Abstract
To investigate the regulatory mechanism(s) of ethylene biosynthesis in fruit, transgenic tomatoes with all known LeEIL genes suppressed were produced by RNA interference engineering. The transgenic tomato exhibited ethylene insensitivity phenotypes such as non-ripening and the lack of the triple response and petiole epinasty of seedlings even in the presence of exogenous ethylene. Transgenic fruit exhibited a low but consistent increase in ethylene production beyond 40 days after anthesis (DAA), with limited LeACS2 and LeACS4 expression. 1-Methylcyclopropene (1-MCP), a potent inhibitor of ethylene perception, failed to inhibit the limited increase in ethylene production and expression of the two 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) genes in the transgenic fruit. These results suggest that ripening-associated ethylene (system 2) in wild-type tomato fruit consists of two parts: a small part regulated by a developmental factor through the ethylene-independent expression of LeACS2 and LeACS4 and a large part regulated by an autocatalytic system due to the ethylene-dependent expression of the same genes. The results further suggest that basal ethylene (system 1) is less likely to be involved in the transition to system 2. Even if the effect of system 1 ethylene is eliminated, fruit can show a small increase in ethylene production due to unknown developmental factors. This increase would be enough for the stimulation of autocatalytic ethylene production, leading to fruit ripening.
Keywords: ACS, ethylene, fruit ripening, LeEIL, tomato
Introduction
Fruit ripening has received considerable attention because of the dramatic changes in a wide range of metabolic processes that occur before and after this event, as well as due to its commercial importance. In climacteric fruit, including tomato, ethylene is known to trigger the onset of ripening and to be essential for the completion of the ripening process throughout the various stages (Abeles et al., 1992; Hiwasa et al., 2003). Two systems, known as system 1 ethylene (system 1) and system 2 ethylene (system 2), have been defined (McMurchie et al., 1972). System 1 represents basal ethylene in unripe fruit and vegetative tissues and is regulated in an autoinhibitory manner. System 2 represents a massive increase in ethylene production associated with fruit ripening and flower senescence, and it is regulated in an autocatalytic manner (Oetiker and Yang, 1995; Lelievre et al., 1998; Nakatsuka et al., 1998; Inaba, 2007). Exogenous ethylene when applied to climacteric fruits in the mature stage stimulates system 2 ethylene biosynthesis, resulting in fruit ripening. Generally, this observation is thought to indicate the autocatalytic manner of system 2 synthesis. The treatment of tomato fruit with 1-methylcyclopropene (1-MCP) at the turning and pink stages suppresses ethylene production, confirming that system 2 is regulated in an autocatalytic manner (Nakatsuka et al., 1998); however, an amount of ethylene (a quarter of the non-treated control) remained in 1-MCP-treated fruit. It was unclear whether the remaining ethylene was because of incomplete inhibition of the ethylene signal in 1-MCP-treated fruit or due to another mechanism.
The main rate-limiting step in the ethylene biosynthetic pathway in plants is the production of 1-aminocyclopropane-1-carboxylic acid (ACC), a reaction catalysed by ACC synthase (ACS), which is followed by the conversion of ACC to ethylene by ACC oxidase (ACO) (Oetiker and Yang, 1995; Bleecker and Kende, 2000). Nakatsuka et al. (1998) and Barry et al. (2000) indicated that system 1 is involved in the expression of LeACS1A, which is ethylene independent, and LeACS6, which is regulated by a negative feedback system. In contrast, system 2 is regulated by LeACS2 and LeACS4, both of which are controlled in a positive feedback manner. However, it is unclear whether system 2 is completely regulated by an autocatalytic system or partly regulated by another mechanism.
One of the most important questions in the physiology of naturally ripening fruit is the mechanism that initially induces system 2. The physiological and molecular pathways that act to initiate the transition from the system 1 to system 2 mode of ethylene synthesis at the onset of ripening remain unknown (Barry and Giovannoni, 2007; Cara and Giovannoni, 2008). One possible explanation is that the cumulative effects of system 1, even if the level is low, reach a certain limit and induce system 2 (Klee, 2004). The second explanation is that there is a change in fruit sensitivity to ethylene; fruit might become more sensitive to low system 1 ethylene as it develops. Barry et al. (2000) suggested that the phase transition from system 1 to system 2 is caused by a change in ethylene sensitivity due to the continuous exposure of the fruit to system 1. These possible explanations include a role for system 1 in the initiation of system 2. The involvement of system 1 in phase transition is supported by the observation that treatment of an immature fruit with ethylene for a short time does not induce system 2 immediately; rather, it shortens the period preceding the onset of system 2 (Yang, 1987). Recently, Kevany et al. (2007) demonstrated that the shortened period to ripening by exogenous ethylene is closely related to the level of the ethylene receptor protein, a negative regulator of the ethylene signal, as ethylene exposure causes a reduction in the ethylene receptor protein. In general, stress such as wounding, water stress, and disease during fruit development induces stress ethylene and shortens the period to the onset of fruit ripening (Abeles et al., 1992; Nakano et al., 2003). These observations indicate that during development, both exogenous and endogenous ethylene increase the physiological age of the fruit and sensitize the fruit to ethylene. However, it is unclear whether system 1 alone is enough to have an effect, as the level is very low.
On the basis of mutant analysis of Arabidopsis, the ethylene signalling pathway has been proposed (Alexander and Grierson, 2002; Klee, 2004; Kendrick and Chang, 2008). Ethylene is perceived by receptors [ETHYLENE RESISTANCE 1 (ETR1) and related proteins; Chang et al., 1993; Hua et al., 1998]. The ethylene signal is transduced to ETHYLENE INSENSITIVE 3 (EIN3) through CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1; Kieber et al., 1993) and ETHYLENE INSENSITIVE 2 (EIN2; Alonso et al., 1999). EIN3 is a transcription factor that plays a crucial role in the regulation of the expression of ethylene-responsive genes (Chao et al., 1997; Solano et al., 1998). Recent studies revealed that in the absence of ethylene, EIN3 protein is quickly degraded through a ubiquitin–proteasome pathway mediated by two F-box proteins, EIN3-binding F box protein 1 and 2 (EBF1 and EBF2), whereas EIN3 protein is stabilized by ethylene (Guo and Ecker, 2003; Potuschak et al., 2003; Gagne et al., 2004). In tomato, four EIN3-like (EIL) genes (LeEIL1–LeEIL4) have been isolated thus far (Tieman et al., 2001; Yokotani et al., 2003). Tieman et al. (2001) demonstrated that the reduced expression of tomato LeEIL genes by antisense technology modulated ethylene responses, including leaf epinasty, flower senescence, and fruit ripening, in a functionally redundant manner. Fu et al. (2005) also showed that the virus-induced gene silencing of LeEIL genes in tomato fruit caused ripening-impaired phenotypes.
In this study, an attempt was made to obtain more insight into the regulatory mechanism of ethylene biosynthesis in tomato fruit using the RNA silencing of LeEIL genes and the application of 1-MCP. Here, evidence is provided that ripening-associated ethylene biosynthesis is regulated by both an autocatalytic system and ethylene-independent developmental factors, and that fruit can initiate system 2 without the cumulative effects of system 1.
Materials and methods
Plant material and transformation
Tomato (Solanum lycopersicum cv Ailsa Craig; Asamizu and Ezura, 2009) was used as the wild-type plant. A near-isogenic line (NIL) of rin in the Ailsa Craig background was obtained from the CM Rick Tomato Genetics Resource Center, University of California, Davis, USA. The plants were grown in a greenhouse at Okayama University, Japan under standard conditions (25/20 °C).
The binary vector for double-stranded RNA interference was constructed according to the protocol of Chuang and Meyerowitz (2000). The conserved region of the LeEIL2 cDNA fragment (655–1046, accession no. AF328785) was used for a double-stranded RNA interference trial. The β-glucuronidase (GUS) fragment from pBI121 (Clontech, Palo Alto, CA, USA) was used as a linker between antisense and sense LeEIL2 fragments (Fig. 1A). Each fragment was cloned into XbaI/SacI-digested pBI121 (Clontech) to place it under the control of the cauliflower mosaic virus 35S promoter (Fang et al., 1989). The recombinant binary vector was introduced into Agrobacterium (Rhizobium radiobacter) strain LBA4404.
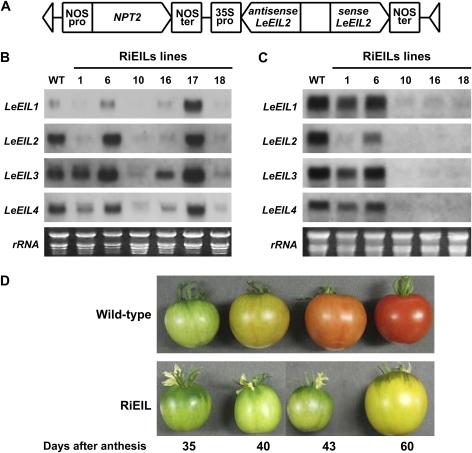
Fig. 1.
Transgenic tomato plants with reduced LeEIL genes. (A) Hairpin construct of RNA interference under the control of the cauliflower mosaic virus 35S promoter. The GUS fragment was used as a linker. (B and C) RNA gel-blot analysis of LeEIL1–LeEIL4 in wild-type and transgenic plants (T0 generation) in leaf (B) and fruit at the mature green stage (C), respectively. (D) Appearance of wild-type and RiEIL (T3 generation) fruit harvested at different maturities.
Transformation of tomato was performed according to a standard procedure (Bird et al., 1988). The cotyledon segments from 7-d-old tomato seedling were used as explants. Explants were pre-cultured on MSZ medium [Murashige and Skoog (MS) medium containing 3% sucrose, 0.8% agar, and 1 mg l−1 zeatin] for 2 d. Pre-cultured explants were immersed in Agrobacterium suspension for 5 min, blotted onto filter paper, and plated on MSZ medium for 2 d. Explants were then cultured and selected on MSZ medium containing 100 mg l−1 kanamycin and 200 mg l−1 carbenicillin. Regenerated shoots were rooted on half-strength MS medium containing 1% sucrose and 0.8% agar, and transferred to compost. The expression of LeEIL genes in transgenic plants was examined by RNA gel-blot analysis. The homozygous progeny of transgenic plants were selected and used for subsequent experiments.
Determination of ethylene biosynthesis
Fruit were harvested at different maturities (30, 35, 40, 45, 50, and 55 DAA) and kept at ambient temperature (24 °C) overnight to reduce harvest shock. Ethylene production from the fruit was measured by enclosing samples in an airtight chamber for 3 h at 24 °C, withdrawing 1 ml of headspace gas from the chamber, and injecting it into a gas chromatograph (model GC-4CM, Shimadzu, Kyoto, Japan) fitted with a flame ionization detector and an activated alumina column. Pericarp tissues from each fruit were frozen in liquid nitrogen and stored at –80 °C until use.
1-MCP treatment
Fruit harvested at 35 DAA were stored in an atmosphere with or without 5 ml l−1 1-MCP generated from Etylbloc® (Rohm and Hass, Philadelphia, PA, USA) in an airtight chamber with soda lime at 24 °C. Fruit were removed from the chamber daily for ventilation. Re-treatment with 1-MCP was performed at 1 d intervals. Ethylene production was monitored during treatment at appropriate intervals. After 20 d of treatment, the fruits were frozen in liquid nitrogen and stored at –80 °C until they were used for real-time reverse transcription-PCR (RT-PCR) analysis.
Triple response assay and epinasty assay
The seedling triple response assay was performed using homozygous T3 seeds as described by Tieman et al. (2001). Surface-sterilized seeds were sown on vermiculite in chambers containing ethylene at the designated concentrations (0, 0.01, 0.1, 1.0, 10, and 100 ppm) and were grown for 12 d in the dark at 24 °C. Chambers were ventilated daily and flushed with air containing the appropriate amount of ethylene. Germination and growth were monitored daily. Petiole epinasty assay was carried out using 1-month-old seedlings of wild-type and RiEIL, and ethylene exposure was carried out at the designed concentrations for 6 h at 24 °C.
RNA gel-blot hybridization
Total RNA was extracted from the tissues by the hot borate method (Wan and Wilkins, 1994). A 5 μg aliquot of RNA was separated by electrophoresis on 1% agarose gels containing 0.66 M formaldehyde, blotted onto nylon membranes (Hybond N+, Amersham Pharmacia Biotech, Piscataway, NJ, USA), and fixed with a UV cross-linker. The probe was generated with the PCR DIG probe synthesis kit (Roche Diagnostics, Mannheim, Germany). The sequences of the primers used for the digoxigenin (DIG)-labelled PCR probe are listed in Supplementary Table S1 available at JXB online. The membranes were hybridized overnight at 45 °C with a DIG-labelled PCR probe in hybridization buffer, 7% (w/v) SDS, 50% (v/v) deionized formaldehyde, 5× SSC, 50 mM sodium phosphate (pH 7.0), 0.1% (w/v) N-lauroylsarcosine sodium salt, and 2% (w/v) blocking reagent (Roche). The membranes were washed twice in 0.1× SSC and 0.1% SDS at 55 °C for 30 min. A hybridized DIG-labelled probe was detected using the DIG chemiluminescent detection system (Roche) according to the manufacturer's instructions.
Real-time quantitative RT-PCR
Total RNA was isolated using an RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA), including a DNA elimination step. First-strand cDNA synthesis was conducted using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). The sequences of the primers used are listed in Supplementary Table S1 at JXB online. Real-time PCRs were performed using cDNA synthesized from 10 ng of total RNA, 0.1 mM of each primer, and 1× iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) in a volume of 30 ml. Reactions for real-time RT-PCR were subjected to 45 cycles of 95 °C for 30 s, 65 °C for 50 s, and 72 °C for 90 s, using an iCycler iQ Real-Time Detection System (Bio-Rad). To reveal the relative amount of each ACS transcript, dilution series of quantified plasmids carrying each ACS cDNA clone were used as a standard. Three independent biological replicates were conducted. The relative amount of each ACS transcript was normalized to LeEF1 in the reaction and expressed as a percentage of LeACS2 at 40 DAA in wild-type fruit.
Results
Transgenic plants with suppressed LeEIL genes
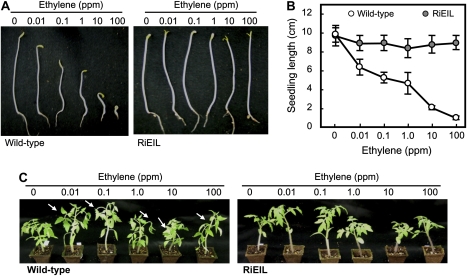
In order to obtain transgenic tomato plants with severely suppressed LeEIL genes, an RNA interference construct using LeEIL2 fragments was employed (Fig. 1A). Six regenerated T0 plants expressed various levels of mRNAs of all four known LeEIL genes (Fig. 1B) in leaf tissue, with only RiEIL-10 and -18 reduced to trace levels of all four LeEIL genes and RiEIL-16 having a slightly higher level of LeEIL3. In fruit, mRNAs of the four LeEIL genes in RiEIL-10, -16, and 18 were reduced to trace levels (Fig. 1C). The three transgenic lines (RiEIL-10, -16, and -18) with severely suppressed LeEIL levels exhibited non-ripening phenotypes, as observed in transgenic tomatoes with antisense constructs (Tieman et al., 2001; Fig. 1D). The RiEIL-10 and -18 lines were selected for further experiments because they not only demonstrated a severe suppression of target genes but they had only a single copy of the transgene (data not shown). Only data from RiEIL-18 are presented in the figures as the results from RiEIL-10 were comparable. Wild-type fruit reached the turning stage at ∼40 DAA. On the other hand, the RiEIL-18 fruit showed no significant colour changes except for a faint yellow colour observed beyond 60 DAA. In addition, the petal remained attached to the fruit even at 60 DAA (Fig. 1D). The triple response assay and epinasty assay revealed distinct ethylene-insensitive phenotypes in the T3 generation of the RiEIL-18 line (Fig. 2). In the transgenic line, no reduction of seedling length or hook formation was observed, even in the presence of 100 ppm ethylene. A symptom of epinasty, petiole twisting, did not appear in the transgenic plants even with 100 ppm ethylene, while it was visible in the wild type with only 0.01 ppm ethylene. In the transgenic line, no colour change of the fruit or flower abscission was observed in the presence of 100 ppm ethylene (data not shown).
Fig. 2.
Response of wild-type and RiEIL (T3 generation) tomatoes to exogenous ethylene. (A and B) Triple response assay in wild-type and RiEIL-18 seedlings (6 d after germination in the dark). Vertical bars are the SD (n=10). (C) Epinasty assay of plants. One-month-old plants were incubated in ethylene for 6 h. Arrows indicate bending points. No symptoms of triple response or epinasty were observed in RiEIL-18 plants even in 100 ppm ethylene.
Ethylene biosynthesis in wild-type, rin, and RiEIL fruit, and effects of 1-MCP
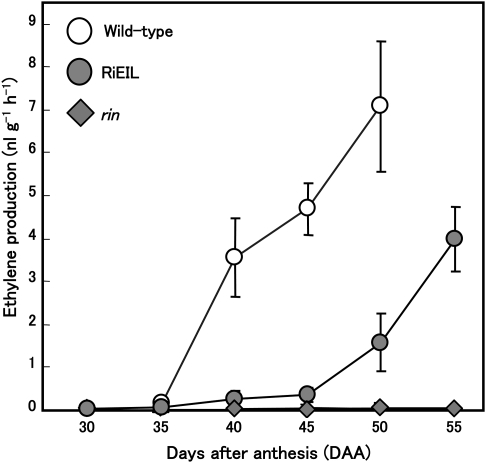
Changes in the ethylene production rate in wild-type, RiEIL-18, and rin fruit during development and ripening were measured. Ethylene production in wild-type fruit increased later than 35 DAA (Fig. 3). Surprisingly, in RiEIL-18 fruit, a significant level of ethylene production (>0.2 nl g−1 h−1) was detected at 40 DAA, which then increased gradually, paralleling that of the wild type from 40 to 50 DAA (Fig. 3). A similar result was shown in an another transgenic line, RiEIL-10 (data not shown). In contrast, ethylene production in rin fruit remained at trace levels up to the end of the experiment (Fig. 3).
Fig. 3.
Changes in the rate of ethylene in wild-type, RiEIL, and rin tomato fruit during development and ripening. Vertical bars are the SE of three replications.
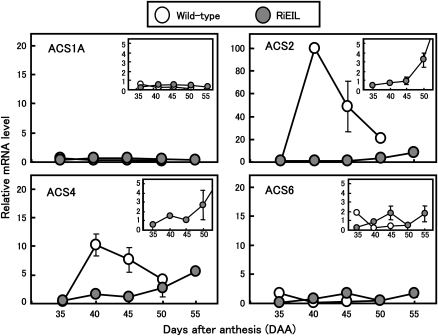
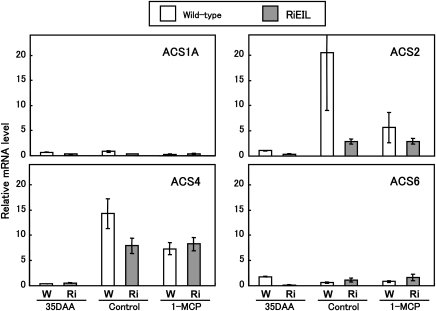
In order to determine the ACS genes responsible for the increase in the ethylene production in transgenic fruit, real-time RT-PCR analysis was performed. Expression levels of LeACS1A and LeACS6 were relatively low in both wild-type and RiEIL-18 fruit (Fig. 4), in agreement with Barry et al. (2000) and Nakatsuka et al. (1998). In wild-type fruit, transcripts of LeACS2 and LeACS4 increased dramatically with a burst of ripening-associated ethylene production (Fig. 4). In transgenic fruit, LeACS2 and LeACS4 transcripts increased with the increase of ethylene production, although the levels were considerably lower than in wild-type fruit.
Fig. 4.
The expression of ACS genes in wild-type and RiEIL tomato fruit during development and ripening. Expression of each ACS gene was measured by real-time quantitative RT-PCR analysis. The relative quantity of each ACS mRNA was expressed as a percentage of LeACS2 at 40 DAA. Vertical bars are the SE of three replications. In some cases, the bars are too small to see.
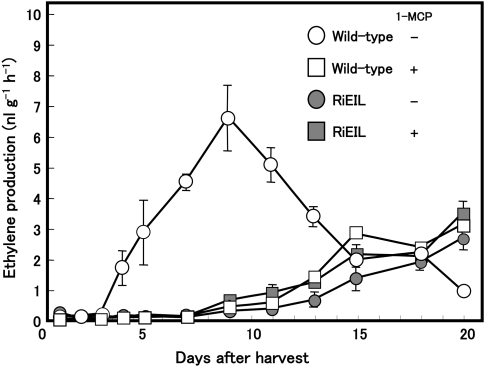
In order to confirm the independence of the increases in ethylene in transgenic fruit from the positive feedback system, 1-MCP was applied continuously to wild-type and transgenic fruit harvested at the mature green stage (35 DAA). Non-treated wild-type fruit exhibited a typical climacteric pattern in ethylene production during storage (Fig. 5). Interestingly, the pattern and level of ethylene production in non-treated transgenic fruit were almost the same as those in 1-MCP-treated transgenic and wild-type fruit. A similar result was obtained from another transgenic line, RiEIL-10 (Supplementary Fig. S1 at JXB online). Incomplete reduction of ripening ethylene by 1-MCP was also observed by Nakatsuka et al. (1998) and Hoeberichts et al. (2002) in wild-type fruit. In all non-treated transgenic and 1-MCP-treated wild-type and transgenic fruit, LeACS2 and LeACS4 transcripts were at trace levels at harvest but were detected at a certain increased level at the end of storage (Fig. 6).
Fig. 5.
Changes in the rate of ethylene production in wild-type and RiEIL fruit. Tomato fruits were harvested 35 d after anthesis and continuously treated with (+) or without (–) 1-MCP. Vertical bars are the SE of three replications.
Fig. 6.
Effect of 1-MCP treatment on the expression of ACS genes in wild-type (W) and RiEIL (Ri) tomato fruit during development and ripening. 35 DAA, fruit harvested 35 d after anthesis; control, fruit harvested at 35 DAA and incubated for 20 d; 1-MCP, fruit harvested at 35 DAA and incubated in 5 ppm 1-MCP for 20 d. Expression of each ACS gene was measured by real-time quantitative RT-PCR analysis. The relative quantity of each ACS mRNA was expressed as a percentage of LeACS2 at 40 DAA, shown in Fig. 4. Vertical bars are the SE of three replications.
Discussion
Ethylene insensitivity in transgenic tomato with suppressed LeEIL genes
By using antisense constructs for each LeEIL (LeEIL1–LeEIL3), Tieman et al. (2001) indicated that LeEIL genes are functionally redundant and positive regulators of multiple ethylene responses; however, even in their strongest suppressed line, the amount of total LeEIL mRNA was 11% of the wild type. Their lines exhibited a partial ethylene response in the triple response assay. In this study, the hairpin RNA-induced gene silencing technique was employed to suppress the total expression of LeEIL1–LeEIL4 to trace levels. The transgenic line with the lowest levels of total mRNA for LeEIL genes, RiEIL-18, displayed non-fruit-ripening phenotypes and severe suppression of petal abscission, even in the late stage of fruit development (Fig. 1). In addition, seedlings of the transgenic line showed no triple response or epinasty in the presence of 100 ppm ethylene (Fig. 2). Therefore, the ethylene insensitivity of the transgenic lines obtained in this study appeared complete. These results indicate that the transgenic line would be a useful tool to study the roles of ethylene in various biological aspects of tomato.
Ripening ethylene biosynthesis is regulated by both autocatalytic and ethylene-independent mechanisms
As transgenic fruit did not develop a red colour, even beyond 50 DAA, no ripening-associated ethylene production was expected. Contrary to expectation, however, RiEIL-18 fruit exhibited a gradual and consistent increase in ethylene production beyond 40 DAA (Fig. 3). Although the levels of ethylene production in transgenic fruit were much lower than in the wild-type fruit, they were significantly higher than the level in system 1 and exceeded 0.1 nl g−1 h−1, the threshold that is sufficient to induce an ethylene response (Abeles et al., 1992). The fruit of mutants that were ripening impaired due to ethylene insensitivity, Nr and Gr, also produced a significant level of ethylene in later developmental stages (Herner and Sink, 1973; Barry et al., 2005). The fruit ethylene production of these mutants is explained by their residual ethylene sensitivity, as these mutants exhibited residual ethylene sensitivity in the triple response assay (Lanahan et al., 1994; Barry et al., 2005). Our transgenic line showed no ethylene sensitivity in the triple response assay; however, we could not exclude the possibility that the low level of ethylene production in the transgenic lines was due to residual ethylene sensitivity caused by leaky LeEIL genes. In order to verify the independence of low-level ethylene production from leaky ethylene sensitivity, a potent inhibitor of ethylene perception, 1-MCP (Sisler and Serek, 1997), was employed. 1-MCP has been shown to bind to ETR1 protein, a receptor of ethylene, and to inhibit the ethylene signal. Ethylene signalling in transgenic fruit is interrupted at LeEIL genes by RNA interference; therefore, in 1-MCP-treated transgenic fruit, the ethylene signal is blocked at two different points, namely upstream and downstream of the ethylene signalling pathway. Thus, the suppressive effect of 1-MCP and the transgene on the signal must be synergistic. Consequently, further suppression of ethylene production would be expected in 1-MCP-treated transgenic fruit if leaky LeEIL mRNA was involved in ethylene production; however, the pattern and level of ethylene production in 1-MCP-treated transgenic fruit were almost identical to those in non-treated transgenic and 1-MCP-treated wild-type fruit (Fig. 5). Despite the double block in the ethylene signal, the residual ethylene was detected, which indicates that the ethylene production is probably not due to leaky ethylene sensitivity, but rather to an ethylene-independent developmental factor. It is concluded that ripening ethylene (system 2) in wild-type tomato fruit consists of two parts: a large part that occurs under autocatalytic regulation and a minor part regulated by an ethylene-independent developmental system (Figs 5, 7).
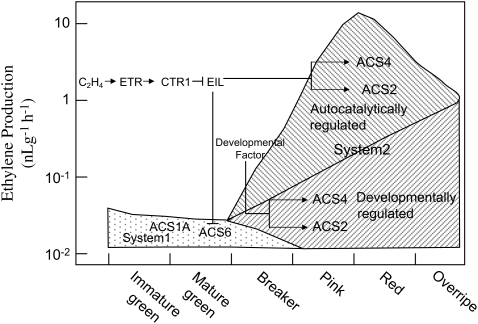
Fig. 7.
Possible model of ethylene biosynthesis in tomato fruit.
Under normal growth conditions, system 2 in the wild-type tomato fruit is induced after 35 DAA. If the cumulative effect of system 1 dictates the transition from system 1 to system 2, the increase of ethylene production in the transgenic fruit must be blocked as ethylene signalling was inhibited in the transgenic fruit throughout the growing stages. In the experiment performed here, ethylene production in transgenic fruit was limited in quantity, and the date of onset of the increase was delayed for several days compared with the wild-type fruit (Figs 3, 5). This suggests that the transition from system 1 to system 2 is dictated to only a small extent by the cumulative effect of system 1, as the effect was severely inhibited in the transgenic fruit.
The reduction of ethylene receptor genes (LeETR4 or LeETR6) by antisense technology causes an early ripening phenotype with increased ethylene sensitivity (Tieman et al., 2000; Kevany et al., 2007, 2008). In addition, Kevany et al. (2007, 2008) reported that the level of ethylene receptor proteins declined in response to ethylene application via 26S proteasome-dependent degradation, resulting in accelerated fruit ripening. They proposed that the level of the ethylene receptor, a negative regulator of ethylene signalling, modulated the timing of the onset of fruit ripening by measuring and memorizing ethylene exposure. On the other hand, from their observations (Kevany et al., 2007), the LeETR protein level in fruit grown under normal conditions increased gradually from the immature stage to the mature green stage and then decreased with the onset of ripening. This suggests that exogenous ethylene at a higher level than the physiologically active level reduces LeETR proteins, but system 1 ethylene might be too low to reduce LeETR proteins during fruit development or to affect the timing of the onset of fruit ripening. Taking the findings of the authors and others together, the cumulative effect of system 1 is less likely to be involved in the transition to system 2 in natural growth conditions.
ACC synthase genes responsible for ethylene biosynthesis
In order to determine the ACS genes responsible for the low level of ethylene production observed in transgenic fruit, real-time quantitative RT-PCR analysis was performed. In transgenic fruit, LeACS2 and LeACS4 transcripts increased gradually with a small increase in ethylene production whereas, in wild-type fruit, a marked increase in LeACS2 and LeACS4 transcripts occurred with the onset of the ripening process (Fig 4). In addition, the levels of LeACS2 and LeACS4 transcripts in transgenic fruit were not affected by 1-MCP treatment and were almost the same as those in 1-MCP-treated wild-type fruit (Fig. 6). On the other hand, LeACS1A and LeACS6 transcripts were much less abundant than LeACS2 and LeACS4 transcripts. These observations suggest that LeACS2 and LeACS4 are involved in both the small increase in ethylene production induced by the developmental factor after 40 DAA and the massive autocatalytic ethylene production.
On the basis of the analysis of wild-type, rin, and Nr fruits, Barry et al. (2000) proposed that LeACS1A and LeACS4 are responsible for initiating system 2 by a combination of LeACS2 and LeACS4. In rin tomato fruit, ethylene production and the expression of LeACS2 and LeACS4 did not increase and were maintained at trace levels, even in later developmental stages (Herner and Sink, 1973; Ng and Tigchelaar, 1977; Barry et al., 2000). These observations suggest that the limited increase in ethylene biosynthesis during ripening requires the competence of RIN, the gene encoding a MADS box transcription factor (Vrebalov et al., 2002; Giovannoni, 2007). Previously, Barry et al. (2000) and Yokotani et al. (2004) demonstrated that exposure of rin fruit to ethylene resulted in the increased accumulation of LeACS2 at a certain level, but not LeACS4. A recent study demonstrated that the RIN protein exhibits transactivator activity and binds to the promoter region of LeACS2 (Ito et al., 2008). Thus, part of the gradual increase in ethylene production observed in RiEIL fruit may be due to the direct up-regulation of the LeACS2 gene by RIN.
To date, in addition to fruit ripening, stress such as wounding and touching has been shown to induce LeACS1A and LeACS2 expression, while LeACS4 is known to be expressed exclusively in ripening fruit (Tatsuki and Mori, 1999; Barry et al., 2000). These observations suggest that the limited increase of LeACS4 observed in transgenic fruit must not be due to mechanical stress but rather to a developmental factor.
Proposed model for the transition of system 1 to system 2
Taking together the present results and those of others, a model is proposed to explain the transition from system 1 to system 2 (Fig. 7). System 1 is produced via LeACS1A and LeACS6, which are regulated by a negative feedback system (Nakatsuka et al., 1998; Barry et al., 2000; Alexander and Grierson, 2002). Transition under natural conditions (absence of exogenous ethylene and stress) occurs mainly via the limited expression of LeACS2 and LeACS4. These are regulated by a developmental factor(s) independent of ethylene, resulting in a limited increase of ethylene biosynthesis. Limited ethylene would play a role as a trigger to stimulate an ethylene burst due to the ethylene-dependent expression of LeACS2 and LeACS4, inducing fruit ripening. System 1 decreases with the onset of system 2, as LeACS6 is regulated by a negative feedback system; therefore, system 2 in tomato fruit consists of both ethylene-dependent (autocatalytic) and ethylene-independent (non-autocatalytic) systems. Even when the effect of system 1 ethylene is eliminated, fruit can initiate system 2, leading to fruit ripening.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Changes in the rate of ethylene production in wild-type and RiEIL-10 fruit. Tomato fruits were harvested 35 days after anthesis and continuously treated with (+) or without (–) 1-MCP. Vertical bars are the SE of three replications.
Acknowledgments
We thank Dr Harry Klee (University of Florida, USA) and Dr Francis M Mathooko (Jomo Kenyatta University of Agriculture and Technology, Kenya) for careful reading of and useful comments on this paper. We also thank the CM Rick Tomato Genetics Center, University of California, Davis, USA for supplying rin tomato seeds and National BioResource Project (NBRP), MEXT, Japan for technical supports. This work was supported in part by a Grant-in-Aid for Scientific Research (grant no. 20380022 to YK) from the Japan Society for the Promotion of Science, Japan.
References
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in plant biology. 2nd edn. San Diego: Academic Press; 1992. [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato; a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Asamizu E, Ezura H. Inclusion of tomato in the genus Solanum as ‘Solanum lycopersicum’ is evident from phylogenetic studies. Journal of the Japanese Society for Horticultural Science. 2009;78:3–5. [Google Scholar]
- Barry CS, Giovannoni JJ. Ethylene and fruit ripening. Journal of Plant Growth Regulation. 2007;26:143–159. [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiology. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, McQuinn RP, Thompson AJ, Seymour GB, Grierson D, Giovannoni JJ. Ethylene insensitivity conferred by the Green-ripe and Never-ripe 2 ripening mutants of tomato. Plant Physiology. 2005;138:267–275. doi: 10.1104/pp.104.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CR, Smith CJS, Ray JA, Moureau P, Bevan MW, Bird AS, Hughes S, Morris PC, Grierson D, Schuch W. The tomato polygalacturonase gene and ripening-specific expression in transgenic plants. Plant Molecular Biology. 1988;11:651–662. doi: 10.1007/BF00017465. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annual Review of Cell and Developmental Biology. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Cara B, Giovannoni JJ. Molecular biology of ethylene during tomato fruit development and maturation. Plant Science. 2008;175:106–113. [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chuang CF, Meyerowitz EM. Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 2000;97:4985–4990. doi: 10.1073/pnas.060034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RX, Nagy F, Sivasubramaniam S, Chua NH. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. The Plant Cell. 1989;1:141–150. doi: 10.1105/tpc.1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB. Virus-induced gene silencing in tomato fruit. The Plant Journal. 2005;43:299–308. doi: 10.1111/j.1365-313X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proceedings of the National Academy of Sciences, USA. 2004;101:6803–6808. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Current Opinion in Plant Biology. 2007;10:283–289. doi: 10.1016/j.pbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Herner RC, Sink LC., Jr Ethylene production and respiratory behavior of the rin tomato mutant. Plant Physiology. 1973;52:38–42. doi: 10.1104/pp.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwasa K, Kinugasa Y, Amano S, Hashimoto A, Nakano R, Inaba A, Kubo Y. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. Journal of Experimental Botany. 2003;54:771–779. doi: 10.1093/jxb/erg073. [DOI] [PubMed] [Google Scholar]
- Hoeberichts FA, Van-Der Plas LHW, Woltering EJ. Ethylene perception is required for the expression of tomato ripening-related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biology and Technology. 2002;26:125–133. [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Inaba A. Studies on the internal feedback regulation of ethylene biosynthesis and signal transduction during fruit ripening, and the improvement of fruit quality. Journal of the Japanese Society for Horticultural Science. 2007;76:1–12. [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T. DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. The Plant Journal. 2008;55:212–223. doi: 10.1111/j.1365-313X.2008.03491.x. [DOI] [PubMed] [Google Scholar]
- Kendrick MD, Chang C. Ethylene signaling: new levels of complexity and regulation. Current Opinion in Plant Biology. 2008;11:479–485. doi: 10.1016/j.pbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Taylor MG, Klee H. Fruit-specific suppression of the ethylene receptor LeETR4 results in early-ripening tomato fruit. Plant Biotechnology Journal. 2008;6:295–300. doi: 10.1111/j.1467-7652.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee H. Ethylene receptor degradation controls the timing of ripening in tomato fruit. The Plant Journal. 2007;51:458–467. doi: 10.1111/j.1365-313X.2007.03170.x. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiology. 2004;135:660–667. doi: 10.1104/pp.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. The Never Ripe mutation blocks ethylene perception in tomato. The Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre JM, Latche A, Jones B, Bouzayen M, Pech JC. Ethylene and fruit ripening. Physiologia Plantarum. 1998;101:727–739. [Google Scholar]
- McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature. 1972;237:235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- Nakano R, Ogura E, Kubo Y, Inaba A. Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiology. 2003;131:276–286. doi: 10.1104/pp.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiology. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TJ, Tigchelaar EC. Action of the non-ripening (nor) mutant on fruit ripening of tomato. Journal of the American Society for Horticultural Science. 1977;102:504–509. [Google Scholar]
- Oetiker JH, Yang SF. The role of ethylene in fruit ripening. Acta Horticulturae. 1995;398:167–178. [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/s0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at receptor level: recent developments. Physiologia Plantarum. 1997;100:577–582. [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuki M, Mori H. Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. Journal of Biological Chemistry. 2001;276:28051–28057. doi: 10.1074/jbc.M101543200. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. The Plant Journal. 2001;26:47–58. doi: 10.1046/j.1365-313x.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proceedings of the National Academy of Sciences, USA. 2000;97:5663–5668. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni JJ. A MADS-box gene necessary for fruit ripening at tomato ripening-inhibitor (rin) locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Analytical Biochemistry. 1994;223:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]
- Yang SF. The role of ethylene and ethylene synthesis in fruit ripening. In: Thompson W, Nothnagel E, Huffaker R, editors. Plant senescence: its biochemistry and physiology. Rockville, MD: The American Society of Plant Physiologists; 1987. pp. 156–165. [Google Scholar]
- Yokotani N, Tamura S, Nakano R, Inaba A, Kubo Y. Characterization of a novel EIN3-like gene (LeEIL4) Journal of Experimental Botany. 2003;54:2775–2776. doi: 10.1093/jxb/erg308. [DOI] [PubMed] [Google Scholar]
- Yokotani N, Tamura S, Nakano R, Inaba A, McGlasson WB, Kubo Y. Comparison of ethylene- and wound-induced responses in fruit of wild-type, rin and nor tomatoes. Postharvest Biology and Technology. 2004;32:247–252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.