Abstract
Rationale: T-regulatory cells (Tregs) are potent immunomodulators in allergic asthma.
Objectives: We evaluated the functional effects of Tregs by adoptively transferring naturally occurring CD4+CD25+ Tregs (NTregs) and CD4+CD25− inducible Tregs (iTregs) from lung and spleens of green fluorescent protein (GFP)-transgenic Balb/c mice into cockroach-sensitized and -challenged mice.
Methods: GFP-labeled NTregs and iTregs were adoptively transferred into cockroach-sensitized and -challenged mice. Airway hyperresponsiveness (AHR) to methacholine was examined using a single-chamber, whole-body plethysmograph and invasive tracheostomy.
Measurements and Main Results: Adoptive transfer of either NTregs or iTregs from lung or spleen reversed airway inflammation and AHR to methacholine, and the effect lasted for at least 4 weeks. GFP-labeled iTregs up-regulated CD25 and forkhead-winged transcriptional factor box protein 3 and migrated to lymph node and lung. Lung CD4+CD25+ T cells isolated from each group of recipient mice were inducible costimulatory molecule–high and programmed death (PD)-1–positive; however, higher expression of PD-1 was found in the spleen iTregs (S25−) and lung iTregs (L25−) groups. Higher levels of transforming growth factor–β and IL-10 mRNA transcripts and bronchoalveolar lavage fluid IL-10 and INF-γ levels were observed in lung CD4+CD25+ cells from the L25− and S25− cell-recipient mice than from lung NTregs (L25+) and spleen NTregs (S25+) cell-recipient mice. Adoptive transfer of either cell type significantly reduced bronchoalveolar lavage fluid IL-4, IL-5, and IL-13 levels.
Conclusions: Tregs reverse AHR and airway inflammation; however iTregs that differentiated into IL-10–producing CD4+ type 1 cells in the lung exert their suppressive activity likely by higher levels of transforming growth factor–β, IL-10, IFN-γ, and elevated levels of PD-1 compared with NTregs. Hence, PD-1 may be a conduit for reversing AHR by Tregs and a plausible target for treating asthma.
Keywords: airway hyperresponsiveness, forkhead winged helix transcription factor box P3, inducible CD4+ CD25− T-regulatory cells, naturally occurring CD4+CD25+ T-regulatory cells, programmed death-1
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Airway hyperresponsiveness and airway inflammation are hallmarks of allergic asthma, which may result from an imbalance in the ratio of Th1 and Th2 cells, and a loss of function and/or absence of CD4+CD25+ T-regulatory cells.
What This Study Adds to the Field
Adoptive transfer of naturally occurring CD4+CD25+ T-regulatory cells and inducible CD4-CD25− T cells of lung and spleen tissue reversed allergic inflammation. This mechanism, at least in part, was due to increased expression of programmed death–1, and secretion of IL-10 and transforming growth factor–β.
Asthma is a chronic airway disease primarily due to dysregulated type 2 immune response to inhaled antigens (1). Airway hyperresponsiveness (AHR) is believed to be mediated by Th2 cells and the release of their signature cytokines, IL-4, IL-5, IL-9, and IL-13 (2). This cytokine milieu causes pulmonary eosinophilia and chronic inflammation, leading to mucus cell hyperplasia, destructive airway tissue remodeling (3), and contraction of smooth muscle cells (4).
The renaissance of CD4+CD25+ T-regulatory cells (Tregs) has been venerated as a plausible strategy to modulate the effects of dysregulated type 2 immune response. This specialized subset of CD4+ T cells is characterized into two categories: naturally occurring CD4+CD25+ Tregs (NTregs) (5, 6) and inducible CD4+CD25− Tregs (iTregs) to include IL-10–producing CD4+ type 1 (TR1) and Th3 cells (7). NTregs constitutively express CD25, the α-chain of the IL-2R (8–10), and suppress autoimmune T-cell responses and maintain peripheral tolerance. NTregs represent approximately 5 to 10% of the peripheral CD4+ T cells in humans and mice (11) and constitutively express the forkhead-winged transcriptional factor box protein 3 (Foxp3) (12). Compelling evidence demonstrates the necessity of Tregs for normal immune response. Mutations in the X-linked Foxp3 gene in scurfy mice leads to an autoimmune disease characterized by multiorgan lymphocytic infiltration that results in enlarged spleen, lymph nodes, and liver, as well as dermatitis and severe runting (13). In humans, mutations in FOXP3 have been linked to autoimmune diseases, including X-linked autoimmunity allergic dysregulation syndrome (14), X-linked autoimmunity immune deficiency syndrome (15, 16), and immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) (12, 17).
TR1 cells were originally derived from patients with severe combined immunodeficiency who had undergone successful HLA-mismatched bone marrow transplantation (18). TR1 cells are spawned from naive CD4+CD25− T cells activated through T-cell receptor, CD28, and IL-2 receptors and can suppress antigen-driven proliferation of CD4+ T cells in vivo and in vitro (19). TR1 cells display a unique profile of cytokine production that is distinct from that of Th0 cells, which can secrete both Th1 and Th2 cytokines albeit at very low levels (20–22); Th1 cells secrete IFN-γ, tumor necrosis factor–β, and IL-2 (23–25), and Th2 cells secrete IL-4, IL-5, IL-6, IL-9, and IL-13 (20, 22). TR1 cells are characterized by the secretion of high levels of IL-10, transforming growth factor (TGF)–β, and mild to moderate levels of IFN-γ, IL-5, and little to no release of IL-2 and IL-4 (5, 24, 25). IL-10–secreting T-regulatory cells are the major T-cell subset that responds to nonpathogenic antigen in healthy individuals. However, in subjects with atopic asthma there is a reduction of TR1 cells and an increase of Th2 cells. Therefore, a skewed Th2/TR1 ratio initiates the development of AHR and airway inflammation in allegoric asthma (26). However, TR1 cells are characterized by their ability to secrete immunosuppressive cytokines, IL-10 and TGF-β, which modulate immune response. In addition, suppression by TR1 cells may occur through the expression of the cell surface marker, programmed death (PD)-1 (26) and possibly other costimulatory molecules. However, the underlying mechanism and the nature of other participating molecules is unknown. Nonetheless, a shift in this delicate balance of T cell subsets may be pivotal in tolerance or immunopathology as seen in allergic asthma. To date, in healthy individuals there are three potential outcomes of effector T cell development: (1) the Th1 response, which is the normal outcome after exposure to viruses or bacteria; (2) the Th2 response, resulting in the milieu of IL-4, IL-5, IL-9, and IL-13 cytokines in the lung or accumulation of eosinophils and mast cells as a reaction from parasite (helminths) or inappropriate Th2 response to nonpathogenic environmental antigens; and (3) the most prevalent response, tolerogenic cells or Tregs producing immunosuppressive cytokines, IL-10 and TGF-β, in steady state conditions (27).
Therefore, using a green fluorescent protein (GFP)-transgenic mouse model we investigated and compared the functional effect of NTregs and iTregs on AHR and allergic airway inflammation, and investigated if there would be any significant divergence if these cell types were derived from spleen and lung tissue. We adoptively transferred indigenous lung and spleen tissue NTregs and iTregs from naive GFP-transgenic balb/c mice into cockroach (CRA)-sensitized and -challenged mice. We found complete reversal of AHR and airway inflammation in all recipients of either Treg type and the effect lasted for at least 4 weeks. In addition, the adoptively transferred CD4+CD25− T cells migrated to the lymph node and lung and differentiated into CD4+CD25+Foxp3+ with significantly higher levels of mRNA transcripts of IL-10, TGF-β, and PD-1 than in lung CD4+CD25+ cells isolated from adoptively transferred NTregs group.
METHODS
Mice
GFP-transgenic balb/c breeder mice were purchased from Jackson Laboratories (Bar Harbor, ME) and 4- to 8-week-old offspring were used in this study. In addition, 4- to 5-week-old Balb/c female mice were purchased from Harlan Laboratories (Indianapolis, IN) and were housed in separate cages according to treatment protocol. Food and water were provided ad libitum. According to National Institutes of Health guidelines, the research protocol of this study was approved by the Institutional Animal Care and Use Committee of Creighton University.
Sensitization and Challenge with Cockroach Antigen
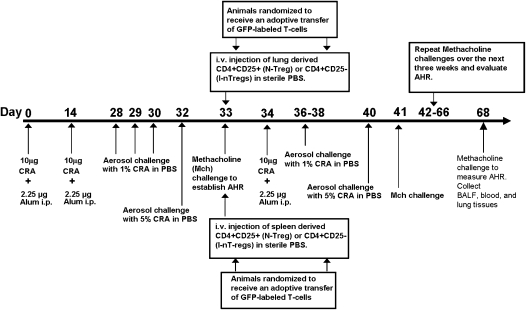
Allergic airway inflammation was induced by intraperitoneal injection of CRA antigen 10 μg, a mixture of (American) Periplaneta Americana (Linnaeus) and (German) Blattella germanica (Linnaeus) (Hollister Stier Laboratories LLC, Spokane, WA) emulsified in alum (Pierce, Rockford, IL) in a total volume of 100 μl per mouse on Days 0 and 14 (Figure 1). Subsequently, animals received aerosol challenge with CRA antigen (1% CRA in phosphate-buffered saline [PBS]) on Days 28 to 30 and 5% CRA in PBS on Day 32.
Figure 1.
Cockroach (CRA) sensitization and adoptive transfer green fluorescent protein (GFP)-labeled T-regulatory cells (Tregs) protocol. Sensitization phase: Day 0 and 14 mice received an intraperitoneal injection of CRA. Challenge phase: Day 28 through 30 and Day 32 mice were aerosol challenged with CRA antigen. Day 33, airway hyperresponsiveness (AHR) to methacholine was evaluated. Adoptive phase: Day 34 animals randomized and received an adoptive transfer of GFP-labeled Tregs. Twenty-four hours later mice began a rigorous 4 weeks of CRA antigen challenge; at the end of each week AHR to methacholine was evaluated. Day 68 mice, animals were killed for analysis.
Measurement of Airway Hyperresponsiveness
AHR to methacholine was examined using a single-chamber, whole-body plethysmograph (Buxco Electronics, Troy, NY) and aerosolized acetyl β-methylcholine (Sigma-Aldrich, St. Louis, MO) in a dose-dependent manner (0.031 g, 0.062 g, 0.125 g, 0.25 g, 0.50 g, and 1.0 g in 10 ml PBS) (Figure 1). On Day 33 and on Day 68 (Figure 1) in a group of representative CRA-sensitized and -challenged mice AHR to methacholine was confirmed by measurement of specific airway resistance in mice anesthetized and cannulated via tracheostomy.
Tissue Preparation and Isolation of GFP-labeled T-regulatory Cells for Adoptive Transfer
To isolate NTregs and iTregs, lungs and spleens were harvested from naive GFP-transgenic Balb/c mice. The tissues were cut into fragments, followed by digestion using collagenase D (Roche Laboratories, Minneapolis, MN) (1 mg/1 ml) and 5 ml of RPMI 1640 (Cambrex, East Rutherford, NJ). The samples were incubated at 37°C in a CO2 incubator for 90 minutes. Tissue was disrupted with a 1-ml syringe. After the tissue was disrupted the cell suspension was poured over a 40-μm filter (BD Bioscience, San Jose, CA) and collected into 15-ml tubes and labeled accordingly. Red blood cells were lysed using TRIS buffered ammonia chloride solution and suspension was neutralized with PBS4 solution. The suspension was centrifuged at 350 × g for 15 minutes. Supernatant was discarded and pellet washed in 10 ml Hanks balanced buffered solution, centrifuged, and resuspended in AutoMACS running buffer. This was followed by isolating Tregs using a two-step process. CD4+ T cells were pre-enriched by depleting unwanted cells by using a cocktail of antibodies. Then the CD25+ cells were positively selected from the enriched CD4+ T-cell fraction (CD4+ CD25+ T-Regulatory Cell Isolation Kit; Miltenyi Biotec, Auburn, CA). Remaining CD4+ were designated as CD4+CD25− cells. Both CD4+CD25+ and CD4+CD25− cells were further purified and sorted by FACSAria (BD Bioscience, San Diego, CA). The purity and viability of the CD4+CD25+ and CD4+CD25− T-cell populations from both lung and spleen tissue were greater than 99.0% and greater than 98%, respectively.
Adoptive Transfer Therapy
Mice with established AHR to methacholine were subjected to adoptive transfer of the cells. AHR was measured by whole-body plethysmography and in randomly selected mice AHR was confirmed by a more rigorous invasive method involving tracheostomy and measurement of specific airway resistance (Figure 2). Starting Day 34, CRA-sensitized mice were randomized into four groups: (1) L25+ group mice that received lung NTregs, (2) L25− group mice that received lung iTregs, (3) S25+ group mice that received spleen NTregs, and (4) S25− group mice that received spleen iTregs. Cells were injected intravenously into the dorsal tail vein of sensitized recipients at 100,000 cells per 50 μl of sterile PBS. Twenty-four hours later these animals received an additional intraperitoneal injection of CRA antigen to prime the adoptively transferred cells (Figure 1). Beginning at Day 37, animals were weekly challenged for 4 weeks with aerosolized CRA antigen (1% CRA and 5% CRA) and AHR to methacholine was evaluated at the end of each week (Figure 1). Nonsensitized control mice were sham treated with sterile PBS as a vehicle.
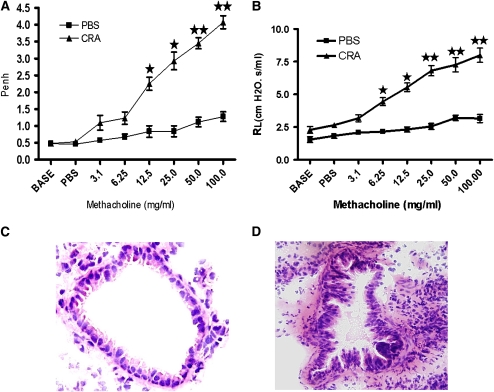
Figure 2.
Day 33 noninvasive and invasive pulmonary analysis. (A) Pulmonary function was evaluated by noninvasive technique of unrestrained single-chamber whole-body plethysmography. All cockroach (CRA)–sensitized and -challenged mice to methacholine exhibited Penh values of 3.94 ± 0.75 (n = 24). (B) (triangles) CRA-sensitized and -challenged mice without adoptive transfer exhibiting elevated lung resistance, (squares) (phosphate-buffered saline [PBS]) control displaying minimal resistance. The results are presented as means ± SE of Penh values of 12 mice per group. (C) At Day 33, several recipient mice were sacrificed, lungs sectioned, then stained with hematoxylin and eosin. PBS control animals displayed normal lung parenchyma. (D) At Day 33, sensitized animals exhibited the cardinal features of asthma.
Flow Cytometry and Antibodies
A FACScan (BD Biosciences, San Jose, CA) was used for analytical flow cytometry and data were processed with CellQuest Pro (Becton Dickinson) using the standard university protocol for cell preparation. Cells were stained with PerCp CD8a (Ly-2) (53.6.7), APC NKR-P113 (PK-136), PerCP CD4 (L3T4), fluorescein isothiocyanate (FITC) CD69 (H12F3), and FITC CD62L (MEL-14), all purchased from BD Bioscience. FITC GITR (DTA-1), APC inducible costimulatory molecule (ICOS) (7e.17G9), and FITC PD-1 (J43) (all purchased from eBioscience, San Diego, CA). In addition, cells were also stained with FITC anti-mouse/rat Foxp3 staining kit (eBioscience).
Mice Euthanization and Lung Preparation
Mice were killed with a lethal injection of 100 μl of pentobarbital. Lungs were removed and a section of the left lobe was placed in 4% formalin. The formalin was removed and tissue was placed in 70% ethanol and paraffin embedded by a Sakura Tissue-Tek VIP paraffin processor (IMEB, San Marcos, CA).
Bronchoalveolar Lavage Fluid and Cytokine Measurements
After killing, lungs were gently lavaged with 1 ml of warm saline (37°C) via a tracheal cannula. Total cell counts were performed with a Coulter counter (Beckman and Coulter, Fullerton, CA). All samples were centrifuged at 400 rpm for 10 minutes, and the supernatant was stored in a −80°C freezer until ELISA assays were performed. Mouse IL-4, IL-5, IL-10, and INF-γ were conducted using a Th1/Th2 and IL-5 ELISA Detection, Ready-Set-Go kits (eBioscience) according to manufacturer's protocol.
Reverse Transcriptase–Polymerase Chain Reaction
To analyze expression of Foxp3, Neuropilin-1, GATA3, and T-bet, the messenger RNA was prepared from isolated Tregs using Trizol (Sigma-Aldrich) reagent protocol. Gene Amp PCR System 2400 (Perkin Elmer, Waltham, MA) was used at 32 cycles for each product, 28 cycles for HPRT, and 30 cycles for Neuropilin-1. Foxp3: Forward 5′-TAC ACC CAG GAA AGA CAG CAA CCT-3′, Reverse 5′-TCT GAA GTA GGC GAA CAT GCG AGT-3′, Tm: 55.0°C. Neuropilin-1: Forward 5′-GAA AGA GGG AAA TAA AGC CA-3′, Reverse 5′-TCC CAC CCT GAA TGA TGA CA-3, Tm: 50.0°C. HPRT: Forward 5′-GAT ACA GGC CAG ACT TTG TTG-3′, Reverse 5′-GGT AGG CTG GCC TAT AGG CT-3′, Tm: 50.0°C. GATA-3: Forward 5′-AGG CAA GAT GAG AAA GAG TGC CTC-3′, Reverse 5′-CTC GAC TTA CAT CCG AAC CCG GTA-3′, Tm: 55.0°C. T-bet: Forward: 5′-GAT CGT CCT GCA GTC TCT CC-3′, Reverse: 5′-AAC TGT GTT CCC GAG GTG TC-3′, Tm: 52.0°C. IL-10: Forward: 5′-ACTGCTATGCTGCCTGCTCTTACT-3′, Reverse: 5′-TGGCCTTGTAGACA CCTTGGTCTT-3′, Tm: 60.2°C. TGF-β: Forward: 5′-TAAAGAGGTCACCCGCGTGCTAAT-3′, Reverse: 5′-GTACTGTGTGTCCAGGCTCCAAA-3′ Tm: 60.2°C.
T-Cell Proliferation Assay
Splenocytes from naive mice were seeded (100,000 cells per well) in anti-CD3 antibody precoated plates (eBioscience) and 2.5 μg/ml anti-CD28 antibody was added (eBioscience). CD4+CD25+ T cells from lung and spleen of adoptively transferred mice and naive balb/c mice were added at varying ratios (1:1, 1:0.5, 1:0.25, and 1:0.125). Cells were incubated for 48 hours at 37°C in RMPI 1640 medium. Bromodeoxyuridine (20 μl) was added to each well for an additional 12 hours followed by analysis of T-cell proliferation using ELISA plate reader.
Data Analysis
Data were analyzed using GraphPad Prism statistical analysis and graphing software. Unpaired Student t test was used to determine differences between two groups by Microsoft Excel. Multiple group comparison was made using analysis of variance. A P value of less than 0.01 was considered significant.
RESULTS
Establishment of AHR in CRA-Sensitized and -Challenged Mice
CRA-sensitized and -challenged mice exhibited AHR on Day 33 after the protocol, shown in (Figure 1). The AHR to methacholine was examined and established with noninvasive whole-body plethysmography and confirmed with a more rigorous invasive method involving tracheostomy and the measurement of specific airway resistance. Administration of 100 mg/ml methacholine exhibited Penh values of 3.94 ± 0.75 (n = 24) (Figure 2A). Specific airway resistance induced by 25 mg/ml methacholine exhibited mean values 20.86 ± 8.55 cm H2O.s/ml (Figure 2B). After tracheostomy several representative animals were killed; lungs were harvested and sectioned, then stained with H&E to demonstrate histological hallmarks of asthmatic airways. The PBS control animals displayed normal airway morphology (Figure 2C). In contrast, histology from the CRA-sensitized and -challenged mice without adoptive transfer (CRAAT−) exhibited inflammatory cell infiltration, epithelial cell hypertrophy, and smooth muscle cell hyperplasia in the airways (Figure 2D).
Effect of Adoptive Transfer of GFP-Labeled NTregs and iTregs Cells
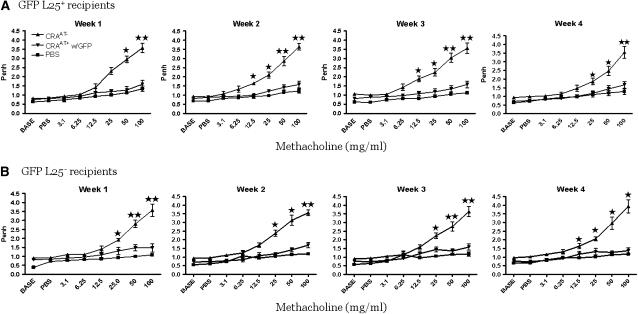
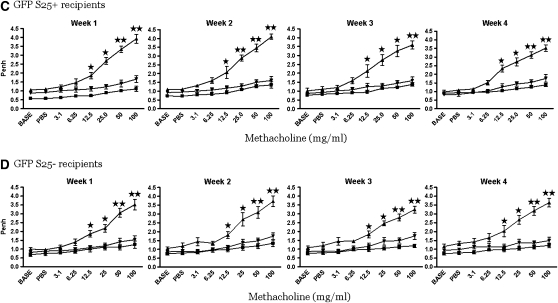
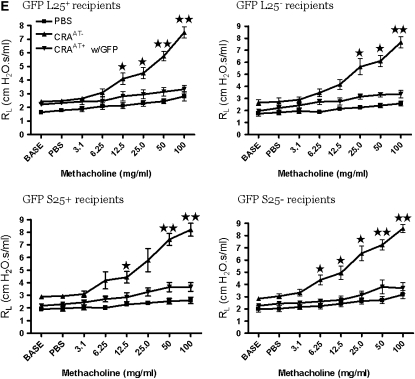
The CRA-sensitized and -challenged mice with established AHR were randomized into their respective groups to receive an adoptive transfer of designated GFP-labeled Tregs (100,000 cells/mouse). The recipient mice of all GFP-labeled Treg subsets completely reversed the AHR and the effect lasted for at least 4 weeks (Figures 3A–3D). This was confirmed by a more rigorous invasive method (Figure 3E).
Figure 3.
Pulmonary evaluation post adoptive transfer. (A–D) In all recipient groups of T-regulatory cells (Tregs), the airway hyperresponsiveness (AHR) to methacholine was significantly reversed to the phosphate-buffered saline (PBS) control level for 4 weeks. These data are presented as means ± SE of Penh values of six mice per group. (E) The reversal of AHR to methacholine was confirmed by invasive tracheostomy and the airway resistance was substantially elevated in cockroach-sensitized and -challenged mice compared with the complete reversal of AHR after adoptive transfer of Tregs in all treatment groups to the PBS control level.
Migration of GFP-Labeled Adoptively Transferred Tregs to Lung, Spleen, and Lymph Nodes
The GFP-labeled lung or spleen iTregs and NTregs migrated to lung, spleen, and lymph nodes of adoptively transferred recipient mice (see Figures E1A–E1I in the online supplement). Interestingly, we adoptively transferred 100,000 GFP-labeled L25+ cells into CRA-sensitized and -challenged mice and after 4 weeks of CRA aerosol challenges we isolated nearly 130,000 of these GFP-labeled NTregs from the lungs of the adoptively transferred recipients (Figure E5A). From the same recipients of GFP-labeled L25+ cells we found fewer than 10,000 of these cells migrated to the spleen (Figure E5B). Moreover, after adoptively transferring 100,000 GFP-labeled L25− cells, we isolated nearly 175,000 of these cells that differentiated into CD4+CD25+ Tregs in the lungs of the adoptively transferred recipients (Figure E5C) and we isolated approximately 5,000 of these differentiated CD4+CD25+ Tregs in the spleen of the same recipient mice (Figure E5D). Furthermore, after adoptive transfer of 100,000 GFP-labeled S25+ cells into CRA-sensitized and -challenged mice, we isolated nearly 90,000 of these GFP-labeled NTregs in the lung (Figure E5E) but more than 20,000 of these GFP-labeled NTregs in the spleen (Figure E5F) of these adoptively transferred recipients. In addition, after adoptively transferring 100,000 GFP-labeled S25− cells, we isolated 90,000 of these cells that differentiated into CD4+CD25+ Tregs in the lung (Figure E5G) and isolated more than 25,000 of these cells that differentiated into CD4+CD25+ Tregs from the spleens (Figure E5H) of the same adoptively transferred recipients. In addition, we found in each experimental group 34,000 to 42,000 GFP-labeled Tregs migrated to the lymph nodes of the recipient mice (Figure E5I). These data suggest that Tregs derived from spleen tissue proliferate less than the Tregs of the lung tissue Also, Tregs from lung or spleen tissue appear to be tissue specific.
Histological Changes in the Lung Tissue Post Adoptive Transfer
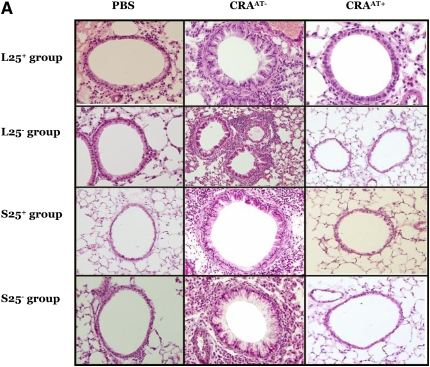
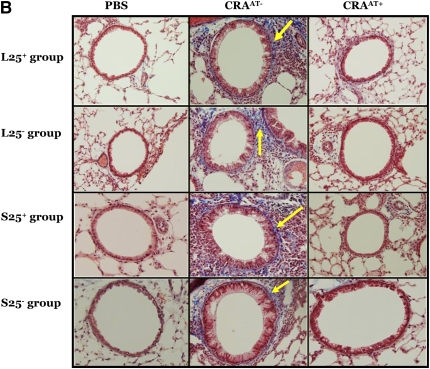
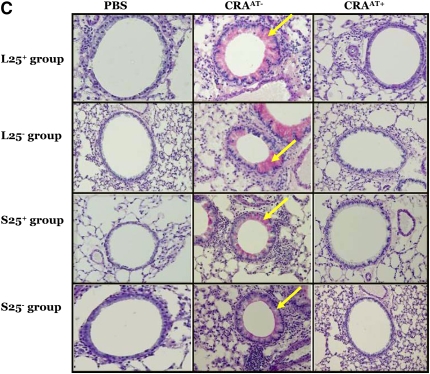
The lungs of all CRAAT− groups exhibited extensive peribronchial and perivascular inflammation, epithelial cell hyperplasia (as shown by hematoxylin and eosin stain; Figure 4A), collagen deposition (as shown by blue color using trichrome stain; Figure 4B), and mucus hypersecretion (as shown by pink color using PAS stain; Figure 4C). However, the adoptive transfer therapy of Tregs in the CRAAT+ mice nearly restored these features (Figures 4A–4C). There was no apparent difference in the lung histology between the PBS control and the CRAAT+ groups.
Figure 4.
Histological examination post adoptive transfer. (A–C) In all experimental groups, phosphate-buffered saline (PBS) control showed normal airway parenchyma. However, the airways of all cockroach (CRA)-sensitized and -challenged mice without adoptive transfer (CRAAT−) groups exhibited the cardinal features of asthma, including clusters of inflammatory cells surrounding the airways, bronchiolar epithelium showing hyperplastic columnar epithelial cells, smooth muscle cell hyperplasia (indicated by hematoxylin and eosin staining), collagen deposition (indicated in blue by trichrome staining, arrows), goblet cell hyperplasia, and mucus hypersecretion (indicated in pink by periodic acid Schiff staining, arrows). All CRA-sensitized and -challenged mice with adoptive transfer (CRAAT+) groups showed restoration of airway inflammation.
Inflammatory Cells in the Bronchoalveolar Lavage Fluid
In the bronchoalveolar lavage fluid (BALF) of all (CRAAT+) there was a significant reduction in eosinophils and lymphocytes compared with CRAAT− group. The number of leukocytes in the BALF of the CRAAT+ group was nearly restored to the levels in PBS control mice (Table 1).
TABLE 1.
DIFFERENTIAL CELL COUNTS IN THE BRONCHOALVIOLAR LAVAGE FLUID OF MICE IN VARIOUS EXPERIMENTAL GROUPS
| Experimental conditions | Absolute Number of Cells in the BALF ×105
|
|||||||
|---|---|---|---|---|---|---|---|---|
| L25+ | L25− | S25+ | S25− | |||||
| PBS | ||||||||
| Eosinophils | 0.0 ± 0.0 | 0.05 ± 0.04 | 0.0 ± 0.0 | 0.01 ± 0.01 | ||||
| Neutrophils | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| Macrophages | 3.4 ± 0.35 | 3.6 ± 0.25 | 4.1 ± 0.10 | 5.9 ± 0.59 | ||||
| Lymphocytes | 0.23 ± 0.14 | 0.25 ± 0.14 | 0.33 ± 0.10 | 0.09 ± 0.08 | ||||
| CRA | ||||||||
| Eosinophils | 5.0 ± 0.43* | 7.5 ± 0.12* | 7.7 ± 0.11* | 8.2 ± 0.16* | ||||
| Neutrophils | 0.14 ± 0.64† | 0.30 ± 0.18† | 0.15 ± 0.05 | 0.36 ± 0.06† | ||||
| Macrophages | 1.5 ± 0.14 | 1.0 ± 0.17 | 1.2 ± 0.24 | 2.3 ± 0.96 | ||||
| Lymphocytes | 0.80 ± 0.34* | 1.3 ± 0.63 | 1.2 ± 0.16* | 1.1 ± 0.16* | ||||
| CRA–adoptive | ||||||||
| Eosinophils | 0.27 ± 0.14* | 0.15 ± 0.03* | 0.41 ± 0.15* | 0.19 ± 0.052* | ||||
| Neutrophils | 0.0 ± 0.0† | 0.0 ± 0.0† | 0.0 ± 0.0 | 0.0 ± 0.0† | ||||
| Macrophages | 3.2 ± 0.14 | 3.5 ± 0.13 | 4.3 ± 0.40 | 4.6 ± 0.18 | ||||
| Lymphocytes | 0.45 ± 0.82† | 0.71 ± 0.072 | 0.36 ± 0.50† | 0.33 ± 0.075† | ||||
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; CRA = cockroach; L25+ = lung naturally occurring CD4+CD25− T-regulatory cells; L25− = lung inducible CD4+CD25− T-regulatory cells; PBS = phosphate-buffered saline; S25+ = spleen naturally occurring CD4+CD25− T-regulatory cells; S25− = spleen inducible CD4+CD25− T-regulatory cells.
P < 0.001. The CRA group values are compared with the PBS group; the CRA–adoptive group values are compared with the CRA group.
P < 0.01. The CRA group values are compared with the PBS group; the CRA–adoptive group values are compared with the CRA group.
Cytokines in the BALF
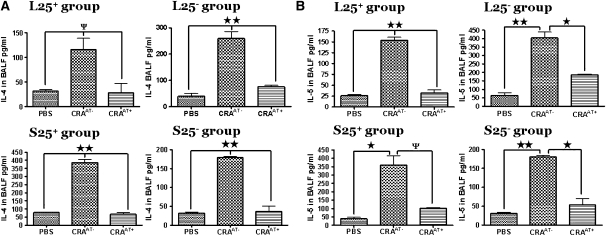
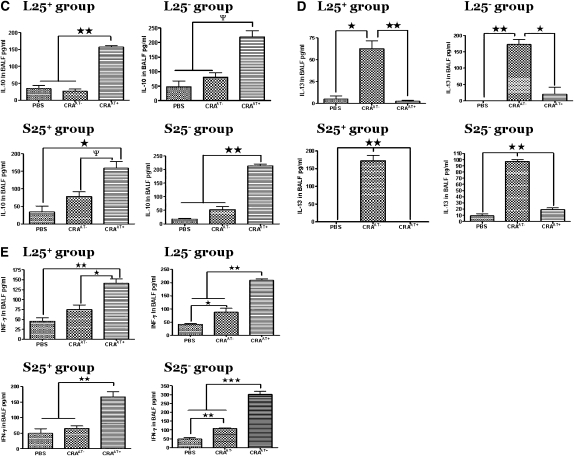
In the BALF of all PBS control and CRAAT+ groups there was a minimal level of IL-4 compared with significantly elevated levels in the CRAAT− groups (P < 0.001) (Figure 5A). In the BALF of mice in all CRAAT− groups we observed substantially high levels of IL-5 compared with significantly low levels in the CRAAT+ group and the PBS control (Figure 5B). These data indicate a higher amount of Th2 cytokines in the lungs of CRAAT− groups than in the PBS control and CRAAT+ groups. High levels of IL-10 were observed in the BALF of all CRAAT+ groups compared with substantially low levels in both CRAAT− and PBS control groups (Figure 5C). IL-10 is produced by Th2 cells, but TR1 cells have been shown to produce IL-10 at significantly high levels. In addition, in the CRAAT− groups there was a significant increase in BALF IL-13 levels compared with significantly low levels in the CRAAT+ and PBS control groups (Figure 5D). The concentration of IFN-γ in the BALF of the CRAAT+ groups was significantly increased compared with the CRAAT− and PBS control groups (Figure 5E).
Figure 5.
Bronchoalveolar lavage fluid (BALF) cytokines that modulate Th2 cell function. (A) In all phosphate-buffered saline (PBS) control and cockroach-sensitized and -challenged mice with adoptive transfer (CRAAT+) groups there were nominal levels of BALF IL-4 detected compared with substantially high levels in the cockroach-sensitized and -challenged mice without adoptive transfer (CRAAT−) groups (P < 0.001). (B) BALF IL-5 was detected at significantly high levels in all CRAAT− groups compared with substantially low levels in all CRAAT+ and PBS control groups. (C) We examined the levels of BALF IL-10 and in all CRAAT+ groups and found high levels of IL-10; all CRAAT− and PBS control groups exhibited drastically low levels. (D) IL-13 was substantially high in all CRAAT−. However, CRAAT+ exhibited very low levels of BALF IL-13 that was comparable to the PBS controls. (E) INF-γ levels were significantly high in the CRAAT+, which paralleled the PBS controls. However, CRAAT− showed substantially high levels of INF-γ. ψP < 0.05, *P < 0.01, **P < 0.001.
Cell Surface Expression of Donor Cells Before Adoptive Transfer
The L25+ donor cells were ICOS−, CD62L+, GITR+, PD-1−, CD69−, and CD25+. L25− donor cells had the same phenotype with the exception of being CD25−. S25+ donor cells expressed low levels of ICOS, CD69−, CD62L−, PD-1−, GITR+, and CD25+. Similarly, S25− donor cells were the same with the exception of being CD25− (data not shown).
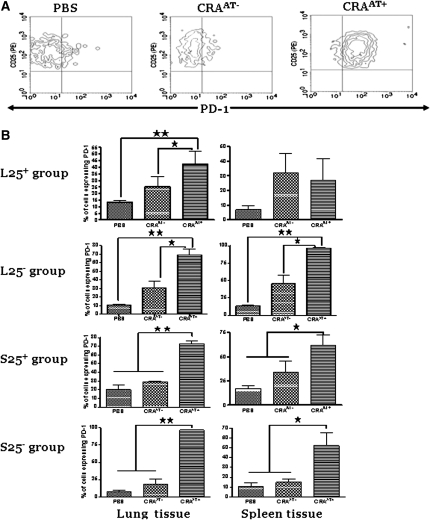
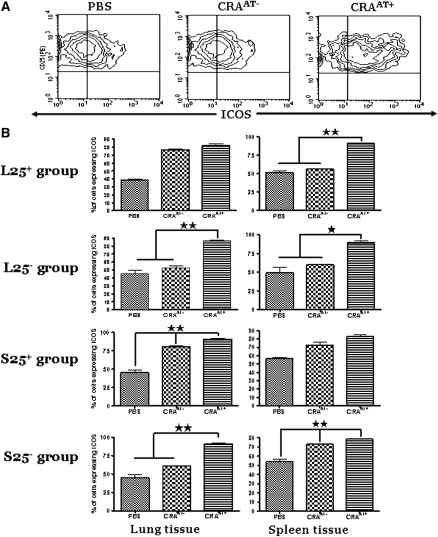
Expression of PD-1 and ICOS in CD4+CD25+ Cells Isolated from Lungs and Spleens of Recipient Mice Post Adoptive Transfer
Lung CD4+CD25+ cells were gated for PD-1 expression to compare between PBS control, CRAAT− group, and the CRAAT+ group. The cells from the recipients of L25− and S25− cells demonstrated a significant increase in both PD-1 or ICOS expression in CRAAT+ group compared with significantly low levels in PBS control and CRAAT− groups (Figures 6A and 7A). The left panels show PD-1 (Figure 6B) or ICOS (Figure 7B) expression in lung CD4+CD25+ cells post adoptive transfer in each experimental group. The lung CD4+CD25+ cells in the CRAAT+ in the recipients of L25− and S25− cells expressed significantly higher levels of PD-1 (Figure 6B) and ICOS (Figure 7B) compared with recipients of L25+ and S25+ cells. Interestingly, lung CD4+CD25+ cells from the recipient mice of S25− cells expressed substantially higher levels of PD-1 than the recipients of S25+ cells (Figure 6B). However, there was no significant difference in the ICOS levels between the S25− and S25+ groups (Figure 7B). The panels on the right show PD-1 (Figure 6B) or ICOS (Figure 7B) expression in spleen CD4+CD25+ cells post adoptive transfer in each experimental group. The expression of PD-1 was increased in all CRAAT+ groups except in the recipients of L25+ cells, compared with PBS control and CRAAT− (Figure 6B). However, the expression of ICOS was increased in all CRAAT+ groups except in the recipients of S25+ cells, compared with PBS control and CRAAT− (Figure 7B).
Figure 6.
The expression of programmed death–1 (PD-1) in lung and spleen CD4+CD25+ T cells post adoptive transfer. (A) Contour plots that are representative of the cells in the lung inducible CD4+CD25− T-regulatory cells (L25−) and spleen inducible CD4+CD25− T-regulatory cells (S25−) groups. PD-1 was robustly expressed in all cockroach-sensitized and -challenged mice with adoptive transfer (CRAAT+) compared with cockroach-sensitized and -challenged mice without adoptive transfer (CRAAT−) and phosphate-buffered saline (PBS) controls. (B) Left panels are lung CD4+CD25+ cells of all experiment groups. PD-1 is expressed in all the CRAAT+ groups compared with CRAAT− and PBS control groups. However, PD-1 was expressed at significantly high levels in the L25− and S25− groups. Right panels are spleen tissue of the same animals that exhibited high expression of PD-1 in all of the CRAAT+ groups compared with CRAAT− and PBS control groups with exception of L25. *P < 0.01, **P < 0.001.
Figure 7.
The expression of inducible costimulatory molecule (ICOS) in lung and spleen CD4+CD25+ T cells post adoptive transfer. (A) Contour plots that are representative of the cells in the lung inducible CD4+CD25− T-regulatory cells (L25−) and spleen inducible CD4+CD25− T-regulatory cells (S25−) groups. ICOS was expressed at substantially high level in all cockroach-sensitized and -challenged mice with adoptive transfer (CRAAT+) groups compared with cockroach-sensitized and -challenged mice without adoptive transfer (CRAAT−) and phosphate-buffered saline (PBS) control. (B) (Left panels) CD4+CD25+ T cells from lung tissue of all experiment groups. ICOS is significantly upregulated in all CRAAT+ groups compared with CRAAT− and PBS control groups with the exception of lung naturally occurring CD4+CD25− T-regulatory cells (L25+) group. The right panels are from spleen tissue of the same animals that showed high expression of ICOS in L25+ and L25− of CRAAT+ compared with low levels in CRAAT− and PBS control. *P < 0.01, **P < 0.001.
Expression of PD-1, ICOS, and Foxp3 in Lung CD4+CD25− Cells from Recipients of L25− and S25− Cells Post Adoptive Transfer
The expression of PD-1, ICOS, and Foxp3 is shown in a representative contour plot for each set of cells in the L25− and S25− groups (Figures E1A–E1C). CD4+CD25− cells in the PBS control were PD-1low compared with PD-1int cells in the CRAAT− and CRAAT+ groups (Figure E1A). In contrast, ICOS expression of CD4+CD25− in the PBS control and CRAAT+ groups were ICOSlow compared with ICOSint in the CRAAT− group (Figure E1B). In addition, the lung CD4+CD25− T cells in the PBS control, CRAAT−, and CRAAT+ groups showed minimal or no expression of intracellular Foxp3 protein (Figure E1C). Statistical bar graphs show the expression of ICOS and PD-1 on CD4+CD25− T cells isolated from the lungs of adoptively transferred mice (Figure E1D).
Foxp3 mRNA Transcripts in Donor Cells Before Adoptive Transfer
L25+ and S25+ cells expressed high levels of Foxp3 compared with low or undetectable levels in L25− and S25− (Figure E2A).
Foxp3 mRNA Transcripts in CD4+CD25+ Cells Isolated from Lungs of Recipient Mice Post Adoptive Transfer
The lung CD4+CD25+ cells in the PBS control of all groups exhibited Foxp3 expression at moderate to high levels, which supports the constitutive expression of Foxp3 in NTregs (Figure E2B). In contrast, the lung CD4+CD25+ cells in the CRAAT− in each group exhibited Foxp3 expression at low to undetectable levels (Figure E2B). However, the lung CD4+CD25+ cells in the CRAAT+ of all groups displayed Foxp3 expression at the PBS control level (Figure E2B). Densitometric analyses confirmed the PCR data (Figures E2A and E2B).
mRNA Transcripts of Neuropilin-1 in Tregs
The lung CD4+CD25+ T cells of each group of CRAAT− and PBS control showed significantly low levels of neuropilin-1 (Nrp-1) mRNA transcripts compared with significantly increased levels in the CRAAT+ groups with the exception of recipients of L25+ cells that exhibited no significant difference between CRAAT− and CRAAT+. However, the lung CD4+CD25+ T cells of CRAAT+ in the S25− group exhibited a higher level of Nrp-1 expression than S25+ group (Figure E3). Densitometric analysis confirmed the PCR findings.
mRNA Transcripts of GATA3 and T-bet Post Adoptive Transfer
We examined mRNA transcripts of GATA3 in CD4+CD25+ cells purified from lungs of mice post adoptive transfer. The lung CD4+CD25+ cells of CRAAT− in each experimental group expressed GATA3 at significantly high levels compared with very low to undetectable levels of GATA3 in the lung CD4+CD25+ cells of CRAAT+ and PBS control groups (Figure E4A).
We also examined mRNA transcripts of T-bet in the same cells used for GATA3 expression. The lung CD4+CD25+ cells of PBS control and CRAAT− groups that were adoptively transferred with L25+ cells exhibited undetectable levels of T-bet. However, lung CD4+CD25+ cells of CRAAT+ group showed moderate expression of T-bet (Figure E4B). The lung CD4+CD25+ cells of PBS control group adoptively transferred with L25− cells exhibited undetectable levels and cells of CRAAT− group adoptively transferred with L25− cells displayed a significant increase compared with the PBS control. Moreover, lung CD4+CD25+ cells of CRAAT+ showed a substantial increase compared with the CRAAT−. The CD4+CD25+ cells from the lungs of mice post-adoptively transferred with S25+ cells exhibited the same pattern, but with lower intensity of the bands, as with the groups post-adoptively transferred with L25− cells (Figure E4B). The lung CD4+CD25+ cells of S25−, PBS control, and CRAAT− exhibited undetectable levels of T-bet. But, lung CD4+CD25+ cells of CRAAT+ group showed a significant increase of T-bet expression compared with PBS control and CRAAT− groups (Figure E4B). Densitometric analyses confirmed the PCR data.
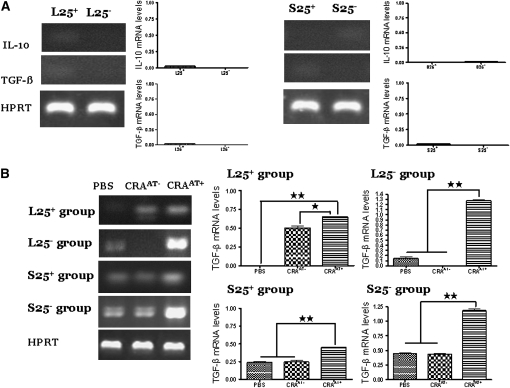
mRNA Expression of IL-10 and TGF-β in Donor Cells
In the S25+ and L25+ cells as well as in S25− and S25− cells isolated from the spleen or lung donor mice, we found very low to undetectable levels in each cell type (Figure 8A). Densitometric analyses confirmed the PCR data.
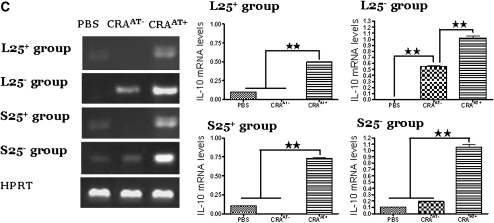
Figure 8.
Expression of TGF-β (400 bp) and IL-10 (610 bp) mRNA transcripts in lung CD4+CD25+ donor cells and in lung CD4+CD25+ cells post adoptive transfer. (A) Donor cells: The cells in the lung naturally occurring CD4+CD25− T-regulatory cells (L25+) and lung inducible CD4+CD25− T-regulatory cells (L25−) groups expressed low to undetectable levels of IL-10 and TGF-β mRNA transcripts. The cells in the spleen inducible CD4+CD25− T-regulatory cells (S25−) group expressed low levels of IL-10 and undetectable levels of TGF-β. The cells in the spleen naturally occurring CD4+CD25− T-regulatory cells (S25+) group expressed low levels of TGF-β and undetectable levels of IL-10. (B) Expression of TGF-β mRNA transcripts in post adoptive CD4+CD25+ T cells: The cells of cockroach-sensitized and -challenged mice with adoptive transfer (CRAAT+) in the L25+ group exhibited higher expression of TGF-β mRNA transcripts compared with cockroach-sensitized and -challenged mice without adoptive transfer (CRAAT−) and phosphate-buffered saline (PBS) control. The cells of CRAAT+ in the L25− group expressed substantially high levels of TGF-β mRNA transcripts compared with undetectable levels in the CRAAT− group and low levels in PBS control. The cells of CRAAT+ in the S25+ and S25− groups showed high expression of TGF-β mRNA transcripts compared with low levels in the CRAAT− group and PBS control. (C) IL-10 mRNA transcripts in post adoptive cells: Cells of the CRAAT+ in the L25+ and S25+ groups showed a significant increase in IL-10 mRNA transcripts compared with undetectable level in the CRAAT− and low levels in PBS control groups. Cells of the CRAAT+ in the L25− group exhibited considerable increase in IL-10 mRNA transcripts compared with undetectable levels in PBS control and moderate levels in CRAAT− group. Cells of the S25− group the CRAAT+ exhibited a substantially high level of IL-10 mRNA transcripts compared with low levels in PBS control and CRAAT− group. Densitometric analyses confirmed the PCR data. *P < 0.01; ** P < 0.001.
TGF-β and IL-10 mRNA Expression in Lung CD4+CD25+ Cells Post Adoptive Transfer
In the L25+ group, cells in the CRAAT+ had higher mRNA expression of TGF-β and IL-10 compared with CRAAT− and PBS control. In the L25− group, cells in the PBS control exhibited low or undetectable levels of either TGF-β or IL-10 and this was undetectable for TGF-β and showed moderate levels for IL-10 in CRAAT−. However, there was a significant increase in TGF-β and IL-10 mRNA transcripts in the CRAAT+ group. In the S25+ group the lung CD4+CD25+ cells showed an increase of both TGF-β and IL-10 mRNA expression in the CRAAT+ group compared with CRAAT− group and PBS control. Interestingly, the cells in the CRAAT+ of the S25− group displayed a substantial increase in both TGF-β and IL-10 mRNA transcripts compared with low to moderate levels expressed in the CRAAT− (Figures 8B and 8C). Densitometric analyses confirmed the PCR data.
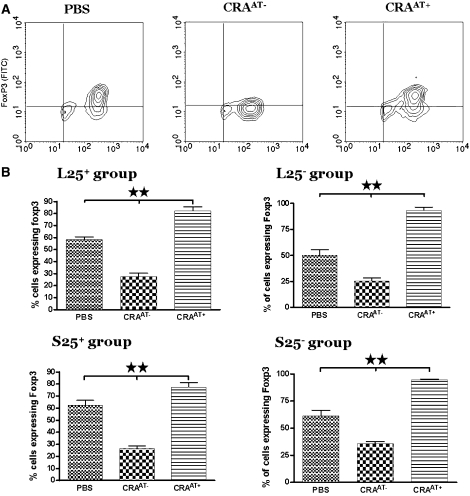
Flow Cytometric Analysis of Foxp3 Expression in CD4+CD25+ T Cells Post Adoptive Transfer
The representative data of intracellular Foxp3 expression in the lung CD4+CD25+ T cells of all experimental groups are shown in the contour plots (Figure 9A). The cells demonstrated positive intracellular expression of Foxp3 in the PBS control. However, the intracellular Foxp3 expression was significantly decreased in the lung CD4+CD25+ T cells of CRAAT− group. The adoptive transfer of the cells in the CRAAT+ group normalized the intracellular Foxp3 expression to the PBS control in all four experimental groups (Figure 9B).
Figure 9.
Intracellular protein expression of Foxp3 in lung CD4+CD25+ cells post adoptive transfer. (A) Contour plots of CD4+CD25+ T cells that are representative of all four groups. Foxp3 expression was significantly expressed in the phosphate-buffered saline (PBS) control compared with a substantial decrease in the cockroach-sensitized and -challenged mice without adoptive transfer, and adoptive transfer of the cells restored Foxp3 expression in the cockroach-sensitized and -challenged mice with adoptive transfer groups. (B) Data in the graphs exhibit statistically significant differences in Foxp3 expression within each group. **P < 0.001.
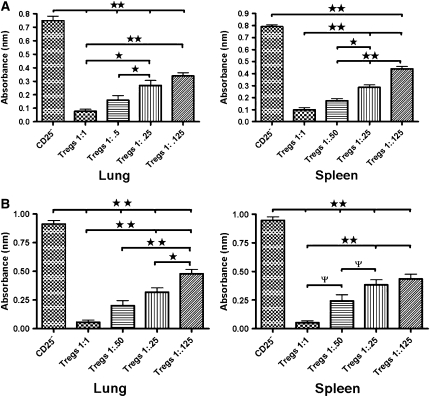
Suppressive Effect of Tregs on the Proliferation of Naive Splenocytes
The 1:1 ratio of adoptively transferred GFP-labeled Tregs or Tregs from naive balb/c mice isolated from lung or spleen tissue substantially suppressed the proliferation of naive splenocytes. The magnitude of the suppression of splenocyte proliferation by lung or spleen GFP-labeled or naive Tregs was dependent on the number of cells but significant even at 1:0.125 ratio (Figures 10A and 10B).
Figure 10.
Suppression of naive splenocytes by lung and spleen green fluorescent protein (GFP)-labeled and naive T-regulatory cells (Tregs). (A) Dose-dependent decrease of CD4+CD25− T-cell proliferation in the presence of decreasing doses of GFP-labeled Tregs with the addition of 20 μL bromodeoxyuridine and incubated for an additional 12 hours at 37°C. (B) The same effect was seen in the presence of decreasing doses of Tregs from naive mice. (ψP < 0.05, *P < 0.01, **P < 0.001)
DISCUSSION
Allergic asthma is characterized by airway hyperresponsiveness and mucosal inflammation mediated by CD4+ Th2 cells. Thus, the suppression of Th2 cytokines (IL-4, IL-5, IL-9, and IL-13) has been shown to be plausible therapy to suppress airway inflammation and hyperresponsiveness (28). In this regard, Tregs have been shown to be potent immunomodulators of a disrupted immune response as seen in asthma. In this study we found that adoptive transfer of GFP-labeled NTregs and iTregs isolated from the lung or spleen of naive GFP-transgenic mice migrated to the lymph nodes and to the lungs, and reversed AHR for at least 4 weeks in CRA-sensitized and -challenged mice. Moreover, we found that GFP-labeled CD4+CD25− Tregs differentiated into CD4+CD25+FoxP3+ Tregs in the lungs of recipients that received adoptive transfer of L25− and S25− cells. The functional difference and suppressive capacity of these TR1 cells compared with NTregs appears to be mediated by the differential expression of PD-1, TGF-β, IL-10, and secretion of IFN-γ.
Several investigators reported that naturally occurring CD4+CD25+ Tregs are anergic or have nominal to absent proliferatory capacity in vitro (29–33). However, we found that NTregs are able to proliferate in the periphery in response to repeated antigen exposure in vivo. This could be supported by the findings of Walker and colleagues, that Tregs are able to divide in the peripheral and suppress effector T cells (34). Moreover, literature has suggested that TR1 cells proliferate poorly with T cell receptor (TCR) stimulation (35), and recent findings suggest that cytokines, such as IL-10 and IL-15, are critical for stimulating their proliferation in vitro (36). However, TR1 cells suppress immune responses in vitro and in vivo and this mechanism is partially dependent on the production of IL-10 and TGF-β (37). Moreover, little is known about the proliferatory capacity of TR1 cells in vivo. In this study, we for the first time report that iTregs differentiate into TR1 cells in vivo under repeated exposure to antigen. These TR1 cells expressed Foxp3 and high levels of mRNA transcripts of IL-10 and TGF-β, and had robust proliferative capacity. Our data also suggest that the S25+ and S25− cells have less proliferative capacity in vivo compared with L25+ or L25− cells, but spleen Tregs have significant capacity to suppress effector T cells under in vivo conditions. Both Treg types from lung and spleen tissue were substantially suppressive even at the one-eighth ratio of the splenocytes and Tregs.
We also found that the GFP-labeled NTregs and iTregs isolated from the lungs of recipients that received GFP-labeled S25+ and S25− cells expressed significantly higher levels of mRNA transcripts of Nrp-1 than GFP-labeled NTregs and iTregs isolated from the lungs of recipients that received GFP-labeled L25+ and L25− cells. These data suggest that the expression of Nrp-1 on these splenic Tregs may serve as a marker and/or a functional mechanism to distinguish this subset of Tregs from lung-derived Tregs. It has been shown that the expression of Nrp-1 on activated Tregs may serve as a unique cell surface marker to define Tregs from other activated T cells (38). Also, the expression of Nrp-1 promotes prolonged cell-to-cell interactions with Tregs and immature dendritic cells than CD4+ T-lymphocytes, resulting in high sensitivity to limited amounts of antigen (39). CD4+CD25+Nrp-1+ Tregs were also found to be highly suppressive in the lymph nodes of patients with cervical cancer (40). The function and characteristics of Tregs may be dependent on anatomical location and the extracellular microenvironment (40). These reports may explain why Tregs from lung tissue proliferate more than spleen-derived Tregs. In addition, our data suggest that Tregs may have inherent tissue-specific capacity to migrate back to the tissue of origin after adoptive transfer. We found that a high percentage of lung adoptively transferred NTregs and iTregs migrated back to the lung and specifically less than 5,000 of the lung iTregs migrated to the spleen (Figure E5A–E5H). Conversely, a high percentage of the adoptively transferred spleen NTregs and iTregs migrated to the lung, but more than 20,000 of these cells were isolated from the spleen of recipient mice. Unraveling these different subsets of Tregs and their function, characteristics, and movement of these cells warrants further investigation, but it elucidates that these cells have high proliferatory capacity in vivo and migrate to sites of inflammation. In addition, we found 34,000 to 42,000 GFP-labeled Tregs migrated to the lymph nodes of adoptively transferred recipients; however, in the lymph nodes of CRA-sensitized and -challenged mice we found 47,000 to 60,000 CD4+CD25+ T cells (Figure E5I). Moreover, these CD4+CD25+ T cells were not Foxp3+ cells, which suggests these cells were probably Th2 and/or Th1 cells (data not shown).
The histological changes in the lung and airway inflammation paralleled the changes in the AHR to methacholine. The CRA-sensitized and -challenged mice exhibited the hallmarks of asthma, including profuse peribronchial and perivascular inflammation, epithelial cell damage resulting from massive inflammatory cell infiltration, goblet cell hyperplasia with mucous hypersecretion, collagen deposition, and smooth muscle cell hyperplasia. The CRAAT+ animals exhibited a reversal of the changes in the histological features with minimal inflammation. Studies have shown that the epithelial layer of the airway can repair and rebuild after asthmatic episodes; however, the time period and how this mechanism occurs remains to be elucidated. The airway remodeling in the CRAAT− resulted from the elevated levels of eosinophils and minimal levels of neutrophils as seen in the BALF. These associated changes in the asthmatic airways appear to be the result of a reduction in the number and/or impaired function of Tregs in the lung of these CRAAT− mice. In normal mice we isolated 40,000 to 43,000 Tregs; however, CRA sensitization resulted in a significant reduction of fewer than 20,000 Tregs in the lung at Day 33. Additionally, at Day 68 there were fewer than 10,000 Tregs in the lungs of CRAAT− mice (Figure E6). Conversely, the adoptive transfer of 100,000 Tregs ameliorated the clinical changes seen in the airways of these allergic asthma-induced mice (Figure E6). The BALF from CRAAT+ mice showed minimal to absence of eosinophils, which was consistent with the histology of normal bronchial parenchyma. The therapeutic effect of the adoptive transfer of Tregs helped to alleviate the characteristics of airway inflammation and AHR, and the morphologic changes were nearly restored to the PBS control level. It is, however, unclear whether the onset of allergic airway inflammation from CRA sensitization impairs the function of Tregs or if this impairment results in a reduction in the number of Tregs in the lung; in all of our sensitized mice, airway inflammation and AHR developed when the number of Tregs was reduced in the airways. Moreover, an adoptive transfer of 100,000 Tregs was very effective in reversing AHR and airway inflammation (Figures E7A and E7B).
Tregs are characterized by a canon of the cell surface expression markers to include: CD4, CD25, CD69, CD62L, ICOS, and GITR. The GFP-labeled iTregs, which upregulated CD25 and Foxp3 isolated from lungs of recipient mice that received S25− and L25− cells, expressed high levels of PD-1. Interestingly, the lung CD4+CD25+ cells obtained from the recipients of S25− group showed nearly 100% PD-1 immunopositive cells compared with about 75% in the S25+ recipient group (Figure 6B). We do not know the number of PD-1–positive cells required for long-lasting reversal of AHR. However, it is tempting to speculate that the differential expression of PD-1 could be one of the reasons underlying the functional differences between these Treg subsets. Therefore, PD-1 could be a strong candidate for characterization of inducible naive T-regulatory cells and critically involved in their suppressive function.
The PD-1 (CD279) receptor is 55-kD type I transmembrane protein of the Ig superfamily, with an extracellular region having one V-like domain. Recently, two new members of the B7 family, B7-H1 (PD-L1) (CD274) and B7-DC (PD-L2) (CD273), were identified as ligands for PD-1. PD-1–PD-L1 belongs to the CD28-B7 signaling family and this interaction leads to downregulation of T cell activity (41). In the studies by Latchman and colleagues the interaction of PD-L1 and PD-1 leads to cell cycle arrest in G0/G1 but does not increase cell death (42). Studies in C57BL/6 mice have shown that mice lacking PD-1 developed a lupus-like arthritis and glomerulonephritis (41–43). In this study, we found that PD-1 is moderately upregulated after antigen exposure in Tregs isolated from lung tissue from CRA-sensitized and -challenged mice without adoptive transfer. In contrast, PD-1 was expressed at significantly higher levels in CD4+CD25+ Tregs isolated from recipient mice of L25− and S25− cells than recipient mice of L25+ and S25+ cells. These data suggest that PD-1 could be a critical mechanism involved in reversal of AHR and airway inflammation by inducible naive T-regulatory cells.
Therefore, in a separate study we examined the role of PD-1 in allergic asthma and Treg function with an adoptive transfer of Tregs and administered an anti PD-1 antibody (aPD-1ab) in CRA-sensitized and -challenged mice. We found that the therapeutic effect of the adoptive transfer of Tregs was blocked after the administration of the aPD-1ab. AHR was exacerbated in these CRA-sensitized and CRA-challenged mice with aPD-1ab and the deleterious effect of airway inflammation paralleled the CRA-sensitized mice without adoptive transfer therapy (unpublished data, H.S.M., D.K.A.). These data suggest that PD-1 has a role with Tregs and their ability to suppress AHR and airway inflammation. The mechanism of how PD-1 works clearly needs more investigation but nevertheless PD-1 may work in concert with other costimulatory molecules, such as CTLA-4 and/or ICOS.
ICOS is a marker that is upregulated on activated T cells in the presence of antigen. ICOS-ICOSL pathways are essential for the secretion of IL-10 (44). In this study, CD4+CD25+ cells isolated from the lungs of the recipients of NTregs and iTregs were ICOShigh and this expression was significantly greater than lung CD4+CD25+ T cells from mice without adoptive transfer. In a recent report, ICOShigh CD4+ T cells were tightly linked to IL-10 production; ICOSmed CD4+ T cells secreted IL-4, IL-5, and IL-13; and ICOSlow CD4+ T cells were associated with secretions of IL-2, IL-3, IL-6, and IFN-γ (45). In our study, donor cells expressed low to undetectable levels of IL-10 and TGF-β, but high expression of ICOS on the lung CD4+CD25+ T cells isolated from the adoptive transfer recipients showed a tight correlation with the high secretion of IL-10 that was seen in the BALF and IL-10 mRNA transcripts. Hence, it is highly likely that high expression of ICOS seen on the TR1 cells isolated from the lungs of adoptive transfer recipients and expression of IL-10 mRNA transcripts and BALF IL-10 may act as a switch mechanism for the secretion of IL-10.
Since the renaissance of T-regulatory cells, the mechanisms that govern the function and characterization of Tregs remain elusive. The most widely accepted marker for Tregs is Foxp3. The precise function of Foxp3 is not known, but an absence of Foxp3 in humans results in IPEX syndrome (46, 47). Patients with IPEX syndrome share many phenotypic features with scurfy mice, exhibiting mutation in Foxp3 and lacking Tregs. However, it remains elusive if these patients and scurfy mice also lack the inducible ability of a naive T cell to differentiate into a TR1 cell.
In this study we sought to evaluate if this perplexing T-cell subset expressed Foxp3 for regulating AHR. Naive Tregs were found to constitutively express Foxp3, and undifferentiated CD4+CD25− T cells did not express Foxp3, However, Foxp3 mRNA transcripts and intracellular protein were upregulated, parallel with CD25+, in the lung CD4+CD25+ cells post adoptive transfer. Lung CD4+CD25+ cells from CRA-sensitized and -challenged mice without adoptive transfer expressed Foxp3 at low to undetectable levels and these mice continued to have the elevated AHR and severe airway inflammation. Foxp3 is crucial for the suppressive capacity of Treg activity in allergic asthma and autoimmune diseases. Subjects with myasthenia gravis have normal density of Tregs similar to healthy subjects, but there was a severe functional defect in their regulatory capacity together with decreased expression of Foxp3 (48). In patients with multiple sclerosis CD4+CD25+ Tregs had reduced levels of Foxp3 expression, which was increased after treatment with a copolymer-1 (49). Thus, Foxp3 appears to be crucial for Treg suppression.
In summary, we delineated a direct role that an adoptive transfer of Tregs has the therapeutic property of reversing the deleterious effects of allergic airway inflammation and AHR in a CRA murine model of allergic asthma. Specifically, the inducible Tregs from either spleen or lungs that have high PD-1 completely reversed AHR to methacholine and lung inflammation. However, the role of Tregs secreting IL-10 and TGF-β and expressing PD-1 and Nrp-1 to modulate allergic asthma remains to be elucidated; nevertheless this specialized subset of T cells clearly provides clinical implications to prevent the pathogenesis of asthma.
Supplementary Material
Acknowledgments
The authors thank Dr. Greg Perry, Director of the Flow Cytometry Laboratory at Creighton, for his constant support and guidance in the flow cytometry studies.
Supported by NIH grant R01 HL070885. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or NIH.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200809-1505OC on May 15, 2009
Conflict of Interest Statement: Neither of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hadeiba H, Locksley RM. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J Immunol 2003;170:5502–5510. [DOI] [PubMed] [Google Scholar]
- 2.McGee HS, Agrawal DK. TH2 cells in the pathogenesis of airway remodeling: regulatory T cells a plausible panacea for asthma. Immunol Res 2006;35:219–232. [DOI] [PubMed] [Google Scholar]
- 3.Pueringer RJ, Hunninghake GW. Inflammation and airway reactivity in asthma. Am J Med 1992;92:32S–38S. [DOI] [PubMed] [Google Scholar]
- 4.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol 2005;116:961–968. [Quiz, p. 969]. [DOI] [PubMed] [Google Scholar]
- 5.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 2002;196:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Garra A, Vieira PL, Vieira P, Goldfeld A. E. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest 2004;114:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji NM, Mizumachi K, Kurisaki J. Antigen-specific, CD4+CD25+ regulatory T cell clones induced in Peyer's patches. Int Immunol 2003;15:525–534. [DOI] [PubMed] [Google Scholar]
- 8.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother 2005;28:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 10.Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med 2003;198:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol 2002;169:6210–6217. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 2006;107:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol 2003;171:1216–1223. [DOI] [PubMed] [Google Scholar]
- 14.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock HM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 2000;106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr 1982;100:731–737. [DOI] [PubMed] [Google Scholar]
- 16.Satake N, Nakanishi M, Okano M, Tomizawa K, Ishizaka A, Kojima K, Onodera M, Ariga T, Satake A, Sakiyama Y. A Japanese family of X-linked auto-immune enteropathy with haemolytic anaemia and polyendocrinopathy. Eur J Pediatr 1993;152:313–315. [DOI] [PubMed] [Google Scholar]
- 17.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001;27:18–20. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA 2006;103:13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol 2000;165:4848–4853. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia M, Gianfrani C, Gregori S, and Roncarolo MG. IL-10-producing T regulatory type 1 cells and oral tolerance. Ann N Y Acad Sci 2004;1029:142–153. [DOI] [PubMed] [Google Scholar]
- 21.Hansson T, Dannaeus A, Klareskog L. Cytokine-producing cells in peripheral blood of children with coeliac disease secrete cytokines with a type 1 profile. Clin Exp Immunol 1999;116:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol 1994;12:635–673. [DOI] [PubMed] [Google Scholar]
- 23.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res 2001;3:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncarolo MG, Gregori S, Levings M. Type 1 T regulatory cells and their relationship with CD4+CD25+ T regulatory cells. Novartis Found Symp 2003;252:115–27. [Discussion p. 127–31, 203–10.] [PubMed] [Google Scholar]
- 25.Wildbaum G, Netzer N, Karin N. Tr1 cell-dependent active tolerance blunts the pathogenic effects of determinant spreading. J Clin Invest 2002;110:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med 2004;199:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermaelen K, Pauwels R. Pulmonary dendritic cells. Am J Respir Crit Care Med 2005;172:530–551. [DOI] [PubMed] [Google Scholar]
- 28.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol 2004;172:5016–5023. [DOI] [PubMed] [Google Scholar]
- 29.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest 2005;115:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, Unutmaz D. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol 2004;2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung YJ, Seoh JY. Feedback loop of immune regulation by CD4+CD25+ Treg. Immunobiology 2009;214:291–302. [DOI] [PubMed] [Google Scholar]
- 32.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol 2006;177:944–949. [DOI] [PubMed] [Google Scholar]
- 33.Matarese G, De Rosa V, La Cava A. Regulatory CD4 T cells: sensing the environment. Trends Immunol 2008;29:12–17. [DOI] [PubMed] [Google Scholar]
- 34.Walker LS. CD4+ CD25+ Treg: divide and rule? Immunology 2004;111:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation 2003;108:1232–1237. [DOI] [PubMed] [Google Scholar]
- 36.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 2003;421:388–392. [DOI] [PubMed] [Google Scholar]
- 37.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med 2001;193:F5–F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol 2004;34:623–630. [DOI] [PubMed] [Google Scholar]
- 39.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 2008;28:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglia A, Buzzonetti A, Monego G, Peri L, Ferrandina G, Fanfani F, Scambia G, Fattorossi A. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology 2008;123:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 2001;22:265–268. [DOI] [PubMed] [Google Scholar]
- 42.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–268. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol 2004;172:2530–2541. [DOI] [PubMed] [Google Scholar]
- 44.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 2002;8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 45.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005;23:515–548. [DOI] [PubMed] [Google Scholar]
- 46.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr 2001;13:533–538. [DOI] [PubMed] [Google Scholar]
- 47.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest 2003;112:1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood 2005;105:735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA 2005;102:6449–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.