Abstract
Rationale: In studies that address health-related quality of life (QoL) and survival, subjects who die are usually censored from QoL assessments. This practice tends to inflate the apparent benefits of interventions with a high risk of mortality. Assessing a composite QoL-death outcome is a potential solution to this problem.
Objectives: To determine the effect of lung volume reduction surgery (LVRS) on a composite endpoint consisting of the occurrence of death or a clinically meaningful decline in QoL defined as an increase of at least eight points in the St. George's Respiratory Questionnaire total score from the National Emphysema Treatment Trial.
Methods: In patients with chronic obstructive pulmonary disease and emphysema randomized to receive medical treatment (n = 610) or LVRS (n = 608), we analyzed the survival to the composite endpoint, the hazard functions and constructed prediction models of the slope of QoL decline.
Measurements and Main Results: The time to the composite endpoint was longer in the LVRS group (2 years) than the medical treatment group (1 year) (P < 0.0001). It was even longer in the subsets of patients undergoing LVRS without a high risk for perioperative death and with upper-lobe-predominant emphysema. The hazard for the composite event significantly favored the LVRS group, although it was most significant in patients with predominantly upper-lobe emphysema. The beneficial impact of LVRS on QoL decline was most significant during the 2 years after LVRS.
Conclusions: LVRS has a significant effect on the composite QoL-survival endpoint tested, indicating its meaningful palliative role, particularly in patients with upper-lobe–predominant emphysema.
Keywords: chronic obstructive pulmonary disease, outcome assessment, palliative care, quality of life, survival, emphysema
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Lung volume reduction surgery (LVRS) improves the chances of survival in some patients with advanced emphysema. The effects of LVRS on patients' health-related quality of life adjusted for the survival time has received less attention.
What This Study Adds to the Field
This study demonstrates the beneficial effect of LVRS on a composite endpoint consisting of the occurrence of death or a clinically meaningful decline in quality of life. The benefit was even stronger in LVRS subsets of patients without high risk for perioperative death and with upper-lobe–predominant emphysema. The results indicate that LVRS provides a meaningful palliative effect beyond the survival benefit.
The choice of methods and endpoints to use in evaluating the quality of life (QoL) trajectory requires careful consideration in chronic obstructive pulmonary disease (COPD). Patients who die during the period of observation from QoL assessments are often censored, as if their outcomes were neither good nor bad (1). Censoring may inflate the apparent benefits of treatment interventions that are associated with increased mortality, such as lung volume reduction surgery (LVRS) (1). One possible solution to this problem is to use a composite endpoint that combines mortality and QoL. For our study, we created a composite endpoint that consisted of the occurrence of death or a clinically meaningful decline in QoL. We tested the effect of LVRS on the selected composite endpoint in patients with severe emphysema who participated in the National Emphysema Treatment Trial (NETT) (2, 3) to define a more meaningful representation of the benefits of the procedure on QoL (palliative effect). Some of the results of these studies have been previously reported in the form of an abstract (4).
METHODS
Database
The design and methods of the NETT have been described previously (2, 5). Briefly, the 1,218 patients with severe emphysema who participated in the NETT underwent pulmonary rehabilitation and then were randomized to receive continued medical treatment or LVRS. The 610 patients in the medical treatment group (38% female) and the 608 patients in the LVRS group (42% female) were asked to complete several measures, including St. George's Respiratory Questionnaire (SGRQ), no more than 3 weeks before randomization (baseline) and at multiple points after randomization (6 mo, 12 mo, and once per year up to 5 y). During the follow-up period, the patients' vital status was assessed through regular phone calls and confirmed in the Social Security death index.
Patients were classified according to predefined parameters in terms of their differential risk of mortality. The initial analysis of NETT data with 24 months of follow-up (2) showed two parameters that were relevant for defining a group in which LVRS produced a survival advantage: (1) emphysema distribution by computed tomography predominantly upper-lobe emphysema versus non–upper-lobe predominant emphysema and (2) exercise capacity (high vs. low, measured in watts). High exercise capacity was defined as a maximum workload achieved during an incremental cycle ergometry study of more than 40 W for men and more than 25 W for women at the postrehabilitation baseline. In our analyses, we used these same definitions (2). A later analysis of NETT data showed a modest but significant benefit of LVRS on survival in all patients without high risk for perioperative mortality at Year 5 of follow-up (3). That report included an analysis of the beneficial effects of LVRS on disease-specific QoL measures at specific time points not adjusted for differential mortality between treatment groups (3).
The group defined as “high risk” of perioperative mortality emerged from the recommendation of the Data and Safety Monitoring Board after interim analysis of the data. This group is characterized by the presence of a FEV1 value of no more than 20% of the predicted value and the presence of a homogeneous distribution of emphysema on computed tomography or a carbon monoxide diffusing capacity that was no more than 20% of the predicted value (2, 6).
Endpoints of Interest
Using the same criteria used in NETT (2, 3), we considered a meaningful decline in a patient's total score on the SGRQ as an increase in at least eight points in the SGRQ score. Eight points represents an unquestionable and meaningful deterioration in QoL (twice a previously identified minimal clinically important difference) (7, 8). The SGRQ version used was the American-English language version with 1-year recall that has a range of scores from 0 to 100 (0 = best; 100 = worst), whereby a drop in score represents an improvement and an increase a worsening.
In our analysis, we considered three endpoints: (1) the occurrence of death, (2) the occurrence of a clinically meaningful decline in QoL, and (3) a composite of the two. We defined the time to decline in QoL as the time to the first observance of at least an eight-point increase in SGRQ (worsening QoL). We defined the time to the composite endpoint as the time until the patient experienced a decline or died, whichever occurred first. This composite endpoint was the main outcome of this study. We used this endpoint to retrospectively examine data from patients in the NETT (2, 3) who were randomized to receive continued medical therapy or medical therapy plus LVRS and were followed for up to 5 years. The other two component endpoints were included to compare the independent influence of LVRS on QoL and death. We censored patients at the earlier follow-up time when a differential follow-up existed for the two components of the composite outcome.
All data except death dates were measured at the scheduled interview time points (time of randomization, 6-month follow-up, and yearly follow-up). Therefore, we performed discrete failure time analyses to investigate time from randomization to the QoL endpoints for the following samples of patients: all patients in the study, patients without a high risk of perioperative mortality, and the four previously defined subsets of patients (predominantly upper-lobe emphysema and high exercise capacity, predominantly upper-lobe emphysema and low exercise capacity, non–upper-lobe predominant emphysema and high exercise capacity, and non–upper-lobe predominant emphysema and low exercise capacity), excluding the high-risk subgroup. The P values for the subgroup analyses were adjusted using the Bonferonni multiple comparison procedure.
We used mixed models to study the progression of QoL over time. Knowing the QoL trajectory in individuals who have different clinical characteristics and have undergone LVRS can help healthcare providers and patients weigh the risks and benefits of this type of surgery and make appropriate decisions concerning whether it should be pursued in particular cases.
Statistical Analyses
We used discrete time analysis to describe the failure probability from the time of randomization until the occurrence of an at least eight-point increase in the total SGRQ score or death in each treatment group (the medical treatment group and the LVRS group). To compare the failure probabilities of the two treatment groups, we used the generalized Wilcoxon test.
To study the natural progression of QoL over time, we used mixed hierarchical models. We fit several functional forms, including polynomials and piecewise polynomials, to select a model that best captured the variation over time. To take possible heterogeneity among patients into account, we included random intercepts and random slopes and tested the significance of these effects.
Because the data involved missing information, we conducted a sensitivity analysis using the method of simultaneously modeling progression of QoL over time and time to missing data.
RESULTS
Patient Characteristics
Of the 1,218 patients in the NETT trial, 610 received continued medical therapy, and 608 underwent LVRS. The two treatment groups had similar characteristics at baseline (i.e., after pulmonary rehabilitation but before randomization) (Table 1).
TABLE 1.
SOCIODEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PATIENTS AT BASELINE (RANDOMIZATION)
| Medical Treatment Group (n = 610)
|
LVRS Group (n = 608)
|
|||
|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD |
| Age, years | 67.2 | 5.9 | 67.0 | 6.3 |
| FEV1, % of predicted value | 26.7 | 7.0 | 26.8 | 7.4 |
| Residual volume, % of predicted value | 223.3 | 48.9 | 220.5 | 49.9 |
| DlCO, % of predicted value | 28.4 | 9.7 | 28.3 | 9.7 |
| Maximum workload, watts | 39.4 | 22.2 | 38.7 | 21.1 |
| Distance walked in 6 min, meters | 368.8 | 96.3 | 367.9 | 94.6 |
| St. George's Respiratory Questionnaire (total score) | 53.6 | 12.7 | 52.5 | 12.6 |
| Quality of Well-Being Questionnaire (total score) | 0.53 | 0.11 | 0.55 | 0.12 |
| UCSD Shortness of Breath Questionnaire (total score) | 63.4 | 18.5 | 61.6 | 18.1 |
Definition of abbreviations: LVRS = lung volume reduction surgery; UCSD = University of California at San Diego.
Endpoints
In the total sample of patients, the median time to the composite event was significantly shorter in the medical treatment group (1 y) than in the LVRS group (2 y) (P < 0.0001) (Table 2). In three of the subsets of patients, based on emphysema distribution and exercise capacity, the difference between the medical treatment group and the LVRS group was also highly significant (Table 2).
TABLE 2.
DISCRETE SURVIVAL TIMES (IN MONTHS) TO THE COMPOSITE EVENT (THE OCCURRENCE OF DEATH OR AN AT LEAST EIGHT-POINT INCREASE IN THE TOTAL SCORE ON ST. GEORGE'S RESPIRATORY QUESTIONNAIRE)
| Patient Group | n | Median Time to the Composite Endpoint (mo) | P Value |
|---|---|---|---|
| All randomized patients | <0.0001 | ||
| Medical treatment | 610 | 12 | |
| LVRS | 608 | 24 | |
| All patients without “high risk” for perioperative mortality | <0.0001 | ||
| Medical treatment | 540 | 12 | |
| LVRS | 538 | 24 | |
| Upper lobe predominant, high exercise capacity | <0.0001 | ||
| Medical treatment | 213 | 12 | |
| LVRS | 206 | 24 | |
| Upper lobe predominant, low exercise capacity | <0.0001 | ||
| Medical treatment | 151 | 12 | |
| LVRS | 139 | 24 | |
| Non–upper lobe predominant, high exercise capacity | 0.35 | ||
| Medical treatment | 111 | 12 | |
| LVRS | 109 | 24 | |
| Non–upper lobe predominant, low exercise capacity | 0.13 | ||
| Medical Treatment | 65 | 12 | |
| LVRS | 84 | 12 |
Definition of abbreviation: LVRS = lung volume reduction surgery.
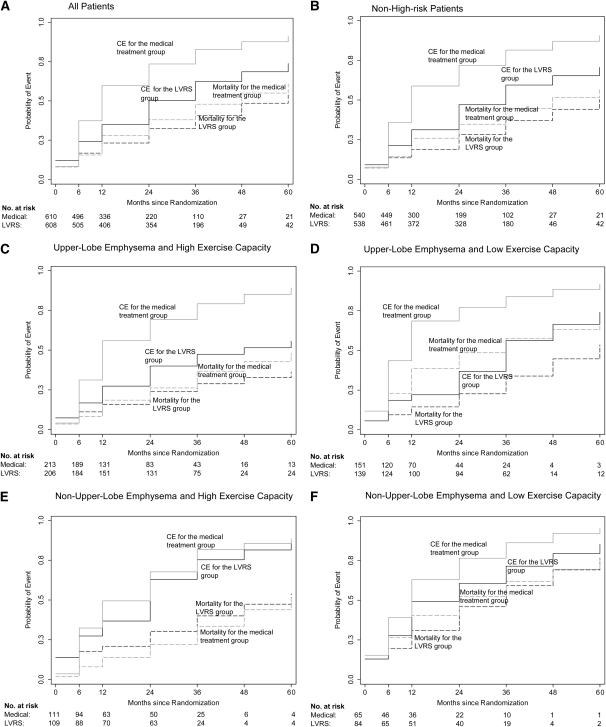
Figure 1 shows the discrete failure functions for the composite endpoint (solid lines) and for mortality alone (dotted lines) in the total sample and in all subsets of the sample. These curves indicate that patients receiving LVRS, particularly those with upper-lobe–predominant emphysema, had combined QoL and survival benefits that exceeded the survival benefits alone, suggesting a slowing of the rate of deterioration.
Figure 1.
Probability of reaching the composite event (CE) or mortality for the lung volume reduction surgery (LVRS) or medical treatment groups. Failure functions, shown as the proportion of patients developing the event over time, in the following samples of patients: (A) All patients in the study. (B) Patients without high risk of perioperative mortality. (C) Patients without high risk for perioperative mortality, upper-lobe–predominant emphysema, and high exercise capacity. (D) Patients without high risk for perioperative mortality, upper-lobe–predominant emphysema, and low exercise capacity. (E) Patients without high risk for perioperative mortality, non–upper-lobe predominant emphysema, and high exercise capacity. (F) Patients without high risk for perioperative mortality, non–upper-lobe predominant emphysema, and low exercise capacity. Solid lines relate to the composite event, which is the occurrence of death or the occurrence of at least an eight-point increase in the total score on St. George's Respiratory Questionnaire, whichever happened first. Gray solid lines represent the medical treatment group, and black solid lines represent the LVRS group. Dotted lines represent the previously reported results of survival analysis for the same sets of patients (n = 9) and are included for comparison. The P value in the upper left corner of each panel refers to the value found when Wilcoxon tests were used to compare the failure functions for the composite event in the medical treatment group with those in the LVRS group. The number at risk refers to the number at risk for the composite event. The failure functions are described for death alone and for the composite event.
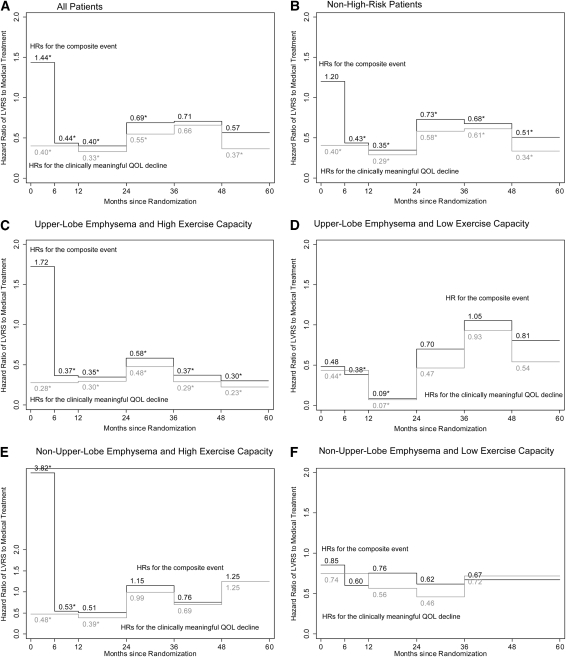
Hazard Ratios
The hazard of reaching each endpoint was determined for the medical treatment group and the LVRS group. Figure 2 shows the results of the hazard ratios (HRs) in the total sample and in all subsets of the sample. If the HR of LVRS to medical treatment was less than 1, it favored the LVRS group. If it was greater than 1, it favored the medical treatment group. The asterisks on the different periods indicate statistical significance (P < 0.05).
Figure 2.
Hazard ratio (HR) of lung volume reduction surgery (LVRS) to medical treatment for decline of at least a nine-point increase in the total St. George's Respiratory Questionnaire (SGRQ) score or composite event. Discrete hazard ratios are shown for the following samples of patients: (A) All patients in the study. (B) Patients without a high risk of perioperative mortality. (C) Patients without high risk for perioperative mortality, and with upper-lobe–predominant emphysema, and high exercise capacity. (D) Patients without high risk for perioperative mortality, upper-lobe–predominant emphysema, and low exercise capacity. (E) Patients without high risk for perioperative mortality, non–upper-lobe predominant emphysema, and high exercise capacity. (F) Patients without a high risk for perioperative mortality, non–upper-lobe predominant emphysema, and low exercise capacity. Black lines indicate HRs for the composite event, which is the occurrence of death or the occurrence of an at least eight-point or higher increase in the total score on the SGRQ, whichever happened first. Gray lines indicate HRs for an at least eight-point increase in the total SGRQ score only. An HR of greater than one favors the medical treatment group for the specific endpoint, and an HR of less than one favors the LVRS group for the specific endpoint. *Statistical significance (P < 0.05).
In each case, two endpoints were modeled separately: time to a decline in QoL (gray line) and time to the composite event (black line). For time to the composite event, the HR favored LVRS in the non–high risk group. The first 6 months favored the medical treatment group, most likely driven by the perioperative mortality. The effect of LVRS was significant in the upper-lobe–predominant emphysema groups but was not significant in the non–upper-lobe predominant emphysema groups. There was a nonsignificant increase in the HR in the fourth and fifth year in the upper-lobe–predominant emphysema low-exercise group that is most likely related to the very low number of subjects in that population at the time of follow-up.
For time to a decline in QoL alone, the HR favored the LVRS group during all 5 years of follow-up, although the effect began to diminish 2 years after randomization.
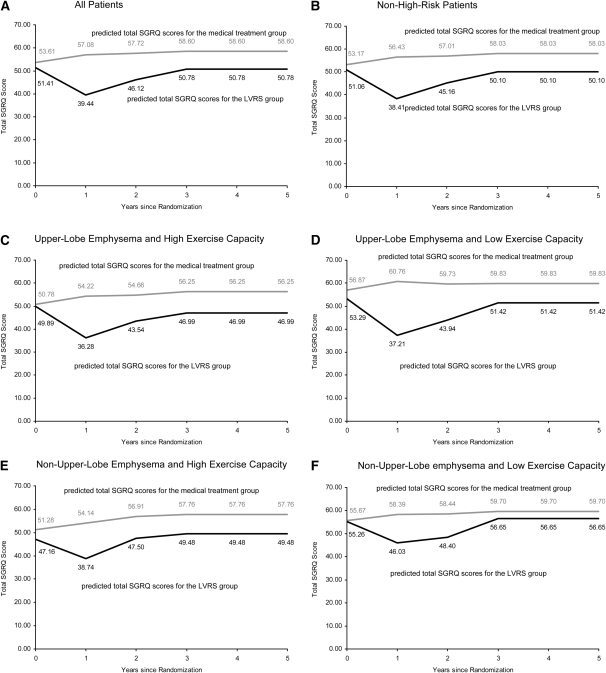
Observed and Predicted Changes in Quality of Life over Time
For the total sample and all subsets of the sample, the observed SGRQ scores are shown in Table E1A of the online supplement, the scores predicted by mixed models with random intercepts and random slopes are shown in Table E1B, and the scores predicted by mixed models with random intercepts are shown in Table E1C. The ML-based likelihood ratio tests revealed that the mixed models with random intercepts and random slopes were the most appropriate, so our main analyses were based on them.
The medical treatment group demonstrated an increase of five to six points over time in the observed and predicted SGRQ scores over the initial 4 years after randomization (a change of four points was the minimal clinically important difference for the SGRQ instrument) (8). In the LVRS group, the observed and predicted scores showed a significant decrease in the initial year after surgery, consistent with an improvement of QoL. The trend thereafter was in the direction of an increase toward the baseline value. However, only in the two non–upper-lobe predominant emphysema subsets (the high-exercise-capacity and low-exercise-capacity groups) did the final score achieve a worse QoL than at baseline.
Figure 3 shows the predicted changes in total SGRQ scores, based on the mixed models with random intercepts and random slopes. For the medical treatment group (black line) and the LVRS group (gray line), testing showed that the slope was constant after year 3 in the total sample and all subsets. Therefore, the final models were linear for each yearly follow-up interval (piecewise).
Figure 3.
Predicted progression of total scores measured in St. George's Respiratory Questionnaire (SGRQ) for the following samples of patients: (A) All patients in the study. (B) Patients without a high risk of perioperative mortality. (C) Patients without high risk for perioperative mortality, and with upper-lobe–predominant emphysema and high exercise capacity. (D) Patients without a high risk for perioperative mortality, upper-lobe–predominant emphysema, and low exercise capacity. (E) Patients without a high risk for perioperative mortality, non–upper-lobe predominant emphysema, and high exercise capacity. (F) Patients without a high risk for perioperative mortality, non–upper-lobe predominant emphysema, and low exercise capacity. Black lines indicate predicted total SGRQ scores for the lung volume reduction surgery (LVRS) treatment group. Gray lines indicate predicted total SGRQ scores for the medical group.
For the first 2 years, the slopes were significantly steeper in the LVRS group than in the medical treatment group (P < 0.01) (Figure 3). For the LVRS group, this reflected a significant improvement in QoL in the first year and then a trend toward the baseline in the second year. After the third year, there was no significant difference between the slopes of the two groups (P = 0.10).
Although the SGRQ includes data on emotional factors and activity, it does not take age and gender into account. When we tested whether age and gender were independent predictors of QoL, we found that neither of these factors was a significant predictor of improvement in the LVRS group during the first year, the period that accounted for most of the palliative effect of LVRS.
To investigate the effect of missing data, we compared the results from two approaches: (1) the mixed hierarchical models and the simultaneous models of QoL progression and (2) time to missing data. The point estimates and the inference tests of the QoL progression were similar using these two approaches. Therefore, the modeling results of QoL progression indicated above were not sensitive to the missing information.
DISCUSSION
Our study demonstrated the beneficial impact of LVRS on a composite outcome that integrates QoL and survival data of patients who had severe emphysema and participated in the NETT. Although the NETT has previously demonstrated (2, 3) that LVRS offers a survival advantage to patients, we have extended these findings by observing that LVRS further offers a palliative effect in these patients by effecting a significant and clinically meaningful improvement in QoL trajectory and that this improvement is most profound in the initial year after LVRS.
We further extended previous QoL analyses of NETT subjects by creating a composite endpoint that accounts for death events, thus accounting for the benefits and risks of LVRS. In our assessment, we used an at least eight-point increase in the total SGRQ score as an indication of a clinically meaningful decline in QOL. The eight-point unit change selected is arbitrary (albeit conservative) and not a product of a formal statistical methodology. Although the four-point threshold has been accepted as a minimal clinically important difference (8), we chose the more robust eight-point threshold because of the nonblinded nature of the NETT trial and the unquestionable significance of an eight-point difference when testing a highly invasive intervention. The original NETT publications also supported the eight-point threshold for its analyses.
The failure probability curves in Figure 1 document large differences between the LVRS group and the medical treatment group in the composite occurrence of death or a clinically meaningful decline in QoL. The beneficial effects of LVRS become more apparent and more pronounced with the composite endpoint analysis compared with an analysis using solely mortality as the endpoint. The benefit for the composite endpoint in the subset of patients with upper-lobe–predominant emphysema and high exercise tolerance is most noteworthy in that a beneficial effect in this group could not be identified using a mortality analysis alone (3).
The results of the HRs for the composite outcome and for the decline of at least eight points in the SGRQ (Figure 2) conclusively demonstrate the palliative effect of LVRS. These analyses show that LVRS tends to significantly decrease the risk of developing a profound decline in QoL (HR < 1) in the total sample of patients with severe emphysema and in all subsets except those who have non–upper-lobe predominant emphysema and a high risk for perioperative mortality.
We believe that our analysis represents the longest trajectory analysis of QoL in patients with severe emphysema with or without LVRS. Other reports have documented QoL in severe emphysema from different series, including NETT, but at specific time points, without modeling QoL progression over time (9–11). The documentation of the progression of QoL in the well-screened and well-characterized NETT patient population should prove particularly helpful in understanding health status progression due to severe emphysema because this population is less confounded by the presence of comorbidities that could dominate any assessment of QOL.
Our study showed that mean SGRQ scores (observed and predicted) were stable in the medical group during the first 4 years and improved in the fifth year (Table E1 of the online supplement). That response could be explained by the survival bias effect and has been previously described with respect to lung function (12) and QoL (13) in cohorts of patients with severe COPD. Our results differ from those of the Inhaled Steroids in Obstructive Lung Disease in Europe (ISOLDE) study, which reported a decline of 3.2 points per year (14). The ISOLDE cohort was significantly less compromised than the NETT cohort, and thus the difference in response was probably influenced less by survival bias effect. In addition, our study showed that the QoL trajectory in the medical group differed significantly from that in the LVRS group (Figure 3). The LVRS group demonstrated a meaningful improvement in QoL (more than eight points) in the first year after surgery. Although this was followed by a trend toward the baseline, the trajectory for the LVRS group remained clinically and statistically different than that of the medical treatment group in the second year and throughout the 5-year period. The authors of an earlier study of patients with moderate to severe COPD found that a four-point increase was associated with a 12.9% increase in COPD-related mortality at 3 years of follow-up (13). Although the slopes of decline for the LVRS and medical treatment groups differed during the first 2 years, the slopes did not differ thereafter. These findings confirm a palliative effect of LVRS, with initial improvement of QoL and subsequent maintenance of the improvement over time.
This analysis has several limitations. First, the absence of cardiovascular comorbidities in the NETT cohort represents a limitation in terms of generalizability of the results to patients with ongoing cardiac disease. Second, the inability to blind patients to the treatment received may have affected the subjective QoL measurements and was the reason for choosing an unquestionable QoL difference of eight points as part of the composite outcome. Finally, we recognize a limitation in the assumption made in the creation of the composite outcome, which arbitrarily considered an eight-point increase in the SGRQ total score as equivalent to death to make an event. Because a strong motivation for patients to undergo such serious surgery is in fact preservation of QoL, such increases in SGRQ score almost certainly represent a serious negative event (13, 15).
The methodology that we used to analyze time-sensitive variables that include unquestionably clinically important differences may become more accepted in the future as a practical and clinically oriented approach to time-dependent analysis of clinical trial outcomes.
We believe that the inclusion of QoL in assessments of LVRS provides further support for the role of this surgical intervention in the clinical care of patients with severe emphysema. QoL measures are highly relevant to patient choices and confirm the rationale for using LVRS as a palliative tool. Our findings are relevant for recommending LVRS for patients with upper-lobe–predominant emphysema and in particular those with high exercise capacity, a subset of patients in whom the survival benefits may be marginal but in whom the combined survival and QoL benefits are pronounced.
Supplementary Material
Acknowledgments
Other acknowledgments: Arthur Gelb, MD, Lakewood Regional Medical Center, Lakewood, CA.
Supported by grant 1K23CA106544 from the National Institutes of Health (R.B.). The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration); and the Agency for Healthcare Research and Quality).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200809-1383OC on May 29, 2009
Conflict of Interest Statement: R.B. received up to $1,000 in consultancy fees from Medacorp. M.H.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C-C.H.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.C. received $1,001 to $5,000 from Boehringer Ingelheim, $1,001 to $5,000 from GlaxoSmithKline, and $1,001 to $5,000 from Aeris in industry-sponsored grants for research studies. R.W. received $5,001 to $10,000 for serving on an advisory board for BIPI, $10,001 to $50,000 as a consultant for GlaxoSmithKline, $10,001 to $50,000 as a consultant for Pfizer, $5,001 to $10,000 as a consultant for Novartis, $1,001 to $5,000 as a consultant for Schering Plough, $10,001 to $50,000 as a consultant for AstraZeneca, $10,001 to $50,000 as a consultant for Emphasys, $5,001 to $10,000 as a consultant for Spiration, $5,001 to $10,000 as a DSMB from Genentech, and $5,001 to $10,000 as a DSMB from Intermune; more than $100,001 from BIPI, more than $100,001 from GlaxoSmithKline, and more than $100,001 from Forest in industry-sponsored grants; and $5,001 to $10,000 as a DSMB member from Centocor and $1,001 to $5,000 as a SAB from Medimmune. B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.L. received $10,001 to $50,000 from U.S. Surgical/Covidien, $1,001 to $5,000 from Stryker, and $1,001 to $5,000 from Axcan in lecture fees; received Institutional–Clinical Trial $295,000 for clinical study, $100,000 per year for 5 years for data management from Accuracy, Institutional–Clinical Trial $375,982 over 3 years from Angiodynamics (Rita Medical), Institutional–nonclinical trial approximately $300,000 over 3 years (2006–2009) from Oncotech, Inc., $10,001 to $50,000 Institutional–nonclincal trial ending June 2008 from Axcan, and Institutional–nonclinical trial $150,000 per year for 3 years (2005–2008) from Stryker; and $350,000 for fellowship training, renewed yearly, institutional from U.S. Surgical/Coviden. A.P.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.C.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Members of the NETT Research Group are as follows: Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, MD (Chair); Betsy Ann Bozzarello; Ameena Al-Amin. Clinical centers: Baylor College of Medicine, Houston, TX: Marcia Katz, MD (Principal Investigator); Carolyn Wheeler, RN, BSN (Principal Clinic Coordinator); Elaine Baker, RRT, RPFT; Peter Barnard, PhD, RPFT; Phil Cagle, MD; James Carter, MD; Sophia Chatziioannou, MD; Karla Conejo-Gonzales; Kimberly Dubose, RRT; John Haddad, MD; David Hicks, RRT, RPFT; Neal Kleiman, MD; Mary Milburn-Barnes, CRTT; Chinh Nguyen, RPFT; Michael Reardon, MD; Joseph Reeves-Viets, MD; Steven Sax, MD; Amir Sharafkhaneh, MD; Owen Wilson, PhD; Christine Young PT; Rafael Espada, MD (Principal Investigator 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, MD (1999–2001); Katherine Hale, MD (1998–2000); Everett Hood, RPFT (1998–2000); Amy Jahn (1998–2000); Satish Jhingran, MD (1998–2001); Karen King, RPFT (1998–1999); Charles Miller III, PhD (1996–1999); Imran Nizami, MD (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998 –2000); Joe Rodarte, MD (Co-Principal Investigator 1996–2000); Robert Teague, MD (Co-Principal Investigator 1999–2000); Kedren Williams (1998–1999). Brigham and Women's Hospital, Boston, MA: John Reilly, MD (Principal Investigator); David Sugarbaker, MD (Co-Principal Investigator); Carol Fanning, RRT (Principal Clinic Coordinator); Simon Body, MD; Sabine Duffy, MD; Vladmir Formanek, MD; Anne Fuhlbrigge, MD; Philip Hartigan, MD; Sarah Hooper, EP; Andetta Hunsaker, MD; Francine Jacobson, MD; Marilyn Moy, MD; Susan Peterson, RRT; Roger Russell, MD; Diane Saunders; Scott Swanson, MD (Co-Principal Investigator, 1996–2001). Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, MD (Principal Investigator); Zab Mohsenifar, MD (Co-Principal Investigator); Carol Geaga, RN (Principal Clinic Coordinator); Manmohan Biring, MD; Susan Clark, RN, MN; Jennifer Cutler, MD; Robert Frantz, MD; Peter Julien, MD; Michael Lewis, MD; Jennifer Minkoff-Rau, MSW; Valentina Yegyan, BS, CPFT; Milton Joyner, BA (1996–2002). Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, MD (Principal Investigator); James Stoller, MD (Co-Principal Investigator); Yvonne Meli, RN,C (Principal Clinic Coordinator); John Apostolakis, MD; Darryl Atwell, MD; Jeffrey Chapman, MD; Pierre DeVilliers, MD; Raed Dweik, MD; Erik Kraenzler, MD; Rosemary Lann, LISW; Nancy Kurokawa, RRT, CPFT; Scott Marlow, RRT; Kevin McCarthy, RCPT; Priscilla McCreight, RRT, CPFT; Atul Mehta, MD; Moulay Meziane, MD; Omar Minai, MD; Mindi Steiger, RRT; Kenneth White, RPFT; Janet Maurer, MD (Principal Investigator, 1996–2001); Terri Durr, RN (2000–2001); Charles Hearn, DO (1998–2001); Susan Lubell, PA-C (1999–2000); Peter O'Donovan, MD (1998–2003); Robert Schilz, DO (1998–2002). Columbia University, New York, NY in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, MD (Principal Investigator); Byron Thomashow, MD (Co-Principal Investigator); Patricia Jellen, MSN, RN (Principal Clinic Coordinator); John Austin, MD; Matthew Bartels, MD; Yahya Berkmen, MD; Patricia Berkoski, MS, RRT (Site coordinator, LIJ); Frances Brogan, MSN, RN; Amy Chong, BS, CRT; Glenda DeMercado, BSN; Angela DiMango, MD; Sandy Do, MS, PT; Bessie Kachulis, MD; Arfa Khan, MD; Berend Mets, MD; Mitchell O'Shea, BS, RT, CPFT; Gregory Pearson, MD; Leonard Rossoff, MD; Steven Scharf, MD, PhD (Co-Principal Investigator, 1998–2002); Maria Shiau, MD; Paul Simonelli, MD; Kim Stavrolakes, MS, PT; Donna Tsang, BS; Denise Vilotijevic, MS, PT; Chun Yip, MD; Mike Mantinaos, MD (1998–2001); Kerri McKeon, BS, RRT, RN (1998–1999); Jacqueline Pfeffer, MPH, PT (1997–2002). Duke University Medical Center, Durham, NC: Neil MacIntyre, MD (Principal Investigator); R. Duane Davis, MD (Co-Principal Investigator); John Howe, RN (Principal Clinic Coordinator); R. Edward Coleman, MD; Rebecca Crouch, RPT; Dora Greene; Katherine Grichnik, MD; David Harpole, Jr., MD; Abby Krichman, RRT; Brian Lawlor, RRT; Holman McAdams, MD; John Plankeel, MD; Susan Rinaldo-Gallo, MED; Sheila Shearer, RRT; Jeanne Smith, ACSW; Mark Stafford-Smith, MD; Victor Tapson, MD; Mark Steele, MD (1998–1999); Jennifer Norten, MD (1998–1999). Mayo Foundation, Rochester, MN: James Utz, MD (Principal Investigator); Claude Deschamps, MD (Co-Principal Investigator); Kathy Mieras, CCRP (Principal Clinic Coordinator); Martin Abel, MD; Mark Allen, MD; Deb Andrist, RN; Gregory Aughenbaugh, MD; Sharon Bendel, RN; Eric Edell, MD; Marlene Edgar; Bonnie Edwards; Beth Elliot, MD; James Garrett, RRT; Delmar Gillespie, MD; Judd Gurney, MD; Boleyn Hammel; Karen Hanson, RRT; Lori Hanson, RRT; Gordon Harms, MD; June Hart; Thomas Hartman, MD; Robert Hyatt, MD; Eric Jensen, MD; Nicole Jenson, RRT; Sanjay Kalra, MD; Philip Karsell, MD; Jennifer Lamb; David Midthun, MD; Carl Mottram, RRT; Stephen Swensen, MD; Anne-Marie Sykes, MD; Karen Taylor; Norman Torres, MD; Rolf Hubmayr, MD (1998–2000); Daniel Miller, MD (1999–2002); Sara Bartling, RN (1998–2000); Kris Bradt (1998–2002). National Jewish Medical and Research Center, Denver, CO: Barry Make, MD (Principal Investigator); Marvin Pomerantz, MD (Co-Principal Investigator); Mary Gilmartin, RN, RRT (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, PT; Enrique Fernandez, MD; Lisa Geyman, MSPT; Connie Hudson; David Lynch, MD; John Newell, MD; Robert Quaife, MD; Jennifer Propst, RN; Cynthia Raymond, MS; Jane Whalen-Price, PT; Kathy Winner, OTR; Martin Zamora, MD; Reuben Cherniack, MD (Principal Investigator, 1997–2000). Ohio State University, Columbus, OH: Philip Diaz, MD (Principal Investigator); Patrick Ross, MD (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, PhD; Mark Gerhardt, MD, PhD; Mark King, MD; David Rittinger; Mahasti Rittinger. Saint Louis University, Saint Louis, MO: Keith Naunheim, MD (Principal Investigator); Robert Gerber, MD (Co-Principal Investigator); Joan Osterloh, RN, MSN (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, DO; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, MD; Gregg Ruppel; Cary Stolar, MD; Janice Willey; Francisco Alvarez, MD (Co-Principal Investigator, 1999–2002); Cesar Keller, MD (Co-Principal Investigator, 1996–2000). Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Satoshi Furukawa, MD (Co-Principal Investigator); Anne Marie Kuzma, RN, MSN (Principal Clinic Coordinator); Roger Barnette, MD; Neil Brister, MD; Kevin Carney, RN, CCTC; Wissam Chatila, MD; Francis Cordova, MD; Gilbert D'Alonzo, DO; Michael Keresztury, MD; Karen Kirsch; Chul Kwak, MD; Kathy Lautensack, RN, BSN; Madelina Lorenzon, CPFT; Ubaldo Martin, MD; Peter Rising, MS; Scott Schartel, MD; John Travaline, MD; Gwendolyn Vance, RN, CCTC; Phillip Boiselle, MD (1997–2000); Gerald O'Brien, MD (1997–2000). University of California, San Diego, San Diego, CA: Andrew Ries, MD, MPH (Principal Investigator); Robert Kaplan, PhD (Co-Principal Investigator); Catherine Ramirez, BS, RCP (Principal Clinic Coordinator); David Frankville, MD; Paul Friedman, MD; James Harrell, MD; Jeffery Johnson; David Kapelanski, MD; David Kupferberg, MD, MPH; Catherine Larsen, MPH; Trina Limberg, RRT; Michael Magliocca, RN, CNP; Frank J. Papatheofanis, MD, PhD; Dawn Sassi-Dambron, RN; Melissa Weeks. University of Maryland at Baltimore, Baltimore, MD in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, MD (Principal Investigator); Henry Fessler, MD (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, MD; Jonathan Orens, MD; Steven Scharf, MD, PhD; David Shade; Stanley Siegelman, MD; Kenneth Silver, MD; Clarence Weir; Charles White, MD. University of Michigan, Ann Arbor, MI: Fernando Martinez, MD (Principal Investigator); Mark Iannettoni, MD (Co-Principal Investigator); Catherine Meldrum, BSN, RN, CCRN (Principal Clinic Coordinator); William Bria, MD; Kelly Campbell; Paul Christensen, MD; Kevin Flaherty, MD; Steven Gay, MD; Paramjit Gill, RN; Paul Kazanjian, MD; Ella Kazerooni, MD; Vivian Knieper; Tammy Ojo, MD; Lewis Poole; Leslie Quint, MD; Paul Rysso; Thomas Sisson, MD; Mercedes True; Brian Woodcock, MD; Lori Zaremba, RN. University of Pennsylvania, Philadelphia, PA: Larry Kaiser, MD (Principal Investigator); John Hansen-Flaschen, MD (Co-Principal Investigator); Mary Louise Dempsey, BSN, RN (Principal Clinic Coordinator); Abass Alavi, MD; Theresa Alcorn, Selim Arcasoy, MD; Judith Aronchick, MD; Stanley Aukberg, MD; Bryan Benedict, RRT; Susan Craemer, BS, RRT, CPFT; Ron Daniele, MD; Jeffrey Edelman, MD; Warren Gefter, MD; Laura Kotler-Klein, MSS; Robert Kotloff, MD; David Lipson, MD; Wallace Miller, Jr., MD; Richard O'Connell, RPFT; Staci Opelman, MSW; Harold Palevsky, MD; William Russell, RPFT; Heather Sheaffer, MSW; Rodney Simcox, BSRT, RRT; Susanne Snedeker, RRT, CPFT; Jennifer Stone-Wynne, MSW; Gregory Tino, MD; Peter Wahl; James Walter, RPFT; Patricia Ward; David Zisman, MD; James Mendez, MSN, CRNP (1997–2001); Angela Wurster, MSN, CRNP (1997–1999). University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD (Principal Investigator); James Luketich, MD (Co-Principal Investigator); Colleen Witt, MS (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, MD; Carl Fuhrman, MD; Robert Hoffman, MD; Joan Lacomis, MD; Joan Sexton; William Slivka; Diane Strollo, MD; Erin Sullivan, MD; Tomeka Simon; Catherine Wrona, RN, BSN; Gerene Bauldoff, RN, MSN (1997–2000); Manuel Brown, MD (1997–2002); Elisabeth George, RN, MSN (Principal Clinic Coordinator 1997–2001); Robert Keenan, MD (Co-Principal Investigator 1997–2000); Theodore Kopp, MS (1997–1999); Laurie Silfies (1997–2001). University of Washington, Seattle, WA: Joshua Benditt, MD (Principal Investigator), Douglas Wood, MD (Co-Principal Investigator); Margaret Snyder, MN (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, MD; Leighton Chan, MD; Cindy Chwalik; Bruce Culver, MD; Thurman Gillespy, MD; David Godwin, MD; Jeanne Hoffman; Andra Ibrahim, MD; Diane Lockhart; Stephen Marglin, MD; Kenneth Martay, MD; Patricia McDowell; Donald Oxorn, MD; Liz Roessler; Michelle Toshima; Susan Golden (1998-2000). Other participants: Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, MD, MPH; Yen-Pin Chiang, PhD; Carolyn Clancy, MD; Harry Handelsman, DO. Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M Berkowitz, PhD; Tanisha Carino, PhD; Joe Chin, MD; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora; Steven Sheingold, PhD (1997–2004). Coordinating Center, The Johns Hopkins University, Baltimore, MD: Steven Piantadosi, MD, PhD (Principal Investigator); James Tonascia, PhD (Co-Principal Investigator); Patricia Belt; Amanda Blackford, ScM; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Vera Edmonds; Gregory L. Foster, MA; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, ScM; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, MD; Michael Smith; Brett Simon, MD; Paul Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Laura Wilson, ScM; Robert Wise, MD. Cost Effectiveness Subcommittee: Robert M. Kaplan, PhD (Chair); J. Sanford Schwartz, MD (Co-Chair); Yen-Pin Chiang, PhD; Marianne C. Fahs, PhD; A. Mark Fendrick, MD; Alan J. Moskowitz, MD; Dev Pathak, PhD; Scott Ramsey, MD, PhD; Steven Sheingold, PhD; A. Laurie Shroyer, PhD; Judith Wagner, PhD; Roger Yusen, MD. Cost Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, MD, PhD (Principal Investigator); Ruth Etzioni, PhD; Sean Sullivan, PhD; Douglas Wood, MD; Thomas Schroeder, MA; Karma Kreizenbeck; Kristin Berry, MS; Nadia Howlader, MS. CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, PhD (Principal Investigator); Janice Cook-Granroth, BS; Angela Delsing, RT; Junfeng Guo, PhD; Geoffrey McLennan, MD; Brian Mullan, MD; Chris Piker, BS; Joseph Reinhardt, PhD; Blake Wood; Jered Sieren, RTR; William Stanford, MD. Data and Safety Monitoring Board: John A. Waldhausen, MD (Chair); Gordon Bernard, MD; David DeMets, PhD; Mark Ferguson, MD; Eddie Hoover, MD; Robert Levine, MD; Donald Mahler, MD; A. John McSweeny, PhD; Jeanine Wiener-Kronish, MD; O. Dale Williams, PhD; Magdy Younes, MD. Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Charles Soltoff, MBA. Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, MD (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, PhD; James Kiley, PhD; Margaret Wu, PhD (1996-2001).
References
- 1.Yusen RD, Littenberg B. Integrating survival and quality of life data in clinical trials of lung disease: the case of lung volume reduction surgery. Chest 2005;127:1094–1096. [DOI] [PubMed] [Google Scholar]
- 2.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 3.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the national emphysema treatment trial research group. Ann Thorac Surg 2006;82:431–443. [DOI] [PubMed] [Google Scholar]
- 4.Benzo R, Kaplan MR, Martinez F, Wise R, Make B, Criner G, Reilly J, Fishman A, Luketich J, Sciurba F. Effect of lung volume reduction surgery on the decline of health related quality of life [abstract]. Am J Respir Crit Care Med 2007;175:A175. [Google Scholar]
- 5.The National Emphysema Treatment Trial Research Group. Rationale and design of the national emphysema treatment trial (NETT): a prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc Surg 1999;118:518–528. [DOI] [PubMed] [Google Scholar]
- 6.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 7.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002;19:398–404. [DOI] [PubMed] [Google Scholar]
- 8.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–1327. [DOI] [PubMed] [Google Scholar]
- 9.Naunheim KS, Wood DE, Krasna MJ, DeCamp MM Jr, Ginsburg ME, McKenna RJ Jr, Criner GJ, Hoffman EA, Sternberg AL, Deschamps C. Predictors of operative mortality and cardiopulmonary morbidity in the national emphysema treatment trial. J Thorac Cardiovasc Surg 2006;131:43–53. [DOI] [PubMed] [Google Scholar]
- 10.Ciccone AM, Meyers BF, Guthrie TJ, Davis GE, Yusen RD, Lefrak SS, Patterson GA, Cooper JD. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513–525. [DOI] [PubMed] [Google Scholar]
- 11.Yusen RD, Lefrak SS, Gierada DS, Davis GE, Meyers BF, Patterson GA, Cooper JD. A prospective evaluation of lung volume reduction surgery in 200 consecutive patients. Chest 2003;123:1026–1037. [DOI] [PubMed] [Google Scholar]
- 12.Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:14–20. [DOI] [PubMed] [Google Scholar]
- 13.Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Felez M, Khalaf A, Marrades RM, Monso E, Serra-Batlles J, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:680–685. [DOI] [PubMed] [Google Scholar]
- 14.Spencer S, Calverley PM, Sherwood Burge P, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:122–128. [DOI] [PubMed] [Google Scholar]
- 15.Conte ME, Pedone C, Forastiere F, Bellia V, Antonelli-Incalzi R. Discriminative and predictive properties of disease-specific and generic health status indexes in elderly COPD patients. BMC Pulm Med 2008;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.