Abstract
The laterally positioned dentate nuclei lie in a key position in the cerebellum to receive input from Purkinje cells in the lateral cerebellar hemisphere participating in both motor and cognitive functions. Although neuropathology of the four cerebellar nuclei using Nissl staining has been qualitatively reported in children and adults with autism, surprisingly the dentate nuclei appeared less affected despite reported reductions in Purkinje cells in the posterolateral cerebellar hemisphere. To determine any underlying abnormalities in the critically important GABAergic system, the rate-limiting GABAsynthesizing enzyme, glutamic acid decarboxylase (GAD) type 65 was measured via in situ hybridization histochemistry in dentate somata. GAD65 mRNA labeling revealed two distinct subpopulations of neurons in adult control and autism post-mortem brains: small-sized cells (about 10–12 µm in diameter, presumed interneurons) and larger-sized neurons (about 18–20 µm in diameter, likely feedback to IO neurons). A mean 51% reduction in GAD65 mRNA levels was found in the larger labeled cells in the autistic group compared to the control group (p=0.009; independent t-test) but not in the smaller cell subpopulation. This suggests a disturbance in the intrinsic cerebellar circuitry in the autism group potentially interfering with the synchronous firing of inferior olivary neurons, and the timing of Purkinje cell firing and inputs to the dentate nuclei. Disturbances in critical neural substrates within these key circuits could disrupt afferents to motor and/or cognitive cerebral association areas in the autistic brain likely contributing to the marked behavioral consequences characteristic of autism.
Keywords: dentate nucleus, cerebellum, autistic, GABA, dysregulation, cerebellar nuclei
Introduction
Autism is a pervasive neurodevelopmental disorder characterized by delayed language development, stereotypical behaviors and impaired socio-emotional interactions (DSM-IV, APA, 1994). Autism is currently viewed as a disorder with multiple etiologies distributed across genetic, neuroanatomical, and behavioral domains that ultimately results in alterations in brain connectivity (Muller, 2007). The cerebellum is an ideal structure to investigate connectivity due to its simple cytoarchitecture, highly ordered topographic circuitry and its multifocal intrinsic GABAergic neurotransmission.
The dentate nuclei are most highly evolved in anthropoid apes and are structurally enlarged and folded in the human brain. The human cerebellar nuclei (CN) consist of the globose, emboliform, fastigial and dentate nuclei, which integrate inputs from the brainstem and spinal cord with Purkinje cell efferents (Batini et al., 1992; Chan-Palay, 1977, 1979). Ramnani (2006) reported that Crus II in the posteriolateral hemisphere is a recipient region for prefrontal cortical inputs via the pons and Crus II Purkinje cells, in turn, project to the dentate. These connections indicate that different parts of the dentate nuclei participate in motor and/or cognitive function (e.g. Sasaki et al., 1979; Leiner et al., 1989, Middleton and Strick, 1997). The rostral aspect of the dentate projects to the motor and premotor cortices, whereas the caudal aspect projects to the frontal eye fields, areas 9 and 46, and to the inferior parietal cortex in primates (Voogd, 2003). Using transneuronal viral techniques in the monkey, the ventral portion of the dentate has been shown to project to classical motor areas, to prefrontal and parietal cortices and to be reciprocally connected with dorsal areas 9 and 46 of the prefrontal cortex (Middleton and Strick, 2001; Dum et al., 2002; Kelly et al., 2003).
Within the dentate nuclei, varying-sized neuronal populations have been reported (Graybiel and Hartwieg, 1974; Tolbert et al., 1978; Chan-Palay, 1977; Mugnaini and Oertel, 1985). In the rat, Chan-Palay (1977) described six subpopulations in the dentate nuclei based on cell size and shape but subsequent investigators defined three populations based on transmitter content and neuronal connectivity (Fredette and Mugnaini, 1991; Batini et al., 1992; Fredette et. al., 1992; Chen and Hillman, 1993). In human tissues however, the existence of subpopulations of dentate neurons has not been well delineated although small and large neurons are found in both the micro- (dorsomedial part) and macro-gyric (dorsolateral and ventral) portions (Voogd, 2004).
In autism, neuropathology of the CN has been reported but interestingly, an inconsistent relationship was seen when relating decreased numbers of Purkinje cells with specific CN. Although the greatest Purkinje cell decrease was reported in the lateral hemisphere in autistic brains (Arin ete al., 1991; see Palmen et al. 2004 for review), the dentate nucleus that normally receives projections from this region was qualitatively the least affected of all the CN (Bauman and Kemper, 1985; Kemper and Bauman, 1998; Bauman and Kemper, 2005). Additionally, the principal olive that projects to the dentate exhibits increased cell size in autistic children but normal neuronal volume in adults (Bauman and Kemper, 1985). In contrast, the accessory inferior olivary nuclei appear least affected in autism and project to the other CN with the most qualitative abnormalities (Bauman and Kemper, 1985). It thus remains a mystery as to why there is a “mismatch” between these relationships especially with regard to whether the dentate nuclei that receive Purkinje cell inputs are or are not altered in autism. One likely neuronal population that could exhibit potential abnormalities in the dentate is the GABAergic subpopulation.
One of the most reliable methods for visualizing these GABAergic subpopulation(s) is to use in situ hybridization histochemistry to label a rate-limiting enzyme, glutamic acid decarboxylase (GAD) (Martin et al., 1991; Martin and Rimvall, 1993). Two main isoforms of GAD, GAD65 and GAD67, have been identified in mammals. The two GAD isoforms exhibit different electrophoretic and kinetic characteristics and their genes are localized on distinct chromosomes, i.e. chromosome 10 for GAD65 and chromosome 2 for GAD67 (Karlsen et al., 1991; Kaufman et al., 1991; Martin and Rimvall, 1993). The GAD65 isoform has been hypothesized to be involved in synapse formation between GABA neurons and their targets during the early postnatal period in the rat cerebellum (Greif et al., 1991) and mediates increased GABA synthesis during intense neuronal activity (Patel et al., 2006). The dentate nuclei, which develop in the early prenatal period, have stronger labeling for GAD65 compared to GAD67 mRNA (Greif et al., 1991; Esclapez et al., 1994). Ji and Obata (1999) showed that in mutant mice lacking GAD67, GABA content in neurites was markedly increased with no disruption in the development of cerebellar Purkinje cells and proposed that the observed preservation of GABAergic function in vivo could be due to the influence of GAD65.
Both GAD isoforms have been shown to be affected in a variety of psychiatric and developmental disorders. GAD67 has been implicated in schizophrenia, bipolar disorder, major depression disorder, and autism (Fatemi et al., 2002; Akbarian and Huang, 2006; Torrey et al., 2005; Yip et al., 2007, 2008). In contrast, higher GAD65 antibody levels were found in the sera of 239 patients with bipolar disorder and 74 with schizophrenia compared to 220 healthy control subjects (Padmos et al., 2004) but GAD65 mRNA levels were significantly decreased (16%) in the dorsolateral prefrontal cortex in patients with schizophrenia (Dracheva et al., 2004). In animal studies, GAD65 is strongly implicated in anxiety (Kash et al., 1999; Stork et al., 2003) and in schizophrenia models (Heldt et al., 2004). A deficiency in GAD65 in adult rats resulted in an average 50% reduction in GABA levels in the hypothalamus and amygdala and accompanied with severe emotional disturbances (Stork et al., 2000). Fatemi et al. (2002) used Western Blot techniques in post-mortem adult autistic tissue and demonstrated a 50% reduction in both GAD65 and GAD67 protein in the cerebellum. Based on this body of evidence we histologically localized one of the key GAD isoforms, GAD65, to determine whether mRNA alterations paralleled protein changes.
Using in situ hybridization, the present investigation revealed at least two distinct subpopulations of dentate neurons that contain GAD65 mRNA present in both autistic and control cases. These include a small sized cell profile (about 10–12 µm) and a larger sized cell subpopulation (about 18–20 µm). Quantification of GAD65 mRNA levels in the sampled groups revealed a significant reduction in GAD65 mRNA in the larger cell population in autistic cases but not in the smaller neurons suggesting heterologous effects in the dentate nuclei potentially affecting specific key aspects of cerebellar circuitry.
Materials and Methods
Subjects and Tissue preparation
Ten fresh-frozen brain blocks, stored at −80oC, were taken from a midrostrocaudal level of the dentate nucleus (see Schmahmann et al., 2000, pp. 101–119) that included both dorsal and ventral lamellae and both micro- and macro-gyric nuclear subdivisions (see Voogd, 2003). Tissue blocks were obtained from the Harvard Brain Tissue Resource Center (HBTRC) via approval from the Autism Tissue Program (ATP) and consisted of two groups (n=5 autism and n=5 control cases) matched for age, postmortem interval (PMI), and pH, as summarized in Table 1. Note that cause of death and seizure history (two autism cases) are indicated. All five autism cases have been diagnosed with moderate to severe autism.
Table 1.
Case information of the study groups
| Case no. | Diagnosis | Gender | Age | PMI | Cause of Death | pH |
|---|---|---|---|---|---|---|
| B4275 | Control | M | 20 | 16 | MVA NA | 6.2 |
| B4272 | Control | M | 19 | 17 | NA | 6.5 |
| B4103 | Control | M | 16 | 13 | GWA | 5.7 |
| B4362 | Control | M | 25 | NA | NA | 6.0 |
| B4271 | Control | M | 19 | 21 | Epiglotitus | 6.2 |
| B3924 | Autistic | F | 16 | 9 | Seizure | 5.9 |
| B3845 | Autistic | M | 30 | 28 | Pancreatitis | 5.7 |
| B1401 | Autistic | F | 21 | 20 | Pneumonia | 5.7 |
| B2825 | Autistic | M | 19 | 9 | Cardiac arrest | 5.7 |
| B4099 | Autistic | M | 19 | 3 | CHF | 6.8 |
Abbreviations: Motor vehicle accident (MVA), Idiopathic dilated cardiomyopathy (IDC), GW abdomen (GWA), Gun shot wound (GSW), Congestive heart failure (CHF). Epileptic patients are 3845 and 2825.
Synthesis of labeled RNA probes
Twenty-µm-thick cryostat-cut coronal sections at −20oC were collected in series onto gelatin-coated slides. In situ hybridization procedures were applied according to a previously described protocol (Yip et al., 2007). In this study, the 35S radiolabeled complementary RNA (cRNA) probe was obtained from a human GAD65 cDNA. Briefly, in vitro transcription of the radioactive GAD65 cRNA probe was performed for 2 hours at 370C in the presence of 2.5 µM of 35S–uracil triphosphate, 10 mM of unlabeled UTP with adenosine triphosphate, cytosine triphosphate (CTP), and guanine triphosphate (GTP) in excess. The labeled cRNA was purified by phenol/chloroform extraction and ethanol precipitation and the length was then reduced to 100–150 nucleotides by partial alkaline hydrolysis. To ensure signal specificity, the GAD65 cRNA hybridization was tested on control sections processed with a sense sequence. The GAD65 mRNA protocol has been reliably established in our laboratory (Soghomonian and Chesselet, 1992; Soghomonian et al., 1994; Soghomonian and Laprade, 1997).
Sections were hybridized for 4 hours at 520C with 4.0 ng of radiolabeled cRNA probe per section (average specific activity: 4.3 × 105 cpm/ng), and diluted in hybridization solution (40% formamide, 10% dextran sulfate, 4 X sodium citrate and sodium chloride buffer [SSC], 10 mM dithiothreitol, 1.0% sheared salmon sperm DNA, 1.0% yeast tRNA, 1 X Denhardt’s solution). The hybridized sections were subsequently washed in 50% formamide at 520C for 5 and 20 minutes, RNAse A (100 mg/ml; Sigma) for 30 minutes at 370C, and in 50% formamide for 5 minutes at 520C, dehydrated and processed for emulsion autoradiography. Slides were coated with Kodak NTB3 nuclear emulsion diluted 1:1 with distilled water containing 300 mM ammonium acetate, air dried for 3 hours, and stored at room temperature in light-tight boxes with desiccant for 7–10 days of exposure and developed in Kodak D-19 for 3.5 minutes before counterstaining with hematoxylin and eosin (H&E).
Quantification
Emulsion radioautographs lightly counterstained with hematoxylin and eosinstained (H&E) were examined on a Nikon E600 microscope and individual neuronal profiles were observed under bright-field illumination with a 60X objective. Microscope images containing GAD65 mRNA-labeled neuronal profiles were digitized using a Sony CCD camera connected to a MacIntosh computer. GAD65 mRNA labeling was measured using the public domain NIH-image software (http://rsb.info.nih.gov/nihimage/download.html). The NIH-image drawing tool was used to outline each individual GAD65-labeled neuronal profile. The slice density function was used to threshold the area covered by silver grains in each profile. The area was calculated and was expressed as a number of pixels per µm2 (group mean ± SEM). Before the quantification was carried out on the control and autism samples, a test was conducted on a few sections to confirm on this material that the relationship between numbers of pixels and numbers of silver grains is linear. Quantitative analysis of radioautographs was carried out on sections processed in parallel and by the same investigator in order to minimize experimental variability. In addition, the experimenter who carried out the quantitative analysis of silver grains was blind to the condition (i.e. control or autistic). The background distribution of silver grains in the autistic and control groups was negligible. The post-mortem interval (PMI; interval between death and freezing the brains) and pH of the tissue was comparable among cases and was not significantly different between the autistic and control group. The level of GAD65 mRNA labeling was expressed as a mean ± SEM of pixels per µm2 of dentate neuronal profile. This was followed by independent t-test statistical analysis to compare mean GAD65 mRNA levels in the autistic and control group for two populations of dentate neurons. The final values were from thirty small- and thirty larger-sized cell profiles from each of the ten cases. The distinction of small and larger-sized neuronal profiles was based on measurements of cross-sectional profiles and was gathered as described below.
Estimation of cell profiles in the dentate nuclei
For each patient case investigated, GAD65 mRNA labeling was only measured in cells that exhibited a distinctly defined nucleus and nucleolus in sections counterstained with hemotoxylin and eosin (H&E). The H&E stain allowed visualization of the boundaries of the cell profiles and was used to estimate their cross-sectional diameter. Sixty cell profiles were quantified from each of the ten cases. In addition to the H&E counterstained GAD65 mRNA series, measurements of cross-sectional cell profiles were also made in an adjacent Nissl series that was not processed for GAD65 in situ hybridization histochemistry. In the Nissl series, a random sampling of neuronal profiles from the mid-rostrocaudal level of the dentate of the control group was performed and each dentate neuronal profile with a visible nucleolus was measured (40 dentate neurons were sampled per subject; 200 total cell number for the control group).
The cross-sectional diameter of the cell profiles was estimated using the Zeiss Axiovision software version 3.1 from photomicrographic images captured on a Zeiss Axioskop 40 microscope under bright-field illumination at 100x. For each cell profile, the picture was focused on the nucleolus and a line horizontal to the field of view was drawn across the cell bisecting the nucleolus. The length of the line spanning from one end of the cell soma membrane to the opposite end was measured and expressed in µm using pre-calibrated length function (in µm). Each cell profile was measured using a line horizontal to the field of view and the orientation of the sections was kept constant to allow comparisons between neuronal profiles and between sections. This method of sampling assumed that the orientation of neuronal profiles relative to the orientation of the dentate nucleus was comparable between tissue sections. The value of the cross sectional diameter for each cell profile in this study was rounded up to its nearest integer, such that a value between 9.50 and 10.49µm was rounded up to 10µm. These measurements were intended to provide a relative estimate of the cell profile size within the dentate rather than the absolute size of the different cell populations. The distribution of cross-sectional size was plotted using the Image J software in order to distinguish different cell populations based on their relative size.
Statistical analysis
The statistical program, SPSS was used to determine the independent samples t-test with significance at the p=0.05 level and the Levene’s test for equality of variance to determine the homogeneity of variance between control and autistic cases.
Results
Distinct dentate subpopulations based on cell profile estimates
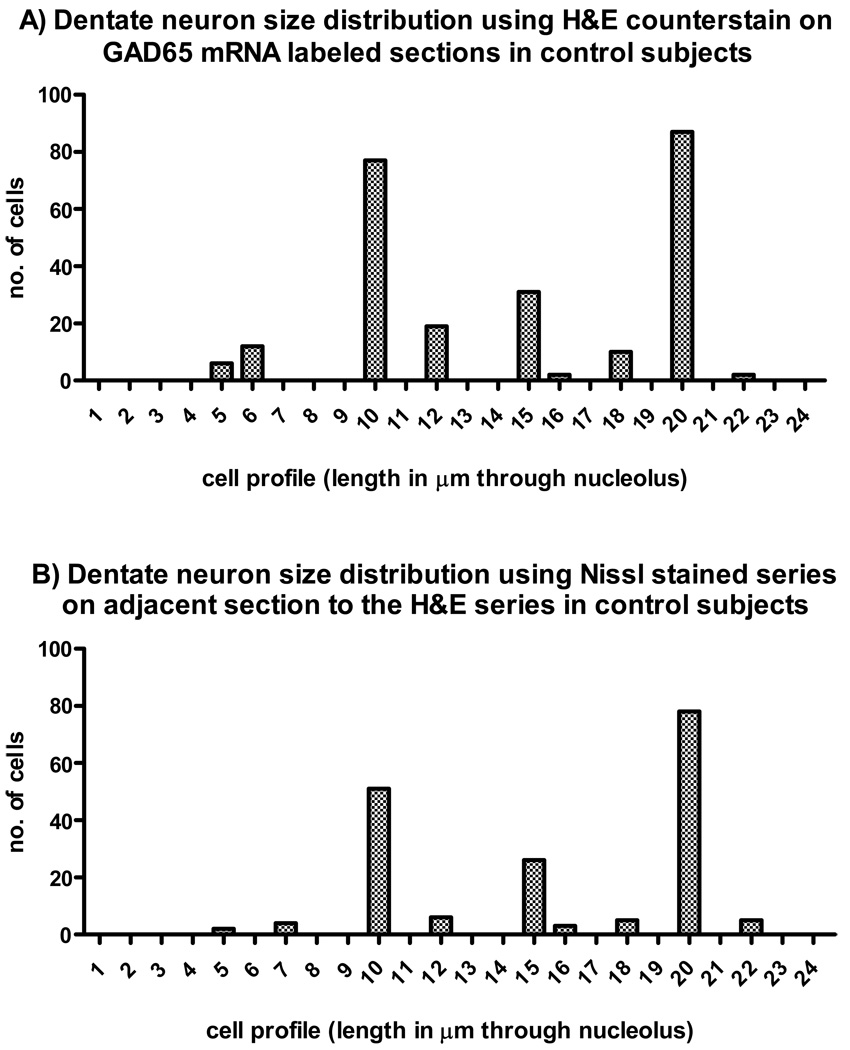
H&E counterstained sections from the control group of GAD65 mRNA-labeled neurons revealed two distinct subpopulations in the dentate nuclei with cell profile measurements averaging 10 µm and 20 µm respectively. A third but less abundant subpopulation averaged 15 µm (Figure 1A). To confirm the cell profile range, an adjacent fresh frozen series of post-fixed sections from the control cases were Nissl stained with similar results (Figure 1B). A photomicrograph of the various Nissl stained cell profiles are shown in Figure 2. Three different sized neurons are shown including (from left to right) one at 10 µm, 20 µm and a rarely found one at 30 µm to illustrate the range of dentate neurons. Note that no dentate neurons in the largest profile range (i.e. 30 µm) contained GAD65 mRNA in the H&E counterstained series. A film autoradiogram of the GAD mRNA-labeling, represented in Figure 3, depicts the dentate nuclei distributed along a thin undulating ribbon of neuropil, stippled by GAD65 mRNA positive neuronal somata.
Figure 1.
Histograms showing the relative distribution of cell profiles (measured length across cell through nucleolus in mm) sampled from dentate neurons from control subjects (60 neurons from each of five cases for the H&E stain and 40 neurons for the Nissl stain). The neurons were estimated from photomicrographs captured from a light microscope (Zeiss axioskop 40) precalibrated for length measurements. A) H&E stained sections processed for in situ hybridization demonstrate two large peaks at 10 µm and 20 µm and a few lower peaks including one at 15 µm. B) Nissl-stained adjacent sections. Note that the distribution, based on neuronal profiles, are very similar in the two methods.
Figure 2.
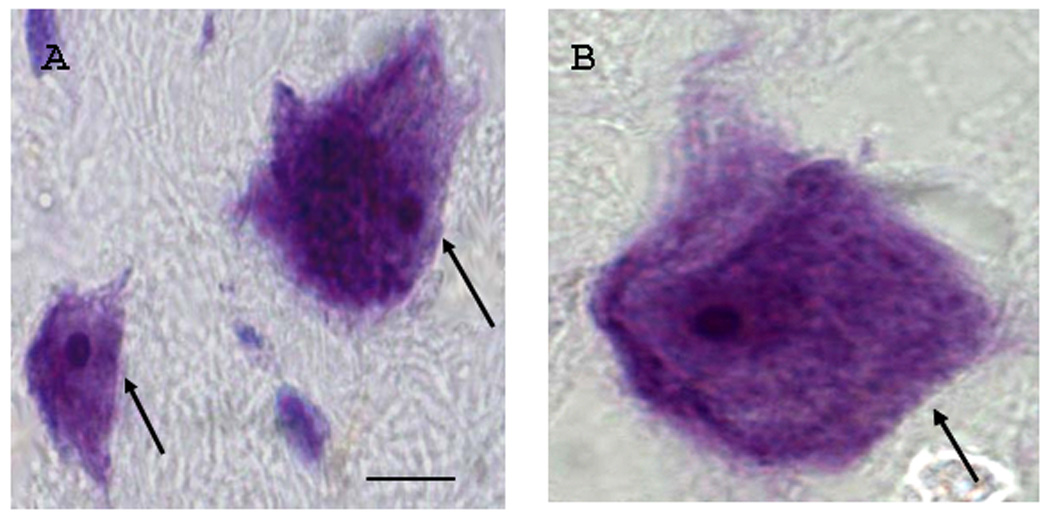
Examples of Nissl-stained sections that were taken from an adjacent series to the in situ hybridization series from a control case illustrating dentate neurons of varying sizes. The photomicrographs show cell profiles (length across nucleolus in µm) in A) approximately 10 µm and 20 µm (arrows) respectively. In this study, these small and larger- sized cells were most abundant in both controls and autistic cases. The largest identified cell is shown in B (approximately 30 µm; arrow) and depicts the ranges of dentate neuronal sizes. Note that measurements were rounded such that a 10 µm cell profile represents measurements ranging from 9.50 to 10.49. Scale bar = 10 µm refers to both panels A and B.
Figure 3.
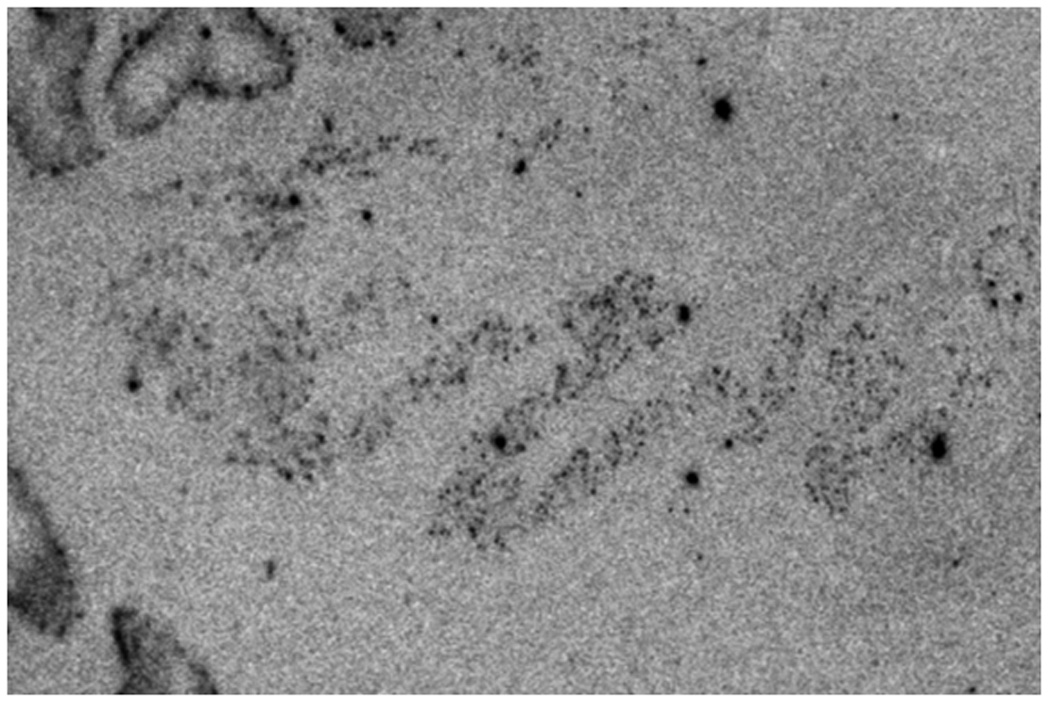
Film radioautogram showing GAD65 mRNA-labeled control cerebellum. The black arrows indicate dentate nuclei. The open arrows show GAD65 mRNA-labeled Purkinje cells.
Relative abundance of GAD65 mRNA in subsets of dentate neurons in control and autistic brains
GAD65 mRNA labeling in the dentate nucleus was visualized as clusters of dark silver grains in and around the dentate cell soma. GAD65 mRNA-labeling was seen in the dentate nucleus in all control and autism cases in neuronal profiles averaging 10 and 20 µm respectively (Figure 4). Note that unlabeled large dentate neurons (20–30 µm) were interspersed amongst the labeled neurons (not shown).
Figure 4.
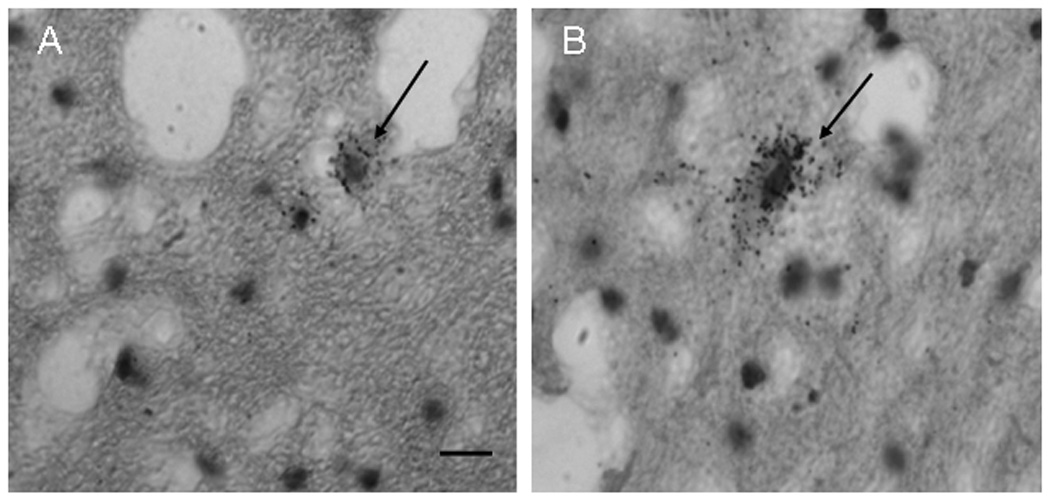
GAD65 mRNA-labeled neurons (arrows) in the dentate nuclei in a control case of a small cell in A) and a larger cell in B). Note the silver grain GAD65-mRNA-positive labeling throughout both cells. Scale bar in = 20 µm and refers to both A and B.
Quantitation of the GAD65 mRNA labeling demonstrated a 51% reduction in signal in the larger-sized GAD65 mRNA labeled subpopulation in the autism group when compared to the control group (175.77 ± 19.60 control, 86.56 ± 17.40 autistic; p=0.009; independent t-test; Levene’s test for equality of variance for homogeneity between variance F = 0.464; Figure 5B). In contrast, there was no significant difference in the mean levels of GAD65 mRNA in the small cell subpopulation between control and autistic groups (155.69 ± 22.69 control; 138.25 ± 21.06 autistic; independent t-test; Levene’s test for equality of variance for homogeneity between variance F = 0.031, p = 0.594; see Figure 5A).
Figure 5.
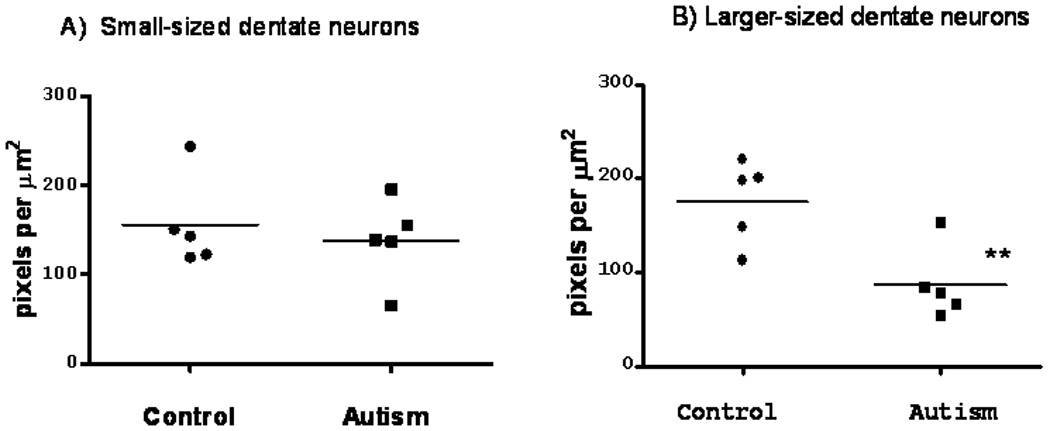
Scatter plot showing the mean levels of GAD65 in the dentate nuclei in five autistic and five control subjects for small 10 µm cells (A) and larger-sized 20 µm cells. Statistical analysis of the larger cell subpopulation showed a significant reduction in GAD65 mRNA levels in the dentate nuclei in the autistic group compared to age-, PMI and pH-matched controls (P** 0.03, independent t-test).
Discussion
Methodological considerations
Major limitations using humans compared to animal tissues include the lack of control over post mortem interval and agonal state. However, large scale gene analysis showed that brain pH and post mortem interval have no significant correlation with RNA integrity (Barton et al., 1993; Popova et al., 2008). Another limitation of this study is that brain blocks through the dentate nuclei were not obtained from intact cerebellum and thus were not sectioned in the same plane. Nevertheless, we did our best to select sections that were visually comparable in level for each of the cases. In future studies, we intend to follow up with more comprehensive comparisons throughout the entire structure. This study is an initial attempt to look at cell profiles of dentate neurons and the level of expression of GAD65 in the subpopulations. Many genes including GAD in the cerebellum are expressed in a cell specific pattern (Wuenschell et al., 1986) and hence differential expression between cell types could be used to reflect both heterogenous expression as well as data variability. Dentate cells were sampled along the morphologically characteristic micro- (dorsomedial part) and macro-gyric (dorsolateral and ventral) portions described by Voogd (2004) to be populated by small and large dentate neurons. GAD mRNA labeling does not occur in glia types in the cerebellum nor in granule cells but is restricted to GABAergic neuronal types as evidenced by in situ hybridization study in the mouse cerebellum (Wuenschell et al., 1986).
Subpopulations of Dentate Neurons
In our human samples, we observed the three major subpopulations of dentate neurons described in animal studies (Oertel et al., 1981; Sultan et al., 2003). These include mixed populations of differing sizes as follows: 1) the larger-sized GABAergic dentate cells that have been previously demonstrated in animals to project exclusively to the inferior olivary complex (Graybiel et al., 1974; Tolbert, 1978; Saint-Cyr and Courville, 1981; Oertel et al., 1981, Mugnaini and Oertel, 1985; De Zeeuw et al., 1988, 1996, 1998; Nelson and Mugnaini, 1989; Ruigrok and Voogd, 1990; Fredette and Mugnaini, 1991), 2) the smaller GABAergic dentate neurons that are either exclusively GABAergic and perhaps additionally glycinergic (Chen and Hillman, 1993; Baurle and Grősser-Cornehls, 1997) and project to other dentate neurons as described in the monkey (Chan-Palay, 1977) and 3) unlabeled neurons that are presumed excitatory glutamatergic population that project to the thalamus, red nucleus and/or brainstem nuclei, (Voogd et al., 1996; Batini et al., 1992; Sultan et al., 2002).
Since a correspondence between GAD65 mRNA and GAD65 protein levels including the dentate nuclei has been reported (Kaufman et al., 1991; Escapalez et al., 1993; Martin, 1993), our results suggest disturbances of GABA transmission in these nuclei. A significant reduction in GAD65 mRNA levels in the larger cell population but not in the small-sized cell subpopulation suggests that GABA synthesis is selectively altered in this subset of neurons. In the human, small GABAergic dentate neurons have also been observed (Mugnaini personal communication in Voogd, 2004).
Possible effects of decreased GAD65 mRNA in larger sized dentate neurons
The larger-sized GAD65 mRNA-labeled subpopulation in this study most likely represents GABAergic dentate neurons that project back to the inferior olive as previously described in animal studies (Tolbert et al., 1978; Chan-Palay et al., 1979; Yu et al., 1985; Teune et al., 1998; Sultan et al., 2002). Inferior olivary neurons are inhibited following dentate nuclei stimulation and are sensitive to even subtle disturbances (Giaquinta et al., 1999; Garifoli et al., 2001; Edge et al., 2003) due to their extensive electrotonically-coupled gap junctions. A disturbance in GABA neurotransmission in the dentate nuclei may have profound effects on the firing synchrony of inferior olivary neurons (Welsh, 2002), which exhibit gross morphological abnormalities in autism (Palmen et al., 2004 for review; Bauman and Kemper, 1985). Isolated autistic cases have demonstrated abbreviated neuronal migration in the inferior olive (Bauman and Kemper, 2005) and some cases had abnormal alignment along the edges of the principal olive ribbon (Bauman and Kemper, 1985; Thevarkunnel et al., 2006). In contrast, Bailey et al. (1998) did not observe this “edge effect” in their six autistic cases but did report an example of olivary dysplasia in one case and bilateral breaks in portions of the olive in two cases. In the Bauman and Kemper study (1985), those cases with abnormal allignment of olivary neurons, might result in a different orientation of their dendrites likely resulting in an asynchrony of firing within the principal olive subpopulation. Changes in inferior olivary cell size might also affect the timing of impulses to the target cerebellar cortex and dentate.
Normal GAD65 mRNA levels in the smaller dentate subpopulation could preserve dentate neurons
The smaller cell dentate subpopulation, known for about two decades in rats (Kumoi et al., 1988; Batini et al., 1989; Buisseret-Delmas et al., 1989) has been reported to either exclusively contain GABA or co-localize with glycine, and they provide synaptic contacts to other dentate neurons that are morphologically distinct from those that receive inputs Purkinje cells or from inferior olive collaterals (Gilerovich, 2000). As described in animal studies, the smaller neurons likely represent the interneuron population, which contains less numerous dendrites, less axon terminals and have a more truncated appearance of dendritic processes (Ramón-Moliner and Nauta, 1966; Chan-Palay, 1977; Sultan et al, 2003). This subpopulation provides stabilization of the internal circuitry and may contribute to the survival of the larger-sized dentate neurons. his view is supported by a comprehensive study in Lurcher mutant mice which have a primary selective loss of PCs (Bäurle and Grüsser-Cornehls, 1997; Bäurle et al., 1997; Sultan et al., 2002; Pedroarena and Schwarz, 2003). Sultan et al (2002) reported in the cerebellar nuclei, a modest loss of the large glutamatergic neurons (20%), a greater loss of large GABA neurons (42%) and inversely, an increase in the small neurons co-labeled with GABA and glycine (40%). Sultan et al. (2002) postulates that a compensatory increase in the small interneuron population, may serve to aid in the survival of the remaining cerebellar nuclei. Relating these findings to the present study, it is likely that the preservation of the small dentate neuronal population may be an underlying reason why the dentate appeared less affected in the neuropathological studies of Bauman and Kemper (1985) despite a significant decrease in Purkinje cells in the lateral hemisphere. It should be noted that although two distinct subpopulations of GAD65 mRNA positive neurons have been described, it would be a mistake to conclude that each of these subpopulations has exclusive projections to specific target areas. Chan-Palay (1977) concludes that it is likely that there is some overlap in connectivity within subpopulations of dentate neurons. Nevertheless, the most likely projections of each subgroup have been described and will serve as a guide to understanding the neuropathology of the dentate nucleus in autism.
Functional Significance
There is now clear support for a GAD expression defect in autism as well as in other neurobiological disorders such as schizophrenia and bipolar disorder that involve a deficiency in both GAD65 and GAD67 levels of protein and transcripts (Dracheva et al., 2004; Akbarian and Huang, 2006; Benes et al., 2007a; Yip et al., 2007, 2008). The gene family that regulates the expression of GAD, the highly conserved Dlx 1/2 (Geschwind et al., 2001) and Dlx 5/6 (IMGSAC, 2001; Geschwind et al., 2001) bigene clusters have been identified within chromosome 2q31.1 (Dlx1/2) and 7q21.3 (Dlx5/6). Both these chromosomosal regions as well as the Dlx2 and Dlx 5 genes have been implicated in autism (Hamilton et al., 2005). In the present study in autism as well as in a recent study by Benes et al. (2007a) investigating GAD in the hippocampus of schizophrenic and bipolar patients, the alterations in GAD levels appeared to be restricted to particular cell types within circumscribed subregions. These observations support the hypothesis that disorders need to be understood at the molecular regulatory level as well as the neuronal circuitry level (Benes, 2007b), as specific afferent-efferent projections and targets may be affected while neighboring circuits remain unaffected.
Clinical research indicates that discrete cerebellar lesions, in otherwise healthy children, cause behavioral and/or cognitive impairments. In autism, however, cerebellar pathology is likely acquired during critical developmental period(s) when the brain is capable of constructing alternate innervation patterns (Zagrebelsky et al., 1997; Sugihara et al., 2003). It is thus possible that there is a “miswiring” of key circuits in the autistic cerebellum with a developmental basis persisting into adulthood. Schmahmann et al. (2004) utilized MRI targeted electrolytic lesions of the dentate nucleus bilaterally in rhesus monkeys, a target for Purkinje cell efferents from the lateral hemisphere, and assessed them on conceptual set shifting tasks, a modified version of the Wisconsin card sorting task. This test determines the monkey’s ability to learn a rule based on abstract principles and then to “shift set” as the rule shifts from one concept condition to another.
Statistically significant greater perseveration was found when the monkeys’ shifted from one abstraction to another. This test has been shown to be sensitive to prefrontal cortical damage, and suggests that the cerebellum is a critical modulator of prefrontal systems mediating executive function (Schmahmann et al., 2004). It is apparent that we are still learning how disturbances in core neural substrates, such as GAD isoforms, in key cerebellar regions, such as the dentate, might affect complex, higher order cortical association areas without necessarily being directly involved in autistic cognitive and behavioral abnormalities.
Acknowledgments
We gratefully thank the Harvard Brain Tissue Resource Center, the Autism Tissue Program (ATP) and the NICHD Brain and Tissue Bank for Developmental Disorders for providing post-mortem brain tissues for our studies.
This study was supported by NIH NICHD R01-HD39459 (GJB, P.I.).
References
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52(2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arin DM, Bauman ML, Kemper TL. The distribution of Purkinje cell loss in the cerebellum in autism. Neurology. 1991;41 Suppl:307. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A Clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Barton AJL, Pearson RCA, Najlerahim A, Harrison PJ. Pre-and postmortem influences on brain RNA. J Neurochemistry. 1993;61(1):1–11. doi: 10.1111/j.1471-4159.1993.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Batini C, Buisseret-Delmas C, Compoint C, Dadiel H. The GABAergic neurons of the cerebellar nuclei in the rat: projections to the cerebellar cortex. Neursoci Lett. 1989;3:251–156. doi: 10.1016/0304-3940(89)90455-2. [DOI] [PubMed] [Google Scholar]
- Batini C, Compoint C, Buisseret-Delmas H, Danile M, Guegan M. Cerebellar nuclei and the nucleocortical projections in the rat: retrograde tracing coupled to GABA and glutatame immunohistochemistry. J Comp Neurol. 1992;315:74–84. doi: 10.1002/cne.903150106. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bäurle J, Grüsser-Cornehls U. Differential number of glycine, and GABAimmunopositive neurons and terminals in the deep cerebellar nuclei of normal and Purkinje cell degeneration mutant mice. J Comp Neurol. 1997;382:443–458. [PubMed] [Google Scholar]
- Bäurle J, Helmchen C, Grüsser-Cornehls U. Diverse effects of Purkinje cell loss on deep cerebellar nuclei and vestibular nuclei neurons in Purkinje cell degeneration mutant mice: a possible compensatory mechanism. J Comp Neurol. 1997;382:580–596. [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. PNAS. 2007a;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Searching for unique endophenotypes for schizophrenia and bipolar disorder within neural circuits and their molecular regulatory mechanisms. Schizophr Bull. 2007b Jun 16;33(4):932–936. doi: 10.1093/schbul/sbm064. Epub 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret-Delmas C, Angaut P. Anatomical mapping of the cerebellar nucleocortical projections in the rat: a retrograde labeling study. J Comp Neurol. 1989;288(2):297–310. doi: 10.1002/cne.902880208. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Cerebellar Dentate Nucleus: Organization, Cytology and Transmitters. Berlin: Springer; 1977. [Google Scholar]
- Chan-Palay V, Palay SL, Wu JY. Gamma-aminobutyric acid pathways in the cerebellum studied by retrograde and anterograde transport of glutamic acid decarboxylase antibody after in vivo injections. Anat Embryol (Berl) 1979;157(1):1–14. doi: 10.1007/BF00315638. [DOI] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Colocalization of neurotransmitters in the dentate cerebellar nuclei. J Neurocytol. 1993;22:81–91. doi: 10.1007/BF01181572. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Holstege JC, Calkoen F, Ruigrok TJ, Voogd J. A new combination of WGA-HRP anterograde tracing and GABA immunocytochemistry applied to afferents of the cat inferior olive at the ultrastructural level. Brain Res. 1988;447(2):369–375. doi: 10.1016/0006-8993(88)91142-0. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Lang EJ, Sugihara I, Ruigrok TJ, Eisenman LM, Mugnaini E, Llinás R. Morphological correlates of bilateral synchrony in the rat cerebellar cortex. J Neurosci. 1996;16(10):3412–3426. doi: 10.1523/JNEUROSCI.16-10-03412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Simpson JI, Casper C, Hoogenraad CC, Galjart N, Koekkoek SKE, Ruigrok TJH. Microcircuitry and function of the inferior olive. Trend Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Elhakem SL, McGurk SR, Davis KL, Haroutunian V. GAD67 and GAD65 mRNA and protein expression in the cerebrocortical regions of elderly patients with schizophrenia. J Neurosci Res. 2004;76(4):581–592. doi: 10.1002/jnr.20122. [DOI] [PubMed] [Google Scholar]
- Dum RP, Li C, Strick PL. Motor and non motor domains in the monkey dentate. Ann NY Acad Sci. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- Edge AL, Marple-Horvat DE, APPs R. Lateral cerebellum: functional localization within Crus I and correspondence to cortical zone. Eur J Neurosci. 2003;18(6):1468–1485. doi: 10.1046/j.1460-9568.2003.02873.x. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between forms. J Neurosci. 1994;14(3 pt 2):1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Tobin AJ, Houser CR. Comparative localization of mRNAs encoding two forms of glutamic acid decarboxylase with nonradioactive in situ hybridization methods. J Comp Neurol. 1993;331:339–362. doi: 10.1002/cne.903310305. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR.“Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices.” Biol Psychiatry 2002528805–810. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol (Berl) 1991;184:225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Adams JC, Mugnaini E. GABAergic neurons in the mammalian inferior olive and ventral medulla detected by glutamate decarboxylase immunohistochemistry. J Comp Neurol. 1992;321(4):501–514. doi: 10.1002/cne.903210402. [DOI] [PubMed] [Google Scholar]
- Garifoli A, Scardilli G, Perciavalle V. Effects of cerebellar dentate nucleus GABAergic cells on rat inferior olivary neurons. Neuroreport. 2001;12(17):3709–3713. doi: 10.1097/00001756-200112040-00021. [DOI] [PubMed] [Google Scholar]
- Geshwind DH, Sowinski J, Lord C, Iversen P, Shestack J, Jones P, Ducat L, Spence SJ. The autism genetic resource exchange: a resource for the study of autism and related neuropsychiatric conditions. Am J Hum Genet. 2001;69:463–466. doi: 10.1086/321292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta G, Casanona A, Smecca G, Bosco G, Perciavalle V. Cortical control of cerebellar dentato-rubral and dentato-olivary neurons. Neuroreport. 1999;10(14):3009–30013. doi: 10.1097/00001756-199909290-00025. [DOI] [PubMed] [Google Scholar]
- Gilerovich EG. Immunohistochemical studies of the structural bases of inhibition in the central cerebellar nuclei in mice. Neurosci Behav Physio. 2000;30(2):201–206. doi: 10.1007/BF02463159. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Hartwieg EA. Some afferent connections of the oculomotor complex in the cat: an experimental study with tracer techniques. Brain Res. 1974;81:543–551. doi: 10.1016/0006-8993(74)90850-6. [DOI] [PubMed] [Google Scholar]
- Greif KF, Erlander MG, Tillakaratne NJ, Tobin AJ. Postnatal expression of glutamate decarboxylases in developing rat cerebellum. Neurochem Res. 1991;16(3):235–242. doi: 10.1007/BF00966086. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Woo JM, Carlson EJ, Ghanem N, Ekker M, Rubenstein JLR. Analysis of four DLX homeobox genes in autistic probands. BMC Genetics. 2005;6:52. doi: 10.1186/1471-2156-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Ji F, Obata K. Development of the GABA system in organotypic culture of hippocampal and cerebellar slices from a 67-kDa isoform of glutamic acid decarboxylase (GAD67)-deficient mice. Neurosci Res. 1999;33(3):233–237. doi: 10.1016/s0168-0102(99)00005-x. [DOI] [PubMed] [Google Scholar]
- Karlsen AE, Hagopian WA, Grubin CE, Dube S, Disteche CM, Adler DA, Barmeier H, Mathewes S, Grant FJ, Foster D. Cloning and primary structure of a human islet isoform of glutamic acid decarboxylase from chromosome 10. Proc Natl Acad Sci. 1991;88(19):8337–8341. doi: 10.1073/pnas.88.19.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65 kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci. 1999;96(4):1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the gammaaminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57(7):645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kumoi K, Saito N, Tanaka C. Immunohistochemical localization of gamma-aminobutyric acid and aspartate-containing neurons in the rat deep cerebellar nuclei. Brain Res. 1988;439(1–2):302–310. doi: 10.1016/0006-8993(88)91487-4. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow R. Reappraising the cerebellum: what does the hindbrain contribute to the forebrain. Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- Martin DL. Short-term control of GABA synthesis in brain. Prog Biophys Mol Biol. 1993;60:17–28. doi: 10.1016/0079-6107(93)90010-h. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60(2):395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Martin DL, Martin SB, Wu SJ, Espina N. Cofactor interactions on the regulation of glutamate decarboxylase activity. Neurochem Res. 1991;16:243–249. doi: 10.1007/BF00966087. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Dentate output channels: motor and cognitive components. Prog Brain Res. 1997;114:555–568. doi: 10.1016/s0079-6123(08)63386-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Bjorklund A, Hokfelt T, editors. GABA and neuropeptides in the CNS: The Handbook of Chemical Neuroanatomy. Part 1. Vol 4. Elsevier: Amsterdam; 1985. pp. 541–543. [Google Scholar]
- Muller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13(1):85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BJ, Mugnaini E. Origins of GABAergic inputs to the inferior olive. In: Strata, editor. The Olivocerebellar System in Motor Control. Vol. 17. Berlin: Experimental Brain Research; 1989. pp. 86–107. [Google Scholar]
- Oertel WH, Schmechel DE, Mugnaini E, Tappaz ML, Kopin IJ. Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neurosci. 1981;6(12):2715–2735. doi: 10.1016/0306-4522(81)90115-9. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Bekris L, Knijff EM, Tiemeier H, Kupka RW, Cohen D, Nolen WA, Lernmark A, Drexhage HA. A high prevalence of organ-specific autoimmunity in patients with bipolar disoder. Biol Psychiatry. 2004;56(7):476–482. doi: 10.1016/j.biopsych.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, Van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127(pt 12):2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM, Schwarz C. Efficacy and short-term plasticity at GABAergic synapses between Purkinje and cerebellar nuclei neurons. J Neurophysiol. 2003;89(2):704–715. doi: 10.1152/jn.00558.2002. [DOI] [PubMed] [Google Scholar]
- Popova T, Mennerich D, Weith A, Quast K. Effect of RNA quality on transcript intensity levels in microarray analysis of human post mortem brain tissues. BMC Genomics. 2008;9:91. doi: 10.1186/1471-2164-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramón-Moliner E, Nauta WJ. The isodendritic core of the brain stem. J Comp Neurol. 1966;126(3):311–335. doi: 10.1002/cne.901260301. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ, Voogd J. Cerebellar nucleo-olivary projections in the rat: an anterograde tracing study with Phaseolus vulgaris-leucoagglutinin (PHA-L) J Comp Neurol. 1990;298(3):315–333. doi: 10.1002/cne.902980305. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Courville J. Sources of descending afferents to the inferior olive from upper brainstem in the cat as revealed by the retrograde transport of horseradish peroxidase. J Comp Neurol. 1981;198(4):567–581. doi: 10.1002/cne.901980403. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Jinnai K, Genba H, Hashimoto S, Mizuno N. Projection of the cerebellar dentate nucleus onto the frontal association cortex in monkeys. Exp Brain Res. 1979;37(1):193–198. doi: 10.1007/BF01474266. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Killiany RJ, Moore TL, DeMong C, MacMore JP, Moss MB. “Cerebellar dentate nucleus lesions impair flexibility but not motor function in monkeys.”. Soc. Neurosci. Abstr. 2004;34:254.12. [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the cerebellum. Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Chesselet MF. Effects of nigrostriatal lesions on the levels of messenger RNAs encoding two isoforms of glutamate decarboxylase in the globus pallidus and ento peduncular nucleus of the rat. Synapse. 1992;11:124–133. doi: 10.1002/syn.890110205. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Laprade N. Glutamate decarboxylase (GAD67 and GAD65) gene expression is increased in a subpopulation of neurons in the putamen of Parkinsonian monkeys. Synapse. 1997;27:122–132. doi: 10.1002/(SICI)1098-2396(199710)27:2<122::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Pedneault S, Audet G, Parent A. Increased glutamate decarboxylase mRNA levels in the striatum and pallidum of MPTP-treated primates. J Neurosci. 1994;14:6256–6265. doi: 10.1523/JNEUROSCI.14-10-06256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865(1):45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Stork O, Yamanaka H, Stork S, Kume N, Obata K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes, Brain and Behavior. 2003;2(2):65–70. doi: 10.1034/j.1601-183x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Lohof AM, Letellier M, Mariani J, Sherrard RM. “Postlesion transcommissural growth of olivary climbing fibres creates functional synaptic microzones.”. Eur J Neurosci. 2003;18(11):3027–3036. doi: 10.1111/j.1460-9568.2003.03045.x. [DOI] [PubMed] [Google Scholar]
- Sultan F, Czubayko U, Their P. Morphological classification of the rat lateral cerebellar nuclear neurons by principal component analysis. J Comp Neurol. 2003;455:139–155. doi: 10.1002/cne.10443. [DOI] [PubMed] [Google Scholar]
- Sultan F, Konig T, Mock M, Their P. Quantitative organization of neurotransmitters in the deep cerebellar nuclei of the Lurcher mutant. J Comp Neurol. 2002;452(4):311–323. doi: 10.1002/cne.10365. [DOI] [PubMed] [Google Scholar]
- Teune TM, Van der Burg J, De Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nulcei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Thevarkunnel S, Gibbs TT, Marcon R, Antzoulatos E, Oblak A, Bauman ML. Altered GABA-A receptor binding in the posterolateral cerebellar hemisphere and the dentate nucleus in autism. Society for Neuroscience Conference. 2006;PP61:692.14. [Google Scholar]
- Tolbert DL, Bantli H, Bloedel JR. Organizational features of the cat and monkey nucleocortical projection. J Comp Neurol. 1978;182(1):39–56. doi: 10.1002/cne.901820104. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Voogd J. Organization of the vestibulocerebellum. Ann NY Acad Sci. 1996;19:553–579. doi: 10.1111/j.1749-6632.1996.tb15728.x. [DOI] [PubMed] [Google Scholar]
- Voogd J. The human cerebellum. J Chemical Neuroanatomy. 2003;26:243–252. doi: 10.1016/j.jchemneu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Voogd J. Cerebellum and Precerebellar Nuclei. In: Paxinos G, Mai JK, editors. The Human Nervous System. Elsevier Academic Press; 2004. pp. 334–336. [Google Scholar]
- Welsh JP. Functional significance of climbing fiber synchrony: a population coding and behavioral analysis. Ann NY Acad Sci. 2002;978:188–204. doi: 10.1111/j.1749-6632.2002.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Wuenschell CW, Fisher RS, Kaufman DL, Tobin AJ. In situ hybridization to localize mRNA encoding the neurotransmitter glutamate decarboxylase in mouse cerebellum. Proc Natl Acad Sci. 1986;83(16):6193–6197. doi: 10.1073/pnas.83.16.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113(5):559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA levels in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- Yu QX, Ebner TJ, Bloedel JR. Electrophysiological study of the corticonuclear projection in the cat cerebellum. Brain Res. 1985;327(1–2):121–134. doi: 10.1016/0006-8993(85)91506-9. [DOI] [PubMed] [Google Scholar]
- Zagrebelsky M, Strata P, Hawkes R, Rossi F. Reestablishment of the olivocerebellar projection map by compensatory transcommissural reinnervation following unilateral transection of the inferior cerebellar peduncle in the inferior cerebellar peduncle in the newborn rat. J. Comp. Neurol. 1997;379:283–299. doi: 10.1002/(sici)1096-9861(19970310)379:2<283::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]