Abstract
Dormancy release in imbibed annual ryegrass (Lolium rigidum Gaud.) seeds is promoted in the dark but inhibited in the light. The role of abscisic acid (ABA) in inhibition of dormancy release was found to be negligible, compared with its subsequent effect on germination of dormant and non-dormant seeds. Inhibitors of ABA metabolism had the expected effects on seed germination but did not influence ABA concentration, suggesting that they act upon other (unknown) factors regulating dormancy. Although gibberellin (GA) synthesis was required for germination, the influence of exogenous GA on both germination and dormancy release was minor or non-existent. Embryo ABA concentration was the same following treatments to promote (dark stratification) and inhibit (light stratification) dormancy release; exogenous ABA had no effect on this process. However, the sensitivity of dark-stratified seeds to ABA supplied during germination was lower than that of light-stratified seeds. Therefore, although ABA definitely plays a role in the germination of annual ryegrass seeds, it is not the major factor mediating inhibition of dormancy release in imbibed seeds.
Keywords: Abscisic acid, dormancy release, fluridone, germination, gibberellins, Lolium rigidum, seed
Introduction
Like many weedy grass species, seeds of annual ryegrass (Lolium rigidum Gaud.) are often dormant when they are shed, and then gradually lose dormancy over a period of months through dry after-ripening (Steadman et al., 2003). However, imbibed seeds can progressively lose dormancy in a matter of weeks if they are kept in constant darkness (Steadman, 2004). Imbibed seeds incubated in the light remain dormant through the actions of cryptochrome and a putative green light receptor (Goggin et al., 2008). Once seeds have lost dormancy through dark stratification, they require light and alternating temperatures to germinate (Steadman, 2004). Depending upon where the theoretical line between dormancy release and stimulation of germination is drawn, this final requirement for light and alternating temperature can be considered as low-level seed dormancy, as opposed to the high level of dormancy exhibited by seeds that still cannot germinate under these ideal conditions (Leon et al., 2007). In the current study, the term ‘dormancy release’ is used to describe the processes occurring in imbibed annual ryegrass seeds during dark stratification, which allow them to germinate subsequently under light and alternating temperature. The biochemical mechanisms by which dormancy release in ryegrass seeds is promoted or inhibited are currently unknown; however, it is highly possible that abscisic acid (ABA) and gibberellin (GA) are involved in mediating these processes, as many studies on a wide variety of plant species have demonstrated the strong influence of these hormones on seed dormancy and germination (reviewed in Hilhorst et al., 2006; Leubner-Metzger, 2006).
In plants, ABA is synthesized through the cleavage and oxidation of carotenoids, and is catabolized via hydroxylation or by conjugation to glucose (Nambara and Marion-Poll, 2005). Dormant seeds treated with fluridone (a compound which inhibits carotenoid and, thus, ABA synthesis) often have similar germination characteristics to non-dormant seeds, indicating that the continued synthesis of ABA is required for dormancy maintenance in imbibed seeds of several species (Ali-Rachedi et al., 2004; Kusumoto et al., 2006; Feurtado et al., 2007). Similarly, Millar et al. (2006) and Gubler et al. (2008) showed that dormancy maintenance in seeds of Arabidopsis and barley depends upon the balance of ABA synthesis and catabolism being shifted towards the former. Expression of the gene encoding ABA 8′-hydroxylase, a cytochrome P450 monooxygenase that catalyses the hydroxylation of ABA to 8′-hydroxy ABA, was higher in imbibed non-dormant seeds compared with dormant seeds, and was inversely correlated with seed ABA concentration (Millar et al., 2006).
As reviewed in Nonogaki (2006), experiments on GA- and testa-deficient mutants of Arabidopsis have shown that GA is required to generate sufficient embryo growth potential to rupture the endosperm and testa in the final stages of germination; however, the influence of GA on dormancy release itself is not as readily apparent. Although GA can stimulate germination of dormant seeds in some species, there are many instances where GA alone is ineffective, and it has been suggested that GA is necessary but not sufficient for dormancy release (Finkelstein et al., 2008). There is also evidence that GA mediates metabolism of ABA and vice versa (Gonai et al., 2004; Gubler et al., 2008). In general, studies on seed dormancy have revealed a highly complex interaction between environmental conditions, various plant growth regulators, and the sensitivity of seeds to these parameters. In the work described below, the potential roles of ABA and GA in dormancy release in stratified L. rigidum seeds were investigated. Seeds were treated with ABA, GA, and various compounds altering their metabolism, and the effects of stratification and chemical treatments on seed germination and endogenous ABA concentrations were measured.
Materials and methods
Seed material
Freshly matured seeds of L. rigidum were collected in October or November of 2000, 2006, and 2007 from a wheat field at Wongan Hills, Western Australia (30°53′S, 116°43′E). Seed viability, tested by tetrazolium staining (Steadman, 2004), was close to 100% for all three populations; moisture content, based on the mass change of seeds upon drying at 103 °C for 24 h, was 13, 15, and 7% for the 2000, 2006, and 2007 populations, respectively. Seeds were stored in sealed foil pouches at –20 °C to maintain their dormancy status. The average basal germination of the 2000, 2006, and 2007 populations [in the presence of 0.1% (v/v) dimethylsulphoxide (DMSO), which was used as a solvent for plant growth regulators] was 44, 43, and 6%, respectively, at 39 d after imbibition. Germination following 21 d dark stratification at 20 °C averaged 73, 76, and 55%, respectively, at 39 d after imbibition.
Plant growth regulators
The plant growth regulators used in this study were ABA, GA3, GA4, fluridone (an inhibitor of phytoene desaturase in the carotenoid biosynthesis pathway and thus of ABA biosynthesis), paclobutrazol (an inhibitor of ent-kaurene oxidase, early in the GA biosynthetic pathway), and diniconazole (an inhibitor of the ABA-catabolizing enzyme ABA 8′-hydroxylase). A comprehensive screening of potential triazole-based chemical inhibitors of ABA 8′-hydroxylase by Kitahata et al. (2005) revealed that diniconazole was a very strong inhibitor, whilst paclobutrazol, although of similar chemical structure, had almost no effect on ABA 8′-hydroxylase activity. In contrast, diniconazole was less effective as a plant growth inhibitor than paclobutrazol (Fletcher et al., 1986). Therefore, for the current study, paclobutrazol and diniconazole were selected as relatively specific inhibitors of GA biosynthesis and ABA catabolism, respectively.
All plant growth regulators were obtained from Sigma-Aldrich (Sydney, Australia). Plant growth regulators were dissolved in DMSO as 50 μM stocks of biologically active isomer, and diluted to the appropriate working concentrations in molten (∼60 °C) 1% (w/v) agar. Controls contained the same volume of DMSO as treatments, and the pH of the agar was unaffected by the addition of the plant growth regulators at the concentrations used in this study. Plates containing plant growth regulators were stored at 4 °C and used within 24 h. Dry seeds were sown on the solidified agar in 90 mm diameter Petri dishes.
Seed stratification and germination conditions
Seeds were stratified either at 20 °C in the dark to decrease the dormancy of the population, or at 20 °C under constant white light (50 W halogen lamps with a fluence rate of 84 μmol m−2 s−1) so that dormancy release was inhibited. A 21 d stratification time was selected based on Steadman (2004), Steadman et al. (2004), and pilot studies on the 2006 and 2007 seed collections. Dormancy release in dark-stratified annual ryegrass seeds is a progressive process, but the rate of dormancy release slows appreciably after 21 d. After stratification, seeds were transferred to germination conditions (25/15 °C day/night cycles with a 12 h photoperiod of combined fluorescent and incandescent white light, fluence rate 20–40 μmol m−2 s−1). Control (non-stratified) seeds were placed directly into germination conditions at the same time. Each treatment consisted of four replicates of 50 seeds. Following transfer to germination conditions, seed germination (defined as protrusion of the radicle from the seed coat) was scored regularly for the next 18–21 d. Although dormancy release is greatly accelerated by dark stratification, it also gradually occurs over a much longer period under normal germination conditions, and so germination counting was ended at 18–21 d post-stratification to avoid complication of the results. Dead or empty seeds, which collapsed upon gentle pinching, were excluded from calculations of percentage germination.
Quantification of endogenous ABA
ABA was extracted, purified, and quantified by an isotope dilution assay using the extraction method of Ferguson et al. (2005). [2H4]ABA (NRC-PBI, Saskatoon, Canada) was added as a quantitative internal standard. Purification included a mixed mode reverse-phase cation-exchange Oasis MCX-SPE column (Waters, Mississauga, Canada), pre-conditioned with 5 ml of methanol followed by 5 ml of 1.0 M formic acid. The sample was loaded in 1.0 M formic acid and ABA was eluted with 5 ml of methanol. Purified ABA was analysed by LC-(+)ESI-MS/MS using a Waters 2680 Alliance HPLC system (Waters, Milford, MA, USA) linked to a Quattro-LC triple quadrupole mass spectrometer (Micromass, Altrincham, UK; see Ferguson et al., 2005). Compounds were quantified by multiple reaction monitoring of the mother (parent) ion and daughter (product) ions, as in Ross et al. (2004), for ABA.
An attempt to measure GA1 and GA4 was made, following the methods of Chiwocha et al. (2003), but levels were too low for consistent quantification within experiments. GA4 was identified in many samples but GA1 was never detected.
Experimental design
Effect of plant growth regulators on dormancy release or germination:
The effects of ABA, GA3, GA4, fluridone, paclobutrazol, and diniconazole on dormancy release in stratified L. rigidum seeds were separated from their effects on germination by transferring seeds to fresh agar at the same time as they were transferred from stratification to germination conditions. Seeds that were stratified in the presence of plant growth regulators were then germinated in their absence (to determine the effects on dormancy release), and vice versa (to determine the effects on germination of dark- and light-stratified seeds). In the initial screening experiment, all plant growth regulators were applied at a concentration of 50 μM, except for GA4 (10 μM). Non-stratified controls were placed directly under germination conditions and also transferred to fresh plates at the same time as the stratified seeds, to give an indication of the basal response of the dormant seed populations to plant growth regulators. Although the three seed collections had different starting levels of dormancy, their responses to plant growth regulators and the magnitude of these responses were almost identical, so the data for the three collections were combined to give an overall picture of the response of L. rigidum seeds to the plant growth regulators. The subsequent experiments described below were performed only on the 2006 and 2007 collections, as seed numbers for the 2000 collection were limited.
Response of germination to high concentrations of ABA supplied during dark stratification:
As dormancy release in the dark was not affected by 50 μM ABA (see Results), the effect of high concentrations of ABA supplied during dark stratification (but not subsequent germination) was tested to determine whether 50 μM ABA is insufficient to inhibit dormancy release, or whether exogenous ABA genuinely has no effect on this process. Seeds were dark stratified for 21 d in the presence of 0, 50, 100, or 500 μM ABA, and then germinated on plain agar, along with a non-stratified control exposed to 0 μM ABA.
Effect of plant growth regulator combinations on dormancy release/germination:
Combinations of (i) fluridone plus diniconazole; (ii) GA4 plus diniconazole; and (iii) fluridone plus GA4 (all at 10 μM) were applied during either stratification or germination, as appropriate. In experiment (i), seeds were stratified on plain agar in the dark or light and then transferred to fluridone plus diniconazole for the germination phase. The rationale behind this experiment is that by inhibiting both ABA synthesis and catabolism just prior to germination, it may be possible to determine if the static ABA concentration at the end of stratification is related to the germination behaviour of the seeds. The individual effects of these compounds on the endogenous ABA concentration were checked by measuring ABA in seeds that were dark or light stratified on 10 μM fluridone or diniconazole for 21 d. Experiment (ii) was designed to determine if inhibiting ABA catabolism with diniconazole during either stratification or germination could be compensated for by supplementing the seeds with GA4. The aim of experiment (iii) was to investigate if the effect of GA4 on dormancy release or on stimulation of germination is enhanced when ABA biosynthesis is inhibited by fluridone.
Sensitivity of dormant and non-dormant seeds to ABA during germination:
To determine if dark-stratified seeds germinate more readily than light-stratified seeds because they are less sensitive to ABA, seeds stratified on plain agar were transferred to germination conditions in the presence of 0, 0.5, 1, 5, 10, 25, or 50 μM ABA. Originally, the experiment included a background of 10 μM fluridone in all treatments so that endogenous ABA synthesis was inhibited and only the known concentration of exogenous ABA was acting on the seeds. However, the results of this experiment were inconsistent and difficult to interpret, possibly because the effects of fluridone were too strong and not necessarily related to inhibition of ABA synthesis (see Results). Therefore, the results presented in this study are from an experiment performed without a fluridone background. The effects of dark and light stratification and subsequent germination on endogenous ABA levels were examined by measuring ABA in dry and stratified seeds.
An attempt was also made to characterize the sensitivity of dark- and light-stratified seeds to GA4 applied during germination, but the low level of response to this hormone resulted in no reproducible differences between treatments. Measurement of endogenous GA4 was also attempted, but the results were inconsistent due to the low levels of GA4 present.
Statistical analyses
Data were analysed by one- or two-factor analysis of variance (ANOVA) at the 5% level of significance. The least significant difference (LSD) test was used to analyse differences between pairs of treatment means.
Results
Screening of seed response to plant growth regulators
The germination requirements of annual ryegrass seeds make them a convenient system for studying the processes involved in dormancy release separately from those involved in germination. Although dormant seeds progressively lose dormancy in the dark (but remain dormant in the light), they subsequently require light to germinate, and so the effects of plant growth regulators on either dormancy or germination can be studied in isolation by transferring the seeds to different media at the time they are transferred to germination conditions.
Effect of plant growth regulators on germination of dark- and light-stratified seeds:
Seeds from the 2000, 2006, and 2007 collections were stratified in the absence of plant growth regulators to allow the normal processes of promotion or inhibition of dormancy release in the dark or light to proceed. These seeds were then placed on media containing plant growth regulators and transferred to germination conditions. Non-stratified control seeds were placed directly in germination conditions in the presence of plant growth regulators. The data from the three seed collections were combined to give an overall representation of the response of L. rigidum seeds to the plant growth regulators.
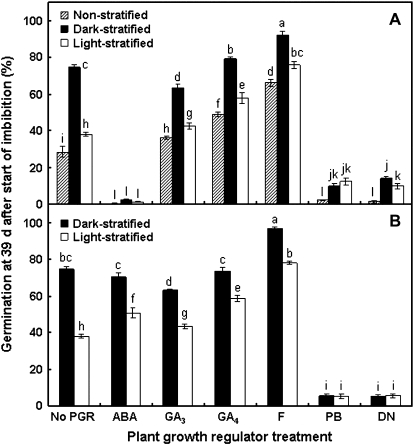
In non-, dark-, and light-stratified seeds, 50 μM ABA almost completely inhibited germination, whilst germination in the presence of paclobutrazol or diniconazole was inhibited by 70–85% (Fig. 1A). The inhibition of germination by paclobutrazol could be reversed by simultaneous application of GA4 (the final germination percentage of non-stratified seeds treated with 10 μM paclobutrazol and 10 μM GA4 was 39±4%, not significantly different from the 28±3% germination of untreated, non-stratified seeds).
Fig. 1.
Effect of plant growth regulators on ryegrass seed dormancy and germination. Seeds were exposed to plant growth regulators (PGR) (A) during germination conditions, either directly upon imbibition (non-stratified) or following 21 d dark or light stratification at 20 °C, or (B) during stratification, after which seeds were germinated in the absence of PGR. All PGRs were applied at 50 μM except for GA4, which was at 10 μM. Values are means ±SE (n=4). Data were analysed by two-factor ANOVA, and different letters above the bars indicate a significant difference at the 5% level. Pooled LSD values are: (A) 4.39, (B) 4.10. ABA, abscisic acid; GA3, gibberellic acid; GA4, gibberellin A4; F, fluridone; PB, paclobutrazol; DN, diniconazole.
Dark- and light- stratified seeds exhibited a minor response to GAs supplied during the germination phase, though GA4 was noticeably more stimulatory for germination than GA3 (Fig. 1A). In contrast, fluridone had a dramatic stimulatory effect: germination of non- and light-stratified seeds was ∼2-fold higher in the presence of fluridone, thus reaching levels similar to those stimulated by standard dark stratification, and dark-stratified seeds reached almost 100% germination when supplied with fluridone (Fig. 1A).
Effect of plant growth regulators on seed dormancy status:
To examine the effects of plant growth regulators on dormancy release in dark- and light-stratified seeds, the seeds from the 2000, 2006, and 2007 collections were stratified in the presence of the plant growth regulators and then transferred to plain agar upon placement in germination conditions.
In contrast to its inhibitory effect on germination, the presence of ABA during stratification had no effect upon the dormancy status of the seeds. Seeds stratified on ABA in the dark reached almost the same germination level as the untreated dark-stratified seeds, whilst germination of light-stratified seeds that were treated with ABA during stratification was actually higher than that of the untreated control (Fig. 1B). This suggests that (i) exogenous ABA is not perceived by the seeds during dormancy release, or is ineffective in inhibition of dormancy release; and (ii) that the exogenous ABA does not persist in the seeds following transfer to plain agar, as it would then be capable of greatly inhibiting germination (Fig. 1A).
As was the case in the germination experiments, GAs were only weakly effective in dormancy release, having a small positive effect when supplied during light stratification (Fig. 1B). Again, seeds responded better to GA4 than to GA3, but the level of dormancy release in light-stratified, GA4-treated seeds was not as high as that achieved by standard dark stratification (Fig. 1B). It is likely that the exogenous GA4 was readily metabolized by the seeds (and thus did not have an influence on the germination phase) once the source was removed. A role for GA4 (a member of the non-C-13-hydroxylated GA pathway) in L. rigidum is consistent with known metabolites from other Lolium species. For example, although L. perenne is known to have mostly forms of the C-13-hydroxylated GA pathway (Morvan-Bertand et al., 2001), other studies with L. temulentum have indicated that there are GAs from both the C-13-hydroxylated and non-C-13-hydroxylated GA pathways, including GA4 (King et al., 2001). Moreover, in attempts to quantify GAs in the present study, detectable amounts of GA4 were found in many samples, but GA1 was never found (data not shown).
The effects of fluridone supplied during stratification were very similar to those observed when the compound was supplied during the germination phase, i.e. germination was significantly increased in both dark- and light-stratified seeds (Fig. 1B). However, the germinated seedlings were unable to synthesize functional chlorophyll (as indicated by their bleached appearance), demonstrating that the fluridone, or its effects on carotenoid and chlorophyll synthesis (Fletcher et al., 1984), persisted in the seeds following transfer to plain agar [also observed by Gianinetti and Vernieri (2007) in red rice seeds]. Therefore, the effects of fluridone during the stratification and germination phases cannot be separated.
The inhibited germination responses of the seeds to paclobutrazol and diniconazole supplied during stratification were very similar to those observed when they were supplied during germination (Fig. 1). The phenotypic effects of paclobutrazol and diniconazole on very young seedlings were not as clear as those of fluridone, but the possibility that the apparent effects of these compounds on dormancy release are actually caused by inhibition of germination due to persistence in the seeds also cannot be discounted.
Effect of high concentrations of ABA on dormancy release
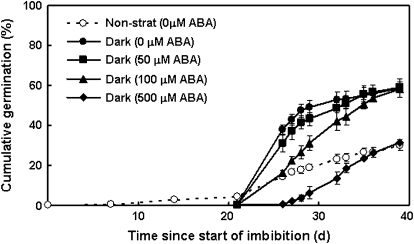
To investigate further the apparent ineffectiveness of exogenous ABA in inhibiting dormancy release, high concentrations of ABA (50, 100, and 500 μM) were supplied to seeds during dark stratification, but not during the subsequent germination phase. Seeds stratified on 50 μM or 100 μM ABA germinated to the same final percentage (at 39 d after imbibition) as seeds stratified in the absence of ABA, although the initial rate of germination of the seeds treated with 100 μM ABA was slower (Fig. 2). Seeds stratified on 500 μM ABA only germinated to basal non-stratified levels (Fig. 2), but this (30%) was still much higher than for seeds germinated in the presence of 500 μM ABA, which did not germinate at all (data not shown). It is likely that germination of the seeds stratified on 500 μM ABA was limited by the rate of catabolism of such a high level of ABA once the source was removed, or possibly that dormancy release is somewhat inhibited by extreme ABA concentrations. These results confirm that exogenous ABA is effective in inhibiting germination of seeds at all levels of dormancy within a population, but does not play a major role in mediating dormancy release in the dark.
Fig. 2.
Response of germination to high concentrations of ABA applied during dark stratification. Seeds were stratified in the dark in the presence of 0, 50, 100, or 500 μM ABA at 20 °C for 21 d and then transferred to plain agar and placed under germination conditions. Control (non-stratified) seeds that were not exposed to ABA were placed directly into germination conditions. Values are means ±SE (n=4).
Role of endogenous ABA in inhibition of dormancy release
Concentration of endogenous ABA in dark- and light-stratified seeds:
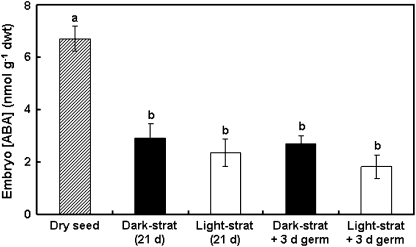
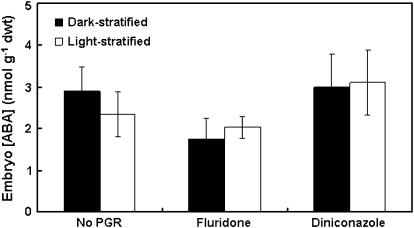
Seeds from the 2006 collection were stratified for 21 d in the dark or light and then transferred to germination conditions for a further 3 d (this time point is just prior to radicle emergence in non-dormant seeds). Subsamples were taken for ABA measurement at 0, 21, and 24 d from the start of imbibition. A preliminary experiment showed that the embryos of dark- and light-stratified L. rigidum seeds contain approximately three times as much ABA as the remainder of the seed tissues (data not shown), so only the embryos were analysed in this study. Dry seeds (0 d after imbibition) contained more than twice as much ABA as imbibed seeds, but there was no difference in ABA concentration between dark- and light-stratified seeds, either before or after transfer to germination conditions (Fig. 3). This indicates that the concentration of ABA in the embryo is not related to the dormancy status of the seeds.
Fig. 3.
Endogenous ABA concentration in embryos of annual ryegrass seeds. Seeds were stratified for 21 d at 20 °C in the dark or light and then transferred to germination conditions for a further 3 d. Embryo ABA concentration was measured in subsamples of seeds at 0, 21, and 24 d after the start of imbibition. Values are means ±SE (n=3). Data were analysed by two-factor ANOVA, and different letters above the bars indicate a significant difference at the 5% level (pooled LSD value=1.5).
Effect of manipulating ABA and GA concentrations during germination or stratification:
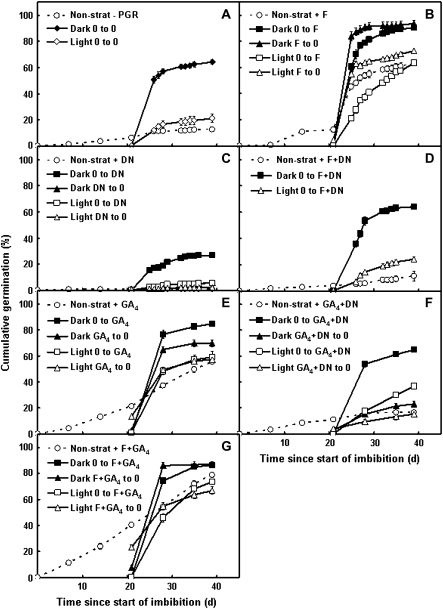
In order to assess whether the dormancy status of seeds following dark or light stratification is mediated by changes in the rate of ABA metabolism, ABA turnover was theoretically halted by placing seeds on 10 μM fluridone (to inhibit ABA synthesis) and 10 μM diniconazole (to inhibit ABA catabolism) simultaneously, upon transfer to germination conditions. The effect of these growth-regulating compounds at 10 μM (as opposed to the 50 μM concentration that was used in the initial experiment shown in Fig. 1) was also evaluated. Fluridone at 10 μM was as effective as at 50 μM in stimulating germination of dark-stratified seeds (90% versus 92% germination at 39 d after imbibition), but slightly less effective in light-stratified seeds (64% versus 76%) (Figs 4B, 1A). Diniconazole at 10 μM permitted germination of dark-stratified seeds to 27%, around the level of basal germination seen in non-stratified seeds (Fig. 4A), and thus was only half as effective as 50 μM (14% germination), but it still strongly inhibited germination of light-stratified seeds (6% versus 9%) (Figs 4C, 1A).
Fig. 4.
Effect of plant growth regulator combinations on seed dormancy release and germination. Seeds were non-stratified, or dark or light stratified at 20 °C for 21 d, and then transferred to germination conditions. Plant growth regulators (PGR) were applied during either the stratification or the germination phases: (A) no PGR; (B) 10 μM fluridone (F); (C) 10 μM diniconazole (DN); (D) 10 μM fluridone plus 10 μM diniconazole; (E) 10 μM GA4; (F) 10 μM GA4 plus 10 μM diniconazole; (G) 10 μM fluridone plus 10 μM GA4. Values are means ±SE (n=4).
Germination of non-stratified, dark-stratified, and light-stratified seeds in the presence of both fluridone and diniconazole was almost identical to that of seeds in their absence (Fig. 4A, D), so it appears that these chemicals effectively nullified one another. Although fluridone and diniconazole (separately) affected germination in a manner that would be expected if ABA synthesis and catabolism were, respectively, inhibited (Fig. 4B, C), seeds stratified in the dark or light for 21 d in the presence of either 10 μM fluridone or 10 μM diniconazole unexpectedly showed no differences in embryo ABA concentration compared with each other, or with untreated control seeds (Fig. 5).
Fig 5.
Endogenous ABA concentration in seeds stratified on fluridone or diniconazole. Seeds were stratified at 20 °C for 21 d in constant darkness or light, in the presence of 10 μM fluridone or diniconazole, or in the absence of plant growth regulators (PGR). Embryos were excised from seeds immediately following stratification and analysed for ABA concentration. Values are means ±SE (n=3). Data were analysed by one-factor ANOVA at the 5% level of significance; there were no differences among samples.
The application of GA4 resulted in an improvement in germination when it was applied at the same time as light, whether in the stratification or germination phase; thus, addition of GA4 during dark stratification was the only treatment to produce little change in response (Fig. 4A, E). Supplemental GA4 was able partially to overcome the inhibitory effects of diniconazole only when both were supplied during germination, whether following dark or light stratification (Fig. 4A, C, F). Germination of seeds supplied with the plant growth regulators during the stratification phase was greatly inhibited, and did not rise above the basal germination level of non-stratified seeds (Fig. 4A, F). This may again indicate that diniconazole and/or GA4 play different roles in stratifying and germinating seeds, and the effects of diniconazole can only be compensated for by GA4 in the latter case.
The response to the combination of fluridone and GA4 supplied during germination was significantly higher than to GA4 alone (Fig. 4E, G), but similar to the response to fluridone alone (Fig. 4B, G), suggesting that the effect of fluridone is so strong that it masks any effects of GA4. The response to fluridone plus GA4 applied during stratification was essentially the same as that observed when applied during germination, again probably because of the persistence of fluridone (or its effects) in the seeds following stratification (Fig. 4G).
Sensitivity of seeds to ABA
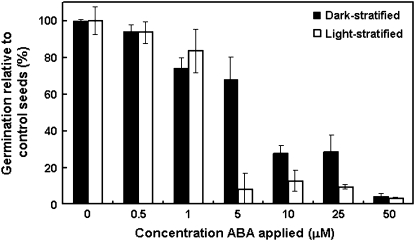
Seeds from the 2006 collection were dark or light stratified and then exposed to various concentrations of ABA upon transfer to germination conditions. The data were adjusted for each stratification treatment so that germination of dark- or light-stratified seeds supplied with 0 μM ABA was 100%, thus allowing comparison between stratification treatments of the sensitivity of seed germination to the inhibitory effects of ABA.
There was no difference in the germination of the two stratification treatments in the presence of 0.5 μM or 1 μM ABA, but the dark-stratified seeds were less sensitive to 5, 10, and 25 μM ABA than the light-stratified seeds (Fig. 6). At 50 μM ABA, germination of both dark- and light-stratified seeds was almost completely inhibited (Fig. 6).
Fig. 6.
Sensitivity of dormant and non-dormant seeds to ABA applied during germination. Seeds were dark or light stratified for 21 d at 20 °C and subsequently transferred to germination conditions for a further 21 d. Upon transfer, seeds were exposed to 0, 0.5, 1, 5, 10, 25, or 50 μM ABA. The percentage germination at 42 d after the start of imbibition is shown. In order to compare sensitivity between stratification treatments, germination under 0 μM ABA was set to 100% for both dark- and light-stratified seeds, and the remaining germination percentages adjusted accordingly. The original germination percentages for seeds treated with 0 μM ABA were: dark-stratified, 80±1%; light-stratified, 30±4%. Values are means ±SE (n=4). Data were analysed by two-factor ANOVA; pooled LSD value=19.2.
Discussion
The role of ABA in inhibition of dormancy release in imbibed L. rigidum seeds is unclear. The results of this study generally support the conclusion of Leon et al. (2007) (in Amaranthus tuberculatus seeds) that ABA and GA play a more important role in the germination process than in dormancy release in mature seeds, and that of Gianinetti and Vernieri (2007) (in red rice seeds), that ABA is not the primary mediator of dormancy in imbibed seeds. In order to make sense of some of the apparently contradictory results in the current study, it is necessary to separate the processes that occur during stratification, where the seeds are unable to germinate due to the absence of light and/or alternating temperature but can undergo changes in dormancy status, from those that occur once the seeds are transferred to ideal germination conditions.
Processes occurring in seeds exposed to ideal germination conditions following dark or light stratification
Exogenous ABA was an efficient germination inhibitor, whereas GA3, which stimulates germination in a wide range of species, was largely ineffective or even slightly inhibitory in ryegrass seed. GA4 was slightly stimulatory, but its effects were completely masked by those of exogenous fluridone (an inhibitor of ABA biosynthesis). The germination-inhibitory effect of diniconazole, which inhibits ABA catabolism, also indicates that a decrease in ABA accumulation is important for germination. Simultaneous application of fluridone and diniconazole saw the neutralization of their individual effects, and the ABA sensitivity of dark-stratified (i.e. lower dormancy) seeds was found to be lower than that of light-stratified seeds. Together, these results indicate that the balance of ABA synthesis/catabolism and the ABA sensitivity of seeds exposed to ideal germination conditions are important determinants of whether germination can proceed. Studies on barley (Benech-Arnold et al., 2006) and wheat (Kawakami et al., 1997) have also shown that non-dormant seeds are insensitive to concentrations of exogenous ABA that inhibit germination of dormant seeds.
The relative magnitude of the responses of the ryegrass seeds to GA4 and ABA/fluridone suggests that ABA is a stronger determinant of germination in annual ryegrass than GA4. It is possible that other GAs are involved in mediation of germination in annual ryegrass; in any case, the inhibition of germination by paclobutrazol showed that GA synthesis during germination is necessary even if ABA is a stronger mediator of germination. Supplemental GA4 could overcome the inhibitory effects of diniconazole on germination, supporting the conclusion that the ABA:GA ratio is also an important determinant of germination (Finch-Savage and Leubner-Metzger, 2006). It must be noted, however, that the null effect observed with simultaneous application of fluridone and diniconazole, or GA4 and diniconazole, could possibly be due to inhibition of GA synthesis by diniconazole, which is structurally similar to paclobutrazol (although apparently not as effective as a GA synthesis inhibitor: Fletcher et al., 1986). The implications of this alternative interpretation of the data are that inhibition of GA synthesis by diniconazole can be compensated for either by exogenous GA4 (as would be expected) or by simultaneous inhibition of ABA synthesis; therefore, the relative amounts of ABA and GA would still be important determinants of germination.
Processes occurring during dark or light stratification of dormant seeds
Exogenous ABA had no effect on dormancy release in annual ryegrass seeds, except that it slowed germination of seeds dark-stratified on an extremely high concentration of ABA (500 μM). Previous studies have shown that exogenous or maternal ABA is ineffective in the establishment of dormancy in developing seeds (reviewed in Kucera et al., 2005), so it is possible that the same is true for inhibition of dormancy release in mature annual ryegrass seeds. Another possibility is that ABA may not be the major mediator of dormancy in imbibed seeds of this species. Supporting this second theory is the fact that there was no difference in endogenous ABA concentration between dark- and light-stratified annual ryegrass seeds. The relationship between endogenous ABA concentration in cereal seeds following a dormancy release treatment and the subsequent germination behaviour of the seeds is apparently variable among species. Dormancy was maintained in light-stratified barley seeds due to higher ABA biosynthetic activity and thus higher ABA concentrations than in dark-stratified seeds (Gubler et al., 2008); in contrast, a dormancy-breaking high-temperature treatment in sorghum seeds did not change the ABA concentration from its low-temperature levels (Benech-Arnold et al., 2003). Gianinetti and Vernieri (2007) found that the ABA concentration in imbibed dormant red rice seeds was actually lower than that in non-dormant seeds which had commenced germination.
The persistence of fluridone, or its effects, in ryegrass seeds following stratification prevented elucidation of its effects on dormancy release, as carotenoid biosynthesis was still inhibited in the seeds following their transfer to germination conditions on plain agar. However, the concentration of ABA in embryos of dark- or light-stratified seeds was unaffected by either fluridone or diniconazole. This was unexpected, as previous studies have shown that fluridone causes a decrease in ABA concentration (Feurtado et al., 2007; Gianinetti and Vernieri, 2007), and the dramatic effects of fluridone and diniconazole on ryegrass seed germination observed in the current study suggest that they are highly effective in altering ABA metabolism. Chae et al. (2004) also found that exogenous fluridone had no effect on the ABA concentration of imbibed Orobanche minor seeds, but that fluridone could still replace dark conditioning and allow dormant seeds to germinate once they were subsequently exposed to strigol, a compound essential for germination of these seeds. Similarly, fluridone did not influence the ABA concentration of sorghum seeds stimulated to germinate at thermal conditions under which dormancy is normally expressed (Benech-Arnold et al., 1999). Therefore, the actions of fluridone and diniconazole under non-germination conditions could be distinct from their actions on ABA metabolism. Alternatively, inhibition of one aspect of ABA metabolism under non-germination conditions could induce a compensatory down-regulation of the opposite process, resulting in an apparently unchanged ABA concentration.
Conclusions
The requirement for darkness to induce dormancy release and then light to stimulate germination makes the L. rigidum seed a useful system for studying these two processes separately. The effects of plant growth regulators which inhibit ABA and GA metabolism, and measurement of endogenous ABA levels in dark-stratified (losing dormancy) and light-stratified (dormant) seeds, have demonstrated that although the process of germination is greatly affected by ABA metabolism and sensitivity and requires GA synthesis, the inhibition of dormancy release during light stratification is, in contrast, apparently most strongly mediated by (unknown) processes distinct from ABA and GA metabolism.
Acknowledgments
The authors would like to thank Ms Mechelle Owen and Ms Roslyn Owen for collection and cleaning of seed. This work was funded by the Australian Research Council.
Glossary
Abbreviations
- ABA
abscisic acid
- ESI
electrospray ionization
- GA
gibberellin
- LC
liquid chromatography
References
- Ali-Rachedi S, Bouinot D, Wagner M-H, Bonnet M, Sotta B, Grappin P, Jullien M. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta. 2004;219:479–488. doi: 10.1007/s00425-004-1251-4. [DOI] [PubMed] [Google Scholar]
- Benech-Arnold RL, Enciso S, Sánchez RA. Fluridone stimulus of dormant sorghum seeds germination at low temperatures is not accompanied by changes in ABA content. In: Weipert D, editor. Proceedings of the VIII International Symposium on Pre-Harvest Sprouting in Cereals, Part II. Germany. 1999. pp. 76–80. [Google Scholar]
- Benech-Arnold RL, Enciso S, Sánchez RA, Rodríguez MV. On the hormonal nature of the stimulatory effect of high incubation temperatures on germination of dormant sorghum (S. bicolor) caryopses. New Phytologist. 2003;160:371–377. doi: 10.1046/j.1469-8137.2003.00900.x. [DOI] [PubMed] [Google Scholar]
- Benech-Arnold RL, Gualano N, Leymarie J, Côme D, Corbineau F. Hypoxia interferes with ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains. Journal of Experimental Botany. 2006;57:1423–1430. doi: 10.1093/jxb/erj122. [DOI] [PubMed] [Google Scholar]
- Chae SH, Yoneyama K, Takeuchi Y, Joel DM. Fluridone and norflurazon, carotenoid-biosynthesis inhibitors, promote seed conditioning and germination of the holoparasite Orobanche minor. Physiologia Plantarum. 2004;120:328–337. doi: 10.1111/j.0031-9317.2004.0243.x. [DOI] [PubMed] [Google Scholar]
- Chiwocha SD, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross AR, Kermode AR. A method for profiling classes of plant hormones and their metabolites using liquid chromatography–electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. The Plant Journal. 2003;35:405–417. doi: 10.1046/j.1365-313x.2003.01800.x. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Wiebe EMK, Emery RJN, Guinel FC. Cytokinin accumulation and an altered ethylene response mediate the pleiotropic phenotype of the pea nodulation mutant R50 (sym 16) Canadian Journal of Botany. 2005;83:989–1000. [Google Scholar]
- Feurtado JA, Yang J, Ambrose SJ, Cutler AJ, Abrams SR, Kermode AR. Disrupting abscisic acid homeostasis in western white pine (Pinus monticola Dougl. Ex D. Don) seeds induces dormancy termination and changes in abscisic acid catabolites. Journal of Plant Growth Regulation. 2007;26:46–54. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annual Review of Plant Biology. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- Fletcher RA, Hofstra G, Gao J. Comparative fungitoxic and plant growth regulating properties of triazole derivatives. Plant and Cell Physiology. 1986;27:367–371. [Google Scholar]
- Fletcher RA, Meru SV, Bhardwaj SN. Reversal of fluridone-induced chlorophyll accumulation in cucumber (Cucumis sativus) cotyledons by stimulatory compounds. Weed Science. 1984;32:722–726. [Google Scholar]
- Gianinetti A, Vernieri P. On the role of abscisic acid in seed dormancy of red rice. Journal of Experimental Botany. 2007;58:3449–3462. doi: 10.1093/jxb/erm198. [DOI] [PubMed] [Google Scholar]
- Goggin DE, Steadman KJ, Powles SB. Green and blue light photoreceptors are involved in maintenance of dormancy in imbibed annual ryegrass (Lolium rigidum) seeds. New Phytologist. 2008;180:81–89. doi: 10.1111/j.1469-8137.2008.02570.x. [DOI] [PubMed] [Google Scholar]
- Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N, Kawaide H, Kamiya Y, Yoshioka T. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. Journal of Experimental Botany. 2004;55:111–118. doi: 10.1093/jxb/erh023. [DOI] [PubMed] [Google Scholar]
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiology. 2008;147:886–896. doi: 10.1104/pp.107.115469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilhorst HWM, Bentsink L, Koornneef M. Dormancy and germination. In: Basra AS, editor. Handbook of seed science and technology. Binghamton, NY: Haworth's Food Products Press; 2006. pp. 271–301. [Google Scholar]
- Kawakami N, Miyake Y, Noda K. ABA insensitivity and low ABA levels during seed development of non-dormant wheat mutants. Journal of Experimental Botany. 1997;48:1415–1421. [Google Scholar]
- King RW, Moritz T, Evans LT, Junttila O, Herlt AJ. Long-day induction of flowering in Lolium temulentum involves sequential increases in specific gibberellins at the shoot apex. Plant Physiology. 2001;127:624–632. [PMC free article] [PubMed] [Google Scholar]
- Kitahata N, Saito S, Miyazawa Y, et al. Chemical regulation of abscisic acid catabolism in plants by cytochrome P450 inhibitors. Bioorganic and Medicinal Chemistry. 2005;13:4491–4498. doi: 10.1016/j.bmc.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- Kusumoto D, Chae SH, Mukaida K, Yoneyama K, Yoneyama K Joel DM, Takeuchi Y. Effects of fluridone and norflurazon on conditioning and germination of Striga asiatica seeds. Plant Growth Regulation. 2006;48:73–78. [Google Scholar]
- Leon RG, Bassham DC, Owen MDK. Thermal and hormonal regulation of the dormancy–germination transition in Amaranthus tuberculatus seeds. Weed Research. 2007;47:335–344. [Google Scholar]
- Leubner-Metzger G. Hormonal interactions during seed dormancy release and germination. In: Basra AS, editor. Handbook of seed science and technology. Binghamton, NY: Haworth's Food Products Press; 2006. pp. 303–341. [Google Scholar]
- Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. The Plant Journal. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- Morvan-Bertrand A, Ernsten A, Lindgård B, Koshioka M, Le Saos J, Boucaud J, Prud'homme M-P, Junttlia O. Endogenous gibberellins in Lolium perenne and influence of defoliation on their contents in elongating leaf bases and leaf sheaths. Physiologia Plantarum. 2001;111:225–231. [Google Scholar]
- Nambara E, Marion-Poll A. Abscisic acid biosynthesis and metabolism. Annual Review of Plant Biology. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- Nonogaki H. Seed germination—the biochemical and molecular mechanisms. Breeding Science. 2006;56:93–105. [Google Scholar]
- Ross ARS, Ambrose SJ, Cutler AJ, Feurtado JA, Kermode AR, Nelson K, Zhou R, Abrams SR. Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high-performance liquid chromatography–electro-spray ionization tandem mass spectrometry with multiple reaction monitoring. Analytical Biochemistry. 2004;329:324–333. doi: 10.1016/j.ab.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Steadman KJ. Dormancy release during hydrated storage in Lolium rigidum seeds is dependent on temperature, light quality, and hydration status. Journal of Experimental Botany. 2004;55:929–937. doi: 10.1093/jxb/erh099. [DOI] [PubMed] [Google Scholar]
- Steadman KJ, Bignell GP, Michael PJ. Stimulating dormancy release and emergence of annual ryegrass (Lolium rigidum) seeds using short-term hydrated storage in darkness. Australian Journal of Agricultural Research. 2004;55:787–795. [Google Scholar]
- Steadman KJ, Crawford AD, Gallagher RS. Dormancy release in Lolium rigidum seeds is a function of thermal after-ripening time and seed water content. Functional Plant Biology. 2003;30:345–352. doi: 10.1071/FP02175. [DOI] [PubMed] [Google Scholar]