Summary
Depletion of EHD3 affects sorting in endosomes by altering the kinetics and route of receptor recycling to the plasma membrane. Here we demonstrate that siRNA knockdown of EHD3, or its interaction partner rabenosyn-5, causes redistribution of sorting nexin 1 (SNX1) to enlarged early endosomes and disrupts transport of internalized Shiga toxin B subunit (STxB) to the Golgi. Moreover, under these conditions, Golgi morphology appears as a series of highly dispersed and fragmented stacks that maintain characteristics of cis-, medial- and trans-Golgi membranes. Although Arf1 still assembled onto these dispersed Golgi membranes, the level of AP-1 γ-adaptin recruited to the Golgi was diminished. Whereas VSV-G-secretion from the dispersed Golgi remained largely unaffected, the distribution of mannose 6-phosphate receptor (M6PR) was altered: it remained in peripheral endosomes and did not return to the Golgi. Cathepsin D, a hydrolase that is normally transported to lysosomes via an M6PR-dependent pathway, remained trapped at the Golgi. Our findings support a role for EHD3 in regulating endosome-to-Golgi transport, and as a consequence, lysosomal biosynthetic, but not secretory, transport pathways are also affected. These data also suggest that impaired endosome-to-Golgi transport and the resulting lack of recruitment of AP-1 γ-adaptin to Golgi membranes affect Golgi morphology.
Keywords: EHD3, Early endosome, Golgi, Retrograde transport, Mannose-6-phosphate receptor
Introduction
The transport of membranes and proteins through the endocytic pathways is a highly regulated process controlled by several proteins (Conner and Schmid, 2003). Upon receptor internalization, either through clathrin-coated pits or in a clathrin-independent manner, the internalized vesicles fuse with sorting organelles known as early endosomes. From early endosomes, receptors undergo various possible fates. They may be targeted to the lysosomal degradation pathway for degradation, or alternatively, they may be returned to the plasma membrane by recycling that can occur either directly from the early endosomes (`fast recycling') or indirectly through tubulovesicular membrane structures localized to the perinuclear region of the cell collectively known as the recycling endosome (Daro et al., 1996; Sheff et al., 1999). However, an important group of intracellular sorting receptors and several extracellular toxins, including the Shiga toxin B subunit (STxB) and Cholera Toxin B subunit (CTxB), undergo transport from endosomes to the Golgi apparatus (Bonifacino and Rojas, 2006). Although the function of several key proteins involved in this retrograde pathway has been characterized, additional regulators await identification.
Anterograde transport from the Golgi to endosomes is crucial for biosynthetic transport and for the delivery of lysosomal hydrolases to the degradatory pathway, mediated by the cation-dependent and cation-independent mannose-6-phosphate receptors (M6PRs). A number of different mechanisms have been identified for retrieval of M6PRs from the endocytic pathway back to the Golgi (Bonifacino and Rojas, 2006). For example, the mammalian retromer complex is comprised of sorting nexin 1 (SNX1) and SNX2, VPS26A and VPS26B, VPS29 and VPS35 (Hierro et al., 2007). The retromer is a major pathway by which M6PRs are recycled to the Golgi (Bujny et al., 2007; Canuel et al., 2008; Popoff et al., 2007; Rojas et al., 2007). AP-1 (Doray et al., 2002; Meyer et al., 2001), GGA proteins (Doray et al., 2002; Puertollano et al., 2001), PACS1 (Crump et al., 2001), Rab9 (Ganley et al., 2004; Lombardi et al., 1993) and TIP47 (Diaz and Pfeffer, 1998), in addition to several SNARE proteins (Mallard et al., 2002), have also been implicated in late endosome and perhaps early-endosome-to-Golgi transport, which is necessary for the recycling of M6PRs. However, endosome-to-Golgi transport is also critical for the internalization of bacterial toxins, such as the STxB (Amessou et al., 2007; Sandvig et al., 1991). Indeed, recent studies show that retromer function is necessary to allow STxB trafficking from endosomes to the Golgi (Bujny et al., 2007; Popoff et al., 2007; Utskarpen et al., 2007).
In addition to the well characterized Rab GTP-binding proteins (Pfeffer, 2001; Zerial and McBride, 2001), another family of proteins recently implicated in the regulation of trafficking through endocytic pathways is the C-terminal Eps15 homology domain-containing (EHD) family (reviewed by Naslavsky and Caplan, 2005; Grant and Caplan, 2008). These proteins contain a nucleotide-binding motif close to their N-terminus that prefers ATP, and a central region implicated in oligomerization (Daumke et al., 2007; Lee et al., 2005; Naslavsky et al., 2006). At their C-terminus, they contain a highly conserved stretch of ∼100 amino acids known as the Eps15 homology (EH) domain (Fazioli et al., 1993), which binds to proteins containing the tripeptide Asp-Pro-Phe (NPF) (de Beer et al., 1998; de Beer et al., 2000). Recently, the solution structure of the EH domain of EHD1 has been solved by NMR (Kieken et al., 2007), and it has been shown that this domain interacts with phosphatidylinositol compounds (Naslavsky et al., 2007).
In mammalian cells, the four C-terminal EHD paralogs share ∼70-85% identity and regulate distinct but partly overlapping endocytic transport events (Naslavsky and Caplan, 2005). Mammalian EHD1 (also known as RME-1) and its Caenorhabditis elegans homolog regulate the recycling of transferrin receptor and yolk receptor, respectively (Grant et al., 2001; Lin et al., 2001). Subsequent studies have provided evidence that EHD1 not only controls the recycling of transferrin and other receptors that are internalized through clathrin-coated pits (Guilherme et al., 2004b; Picciano et al., 2003), but also receptors that are internalized independently of clathrin, such as major histocompatibility class I (MHCI) (Caplan et al., 2002; Shi et al., 2007) and β1 integrins (Jovic et al., 2007).
The major site of EHD1 action is at the recycling endosome [also termed the endocytic recycling compartment (Maxfield and McGraw, 2004)], in regulating transport of receptors to the plasma membrane (Caplan et al., 2002; Grant et al., 2001; Lin et al., 2001). Although the exact mechanism by which it functions is only partly understood, EHD1 cooperates with the Arf6 to promote recycling via an array of perinuclear tubular and vesicular structures (Caplan et al., 2002). EHD1 intersects with Rab-dependent regulation by interacting with at least two Rab effector proteins, rabenosyn-5 (Naslavsky et al., 2004) and Rab11-FIP2 (Naslavsky et al., 2006). EHD2, whose crystal structure was also recently solved (Daumke et al., 2007), links internalization with the actin microfilament system through an actin-binding interaction partner known as EH-binding protein 1 (EHBP1) (Guilherme et al., 2004a) and is involved in myoblast fusion (Doherty et al., 2008). EHD4 is primarily involved in the regulation of early endosome transport (George et al., 2007) (Sharma et al., 2008). EHD3, the closest paralog of EHD1, has been implicated in the regulation of early-endosome-to-recycling-endosome transport, raising the question that it might have a general role in regulating trafficking between the early endosome and other organelles (Naslavsky et al., 2006). In the current study, we have identified EHD3 as a regulator of endosome-to-Golgi transport, and provide evidence for a mechanism by which it controls retrograde transport.
Results
EHD3 depletion causes redistribution of SNX1 and impairs accessibility of internalized Shiga toxin B subunit to the Golgi
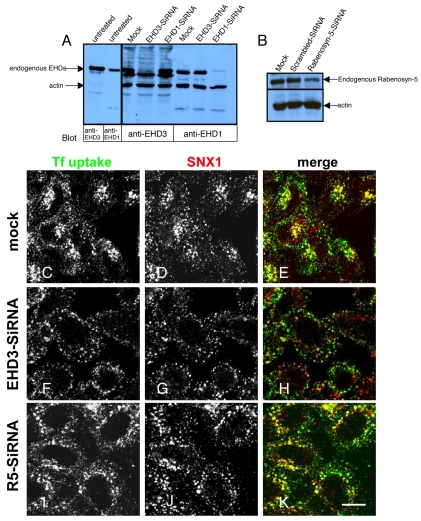
We have previously shown that depletion of EHD3 impairs transport from early endosome to the perinuclear recycling endosome (Naslavsky et al., 2006). To determine whether EHD3 is also involved in the regulation of endosome-to-Golgi transport, we first assayed the distribution of the retromer complex subunit SNX1, which is involved in this transport pathway (Mari et al., 2008). Accordingly, we first established an efficient and specific depletion of EHD3. As indicated (Fig. 1A), untreated, mock-treated, and HeLa cells treated with EHD1 siRNA showed clearly discernable levels of endogenous EHD3 expression, whereas endogenous EHD3 expression was reduced to undetectable levels in EHD3 siRNA-treated cells. To further confirm specificity, we also demonstrated that endogenous EHD1 expression remained unaffected upon EHD3 siRNA treatment (Fig. 1A).
Fig. 1.
SNX1 is retained in enlarged early endosomes upon knockdown of EHD3 or rabenosyn-5. (A) HeLa cells were subjected to mock or siRNA treatment for 48 hours to knock down EHD3 or EHD1. The two left lanes are lysate of untreated HeLa cells, denoting the size of the corresponding endogenous proteins, as detected by specific antibodies. (B) HeLa cells were mock-treated or treated with siRNA for 48 hours to knock down endogenous rabenosyn-5 (R5). In both immunoblots, equal amounts of protein from each sample were run on SDS-PAGE and immunoblotted with polyclonal anti-rabenosyn-5. Actin was used in all gels as a control for protein content. (C-E) Mock-treated cells, (F-H) EHD3 siRNA-treated cells and (I-K) rabenosyn-5 siRNA-treated cells were starved for 30 minutes and allowed to internalize Alexa Fluor 488-conjugated-Tf for 7 minutes, followed by a `chase' of 8 minutes (37°C) in complete medium. After fixation, immunostaining of endogenous SNX1 was performed with a monoclonal antibody followed by Alexa Fluor 568 goat-anti mouse secondary antibody. Scale bar: 10 μm.
To confirm the functional efficacy of the EHD3 siRNA, cells were pulsed and chased with Transferrin-568 (Tf-568). In mock-treated cells, Tf-568 reached the recycling endosome and also was localized within early endosome after 8 minutes of chase (Fig. 1C). In these cells, SNX1 extensively colocalized with internalized Tf-568 at the perinuclear region (containing the Golgi and recycling endosome), as well as in peripheral early endosomes (Fig. 1D,E). By contrast, the Tf-568 in EHD3 siRNA cells was not observed in the recycling endosome, but was contained in large peripheral structures previously shown to be early endosomes (Naslavsky et al., 2006), consistent with our earlier studies (Fig. 1F). In EHD3-depleted cells, SNX1 was also absent from the perinuclear Golgi-recycling-endosome region, and appeared to be retained in Tf-568-positive peripheral early endosomes (Fig. 1G,H).
Rabenosyn-5 (RBNS5) is an early endosomal divalent Rab4/5 effector that interacts with EHD3 and EHD1. To determine whether rabenosyn-5 also regulates SNX1 subcellular distribution, we performed similar siRNA knockdown experiments. As indicated, rabenosyn-5 siRNA reduced endogenous levels of the protein to less than 20% of that in the mock-treated cells (Fig. 1B). Indeed, as previously reported, rabenosyn-5 depletion had a similar effect on Tf-568 to that of EHD3 depletion; Tf-568 was retained in large peripheral early endosomes and could rarely be seen in the perinuclear recycling endosome region, unlike normal Tf-568 distribution in mock-treated cells (Fig. 1I). Moreover, SNX1 was redistributed from the perinuclear region to peripheral early endosome structures in a manner similar to that observed for EHD3-depleted cells (Fig. 1J,K). A similar redistribution was observed with the retromer complex subunit SNX2 (our unpublished observations). These data suggest that EHD3 and its early endosomal interaction partner, rabenosyn-5, affect SNX1 and SNX2 localization, and thus may regulate transport from early endosomes to the recycling endosome or Golgi.
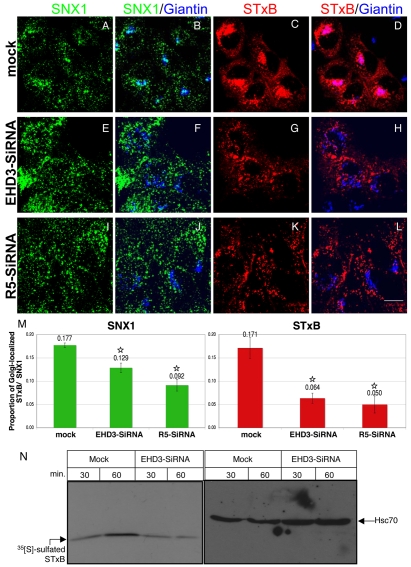
Given the altered distribution of SNX1 and SNX2 at steady state, we next questioned whether traffic from endosomes to the Golgi is affected by EHD3 depletion by investigating internalization of fluorescently tagged STxB. In HeLa cells, as few as 18 minutes of internalization at 37°C was sufficient to concentrate most of the STxB at the Golgi (our unpublished observations). With a longer internalization period of 35 minutes, STxB could be observed accessing the perinuclear area and colocalizing with giantin (GOLGB1), a marker for cis or medial Golgi (Fig. 2C,D; colocalization is pink in D). A pool of SNX1 also colocalized with giantin (Fig. 2A,B; colocalization is light blue in B). SNX1 and STxB also demonstrated some colocalization (merge not shown). By contrast, loss of EHD3 expression caused accumulation of STxB in the periphery (Fig. 2G). Compared with mock-treated cells under these conditions, only 37% of the STxB reached the Golgi (Fig. 2G,H, quantified in Fig. 2M). The lack of pink overlay in Fig. 2H demonstrates limited STxB access to the Golgi. Importantly, SNX1 levels at the Golgi were also reduced in EHD3-depleted cells (Fig. 2E,F; note the lack of light blue color in Fig. 2F indicating poor colocalization, results quantified in Fig. 2M). Since depletion of rabenosyn-5 also altered the SNX1 distribution pattern at steady state, we monitored internalized STxB transport to the Golgi upon rabenosyn-5 depletion. As shown, STxB transport to the Golgi was again delayed, with only 29% of the level in mock-treated cells reaching the Golgi (Fig. 2K,L, quantified in Fig. 2M), and likewise SNX1 was largely absent from the Golgi (Fig. 2I,J, quantified in Fig. 2M). The mis-sorted STxB-containing vesicles observed in EHD3- and rabenosyn-5-depleted cells did not redistribute into classical early endosomes or recycling endosomes, because they were negative for Rab11 (supplementary material Fig. S1C), displayed weak overlap with the early endosome marker EEA1 (supplementary material Fig. S1B), but showed significant colocalization with the retromer subunit Vps35 (supplementary material Fig. S1A). This distribution pattern suggests that the STxB was trapped in a post-early-endosomal transport carrier.
Fig. 2.
Depletion of EHD3 or rabenosyn-5 traps internalized STxB in endosomes and delays its progression to the Golgi. HeLa cells were mock treated (A-D) or treated with siRNA for 48 hours to reduce EHD3 (E-H) or rabenosyn-5 (R5) (I-L). 546-STxB was bound to cells on ice (30 minutes) and uptake was accomplished by shifting the cells to 37°C in complete medium for an additional 35 minutes. After fixation, endogenous SNX1 and giantin were co-stained with their respective antibodies, followed by Alexa Fluor 488 goat anti-mouse and Alexa Fluor 405 goat anti-rabbit, respectively. (M) The colocalization of SNX1 at the Golgi is shown in a quantitative analysis (left graph shows results from B,F,J). Likewise, the influx of STxB into the Golgi is summarized in M (right graph shows results from D,H,L). *P<0.01. (N) Sulfation analysis of the STxB variant as a measure of its arrival at the TGN. Sulfation-site-tagged STxB was allowed to bind to mock-treated and EHD3 siRNA-treated cells on ice. Cells were then shifted to 37°C in sulfate-free medium containing [35S]sulphate for 30 and 60 minutes. Samples were immunoprecipitated with anti STxB (right blot). The same samples were precipitated with TCA and immunoblotted with anti-Hsc70 to show equivalent loading in each lane (left blot). Overall sulfation levels of cellular proteins remained largely unchanged (data not shown). Scale bar: 10 μm.
These findings were further supported by our biochemical analysis, exploiting trans-Golgi network (TGN)-localized sulfotransferase activity to detect arrival of the sulfation-site-tagged STxB variant at the TGN (Mallard and Johannes, 2003) (Fig. 2N). In mock-treated cells, the level of sulfated (TGN-localized) STxB increased two- to threefold between 30 and 60 minutes after internalization (Fig. 2N, left panel). Specificity and loading in this experiment was controlled by immunoblotting with antibodies that recognize the ubiquitous 70 kDa heat-shock protein (Hsc70) (Fig. 2N, right panel). However, upon EHD3 depletion, no increase in sulfated STxB was detected, indicating that endosome-to-Golgi transport is impaired in the absence of EHD3.
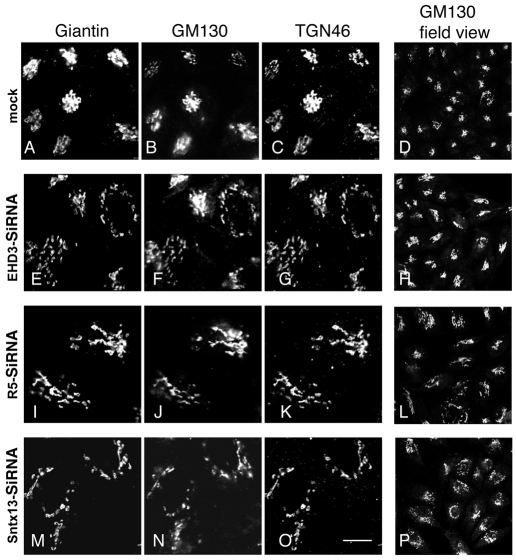
EHD3 depletion alters Golgi morphology by fragmenting and dispersing Golgi cisternae
We hypothesized that proteins that cycle between early endosomes and the Golgi might be trapped in the peripheral vesicles upon EHD3 depletion. These proteins sustain formation and maintenance of the Golgi, thus Golgi morphology would be affected by their absence. To determine whether Golgi morphology was indeed influenced by the loss of EHD3 expression, HeLa cells were either mock treated (Fig. 3A-D), or treated with EHD3 siRNA (Fig. 3E-H), rabenosyn-5 siRNA (Fig. 3I-L) or siRNA for the early endosomal SNARE protein, syntaxin13 (STX13) (Fig. 3M-P), which has been implicated in endocytic recycling (Prekeris et al., 1998). By immunostaining with the cis- and medial-Golgi markers GM130 and giantin, as well as the trans-Golgi marker TGN46, it was evident that the usual tightly packed Golgi stacks observed in mock-treated cells (Fig. 3A-D) became highly fragmented and dispersed, with structures observed primarily in the periphery upon depletion of the endosomal regulatory proteins (Fig. 3E-P). Morphological alterations in the Golgi were observed in almost 100% of the treated cells, with most cells displaying a Golgi that acquired a more vesiculated pattern. However, some treated cells displayed `stretched' or elongated peripheral stacks. No significant difference was observed in the phenotype induced by the three early endosomal proteins, with each causing a wide array of heterogeneous phenotypic abnormalities. Close examination of the loss of EHD3, rabenosyn-5 or STX13 indicated a mild enhancement of the number of actin stress fibers observed, as well as a dispersal of the MTOC (Microtubule organizing center) to a less dense and more ring-like structure surrounding the nucleus (supplementary material Fig. S2), but the potential significance of these findings is not known. However, we observed no evidence of Golgi merging with early endosomes, because these vesiculated stacks did not acquire the early endosomal marker EEA1 (our unpublished observations). Overall, these data point to a regulatory role for EHD3 and the early endosomal proteins rabenosyn-5 and STX13 in the control of Golgi maintenance and morphology.
Fig. 3.
Golgi stacks undergo morphological alterations upon knockdown of EHD3, rabenosyn-5 and Syntaxin13, but retain cis- and trans-Golgi markers. Mock-treated HeLa cells (A-D), or siRNA treatment for EHD3 (E-H), rabenosyn-5 (I-L) or STX13 (M-P) were fixed and permeabilized. Cis and medial Golgi was visualized with mouse anti-GM130 and rabbit anti-giantin and the trans Golgi was detected with sheep anti-TGN46. Secondary antibodies were: Alexa Fluor 405 goat anti-mouse, Alexa Fluor 488 goat anti-rabbit and Cy3 donkey anti-sheep, respectively). D, H, L and P are field views of cells stained with anti-GM130. Scale bar, 10 μm.
Depletion of EHD3 or its early endosomal interaction partner rabenosyn-5 affects lysosomal biosynthetic transport from the Golgi, but does not impair secretion
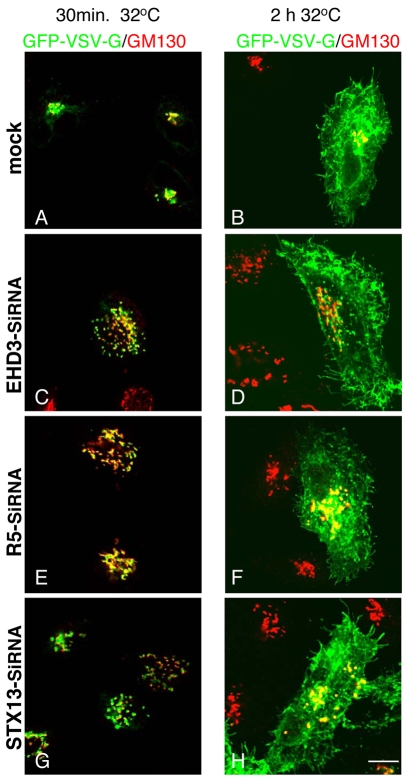
We next addressed the potential consequences of EHD3 knockdown on Golgi function. First, we tested whether EHD3 depletion affected the secretory pathway from the fragmented Golgi structures to the plasma membrane. We therefore transfected the temperature-sensitive GFP-VSV-G as a model to monitor transport through the secretory pathway. Within 30 minutes of shifting the cells from 40°C to 32°C, most of the GFP-VSV-G could be detected in a compact GM130-positive Golgi in mock-treated cells (Fig. 4A), or within dispersed Golgi stacks upon depletion of EHD3, rabenosyn-5 or STX13 (Fig. 4C,E,G), demonstrating that the ER-to-Golgi portion of the anterograde secretory pathway remained unaffected. Moreover, in mock-treated and all the siRNA-treated cells, GFP-VSV-G was able to reach the plasma membrane within 2 hours at 32°C (Fig. 5B,D,F,H), indicating that the impaired early-endosome-to-Golgi transport and fragmentation of the Golgi had little or no effect on the trafficking of GFP-VSV-G through the latter half of the secretory pathway.
Fig. 4.
EHD3 depletion does not impair the secretory route of VSV-G from vesiculated Golgi to the plasma membrane. HeLa cells were transfected with the temperature-sensitive GFP-VSV-G and concurrently mock treated (A,B) and siRNA treated to knock down EHD3 (C,D), rabenosyn-5 (R5) (E,F) or STX13 (G,H) for 48 hours. In the last 18 hours the cells were shifted to 40°C, leading to accumulation of GFP-VSV-G in the endoplasmic reticulum. To induce transport of GFP-VSV-G into the Golgi, cells were then transferred to 32°C for 30 minutes and fixed (A,C,E,G). To allow transport via the secretory pathway from the Golgi to the plasma membrane, cells were kept at 32°C for an additional 2 hours (B,D,F,H). Golgi cisternae were detected with anti-GM130 (in red). Scale bar, 10 μm.
Fig. 5.
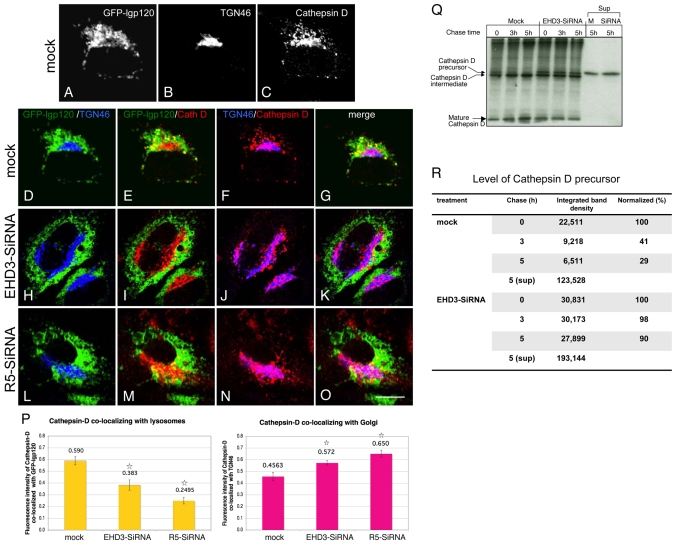
Depletion of EHD3 or rabenosyn-5 causes impaired biosynthetic transport of cathepsin-D to lysosomes. HeLa cells, transfected with GFP-lgp120, were mock treated (A-G) or treated with EHD3 siRNA (H-K) or rabenosyn-5 (L-O) for 48 hours before fixation. TGN was detected with rabbit anti-TGN46 followed by Alexa Fluor 405-conjugated goat anti-rabbit. Anti-cathepsin-D was used to visualize endogenous cathepsin-D, followed by Alexa Fluor 568-conjugated goat anti-mouse. A-C are single-stain images of a mock-treated cell. Triple-stained images are split into pairs of colors to facilitate visualization of the distribution patterns, with the corresponding merged images (G,K,O). The graphs in P are a quantitative analysis of colocalization (see Materials and Methods) between cathepsin-D and GFP-lgp120 (left graph, representing E, I and M), whereas the right graph quantifies the colocalization of cathepsin-D with TGN46, representing F, J and N. *P<0.01 compared with mock-treated cells. (Q) Mock- and EHD3-siRNA-treated HeLa cells were pulsed with [35S]cysteine/methionine and chased for the indicated times. The cells were lysed, and immunoprecipitated with anti-cathepsin D antibody. At the 5 hour time point, the entire supernatant was collected and immunoprecipitated with anti-cathepsin-D, to detect levels of mis-sorted cathepsin D that has been secreted from the TGN. Samples were separated by SDS-PAGE prior to exposure of the gels to autoradiograhic film. (R) Levels of cathepsin-D precursor bands were quantified by densitometry from the representative gel displayed in Q. Scale bar: 10 μm.
We then analyzed the effect of EHD3-and rabenosyn-5-depletion on biosynthetic transport to late endosomes and lysosomes. We monitored the transport of cathepsin D, a lysosomal lumenal hydrolase. Cathepsin D normally interacts with M6PR at the TGN and is escorted to the late endosomal pathway, from which it accesses the lysosomal lumen. Cells were transfected with GFP-lgp120, a lysosomal membrane marker, and co-stained for trans Golgi (TGN46) and cathepsin D. In mock-treated cells, as expected, the GFP-lgp120 showed little overlap with the Golgi (Fig. 5A,B,D, Golgi in blue). Cathepsin D in these cells overlapped with GFP-lgp120 (Fig. 5A,C,E, overlap in yellow), because both proteins are generally lysosomal residents. A small proportion of cathepsin D was also present in the TGN (Fig. 5B,C,F, overlap in pink), which is presumably newly synthesized enzyme not yet transported to the lysosomal lumen. Upon depletion of either EHD3 (Fig. 5H-K) or rabenosyn-5 (Fig. 5L-O), the Golgi was distended and decentralized (Fig. 5H,L, Golgi in blue) as previously shown in Fig. 3. However, a significantly larger proportion of cathepsin D was now retained in the Golgi (Fig. 5J,N, overlap displayed in pink). At the same time, less cathepsin D colocalized with lgp120 (Fig. 5, compare E with I and M). Quantitative analysis measurements using Pearson's correlation (Bolte and Cordelieres, 2006) demonstrated a statistically significant reduction of cathepsin D localized to lgp120-positive lysosomes upon EHD3 depletion (35%) or rabenosyn-5 depletion (58%) (Fig. 5P, left graph). These data also correlated with increases of ∼21% and ∼30% in cathepsin D retained at the TGN in cells lacking EHD3 or rabenosyn-5, respectively (Fig. 6P, right graph).
Fig. 6.
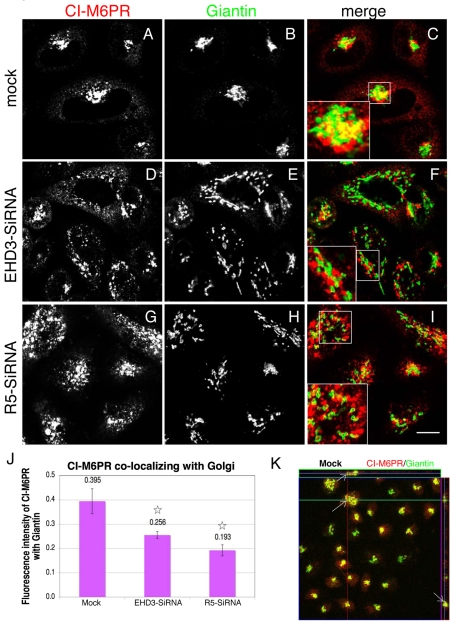
Depletion EHD3 and rabenosyn-5 cause impaired mannose-6-phosphate receptor retrieval to the Golgi. Mock-(A-C and K), EHD3 siRNA (D-F) or rabenosyn-5 siRNA-treated cells (G-I) were fixed and stained with antibodies against the cation-independent mannose 6-phosphate receptor (CI-M6PR) and giantin, followed by Alexa Fluor 568 goat anti-mouse and Alexa Fluor 488 goat anti-rabbit secondary antibodies, respectively. Insets depict co-staining for CI-M6PR and giantin. Colocalization of CI-M6PR and giantin is quantified in J and represents 70-90 cells (see Materials and Methods). *P<0.01 compared with mock-treated controls. (K) Z-section analysis was performed to assess three-dimensional colocalization between CI-M6PR and giantin in mock-treated cells. Arrows denote specific puncta at the Golgi, whose colocalization is analyzed in three dimensions. The green horizontal line in the image represents the slice that was analyzed, shown to the right of the image as the z-axis. The slice above the image represents the x-y axis. In both slices, a middle line is seen, representing the actual focal plane for this image. Scale bar: 10 μm.
To provide additional biochemical support for our findings that cathepsin D is impaired in its ability to traffic from the TGN to lysosomes, we pulse-labeled mock- and EHD3 siRNA-treated cells with [35S]cysteine/methionine and monitored the rate of processing of precursor cathepsin D (∼50 kDa) to its intermediate (∼47 kDa) and mature (∼31 kDa) forms (Fig. 5Q). The proteolytic processing of cathepsin D generally reflects its transport from the TGN to the lysosomal lumen. As indicated, even at steady state (Fig. 5Q, 0 time), a greater proportion of precursor was detected in the EHD3 siRNA-treated cells (quantified in Fig. 5R). Moreover, whereas the mock-treated cells showed an almost complete loss of the precursor cathepsin D form and a two- to threefold increase in the level of mature cathepsin D after 5 hours of chase, EHD3 depletion led to little or no change in the levels of precursor, intermediate and mature cathepsin D forms. In addition, ∼35% more precursor cathepsin D was found to be secreted to the supernatant upon EHD3 depletion after 5 hours (Fig. 5Q and quantified in R). Collectively, these data demonstrate that the biosynthetic transport of cathepsin D from the Golgi to the lysosomal lumen is impaired upon knockdown of EHD3 or rabenosyn-5.
Retention of cathepsin D in the TGN results from an altered subcellular distribution of CI-M6PR
Mannose-6-phosphate receptors (M6PRs) play a crucial role in targeting lysosomal hydrolases to the endocytic pathway, and ultimately to the lysosomal lumen. We next assessed whether the mechanism of cathepsin D retention at the TGN results, at least in part, from impaired trafficking and/or altered subcellular distribution of the cation-independent mannose-6-phosphate receptor (CI-M6PR). In mock-treated cells, CI-M6PR was concentrated in the perinuclear region and displayed significant overlap with the Golgi marker giantin (Fig. 6A-C). To confirm that this overlap occurs in three dimensions, serial z-sections were obtained and a representative micrograph shows overlap between CI-M6PR and giantin in all three dimensions (Fig. 6K). As we have previously discussed, loss of EHD3 expression (Fig. 6D-F) or rabenosyn-5 (Fig. 6G-I) led to the fragmentation and dispersal of the Golgi (Fig. 6E,H). Interestingly, in these cells, CI-M6PR was also dispersed to the periphery and now showed only weak overlap with the Golgi, suggesting that CI-M6PR could not be efficiently recycled back to the Golgi (Fig. 6D-I). The CI-M6PR in these structures was partially colocalized with EEA1, but only displayed minor overlap with TGN46 (supplementary material Fig. S1F,G). Quantitative analysis (Fig. 6J) shows a statistically significant decrease of CI-M6PR in the Golgi by 35% and 51% for EHD3- and rabenosyn-5-depleted cells, respectively. These data support a model by which CI-M6PR recycling from endosomes to the Golgi is impaired in EHD3-depleted cells, therefore leading to the retention of cathepsin D in the TGN.
Recruitment of γ-adaptin to the Golgi is impaired upon depletion of EHD3, rabenosyn-5 or STX13
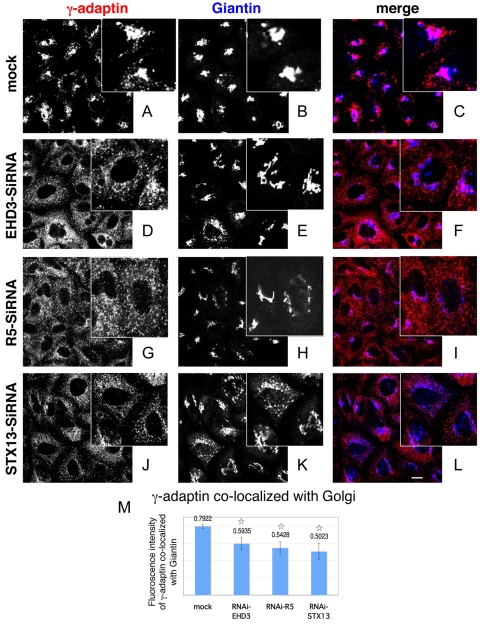
It has been found that retrograde retrieval of CI-M6PR from the endosomal pathway to the Golgi is mediated by the AP-1 adaptor complex (Meyer et al., 2000). Therefore, to further our understanding of the mechanism by which biosynthetic transport is impaired upon knockdown of EHD3, rabenosyn-5 (R5) or syntaxin13 (STX13), we assessed the ability of the AP-1 complex protein γ-adaptin (AP1G1) to be recruited to the Golgi. As expected, in mock-treated HeLa cells, γ-adaptin displayed a distinct concentration in the giantin-positive Golgi region (Fig. 7A-C, colocalization at the Golgi indicated in pink). However, upon depletion of EHD3, rabenosyn-5 or STX13, γ-adaptin was dramatically relocalized to a highly scattered pattern throughout the cells, which showed a significant decrease in colocalization with the dispersed Golgi (Fig. 7D-L) (note the decreased pink color in the merges, indicating diminished colocalization of γ-adaptin with giantin). The γ-adaptin in these structures partially colocalized with Vps35, but showed only very weak overlap with EEA1 (supplementary material Fig. S1D,E, summarized in Fig. S1H). Quantification analysis (Fig. 7M) demonstrates that recruitment of γ-adaptin to giantin-containing Golgi was reduced by 25-30% compared with that in mock-treated cells, probably as a consequence of reduced AP-1 recruitment under these conditions.
Fig. 7.
Depletion of EHD3, rabenosyn-5 (R5) or STX13 leads to diminished recruitment of γ-adaptin to fragmented Golgi complexes. Mock-treated (A-C) and siRNA-treated (D-L) cells were fixed and stained with mouse anti-γ-adaptin (red) and giantin (blue). Note the differences in the pattern of γ-adaptin in mock-treated compared with siRNA-treated cells (insets). The merged images facilitate visualization of colocalization observed in mock (pink) compared with a distinct red and blue pattern in siRNA-treated cells. (M) 70-90 cells from each sample from the above experiment (A-L) were assessed for colocalization of γ-adaptin and giantin. Values above bars give fluorescence intensity. *P<0.01 compared with mock-treated cells. Scale bar: 10 μm.
Dissociation of Arf1 and γ-adaptin upon depletion of EHD3 or rabenosyn-5
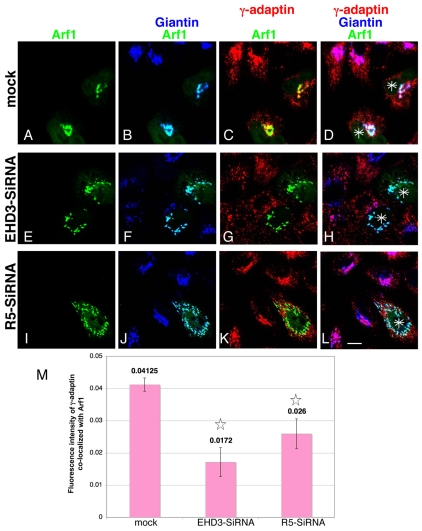
Since Arf1 is an important factor in AP-1 recruitment to the Golgi, we next assessed whether loss of EHD3 or rabenosyn-5 affects localization of Arf1 to the Golgi. In mock-treated cells (Fig. 8A-D), transfected GFP-Arf1 (green) (Fig. 8A) was highly colocalized with the Golgi marker endogenous giantin (colocalization in light blue) (Fig. 8B), as well as with endogenous γ-adaptin (red) (Fig. 8C, colocalization represented in yellow and quantified in Fig. 8M). All three proteins showed significant colocalization at the Golgi (Fig. 8D, white indicates triple stain, asterisks indicate GFP-Arf1-transfected cells). Upon depletion of either EHD3 (Fig. 8E-H) or rabenosyn-5 (Fig. 8I-L), it was evident that Arf1 maintained its binding to the dispersed Golgi cisternae, unlike γ-adaptin (Fig. 8F,J, light blue color indicates its colocalization with giantin). However, the levels of colocalized Arf1 and γ-adaptin were greatly reduced upon EHD3 and rabenosyn-5 depletion (Fig. 8, compare yellow color in C with G and K, and see quantification in M), with most γ-adaptin now dissociated from the Golgi (Fig. 8, compare white color in D with H and L). A brief treatment of mock- and siRNA-treated cells with Brefeldin A rendered a cytosolic pattern of GFP-Arf1, demonstrating its ability to dissociate from the fragmented/dispersed Golgi (our unpublished observations). Having observed that Arf1 continued to be recruited to the Golgi membranes upon depletion of EHD3, we also tested whether that was the case for the coatomer COP1, which facilitates retrograde transport from the Golgi to the endoplasmic reticulum, assembling onto non-clathrin-coated vesicles in an Arf1-dependent manner. As with Arf1, βCOP, a subunit of the COP1 complex, continued to be assembled onto EHD3-induced fragmented Golgi (our unpublished observations). Collectively, these data strongly support a role for EHD3 in the regulation of endosome-to-Golgi transport, and consequently facilitate in the recruitment of γ-adaptin to the Golgi. As a consequence, depletion of EHD3 and rabenosyn-5 has profound effects on biosynthetic transport from the Golgi to the lysosomes, but no observable effects on secretion from the Golgi.
Fig. 8.
Dissociation between Arf1 and γ-adaptin upon knockdown of EHD3 or rabenosyn-5. GFP-Arf1-transfected HeLa cells were treated with EHD3 siRNA (E-H) or rabenosyn-5 siRNA (R5-siRNA) (I-L), or left untreated (A-D) for 48 hours. After fixation, endogenous γ-adaptin and giantin were stained with specific antibodies, followed by Alexa Fluor 568 goat anti-mouse and Alexa Fluor 405 goat anti-rabbit, respectively. The triple-colored images are presented here as follows: GFP-Arf1 only (A,E,I); GFP-Arf1 and giantin (B,F,J; light blue represents their colocalization); GFP-Arf1 and γ-adaptin (C,G,K; yellow depicts their colocalization); merge of all three colors (D,H,L; white is the merge of the three colors; asterisks depict the GFP-Arf1 transfected cells). (M) The bar graph represents a quantitative analysis of 70-90 cells per sample from the experiment above, measuring the colocalization between γ-adaptin and GFP-Arf1 (bars represent results from C,G,K). *P<0.01 compared with mock-treated cells. Scale bar: 10 μm.
Discussion
Sorting and transport from the Golgi has major physiological significance for cells, as do the mechanisms involved in retrieval of regulatory proteins back to the Golgi. To date, a large number of proteins have been identified that mediate endosome-to-Golgi trafficking. These proteins have been categorized as being involved in: (1) recruitment, (2) budding and sorting, (3) tethering, and (4) fusion processes (reviewed by Bonifacino and Rojas, 2006). In this study, we highlight a previously undescribed role for the C-terminal EHD protein, EHD3, in the regulation of endosome-to-Golgi transport. EHD3 would most likely be categorized as being involved in recruitment because: (1) its C-terminal EH domain, present in other EHD family members, is known to bind to proteins containing NPF motifs (reviewed by Naslavsky and Caplan, 2005; Grant and Caplan, 2008); (2) EHD3 is an ATP-binding protein (Daumke et al., 2007; Lee et al., 2005; Naslavsky et al., 2006); and (3) EHD3 can bind to lipids and membranes directly (Blume et al., 2007; Naslavsky et al., 2007). Indeed, not only EHD3, but its early endosomal interaction partner, rabenosyn-5, as well as STX13, an endosomal SNARE protein, also control early-endosome-to-recycling-endosome and endosome-to-Golgi trafficking.
We have demonstrated that retromer complex proteins SNX1 and SNX2, both of which are involved in budding and sorting at the early endosome (Bujny et al., 2007; Carlton et al., 2004; Mari et al., 2008; Rojas et al., 2007; Utskarpen et al., 2007), are redistributed and apparently incapable of cycling from endosomes to the Golgi in the absence of EHD3 or rabenosyn-5. Moreover, internalized proteins, such as STxB, which normally reach the Golgi from early endosomes, display a marked delay in transport to the Golgi, indicating an overall impairment in this pathway.
In the absence of EHD3, Golgi morphology is radically altered, with fragmentation and dispersal of Golgi cisternae to the periphery. Other studies have previously reported impairment of endosome-to-Golgi transport with consequences on Golgi architecture. For example, cholesterol overloading affects Golgi vesiculation and trafficking to this organelle, in a mechanism dependent upon actin, dynamin I, dynamin II and the cytosolic calcium-independent phospholipase A2 (PLA2 or PLA2G6) (Grimmer et al., 2005). By contrast, Golgi fragmentation and dispersal observed in EHD3-depleted cells is independent of cytosolic PLA2, dynamin (supplementary material Fig. S3) or Rab11 (our unpublished observations). Moreover, both specific inhibitors (methyl arachidonyl fluorophosphonate; MAFP) and general inhibitors (ONO-R5-082; our unpublished observations) of PLA2 did not prevent fragmentation and Golgi scattering upon EHD3 depletion (supplementary material Fig. S3).
Lipid modifiers also cycle between early endosomes and the TGN and are involved in Golgi maintenance. An elegant study showed that depletion of PI4KIIα culminated in a dramatic reduction in γ-adaptin association with the Golgi (Wang et al., 2003). Thus, in the case of EHD3 depletion, it is possible that certain lipid modifiers and/or enzymes such as PI4KIIα or cytosolic PLA2 might be retained in endosome-derived carriers, which fail to reach the Golgi membranes. This could potentially lead to a change in Golgi lipid membrane composition, influencing fission processes and interactions with motor proteins and the cytoskeleton. This suggests the possibility that depletion of EHD3 affects endosome-to-Golgi transport at a stage upstream of that observed in cholesterol-overloaded cells, and is probably related directly to trafficking from endosomes to the Golgi. Supporting this notion is the finding that VSV-G secretion is diminished upon cholesterol overload, as well as by the addition of manganese (another treatment that induces Golgi fragmentation) (Towler et al., 2000), but not upon depletion of EHD3, rabenosyn-5 or STX13.
Of the Golgi-tethering factors that have a role in endosome-to-Golgi transport, two golgins, GCC185 and GCC88, regulate trafficking between Golgi and endosomes (Ganley et al., 2008; Lieu et al., 2007; Reddy et al., 2006; Derby et al., 2007). siRNA knockdown of either golgin led to Golgi fragmentation, altered M6PR distribution and impaired STxB transport to the Golgi, similarly to that seen upon depletion of EHD3, rabenosyn-5 or STX13. Whereas knockdown of either golgin or EHD3 induced accumulation of STxB in transferrin-positive, EEA1-negative vesicles (presumably derived from the recycling endosome), golgin knockdown caused STxB accumulation in Rab11-containing vesicles, but depletion of EHD3 did not. This is consistent with a model in which carrier vesicles derived from the early endosome would not reach the recycling endosome when EHD3 is absent. Although GCC185 knockdown dispersed M6PR to fragmented Golgi (Derby et al., 2007; Lieu et al., 2007; Reddy et al., 2006), loss of EHD3, rabenosyn-5 or STX13 generally prevented M6PR from reaching even the dispersed or fragmented Golgi, implicating EHD3 in the regulation of an earlier stage of retrograde transport. However, depletion of GP130, a cis-Golgi protein that normally cycles between the Golgi and endosomes, resulted in the same phenotype as EHD3 depletion, so that STxB was retained in transferrin-containing early endosome vesicles (Natarajan and Linstedt, 2004). Although GP130 is primarily localized to the Golgi at steady state, it appears to carry out a sorting role similar to that of EHD3 for retrieval of Golgi-targeted proteins from the endosomal system (Natarajan and Linstedt, 2004). Collectively, these studies support an important role for recycling of Golgi proteins from the early endosome, perhaps via recycling endosomes.
In addition to Golgi-tethering factors, SNARE-mediated fusion events are crucial for Golgi maintenance and function. Our data support a role for the SNARE protein STX13, in controlling endosome-to-Golgi transport. We observed substantial overlap of STX13 with both rabenosyn-5 and EHD3 on vesicular structures (our unpublished observations). In addition, endogenous STX13 partially colocalized with the TGN and TGN46-containing vesicles. Although these two pools might not necessarily communicate directly, it is likely that STX13 is important for both exit from early endosomes and fusion to the TGN. Here, we have demonstrated that depletion of STX13 delays endosome-to-Golgi transport and alters Golgi morphology in a manner similar to that observed upon depletion of either EHD3 or rabenosyn-5. Indeed, STX13 is a crucial player in the regulation of recycling of endocytosed cargo, along the early endosomes to the recycling endosome axis (Advani et al., 1998; Prekeris et al., 1998). Moreover, other SNAREs such as STX5 and STX16 are required for STxB trafficking from endosomes to the Golgi, and retrograde transport, respectively (Amessou et al., 2007). STX16 depletion also leads to the accumulation of STxB in transport vesicles containing transferrin receptor (Amessou et al., 2007). Furthermore, since neither STX13 depletion (our study), nor the loss of STX16 expression (Amessou et al., 2007) had any effect on VSV-G secretion, this highlights the specificity of the roles that these SNAREs have in endosome-to-Golgi trafficking, but not in secretory transport. The potential interplay between these SNAREs will need to be addressed in future studies.
By what mechanism do EHD3, rabenosyn-5 and STX13 control endosome-to-Golgi transport? In examining the functional capabilities of the decentralized Golgi complexes resulting from depletion of EHD3, rabenosyn-5 or STX13, we found that although secretion remained largely unaffected, biosynthetic transport of cathepsin D to lysosomes was seriously impaired. Since cathepsin D remained primarily at the Golgi, we reasoned that the poor retrieval of CI-M6PR from endosomes to the Golgi that we observed in EHD3-depleted cells was responsible for this phenomenon. CI-M6PR is scattered in peripheral early endosomes in embryonic fibroblasts from mice deficient in the AP-1 μ1A subunit (Meyer et al., 2000). Therefore, the compromised CI-M6PR retrieval observed upon EHD3 depletion might result from impaired endosome-to-Golgi transport and consequently, poor recruitment of γ-adaptin to the Golgi. Given that Arf1 is still recruited to the dispersed or fragmented Golgi membranes, the step involved in CI-M6PR mislocalization is likely to be `downstream' of Arf1, allowing cycling out of the Golgi to the endosomal system, but no return into the Golgi. The concentration of Arf1, GGA and AP-1 at a TGN vesicle-budding site provides a handy platform for clathrin assembly and priming of CI-M6PR for escorting biosynthetic lysosomal cargo molecules out of the TGN. Reduction in the level of the AP-1 γ-adaptin subunit recruitment to the Golgi might prevent formation of such budding sites, where CI-M6PR would have otherwise assembled, although it was demonstrated that unlike EHD3 depletion, γ-adaptin depletion does not affect Golgi architecture (Ghosh et al., 2003; Meyer et al., 2000). Its localization to peripheral non-Golgi membranes could be due to interactions with Rabaptin5α/Rabaptin4, which is localized to endosomal membranes (Deneka et al., 2003). These data suggest that proteins cycling between endosomes and the Golgi are affected by EHD3 depletion. However, GTP-binding proteins, such as Arf1 and dynamin II, which cycle between the cytosol and Golgi membranes depending on their GTP-status, remain unaffected.
Our data support a model by which EHD3, rabenosyn-5 and STX13 have important roles in transport events from the early endosomes. Indeed, our knockdown experiments have demonstrated decreased Golgi localization of the retromer subunits SNX1 and SNX2. Although SNX1 and SNX2 partially colocalize with EEA1 under normal conditions, upon EHD3 depletion these proteins relocalized to enlarged peripheral EEA1- and Rab11a-negative endosomes that also contain aberrant levels of M6PR (our unpublished observations). This suggests a build-up of `post-early-endosome carriers' that have undergone some sorting or maturation before their arrested transport. It is noteworthy that internalized STxB also accumulated in a separate population of `post-early-endosome carriers', containing transferrin receptor, γ-adaptin, low levels of SNX1 and SNX2, and no detectable Rab11a.
Since it has been demonstrated that the SNX1 retromer subunit is required for efficient retrograde transport of STxB from endosomes to the Golgi (Bujny et al., 2007), it is of particular interest to note that a recent study has demonstrated that EHD1 can interact with the retromer subunit VPS35, and that overexpression of EHD1 mutants that are incapable of proper ATP hydrolysis causes redistribution of the retromer (Gokool et al., 2007). Although EHD1 and EHD3 are 86% identical, our studies suggest that EHD3 has a more prominent role at the early endosome than EHD1 (Naslavsky et al., 2006), although both proteins are necessary for transport from early endosomes to recycling endosomes to the Golgi. This supports our contention that, in addition to the role played by EHD1 (Gokool et al., 2007), EHD3 is also a key regulator of retromer-mediated endosome-to-Golgi transport. Future studies will need to dissect the roles that individual EHD proteins carry out in endosome-to-Golgi trafficking.
Materials and Methods
Cell culture, silencing RNA and transfection
HeLa cells were cultured as described (Jovic et al., 2007). siRNA treatment was typically for 48 hours and performed with Oligofectamine in Optimem (Invitrogen, Carlsbad, CA) using 2.4 μg oligonucleotide duplexes (Dharmacon, Lafeyette, CO) per 35 mm dish. The sequences used were: EHD3 (base pairs 579-599); EHD1 (base pairs 943-963) rabenosyn-5 (base pairs 1559-1579); STX13 (base pairs 224-246). Mock treatment omitted the addition of the oligonucleotide. FuGene HD was used for cDNA transfection of siRNA-treated cells, whereas other transfections were performed with FuGene-6 (Roche Applied Science, Indianapolis, IN) for >24 hours.
SDS-PAGE analysis of siRNA efficiency
HeLa cells were treated with siRNA against EHD1, EHD3, rabenosyn-5, or Syntaxin13, or mock-treated as described above, and then lysed. Protein measurement was performed on each sample (Bio-Rad Protein Assay, Bio-Rad, Hercules, CA) in order to load an equal amount of protein for SDS-PAGE. After transfer to nitrocellulose, the filters were immunoblotted with the corresponding antibodies. Actin detection was used to confirm equal loading of protein.
Recombinant DNA constructs
GFP-EHD1 and GFP-EHD3 have been previously described (Caplan et al., 2002; Naslavsky et al., 2006). The dominant mutant of GFP-DynII (K44A) and the lysosomal glycoprotein GFP-lgp120 were kind gifts from R. Lodge (INRS, Quebec, Canada). Temperature-sensitive GFP-vesicular stomatitis virus glycoprotein (GFP-VSV-G) and GFP-Arf1 were kindly provided by J. Donaldson (NHLBI, NIH, MD).
Antibodies and reagents
Rabbit anti-rabenosyn-5, EHD1 and EHD3 were previously described (Naslavsky et al., 2004); mouse anti-actin, rabbit anti-giantin, mouse anti-CI-M6PR (2G11), rabbit anti-βCOP and rabbit anti-TGN46 were purchased from Affinity Bio Reagents (Golden, CO); mouse anti-SNX1, mouse anti-GM130, mouse anti-DynII were from Transduction Labs (Franklin Lakes, NJ); sheep anti-TGN46 (Serotec, Raleigh, NC); DAPI (Molecular Probes, Invitrogen, Carlsbad, CA); mouse anti γ-tubulin (clone GTU-88) and γ-adaptin (clone 100/3) were from Sigma (St Louis, MO). Other antibodies used include: β-COP (Affinity BioReagents), mouse anti-STxB (clone 13C4; Santa Cruz, CA) and rat anti-Hsc70 (StressGen, Ann Arbor, MI). Secondary anti-mouse and anti-rabbit Alexa Fluor 405, Alexa Fluor 488 or Alexa Fluor 568 antibodies were from Molecular Probes. HRP-conjugated goat anti-mouse IgG and Cy3 donkey anti-sheep (Jackson ImmunoResearch, West Grove, PA) was used in immunoblotting. The PLA2 inhibitor MAFP (methyl arachidonyl fluorophosphonate) was from Biomol Research Laboratories (Plymouth Meeting, PA).
STxB uptake
Alexa Fluor 546-STxB was prepared as previously described (Johannes et al., 1997; Mallard et al., 1998). STxB plasmid was a kind gift from Ludger Johannes (Institut Curie, Paris, France). STxB was expressed in DH5α E. coli, and purified on a mono-Q anion exchange column (GE Healthcare, Milwaukee, WI). Purified STxB was labeled using the Alexa Fluor 546 labeling kit (Invitrogen) according to the manufacturer's instructions. siRNA-treated HeLa cells were cooled on ice and incubated with ice-cold 546-STxB for 40 minutes. Unbound toxin was washed with cold PBS and the cells were shifted to 37°C in complete medium for various time points, before fixation.
STxB sulfation analysis
Analysis of sulfation on sulfation-site-tagged STxB variant was done as previously described (Johannes et al., 1997) (Mallard et al., 1998). Briefly, siRNA-treated cells were starved of sulfate for 2 hours at 37°C in sulfate-free buffer [DPBS (14080 Gibco), vitamins, MEM amino acids, 0.5% dialysed bovine serum and no antibiotics]. Cells were cooled on ice in cold sulfate-free buffer, then incubated with cold 6 μg/ml STxB (in sulfate-free buffer) for 30 minutes. Unbound sulphation-site-tagged STxB was extensively washed and incubated in sulfate-free buffer containing 0.3 mCi/ml of [35S]sulfate (MP Biomedicals, Solon, OH) at 37°C for the indicated times. STxB was immunoprecipitated from cell lysates with anti-STxB (mAb 13C4) and Sepharose bound to protein G. Flow-through lysate (unbound to anti-STxB) was separated by SDS-PAGE and blotted with anti Hsc-70, (a `housekeeping' protein) to monitor equal levels of protein.
Transferrin internalization
HeLa cells treated with siRNA were starved for 30 minutes with DMEM supplemented with 0.5% BSA at 37°C. They were then allowed to internalize 488-Transferrin (Tf) for 7 minutes, followed by a chase (in complete medium) for another 8 minutes before fixation. This timing has been shown to label both early endosome and recycling endosome compartments (Sheff et al., 1999; Naslavsky et al., 2006; Sheff et al., 2002).
VSV-G trafficking
HeLa cells grown on glass coverslips, were treated with the selected siRNA for 48 hours and were, in parallel, transfected with the temperature-sensitive mutant of GFP-VSV-G as detailed above. During the last 18 hours, cells were transferred to 40°C to allow accumulation of newly synthesized GFP-VSV-G in the ER. For anterograde passage to the Golgi and into the secretory pathway, cells were shifted to 32°C in complete media supplemented with 20 mM HEPES and 100 μg/ml cycloheximide (Ying et al., 2003) for 30 minutes (reaching the Golgi), or up to 2 hours (reaching the plasma membrane, with a small pool of the GFP-VSV-G still observed at the Golgi).
Cathepsin-D maturation assay with metabolic labeling
Metabolic labeling of siRNA-treated HeLa cells was performed as described by Perez-Victoria et al. (Perez-Victoria et al., 2008). Briefly, Cells grown on 35 mm dish were starved for methionine and cysteine for 30 minutes with starvation medium (20 mM HEPES, glutamine, 5% dialyzed bovine serum), and pulsed for 2 hours at 37°C in this medium, containing 0.3 mCi/ml [35S]-methionine-cysteine (Amersham, Piscataway NJ), then chased at 37°C with complete medium (supplemented with 10% bovine serum). At 5 hours chase, supernatant was collected prior to lysis of the cells. Cells were washed with PBS and lysed with 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 1.8 mg/ml iodoacetamide and protease inhibitors. Cell extracts and supernatants were immunoprecipitated with anti cathepsin-D, and analyzed by SDS-PAGE and fluorography. Quantification of bands on the films was done using Adobe Photoshop software.
Quantification of immunofluorescent images
HeLa cells were fixed and permeabilized as previously described (Caplan et al., 2001). Images were obtained on a Zeiss LSM 5 Pascal confocal microscope, utilizing a ×63 lens with 1.4 numerical aperture. Quantification of colocalization was performed with ImageJ software, utilizing JACoP (http://rsb.info.nih.gov/ij/plugins/track/jacop.html) (Bolte and Cordelieres, 2006) to calculate Pearson's coefficients. Each pair-wise comparison was done on eight sets of images acquired with the same optical settings. Bars represent the mean ± s.e. calculated from eight sets of images. One-tailed student's t-tests were performed to assess the statistical significance of the findings.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/3/389/DC1
Support for this research was provided by grants from the National Institutes of Health (1R01GM074876 to S.C.) and the Nebraska Department of Health (S.C.). Deposited in PMC for release after 12 months.
References
- Advani, R. J., Bae, H. R., Bock, J. B., Chao, D. S., Doung, Y. C., Prekeris, R., Yoo, J. S. and Scheller, R. H. (1998). Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273, 10317-10324. [DOI] [PubMed] [Google Scholar]
- Amessou, M., Fradagrada, A., Falguieres, T., Lord, J. M., Smith, D. C., Roberts, L. M., Lamaze, C. and Johannes, L. (2007). Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J. Cell Sci. 120, 1457-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume, J. J., Halbach, A., Behrendt, D., Paulsson, M. and Plomann, M. (2007). EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp. Cell Res. 313, 219-231. [DOI] [PubMed] [Google Scholar]
- Bolte, S. and Cordelieres, F. P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213-232. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J. S. and Rojas, R. (2006). Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell. Biol. 7, 568-579. [DOI] [PubMed] [Google Scholar]
- Bujny, M. V., Popoff, V., Johannes, L. and Cullen, P. J. (2007). The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J. Cell Sci. 120, 2010-2021. [DOI] [PubMed] [Google Scholar]
- Canuel, M., Lefrancois, S., Zeng, J. and Morales, C. R. (2008). AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem. Biophys. Res. Commun. 366, 724-730. [DOI] [PubMed] [Google Scholar]
- Caplan, S., Hartnell, L. M., Aguilar, R. C., Naslavsky, N. and Bonifacino, J. S. (2001). Human Vam6p promotes lysosome clustering and fusion in vivo. J. Cell Biol. 154, 109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, S., Naslavsky, N., Hartnell, L. M., Lodge, R., Polishchuk, R. S., Donaldson, J. G. and Bonifacino, J. S. (2002). A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 21, 2557-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton, J., Bujny, M., Peter, B. J., Oorschot, V. M., Rutherford, A., Mellor, H., Klumperman, J., McMahon, H. T. and Cullen, P. J. (2004). Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 14, 1791-1800. [DOI] [PubMed] [Google Scholar]
- Conner, S. D. and Schmid, S. L. (2003). Regulated portals of entry into the cell. Nature 422, 37-44. [DOI] [PubMed] [Google Scholar]
- Crump, C. M., Xiang, Y., Thomas, L., Gu, F., Austin, C., Tooze, S. A. and Thomas, G. (2001). PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20, 2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro, E., van der Sluijs, P., Galli, T. and Mellman, I. (1996). Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci. USA 93, 9559-9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumke, O., Lundmark, R., Vallis, Y., Martens, S., Butler, P. J. and McMahon, H. T. (2007). Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449, 923-927. [DOI] [PubMed] [Google Scholar]
- de Beer, T., Carter, R. E., Lobel-Rice, K. E., Sorkin, A. and Overduin, M. (1998). Structure and Asn-Pro-Phe binding pocket of the Eps15 homology domain. Science 281, 1357-1360. [DOI] [PubMed] [Google Scholar]
- de Beer, T., Hoofnagle, A. N., Enmon, J. L., Bowers, R. C., Yamabhai, M., Kay, B. K. and Overduin, M. (2000). Molecular mechanism of NPF recognition by EH domains. Nat. Struct. Biol. 7, 1018-1022. [DOI] [PubMed] [Google Scholar]
- Deneka, M., Neeft, M., Popa, I., van Oort, M., Sprong, H., Oorschot, V., Klumperman, J., Schu, P. and van der Sluijs, P. (2003). Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J. 22, 2645-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby, M. C., Lieu, Z. Z., Brown, D., Stow, J. L., Goud, B. and Gleeson, P. A. (2007). The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic 8, 758-773. [DOI] [PubMed] [Google Scholar]
- Diaz, E. and Pfeffer, S. R. (1998). TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93, 433-443. [DOI] [PubMed] [Google Scholar]
- Doherty, K. R., Demonbreun, A., Wallace, G. Q., Cave, A., Posey, A. D., Heretis, K., Pytel, P. and McNally, E. M. (2008). The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J. Biol. Chem. 283, 20252-20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray, B., Ghosh, P., Griffith, J., Geuze, H. J. and Kornfeld, S. (2002). Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297, 1700-1703. [DOI] [PubMed] [Google Scholar]
- Fazioli, F., Minichiello, L., Matoskova, B., Wong, W. T. and Di Fiore, P. P. (1993). eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol. Cell. Biol. 13, 5814-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley, I. G., Carroll, K., Bittova, L. and Pfeffer, S. (2004). Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol. Biol. Cell 15, 5420-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley, I. G., Espinosa, E. and Pfeffer, S. R. (2008). A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J. Cell Biol. 180, 159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, M., Ying, G., Rainey, M. A., Solomon, A., Parikh, P. T., Gao, Q., Band, V. and Band, H. (2007). Shared as well as distinct roles of EHD proteins revealed by biochemical and functional comparisons in mammalian cells and C. elegans. BMC Cell Biol. 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, P., Griffith, J., Geuze, H. J. and Kornfeld, S. (2003). Mammalian GGAs act together to sort mannose 6-phosphate receptors. J. Cell Biol. 163, 755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokool, S., Tattersall, D. and Seaman, M. N. (2007). EHD1 interacts with retromer to stabilise SNX1-tubules and facilitate endosome-to-Golgi retrieval. Traffic 8, 1873-1886. [DOI] [PubMed] [Google Scholar]
- Grant, B. D. and Caplan, S. (2008). Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic 9, 2043-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, B., Zhang, Y., Paupard, M. C., Lin, S. X., Hall, D. H. and Hirsh, D. (2001). Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat. Cell Biol. 3, 573-579. [DOI] [PubMed] [Google Scholar]
- Grimmer, S., Ying, M., Walchli, S., van Deurs, B. and Sandvig, K. (2005). Golgi vesiculation induced by cholesterol occurs by a dynamin- and cPLA2-dependent mechanism. Traffic 6, 144-156. [DOI] [PubMed] [Google Scholar]
- Guilherme, A., Soriano, N. A., Bose, S., Holik, J., Bose, A., Pomerleau, D. P., Furcinitti, P., Leszyk, J., Corvera, S. and Czech, M. P. (2004a). EHD2 and the Novel EH Domain Binding Protein EHBP1 Couple Endocytosis to the Actin Cytoskeleton. J. Biol. Chem. 279, 10593-10605. [DOI] [PubMed] [Google Scholar]
- Guilherme, A., Soriano, N. A., Furcinitti, P. S. and Czech, M. P. (2004b). Role of EHD1 and EHBP1 in perinuclear sorting and insulin-regulated GLUT4 recycling in 3T3-L1 adipocytes. J. Biol. Chem. 279, 40062-40075. [DOI] [PubMed] [Google Scholar]
- Hierro, A., Rojas, A. L., Rojas, R., Murthy, N., Effantin, G., Kajava, A. V., Steven, A. C., Bonifacino, J. S. and Hurley, J. H. (2007). Functional architecture of the retromer cargo-recognition complex. Nature 449, 1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes, L., Tenza, D., Antony, C. and Goud, B. (1997). Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272, 19554-19561. [DOI] [PubMed] [Google Scholar]
- Jovic, M., Naslavsky, N., Rapaport, D., Horowitz, M. and Caplan, S. (2007). EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J. Cell Sci. 120, 802-814. [DOI] [PubMed] [Google Scholar]
- Kieken, F., Jovic, M., Naslavsky, N., Caplan, S. and Sorgen, P. L. (2007). EH domain of EHD1. J. Biomol. NMR 39, 323-329. [DOI] [PubMed] [Google Scholar]
- Lee, D. W., Zhao, X., Scarselletta, S., Schweinsberg, P. J., Eisenberg, E., Grant, B. D. and Greene, L. E. (2005). ATP Binding regulates oligomerization and endosome association of RME-1 family proteins. J. Biol. Chem. 280, 280-290. [DOI] [PubMed] [Google Scholar]
- Lieu, Z. Z., Derby, M. C., Teasdale, R. D., Hart, C., Gunn, P. and Gleeson, P. A. (2007). The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-golgi network. Mol. Biol. Cell 18, 4979-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S. X., Grant, B., Hirsh, D. and Maxfield, F. R. (2001). Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat. Cell Biol. 3, 567-572. [DOI] [PubMed] [Google Scholar]
- Lombardi, D., Soldati, T., Riederer, M. A., Goda, Y., Zerial, M. and Pfeffer, S. R. (1993). Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 12, 677-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F. and Johannes, L. (2003). Shiga toxin B-subunit as a tool to study retrograde transport. Methods Mol. Med. 73, 209-220. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B. and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 143, 973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., Tang, B. L., Galli, T., Tenza, D., Saint-Pol, A., Yue, X., Antony, C., Hong, W., Goud, B. and Johannes, L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari, M., Bujny, M. V., Zeuschner, D., Geerts, W. J., Griffith, J., Petersen, C. M., Cullen, P. J., Klumperman, J. and Geuze, H. J. (2008). SNX1 defines an early endosomal recycling exit for Sortilin and Mannose 6-Phosphate receptors. Traffic 9, 380-393. [DOI] [PubMed] [Google Scholar]
- Maxfield, F. R. and McGraw, T. E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5, 121-132. [DOI] [PubMed] [Google Scholar]
- Meyer, C., Zizioli, D., Lausmann, S., Eskelinen, E. L., Hamann, J., Saftig, P., von Figura, K. and Schu, P. (2000). mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19, 2193-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, C., Eskelinen, E. L., Guruprasad, M. R., von Figura, K. and Schu, P. (2001). Mu 1A deficiency induces a profound increase in MPR300/IGF-II receptor internalization rate. J. Cell Sci. 114, 4469-4476. [DOI] [PubMed] [Google Scholar]
- Naslavsky, N. and Caplan, S. (2005). C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH? J. Cell Sci. 118, 4093-4101. [DOI] [PubMed] [Google Scholar]
- Naslavsky, N., Boehm, M., Backlund, P. S., Jr and Caplan, S. (2004). Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Mol. Biol. Cell 15, 2410-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky, N., Rahajeng, J., Sharma, M., Jovic, M. and Caplan, S. (2006). Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol. Biol. Cell 17, 163-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky, N., Rahajeng, J., Chenavas, S., Sorgen, P. L. and Caplan, S. (2007). EHD1 and Eps15 interact with phosphatidylinositols via their Eps15 homology domains. J. Biol. Chem. 282, 16612-16622. [DOI] [PubMed] [Google Scholar]
- Natarajan, R. and Linstedt, A. D. (2004). A cycling cis-Golgi protein mediates endosome-to-Golgi traffic. Mol. Biol. Cell 15, 4798-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Victoria, F. J., Mardones, G. A. and Bonifacino, J. S. (2008). Requirement of the human GARP complex for Mannose 6-phosphate-receptor-dependent sorting of Cathepsin D to lysosomes. Mol. Biol. Cell 19, 2350-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S. R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487-491. [DOI] [PubMed] [Google Scholar]
- Picciano, J. A., Ameen, N., Grant, B. D. and Bradbury, N. A. (2003). Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am. J. Physiol., Cell Physiol. 285, C1009-C1018. [DOI] [PubMed] [Google Scholar]
- Popoff, V., Mardones, G. A., Tenza, D., Rojas, R., Lamaze, C., Bonifacino, J. S., Raposo, G. and Johannes, L. (2007). The retromer complex and clathrin define an early endosomal retrograde exit site. J. Cell Sci. 120, 2022-2031. [DOI] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., Chen, Y. A. and Scheller, R. H. (1998). Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 143, 957-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano, R., Aguilar, R. C., Gorshkova, I., Crouch, R. J. and Bonifacino, J. S. (2001). Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 292, 1712-1716. [DOI] [PubMed] [Google Scholar]
- Reddy, J. V., Burguete, A. S., Sridevi, K., Ganley, I. G., Nottingham, R. M. and Pfeffer, S. R. (2006). A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol. Biol. Cell 17, 4353-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, R., Kametaka, S., Haft, C. R. and Bonifacino, J. S. (2007). Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol. Cell. Biol. 27, 1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., Prydz, K., Ryd, M. and van Deurs, B. (1991). Endocytosis and intracellular transport of the glycolipid-binding ligand Shiga toxin in polarized MDCK cells. J. Cell Biol. 113, 553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, M., Naslavsky, N. and Caplan, S. (2008). A Role for EHD4 in the regulation of early endosomal transport. Traffic 9, 995-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, D. R., Daro, E. A., Hull, M. and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, D., Pelletier, L., O'Connell, C. B., Warren, G. and Mellman, I. (2002). Transferrin receptor recycling in the absence of perinuclear recycling endosomes. J. Cell Biol. 156, 797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, A., Pant, S., Balklava, Z., Chen, C. C., Figueroa, V. and Grant, B. D. (2007). A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr. Biol. 17, 1913-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler, M. C., Prescott, A. R., James, J., Lucocq, J. M. and Ponnambalam, S. (2000). The manganese cation disrupts membrane dynamics along the secretory pathway. Exp. Cell Res. 259, 167-179. [DOI] [PubMed] [Google Scholar]
- Utskarpen, A., Slagsvold, H. H., Dyve, A. B., Skanland, S. S. and Sandvig, K. (2007). SNX1 and SNX2 mediate retrograde transport of Shiga toxin. Biochem. Biophys. Res. Commun. 358, 566-570. [DOI] [PubMed] [Google Scholar]
- Wang, Y. J., Wang, J., Sun, H. Q., Martinez, M., Sun, Y. X., Macia, E., Kirchhausen, T., Albanesi, J. P., Roth, M. G. and Yin, H. L. (2003). Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299-310. [DOI] [PubMed] [Google Scholar]
- Ying, M., Grimmer, S., Iversen, T. G., Van Deurs, B. and Sandvig, K. (2003). Cholesterol loading induces a block in the exit of VSVG from the TGN. Traffic 4, 772-784. [DOI] [PubMed] [Google Scholar]
- Zerial, M. and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.