Abstract
Roots of some gramineous plants secrete phytosiderophores in response to iron deficiency and take up Fe as a ferric–phytosiderophore complex through the transporter YS1 (Yellow Stripe 1). Here, this transporter in maize (ZmYS1) and barley (HvYS1) was further characterized and compared in terms of expression pattern, diurnal change, and tissue-type specificity of localization. The expression of HvYS1 was specifically induced by Fe deficiency only in barley roots, and increased with the progress of Fe deficiency, whereas ZmYS1 was expressed in maize in the leaf blades and sheaths, crown, and seminal roots, but not in the hypocotyl. HvYS1 expression was not induced by any other metal deficiency. Furthermore, in maize leaf blades, the expression was higher in the young leaf blades showing severe chlorosis than in the old leaf blades showing no chlorosis. The expression of HvYS1 showed a distinct diurnal rhythm, reaching a maximum before the onset of phytosiderophore secretion. In contrast, ZmYS1 did not show such a rhythm in expression. Immunostaining showed that ZmYS1 was localized in the epidermal cells of both crown and lateral roots, with a polar localization at the distal side of the epidermal cells. In maize leaves, ZmYS1 was localized in mesophyll cells, but not epidermal cells. These differences in gene expression pattern and tissue-type specificity of localization suggest that HvYS1 is only involved in primary Fe acquisition by barley roots, whereas ZmYS1 is involved in both primary Fe acquisition and intracellular transport of iron and other metals in maize.
Keywords: Barley, expression pattern, iron, localization, maize, phytosiderophore, transporter
Introduction
Some gramineous plants such as barley and wheat have a distinct strategy (Strategy II) for iron (Fe) acquisition, which is different from that of non-grass species (Marschner and Römheld, 1994; Curie and Briat, 2003; Walker and Connolly, 2008). Iron acquisition by gramineous plants includes (i) biosynthesis of phytosiderophores (mugineic acids) inside the roots; (ii) secretion of phytosiderophores to the rhizosphere; (iii) solubilization of insoluble iron in soils by chelation of phytosiderophores; and (iv) uptake of the ferric–phytosiderophore complex by the roots (Ma, 2005). It has been demonstrated that the last step is mediated through a transporter for the ferric–phytosiderophore complex. The first gene (YS1, Yellow Stripe 1) encoding the ferric–phytosiderophore transporter was identified in maize (Curie et al., 2001). YS1 belongs to the oligopeptide transporter (OPT) family (Yen et al., 2001), but quite distantly as it shares only ∼10% similarity and does not contain the OPT consensus domain (Koh et al., 2002). There are eight and 19 homologues (YSL, Yellow Stripe-like proteins) of YS1 in the Arabidopsis and rice genome, respectively (Curie et al., 2001, Jean et al., 2005). Some homologues have also been identified in barley (Murata et al., 2006) and in a dicot species, Thlaspi caerulescens (Gendre et al., 2007). The presence of YSL in non-grass species suggests that YSLs are involved not only in iron acquisition, but also in the internal transport of iron.
Previous studies suggest that there are at least two distinct groups of YSLs based on their expression patterns and transport substrates. One group transports the Fe(III)–phytosiderophore complex from the rhizosphere to the root cells, and includes ZmYS1, HvYS1 from barley, and OsYSL15 from rice (Curie et al., 2001; Murata et al., 2006; Inoue et al., 2009). The other group is responsible for the intracellular transport of iron and other metals complexed with nicotianamine (NA), and includes OsYSL2, AtYSL1, 2, 3, and TcYSL3. In contrast to the known transporters for ferric–phytosiderophore in roots (e.g. ZmYS1, HvYS1, and OsYSL15), transporters such as OsYSL2 from rice and AtYSL2 from Arabidopsis did not transport the Fe(III)–phytosiderophore complex in yeast and/or Xenopus oocyte assay (DiDonato et al., 2004; Koike et al., 2004). Instead, OsYSL2 transports Fe(II)–NA and Mn–NA complexes (Koike et al., 2004), and AtYSL2 transports Fe(II)–NA and Cu–NA complexes in yeast (DiDonato et al., 2004), although contrasting results also have been reported (Schaaf et al., 2005). OsYSL2 is expressed in the companion cells of leaf blades and sheaths, and has been suggested to be involved in phloem transport of Fe and Mn, while AtYSL2 is expressed in many cell types in both roots and shoots and is suggested to be involved in the lateral movement of metals in the vasculature and in Fe and Zn homeostasis (DiDonato et al., 2004; Schaaf et al., 2005). The transport substrates for AtYSL1 and AtYSL3 have not been identified, but analysis with single and double mutants suggested that these genes are responsible for mobilization of micronutrients such as Mn, Zn, Cu, and Fe from leaves and for loading of Fe–NA into the seeds (Jean et al., 2005; Waters et al., 2006).
HvYS1 is the closest homologue of ZmYS1, sharing 72.7% identity and 95.0% similarity, but they have different expression patterns and transport substrates. HvYS1 is only expressed in the roots (Murata et al., 2006), whereas ZmYS1 is expressed in both roots and shoots (Curie et al., 2001). Both transporters are greatly up-regulated by Fe deficiency. Furthermore, in contrast to HvYS1, which transports the ferric–phytosiderophore complex only (Murata et al., 2006), ZmYS1 also transports other substrates including zinc–, copper–, and nickel–phytosiderophore complexes, and nickel, Fe(II), and Fe(III) complexes with NA in yeast and Xenopus oocytes (Roberts et al., 2004; Schaaf et al., 2004). These results suggest that ZmYS1 and HvYS1 may have different roles in iron and metal transport. However, since HvYS1 and ZmYS1 were characterized by different groups previously, results may not be comparable. For example, the localization of HvYS1 activity has been examined, but that of ZmYS1 is unknown. In the present study, to make ZmYS1 and HvYS1 comparable, they were further characterized in terms of expression pattern and tissue localization.
Materials and methods
Plant materials and growth condition
Seeds of barley (Hordeum vulgare L., cv. Morex) and maize (Zea mays L., cv. B73) were soaked in deionized water for 2 h, and then incubated overnight on moist paper in the dark at 20°C for barley or 25°C for maize. Germinated seeds were transferred to a net floated on a continuously aerated solution containing 0.5 mM CaCl2 (pH 5.6) in the dark. Seedlings were then transferred into the aerated 1/5 Hoagland's solution (pH 6.0) containing 1 mM KNO3, 1 mM Ca(NO3)2, 0.4 mM MgSO4, and 0.2 mM KH2PO4, and the micronutrients comprising 20 μM Fe-EDTA, 3 μM H3BO3, 1.0 μM (NH4)6Mo7O24, 0.5 μM MnCl2, 0.4 μM ZnSO4, and 0.2 μM CuSO4. The nutrient solution was changed once every 3 d. At least three biological replicates were conducted for all experiments.
Time-dependent and metal-responsive expression of HvYS1
Barley seedlings (12-d-old) were subjected to a nutrient solution without Fe in a growth chamber (22°C day/18°C night, 12 h/12 h). On days 0, 1, 2, 4, and 7, roots and shoots were sampled and subjected to RNA extraction as described below. The expression of HvYS1 in different tissues was examined by real-time RT-PCR as described below. To investigate the response of HvYS1 to different metal deficiency, barley seedlings (12-d-old) were grown in a nutrient solution without either Fe, Mn, Cu, or Zn for 7 d. Roots and shoots were then sampled and subjected to RNA extraction and quantitative analysis of gene expression as described below.
Tissue-specific expression of ZmYS1
Maize seedlings (14-d-old) were cultivated in a nutrient solution without Fe in a glasshouse. After 5 d the leaf blade, leaf sheath, hypocotyl, crown roots, and seminal roots were separated and used for RNA extraction. The expression of ZmYS1 in different tissues was examined by RT-PCR as described below.
To investigate the expression level of ZmYS1 in different leaf blades with different Fe status, the maize seedlings were first grown in a nutrient solution with Fe in a glasshouse. When the second leaf was fully expanded, the seedlings (7-d-old) were transferred to a nutrient solution without Fe. After 4 d, when the fourth leaf was expanded, each leaf blade was sampled and used for RNA extraction and quantitative analysis of ZmYS1. The SPAD value of each leaf blade was measured with the chlorophyll meter, SPAD-502 (Konica Minolta, Tokyo, Japan).
Time-dependent expression of ZmYS1 in different tissues
To investigate time-dependent change of ZmYS1 expression in different tissues, the maize seedlings were pre-cultured with Fe for 10 d in a glasshouse. The seedlings were then grown in a nutrient solution without Fe for 0, 1, 2, 4, or 7 d. The youngest leaf blade and seminal roots were harvested separately, and used for RNA extraction and quantitative analysis of ZmYS1 as described below.
Collection and quantitative analysis of phytosiderophore
The root exudates were collected from Fe-deficient barley and maize plants. On the day before exudate collection, roots were washed with deionized water twice, and then placed in 1.2 l of deionized water. Collection of the root exudates was started before sunrise (∼7:00 h for barley and 5:00 h for maize) in a glasshouse. At 3 h intervals over the 24 h period, the exudate solution was removed and roots were transferred to fresh deionized water. The experiments were performed in November for barley and in July for maize. The root exudates collected were immediately passed through a cation-exchange column (16 mm×14 cm) filled with Amberlite IR 120B (H+ form, Organo Co., Tokyo, Japan) and eluted with 2 M NH4OH (Ma et al., 2003). The eluates were concentrated by using a rotary evaporator at 40°C. After the residues were dissolved with 1 ml of distilled water, the amounts of phytosiderophores were determined by measuring their iron-solubilizing capacity according to Takagi (1976), with some modifications (Ma et al., 2003), for barley, and by HPLC according to Ueno et al. (2007) for maize.
At each sampling time point, the roots and the shoots were also sampled and frozen in liquid nitrogen for RNA extraction as described below.
RNA extraction and gene expression quantification
Total RNA was extracted from frozen plant samples by using an RNeasy plant mini kit (Qiagen) and then converted to cDNA followed by DNase I treatment using the protocol supplied by the manufacturers of SuperScript II (Invitrogen). Specific cDNAs were amplified by SYBR pre-mix EX Taq™ (Takara) and real-time RT-PCR (ABI Prism 7500, Applied Biosystems) with the following primer sets; 5′-AAAAAATGCGGACGACACTGT-3′ and 5′-AGGCATAACCAGCGTATGCC-3′ for HvYS1, 5′-GTTCAGTTTCCTACGTTCGGTC-3′ and 5′-TCTCCACCGTTTGCCACTCTG-3′ for ZmYS1, and 5′-GACTCTGGTGATGGTGTCAGC-3′ and 5′-GGCTGGAAGAGGACCTCAGG-3′ for Actin as an internal standard.
Immunostaining of ZmYS1
Antibody against ZmYS1 was prepared by immunizing rabbits with a synthetic peptide C-DEMAALDDLQRDEI (positions 377–390 of ZmYS1). ZmYS1 immunostaining was performed in both seminal and crown roots and leaf blades of Fe-deficient maize as described previously (Murata et al., 2006). Tissues were sliced into 100 μm thick sections for immunostaining. The Axio Imager with Apotome (Carl Zeiss) was used for observation.
Statistical analysis
Analysis of variance (ANOVA) was performed on all data sets.
Results and discussion
Comparison of the expression pattern of ZmYS1 and HvYS1
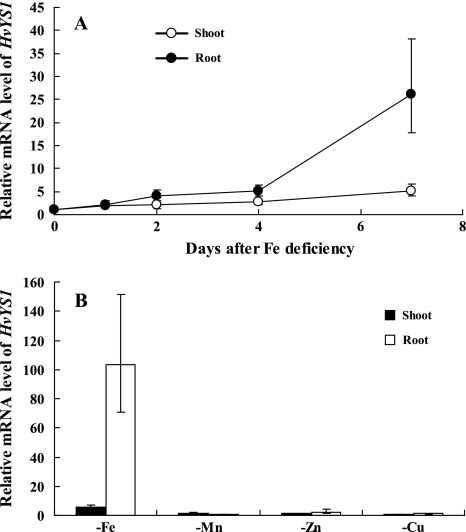
Previous studies have shown that the expression of both ZmYS1 and HvYS1 was induced by Fe deficiency (Curie et al., 2001; Murata et al., 2006). Northern blot hybridization analysis showed that the expression of ZmYS1 in maize roots increased with time since the initiation of Fe deprivation (Curie et al., 2001). In the present study, the expression of HvYS1 was determined at different time points after iron deficiency by using real-time RT-PCR. Similar to ZmYS1 (Curie et al., 2001), the expression of HvYS1 increased with the time of Fe deficiency treatment (Fig. 1A). On day 7 after Fe deficiency treatment, the HvYS1 mRNA abundance in the roots increased 25-fold compared with that on day 0 (P <0.01; Fig. 1A). However, in contrast to ZmYS1, the expression levels of HvYS1 in the shoots were significantly lower than those in the root at each time point (P <0.01; Fig. 1A).
Fig. 1.
Expression of HvYS1 in the roots and shoots of barley. (A) Time-dependent expression of HvYS1. Barley seedling were grown in Fe-free nutrient solution and sampled on different days. (B) Response of HvYS1 to deficiency of different metals. Barley seedlings were grown in a nutrient solution without Fe, Mn, Cu, or Zn for 7 d. The expression level of HvYS1 on different days or in different tissues was determined by quantitative RT-PCR. The mRNA expression level as relative mRNA levels was, respectively, normalized based on the expression level of different tissues of Fe-sufficient plants. The Actin mRNA level was used as an internal control. Data are means ±SD (n=3).
The response of ZmYS1 to metal deficiency has been investigated in maize. The expression of ZmYS1 was up-regulated in roots by Fe deficiency, but not by Cu or Zn deficiency (Roberts et al., 2004). A similar experiment was conducted with HvYS1. As shown in Fig. 1B, deficiency of Mn, Cu, or Zn for 7 d did not induce the expression of HvYS1 (P >0.05). These results indicate that like ZmYS1, HvYS1 is not involved in the primary acquisition of Mn, Zn, and Cu.
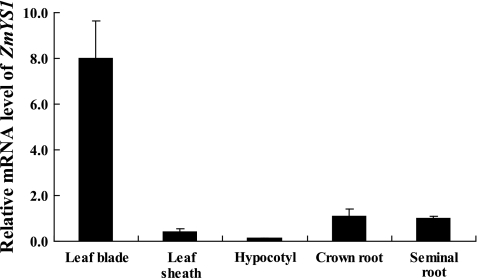
Since ZmYS1 is also expressed in the shoots (Curie et al., 2001; Roberts et al., 2004), the expression of ZmYS1 was further examined in different tissues of Fe-deficient maize. The highest abundance of ZmYS1 was found in the leaf blades (Fig. 2). The expression of ZmYS1 was much lower in the leaf sheaths, and almost no expression was detected in the hypocotyl. In crown and seminal roots, the expression of ZmYS1 was similar (P >0.05) and lower than that in the leaf blades (P <0.01; Fig. 2).
Fig. 2.
Tissue-specific expression of ZmYS1. Maize seedlings were grown in Fe-free nutrient solution for 14 d and then separated into different tissues. The expression level of ZmYS1 in different tissues was determined by quantitative RT-PCR. The mRNA expression level as relative mRNA levels was normalized based on the expression level of the seminal roots of Fe-deficient plants. The Actin mRNA level was used as an internal control. Data are means ±SD (n=3).
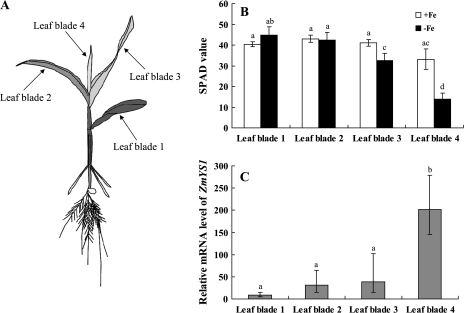
The expression of ZmYS1 was then further examined in different leaf blades with different Fe status. To generate leaf blades with different Fe status, maize seedlings were first grown in a nutrient solution containing Fe and then transferred to a solution without Fe. After 4 d in the –Fe solution, the younger leaves showed Fe deficiency symptoms of chlorosis, while the older leaves stayed green (Fig. 3A). The SPAD value of the youngest leaf without Fe supply was only half of that in plants with continuous Fe supply (P <0.01; Fig. 3B), whereas the oldest leaf had similar SPAD values in the +Fe- and –Fe-treated plants (P >0.05). Notably, the expression level of ZmYS1 tended to increase with decreasing SPAD value in leaf blades, with >20-fold difference between the youngest and the oldest leaf blade (P <0.01; Fig. 3C). These results indicate that the expression of ZmYS1 is regulated by Fe status in leaf blades.
Fig. 3.
Effect of Fe status on the expression level of ZmYS1 in different leaf blades. Maize seedlings were grown in a nutrient solution with Fe for 7 d and then in a solution without Fe for 4 d. (A) A maize plant showing Fe deficiency symptoms in the young leaves. (B) SPAD value of different leaf blades with or without Fe supply. (C) Expression of ZmYS1 in different leaf blades. The expression level of ZmYS1 in different tissues was determined by quantitative RT-PCR. The mRNA levels are relative to those of the +Fe plants. Different letters indicate a significant difference at P <0.05. Data are means ±SD (n=3).
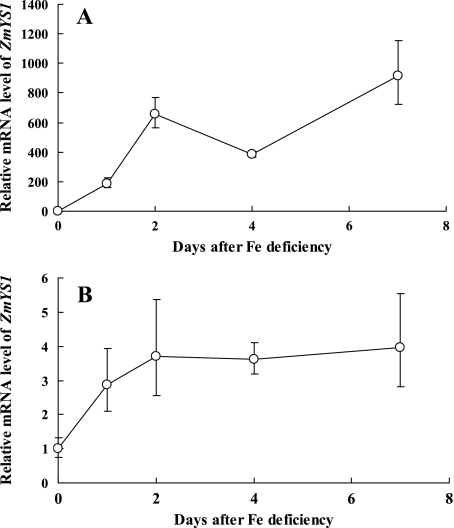
The time-dependent changes of ZmYS1 expression were further investigated in the youngest leaf blade and roots. The expression of ZmYS1 increased rapidly from day 1 after Fe deficiency, and reached 600 times higher on day 2 compared with the expression level of the leaf blade from the +Fe plants (Fig. 4A). The expression in the seminal roots was also enhanced, reaching a plateau 1 d after the Fe deficiency treatment (P <0.01), but the expression levels were only 3- to 4-fold higher than those of the Fe-sufficient plants. (Fig. 4B). All these results indicate that the expression of ZmYS1 responds to the Fe deficiency more in the youngest leaf blade than in the roots, which is different from HvYS1 (Fig. 1A).
Fig. 4.
Time-dependent expression of ZmYS1 in the youngest leaf blades (A) and seminal roots (B). Maize seedlings were grown in a nutrient solution without Fe for different numbers of days. The expression level of ZmYS1 in each tissue on different days was determined by quantitative RT-PCR. The mRNA expression level as relative mRNA levels was normalized, respectively, based on the expression level of different tissues of Fe-sufficient plants. Data are means ±SD (n=3)
Diurnal rhythm of ZmYS1 and HvYS1 expression
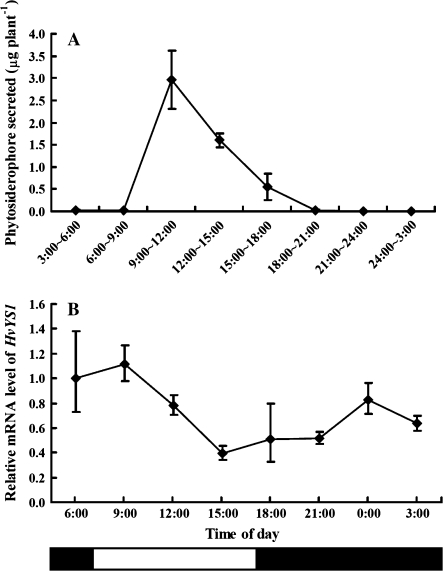
Secretion of phytosiderophore from the roots is characterized by a diurnal rhythm in some gramineous plants such as barley, wheat, and Festuca rubra (Takagi et al., 1984; Ma et al., 2003; Reichman and Parker, 2007). Generally, plants start to secrete phytosiderophores in the morning from the third hour after sunrise or the onset of the light period, and secretion continues for ∼3 h. However, gramineous plants such as maize do not show a diurnal rhythm in secretion (Yehuda et al., 1996). To investigate whether the expression of ZmYS1 and HvYS1 has a diurnal rhythm, the time-dependent expression of HvYS1 and ZmYS1 as well as phytosiderophore secretion were determined in Fe-deficient barley and maize. In Fe-deficient barley, the secretion of phytosiderophore showed a diurnal rhythm (P <0.01; Fig. 5A). With sunrise occurring at 7:00 h, the secretion reached a peak during 9:00–to 12:00 h. This result is in agreement with previous findings (Takagi et al., 1984). In contrast to the phytosiderophore secretion pattern, HvYS1 was expressed all day in the Fe-deficient barley roots (Fig. 5B). However, there was a significant fluctuation in the expression level (P <0.01). The peak of expression was found at 6:00 h, which was before sunrise (Fig. 5B), and remained at a constant level at 9:00 h, during the peak of phytosiderophore secretion. This result is not completely consistent with that of Nagasaka et al. (2009). They found that the expression peak of HvYS1 was at the start of illumination. Since they did not monitor the secretion of phytosiderophore at the same time, it is difficult to compare their results directly with the present results. Phytosiderophore is easily degraded by microorganisms (Watanabe and Wada, 1989); therefore, the expression of HvYS1 before the secretion of phytosiderophore will help the roots to take up the Fe(III)–phytosiderophore complex efficiently.
Fig. 5.
Diurnal rhythm of phytosiderophore secretion (A) and HvYS1 expression (B) in barley. Root exudates were collected from Fe-deficient barley seedlings every 3 h in a glasshouse (sunrise 7:00 h) and roots were sampled at the same time point. The amount of phytosiderophore secreted was determined according to Ma et al. (2003). The expression level of HvYS1 at different times was determined by quantitative RT-PCR. The relative mRNA levels in the roots at 6:00 h are shown. Data are means ±SD (n=3).
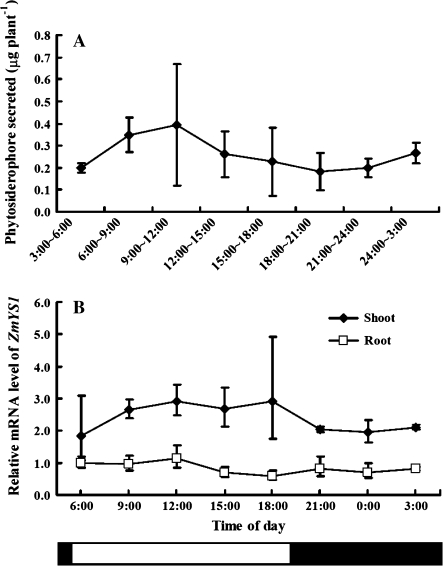
In contrast, the secretion of phytosiderophore in the Fe-deficient maize roots did not show a distinct diurnal rhythm as seen in barley (P >0.05; Fig. 6A). This is in agreement with previous findings (Yehuda et al., 1996). Interestingly, there was also no diurnal change in the expression of ZmYS1 in either the roots or shoots (P >0.05; Fig. 6B). Although the mechanism controlling the diurnal rhythm of phytosiderophore secretion and YS1 expression is still unknown, it would be interesting to compare the promoter regions of HvYS1 and ZmYS1 in the future.
Fig. 6.
Diurnal rhythm of phytosiderophore secretion (A) and ZmYS1 expression (B) in maize. Root exudates were collected from Fe-deficient maize seedlings every 3 h (sunrise 5:00 h) and roots and shoots were sampled at the same time point. The amount of phytosiderophore secreted was determined by HPLC according to Ueno et al. (2007). The expression level of ZmYS1 at different times was determined by quantitative RT-PCR. The relative mRNA levels in the roots at 6:00 h are shown. Data are means ±SD (n=3).
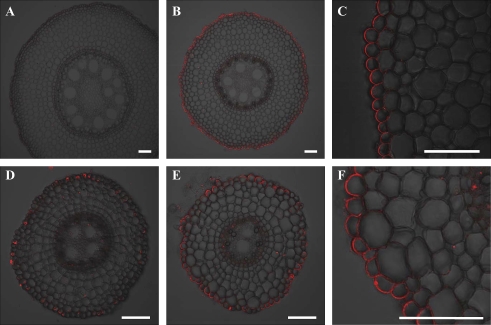
Tissue-type specificity of localization of ZmYS1
Immunostaining showed that HvYS1 is localized to the epidermal cells in the Fe-deficient barley roots (Murata et al., 2006). However, in maize, the tissue-type specificity of localization of ZmYS1 is unknown. The localization was investigated by immunostaining with anti-ZmYS1 polyclonal antibody. In the Fe-sufficient maize, the signal of ZmYS1 was hardly observed in both the crown and lateral roots (Fig. 7A, D). However, in the Fe-deficient maize, ZmYS1 was found to be localized at the epidermal cells of crown and lateral roots (Fig. 7B. E). This localization is similar to HvYS1 (Murata et al., 2006). However, different from HvYS1, ZmYS1 showed polar localization at the distal side of the epidermal cells. Polar localization of some mineral transporters has been reported recently. For example, an influx silicon transporter Lsi1 is localized at the distal side of the exodermal and endodermal cells of rice roots (Ma et al., 2006), while an efflux silicon transporter Lsi2 is localized at the proximal side of the same cells (Ma et al., 2007). In maize roots, the Si influx transporter ZmLsi1 also showed polar localization at the distal side of epidermal and cortical cells (Mitani et al., 2009). To our knowledge, this is the first time that polar localization has been described for a metal complex transporter. The mechanism controlling polar localization of transporters has not been described. It will be interesting to elucidate the mechanism of polar localization by comparing HvYS1 and ZmYS1 in the future.
Fig. 7.
Tissue-specific localization of ZmYS1 in maize roots. (A, D) Immunostaining with anti-ZmYS1 antibody in the crown (A) and lateral roots (D) of maize grown in a nutrient solution containing Fe. (B, C, E, and F), Immunostaining in the crown (B and C) and lateral roots (E and F) of maize grown in Fe-free nutrient solution. Scale bars indicate 100 μm.
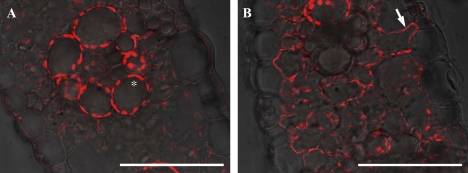
In the leaf blade, ZmYS1 was not localized in the epidermal cells, but was detected on the peripheries of all mesophyll cells (Fig. 8). Heterologous studies in yeast and Xenopus oocytes have shown that ZmYS1 transports not only the Fe(III)–phytosiderophore complex, but also zinc–, copper–, and nickel–phytosiderophore complexes, and nickel, Fe(II), and Fe(III) complexes with NA (Roberts et al., 2004; Schaaf et al., 2004). The localization of ZmYS1 in the leaf blade suggests that ZmYS1 is also involved in the intracellular transport of these metals. However, since the expression of ZmYS1 in both roots and shoots only responded to Fe deficiency, but not to Cu and Zn deficiency at both the mRNA and protein levels (Roberts et al., 2004), it would be interesting in the future to examine whether ZmYS1 is also involved in intracellular transport of Zn and Cu in planta.
Fig. 8.
Localization of ZmYS1 in maize leaf blade. A leaf blade of plants grown in Fe-sufficient (A) or Fe-deficient conditions with chlorosis (B) was used for immunostaining. The asterisk and arrow indicate autofluorescence of the chloroplast and ZmYS1 localization at the plasma membrane of the mesophyll cell, respectively. Scale bars indicate 100 μm.
The present results together with previous findings indicate that HvYS1 is only involved in primary uptake of iron from the rhizosphere to the root epidermal cells in barley, while ZmYS1 has dual roles, both iron acquisition from the rhizosphere and the intracellular transport of iron and possibly other metals in the leaf blades. Since the intracellular transport of iron in barley leaves is also required, it will be interesting to identify Fe transporter(s) in barley leaves in the future. According to the database, there are >10 expressed sequence tags showing homology to HvYS1. Functional analysis of these homologues in the future will help understand the iron transport system in whole plants.
Acknowledgments
This work was mainly supported by the Program of Promotion of Basic Research Activities for Innovative Biosciences (BRAIN). The work was also supported by a Sunbor grant and the Ohara Foundation for Agricultural Science. We thank Jared Westbrook and Fangjie Zhao for their critical reading of this manuscript.
References
- Curie C, Briat JF. Iron acquisition and signaling in plants. Annual Review of Plant Biology. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe 1 encodes a membrane protein directly involved in Fe (III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- DiDonato RJ, Roberts LA, Sanderson T, Eisley RB, Walker EL. Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine–metal complexes. The Plant Journal. 2004;39:403–414. doi: 10.1111/j.1365-313X.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- Gendre D, Czernic P, Conejero G, Pianelli K, Briat JF, Lebrun M, Mari S. TcYSL3 a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens encodes a nicotianamine Ni/Fe transporter. The Plant Journal. 2007;49:1–15. doi: 10.1111/j.1365-313X.2006.02937.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa NK. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. Journal of Biological Chemistry. 2009;284:3470–3479. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- Jean ML, Schikora A, Mari S, Briat JF, Curie C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. The Plant Journal. 2005;44:769–782. doi: 10.1111/j.1365-313X.2005.02569.x. [DOI] [PubMed] [Google Scholar]
- Koh S, Wiles AM, Sharp JS, Naider FR, Becker JM, Stacey G. An oligopeptide transporter gene family in Arabidopsis. Plant Physiology. 2002;128:21–29. [PMC free article] [PubMed] [Google Scholar]
- Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. OsYSL2 is a rice metal–nicotianamine transporter that is regulated by iron and expressed in the phloem. The Plant Journal. 2004;39:415–424. doi: 10.1111/j.1365-313X.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- Ma JF. Plant root responses to three abundant soil mineral: silicon, aluminum and iron. Critical Reviews in Plant Sciences. 2005;24:267–281. [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. Silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Ma JF, Ueno H, Ueno D, Rombolà AD, Iwashita T. Characterization of phytosiderophore secretion under Fe deficiency stress in Festuca rubra. Plant and Soil. 2003;256:131–137. [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature. 2007;448:209–211. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- Marschner H, Römheld V. Strategies of plants for acquisition of iron. Plant and Soil. 1994;165:261–274. [Google Scholar]
- Mitani N, Yamaji N, Ma JF. Identification of maize silicon influx transporters. Plant and Cell Physiology. 2009;50:5–12. doi: 10.1093/pcp/pcn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T. A specific transporter for iron(III)–phytosiderophore in barley roots. The Plant Journal. 2006;46:563–572. doi: 10.1111/j.1365-313X.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- Nagasaka S, Takahashi M, Nakanishi-Itai R, Bashir K, Nakanishi H, Mori S, Nishizawa NK. Time course analysis of gene expression over 24 hours in Fe-deficient barley roots. Plant Molecular Biology. 2009;69:621–631. doi: 10.1007/s11103-008-9443-0. [DOI] [PubMed] [Google Scholar]
- Reichman SM, Parker DR. Probing the effects of light and temperature on diurnal rhythms of phytosiderophore release in wheat. New Phytologist. 2007;174:101–108. doi: 10.1111/j.1469-8137.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Pierson AJ, Panaviene Z, Walker EL. Yellow Stripe1. expanded roles for the maize iron–phytosiderophore transporter. Plant Physiology. 2004;135:115–120. doi: 10.1104/pp.103.037572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. Journal of Biological Chemistry. 2004;279:9091–9096. doi: 10.1074/jbc.M311799200. [DOI] [PubMed] [Google Scholar]
- Schaaf G, Schikora A, Häberle J, Vert GA, Ludewig U, Briat JF, Curie C, von Wirén N. A putative function of the Arabidopsis Fe–phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant and Cell Physiology. 2005;46:762–774. doi: 10.1093/pcp/pci081. [DOI] [PubMed] [Google Scholar]
- Takagi S. Naturally occurring iron-chelating compounds in oat- and rice-root washing. Soil Science and Plant Nutrition. 1976;22:423–433. [Google Scholar]
- Takagi S, Nomoto K, Takemoto T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. Journal of Plant Nutrition. 1984;7:469–477. [Google Scholar]
- Ueno D, Rombolá A, Iwashita T, Nomoto K, Ma JF. Identification of two novel phytosiderophores secreted from perennial grasses. New Phytologist. 2007;174:304–310. doi: 10.1111/j.1469-8137.2007.02056.x. [DOI] [PubMed] [Google Scholar]
- Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Current Opinion in Plant Biology. 2008;11:530–535. doi: 10.1016/j.pbi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Wada H. Mugineic acid-decomposing bacteria isolated from rhizosphere of iron-deficient barley. Japanese Journal of Soil Science and Plant Nutrition. 1989;60:413–417. [Google Scholar]
- Waters BM, Chu HH, DiDonato RJ, Roberts LA, Eisley RB, Lahner B, Salt DE, Walker EL. Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiology. 2006;141:1446–1458. doi: 10.1104/pp.106.082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda Z, Shenker M, Römheld V, Marschner H, Hadar Y, Chen Y. The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiology. 1996;112:1273–1280. doi: 10.1104/pp.112.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen MR, Tseng YH, Saier MH. Maize Yellow Stripe1, an iron–phytosiderophore uptake transporter, is a member of the oligopeptide transporter (OPT) family. Microbiology. 2001;147:2881–2883. doi: 10.1099/00221287-147-11-2881. [DOI] [PubMed] [Google Scholar]