Summary
In the mammalian cecum and colon, a single layer of absorptive, mature enterocytes are a crucial element of the physical barrier to the contents of the lumen. Enterocytic differentiation involves expansion of cytoplasmic cytoskeletal networks, which have been proposed to maintain structural integrity of individual cells and thus the entire epithelial barrier. We sought molecular tools to test this hypothesis in vivo, because in vitro systems displaying full intestinal epithelial differentiation have not yet been developed. Vav proteins are RhoGEFs that modulate cytoskeletal networks in immune cells. We found that Vav proteins were preferentially expressed in terminally differentiating cecal and colonic enterocytes. Loss of Vav protein expression in triple-knockout mice (Vav1–/–;Vav2–/–;Vav3–/–) resulted in defective expansion of microtubule cytoskeletons, a significant decrease in cell height and diminished expression of differentiation markers. Despite these changes, enterocytes in the triple-mutant mice did not contain measurable alterations in actin cytoskeleton, apical cell-cell junctions, nuclear position or global polarized delivery of proteins involved in terminal differentiation. Aged triple-mutant mice spontaneously developed ulcerative lesions that were, in part, a result of defective wound repair. These studies show that Vav proteins are required for enterocytic differentiation and colonic epithelial barrier integrity.
Keywords: Microtubules, Cell size, Epithelium, Vav proteins
Introduction
The mouse cecum and colon are adjacent structures with very similar anatomic properties and are the most distal organs of the gastrointestinal tract (Popesko et al., 2003). Both organs must provide and maintain a barrier separating hundreds of billions of microbes in the lumen from accessing the robust microvascular network within their mesenchyme (Savage, 1977; Stappenbeck et al., 2002). One important physical aspect of this barrier is a single layer of terminally differentiated epithelial cells, composed mostly of absorptive enterocytes that are located at the interface of the host and luminal compartments. These enterocytes are continuously replaced by differentiating cells that migrate out of adjacent invaginations of the epithelium, called the crypts of Lieberkühn. The basal third of each crypt contains tripotent stem cells and committed proliferative daughters that give rise to enterocytes as well as two other epithelial lineages, which also participate in barrier function: mucus-secreting goblet cells and hormone-secreting enteroendocrine cells (Chang and Leblond, 1971). The barrier function of the epithelium is constantly maintained even while the cells that form it are replaced in a rapid and continuous fashion (Chang and Leblond, 1971; Chang and Nadler, 1975).
The Wnt, Notch, Hedgehog and BMP signaling pathways all participate in intestinal epithelial homeostasis. Both Wnt and Notch signaling are required to maintain epithelial proliferation of intestinal stem cells and their immediate proliferative daughters (Korinek et al., 1998; Pinto et al., 1999; Sancho et al., 2003; Kuhnert et al., 2004; van Es et al., 2005). In addition, Notch signaling is required for the fate specification of the enterocytic lineage (van Es et al., 2005). BMP signaling also functions as a key negative regulator of proliferation and stem cell census: inhibition of BMP signaling leads to ectopic crypt formation (Haramis et al., 2004). The Hedgehog pathway has been suggested to antagonize Wnt signals as cells exit the crypt and to be required for enterocytic differentiation. Loss of Hedgehog function results in lower expression of certain markers of terminal differentiation of the surface colonic epithelium, but no morphologic abnormalities in these cells (van den Brink et al., 2004).
An important component of enterocyte terminal differentiation during the migration to the surface is their cytoplasmic enrichment of cytoskeletal networks. A primary example is the actin-based microfilament network, whose robust expression in the apex of surface epithelial cells (Drenckhahn and Dermietzel, 1988) underlies an increase in length and density of their microvilli, as well as enhancement of associated junctional complexes (zonula occludens and zonula adherens) that form band-like structures around the apex of the cell. A second prominent cytoplasmic cytoskeletal network in these cells consists of microtubules. In post-mitotic cells, this network is critical for polarized delivery of proteins to and from the cell surface and has been proposed to have a role in the structural integrity of the cells. Proteins controlling the maturation of the cytoskeleton in the surface absorptive cells have long been hypothesized to be important for maintaining the integrity of the barrier that these cells form (Farquhar and Palade, 1963; Hull and Staehelin, 1979; Fey et al., 1984; Ingber, 2003).
No in vitro experimental system that completely recapitulates the differentiation program of non-transformed, post-mitotic intestinal enterocytes is available to test this hypothesis. We have previously attempted to address this question in vivo by overexpressing dominant-active and dominant-negative forms of Rac GTPases, which are well known to modulate cytoskeletal components in the intestinal epithelium of chimeric mice (Stappenbeck and Gordon, 2000; Stappenbeck and Gordon, 2001). We found that Rac has a role in intestinal epithelial homeostasis and enterocyte differentiation, but these studies were limited by the perinatal lethality of mice that expressed high levels of these Rac GTPase transgenes. In addition, Rac activity was modulated throughout the intestinal epithelium. Therefore, separating out the effects on differentiation from those on the epithelial progenitor compartment was also difficult.
Rho guanine nucleotide exchange factors (GEFs) activate Rho GTPases, comprise a much larger family of enzymes (at least 22 genes in mice and man) than the Rho GTPases they modify, and display varying tissue and cellular expression patterns (Jaffe and Hall, 2005). Therefore, we surmised that specific members or subfamilies might be expressed preferentially in differentiated intestinal epithelial cells and thus would be more amenable targets for a genetic approach to test this hypothesis. In this report, we investigated the role of mammalian Vav proteins, a sub-family of three related GEFs that modulate actin and microtubules by activating Rho GTPases (including subfamilies of Rho, Rac and Cdc42) (Bustelo, 2001; Swat and Fujikawa, 2005). Vav expression and function has been best characterized in hematopoietically derived cells. In these lineages, individual Vav proteins have both redundant and unique functions. For example, recent findings show that osteoclasts in Vav3–/– mice contain an abnormal actin cytoskeleton and are defective in bone resorption (Faccio et al., 2005). In lymphocytes, Vav proteins localize to the actin cytoskeleton within the immune synapse (Faure et al., 2004) and are required for plasma cell differentiation (Stephenson et al., 2006). Previous studies have shown that loss of Vav2 function results in defective B cell activation, whereas loss of Vav1 and Vav3 results in abnormal neutrophil adhesion and phagocytosis (Gakidis et al., 2004; Doody et al., 2001).
The role of Vav proteins in non-bone-marrow-derived cell types has not been described. We found that the normal expression of all three Vav proteins was enhanced in surface cecal and colonic enterocytes, suggesting that they could have a role in the terminal differentiation of these cells. We used knockout mice to show that these three genes are in fact crucial for specific aspects of enterocyte differentiation that involve cell height and the robustness of the cytoplasmic microtubule network.
Results
Vav proteins are preferentially expressed in enterocytes in the adult mouse colonic epithelium
The microanatomy of a cecal crypt-surface unit (defined as a single crypt and the surface epithelial cells that emanate from it) can be divided into three equally sized functional zones (Fig. 1A; upper, middle and lower zones). The epithelium of the lower zone, the crypt base, contains dividing epithelial progenitors. The middle zone epithelium consists of post-mitotic cells that are predominately immature enterocytes. The upper zone consists of terminally differentiated enterocytes that line the crypt orifice (the opening of the crypt to the lumen) and the surface. Mucous-secreting goblet cells, the second-most predominant differentiated epithelial cell, are distributed throughout the crypt in WT cecums (Fig. 1B).
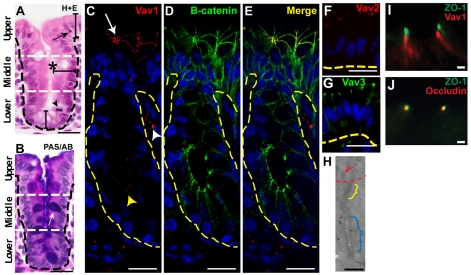
Fig. 1.
Vav proteins are expressed in upper zone enterocytes of the mouse cecum and localize near adherens junctions. (A,B) Sections of WT adult mouse cecum stained with hematoxylin and eosin (H+E) (A) and PAS/alcian blue (B). A single crypt-surface epithelial unit is shown. Black dashed lines indicate the basal epithelial surface. White dashed lines demarcate three epithelial zones. In A, the asterisk denotes the crypt lumen, the arrowhead indicates an M-phase cell in the lower zone and the arrow denotes an apoptotic body in the upper zone. In B, the white arrow denotes a goblet cell (purple). (C-E) Sections of a WT mouse cecum double-labeled with rabbit anti-Vav1, Alexa Fluor 594-conjugated donkey anti-rabbit (red), mouse anti-β-catenin, Alexa Fluor 488-conjugated sheep anti-mouse (green) antibodies and bis-benzimide. Vav1 protein expression was most robust in upper zone enterocytes (arrow; the tangential orientation shows a crypt orifice). The white and yellow arrowheads indicate cells with Vav protein expression in the mesenchyme and crypt epithelium, respectively. (F,G) Sections of WT upper zone cecal enterocytes labeled with anti-Vav2 (F, red) and anti-Vav3 (G, green) antibodies. The yellow dashed lines in C-G indicate the epithelial-mesenchymal junction. (H) Immunogold labeling for Vav1 in an apical junctional complex from a WT upper zone enterocyte. The red arrow denotes microvilli. The yellow and blue brackets indicate tight and adherens junctions, respectively. Vav1 was detected near adherens junctions. (I,J) Sections of a WT upper zone cecal enterocyte double-labeled with anti-ZO-1 (I, green) and anti-Vav1 (I, red) or anti-ZO-1 (J, green) and anti-occludin (J, red) antibodies. Scale bars: A-G, 15 μm; H-J, 200 nm.
Our initial goal was to find RhoGEF(s) that showed enhanced expression in differentiated, upper zone enterocytes of the intestine, so that we could potentially use genetic manipulation to specifically investigate the role of Rho GTPases in epithelial differentiation and barrier function. We found that the distribution of protein expression of the three murine Vav proteins, met this requirement in the cecum and also in the proximal colon. Immunohistochemical analysis of WT adult mouse cecums showed that Vav proteins were enriched in a cellular and subcellular location where they could potentially mediate epithelial alterations in actin and/or microtubule cytoskeletal networks during differentiation. Vav1 protein was most robustly detected at the apical cell-cell interfaces of enterocytes in the upper zone of the crypt-surface axis (Fig. 1C). In these epithelial cells, Vav1 colocalized with markers of apical junctional proteins such as β-catenin (Fig. 1C-E). The staining pattern for Vav1 was identical in the proximal (also known as the ascending) colon (supplementary material Fig. S1A). Antibodies specific for Vav2 and Vav3 showed robust expression in upper zone enterocytes of WT mouse cecums (Fig. 1F,G) and colons (not shown) in a similar subcellular distribution as Vav1. No staining for any of the three Vav proteins was detected in the small intestinal epithelium of WT mice and from cecum and colons of mice that lacked all three Vav genes [not shown; genotype of these mice is Vav1–/–;Vav2–/–;Vav3–/–, abbreviated to Vav1/2/3null; see Stephenson et al. (Stephenson et al., 2006) for their production and validation].
The location of Vav expression at apical cell-cell borders of cecal and colonic enterocytes was consistent with either association with tight junctions and/or adherens junctions. An association with tight junctions would suggest that Vav proteins function in the regulation of paracellular transport. Alternatively, an association with adherens junctions would suggest possible roles in cell adhesion, polarity or interaction with cytoplasmic cytoskeletal networks such as actin and/or microtubules. We performed immuno-gold staining for Vav1 that showed the majority of the labeling localized to a region adjacent to the outer plaque of the adherens junction with no detectable labeling of tight junctions (Fig. 1H).
To verify the location of the Vav proteins, we performed double-label immunofluorescence with antisera directed against the Vav proteins and ZO-1 (a marker of tight junctions) and visualized the localization of these proteins using confocal microscopy at ×630. The limit of resolution for fluorescence visualization at this magnification is ∼200 nm (Gustafsson, 1999), which is approximately the distance between these two apical junctions. We could easily distinguish non-overlapping staining for all three Vav proteins and ZO-1 (Fig. 1I). As a control, we performed double-label staining with two tight junction markers (ZO-1 and occludin) and found completely overlapping staining patterns (Fig. 1J). These findings suggest that Vav proteins have a functional role in adherens junctions.
Vav-deficient colonic enterocytes contain abnormal microtubule networks
Based on the localization of the Vav proteins in the epithelium of the mouse cecum and colon, we predicted that their absence might affect the cytoskeletal organization of terminally differentiated colonic enterocytes. Actin and microtubules are two prominent cytoskeletal networks in the intestinal epithelium (Fig. 2A,C). Actin was robustly expressed at the apical surface of upper zone enterocytes and is expressed at far lower levels in all epithelial cells of the middle and lower zones in crypts (Fig. 2A). Cytoplasmic microtubule networks (Fig. 2C) undergo a similar change in expression pattern in the colonic epithelium. Less-differentiated epithelial cells in the lower and middle epithelial zones contained an accumulation of polymerized microtubules in the cell apex (this is more pronounced in the middle zone). As cells migrated into the upper zone, the microtubule networks became robust throughout the cytoplasm. The apical cap of microtubules (such as in the middle zone) is considered to be the nucleation site for polymerization of this network in simple epithelial cells, with microtubules forming towards the base of the cells (Musch, 2004).
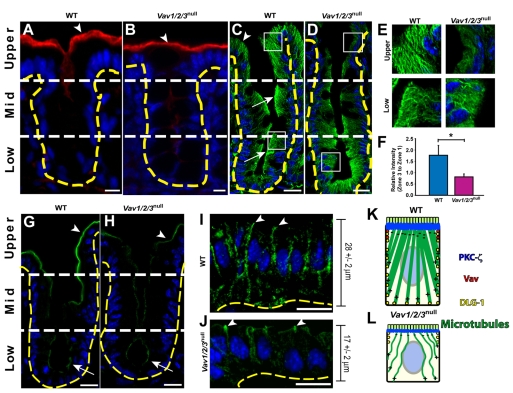
Fig. 2.
Surface enterocytes of Vav1/2/3null mice contain abnormal microtubule networks. (A-D) Sections of cecum from (A,C) WT and (B,D) Vav1/2/3null mice stained with rabbit anti-actin and Cy3-conjugated donkey anti rabbit (red) antibodies and bis-benzimide (blue) (A,B), and FITC-conjugated rabbit anti-α-tubulin (green) and bis-benzimide (C,D). The arrowheads in A and B denote the high level of actin staining in the apex of upper zone enterocytes of both Vav1/2/3null and WT mice. Arrows in C indicate the apical accumulation of polymerized α-tubulin at the cell apex of the lower and middle zones, which is similar to Vav1/2/3null cells in these regions; the arrowhead denotes increased cytoplasmic microtubules in upper zone enterocytes compared with their counterparts in the Vav1/2/3null mice. (E) Insets of boxed regions in C and D from upper and lower zone epithelial cells. (F) Ratio (± s.e.m.) of mean fluorescent intensity of α-tubulin staining in the apical cytoplasm (upper zone/lower zone) of WT and Vav1/2/3null mice. *P<0.01, Student's t-test comparing WT and Vav1/2/3null mice. (G-J) Sections of cecums from WT (G,I) and Vav1/2/3null (H,J) mice stained with rabbit anti-PKCζ and FITC-conjugated donkey anti rabbit (green) antibodies (G,H) and rabbit anti-Dlg1 and FITC-conjugated donkey anti rabbit (green) antibodies (I,J). (K,L) Summary of cellular localization and microtubule networks in upper zone enterocytes in WT and Vav1/2/3null mice. Scale bars: A,C,D,G-J, 15 μm; B, 7.5 μm.
We utilized mice in which all three Vav genes were knocked out to evaluate a number of epithelial parameters, including their role in actin and microtubule networks of the cecal and colonic epithelium. In all experiments reported here, the abnormities uncovered in the epithelium occurred only in Vav1/2/3null mice. Extensive controls using knockouts of single and combinations of two Vav genes were evaluated as well as WT littermate controls. In all experiments, we found that mice with at least one functioning Vav gene were indistinguishable from WT mice. For simplicity, we will describe the WT data as controls for all experiments.
We found no detectable differences in actin staining intensity or distribution when we compared WT and Vav1/2/3null mice for each of the three epithelial zones of the crypt-surface axis (Fig. 2A,B). In similar comparisons of Vav1/2/3null and WT mice, we found indistinguishable intracellular distributions of polymerized microtubules in the lower and middle epithelial zones. However, the upper zone epithelial cells in Vav1/2/3null mouse cecums and colons contained much fewer elaborate cytoplasmic microtubular networks compared with cells in the same zone of WT mice (Fig. 2C-E) (see supplementary material Fig. S1B-E for higher magnification images).
To quantify the extent of the underdeveloped microtubule network observed in upper zone epithelial cells of Vav1/2/3null mice, we used the lower zone microtubule networks as internal controls for each image. We reasoned that this was an appropriate baseline because we observed no apparent differences in the density of the lower zone microtubule networks between WT and Vav1/2/3null mice. Therefore, we compared the mean average ratio of fluorescence intensities in the supranuclear cytoplasm of upper zone versus lower zone epithelial cells for both WT and Vav1/2/3null mice. In WT mice, this ratio was 1.8±0.3, indicating an increase in microtubules as cells move from the lower zone to the upper zone. In Vav1/2/3null mice the ratio was 0.8±0.1, which was statistically significantly less than WT (n=5 mice per group; 100 cells/mouse were analyzed; P<0.01 using Student's t-test) (Fig. 2F). Similar differences in the ratios of epithelial microtubule density were also observed in the proximal colon for WT and Vav1/2/3null mice (data not shown). We evaluated the localization of γ-tubulin, a known microtubule-organizing center for polarized epithelial cells, and found similar sites of apical expression in both control and Vav1/2/3null upper zone epithelial cells, although the expression appeared to be diminished in the Vav1/2/3null mice (supplementary material Fig. S1F,G).
The link between the Rho GTPase Cdc42 and microtubule polymerization was made in migrating astrocytes (Cau and Hall, 2005). These authors found that Cdc42 stimulated the PKCζ to recruit APC (adenomatous polyposis coli) to the plus ends of microtubules and also stimulated Dlg1 to localize to the cell periphery, where growing microtubules terminated. These processes were essential for microtubule formation in migrating cells. The current model of microtubule polymerization in polarized epithelial cells shows that this process occurs from a diffuse apical microtubule-organizing center, located in the cell apex, whereas plus ends of microtubules grow towards the periphery (Musch, 2004).
We found that PKCζ localized to the cell apex of all WT cecal epithelial cells and showed increased staining in upper zone enterocytes (Fig. 2G). In upper zone WT enterocytes, Dlg1 protein localized along basolateral cell-cell borders (Fig. 2I). The intracellular distribution of PKCζ and Dlg1 in enterocytes places them in a position to mediate polymerization from the apex and microtubule interactions at the periphery of the cell. Interestingly, in Vav1/2/3null mice, PKCζ localized to the cell apex of all cecal epithelial cells, but did not demonstrate the robust increase in the upper zone as observed in WT mice (Fig. 2G,H). In addition, in Vav1/2/3null mice, Dlg1 was primarily located at the apex of upper zone enterocytes (Fig. 2J). Taken together, the abnormal cytoplasmic microtubules, PKCζ expression and distribution of Dlg1 in Vav1/2/3null upper zone enterocytes are consistent with the current model of microtubule polymerization in enterocytes and is consistent with the molecules previously identified to augment microtubule formation in neurons (Fig. 2K,L) (Cau and Hall, 2005).
Vav proteins are required for the correct height of colonic enterocytes
Microtubules have been proposed to have a role in cell shape (Ingber, 2003). Therefore, we compared the cell size of the cecal and ascending colonic epithelial cells of adult WT with Vav1/2/3null mice. We found that the upper zone surface epithelial cells in Vav1/2/3null mice were notably shorter than their counterparts in WT mice (Fig. 3A-C; supplementary material Fig. S1H,I). To quantify this observation, we measured epithelial cell heights in all three zones of the cecums from both WT and Vav1/2/3null mice using well-oriented histological sections (defined as the presence of a crypt opening that is visualized in all three zones) (see Fig. 1A for WT and Fig. 3C for Vav1/2/3null). In WT mice, the height of epithelial cells showed a statistically significant increase as cells transited from the lower zone (17±2 μm; n=4 mice/group; 100 crypt units analyzed/mouse) to the middle zone (22±3 μm; P<0.01) and then from the middle to the upper zone (28±2 μm; P<0.01) (Fig. 1A and Fig. 3D). Although epithelial cell height of Vav1/2/3null mice showed a similar statistically significant increase when comparing the lower (17±2 μm) to the middle zone (23±2 μm; P<0.01, Student's t-test, n=4 mice/group; 100 crypt units analyzed/mouse), cell height decreased as epithelial cells transited into the upper zone (18±2 μm) of Vav1/2/3null mice (Fig. 3D).
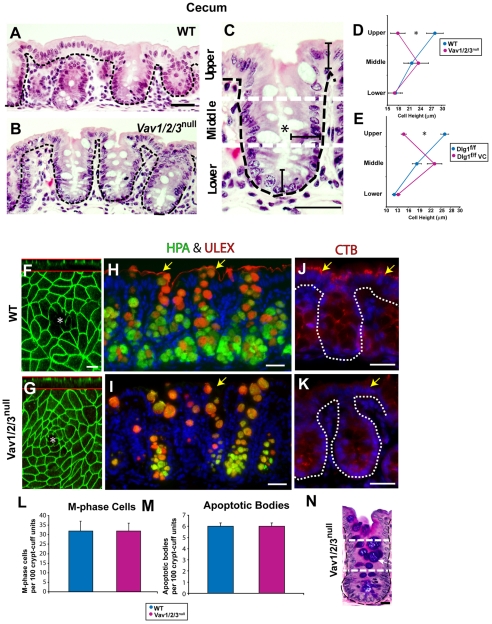
Fig. 3.
Upper zone enterocytes of Vav1/2/3null mice are short. (A-C) H+E-stained sections of (A) WT and (B,C) Vav1/2/3null mouse cecums. The upper zone WT epithelial cells were taller than the corresponding Vav1/2/3null surface epithelial cells. The black brackets in C highlight the epithelial cell heights in all three zones (compare with Fig. 1A for WT). (D,E) Quantification of cell height in all three zones. Asterisks indicate a statistically significant difference between the upper zone enterocytes of (D) WT and Vav1/2/3null mice and (E) Dlg1flox/flox and Dlg1flox/flox; Villin-Cre mice (P<0.001) using a Student's t-test (means for each group ± s.e.m., n=3-4 mice/group, n=100 cells measured per zone per mouse). (F,G) Confocal analysis of the mouse cecal whole mounts from a WT and Vav1/2/3null mouse stained with rabbit anti-ZO-1 to label tight junctions of the upper zone enterocytes at the cell apex. This staining highlights the shape and size of surface epithelial cells present around a single crypt opening (asterisks). A confocal Z-stack is shown above the red line for each image. (H,I) Staining of WT and Vav1/2/3null ascending colons with Cy3-conjugated UEA lectin (Ulex, red) and FITC-conjugated HPA lectin (green). UEA and HPA stain crypt goblet cell mucin. UEA stains the apical surface of enterocytes in WT but not Vav1/2/3null mice (indicated by yellow arrows). (J,K) Staining of WT and Vav1/2/3null ascending colons with Cy3-conjugated CTB lectin. CTB detects a more robust Golgi-type pattern in the enterocytes of WT compared with Vav1/2/3null upper zone enterocytes (yellow arrows). (L,M) Quantification of M-phase cells (L) and apoptotic bodies (M) per 100 crypt-surface units showed no statistically significant differences when comparing WT and Vav1/2/3null mice. (N) PAS/AB stained section of a Vav1/2/3null mouse colon. Scale bars: A-C, 30 μm; F,G, 5 μm; H-K, 30 μm; N, 15 μm.
The lower and middle zone epithelial cell height was not significantly different between WT and Vav1/2/3null mice. However, comparison of upper zone enterocytes between these two groups of mice showed a statistically significant difference (P<0.001, Student's t-test). The effects of loss of function of Vav were also observed in the proximal colon (data not shown). Intestinal sites where Vav proteins were not expressed (e.g. the small intestine) showed similar epithelial heights when comparing Vav1/2/3null and WT mice. We only found an effect on cell height in the Vav1/2/3null mice, because mice containing knockouts of individual Vav proteins or various combinations of double knockouts showed similar profiles of epithelial cell height as those in WT mice (data not shown).
The striking finding of short upper zone epithelial cells in the cecum and colon of Vav1/2/3null mice, led us to investigate whether the colonic epithelial Vav proteins were part of a pathway that determines height of these cells. A candidate downstream target of Vav in such a pathway is Dlg1, based on its altered localization in the upper zone cecal and colonic epithelial cells of the Vav1/2/3null mice (Fig. 2I,J). To test this hypothesis, we evaluated the epithelial cell heights in mice lacking expression of Dlg1 because no current culture model can recapitulate the features of postmitotic enterocytes.
We could not use Dlg1–/– mice, because they die at birth as a result of urinary tract abnormalities before the colon is fully developed (Mahoney et al., 2006). Therefore, we evaluated mice with loss of Dlg1 that was restricted to the intestinal epithelium by crossing Dlg1flox/flox mice (Stephenson et al., 2007) with Villin-Cre mice (Madison et al., 2002). Dlg1flox/flox; Villin-Cre mice were viable to adulthood and showed loss of Dlg1 in intestinal epithelial cells (supplementary material Fig. S2A,B). Analysis of epithelial cell heights in the crypt-surface units of Dlg1flox/flox; Villin-Cre mice showed a strikingly similar phenotype to that in Vav1/2/3null mice. Dlg1-deficient upper zone epithelial cells were significantly shorter than controls (Fig. 3E; supplementary material Fig. S2C,D). Interestingly, loss of Dlg1 only had this effect in the upper zone where the Vav proteins are detectable.
We next compared the apical cell surface of upper zone cecal epithelial cells from WT and Vav1/2/3null mice. We stained cecal whole-mount preparations with markers of apical junctions (e.g. ZO-1 in Fig. 3F,G) and visualized the surface of the upper zone cells by confocal microscopy. In both sets of mice, the cell shape of the surface enterocytes varied from rectangular to hexagonal. However, the average cell surface area of WT and Vav1/2/3null mice was not statistically significantly different (WT, 37±4 μm2 and Vav1/2/3null, 35±4 μm2; n=4 mice/group). Therefore, the cell size abnormality of the upper zone epithelial cells in Vav1/2/3null mice was confined to one dimension (cell height). In addition, the localization of tight junction components to the cell apex suggested that tight junctions were not affected by the absence of Vav proteins (Fig. 3F,G) (Clayburgh, 2004).
We next evaluated whether the short upper zone epithelial cells displayed additional defects in differentiation. We previously found that inhibition of Rac GTPase activity within intestinal epithelial cells affected differentiation through diminished expression of specific glycans recognized by lectins such as Ulex europaeus agglutinin (UEA) (Stappenbeck and Gordon, 2000). Therefore, we evaluated the expression of a panel of lectins with known specificity to specific cell types and cellular compartments within them (Falk et al., 1994). Goblet cell markers such as the lectins Helix pomatia agglutinin (HPA) and UEA labeled these cells in the crypts of both WT and Vav1/2/3null mice (Fig. 3H,I). However, upper zone enterocytes from Vav1/2/3null mice showed markedly diminished staining with both UEA and the cholera toxin β-subunit (CTB) compared with WT mice (Fig. 3H-K), indicating incomplete differentiation of these epithelial cells.
The absence of Vav proteins did not create any perceptible alterations in colonic crypts. When we compared WT to Vav1/2/3null mice, no statistically significant differences in either epithelial proliferation or apoptosis were found (Fig. 3L,M). In addition, differentiation of addition cell types was not altered (e.g. goblet cells) (Fig. 3N) (enteroendocrine cells, not shown).
Absence of Vav proteins does not affect cell-cell junctions, nuclear position or epithelial polarity
In addition to cell size, microtubules might have a role in nuclear position, maintenance of apical junctions and epithelial polarity. We investigated all three of these possibilities in Vav1/2/3null mice. Microtubules have been proposed to position the nucleus in the cell (Ingber, 2003). Therefore, we next tested whether the deficiency of microtubules in upper zone cecal enterocytes of Vav1/2/3null mice correlated with a broader nuclear localization in the cytoplasm. We quantified nuclear position by measuring the minimum distance from the apex of the nucleus to the cell surface for epithelial cells in all three zones of WT and Vav1/2/3null mice. Surprisingly, the pattern of variability in nuclear position was virtually the same when comparing equivalent zones of WT and Vav1/2/3null mice (s.d. 1.3 μm lower zone; 1.6 μm, middle zone; 2.2 μm, upper zone for WT) and (s.d. 1.4 μm lower zone; 1.6 μm, middle zone; 2.2 μm, upper zone for Vav1/2/3null mice). This finding suggests that nuclear positioning was not affected by the diminished microtubules in the upper zone epithelium of Vav1/2/3null mice.
When epithelial nuclear positions were plotted for each zone (Fig. 4A), we found that WT cells in each zone contained two areas of cytoplasm (apical and basal) where the nucleus was excluded. Both of these areas were markedly truncated in Vav1/2/3null upper zone enterocytes. When we plotted the ranges of nuclear position for all three zones (Fig. 4B), we found that as cells transited from the lower to the middle zones, the basal nuclei exclusion area increased from ∼2 μm to ∼5.5 μm in both WT and Vav1/2/3null mice. During this same transition, the apical nuclei exclusion area not significantly changed. As epithelial cells transited to the upper zone in WT mice, the height of the basal area was unchanged whereas the apical nuclei exclusion area increased to ∼8 μm. Vav1/2/3null mice contained statistically significant truncations of both the apical and basal nuclei exclusion areas. This finding suggests that Vav proteins are required to increase the height of the cytoplasm during terminal differentiation of cecal enterocytes.
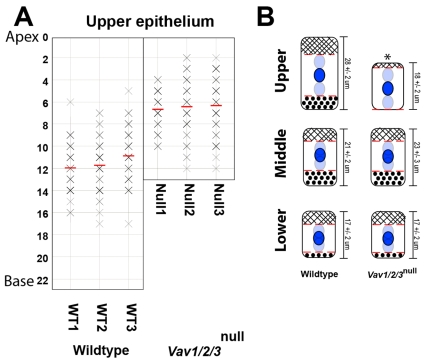
Fig. 4.
Vav proteins are required for cytoplasmic `nuclei exclusion zones' in mature enterocytes. (A) Supranuclear cytoplasm lengths (distance from the top of the nucleus to the cell apex) were plotted for upper zone of cecal enterocytes (n=3 per genotype). A measurement of zero (y-axis) indicates the apex of a nucleus was located at the cell surface. The red bar denotes the average distance per mouse. The intensity of each X correlates with the number of cells of a given measurement (e.g. darker intensity indicates more cells; n=50 cells measured per zone per mouse). (B) The range of nuclear position in all three zones for WT and Vav1/2/3null mice depicted in a cartoon. The average cell heights (from Fig. 3D) are indicated to the right of each cell. The mean nuclear position (± s.e.m.) is in dark blue. The upper and lower limits of nuclear position are in light blue (abutting the dotted red lines). The apical nuclei free area is hashed. The basal nuclei free area is dotted. The asterisk indicates that both the supra- and subnuclear areas of cytoplasm are statistically significantly different in the upper zone enterocytes of Vav1/2/3null compared with WT mice (P<0.001 determined by Student's t-test).
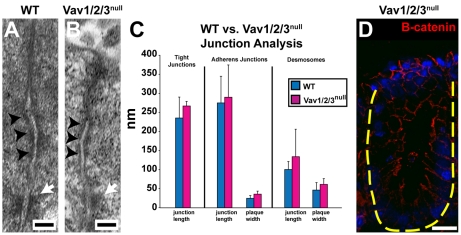
We next evaluated the morphology of the apical junctional complexes of surface enterocytes with and without the presence of Vav proteins. Transmission electron microscopy of WT and Vav1/2/3null mouse cecums showed similar morphology of all apical junctions (Fig. 5A,B). Quantification of junction length and plaque width showed no statistically significant differences in these parameters (Fig. 5C). β-catenin, a component of adherens junctions, was localized normally in Vav1/2/3null mice (Fig. 5D). These data are consistent with maintenance of cell-cell adhesion in the absence of Vav proteins.
Fig. 5.
Vav1/2/3null apical junctions are not obviously altered in the cecal upper zone enterocytes. (A,B) Transmission electron microscopic images of the apical junctional complex from cecal upper zone enterocytes of WT and Vav1/2/3null mice show no detectable differences. Black arrowheads denote adherens junctions and white arrows denote desmosomes. (C) Quantification of junction lengths and plaque widths. (D) Section of Vav1/2/3null cecum stained with anti-β-catenin antibody (red). Scale bars: A,B, 100 nm; D, 15 μm.
The mislocalization of two proteins, Dlg1 and PKCζ, which are known to have a role in the establishment of epithelial cell polarity (Bilder and Perrimon, 2000; Crean et al., 2004), suggests an overall defect in this process in Vav1/2/3null colonic epithelial cells. To test whether loss of Vav proteins affected polarized delivery of proteins to the cell surface of cecal enterocytes, we examined relative staining patterns of apical surface markers (actin, villin, carbonic anhydrase 4 and γ-tubulin), and a basolateral marker (integrin α6) (Fujimoto et al., 2002) by immunofluorescence microscopy (Fig. 6A-D; supplementary material Fig. S1F,G). No significant differences in staining between Vav1/2/3null and WT mice were observed for any of these proteins. Thus, there does not appear to be a global defect in the delivery of proteins to the correct cellular location in the absence of Vav proteins. However, it remains a possibility that a subset of proteins (in particular those associated with Dlg1 and PKCζ) are mislocalized.
Fig. 6.
Vav1/2/3null cecal surface epithelial cells do not demonstrate defects in polarity. (A,B) Sections of a WT and Vav1/2/3null cecums stained with anti-carbonic anhydrase 4 (Car4; red). (C,D) Sections showing the upper zone epithelium of WT and Vav1/2/3null cecums stained with anti-integrin α6 (epithelial basal surface, red), anti-villin (apex of the epithelial cells, green) antibodies and bis-benzimide. (E,F) Scanning electron microscopy of WT (E) and Vav1/2/3null (F) cecums. Vav1/2/3null mice contain denser microvilli on their cell surface than WT mice. The yellow dashed lines denote cell borders. (G,H) Transmission EM of cecums from WT (G) and Vav1/2/3null (H) mice showing microvillus morphology (shape, length and caliber) was not significantly different between the two groups of mice. Scale bars: A,B, 10 μm; C-F, 5 μm; G,H, 100 nm.
We did detect a subtle difference in the density of microvilli on the upper zone enterocytes of Vav1/2/3null compared with WT mice using scanning electron microscopy. Vav1/2/3null enterocytes had a denser configuration of microvilli on their apical cell surface because there was no discernable space between individual microvilli. By contrast, individual microvilli could be distinguished on the surface of WT enterocytes (Fig. 6E,F). However, quantitative analysis of microvillus density in transmission EM micrographs showed no statistically significant difference between the WT and Vav1/2/3null mice (data not shown). Other parameters of the microvilli evaluated by transmission EM, such as the length and caliber of microvilli, were also similar in the knockout and control groups (Fig. 6G,H). We also examined other potential downstream molecular mediators of Vav proteins, such as ERM, which has been shown to have a role in maintaining the correct density and orientation of microvilli (Morales et al., 2004; Saotome et al., 2004). We found no differences in expression of phosphorylated ERM on the apical surface when comparing WT and Vav1/2/3null mice (data not shown).
Spontaneous mucosal ulceration and defective wound repair in Vav1/2/3null mice
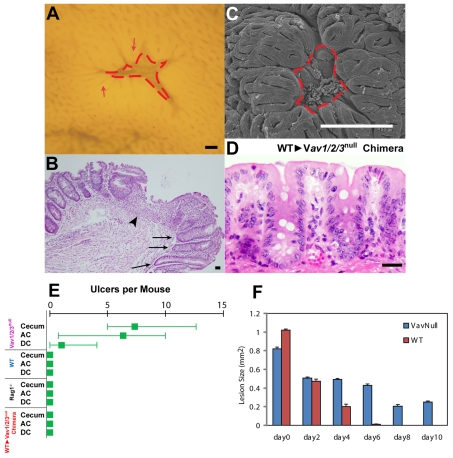
Loss of Vav function had functional consequences because mice lacking Vav proteins contained focal areas of barrier destruction in the colon. As Vav1/2/3null mice aged beyond 6 weeks, they all developed spontaneous mucosal ulcers in the cecum and colon as documented by analysis of whole-mount preparations, scanning electron microscopy and histological sections (Fig. 7A-C). The central area of ulcer crypts was replaced by granulation tissue consisting of myofibroblasts, capillaries and a mixed inflammatory infiltrate. The epithelium bordering the ulcers displayed elongated crypts with increased fractional representation of undifferentiated, proliferating epithelial progenitors [see Pull et al. (Pull et al., 2005) for characterization of this type of lesion]. The ulcers were most prominent in the cecum (Fig. 7E) (n=7.3±5 ulcers per Vav1/2/3null mouse; n=6 mice) and proximal (or ascending) colon (n=6±5 ulcers per Vav1/2/3null mouse), and were less prominent in the distal half of the colon (descending colon and rectum; n=1.3±2.3 ulcers per Vav1/2/3null mouse). No ulcers were observed in either the stomach or small intestine of Vav1/2/3null mice. No colonic ulcers were detected in mice containing at least one normal Vav gene as well as age-matched WT mice from the same colony.
Fig. 7.
Spontaneous mucosal ulceration in the cecum and colons of adult Vav1/2/3null mice required Vav expression in bone marrow derived cells. (A,C) Whole-mount of the cecal mucosal surface of an adult Vav1/2/3null mouse showing an ulcer by light microscopy (A) and scanning EM (C). Dashed lines outline ulcer beds surrounded by crypts with enlarged openings at the surface (indicated by arrows). (B) H+E-stained section of an ulcer from a Vav1/2/3null mouse. Arrowhead indicates granulation tissue in the center of the ulcer and arrows indicate enlarged crypts that immediately surround it. (D) H+E-stained section of a cecum from a Vav1/2/3null-WT chimera. The epithelial cells in the upper zone are similar in height to lower zone cells as found in Vav1/2/3null mice. (E) Quantification of ulcers by anatomic region and genotype (n=6-10 mice per group). (F) Quantification of wound healing in biopsy-injured WT and Vav1/2/3null mice. The average surface area of lesions (± s.d.) is plotted for each group at the time of injury, and thereafter every 2 days (n=5 mice per group and 2-3 lesions per mouse were evaluated). Scale bars: A, 200 μm; B, 500 μm; C, 50 μm; D, 15 μm.
An autoimmune etiology is not likely given the previously described profound immunodeficiency in Vav1/2/3null mice at the level of both T cells and B cells (Fujikawa et al., 2004; Stephenson et al., 2006). We examined the cecum and proximal colonic mucosa of Vav1/2/3null mice in areas distant and relatively spared from ulceration. Neither an influx of immune cells in the lamina propria nor epithelial hyperplasia within crypts was detected (as shown in Fig. 3) as typically occurs in autoimmune colitis (Kang et al., 2008). These findings support the idea that a generalized autoimmune colitis is not the stimulus for mucosal ulceration in the Vav1/2/3null mice.
We next tested whether the ulcerations of the Vav1/2/3null mice were solely the result of the absence of Vav protein expression in the epithelium. We generated bone marrow chimeric animals by giving bone marrow transplants from WT mice to irradiated Vav1/2/3null mice. The colonic surface epithelium of these chimeric mice 12 weeks after transplant displayed the same epithelial defect in the upper zone as in Vav1/2/3null mice (Fig. 7D) (epithelial cell heights, 17±2 for the lower zone, 23±3 for the middle zone and 18±2 for the upper zone). However, no intestinal ulcers were detected (Fig. 7E) (n=6 mice). Therefore, the decreased cell height of the surface epithelium was dependent on Vav protein expression in the epithelium and was not secondary to the mucosal ulcerations.
Vav1/2/3null mice are immunodeficient because they produce no functional B cells or T cells (Fujikawa et al., 2004; Stephenson et al., 2006). Mice defective for correct lymphocyte function (e.g. Rag1–/– mice) did not develop spontaneous colonic ulcers in our colony (Fig. 7E). Taken together, these findings suggest that the formation of ulcers in Vav1/2/3null mice might require the absence of Vav proteins in both the epithelium and in some as yet undefined bone-marrow-derived lineage(s).
We next modeled the process of wound formation and subsequent repair in Vav1/2/3null mice. Under the visual guidance of a miniature endoscope (see Becker et al., 2004), we performed superficial biopsy injuries to the colonic mucosa of mice. We then monitored the progress of healing of each wound by serial visual inspection every 2 days. Wounds of ∼1 mm2 in control mice showed reproducible healing within 6 days. However, similarly sized injuries in the Vav1/2/3null mice failed to show complete healing even at 10 days after injury (Fig. 7F; supplementary material Fig. S3). These results suggest that defective wound repair might have a role in the spontaneous ulcers that we observed in the Vav1/2/3null mice. This experimental system will be useful to further dissect the role of both epithelial and bone-marrow-derived cells in colonic mucosal injuries.
Discussion
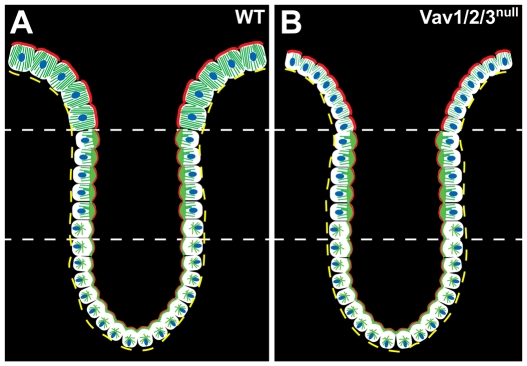
We show that all three Vav proteins are expressed in cecal and colonic upper zone enterocytes. The Vav proteins are required in these cells for several aspects of differentiation, including elaboration of apical microtubular networks, cell height and expression of differentiation-associated lectins (Fig. 8). Interestingly, the loss Vav protein function was not associated with any detectable defects in apical junctions or global shifts in epithelial polarity. In addition, we found that Vav-deficient mice developed spontaneous ulcers in the cecum and colon, which are due in part to delayed wound repair. Using bone marrow chimeric mice, we confirmed that the epithelial defect in cell height was intrinsic to epithelial cells, but that spontaneous ulcerations required loss of Vav protein in the immune system.
Fig. 8.
Model of the role of Vav proteins in apical maturation. (A,B) The crypt-surface epithelium from a WT and Vav1/2/3null mouse cecum is depicted. The cytoplasmic microtubule networks are depicted in green, actin networks in red and nuclei in blue. The lower and middle zones contain similar distributions and organization of actin and microtubules in WT and knockout mice. The upper zone enterocytes of the Vav1/2/3null mice are shorter and contain less-robust microtubule networks than the WT.
Vav proteins, when evaluated either together as an entire family or as individual members, are known to have a role in the functional differentiation of several hematopoietically derived cell types. In particular, osteoclasts, natural killer cells and B cells and T cells all display key defects in their cytoskeletal organization of actin and/or microtubules that result in loss of function. For example, B cells do not mature into immunoglobulin-secreting plasma cells (Doody et al., 2001; Stephenson et al., 2006), natural killer cells do not make functional cytolytic synapses (Graham et al., 2006) and osteoclasts undergo bone resorption inefficiently (Faccio et al., 2004). In the cecum, Vav expression is well positioned to affect terminal differentiation of surface enterocytes. Here, the major effect of Vav protein is to facilitate correct glycosylation of extra- and intracellular proteins, as well as to promote the increase in cell height and concurrent expansion of cytoplasmic microtubules that occurs as these cells differentiate. As this cell elongation is part of the normal terminal differentiation program of these cells, we interpret a defect in cell height in the Vav-deficient mice as a defect in the final functional differentiation of these cells, just as found in bone-marrow-derived cell types.
Vav1/2/3null upper zone enterocytes appear to contain a deficiency rather than a complete ablation of microtubule polymerization. Complete depolymerization of microtubules, either for brief time in vivo or for longer periods in vitro, results in an obvious loss of epithelial polarity as characterized by the formation of microvilli on the basolateral surface of enterocytes (Achler et al., 1989; Gilbert et al., 1991) and the abnormal nuclear position in the cell (Ingber, 2003). Vav1/2/3null upper zone enterocytes contained neither of these defects. The major consequence of the microtubule network deficiency of Vav1/2/3null colonic epithelium appears to be short enterocytes. Cellular tensegrity models have suggested that microtubules are akin to structural beams that bear external compression loads, whereas actin bears the tensional forces (Wang et al., 2003; Ingber, 2003). Thus, an absence of the normally robust microtubular network in the surface epithelium might account for the observed cell size defect through the loss of apical and basal cytoplasm.
We found that the normal increase in cell height of upper zone enterocytes also required Dlg1, a protein that we investigated because of its mislocalization in Vav1/2/3null enterocytes. We propose that Vav proteins and Dlg1 are part of a novel pathway that determines cell size, because their affects are distinct from cell-size changes previously described. For example, cellular elongation with apical constriction that occurs during invagination of cells at the base of epithelial or neuroepithelial cell sheets requires alterations of actin and microtubules (Fristrom, 1988), and is mediated by the Shroom family of PDZ-containing proteins (Haigo et al., 2003; Lee et al., 2007). Similarly, the transcription factor Mist1 appears to regulate the process of cell-shape change in zymogenic cells, because they differentiate and migrate to the base of invaginations in the mouse stomach epithelium (Ramsey et al., 2007). Colonic enterocytes probably do not need to undergo apical constriction because they are not part of an invagination process. Accordingly, we found that loss of Vav proteins was strictly in one dimension (height) and did not result in alteration in the cross-sectional area (cell dimensions in a plane parallel to the basement membrane) of colonic enterocytes, and these cells displayed only subtle alterations in actin-associated structures (density of microvilli). Therefore, it appears that colonic enterocytes utilize primarily microtubule alterations to mediate elongation (or height).
Another well-described pathway controlling cell size and shape is the S6-kinase-mTOR pathway, which senses nutrient availability and adjusts cell mass and proliferation (Ruvinsky and Meyuhas, 2006). Defects in S6 kinase, for example, lead to small cells in three dimensions. The Vav-mediated alterations in cell height are one-dimensional and do not occur in dividing cells, and thus appear to be distinct from this pathway.
Our studies suggest that loss-of-function mutations (such as Vav knockout) can be compatible with life and create subtle but reproducible epithelial defects that leave the animal susceptible to spontaneous mucosal damage over time. The challenge now is to determine the contribution of these novel epithelial defects in our mouse models and ultimately in human disease. Our initial studies suggest that wound formation and repair are defective in Vav1/2/3null mice. Our future goals include determining the relative contribution of epithelial cell height to wound repair and whether this process has a role in the development of inflammatory bowel diseases in humans.
Materials and Methods
Mice
All experiments involving animals were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Germline Vav1–/– and Vav2–/– mice were described previously (Turner et al., 1997; Tedford et al., 2001) and were gifts from Victor Tybulewicz (National Institute for Medical Research, London, UK) and Martin Turner (The Babraham Institute, Cambridge, UK). These mice were bred with Vav3–/– mice in a mixed C57BL/6-129Sv background. Dlg1flox/flox mice were previously described (Stephenson et al., 2007). Villin-Cre mice (Madison et al., 2002) were obtained from the Jackson Laboratories. All mice were maintained free of specified pathogens in a barrier facility under a 12-hour light cycle. All mice analyzed in this report were littermates derived from mating heterozygous animals. Wild-type Vav1/2/3null bone marrow chimeras were produced according to published procedures (Edelson and Unanue, 2002).
Endoscopic procedures
We used a high-resolution miniaturized colonoscope (Becker et al., 2004) consisting of a miniature rigid endoscope (1.9 mm outer diameter), xenon light source, and triple-chip high-resolution CCD camera (Karl Storz, Goleta, CA). Mice were anesthetized with ketamine/xylazine. Single full-thickness areas of the entire mucosa and submucosa were removed with flexible 3 Fr. biopsy forceps. 3-5 biopsies/mouse were taken along the dorsal side of the colon (spacing was >5 mm). To quantify the surface area of the excised mucosa immediately and every 2 days thereafter, we photographed each lesion and measured the diameter of the major and minor axis of each lesion. We calibrated images with a 0.5-mm-diameter rod. Lesions measuring 1.75±0.25 mm at major axis and 0.25±0.05 mm at minor axis at day 0 were selected for further analysis. The surface area of each lesion was determined using the measurements of the two axes.
Whole-mount analysis
The mouse gastrointestinal tract was flushed with PBS and pinned in Bouin's fixative for 4 hours at 4°C. The mucosal surface was viewed on an Olympus XZS22 microscope and photographed with an Olympus DP-70 digital camera.
Histochemical and immunohistochemistry analysis
For morphometric histological analysis, the small intestine, cecum and colon was embedded in 2% agar for routine paraffin processing. Serial 5-μm-thick sections were cut perpendicular to the crypt-surface axis and parallel to the rostral-caudal axis and stained with hematoxylin and eosin. For a subset of immunohistochemical studies, the intestine was dissected and frozen in OCT compound. Serial 5-μm-thick cryosections were cut, fixed in methanol (5 minutes at –20°C) and rehydrated in PBS. Sections were blocked in SNIPER (Biocare Medical) and incubated with the following antibodies (all analyzed in serial dilution from 1:100 to 1:1000): rabbit anti-human/mouse Vav1 (Santa Cruz Biotechnology); rabbit anti-human/mouse Vav2 (Santa Cruz Biotechnology); rabbit anti-mouse Vav3 (Upstate); rat anti-human/mouse CD49f (Integrin-Alpha-6) (Chemicon International, clone MA6); mouse anti-chicken villin (Serotec; clone 1D2C3); rabbit anti-actin (Sigma, C-terminus immunogen); mouse IgG1 anti-mouse β-catenin (Becton Dickinson, clone 14); mouse IgG2 anti-human E-cadherin (Becton Dickinson, clone 36); rabbit anti-CA4 (obtained from William Sly, St Louis University, MO); rabbit anti-PKCζ (Santa Cruz); and rabbit anti-occluden (Zymed); rabbit anti-ZO-1 (Zymed). Antigen-antibody complexes were visualized with Alexa Fluor 594- or Alexa Fluor 488-conjugated donkey anti-rabbit or anti-mouse IgG (Molecular Probes, 1:500). Nuclei were stained with bis-benzimide (50 ng/ml in PBS). Stained sections were mounted in 50% glycerol/PBS and viewed on a Zeiss Axiovert 200 with Axiocam MRM camera and with Apotome optical sectioning slider.
To visualize microtubules, dissected cecum and colons were gently flushed with PBSat 37°C, followed by 2% paraformaldehyde, 75 mM lysine, 25 mM taxol, 0.1% Triton X-100 in PBS. The intestine was opened along the mesenteric side, pinned mucosal side up and fixed for 20 minutes at 37°C. The whole mount was washed with 5% sucrose in PBS at 37°C and embedded in OCT. 5-μm-thick cryosections were cut along the cephalo-caudal axis. Sections were rehydrated in PBS (5 minutes) and pretreated with blocking buffer for 15 minutes, then incubated with FITC-conjugate mouse anti-chicken α-tubulin (Sigma, clone DM1A, 1:2000).
The relative intensity of α-tubulin staining in the apical cytoplasm of the surface to the crypt base epithelium was quantified using ApoTome to generate 3-μm-thick images for each slide. Mean intensity in each cellular compartment was measured using a single channel in the histogram viewer of Adobe Photoshop. Mean intensities of surface and crypt cells were only compared in the same image (n=3 mice per group and 20 images per mouse analyzed).
Sections of Bouins-fixed intestine were also incubated with a series of FITC-tagged lectins (all at a final concentration of 5 μg/ml blocking buffer) (Falk et al., 1994). The lectin panel consisted of the following members: (i) Helix pomatia agglutinin (Sigma, α-GalNAc/GalNAcβ4Gal-glycans), (ii) cholera toxin B-subunit [Sigma, GalNAcβ4(Neu5Acα2,3)Galβ] and (iii) Ulex europeaus agglutinin-1 (Sigma, Fucα1,3Galβ).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/3/324/DC1
We thank Emil Unanue and Andrey Shaw for helpful discussions and Tracie Kloeppel for technical assistance. This work was funded in part by National Institutes of Health Grants P30-DK52574, P30-AR048335, AI061077 (W.S.), DK071619 (T.S.), the Pew Scholars Program (T.S.) and the Washington University Digestive Diseases Research Center (P30 DK52574, T.S.). The authors have no conflicting financial interests. Deposited in PMC for release after 12 months.
References
- Achler, C., Filmer, D., Merte, C. and Drenckhahn, D. (1989). Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. J. Cell Biol. 109, 179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, C., Fantini, M. C., Schramm, C., Lehr, H. A., Wirtz, S., Nikolaev, A., Burg, J., Strand, S., Kiesslich, R., Huber, S. et al. (2004). TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491-501. [DOI] [PubMed] [Google Scholar]
- Bilder, D. and Perrimon, N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676-680. [DOI] [PubMed] [Google Scholar]
- Bustelo, X. R. (2001). Vav proteins, adaptors and cell signaling. Oncogene 20, 6372-6381. [DOI] [PubMed] [Google Scholar]
- Cau, J. and Hall, A. (2005). Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 118, 2579-2587. [DOI] [PubMed] [Google Scholar]
- Chang, W. W. and Leblond, C. P. (1971). Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am. J. Anat. 131, 73-99. [DOI] [PubMed] [Google Scholar]
- Chang, W. W. and Nadler, N. J. (1975). Renewal of the epithelium in the descending colon of the mouse. IV. Cell population kinetics of vacuolated-columnar and mucous cells. Am. J. Anat. 144, 39-56. [DOI] [PubMed] [Google Scholar]
- Clayburgh, D. R. (2004). A porous defense: the leaky epithelial barrier in intestinal disease. Lab. Invest. 84, 282-291. [DOI] [PubMed] [Google Scholar]
- Crean, J. K., Furlong, F., Finlay, D., Mitchell, D., Murphy, M., Conway, B., Brady, H. R., Godson, C. and Martin, F. (2004). Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J. 18, 1541-1543. [DOI] [PubMed] [Google Scholar]
- Doody, G. M., Bell, S. E., Vigorito, E., Clayton, E., McAdam, S., Tooze, R., Fernandez, C., Lee, I. J. and Turner, M. (2001). Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2, 542-547. [DOI] [PubMed] [Google Scholar]
- Drenckhahn, D. and Dermietzel, R. (1988). Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J. Cell Biol. 107, 1037-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson, B. T. and Unanue, E. R. (2002). MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169, 3869-3875. [DOI] [PubMed] [Google Scholar]
- Faccio, R., Teitelbaum, S. L., Fujikawa, K., Chappel, J., Zallone, A., Tybulewicz, V. L., Ross, F. P. and Swat, W. (2005). Vav3 regulates osteoclast function and bone mass. Nat. Med. 11, 284-290. [DOI] [PubMed] [Google Scholar]
- Falk, P., Roth, K. A. and Gordon, J. I. (1994). Lectins are sensitive tools for defining the differentiation programs of mouse gut epithelial cell lineages. Am. J. Physiol. 266, G987-G1003. [DOI] [PubMed] [Google Scholar]
- Farquhar, M. G. and Palade, G. E. (1963). Junctional complexes in various epithelia. J. Cell Biol. 17, 375-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure, S., Salazar-Fontana, L. I., Semichon, M., Tybulewicz, V. L., Bismuth, G., Trautmann, A., Germain, R. N. and Delon. J. (2004). ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat. Immunol. 5, 272-279. [DOI] [PubMed] [Google Scholar]
- Fey, E. G., Wan, K. M. and Penman, S. (1984). Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J. Cell Biol. 98, 1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristrom, D. (1988). The cellular basis of epithelial morphogenesis. Tissue Cell 20, 645-690. [DOI] [PubMed] [Google Scholar]
- Fujikawa, K., Miletic, A. V., Alt, F. W., Faccio, R., Brown, T., Hoog, J., Fredericks, J., Nishi, S., Mildiner, S., Moores, S. L. et al. (2004). Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 198, 1595-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto, K., Beauchamp, R. D. and Whitehead, R. H. (2002). Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology 123, 1941-1948. [DOI] [PubMed] [Google Scholar]
- Gakidis, M. A., Cullere, X., Olson, T., Wilsbacher, J. L., Zhang, B., Moores, S. L., Ley, K., Swat, W., Mayadas, T. and Brugge, J. S. (2004). Vav GEFs are required for 2 integrin-dependent functions of neutrophils. J. Cell Biol. 166, 273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, T., Le Bivic, A., Quaroni, A. and Rodriguez-Boulan, E. (1991). Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J. Cell Biol. 113, 275-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, D. B., Cella, M., Giurisato, E., Fujikawa, K., Miletic, A. V., Kloeppel, T., Brim, K., Takai, T., Shaw, A. S., Colonna, M. et al. (2006). Vav1 controls DAP-10 mediated natural cytotoxicity by regulating actin and microtubule dynamics. [DOI] [PubMed]
- Gustafsson, M. G. L. (1999). Extended resolution fluorescence microscopy. Curr. Opin. Struct. Biol. 9, 627-634. [DOI] [PubMed] [Google Scholar]
- Haigo, S. L., Hildebrand, J. D., Harland, R. M. and Wallingford, J. B. (2003). Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr. Biol. 24, 2125-2137. [DOI] [PubMed] [Google Scholar]
- Haramis, A. P., Begthel, H., van den Born, M., van Es, J., Jonkheer, S., Offerhaus, G. J. and Clevers, H. (2004). De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303, 1684-1686. [DOI] [PubMed] [Google Scholar]
- Hull, B. E. and Staehelin, J. L. (1979). The terminal web: a reevaluation of its structure and function. J. Cell Biol. 81, 67-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber, D. E. (2003). Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 116, 1157-1173. [DOI] [PubMed] [Google Scholar]
- Jaffe, A. B. and Hall, A. (2005). Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247-269. [DOI] [PubMed] [Google Scholar]
- Kang, S. S., Bloom, S. M., Norian, L., Geske, M. J., Flavell, R. A., Stappenbeck, T. S. and Allen, P. M. (2008). An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Med. 5, e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek, V., Barker, N., Moerer, P., van Donselaar, E., Huls, G., Peters, P. J. and Clevers, H. (1998). Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379-383. [DOI] [PubMed] [Google Scholar]
- Kuhnert, F., Davis, C. R., Wang, H. T., Chu, P., Lee, M., Yuan, J., Nusse, R. and Kuo, C. J. (2004). Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA 101, 266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C., Scherr, H. M. and Wallingford, J. B. (2007). Shroom family proteins regulate {gamma}-tubulin distribution and microtubule architecture during epithelial cell shape change. Development 7, 1431-1441. [DOI] [PubMed] [Google Scholar]
- Madison, B. B., Dunbar, L., Qiao, X. T., Braunstein, K., Braunstein, E. and Gumucio, D. L. (2002). Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275-33283. [DOI] [PubMed] [Google Scholar]
- Mahoney, Z. X., Sammut, B., Xavier, R. J., Cunningham, J., Go, G., Brim, K. L., Stappenbeck, T. S., Miner, J. H. and Swat, W. (2006). Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc. Natl. Acad. Sci. USA 103, 19872-19877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, F. C., Takahashi, Y., Kreimann, E. L. and Georgescu, M. M. (2004). Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci. USA 101, 17705-17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch, A. (2004). Microtubule organization and function in epithelial cells. Traffic 5, 1-9. [DOI] [PubMed] [Google Scholar]
- Pinto, D., Robine, S., Jaisser, F., El Marjou, F. E. and Louvard, D. (1999). Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J. Biol. Chem. 274, 6476-6482. [DOI] [PubMed] [Google Scholar]
- Popesko, P., Rajtová, V. and Horák, J. (2003). A Colour Atlas of the Anatomy of Small Laboratory Animals. St Louis, MO: Saunders.
- Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. and Stappenbeck, T. S. (2005). Activated macrophages are an adaptive element to the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 102, 99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey, V. G., Doherty, J. M., Chen, C. C., Stappenbeck, T. S., Konieczny, S. F. and Mills, J. C. (2007). The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134, 211-222. [DOI] [PubMed] [Google Scholar]
- Ruvinsky, I. and Meyuhas, O. (2006). Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 6, 342-348. [DOI] [PubMed] [Google Scholar]
- Sancho, E., Batlle, E. and Clevers, H. (2003). Live and let die in the intestinal epithelium. Curr. Opin. Cell Biol. 15, 763-770. [DOI] [PubMed] [Google Scholar]
- Saotome, I. M., Curto and A. I., McClatchey. (2004). Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 6, 855-864. [DOI] [PubMed] [Google Scholar]
- Savage, D. C. (1977). Microbial ecology of the gastrointestinal tract. Ann. Rev. Microbiol. 31, 107-133. [DOI] [PubMed] [Google Scholar]
- Stappenbeck, T. S. and Gordon, J. I. (2000). Rac1 mutations produce aberrant epithelial differentiation in the developing and adult mouse small instestine. Development 127, 2629-2642. [DOI] [PubMed] [Google Scholar]
- Stappenbeck, T. S. and Gordon, J. I. (2001). Extranuclear sequestration of phosphor-N-terminal kinase and distorted villi produced by activated Rac1 in the intestinal epithelium of chimeric mice. Development 128, 2603-2614. [DOI] [PubMed] [Google Scholar]
- Stappenbeck, T. S., Hooper, L. V. and Gordon, J. I. (2002). Developmental regulation of intestinal vasculogenesis by indigenous microbes via Paneth cells. Proc. Natl. Acad. Sci. USA 99, 15451-15456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, L. M., Miletic, A. V., Kloeppel, T., Kusin, S. and Swat, W. (2006). Vav proteins regulate the plasma cell program and secretory Ig production. J. Immunol. 177, 8620-8625. [DOI] [PubMed] [Google Scholar]
- Stephenson, L. M., Sammut, B., Graham, D. B., Chan-Wang, J., Brim, K. L., Huett, A. S., Miletic, A. V., Kloeppel, T., Landry, A., Xavier, R. et al. (2007). DLGH1 is a negative regulator of T-lymphocyte proliferation. Mol. Cell. Biol. 27, 7574-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swat, W. and Fujikawa, K. (2005). The vav family: at the crossroads of signaling pathways. Immunol Res. 32, 259-266. [DOI] [PubMed] [Google Scholar]
- Tedford, K., Nitschke, L., Girkontaite, I., Charlesworth, A., Chan, G., Sakk, V., Barbacid, M. and Fischer, K. D. (2001). Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2, 548-555. [DOI] [PubMed] [Google Scholar]
- Turner, M., Mee, P. J., Walters, A. E., Quinn, M. E., Mellor, A. L., Zamoyska, R. and Tybulewicz, V. L. (1997). A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7, 451-460. [DOI] [PubMed] [Google Scholar]
- van den Brink, G. R., Bleuming, S. A., Hardwick, J. C., Schepman, B. L., Offerhaus, G. J., Keller, J. J., Nielsen, C., Gaffield, W., van Deventer, S. J., Roberts, D. J. et al. (2004). Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 36, 277-282. [DOI] [PubMed] [Google Scholar]
- van Es, J. H., van Gijn, M. E., Riccio, O., van den Born, M., Vooijs, M., Begthel, H., Cozijnsen, M., Robine, S., Winton, D. J., Radtke, F. et al. (2005). Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959-963. [DOI] [PubMed] [Google Scholar]
- Wang, N., Butler, J. P. and Ingber, D. E. (1993). Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124-1127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.