Abstract
The asymmetrical distribution of F-actin directed by cell polarity has been observed during the migration of monospores from the red alga Porphyra yezoensis. The significance of Ca2+ influx and phosphoinositide signalling during the formation of cell polarity in migrating monospores was analysed pharmacologically. The results indicate that the inhibition of the establishment of cell polarity, as judged by the ability of F-actin to localize asymmetrically, cell wall synthesis, and development into germlings, occurred when monospores were treated with inhibitors of the Ca2+ permeable channel, phospholipase C (PLC), diacylglycerol kinase, and inositol-1,4,5-trisphosphate receptor. Moreover, it was also found that light triggered the establishment of cell polarity via photosynthetic activity but not its direction, indicating that the Ca2+ influx and PLC activation required for the establishment of cell polarity are light dependent. By contrast, inhibition of phospholipase D (PLD) prevented the migration of monospores but not the asymmetrical localization of F-actin. Taken together, these findings suggest that there is functional diversity between the PLC and PLD signalling systems in terms of the formation of cell polarity; the former being critical for the light-dependent establishment of cell polarity and the latter playing a role in the maintenance of established cell polarity.
Keywords: Ca2+ influx, cell polarity, cell wall, F-actin, monospore, phosphatidylinositol, phospholipase C, phospholipase D, Porphyra yezoensis
Introduction
The asymmetrical distribution of intracellular molecules defines cell polarity, which, in turn, governs directional cell migration and elongation, cell differentiation, and other important cellular regulations in eukaryotes (Feijó et al., 1995; Holdaway-Clarke and Hepler, 2003; Homblé and Léonetti, 2007). It is well known that spatio-temporal increases in cytoplasmic free Ca2+ ([Ca2+]cyt) are mainly generated by an influx of extracellular Ca2+ through membrane Ca2+ permeable channels, allowing regulation of a variety of Ca2+-dependent signalling systems in plants (Berridge et al., 2000; Sanders et al., 2002; Wheeler and Brownlee, 2008). It has also been reported that calcium gradients and calcium-dependent proteins are spatially and temporally regulated in tip-growing cells (Hepler et al., 2001; Yoon et al., 2006). Indeed, a tip high [Ca2+]cyt gradient is required for the polarized growth of pollen tubes and root hairs in higher plants and rhizoids in Fucoid brown algae (Brownlee and Pulsford, 1988; Pierson et al., 1994, 1996; Wymer et al., 1997; Homblé and Léonetti, 2007). Thus, Ca2+ influx is fundamental in the formation of cell polarity. It is also well known that a tip-polarized increase in [Ca2+]cyt, which is controlled by the direction of light, regulates the formation of the apical–basal axis during rhizoid development in Fucoid zygotes (Brownlee and Pulsford, 1988; Taylor et al., 1996).
A rise in [Ca2+]cyt is sensed by proteins such as Ca2+-dependent kinases and phospholipase C (PLC), which subsequently activate downstream signalling cascades (Bush, 1993; Saimi and Kung, 2002; Yoon et al., 2006; Kim et al., 2007). PLC hydrolyses phosphatidylinositol-4,5-bisphosphate [PtdIns(4,5)P2] in a Ca2+-dependent manner to produce two second messengers, diacylglycerol (DG) and inositol-1,4,5-trisphoshphate (IP3), which, in turn, activate protein kinase C (PKC) and Ca2+ release from intracellular stores via the IP3 receptor (IP3R), respectively (Berridge and Irvine, 1984). Subsequently, DG is immediately phosphorylated by diacylglycerol kinase (DGK) to produce phosphatidic acid (PA), an important second messenger in plant cells involved in various physiological processes (Munnik, 2001; Meijer and Munnik, 2003). PA activates phosphatidylinositol phosphatase kinase (PIPK) to produce PtdIns(4,5)P2 as a substrate of PLC in both animals and plants (Oude Weernink et al., 2007; Saavedra et al., 2009). PtdIns(4,5)P2 then activates phospholipase D (PLD), which hydrolyses phosphatidylcholine (PC) to produce PA (Moritz et al., 1992; Jenkins et al., 1994; Ishihara et al., 1998; Jones et al., 2000). Thus, it appears that both PLC and PLD exert control over PA concentrations in plant cells. However, there are differences in the roles of PLC and PLD in the formation of cell polarity in plants. For instance, PLC regulates F-actin dynamics, vesicle trafficking, and ion transport in pollen tubes (Hunt et al., 2003; Dowd et al., 2006; Helling et al., 2006), whereas PLD regulates the organization of microtubules in Fucoid embryos, and seed germination and root elongation in green plants (Gardiner et al., 2001, 2003; Dhonukshe et al., 2003; Peters et al., 2007).
Despite the importance of phospholipases and phosphoinositides (PIs) in the formation of cell polarity in plants, the functional significance of PI signalling remains largely unknown in red algae. The only exception is our study using the red alga Porphyra yezoensis (Li et al., 2008), a model for fundamental and applied studies of marine plants (Saga and Kitade, 2002). P. yezoensis has a biphasic heteromorphic life cycle based on sexual propagation that consists of microscopic filamentous sporophytes and macroscopic leafy gametophytes. In addition, this species also undergoes asexual propagation through the production of monospores in monosporangia in the marginal region of the leaf thallus, which then proceeds to form new leaf thalli (Miura, 1985). Recently, it was found that, before attachment and germling formation, monospores migrate with the accumulation of F-actin at the leading edge (Li et al., 2008) as reported in Dictyostelium cells and leukocytes (Affolter and Weijer, 2005; Bagorda et al., 2006). The involvement of D-3-phosphorylated PIs, such as PtdIns3P, PtdIns(4,5)P2, and PtdIns(3,4,5)P3, in polarity establishment via PI3K activity has also been demonstrated (Li et al., 2008). In addition, recent extensive research clearly demonstrated that the asymmetric and non-overlapping distribution of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 generate the force required for the migration of leukocytes and Dictyostelium cells (Harris et al., 2008; Kölsch et al., 2008). Moreover, the importance of the similar distributions of two PIs is well known in the regulation of cell division and the formation of cell polarity in epithelial and tubular cells (Gassama-Diagne et al., 2006; Comer and Parent, 2007; Martin-Belmonte et al., 2007). Based on the involvement of PtdIns(3,4,5)P3 in the formation of the asymmetrical distribution of F-actin at the leading edges in leukocytes and Dictyostelium cells (Harris et al., 2008; Kölsch et al., 2008), the presence of PtdIns(3,4,5)P3 in monospores was examined using the PH domain from human Akt 1, which specially binds to PtdIns(3,4)P2 and PtdIns(3,4,5)P3 (James et al., 1996; Frech et al., 1997). Since a fusion protein consisting of the Akt1 PH domain and cyan fluorescent protein was observed localizing at the plasma membrane, it is possible that P. yezoensis contains both PtdIns(3,4)P2 and PtdIns(3,4,5)P3 (Mikami et al., 2009). Animal genomes encode multiple PI3Ks that are classified into classes I, II, and III (Vanhaesebroeck and Waterfield, 1999) with only class I PI3K known to be involved in the production of PtdIns(3,4,5)P3 (Funamoto et al., 2002). By contrast, genomes of land plants and yeast only contain class III PI3K, which produces only PtdIns3P (Stack and Emr, 1994; Choi et al., 2008). However, an ability to produce PtdIns(3,4,5)P3 has recently been demonstrated in a type III PI3K (Vsp43)-dependent manner in the yeast Schizosaccharomyces pombe (Mitra et al., 2004). Thus, it is possible that PtdIns(3,4,5)P3 is also produced by class III PI3K under the appropriate growth conditions in P. yezoensis, although the biochemical detection of PtdIns(3,4,5)P3 is necessary to conclude the presence of this PI.
In contrast to accumulating knowledge concerning PI3K in P. yezoensis, little is known about the contribution of other factors involved in PI signalling during the formation of cell polarity in monospores. The aim of this study was to access the function of Ca2+ influx, PLC, DGK, IP3R, and PLD during the establishment and maintenance of cell polarity in monospores from P. yezoensis. All experiments were conducted with pharmacological reagents, namely, inhibitors of PLC, PLD, DGK, and Ca2+ influx, and the effects were examined by the observation of migration and the development of monospores, the subcellular distribution of F-actin, and cell wall synthesis. Here, evidence is presented of the involvement of Ca2+ influx, PLC, DGK, and IP3R-like protein in the establishment of cell polarity and of PLD in polarity maintenance. It is also shown that light regulates the establishment of cell polarity. These results will help enhance our understanding of the role of the PI signalling system in the formation of cell polarity in plants.
Materials and methods
Plant material
Gametophytic blades and monospores of P. yezoensis strain TU-1 were used in the present study. The cultivation of blades and the collection of monospores were performed as described by Li et al. (2008).
Pharmacological studies
Pharmacological reagents were dissolved in DMSO to create stock solutions of 10 mM U73122 (Sigma, St Louis, USA), 5 mM U73343 (Sigma), 100 mM 2-aminoethyl diphenyl borate (2-APB; Sigma), 10 mM calcium ionophore A23187 (Sigma), and 30 mM R59022 (Calbiochem, USA). LaCL3 (Sigma) was dissolved in deionized water (DW) to create a 1 M stock solution. EGTA (Dojindo Laboratories, Japan) was dissolved in enriched sea life (ESL) to create a 0.5 M stock solution and it was adjusted to pH 8.0 with NaOH. Dilution of 1-butanol and t-butanol (Wako Pure Chemical Industries, Japan) 0.05–0.4% (v/v) were freshly prepared by resolving in ESL medium. They were then added to the ESL medium to treat monospores at working concentrations, which were created by the dilution of stock solutions in which the concentrations of DMSO and DW did not exceed 0.5% and 0.04%, respectively. At the same time, appropriate control experiments were performed with DMSO or DW at concentrations corresponding to the maximum volume of the reagents.
Staining of F-actin
In order to study the organization of F-actin in monospores, F-actin was stained using Alexa Fluor phalloidin 488 (Molecular Probes, Eugene, Oregon, USA) according to the protocol described by Li et al. (2008). Since most monospores treated with pharmacological reagents cannot adhere tightly to cover glasses, they were rinsed only once in PBS and then mounted on a slide with 4% n-propyl gallate resolved in 90% glycerol and 10% PBS. The stained F-actin was observed using a Leica DM 5000 B fluorescence microscope equipped with a Leica DFC 300 FX camera. All images were obtained using a ×100 oil immersion objective with filter set L5 (excitation at 480/40 nm and emission at 527/30 nm; Medical Agent Co., Japan) for Alexa Fluor 488 phalloidin. Photomicrographs were taken using the Leica DFC 300 FX camera system and images were collected and processed using the Adobe Photoshop 7.0 software package.
Staining of renascent cell wall
Fluorescent Brightener 28 (0.01%; Sigma) was obtained by resolving in sea life and filtering with a 0.2 μm millipore filter (Whatman, Germany); it was then stored at 4 °C in the dark until use. Renascent cell wall existing in monospores treated with the various pharmacological reagents was stained directly. The incubation medium was replaced by 0.01% Fluorescent Brightener 28, and then cover glasses were mounted on a slide glass. Cell wall was observed using a Leica DM 5000 B fluorescence microscope equipped with a Leica DFC 300 FX camera. All images were obtained using a ×40 objective with filter set A (excitation at 340–380 nm and emission at 425 nm; Medical Agent Co., Japan) for Fluorescent Brightener 28. Photomicrographs were processed as described above.
Results
Calcium influx is critical for the initiation of monospore early development
As previously reported (Li et al., 2008), migration of monospores required the pre-establishment of cell polarity for the asymmetrical localization of F-actin on the front side of migrating cells (Fig. 1Aa); this followed the synthesis of renascent cell wall during migration (Fig. 1Ab). The factors involved in the formation of cell polarity in monospores, as judged by the localization of F-actin and cell wall synthesis, are investigated further here. It was evident when F-actin was observed that treatment with phalloidin had resulted in monospore frangibility and a change in colour of the red chloroplasts to pale or green; the monospores were weakened to the extent that they could not bear the weight of the cover glass. In addition, crushing of the monospores into a flat shape by the weight of the cover-glass sometimes produced autofluorescence from the chloroplasts. However, phalloidin treatment itself did not affect the organization of F-actin and the images of F-actin that correctly exhibited the effect of the inhibitors used in this study. Fluorescent Brightener 28 treatment for the visualization of the cell wall did not create such a problem.
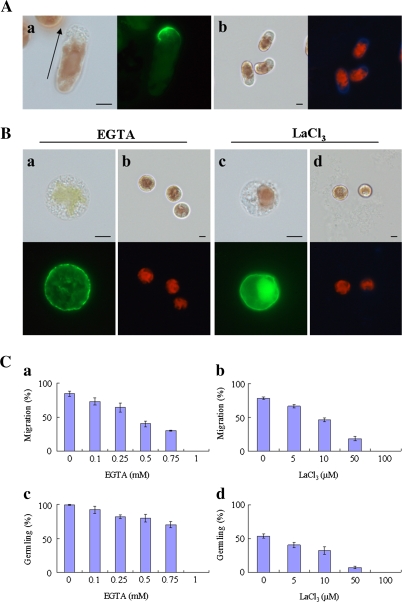
Fig. 1.
Calcium influx plays an important role in the establishment of cell polarity in monospores. (A) The polarized F-actin and renascent cell wall synthesis in monospores incubated in ESL for 3 h after release from the gametophyte. The polarized F-actin accumulated in the front of the migrating monospores (a) and the renascent cell wall synthesis in migrating monospores (b). The arrow in (a) indicates the direction of migration of the monospore. Left and right photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm. (B) Effects of calcium chelator (EGTA) and calcium channel blocker (LaCl3) on the F-actin organization and renascent cell wall synthesis. Freshly released monospores incubated with 1 mM EGTA (a, b) and 100 μM LaCl3 (c, d) for 3 h, which indicate the symmetrical organization of F-actin (a, c) and no renascent cell wall synthesis (b, d). Upper and lower photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm. (C) Dose-dependent inhibition of the motility and development of monospores by calcium chelator and channel blocker. Freshly released monospores incubated with an increasing concentration of EGTA (a, c) and LaCl3 (b, d) for 3 h (a, b) and 24 h (c, d). Columns and vertical bars represent the mean and SD, respectively (n=3).
It is well known that Ca2+ influx is essential for the migration of leukocytes, fibroblasts, and macrophages (Mandeville et al., 1995; Yang and Huang, 2005; Evans and Falke, 2007), which led us to investigate whether such influx is also involved in cell polarity formation in monospores. As a result, monospores treated with 1 mM EGTA for 3 h presented symmetrical distribution of F-actin (Fig. 1Ba) and a lack of a renascent cell wall (Fig. 1Bb). Moreover, this occurred in a dose-dependent manner; that is, when the EGTA concentration was increased from 0.1 to 1 mM, the rate of migrating monospores and the formation of germlings decreased (Fig. 1Ca,c). This inhibitory effect was suppressed by removing the EGTA (data not shown).
Next, to examine the effect of a channel-mediated Ca2+ influx, the inorganic Ca2+ channel blocker La3+ was applied to freshly released monospores. When monospores were treated with 100 μM LaCl3, the F-actin was symmetrically localized (Fig. 1Bc) and these cells remained cell wall-free (Fig. 1Bd). Moreover, as shown in Fig. 1Cb and d, the rate of monospore migration and the formation of germlings also decreased in a dose-dependent manner.
These results indicate that the inhibition of Ca2+ channel activity prevent the asymmetrical distribution of F-actin, monospore migration, and germling formation. Thus, it is concluded that Ca2+ influx mediated by Ca2+ permeable channels and the resultant increase in [Ca2+]cyt are indispensable for the establishment of cell polarity in monospores and for subsequent migration and development. The importance of Ca2+ influx on the early development of monospores was supported by an acceleration of migration when monospores were treated with 1 μM calcium ionophore A23187 (Table 1).
Table 1.
Acceleration of monospore migration by calcium ionophore A23187
| Percentage of migrating monospores (%)a |
|||
| 1 h | 2 h | 3 h | |
| ESL | 24.5±3.7 | 56.1±2.3 | 84.2±2.4 |
| A23187 (1 μM) | 61.9±0.9 | 71.7±3.2 | 80.1±3.0 |
Freshly released monospores were treated with or without calcium ionophore A23187, and then the migrating and spherical monospores were counted at 1 h, 2 h, and 3 h after incubation. Three replicate experiments performed with more than 50 monospores independently.
Values indicate mean±SD.
PLC is critical for the establishment of cell polarity in monospores
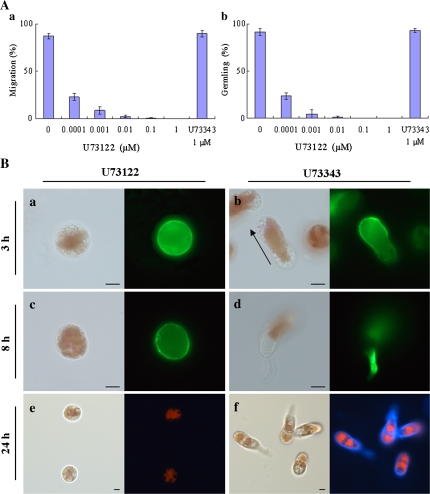
Since the activity of PLC depends on [Ca2+]cyt in eukaryotic cells (Staxén et al., 1999; Pan et al., 2005), the effects of U73122, a specific inhibitor of PLC (Smith et al., 1990), were tested on motility and germling formation in freshly released monospores. In the presence of U73122, monospores did not start moving or develop into germlings at concentrations ranging from 10 nM to 1 μM (Fig. 2Aa, b), concentrations that are much lower than those used in studies of other higher plants and animals (10–100 μM). Figure 2B shows that F-actin distributed symmetrically after incubation with 1 μM U73122 for 3 h and 8 h (Fig. 2Ba, c), and, moreover, no cell wall was observed in these cells after 24 h incubation (Fig. 2Be). The effects of U73122 were not reversible at either 1 μM or 0.1 μM after washing (data not shown). By contrast, the inactive analogue U73343 showed no effect on motility or further development of the freshly released monospores at 1 μM (Fig. 2Aa, b). In this case, monospores started to move and form germlings normally, with polarized F-actin observed at the leading edge of the migrating monospores (Fig. 2Bb) and at the bottom of 1-celled germlings (Fig. 2Bd). Moreover, a thick renascent cell wall was found in germlings after 24 h incubation with U73343 (Fig. 2Bf). These results indicate that PLC is involved in the establishment of cell polarity to direct the asymmetrical localization of F-action and the subsequent migration of monospores.
Fig. 2.
Involvement of PLC activity in the establishment of cell polarity in monospores. (A) Effects of PLC inhibitor on the motility and development of monospores. Freshly released monospores incubated with an increasing concentration of U73122 from 0.1 nM to 1 μM and 1 μM U73343 for 3 h (a) and 24 h (b). Columns and vertical bars represent the mean and SD, respectively (n=3). (B) Effects of PLC inhibitor on the F-actin organization and renascent cell wall synthesis. Freshly released monospores incubated with 1 μM U73122 (a, c, e) and 1 μM U73343 (b, d, f) for 3 h (a, b), 8 h (c, d), and 24 h (e, f). The organization of F-actin in U73122 and U73343 treated monospores are indicated in (a, c) and (b, d). Renascent cell wall synthesis in U73122 and U73343 treated monospores are indicated in (e) and (f). Arrow in (b) indicates the direction of migration of the monospore. Left and right photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm.
Metabolites of PtdIns(4,5)P2 play crucial roles in the establishment of cell polarity in monospores
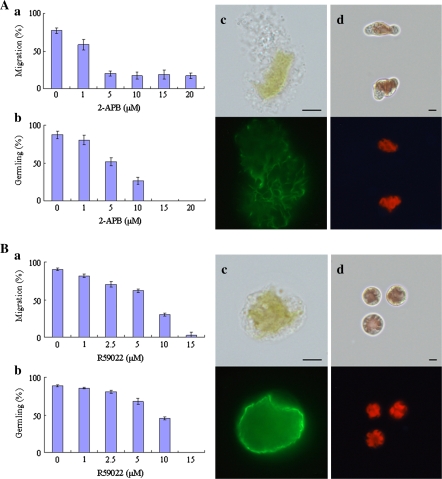
PLC hydrolyses PtdIns(4,5)P2 into two second messengers, IP3 and DG (Katan, 1998). Since it was recently shown that IP3R exists in green algae (Wheeler and Brownlee, 2008), it was also examined whether IP3R-like activity is required for the early development of monospores using an IP3R antagonist, 2-APB, which inhibits IP3R activity on the ER membrane in animal cells (Maruyama et al., 1997). Treatment of monospores with 2-APB prevented the migration of monospores but no difference was observed with a concentration ranging from 5 μM to 20 μM (Fig. 3Aa). In addition, inhibition of germling development was observed in a dose-dependent manner (Fig. 3Ab). When freshly released monospores were treated with 20 μM 2-APB for 3 h, symmetrical distribution of F-actin and cell wall-free monospores were observed in erratic monospores without amoeboid movement (Fig. 3Ac, d). After 24 h incubation, monospores returned to a spherical shape or remained grotesque until death (data not shown). These effects were recovered following 2-APB removal (data not shown). However, since specificity of 2-APB for IP3R is not high, further experiments are needed to confirm the presence of IP3R in P. yezoensis.
Fig. 3.
Involvement of PtdIns(4,5)P2 metabolites in the establishment of cell polarity in monospores. (A) Effects of 2-APB on the motility and development of monospores. Freshly released monospores incubated with an increasing concentration of 2-APB for 3 h (a) and 24 h (b). Columns and vertical bars represent the mean and SD, respectively (n=3). The organization of F-actin and renascent cell wall synthesis in 20 μM 2-APB treated monospores are indicated in (c) and (d), respectively. Upper and lower photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm. (B) Effects of R59022 on the motility and development of monospores. Freshly released monospores incubated with an increasing concentration of R59022 for 3 h (a) and 24 h (b). Columns and vertical bars represent the mean and SD, respectively (n=3). The organization of F-actin and renascent cell wall synthesis in 15 μM R59022 treated monospores are indicated in (c) and (d), respectively. Upper and lower photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm.
The other second messenger that DG produces through DGK in plants is PA (Meijer and Munnik, 2003). Since PA is a main second messenger in plants, the role of DGK in the polarity formation of monospores was tested using R59022, a DGK inhibitor, at an increasing concentration of 1 μM to 15 μM. Treatment of freshly released monospores with R59022 inhibited migration and germling formation in a dose-dependent manner (Fig. 3Ba, b). Symmetrical distribution of F-actin and inhibition of cell wall synthesis were also observed in monospores treated with 15 μM R59022 for 3 h (Fig. 3Bc, d). These effects of R59022 were completely removed by washing (data not shown).
From these results indicating the disruption of F-actin asymmetry and a decrease in germling formation by both 2-APB and R59022, it is possible to suggest that IP3R-like protein and DGK are involved in the establishment of cell polarity in monospores, consistent with the effects of PLC shown in Fig. 2.
PLD participates in the maintenance of cell polarity in monospores
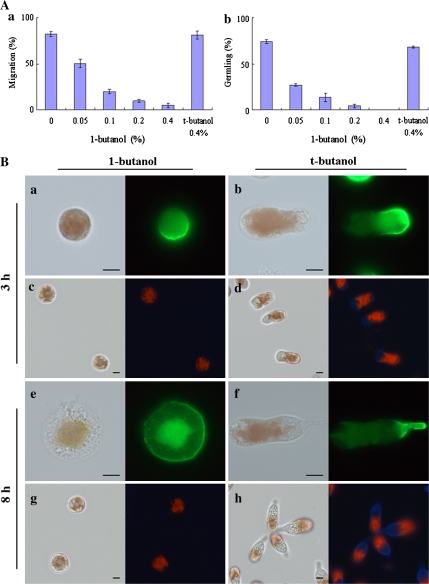
The effects of R59022 indicate the importance of PA in polarity establishment in monospores (Fig. 3Ba, b). Since PA is also produced by PLD from PC (Munnik, 2001), it was examined if PLD-dependent PA production is required for the formation of cell polarity in monospores. When monospores were treated with 1-butanol at an increasing concentration from 0.05% to 0.4% (v/v), the rate of migration and germling formation decreased in a dose-dependent manner (Fig. 4Aa, b). Moreover, in monospores treated with 0.4% 1-butanol for 3 h, F-actin was asymmetrically localized (Fig. 4Ba); however, cells presented as a round shape without a cell wall (Fig. 4Bc). On the other hand, 8 h treatment of monospores resulted in symmetrically distributed F-actin and no cell wall (Fig. 4Be, g). Incubation with 0.4% 1-butanol did not kill monospores even after 24 h incubation and the inhibitory effect of 1-butanol was recovered after washout with ESL (data not shown). By contrast, monospores treated with 0.4% t-butanol were able to migrate normally and form germlings (Fig. 4Aa,b); after 3 h treatment, monospores migrated with polarized F-actin and a renascent cell wall (Fig. 4Bb, d), while 8 h treatment resulted in adhesion to the substrate and the development of germlings with an accumulation of F-actin in the bottom and also with a thick cell wall (Fig. 4Bf, h). From these results, since inhibition of PLD activity did not disrupt the formation of F-actin asymmetry but prevented its maintenance, it was concluded that PLD participates in the maintenance, but not in the establishment, of cell polarity during the early development of monospores.
Fig. 4.
PLD participates in the maintenance of cell polarity in monospores. (A) Effects of PLD inhibitor on the motility and development of monospores. Freshly released monospores incubated with an increasing concentration of 0.05–0.4% 1-butanol and 0.4% t-butanol for 3 h (a) and 24 h (b). Columns and vertical bars represent the mean and SD, respectively (n=3). (B) Effects of PLD inhibitor on the F-actin organization and renascent cell wall formation. Freshly released monospores incubated with 0.4% 1-butanol (a, c, e, g) and its analogue t-butanol (b, d, f, h) for 3 h (a–d) and 8 h (e–h). The organization of F-actin in 1-butanol and t-butanol treated monospores are indicated in (a, e) and (b, f). Renascent cell wall synthesis in 1-butanol and t-butanol treated monospores are indicated in (c, g) and (d, h). Left and right photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm.
Light triggers the regulatory system of cell polarity establishment
In Fucoid zygotes, the direction of light influences the establishment of the cell axis, as rhizoids grow away from light (Kropf, 1992; Brownlee et al., 2001). However, sperm entry triggers a default axis formation in darkness with an F-actin patch, adhesive secretion, and rhizoid outgrowth found at the position of sperm entrance, although the default axis is overridden by the unilateral light (Henderson et al., 1998; Hable and Kropf, 2000). Thus, establishment of cell polarity in Fucoid zygotes is initiated under both dark and light. Based on these findings, it was next examined whether the presence and direction of light is also required for the formation of cell polarity in P. yezoensis monospores.
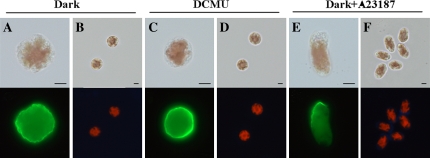
As shown in Fig. 5A and B, it was found that migration was prevented in dark-treated monospores in which F-actin was symmetrically distributed and the cell wall was not synthesized. Such effects of darkness were recovered by irradiation with light (data not shown). Moreover, when monospores were irradiated with unilateral light, the directions of migration and light were not correlated (data not shown), indicating that migration and the early development of germlings do not depend on the direction of light. Thus, the regulatory mechanism to establish cell polarity in monospores is different from that of Fucoid zygotes.
Fig. 5.
Effects of light illumination on the early development of monospores. The organization of F-actin (A, C, E) and renascent cell wall synthesis (B, D, F) in monospores incubated in darkness (A, B), with 100 μM DCMU (C, D) and with 1 μM calcium ionophore A23187 in darkness (E, F) for 3 h are indicated. Upper and lower photographs in each panel show bright-field and fluorescent images, respectively. Scale bars=5 μm.
Next, the involvement of photosynthetic activity in polarity formation was examined using DCMU, an inhibitor of electron transport on the acceptor side of photosystem II (PSII). When monospores were treated with 100 μM DCMU for 3 h, F-actin was symmetrically distributed in these cells (Fig. 5C). In addition, cell wall synthesis was prevented (Fig. 5D). It was therefore concluded that light triggers the establishment of cell polarity via photosynthetic activity based on the inhibition of F-actin asymmetry and migration by DCMU. This hypothesis is supported by the polarized accumulation of F-actin and renascent cell wall in monospores treated with 1 μM calcium ionophore A23187 in the absence of light irradiation for 3 h (Fig. 5E, F), indicating that the increase in [Ca2+]cyt via Ca2+ influx activates PLC and PLD signalling cascades even in the dark.
Discussion
The data presented above reveal that Ca2+ influx, the PI signalling system, and light are essential for the establishment and maintenance of cell polarity during the early development of monospores from the marine red alga P. yezoensis. The formation of cell polarity in directional cell migration or chemotaxis has been extensively studied in mammalian leukocytes and Dictyostelium cells (Affolter and Weijer, 2005; Bagorda et al., 2006), indicating the importance of signalling systems involving phosphatidylinositol kinases and phospholipases (Harris et al., 2008; Kölsch et al., 2008). In plants, however, knowledge about the importance of the PI signalling system in cell polarity is restricted to tip growth of pollen tubes, root hairs, and rhizoids (Gardiner et al., 2003; Helling et al., 2006; Peters et al., 2007). Our findings on migrating monospores using pharmacological inhibitors therefore provide new evidence of the critical roles of PI signalling in cell polarity formation in plants. Based on our findings, together with the involvement of light in the establishment of cell polarity (Fig. 5), it is hypothesized that light triggers the activation of Ca2+ permeable channels and/or PI3K, which follows PLC activation to establish the cell polarity required for the asymmetrical distribution of F-actin and PLD activation for the maintenance of cell polarity (Fig. 6). Similar functional diversity between PLC and PLD in polarity formation has recently been found in zygotes of a brown alga Silvetia compressa, in which inhibition of PLC signalling by R59022 disrupted polarization and the subsequent polar growth, including germination and cell division, with the formation of microtubule arrays, whereas inhibition of PLD with 1-butanol only affected cell division during polar growth (Peters et al., 2008).
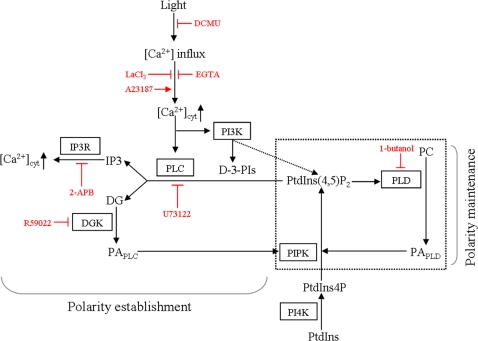
Fig. 6.
Proposed model of the relationship between the PI signalling system and formation of cell polarity in monospores. PtdIns(4)P produced by PI4K from PtdIns is phosphorylated by PIPK to generate PtdIns(4,5)P2. PtdIns(4,5)P2 can be hydrolysed by PLC to generate the second messengers IP3 and DG. IP3 then binds IP3R, which results in the release of Ca2+ from the cytoplasm. The inhibition of PLC, DGK, and IP3R in addition to Ca2+ influx prevents the establishment of cell polarity. The catalysis of PLC and PI3K depends on Ca2+ influx, which is triggered by light irradiation. DG is converted to PA by DGK. PA is also produced from PC by PLD. PA activates PIPK to produce PtdIns(4,5)P2 as a precursor of the substrate of PLC and PtdIns(4,5)P2 activates PLD which hydrolyses PC to produce PA. According to the function of PLD, the positive regulatory circuit indicated by the box drawn with a dashed line is proposed for the maintenance of cell polarity. Pharmacological reagents and their actions are indicated by red characters and bars.
It has been demonstrated that Ca2+ influx leads to migration with an asymmetrical distribution of F-actin and synthesis of the cell wall in monospores (Fig. 1), both of which are critical for the formation of cell polarity and the development of monospores. Involvement of Ca2+ influx in cell migration has also been observed in leukocytes and macrophages (Evans and Falke, 2007; Oh-hora and Rao, 2008). Similarly, the importance of Ca2+ influx in the establishment of cell polarity has also been demonstrated in Fucoid embryos (Robinson and Cone, 1980; Roberts et al., 1993; Taylor et al., 1996), and an extracellular Ca2+ influx is considered to play an important role in the regulation of germination and tip-growth pollen tube cells in land plants (Rathore et al., 1991; Pierson et al., 1994; Holdaway-Clarke and Hepler, 2003). Alternatively, there was evidence that transcellular ion currents, characterized by delocalized influx and efflux of ions including Ca2+, play a central role in the establishment of cell polarity via the generation of cytoplasmic ion gradients in Fucoid zygotes, in which the gradient is high at the site of Ca2+ influx (Kropf, 1992; Homblé and Léonetti, 2007). Although Ca2+ channel blocker experiments suggest the existence of Ca2+ channels in P. yezoensis (Fig. 1), the nature of the Ca2+ channel responsible for the extracellular Ca2+ influx is still unclear.
PLC is involved in chemotaxis in T cells via an increase in Ca2+ from intracellular stores by IP3R (Bach et al., 2007). In addition, during cAMP-dependent chemotaxis in Dictyostelium cells, PLC is thought to control the concentration of PtdIns(4,5)P2 that is phosphorylated by PI3K to produce PtdIns(3,4,5)P3, which is involved in chemotaxis (Kortholt et al., 2007). Thus, PLC has two different roles: the regulation of Ca2+-dependent downstream signalling via IP3R and the determination of the PtdIns(4,5)P2 concentration involved in the activation of PI3K signalling. Since PtdIns(3,4,5)P3 recruits factors as PLD activators such as Rho and Arf (ADP-ribosylation factor)-GTPases and PKC (Henage et al., 2006), it is possible that there is a PI3K-PLD cascade for regulating chemotaxis, which is indirectly activated by PLC via control of the PI3K substrate concentration. In monospores, the importance of PLC in the establishment of cell polarity is demonstrated, while PLD maintains polarity during migration (Figs 2, 4). Since PI3K activity regulates the establishment of cell polarity in monospores (Li et al., 2008), it is possible that PLD acts downstream of the relationship between PLC and PI3K (Fig. 6).
Although the function of PLD in polarity determination is not fully understood, inhibition of PLD resulted in a rapid decrease in PtdIns(4,5)P2 synthesis, and, thereby, defects in actin-based motility in Dictyostelium cells (Zouwail et al., 2005). In addition, PLD activity has been shown to regulate microtubule organization for cell polarity determination in Fucoid zygotes (Peters et al., 2007). PtdIns(4,5)P2-dependent PLD activity is also involved in the tip growth of pollen tubes (Potocký et al., 2003). These findings suggest that PtdIns(4,5)P2-dependent activation of PLD is important for cell polarity. PLD catalyses the production of PA from PC (Oude Weernink et al., 2007), while PA is also produced from DG by GDK (Munnik, 2001; Meijer and Munnik, 2003). It is notable that PtdIns(4,5)P2 synthesis is catalysed by PIPK, the activity of which is positively regulated by PA produced by both DGK and PLD (Moritz et al., 1992; Jenkins et al., 1994; Jones et al., 2000). Therefore, a positive regulatory circuit consisting of PA, PIPK, PtdIns(4,5)P2, and PLD is hypothesized for the maintenance of cell polarity in monospores (Fig. 6), the trigger of which is proposed to be PA produced by DGK according to the effect of the DGK inhibitor (Fig. 3). To confirm this hypothesis, it is necessary to analyse both PA-dependent activation of PIPK and PtdIns(4,5)P2-dependent activation of PLD in the maintenance of cell polarity in P. yezoensis cells.
The presence and nature of IP3R, which acts as an IP3-dependent Ca2+ channel on vacuolar and/or ER membranes, have yet to be determined in land plants. To date, numerous physiological findings have indicated the functional significance of IP3 in pollen tube elongation, stomatal closure, and responses to a number of environmental stimuli in many species (Gilroy et al., 1990; Franklin-Tong et al., 1996; Krinke et al., 2007), which strongly led us to propose the presence of IP3R in plants. However, no IP3R genes bearing a homology to animal genes have so far been found in the genomes of Arabidopsis thaliana, rice, and Physcomitrella patens. Moreover, since the importance of inositol hexakisphosphate (IP6) over IP3 has been demonstrated in guard cells (Lemtiri-Chlieh et al., 2003), it is possible that a structurally novel IP6 receptor rather than IP3R is functional in plants. In contrast to land plants, IP3R homologues have been identified in green algae Chlamydomonas reinhardtii and Volvox carterii, suggesting the loss of IP3R by land plants when they diverged (Wheeler and Brownlee, 2008). Therefore, it is possible that red algae also have orthotic IP3R, since green and red algae originated from the same single ancestor (Palmer, 2000; McFadden and van Dooren, 2004). Indeed, our results suggest the presence of IP3R-like protein in P. yezoensis cells (Fig. 3). Thus, identification of IP3R in P. yezoensis will be of further importance in understanding the PI signalling system in red algae.
Finally, although the involvement of light in the establishment of cell polarity in monospores has been demonstrated (Fig. 5), it remains unclear how PSII activity controls PI3K and/or Ca2+ channels. It is generally accepted that light stimulates an influx of ions such as Ca2+, K+, and H+ (Takagi and Nagai, 1988; Spalding and Goldsmith, 1993; Živanović et al., 2005, 2007). The significance of Ca2+ influx and photosynthetic activity in the establishment of cell polarity in monospores is observed here (Figs 1, 5); however, the relationship between the two remains largely unexplored. In maize leaves, the influx of K+ and H+ is largely photosynthesis-dependent because of inhibition by DCMU, whereas Ca2+ uptake is stimulated by red light rather than photosynthetic activity (Živanović et al., 2005, 2007). Although red light-inducible Ca2+ influx has been observed in many other plant species such as oat, moss, and green alga (Ermolayeva et al., 1997; Johannes et al., 1997; Chae et al., 1990; Dreyer and Weisenseel, 1979), blue light also stimulates Ca2+ influx in maize leaves (Živanović et al., 2005, 2007). Thus, it appears that light-inducible Ca2+ influx is mediated by non-photosynthetic machinery via photoreceptors in plants. In the present study, since Ca2+ influx was not monitored, it is unclear whether DCMU has an inhibitory effect on Ca2+ influx in monospores. Monitoring the changes in [Ca2+]cyt by light is therefore necessary to determine the relationship between PSII activity and Ca2+ influx. Moreover, elucidation of the effects of red and blue light on the increase in [Ca2+]cyt and the formation of cell polarity in monospores should also be addressed to understand further how light regulates the initiation of monospore development. Since it has already been determined that translational activity is not required for the establishment of cell polarity in monospores (Li et al., 2008), it is possible that the targets of light are pre-existing PLC, PI3K, and/or Ca2+ channels.
In conclusion, this study demonstrates the pivotal function of Ca2+ influx and PI signalling during the formation of cell polarity in monospores from P. yezoensis. In the light of our findings and the related literature, it appears that the mechanisms mediating the formation of cell polarity in migrating eukaryotic cells converge into a common PI signalling pathway. However, important questions about the presence of PtdIns(3,4,5)P3 and IP3R in P. yezoensis cells remain to be determined. Further study using both physiological and molecular biological approaches should reveal whether the PI signalling systems required for migration are in fact conserved in migrating eukaryotic cells.
Acknowledgments
We are grateful to Dr Hajime Yasui (Hokkaido University, Japan) for kindly providing the microscopes and to our colleagues for helpful discussions. This study was supported in part by a grant from the Sumitomo Foundation (to KM) and by Grants-in-Aid for the 21st COE (Center of Excellence) Program ‘Marine Bio-Manipulation Frontier for Food Production’ and the City Area Program in Industry–Academia–Government Joint Research (Hakodate area) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to NS).
References
- Affolter M, Weijer CJ. Signalling to cytoskeletal dynamics during chemotaxis. Developmental Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bach TL, Chen QM, Kerr WT, et al. Phospholipase Cβ is critical for T cell chemotaxis. Journal of Immunology. 2007;179:2223–2227. doi: 10.4049/jimmunol.179.4.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagorda A, Mihaylov VA, Parent CA. Chemotaxis: moving forward and holding on to the past. Thrombosis and Haemostasis. 2006;95:12–21. [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brownlee C, Bouget F-Y, Corellou F. Choosing sides: establishment of polarity in zygotes of fucoid algae. Seminars in Cell and Developmental Biology. 2001;12:345–351. doi: 10.1006/scdb.2001.0262. [DOI] [PubMed] [Google Scholar]
- Brownlee C, Pulsford AL. Visualization of the cytoplasmic Ca2+ gradient in Fucus serratus rhizoids: correlation with cell ultrastructure and polarity. Journal of Cell Science. 1988;91:249–256. [Google Scholar]
- Bush DS. Regulation of cytosolic calcium in plants. Plant Physiology. 1993;103:7–13. doi: 10.1104/pp.103.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Q, Park HJ, Hong SD. Loading of quin2 into the oat protoplast and measurement of cytosolic calcium ion concentration changes by phytochrome action. Biochimica et Biophysica Acta. 1990;1051:115–122. doi: 10.1016/0167-4889(90)90182-d. [DOI] [PubMed] [Google Scholar]
- Choi Y, Lee Y, Jeon BW, Staiger CJ, Lee Y. Phosphatidylinositol 3- and 4-phosphate modulate actin filament reorganization in guard cells of day flower. Plant, Cell and Environment. 2008;31:366–377. doi: 10.1111/j.1365-3040.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128:239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Laxalt AM, Goedhart J, Gadella TWJ, Munnik T. Phospholipase D activation correlates with microtubule reorganization in living plant cells. The Plant Cell. 2003;15:2666–2679. doi: 10.1105/tpc.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd PE, Coursol S, Skipan AL, Kao TH, Gilroy S. Petunia phospholipase C1 is involved in pollen tube growth. The Plant Cell. 2006;18:1438–1453. doi: 10.1105/tpc.106.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer EM, Weisenseel MH. Phytochrome-mediated uptake of calcium in Mougeotia cells. Planta. 1979;146:31–39. doi: 10.1007/BF00381252. [DOI] [PubMed] [Google Scholar]
- Ermolayeva E, Sanders D, Johannes E. Ionic mechanism and role of phytochrome-mediated membrane depolarization in caulonemal side branch initial formation in the moss Physcomitrella patens. Planta. 1997;201:109–118. [Google Scholar]
- Evans JH, Falke JJ. Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proceedings of the National Academy of Sciences, USA. 2007;104:16176–16181. doi: 10.1073/pnas.0707719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó JA, Malhó R, Obermeyer G. Ion dynamics and its possible role during in vitro pollen germination and tube growth. Protoplasma. 1995;187:155–167. [Google Scholar]
- Franklin-Tong VE, Drøbak BK, Allan AC, Watkins PAC, Trewavas AJ. Growth of pollen tubes of Papaver rhoeas is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-trisphosphate. The Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. Journal of Biological Chemistry. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Gardiner J, Collings DA, Harper JDI, Marc J. The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organization in Arabidopsis. Plant and Cell Physiology. 2003;44:687–696. doi: 10.1093/pcp/pcg095. [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Harper JDI, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J. A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. The Plant Cell. 2001;13:2143–2158. doi: 10.1105/TPC.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nature Cell Biology. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Hable WE, Kropf DL. Sperm entry induces polarity in focoid zygotes. Development. 2000;127:493–501. doi: 10.1242/dev.127.3.493. [DOI] [PubMed] [Google Scholar]
- Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signalling in T lymphocytes. Journal of Biological Chemistry. 2008;283:2465–2469. doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. The Plant Cell. 2006;18:3519–3534. doi: 10.1105/tpc.106.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henage LG, Exton JH, Brown HA. Kinetic analysis of a mammalian phospholipase D: allosteric modulation by monomeric GTPases, protein kinase C, and polyphosphoinositides. Journal of Biological Chemistry. 2006;281:3408–3417. doi: 10.1074/jbc.M508800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DC, Bisgrove SR, Hable WE, Alessa L, Kropf DL. Division patterns in the thallus of Pelvetia compressa embryos and the effects of gravity. Protoplasma. 1998;203:112–117. [Google Scholar]
- Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annual Review of Cell and Developmental Biology. 2001;17:159–187. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Hepler PK. Control of pollen tube growth: role of ion gradients and fluxes. New Phytologist. 2003;159:539–563. doi: 10.1046/j.1469-8137.2003.00847.x. [DOI] [PubMed] [Google Scholar]
- Homblé F, Léonetti M. Emergence of symmetry breaking in fucoid zygotes. Trends in Plant Science. 2007;12:253–259. doi: 10.1016/j.tplants.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hunt L, Mills LN, Pical C, et al. Phospholipase C is required for the control of stomatal aperture by ABA. The Plant Journal. 2003;34:47–55. doi: 10.1046/j.1365-313x.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Shibasaki Y, Kizuki N, Wada T, Yazaki Y, Asano T, Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases. Cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. Journal of Biological Chemistry. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochemical Journal. 1996;315:709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. Journal of Biological Chemistry. 1994;269:11547–11554. [PubMed] [Google Scholar]
- Johannes E, Ermolayeva E, Sanders D. Red light-induced membrane potential transients in the moss Physcomitrella patens: ion channel interaction in phytochrome signalling. Journal of Experimental Botany. 1997;48:599–608. doi: 10.1093/jxb/48.Special_Issue.599. [DOI] [PubMed] [Google Scholar]
- Jones DR, Sanjuan MA, Mérida I. Type Iα phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Letters. 2000;476:160–165. doi: 10.1016/s0014-5793(00)01702-6. [DOI] [PubMed] [Google Scholar]
- Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. Biochimica et Biophysica Acta. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- Kim B-G, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schültke S, Lee SC, Kudla J, Luan S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. The Plant Journal. 2007;52:473–484. doi: 10.1111/j.1365-313X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signalling. Journal of Cell Science. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortholt A, King JS, Keizer-Gunnink I, Harwood AJ, Van Haastert PJM. Phospholipase C regulation of phosphatidylinositol 3,4,5-trisphosphate-mediated chemotaxis. Molecular Biology of the Cell. 2007;18:4772–4779. doi: 10.1091/mbc.E07-05-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinke O, Novotná Z, Valentová O, Martinec J. Inositol trisphosphate receptor in higher plants: is it real? Journal of Experimental Botany. 2007;58:361–376. doi: 10.1093/jxb/erl220. [DOI] [PubMed] [Google Scholar]
- Kropf DL. Establishment and expression of cellular polarity in Fucoid zygotes. Microbiological Reviews. 1992;56:316–339. doi: 10.1128/mr.56.2.316-339.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA. Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proceedings of the National Academy of Sciences, USA. 2003;100:10091–10095. doi: 10.1073/pnas.1133289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Saga N, Mikami K. Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. Journal of Experimental Botany. 2008;59:3575–3586. doi: 10.1093/jxb/ern207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JT, Ghosh RN, Maxfield FR. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophysical Journal. 1995;68:1207–1217. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-permeable modulator of Ins(1,4,5)P3-induced Ca2+ release. Journal of Biochemistry. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- McFadden GI, van Dooren GG. Evolution: red algal genome affirms a common origin of all plastids. Current Biology. 2004;14:R514–R516. doi: 10.1016/j.cub.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Meijer HJG, Munnik T. Phospholipid-based signalling in plants. Annual Review of Plant Biology. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- Mikami K, Uji T, Li L, Takahashi M, Yasui H, Saga N. Visualization of phosphoinositides via the development of the transient expression system of a cyan fluorescent protein in the red alga Porphyra yezoensis. Marine Biotechnology. 2009 doi: 10.1007/s10126-008-9172-z. 10.1007/s10126-008-9172-z. [DOI] [PubMed] [Google Scholar]
- Mitra P, Zhang Y, Rameh LE, et al. A novel phosphatidylinositol(3,4,5)P3 pathway in fission yeast. Journal of Cell Biology. 2004;166:205–211. doi: 10.1083/jcb.200404150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A. Genetic analysis of the variant color types of light red, light green and light yellow phenotypes of Porphyra yezoensis (Rhodophyta, Bangiaceae) In: Hara H, editor. Origin and evolution of diversity in plants and plant communities. Tokyo: Academia Scientific Book Inc; 1985. pp. 270–284. [Google Scholar]
- Moritz A, Graan PN De, Gispen WH, Wirtz KW. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. Journal of Biological Chemistry. 1992;267:7207–7210. [PubMed] [Google Scholar]
- Munnik T. Phosphatidic acid: an emerging plant lipid second messenger. Trends in Plant Science. 2001;6:227–233. doi: 10.1016/s1360-1385(01)01918-5. [DOI] [PubMed] [Google Scholar]
- Oh-hora M, Rao A. Calcium signalling in lymphocytes. Current Opinion in Immunology. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Weernink PA, Han L, Jakobs KH, Schmidt M. Dynamic phospholipid signalling by G protein-coupled receptors. Biochimica et Biophysica Acta. 2007;1768:888–900. doi: 10.1016/j.bbamem.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Palmer JD. Molecular evolution: a single birth of all plastids? Nature. 2000;405:32–33. doi: 10.1038/35011184. [DOI] [PubMed] [Google Scholar]
- Pan Y-Y, Wang X, Ma L-G, Sun D-Y. Characterization of phosphatidylinositol-specific phospholipase C (PI-PLC) from Lilium davidii pollen. Plant and Cell Physiology. 2005;46:1657–1665. doi: 10.1093/pcp/pci181. [DOI] [PubMed] [Google Scholar]
- Peters NT, Logan KO, Miller AC, Kropf DL. Phospholipase D signalling regulates microtubule organization in the fucoid alga Silvetia compressa. Plant and Cell Physiology. 2007;48:1764–1774. doi: 10.1093/pcp/pcm149. [DOI] [PubMed] [Google Scholar]
- Peters NT, Pol SU, Kropf DL. Phospholipid signalling during stramenopile development. Plant Signalling and Behavior. 2008;3:398–400. doi: 10.4161/psb.3.6.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient, effect of BAPTA-type buffers and hypertonic media. The Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK. Tip-localized calcium entry fluctuates during pollen tube growth. Developmental Biology. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- Potocký M, Eliás M, Profotová B, Novotná Z, Valentová O, Zárský V. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003;217:122–130. doi: 10.1007/s00425-002-0965-4. [DOI] [PubMed] [Google Scholar]
- Rathore KS, Cork RJ, Robinson KR. A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Developmental Biology. 1991;148:612–619. doi: 10.1016/0012-1606(91)90278-b. [DOI] [PubMed] [Google Scholar]
- Roberts SK, Berger F, Brownlee C. The roles of Ca2+ in signal transduction following fertilization in Fucus serratus. Journal of Experimental Biology. 1993;184:197–212. [Google Scholar]
- Robinson KR, Cone R. Polarization of fucoid eggs by a calcium ionophore gradient. Science. 1980;207:77–78. doi: 10.1126/science.207.4426.77. [DOI] [PubMed] [Google Scholar]
- Saavedra L, Balbi V, Dove SK, Hiwatashi Y, Mikami K, Sommarin M. Characterization of phosphatidylinositol phosphate kinase from the moss physcomitrella patens: PpPIPK1 and PpPIPK2. Plant and Cell Physiology. 2009;50:595–609. doi: 10.1093/pcp/pcp018. [DOI] [PubMed] [Google Scholar]
- Saga N, Kitade Y. Porphyra: a model plant in marine science. Fisheries Science. 2002;68:S1075–S1078. [Google Scholar]
- Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annual Review of Physiology. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the cross roads of signalling. The Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. Journal of Pharmacology and Experimental Therapeutics. 1990;253:688–697. [PubMed] [Google Scholar]
- Spalding EP, Goldsmith MHM. Activation of K+ channels in the plasma membrane of Arabidopsis by ATP produced photosynthetically. The Plant Cell. 1993;5:477–484. doi: 10.1105/tpc.5.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack JH, Emr SD. Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. Journal of Biological Chemistry. 1994;269:31552–31562. [PubMed] [Google Scholar]
- Staxén I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proceedings of the National Academy of Sciences, USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Nagai R. Light-affected Ca2+ fluxes in protoplasts from Vallisneria mesophyll cells. Plant Physiology. 1988;88:228–232. doi: 10.1104/pp.88.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Manison NFH, Fernadez C, Wood J, Brownlee C. Spatial organization of calcium signalling involved in cell volume control in the Fucus rhizoid. The Plant Cell. 1996;8:2015–2031. doi: 10.1105/tpc.8.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. Signalling by distinct classes of phosphoinositide 3-kinases. Experimental Cell Research. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Brownlee C. Ca2+ signalling in plants and green algae-changing channels. Trends in Plant Science. 2008;13:506–514. doi: 10.1016/j.tplants.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. The Plant Journal. 1997;12:427–439. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]
- Yang S, Huang X-Y. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. Journal of Biological Chemistry. 2005;280:27130–27137. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- Yoon GM, Dowd PE, Gilroy S, McCubbin AG. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. The Plant Cell. 2006;18:867–878. doi: 10.1105/tpc.105.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Živanović BD, Pang J, Shabala S. Light-induced transient ion flux responses from maize leaves and their association with leaf growth and photosythesis. Plant, Cell and Environment. 2005;28:340–352. doi: 10.1111/j.1365-3040.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Živanović BD, Cuin TA, Shabala S. Spectral and dose dependence of light-induced ion flux responses from maize leaves and their involvement in leaf expansion growth. Plant and Cell Physiology. 2007;48:598–605. doi: 10.1093/pcp/pcm032. [DOI] [PubMed] [Google Scholar]
- Zouwail S, Pettitt TR, Dove SK, Chibalina MV, Powner DJ, Haynes L, Wakelam MJ, Insall RH. Phospholipase D activity is essential for actin localization and actin-based motility in Dictyostelium. Biochemical Journal. 2005;389:207–214. doi: 10.1042/BJ20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]