 [18].
Inactivation of csuE, which codes for the putative tip adhesin, results in the abolition of pili production as well as biofilm formation [17]. This observation suggests that CsuA/ BABCDE-mediated pili play a role in the initial steps of biofilm formation by allowing bacterial cells to adhere to abiotic surfaces and initiate the formation of microcolonies that precede the full development of biofilm structures. The expression of this operon is regulated by a two-component regulatory system comprised of a sensor kinase encoded by bfmS and a response regulator encoded by bfmR. Transcriptional and translational analyses show that the inactivation of bfmR results in a loss of expression of the csu operon and the consequent abolition of both pili production and biofilm formation on plastic when cells are cultured in rich medium [21]. However, inactivation of the open-reading frame encoding the BfmS sensor kinase results in an attenuation but not abolition of biofilm formation. This indicates that there could be cross-talk between other sensing components and the BfmR response regulator, a hypothesis that suggests that multiple and different environmental stimuli could control biofilm formation via the BfmRS regulatory pathway. Interestingly, the function of this regulatory system is not confined to biofilm initiation, but is also involved in cell morphology. Equally interesting is the fact that the composition of the culture medium and the interaction of cells with abiotic surfaces play a significant role when the BfmRS system is not expressed [21]. Taken together, these observations underscore the importance of extracellular signals, not only in biofilm initiation and development but also in the morphology of cells interacting with abiotic surfaces.
In the case of glass surfaces, transposon inactivation of a homolog of the staphylococcal biofilm-associated protein (Bap) in A. baumannii strain 307-0294 resulted in a destabilization of the mature biofilm [22]. This destabilization was measured as a reduction of both biofilm volume and thickness upon imaging of cells producing green fluorescent protein under confocal laser-scanning microscopy. Bap is a protein exposed on the surface of the bacterial cell, as was evidenced by flow-cytometry analysis of immunofluores-cent-labeled whole bacterial cells. Based on this cellular location and its participation in biofilm development, it is hypothesized that this protein is involved in cell–cell interactions that support biofilm maturation. Furthermore, this protein is conserved among a panel of 98 Acinetobacter strains [22], a finding that underscores the importance of this protein in adhesion and biofilm formation. However, at present, there are no reports describing the potential environmental factors and conditions that could control the differential expression of the bap gene.
Another factor that has been observed to control the formation of biofilms by A. baumannii is the presence of metal cations. The A. baumannii 19606-type strain forms more biofilms on plastic when cultured in a chemically-defined medium under iron-chelated conditions, which were imposed by the presence of the synthetic iron chelators 2,2′-dipyridyl and ethylenedi-amine-di-(o-hydroxyphenyl) acetic acid [17]. Such a response is in line with the well-known role that iron in particular, and metals in general, play in the differential expression of bacterial genes, some of which are central to the virulence of relevant pathogenic microorganisms. The analysis of a bank of multidrug-resistant A. baumannii clinical isolates also showed that the presence of the chelating agent ethylene-diaminetetraacetic acid caused a significant reduction in bacterial attachment and biofilm formation on human respiratory epithelial cells as well as on plastic surfaces [11]. The molecular mechanism by which these two cations, particularly in the case of iron where the Fur repressor proteins plays a central role in gene expression, remains to be elucidated.
Another pathway by which A. baumannii senses extracellular signals and directs biofilm formation is quorum sensing. The A. baumannii M2 strain produces an acyl-homoserine lactone molecule, a product of the abaI autoinducer synthase gene, the expression of which is positively upregulated by a feedback loop [23]. Mutagenesis of the abaI gene showed that this secreted product plays a role in the later stages of biofilm formation. Its inactivation results in a 30–40% biofilm reduction when compared with the isogenic parental strain. Exogenous addition of purified M2 acyl-homoserine lactone restored biofilm maturation of the abaI mutant. Thus, communication between bacterial cells with respect to cell density is integral to the maturation of A. baumannii biofilm as described for other relevant human bacterial pathogens [24].
In addition to abiotic surfaces, A. baumannii has evolved sophisticated methods to attach to biotic surfaces such as epithelial cells [11]. Although the mechanisms of attachment to human bronchial epithelial cells remain obscure, there is a positive correlation between the level of expression of the blaPER-1 broad-range β-lactamase gene and the level of bacterial adhesion and biofilm formation on this type of cell. Such a correlation may explain the ability of A. baumannii to persist and disseminate in medical environments and the human host even in the presence of a wide range of antibiotics.
Our preliminary work has also shown that the A. baumannii 19606-type strain attaches to, and forms biofilms on, human alveolar epithelial cells and Candida albicans filaments but not yeast cells (Figure 1) [25]. This process does not require the expression of the CsuA/BABCDE operon since an isogenic derivative with a disruption in the csuE coding region showed the same biofilm phenotype as the parental strain. This is in contrast to the observations described earlier in which the expression of this gene and the subsequent assembly of pili are essential steps for biofilm formation on polystyrene. Equally contrasting is the recent observation that the A. baumannii 19606 CsuE mutant is able to adhere to human respiratory epithelial cells [26]. Based on this information and the role BfmRS plays in biofilm formation on abiotic surfaces, it is tempting to speculate that this two-component regulatory system may not play a role in the interaction of A. baumannii with a biotic surface such as that of C. albicans filaments. However, the extent and components of the BfmRS regulon are still unknown and it is possible that this regulatory system does infact play a role when bacterial and eukaryotic cells interact independently of its Csu-regulatory functions. It is interesting to note that a recent report also examined the interaction of A. baumannii with C. albicans although in a different experimental context since it included the nematode Caenorhabditis elegans [27]. This work, which does not provide direct evidence of bacterial attachment to, and biofilm formation on, fungal filaments, observes that inactivation of a homolog of the gacS sensor kinase attenuates the virulence of A. baumannii when tested using the tri-partite C. elegans–A. baumannii–C. albicans experimental model. Taken together, these observations suggest that biofilm formation on fungal filaments and their killing by A. baumannii are processes that are controlled by different regulatory systems that may respond to different environmental cues.
Figure 1. Microscopy analysis of Acinetobacter baumannii 19606 cells attached to eukaryotic cells.
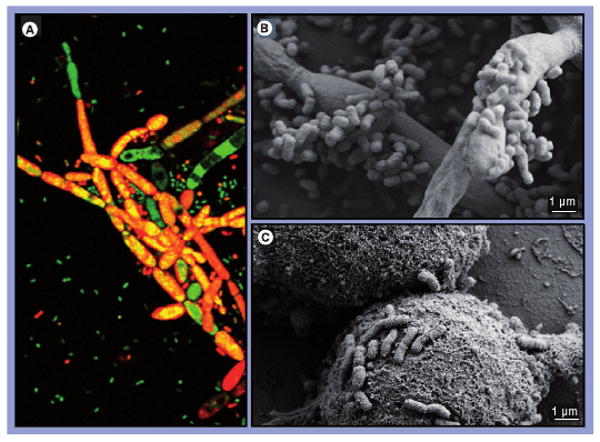
(A) Laser-scanning confocal microscopy of live/dead-stained A. baumannii 19606 cells attached to Candida albicans tup1 filaments. Live bacterial cells, stained green, attached to the surface of dead fungal filaments, stained red, appear as areas of yellow co-fluorescence. The micrograph was taken at 400× magnification. (B) Scanning electron microscopy (SEM) of bacterial cells attached to C. albicans tup1 filaments. (C) SEM of bacterial cells attached to A549 human alveolar epithelial cells.
Conclusion & future perspective
The ability of A. baumannii to form biofilms is multifactorial and diverse, dependant upon the surface with which the cells are interacting. The expression of bacterial-associated factors in biofilm development is dependent upon nutrients and sensing of the environment by either the BfmS sensor kinase, cross-talk with other kinases or substrate-level phosphorylation of the cognate response regulators such as BfmR. In addition to these factors, surface proteins such as a Bap homolog could be involved in stabilizing the mature biofilm on abiotic or biotic surfaces. The presence of metal cations and the expression of resistance to broad-spectrum antibiotics can also increase the ability of A. baumannii to adhere to, and form biofilms on, a surface. However, many of the molecular mechanisms by which these bacteria adhere to diverse, medically relevant surfaces and human host cells remain obscure. Elucidating these mechanisms using modern and global approaches could provide missing basic information on these processes, which could be novel targets for future antimicrobial strategies as the age of antibiotics begins to wane. These are realistic and achievable goals since A. baumannii has entered the genomic and postgenomic era after several genomes were fully sequenced and annotated or are close to completion. Comparative genomics has already shed light on the common and unique genetic features of different clinical isolates, such as the presence of a unique resistance island in a multidrug-resistant nosocomial isolate [28]. These advances, together with the possibility of conducting global gene-expression analyses and testing virulence with appropriate experimental models, should provide a quantum leap in our understanding of not only biofilm-formation functions but also how these functions correlate with other cellular factors that contribute to the virulence of A. baumannii and its ability to cause severe infections in humans.
Executive summary
Acinetobacter baumannii strains have emerged as problematic pathogens due to multi- and pandrug resistance, making treatment difficult.
Prevention of colonization and persistence is key, thus a better understanding of microbial survival in healthcare settings could facilitate lower colonization rates.
This survivability characteristic of Acinetobacter is hypothesized to be mediated by biofilm formation in the healthcare setting.
Biofilm structure & function
Biofilms are structural arrangements of cells on a surface that are encased in an extracellular matrix. These structures can confer resistance to antimicrobial compounds.
A. baumannii clinical isolates survive following long periods of desiccation and form biofilms on abiotic and biotic surfaces.
Multidrug resistance correlates with the ability to attach to polystyrene and epithelial cells. Furthermore, expression of blaPER-1 correlates with cell adherence and biofilm formation.
Regulatory networks tightly control the expression of biofilm-associated factors such as cellular appendages, adhesions and cell density-sensing molecules.
Acinetobacter baumannii biofilm formation & regulation
The 19606-type strain forms biofilms on polystyrene. The initiation of these structures is mediated by somatic pili produced by the CsuA/BABCDE chaperone-usher secretion system, the expression of which is regulated by the two-component system BfmRS.
A homolog of staphylococcal biofilm-associated protein has been identified as a surface component of A baumannii that is involved in biofilm stabilization and maturation.
Several environmental signals have been identified that influence biofilm formation including the presence of metal cations and quorum-sensing molecules.
The 19606-type strain attaches to, and forms biofilms on, biotic surfaces such as Candida albicans filaments and human alveolar epithelial cells. These interactions result in the killing of the eukaryotic cells.
Conclusion & future perspective
The regulation of biofilm formation is multifactorial including a variety of environmental signals and sensing molecules that result in the production of a variety of bacterial factors, including surface proteins and pili that play a role in adhesion and biofilm development.
Although A. baumannii has been observed to form biofilms on both abiotic and biotic surfaces, many of the molecular mechanisms remain unidentified.
The advent of sequenced and annotated genomes for clinically relevant strains provides excellent resources for the investigation of genes and gene products involved in these processes.
Global gene-expression analysis and animal model experiments can shed light on the virulence properties of this pathogen.
Acknowledgments
The authors' work was supported by Miami University research Funds and by NIH R01-AI070174 and NSF MRI-0420479 grants.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Jennifer A Gaddy, Department of Microbiology, Miami University, 32 Pearson Hall, Oxford, OH 45056, USA, Tel.: +1 513 529 5439; gaddyja@muohio.edu.
Luis A Actis, Department of Microbiology, Miami University, 32 Pearson Hall, Oxford, OH 45056, USA, Tel.: +1 513 529 5424; Fax: +1 513 529 2431; actisla@muohio.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Juni E. Genus II Acinetobacter. In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey's Manual of Systematic Bacteriology. Springer Science & Business Media, Inc.; New York, USA: 2005. pp. 425–437. [Google Scholar]; ▪▪ Overview of Acinetobacter taxonomy and the clinical significance of the genus.
- 2.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]; ▪▪ Excellent overview of methods used for the taxonomy of acinetobacters with particular emphasis on clinical isolates.
- 3.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Updated review of the clinical significance of Acinetobacter.
- 4.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charnot-Katsikas A, Dorafshar AH, Aycock JK, David MZ, Weber SG, Frank KM. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J Clin Microbiol. 2009;47:258–263. doi: 10.1128/JCM.01250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronson NE, Sanders JW, Moran KA. In harm's way: infections in deployed American military forces. Clin Infect Dis. 2006;43:1045–1051. doi: 10.1086/507539. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 2007;11:134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wendt C, Dietze B, Dietz E, Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Bano J, Marti S, Soto S, et al. Biofilm formation in Acinetobacter baumannii: associated features and clinical implications. Clin Microbiol Infect. 2008;14:276–278. doi: 10.1111/j.1469-0691.2007.01916.x. [DOI] [PubMed] [Google Scholar]; ▪ Describes the clinical importance of Acinetobacter baumannii biofilm formation.
- 11.Lee HW, Koh YM, Kim J, et al. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect. 2008;14:49–54. doi: 10.1111/j.1469-0691.2007.01842.x. [DOI] [PubMed] [Google Scholar]; ▪ First publication exploring A. baumannii biofilm formation and attachment to eukaryotic cells.
- 12.Rao RS, Karthika RU, Singh SP, et al. Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol. 2008;26:333–337. doi: 10.4103/0255-0857.43566. [DOI] [PubMed] [Google Scholar]
- 13.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 14.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghannoum M, O'Toole GA. Microbial Biofilms. ASM Press; Washington DC, USA: 2004. [Google Scholar]
- 16.Stanley NR, Lazazzera BA. Environmental signals and regulatory pathways that influence biofilm formation. Mol Microbiol. 2004;52:917–924. doi: 10.1111/j.1365-2958.2004.04036.x. [DOI] [PubMed] [Google Scholar]; ▪ Good overview of factors controlling bacterial biofilm formation.
- 17.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]; ▪▪ First paper demonstrating A. baumannii biofilm formation on polystyrene requires the production of pili.
- 18.Vallenet D, Nordmann P, Barbe V, et al. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS ONE. 2008;3:e1805. doi: 10.1371/journal.pone.0001805. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First report describing the comparative analysis of Acinetobacter genomes.
- 19.Smith MG, Gianoulis TA, Pukatzki S, et al. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007;21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First report of the nucleotide sequence of an A. baumannii strain.
- 20.Iacono M, Villa L, Fortini D, et al. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob Agents Chemother. 2008;52:2616–2625. doi: 10.1128/AAC.01643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology. 2008;154:3398–3409. doi: 10.1099/mic.0.2008/019471-0. [DOI] [PubMed] [Google Scholar]; ▪▪ Presents a two-component regulatory system involved in A. baumannii biofilm formation.
- 22.Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036–1044. doi: 10.1128/JB.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ First identified example of a biofilm associated protein in A. baumannii.
- 23.Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irie Y, Parsek MR. Quorum sensing and microbial biofilms. Curr Top Microbiol Immunol. 2008;322:67–84. doi: 10.1007/978-3-540-75418-3_4. [DOI] [PubMed] [Google Scholar]
- 25.Gaddy JA, Tomaras AP, Actis LA. Acinetobacter baumannii outer membrane protein A is involved in attachment to biotic and abiotic surfaces and killing of Candida albicans filaments. Presented at: 108th American Society for Microbiology General Meeting; Boston, MA, USA. 1–5 June 2008. [Google Scholar]
- 26.de Breij A, Gaddy J, van der Meer J, et al. CsuA/BABCDE-dependent pili are not involved in the adherence of Acinetobacter baumannii ATCC19606T to human airway epithelial cells and their inflammatory response. Res Microbiol. 2009 doi: 10.1016/j.resmic.2009.01.002. In Press. [DOI] [PubMed] [Google Scholar]
- 27.Peleg AY, Tampakakis E, Fuchs BB, Eliopoulos GM, Moellering RC, Jr, Mylonakis E. Prokaryote–eukaryote interactions identified by using Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:14585–14590. doi: 10.1073/pnas.0805048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournier PE, Vallenet D, Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Describes the comparative analysis of the genomes of A. baumannii clinical isolates.
Website
- 101.Genoscope: French National Sequencing Center; BaumannoScope Project. www.genoscope.cns.fr/agc/mage/baumannoscope. [Google Scholar]


