Abstract
Portal hypertension and its complications account for the majority of morbidity and mortality that occurs in patients with cirrhosis. In addition to portal hypertension, a number of other vascular syndromes are also of great importance especially the ischemia-reperfusion (IR) injury. With the identification of major vascular defects that could account for many of the clinical sequelae of these syndromes, the liver vasculature field has now integrated very closely with the broader vascular biology discipline. In that spirit, the Henry and Lillian Stratton Basic Research Single Topic Conference was held on the topic of Vascular Biology and Pathobiology of the Liver. The course took place approximately 10 years after the first AASLD-sponsored conference on this topic that occurred in Reston, Virginia. The conference initiated with an introduction to basic vascular cell signaling and then explored vascular biology specifically as it relates to liver cells. Subsequently, specific disease syndromes were discussed in more detail including portal hypertension and IR injury. Finally, clinical and translational sessions focused on emerging therapies and technologies to treat vascular diseases of the liver.
Principles of Vascular Biology in the Context of Hepatic Circulation
Many of the advances in liver vascular biology and treatment of liver disease have occurred through translation of basic discoveries in the broader field of vascular biology. One of the key pathways that transduce vascular cell signals is nitric oxide (NO). The first part of this review, describes recent advances in NO signaling pathways, identifies recent important vascular studies in model organisms, and applies this information to vascular cells in hepatic circulation.
Vascular cell signaling mediated by NO
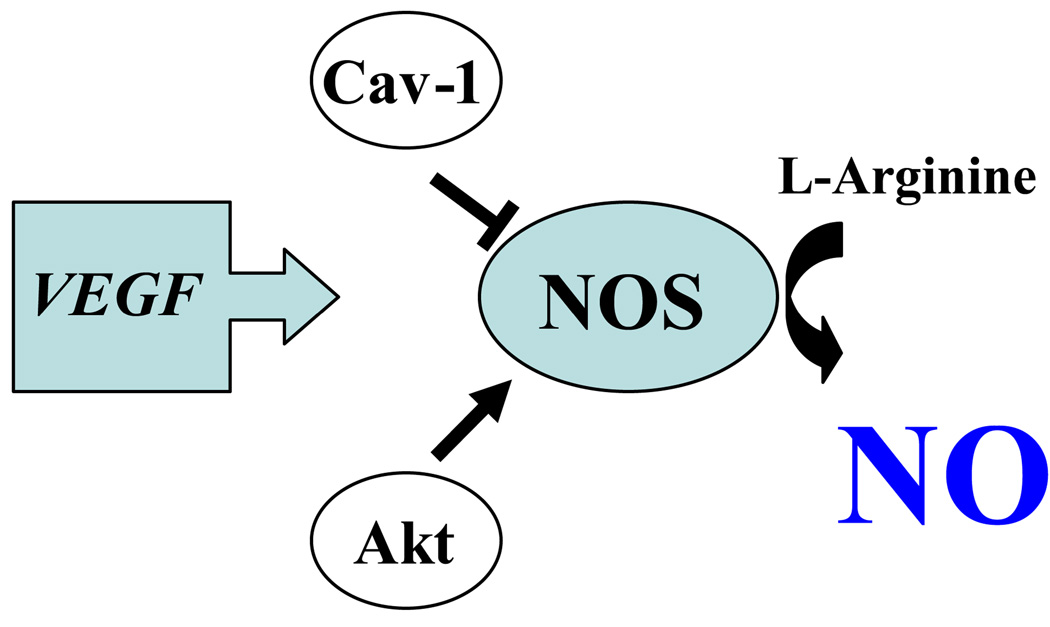
Endothelial cells (EC) produce NO through the enzyme endothelial NO synthase (eNOS). NO then regulates important vascular functions, such as vascular tone, angiogenesis and arterial remodeling (1). eNOS is regulated in a multi-protein complex, (Figure 1) which includes the activating kinase, Akt (1) and putative negative regulator caveolin-1 (Cav-1). While eNOS(−/−) mice evidence impaired angiogenesis, overexpression of a constitutively active allele of eNOS that mimics the phosphorylated and active state, rescues these angiogenic defects (2). Since eNOS is an EC specific Akt substrate, EC from mice lacking the Akt1 isoform also have defects in eNOS phosphorylation, NO production, and angiogenesis which are correspondingly rescued by Akt1 overexpression (3). One key mechanism by which the Akt-eNOS axis promotes angiogenesis in vivo is through mobilization of endothelial progenitor cells and EC migration to sites of vascular injury. In contradistinction to AKT, Cav-1 is a negative regulator of eNOS. Mice deficient in Cav-1 have defective mechano-transduction, calcium signaling, prostacyclin production and elevated NO production indicating that endothelial Cav-1 also importantly regulates vascular function by regulating eNOS and other signaling pathways (4).
Figure 1. eNOS regulation by AKT and caveolin.
One of the key pathways that transduce vascular cell signals is NO. Endothelial cells (EC) produce NO through the enzyme endothelial NO synthase (eNOS). eNOS is regulated in a multi-protein complex, which includes the activating kinase, Akt and putative negative regulator caveolin-1 (Cav-1). Vascular endothelial growth factor (VEGF) is one of the most potent activators of eNOS and especially relevant in the context of splanchnic vasodilation and portal hypertension.
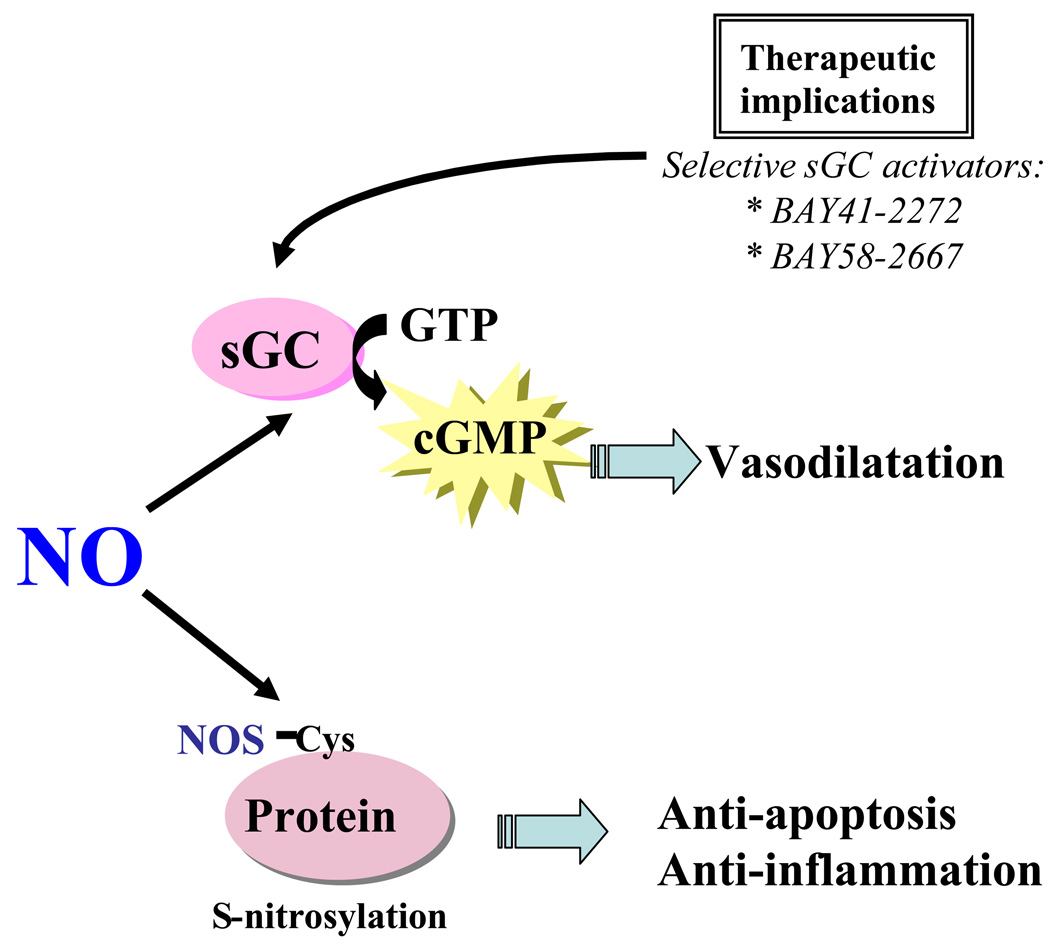
Upon its generation in EC, NO also acts on adjacent vascular effector cells to modulate vascular tone, cellular proliferation, apoptosis, migration, and synthesis of extracellular matrix (Figure 2). NO acts in part by stimulating soluble guanylate cyclase (sGC) to produce intracellular second messenger cyclic guanosine monophosphate (cGMP), which in turn mediates vasodilatation (Figure 2). Local or systemic administration of NO donors and transfer of genes encoding NOS attenuate vascular injury through sGC activation (5, 6). Interestingly, gene transfer of the individual sGC subunits, sGC α1 and β1, further increase NO responsiveness in vascular injury models (7). New NO-independent pharmacologic activators of sGC, such as BAY41-2272 and BAY 58-2667, also have a remarkable ability to stimulate sGC and thus to selectively dilate blood vessels (8). Thus, direct activation of sGC may provide an alternate therapeutic target downstream from NO in vascular injury.
Figure 2. NO signaling mechanisms.
NO acts in part by stimulating soluble guanylate cyclase (sGC) to produce intracellular second messenger cyclic guanosine monophosphate (cGMP), which in turn mediates vasodilatation. Direct activation of sGC through drugs such as BAY41-2272 and BAY58-2667 has been shown to have a remarkable ability to dilate blood vessels, and thus may provide an alternate therapeutic target downstream from NO in vascular diseases that benefit from enhanced NO signaling. Besides the conventional NO signaling via cGMP, NO induced S-nitrosylation, the coupling of an NO moiety to a reactive thiol side chain of cysteine to form an S-nitrosothiol, has been proposed as a specific post-translational regulatory mechanism for many proteins and may mediate diverse biological functions.
Besides the conventional NO signaling via cGMP, NO induced S-nitrosylation, has received considerable attention recently. S-nitrosylation is the coupling of an NO moiety to a reactive thiol side chain of cysteine to form an S-nitrosothiol, and has been proposed as a specific post-translational regulatory mechanism for many proteins (9). One of the important factors that determine the specificity of S-nitrosylation is the compartmentalization of eNOS with its target proteins for S-nitrosylation. eNOS is unique among the NOS family members as it is localized mainly to specific intracellular membrane domains including the cytoplasmic aspect of the Golgi apparatus and the plasma membrane caveolae (1). Interestingly, high NO concentrations are formed at these sites and correspond with increased S-nitrosylation of proteins at these sites. eNOS based nitrosylation at sites of eNOS localization is important for EC biology since nitrosylation of specific targets has recently been demonstrated to regulate exocytosis in EC (1, 10). Collectively, these observations suggest that the location of NOS determines S-nitrosylation of specific proteins by creating a high NO concentration near the target proteins, thereby favoring the S-nitrosylation reaction (10).
Model organisms for the study of vascular biology
The zebrafish (Danior rerio) is a powerful model organism for the study of vascular biology due to a rapid development cycle that is easily visualized and readily amenable to morpholino-based gene knockdown technology (11). Several genes have recently been found to be important for zebrafish vasculature pathways that are conserved in mammals; including the key angiogenic molecule, vascular endothelial growth factor (VEGF) (12), syndecan-2, a heparan sulfate proteoglycan that functions genetically as a VEGF co-receptor, and MAGP-1, a gene required for development of the vascular matrix (13, 14). Thus, the zebrafish can provide a unique genetic model for screening and discovery of new vascular targets.
Liver vascular cells important in hepatic circulation
Hepatic circulation is unique from most other vascular beds owing to complex interactions between several liver specific cell-types, including specialized sinusoidal endothelial cells (SEC), pericyte-like hepatic stellate cells (HSC), macrophage-like Kupffer cells and additional blood-derived cells. Signaling between these cells maintains sinusoidal homeostasis and conversely, alterations in signaling lead to sinusoidal pathobiology.
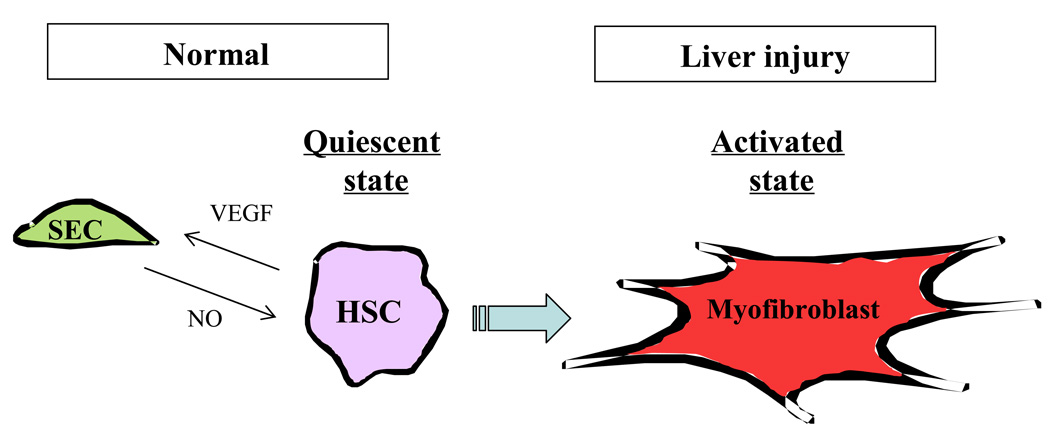
SEC in liver have a unique morphology that facilitates their function. Anatomically they maintain open, non-diaphragmed fenestrae and also lack an organized basement membrane in order to facilitate oxygenation of hepatocytes and transport of macromolecules into the space of Disse. It follows that alterations in SEC greatly influence hepatic microcirculation and functions (Figure 3). For example, morphological changes of SEC, including capillarization, the loss of SEC fenestration with formation of organized basement membrane, have been observed in chronic liver disease and aging (15). SEC also signal in important ways with HSC. HSC produce VEGF, which has important stimulatory effects on SEC (16). Furthermore, when SEC and HSC are co-cultured, α-smooth muscle actin (α-SMA) expression is decreased in HSC, suggesting that soluble mediators such as NO from SEC can maintain HSC in a quiescent state (unpublished data, DeLeve). This cross-talk is disrupted in chronic liver disease and contributes to alterations in the hepatic sinusoid including HSC activation.
Figure 3. Interactions among hepatic cells and myofibroblast activation.
Hepatic circulation is unique from most other vascular beds owing to complex interactions between several liver specific cell-types, including specialized sinusoidal endothelial cells (SEC), pericyte-like hepatic stellate cells (HSC), macrophage-like - Kupffer cells (Mϕ) and additional blood-derived cells. Signaling between these cells maintains sinusoidal homeostasis and conversely, alterations in signaling lead to sinusoidal pathobiology. In particular, SEC can maintain HSC in a quiescent state and limit HSC mass through NO generation, conversely HSC (and other cells) produce VEGF that maintains SEC phenotype and specialized function. This cross-talk between SEC and HSC is disrupted in chronic liver disease and contributes to alterations in the hepatic sinusoid including HSC activation.
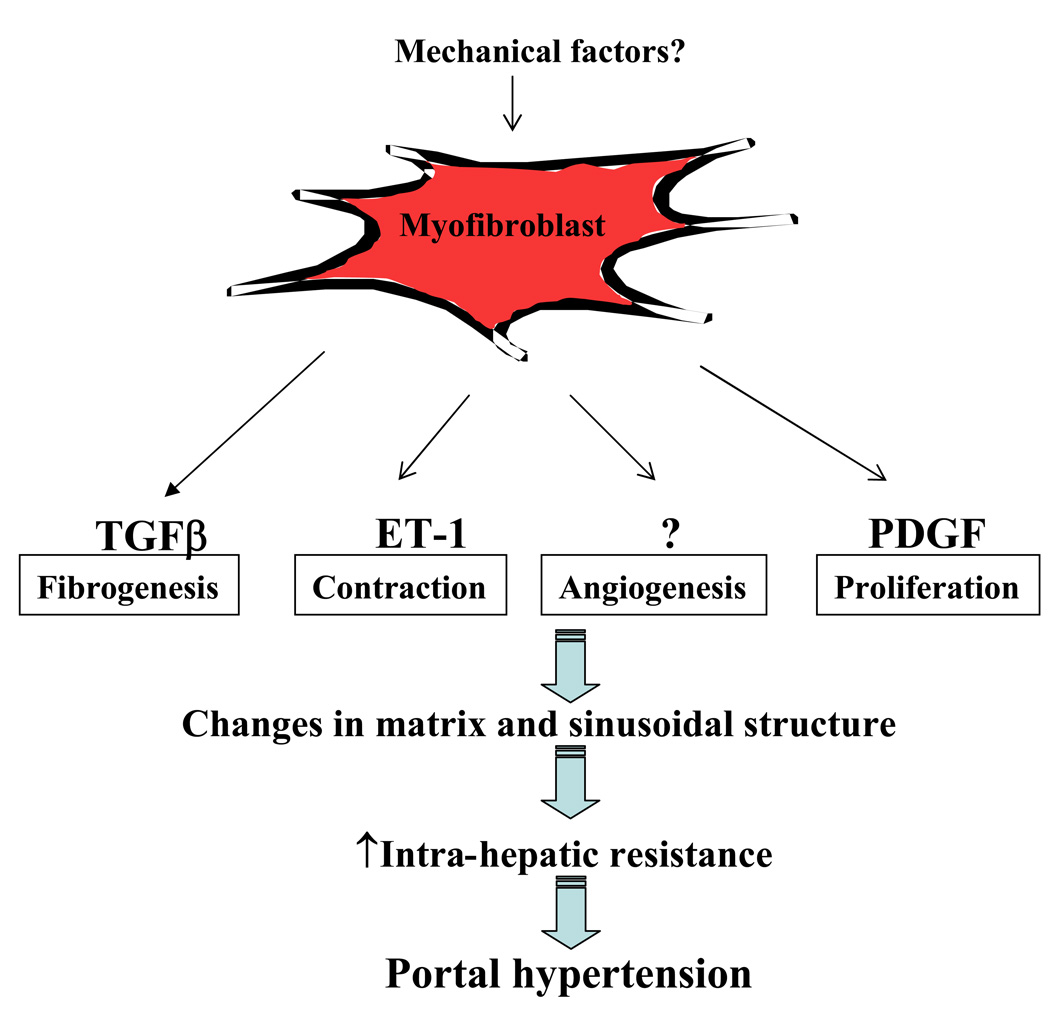
It is becoming increasingly recognized that HSC are not the only liver myofibroblast-like cell that undergo activation; portal fibroblasts are being recognized as a distinct and important cell type in liver injury response as well (Figure 4) (17). Differentiation of myofibroblast cells in liver requires a complex cytokine profile that includes transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), chemokines, adipokines, components of the renin-angiotensin system, lectins, and others. In addition, mechanical factors such as stretch and other dynamic mechanical changes are also being increasingly recognized to contribute to HSC and portal fibroblast phenotype and activation (18). In addition to their important role in matrix deposition during fibrogenesis, HSC regulation of sinusoidal structure and function is also of great importance. Indeed, HSC have been termed liver specific pericytes owing to their putative role in neovascularization and sinusoidal remodeling (19).
Figure 4. Myofibroblast phenotypes in portal hypertension.
Differentiation of myofibroblast cells in liver requires a complex cytokine profile that includes transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), chemokines, adipokines, components of the renin-angiotensin system, lectins, and others. Mechanical factors such as stretch and other dynamic mechanical changes are also being increasingly recognized to contribute to HSC and portal fibroblast phenotype and activation. This activation phenotype is multifaceted and includes components of proliferation, contractility, fibrogenesis, and also perhaps angiogenesis. The role of HSC in liver angiogenesis is an area of active investigation especially as it may relate to cirrhotic vascular changes and portal hypertension.
New Concepts in the Vascular Biology of Cirrhosis and Portal Hypertension
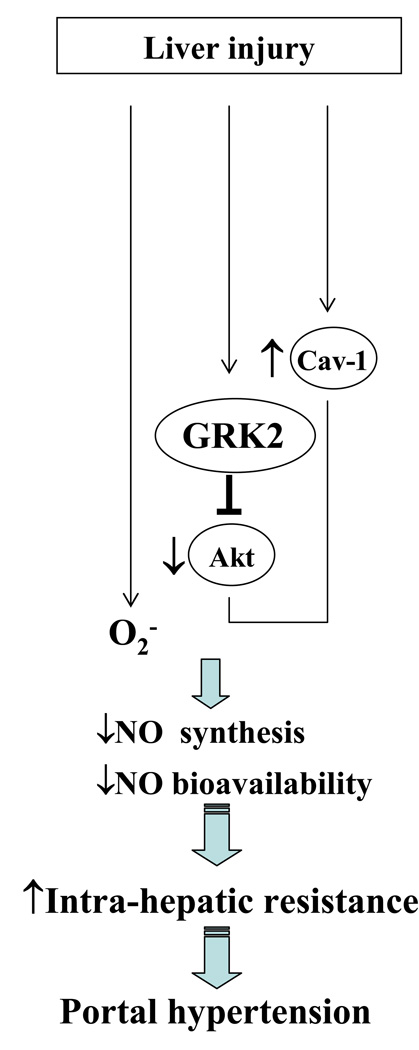
Portal hypertension and its complications account for the majority of morbidity and mortality that occurs in patients with cirrhosis. Despite advances in treatment and improvements in survival rates for some complications of portal hypertension, this syndrome continues to remain a management challenge. Furthermore, despite many major advances over the years, the pathogenesis of portal hypertension continues to be enigmatic. It is now well recognized that increased intrahepatic resistance is the primary initiator in the development of portal hypertension and that increased resistance occurs due to fibrosis and through dynamic changes in sinusoidal tone and structure. SEC-HSC crosstalk is altered in liver injury and leads to these defects in part through increased HSC contractility (Figure 5). One of the most well established defects in SEC-HSC crosstalk in liver injury is the decreased bioavailability of the vasodilator NO, concurrent with increased production of the constrictor, ET-1. ET-1 promotes alpha-SMA-containing stress fibers and increased contractility thereby promoting increased intrahepatic resistance (20). In addition to the well established upregulation of ET-1 production by TGF-β, overproduction of endothelin-1 in cirrhotic liver is also due to dysregulation of the converting enzyme that cleaves precursor endothelin-1 to the mature peptide (21). Interestingly, endothelin converting enzyme-1 is up-regulated in HSC, rather than SEC, indicating that the major cellular source of endothelin in the injured liver are HSC rather than SEC, thereby highlighting an important autocrine activation loop in HSC. In addition to alterations in ET-1, eNOS derived NO is reduced after liver injury, independent of changes in eNOS expression and through defects in post-translational regulation of the enzyme within EC (22). One recently recognized protein that contributes to this process is the G-protein-coupled receptor kinase 2 (GRK2), which directly interacts with and inhibits Akt, thereby decreasing eNOS activity (22). Thus a dynamic interplay of vasoconstrictive and vasorelaxing compounds within the hepatic microcirculatory unit regulates intrahepatic vascular resistance.
Figure 5. Regulation of intrahepatic NO generation in liver cirrhosis and portal hypertension.
One of the most well established defects in SEC-HSC crosstalk in liver injury is the decreased bioavailability of the vasodilator NO. which occurs independent of changes in eNOS expression and through defects in post-translational regulation of the enzyme within endothelial cells. During the process of liver injury, reduction of NO bioavailability occurs due to decreased synthesis of NO, enhanced inactivation of NO by the overproduction of superoxide (O2−) or both. An inactivation of eNOS occurs through an increase in cav-1 expression as well as one recently recognized protein, the G-protein-coupled receptor kinase 2 (GRK2), which directly interacts with and inhibits Akt, thereby decreasing eNOS activity.
In addition to their regulation of resistance in cirrhosis, HSC are also critical to changes in sinusoidal structure in liver injury. Recent studies have highlighted a link between angiogenesis and fibrosis whereby changes in sinusoidal structure and blood flow may be required for collagen deposition and scar formation, an extension of the prevalent concept of angiogenesis and tumor growth. The migratory capacity of myofibroblasts promotes these pathways as recently demonstrated in lung fibrosis (23). Changes in sinusoidal structure and matrix that occur in response to HSC activation and matrix deposition are now recognized to be reversible in some circumstances (24). Interestingly, macrophages contribute to both liver injury and recovery from sinusoidal matrix deposition through distinct interactions with HSC and their activation and quiescence (25). Two key elements for the resolution of altered sinusoidal structure include apoptosis of HSC and degradation of deposited matrix through matrix metalloproteinases (MMPs) (24). A number of potential therapies are focused on modulating these pathways. For example, recent studies have demonstrated that treatment of rats with experimental cirrhosis with PDGF receptor tyrosine kinase inhibitor, imatinib resulted in reductions in portal pressure and attenuation of the numbers of HSC covering the sinusoids (unpublished data, Semela and Shah). Thus, antiangiogenic therapies may be effective for chronic liver diseases in which enhanced HSC coverage of sinusoids may be playing a pathological role.
In addition to increased intrahepatic resistance, a hyperdynamic splanchnic and systemic circulation is also typical of cirrhotic patients. It is likely initiated by arterial vasodilatation, leading to central hypovolemia, sodium retention and an increased intravascular volume (26). NO appears to be the primary vasodilator in the arterial vasodilatation and the hyperdynamic circulatory state of liver cirrhosis. Degree of portal hypertension correlates with eNOS activation in the splanchnic circulation, in an animal model of portal hypertension (27) and is initiated by physical stimuli that induce Akt-dependent eNOS activation as well as through increased production of VEGF (27–29). Thus, portal pressure itself, may be an important factor that regulates vasodilatation in the splanchnic arterial circulation by means of eNOS activation in the splanchnic circulation (27).
The formation of porto-systemic collateral vessels and gastroesophageal varices are another important feature of portal hypertension that result in esophageal variceal hemorrhage. Recent studies have focused on the potential role of angiogenic factors in the formation of porto-systemic collateral vessels in portal hypertension as well as therapeutic implications of anti-angiogenic therapies (30–32). Splanchnic neovascularization in portal hypertensive rats is inhibited by the administration of a VEGF receptor-2 blocker. This VEGF-dependent process may be mediated by NADPH oxidase dependent signaling. Besides VEGF other pro-angiogenic factors, such as heme oxygenase and PDGF are also involved in this process (30–32). This new and important line of work may have important implications for treatment of portal hypertension and its complications.
Ischemia and Reperfusion Injury
Liver I/R-induced injury is a major cause of morbidity and mortality associated with liver transplantation and resectional surgery as well as septic and hemorrhagic shock. Indeed, there is a large body of experimental and clinical evidence suggesting that I/R induced by these surgical procedures or pathophysiological events injures the liver and may ultimately lead to tissue dysfunction and possibly liver failure. It is clear that post-ischemic heptocellular injury occurs in two distinct phases consisting of an acute phase occurring within the first 6 hours of reperfusion followed by a later, sub-acute phase occurring from 6 to 24 hours post-ischemia (33). One of the earliest events that occur in the acute, polymorphonuclear leukocyte (PMN)-independent phase is remarkable endothelial dysfunction characterized by profound decreases in eNOS-derived NO. Reductions in NO bioavailability occur very quickly and appear to be due to decreased synthesis of NO, enhanced inactivation of NO by the overproduction of superoxide (O2−) or both. Since NO is a potent vasodilator, the net result of decreased steady state levels of NO during the acute phase of I/R is significant alterations in the vascular reactivity of the microvasculature. Intriguing new data demonstrates that eNOS activity is inhibited in response to liver injury thereby promoting ET-1/ET-B receptor-mediated contraction of HSC and sinusoid constriction (34). The resulting vasoconstriction within the liver microcirculation produces heterogeneous blood flow patterns resulting in focal hypoxia and potentiation of liver injury (35). In addition, interesting new evidence suggests that microvascular dysfunction represents an important pathophysiological mechanism responsible for the enhanced I/R liver injury observed in steatotic livers. This enhanced sensitivity to post-ischemic liver injury in genetically obese mice (ob/ob) correlates well with a dramatic dysfunction of the microcirculation (36). Surprisingly however, both inflammatory gene expression and leukocyte infiltration are actually reduced in these post-ischemic steatotic livers suggesting that much of the enhanced I/R liver injury in this model of steatosis is due to dysfunction of the liver microcirculation rather than the resulting inflammatory response (36). However, in a second model of alcohol-induced steatosis, the enhanced liver injury induced by I/R is associated with increased PMN infiltration, yet the microvascular dysfunction in these steatotic mice is minimal compared to the ob/ob mice. Taken together, these data suggest that steatosis enhances post-ischemic hepatocellular injury by multiple mechanisms that depend upon the degree of steatosis as well as the etiology behind the steatosis.
In addition to its potent vasodilatory activity, NO is also a potent scavenger of oxygen-derived free radical species, suggesting that the rapid reduction in NO bioavailability following liver I/R will result in alterations in the redox state of the tissue in favor of a more oxidative environment (33). It is well appreciated that oxidative stress promotes the NF-κB (and AP-1)-dependent expression of certain inflammatory cytokines known to be involved in the pathophysiology of I/R-induced injury. Indeed, NFκ-B-dependent expression of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-12 by Kupffer cells and/or hepatocytes have been implicated as important mediators of reperfusion injury in the liver (37). However, recent evidence suggests that NF-κB activation may also act to protect hepatocytes from the injurious effects of I/R (38). The mechanisms by which NF-κB activation protects the post-ischemic liver are poorly understood. It is known that NF-κB activation induces several protective or “antiapoptotic” genes including MnSOD, a zinc finger protein termed A20, cellular inhibitor of apoptosis-2 (c-IAP-2), and Bcl-2 family members such as A1. Recent provocative data suggests that complete inhibition of NF-κB worsens liver I/R injury suggesting a protective role for this transcription factor. These data agree well with those of Beraza et al who recently reported that genetic ablation of the structural component (NEMO) of the IKK complex enhances hepatocellular damage in response to liver I/R (38). These investigators came to a similar conclusion as did Dr. Lentsch, which complete inhibition of NF-κB activation within hepatocytes actually worsens post-ischemic liver injury. However, the mechanisms by which this happens have not been identified.
In contrast to the acute PMN-independent phase of I/R-induced liver injury, the later, sub-acute phase is characterized by a PMN-dependent process in which I/R-induced reactive oxygen species generation is associated with cytokine and chemokine expression and complement activation (39). There is good evidence to suggest that these inflammatory mediators promote the invasion of PMNs into the interstitium via the up-regulation of adhesion molecules and formation of chemotactic gradients. Although a number of different adhesion molecules (P-and E-selectin, β2 integrins, α4, ICAM-1, VCAM-1) have been shown to play important roles in the pathophysiology of splanchnic organ injury subjected to I/R, virtually none of these adhesion molecules have been demonstrated to be used by PMNs to bind to the sinusoidal endothelium (SEC) during liver I/R (40). Newer data demonstrates, for the first time, that PMNs use CD44 to bind to hyuloronan expressed by SEC to promote their adhesion and extravasation during inflammation. These studies may have important therapeutic ramifications as it is known that adherent PMNs become metabolically activated and transmigrate through SEC to the underlying hepatocytes where they generate additional reactive oxygen metabolites in conjunction with the release of extracellular matrix degrading enzymes such as collagenase and matrix metalloproteases. The net result is an amplification of the acute responses resulting in extensive inflammatory tissue injury. P-selectin is a key cell adhesion molecule involved in mediating the recruitment, rolling, and interaction of leukocytes (and platelets) under inflammatory conditions through its ligand P-selectin glycoprotein ligand-1 (PSGL-1) (41–44). These interactions likely contribute to impaired vascular function in I/R injury as well as other vasculopathies as well (45, 46).
Kupffer cells also can contribute to IR injury through their ability to produce cytokines and reactive oxygen species (ROS) which contributes to SEC apoptosis (47, 48). A confounding factor in the I/R injury response is the existence of hepatic zonation. A recent study demonstrated that SEC apoptosis was significantly enhanced in the presence of Kupffer cells, and that this was blocked by inhibition of ROS and inhibitors of caspase 3 and 9, suggesting that Kupffer cell derived ROS contributes to SEC apoptosis. Furthermore, perivenous SEC were more susceptible to apoptosis than periportal-SEC, suggesting zonal variability in SEC injury responses (49).
Emerging Therapies for Vascular Diseases of the Liver
A major directive in hepatic vascular biology is to identify new and emerging targets for therapy for vascular diseases of the liver relating to vasoregulation, angiogenesis, and sinusoidal remodeling. There are a number of new signaling pathways that may be amendable to pharmacologic approaches. Furthermore, new molecular techniques to deliver compounds to liver vasculature and even to reconstitute liver vascular cells are on the horizon.
Angiogenic and vasoactive signaling pathways amenable to pharmacologic therapy (summarized in Table 1)
Table 1.
New Targets for Portal Hypertension Therapy
| • | Hydrogen sulfide |
| • | Nuclear receptors |
| • | HSC apoptosis |
| • | Anti-angiogenic therapy |
Hydrogen sulfide (H2S) is a gaseous neuromodulator that exerts potent vasodilatory effects on the circulation (50, 51). The major sources of H2S in the liver are from hepatocytes and HSC where it is generated from methionine and L-cysteine by the activity of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (50, 52). H2S has interesting parallels to NO; both are gases that counteract HSC contraction and attenuate hepatic vasoconstriction, thus supporting a parallel role of alterations in the H2S pathway in liver cirrhosis (51, 52). Indeed, inhibition of CBS and CSE is a common finding in patients with chronic liver disorders and leads to hyperhomocysteinemia in many cirrhotic patients (50). Homocysteine, in turn, promotes endothelial dysfunction by inactivating the generation of NO by SEC (52). Furthermore, H2S can compensate for defective NO production in rodent models of portal hypertension providing evidence that H2S and NO pathways may synergize in regulating hepatic vasoconstriction (51, 52). Current research directions are focused on modulation of H2S generation in the liver and/or pharmacologic compounds that may deliver H2S within the intrahepatic circulation in various liver diseases.
Nuclear receptors are another emerging signaling pathway in the context of EC biology that are a major therapeutic target for the pharmaceutical industry. FXR nuclear receptor has received significant attention in parenchymal liver cells however recent work has revealed the expression and activation of FXR within the vasculature (53). FXR pathway appears to be important in the process of smooth muscle cell apoptosis (53) and more recent studies have also delineated a role for FXR in HSC and EC as well (54, 55). For example, a recent study demonstrated that FXR was important in regulation of matrix gene regulation in HSC with important effects on liver fibrosis (54). Recent studies have also delineated an important role for FXR in EC function (55), including effects of FXR on endothelin production and cell migration through activation and upregulation of MMP-9 (55, 56). In total, these studies suggest a potentially important role for FXR in the intrahepatic circulation. Particularly, as bile acid gradients are highest in liver, especially in chronic liver disease states, activation of the FXR pathway may result in numerous, unexpected, and yet-to-be discovered effects of bile acids on liver nonparenchymal cells.
Angiogenesis is emerging as an important process within the liver circulation in numerous diseases including liver cancer, liver fibrosis, and others (19). One of the dominant angiogenic pathways is the VEGF pathway (19). VEGF is regulated by hypoxia inducible factor (HIF) (57); a family of basic Helix Loop Helix transcription factors that activate gene transcription through binding to hypoxia response elements (HREs) contained within regulatory regions of hypoxia sensitive genes such as VEGF. Elegant studies using conditional knockout of specific HIF subunits and regulators have demonstrated that different HIF isoforms play distinct roles in the regulation of hypoxia-inducible transcriptional programs in the liver. For example, while HIF target genes involved in glycolysis, such as PGK, are regulated by HIF-1 isoform, the hypoxic induction of VEGF and liver angiogenesis appear to be predominantly HIF-2 isoform dependent (58–61). Thus, targeting HIF could be an important pathway by which to regulate VEGF and angiogenesis in liver.
There are also other important pathways that regulate liver angiogenesis. Hepatocellular cancer (HCC) has a characteristic hypervascularity and depends on angiogenesis for tumor growth (62). Pharmacological inhibition of the serine-threonine kinase mammalian target of rapamycin (mTOR) with sirolimus impairs tumor growth by an antiangiogenic mechanism (63–65). In a rat model of HCC, animals treated with mTOR inhibitors, developed smaller tumors, fewer extrahepatic metastases, less ascites and had a longer survival. Further, analysis of tumor vasculature in sirolimus treated animals by scanning electron microscopy revealed inhibition of sprouting vessel formation. There are several other promising kinase inhibitors involved in angiogenesis (i.e. sorafenib, sunitinib, imatinib), which are currently being evaluated in clinical trials for HCC as well (66).
Molecular approaches towards modulating and reconstituting the vascular endothelium (summarized in Table 2)
Table 2.
Novel Delivery Approaches for Portal Hypertension
| • | Bone marrow transplantation |
| • | Endothelial cell transplantation |
| • | Sleeping Beauty Transposon |
| • | Nanoparticle-based delivery |
Recent studies have established the proof-in-concept of modulating SEC structure and function in vivo through cell transplantation-based endothelial reconstitution. Various liver diseases are associated with dysfunctional SEC including hemophilia A (67) and could be amenable to SEC transplantation. To address whether defective SEC could be replaced with transplanted ones, SEC from transgenic donor mice where a green fluorescent protein (GFP) reporter was expressed in SEC by virtue of the Tie2 endothelial promoter, were injected into recipient mice to assess engraftment in the hepatic sinusoidal bed (68). Transplanted LSEC proliferated over time with progressive replacement of the liver endothelium, such that approximately 20% of the endothelial compartment was replaced after 3 months (69). Transplanted cells became integrated in the liver structure, were distinct from other sinusoidal liver cells and retained normal function, including DiI-Ac-LDL incorporation and production of coagulation Factor VIII (67). Thus, cell transplantation may not be limited to hepatocyte-based therapies and SEC transplantation could one day have the potential for resolving numerous vascular diseases such as veno-occlusive disease, portal hypertension, and hemophilia (67).
In addition to SEC transplantation, SEC specific targeting of nanocapsules into the liver is also an exciting approach for future therapeutics especially owing to well-documented limitations of adenoviral vectors in humans. Although many vector systems and particles have a propensity to home to the liver in vivo, the cell type which takes them up may be variable. However, SEC may be targeted in vivo through their expression of hyaluronan receptors (HAR) allowing nanocapsules coated with the HAR ligand, to target SEC in vivo. Proof-in-concept was first demonstrated by using reporter genes encoding red fluorescent protein that were encapsulated in the nanoparticles, and could be delivered selectively to SEC when injected in mice in vivo (unpublished data, Kren and Steer). This work was extended by targeting transgenic hemophilia A mice with nanocapsules containing Sleeping Beauty (SB) transposon construct. Transposons are sequences of DNA that can translocate within the genome of cells and promote mutations of endogenous DNA and integrations of transgene DNA. Although still under development, this system may be a powerful tool for gene therapy and gene discovery in the future (70). Indeed, these particles targeted liver EC and provided long-term expression of clinically relevant gene products (unpublished data, Kren and Steer). Thus, new technologies that can result in liver EC specific expression of transgenes may be on the horizon.
Summary
In conclusion, advances in basic vascular cell signaling are being translated into advances in hepatic vascular biology, in the context of molecular signal transduction and subsequently towards experimental therapeutics. Indeed delineation of such pathways may provide a pipeline of new potential therapies for patients with vascular diseases of the liver.
Acknowledgments
The course directors wish to especially thank the authors listed below for their outstanding talks and written contributions which facilitated the generation of this manuscript… William Sessa (Endothelial Cell Signal Transduction and Nitric Oxide - Yale University), Ken Bloch (Nitric Oxide/cGMP in Vascular Biology – Harvard University), Yasuko Iwakiri (S-nitrosylation: How is Specificity of Signaling Achieved - Yale University), Steve Ekker (Insights into Vascular Development from Model Organisms - University of Minnesota), Laurie DeLeve (Sinusoidal Endothelial Cell: Structure and Function - University of Southern California), Rebecca Wells (Biology of Hepatic Stellate Cells - University of Pennsylvania), Don Rockey (Novel Mediators of the Functional Circulatory Disturbances in the Cirrhotic Liver - UT Southwestern), John Iredale (Sinusoidal Remodeling - Queen’s Medical Research Institute-Edinburgh UK), Roberto J Groszmann (The Genesis of Hyperdynamic Circulation: Role of Mechanical Forces and Cytokines - Yale University), Jaime Bosch (University of Barcelona), Mark Clemens (Vascular Injury from Drugs and Shock - UNC Charlotte), Hartmut Jaeschke (Mechanisms of Inflammatory Injury and Microvascular Dysfunction in Lean and Steatotic Livers during Hepatic Ischemia-reperfusion - University of Kansas), Alex Lentsch (NF-κB in Ischemia/Reperfusion - University of Cincinnati), Paul Kubes (Mechanisms of Neutrophil Recruitment in the Inflamed Liver - University of Calgary), Neil Granger (Role of Leukocytes and Platelets in Vascular Biology - LSU-Shreveport), Tak Yee Aw (Role of Kupffer Cells in Hepatic Vascular Function/Dysfunction – LSU-Shreveport), Stefano Fiorucci (Novel Gas-based Therapies: NO and H2S - University of Perugia), David Bishop-Bailey (Nuclear Receptors as a Novel Target in Vascular Function and Homeostasis - William Harvey Research Institute), Volker Haase (The HIF Pathway in Liver Angiogenesis: Therapeutic Implications Gleaned from Transgenic Mice - University of Pennsylvania), David Semela (Beyond VEGF: Other Anti-angiogenic Basel University Hospital), Sanjeev Gupta (Cell Transplantation Based Endothelial Reconstitution - Albert Einstein College of Medicine), Betsy Kren (Cell Specific Targeting of Gene Expression in Liver: Therapeutic Opportunities for Hepatic Vascular Diseases - University of Minnesota).
References
- 1.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks DS, Vita JA, Folts JD, Keaney JF, Jr, Welch GN, Loscalzo J. Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxide. J Clin Invest. 1995;96:2630–2638. doi: 10.1172/JCI118328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki J, Nishio M, Kato Y, Motoyama Y, Yoshida K. FK409, a new nitric-oxide donor, suppresses smooth muscle proliferation in the rat model of balloon angioplasty. Atherosclerosis. 1995;117:97–106. doi: 10.1016/0021-9150(95)05563-c. [DOI] [PubMed] [Google Scholar]
- 7.Sinnaeve P, Chiche JD, Nong Z, Varenne O, Van Pelt N, Gillijns H, Collen D, et al. Soluble guanylate cyclase alpha(1) and beta(1) gene transfer increases NO responsiveness and reduces neointima formation after balloon injury in rats via antiproliferative and antimigratory effects. Circ Res. 2001;88:103–109. doi: 10.1161/01.res.88.1.103. [DOI] [PubMed] [Google Scholar]
- 8.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 10.Iwakiri Y, Satoh A, Chatterjee S, Shah V, Chalouni C, Toomre D, Fulton D, Groszmann RJ, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. PNAS. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 12.Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen E, Hermanson S, Ekker SC. Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood. 2004;103:1710–1719. doi: 10.1182/blood-2003-06-1783. [DOI] [PubMed] [Google Scholar]
- 14.Chen E, Larson JD, Ekker SC. Functional analysis of zebrafish microfibril-associated glycoprotein-1 (Magp1) in vivo reveals roles for microfibrils in vascular development and function. Blood. 2006;107:4364–4374. doi: 10.1182/blood-2005-02-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean AJ, Cogger VC, Chong GC, Warren A, Markus AM, Dahlstrom JE, Le Couteur DG. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200:112–117. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 16.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 17.Chan EP, Wells RG. Today's hepatic stellate cells: not your father's sternzellen. Hepatology. 2007;45:1326–1327. doi: 10.1002/hep.21725. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007 doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 20.Rockey DC, Shah V. Nitric oxide biology and the liver: report of an AASLD research workshop. Hepatology. 2004;39:250–257. doi: 10.1002/hep.20034. [DOI] [PubMed] [Google Scholar]
- 21.Shao R, Shi Z, Gotwals PJ, Koteliansky VE, George J, Rockey DC. Cell and molecular regulation of endothelin-1 production during hepatic wound healing. Mol Biol Cell. 2003;14:2327–2341. doi: 10.1091/mbc.02-06-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 23.Douglas IS, Nicolls MR. Chemokine-mediated angiogenesis: an essential link in the evolution of airway fibrosis? J Clin Invest. 2005;115:1133–1136. doi: 10.1172/JCI25193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 26.Groszmann R, Loureiro-Silva M, Tsai M. The biology of portal hypertension. 4th ed. New York: Lippincott Williams & Wilkins; 2001. pp. 679–697. [Google Scholar]
- 27.Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980–G987. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 28.Tsai M, Iwakiri Y, Cadelina G, Sessa W, Groszmann R. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452–1461. doi: 10.1016/j.gastro.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Iwakiri Y, Tsai M, McCabe T, Gratton J, Fulton D, Groszmann R, Sessa W. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol. 2002;282:H2084–H2090. doi: 10.1152/ajpheart.00675.2001. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia-Pagan JC, Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007 doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]
- 31.Angermayr B, Fernandez M, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J. NAD(P)H oxidase modulates angiogenesis and the development of portosystemic collaterals and splanchnic hyperaemia in portal hypertensive rats. Gut. 2007;56:560–564. doi: 10.1136/gut.2005.088013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angermayr B, Mejias M, Gracia-Sancho J, Garcia-Pagan JC, Bosch J, Fernandez M. Heme oxygenase attenuates oxidative stress and inflammation, and increases VEGF expression in portal hypertensive rats. J Hepatol. 2006;44:1033–1039. doi: 10.1016/j.jhep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Hines IN, Hoffman JM, Scheerens H, Day BJ, Harada H, Pavlick KP, Bharwani S, et al. Regulation of postischemic liver injury following different durations of ischemia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G536–G545. doi: 10.1152/ajpgi.00400.2002. [DOI] [PubMed] [Google Scholar]
- 34.Ashburn JH, Baveja R, Kresge N, Korneszczuk K, Keller S, Karaa A, Yokoyama Y, et al. Remote trauma sensitizes hepatic microcirculation to endothelin via caveolin inhibition of eNOS activity. Shock. 2004;22:120–130. doi: 10.1097/01.shk.0000127683.26493.e4. [DOI] [PubMed] [Google Scholar]
- 35.Kamoun WS, Karaa A, Kresge N, Merkel SM, Korneszczuk K, Clemens MG. LPS inhibits endothelin-1-induced endothelial NOS activation in hepatic sinusoidal cells through a negative feedback involving caveolin-1. Hepatology. 2006;43:182–190. doi: 10.1002/hep.20940. [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa T, Ito Y, Wijeweera J, Liu J, Malle E, Farhood A, McCuskey RS, et al. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1385–G1395. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des. 2006;12:2867–2873. doi: 10.2174/138161206777947597. [DOI] [PubMed] [Google Scholar]
- 38.Beraza N, Ludde T, Assmus U, Roskams T, Vander Borght S, Trautwein C. Hepatocyte-specific IKK gamma/NEMO expression determines the degree of liver injury. Gastroenterology. 2007;132:2504–2517. doi: 10.1053/j.gastro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 40.Bonder CS, Kubes P. The future of GI and liver research: editorial perspectives: II. Modulating leukocyte recruitment to splanchnic organs to reduce inflammation. Am J Physiol Gastrointest Liver Physiol. 2003;284:G729–G733. doi: 10.1152/ajpgi.00023.2003. [DOI] [PubMed] [Google Scholar]
- 41.Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, Totani L, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- 42.Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb Haemost. 1999;81:1–7. [PubMed] [Google Scholar]
- 43.Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–680. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 44.Tailor A, Granger DN. Hypercholesterolemia promotes leukocyte-dependent platelet adhesion in murine postcapillary venules. Microcirculation. 2004;11:597–603. doi: 10.1080/10739680490503393. [DOI] [PubMed] [Google Scholar]
- 45.Kim MH, Granger DN, Harris NR. Mediators of CD18/P-selectin-dependent constriction of venule-paired arterioles in hypercholesterolemia. Microvasc Res. 2007;73:150–155. doi: 10.1016/j.mvr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stokes KY, Calahan L, Russell JM, Gurwara S, Granger DN. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation. 2006;13:377–388. doi: 10.1080/10739680600745877. [DOI] [PubMed] [Google Scholar]
- 47.Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- 48.Horie Y, Wolf R, Russell J, Shanley TP, Granger DN. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology. 1997;26:1499–1505. doi: 10.1002/hep.510260617. [DOI] [PubMed] [Google Scholar]
- 49.Taniai H, Hines IN, Bharwani S, Maloney RE, Nimura Y, Gao B, Flores SC, et al. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39:1544–1552. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- 50.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 51.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 52.Distrutti E, Mencarelli A, Santucci L, Renga B, Rizzo G, Orlandi S, Donini A, et al. Evidence That Homocysteine and H2S Exert Opposite Effects On Hepatic Microcirculation in Normal and Cirrhotic Rat Livers. Hepatology. 2007 doi: 10.1002/hep.22037. Submitted. [DOI] [PubMed] [Google Scholar]
- 53.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Das A, Fernandez Zapico M, Cao S, Yao J, Fiorucci S, Hebbel R, Urrutia R, et al. Disruption of an SP2/KLF6 Repression Complex by SHP is Required for Farnesoid X Receptor-Induced Endothelial Cell Migration. J Biol Chem. 2006;281:39105–39113. doi: 10.1074/jbc.M607720200. [DOI] [PubMed] [Google Scholar]
- 56.He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, et al. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192–199. doi: 10.1161/01.RES.0000200400.55539.85. [DOI] [PubMed] [Google Scholar]
- 57.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 58.Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haase VH. The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin Cell Dev Biol. 2005;16:564–574. doi: 10.1016/j.semcdb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Sieghart W, Fuereder T, Schmid K, Cejka D, Werzowa J, Wrba F, Wang X, et al. Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation. 2007;83:425–432. doi: 10.1097/01.tp.0000252780.42104.95. [DOI] [PubMed] [Google Scholar]
- 65.Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 66.Ribatti D, Vacca A, Nico B, Sansonno D, Dammacco F. Angiogenesis and anti-angiogenesis in hepatocellular carcinoma. Cancer Treat Rev. 2006;32:437–444. doi: 10.1016/j.ctrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Kumaran V, Benten D, Follenzi A, Joseph B, Sarkar R, Gupta S. Transplantation of endothelial cells corrects the phenotype in hemophilia A mice. J Thromb Haemost. 2005;3:2022–2031. doi: 10.1111/j.1538-7836.2005.01508.x. [DOI] [PubMed] [Google Scholar]
- 68.Benten D, Follenzi A, Bhargava KK, Kumaran V, Palestro CJ, Gupta S. Hepatic targeting of transplanted liver sinusoidal endothelial cells in intact mice. Hepatology. 2005;42:140–148. doi: 10.1002/hep.20746. [DOI] [PubMed] [Google Scholar]
- 69.Follenzi A, Ulla M, Novikoff P, Gupta S. Reconstitution of the mouse liver with genetically modified liver sinusoidal endothelial cells (LSEC) for biological studies and cell and gene therapy applications. Hepatology. 2006;44:255A. [Google Scholar]
- 70.Hackett PB, Ekker SC, Largaespada DA, McIvor RS. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv Genet. 2005;54:189–232. doi: 10.1016/S0065-2660(05)54009-4. [DOI] [PubMed] [Google Scholar]