Abstract
Hospital- and community-based studies in sub-Saharan Africa document a high prevalence of cryptosporidiosis in children aged 6–36 months, particularly among those who are malnourished or positive for human immunodeficiency virus (HIV) infection and during rainy seasons. This is despite advances in developed countries that have curbed the incidence of cryptosporidiosis in the general and HIV-positive populations. Transmission in sub-Saharan Africa appears to occur predominantly through an anthroponotic cycle. The preponderance of Cryptosporidium hominis, given its limited host range, and the dominance of the more ubiquitous Cryptosporidium parvum after coexposure to both species, however, suggest that the current knowledge of transmission is incomplete. Given the poor sanitation and hygiene, limited availability of antiretrovirals, and the high prevalence of cryptosporidiosis in children—independent of HIV infection—in this region, effective control measures for cryptosporidiosis are desperately needed. Molecular targets from the recently sequenced parasite genome should be exploited to develop an effective and safe treatment for children.
Cryptosporidium species are recognized globally as important causes of diarrhea in children and adults. The literature implicates these protozoan parasites in 3 main epidemiological scenarios—namely, (1) sporadic, often water-related, outbreaks of self-limiting diarrhea in otherwise healthy persons; (2) chronic, life-threatening illness in immunocompromised patients, most notably those with HIV/AIDS; and (3) diarrhea and malnutrition in young children in developing countries. In industrialized nations, improved water-management practices have resulted in a decline in cryptosporidiosis in the general population [1], and antiretrovirals have curbed the incidence and severity in patients with HIV/AIDS [2, 3]. In the absence of these interventions, the burden of cryptosporidiosis continues to fall heavily on developing regions, where infection is both more ubiquitous and clinically consequential.
In developing countries, cryptosporidiosis is most prevalent during early childhood, with as many as 45% of children experiencing the disease before the age of 2 years [4]. Infection is characterized by watery diarrhea, which persists for >2 weeks in a substantial proportion of children [5–8]. Although chronicity in adults is usually predisposed by HIV-related immunodeficiency, children in developing countries are uniquely vulnerable to persistent infection because of the independent and synergistic effects of immune naivete, malnutrition, and HIV infection. Cryptosporidium also plays a causal role in childhood malnutrition in these areas [9–11] and has been linked to impaired physical fitness in late childhood [12]. In addition, cryptosporidiosis has been found to be a significant and independent predictor of childhood death in sub-Saharan Africa [6, 7, 13].
Although the epidemiological and clinical picture is similar in all developing regions, sub-Saharan Africa has been the site of extensive research on cryptosporidiosis in children. We review these studies and draw on literature from other regions, to highlight the ongoing importance of Cryptosporidium in child health, the lingering questions regarding transmission, and the need for an effective intervention in this and other developing regions.
EPIDEMIOLOGY
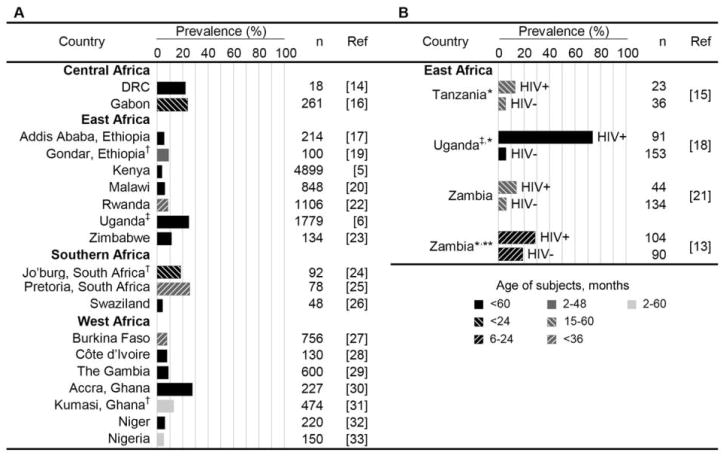
Cryptosporidiosis prevalence in children varies considerably across sub-Saharan Africa and within certain subsets of the population. Figure 1 summarizes cryptosporidiosis prevalence in children aged <5 years who visit health care facilities with diarrhea. Although rarely diagnosed in this setting, coinfection with other enteric pathogens occurs frequently, because of common exposure through poor sanitation and hygiene and because of immune predisposition due to HIV infection.
Figure 1.
Prevalence of cryptosporidiosis among children in sub-Saharan Africa [5, 6, 13–33]. Estimates shown are derived from studies of children aged <5 years attending health care facilities with diarrhea. Prevalence is reported separately for children with unknown or unreported (A) and known (B) HIV serostatus. The age range and number of children providing specimens for each study is indicated. Unless otherwise noted, acute and persistent cases of diarrhea were included in the original studies, and primary detection of Cryptosporidium was performed using acid-fast or immunofluorescence staining. DRC, Democratic Republic of the Congo; Jo’burg, Johannesburg. *Children with persistent diarrhea only. **Malnourished children only. †Children with acute diarrhea only. ‡Primary detection with use of PCR.
In most sub-Saharan countries, cryptosporidiosis prevalence peaks among children aged 6–12 months and decreases thereafter. Breast-feeding is speculated to afford some protection, either through conferment of immunoglobin or avoidance of contaminated water. This may explain why infection is delayed until after the age of 6 months, an age that is commonly marked by the introduction of complementary foods. Children likely experience infection throughout childhood and adolescence, although the clinical significance becomes less apparent with age. This is probably attributable to development of immunity following frequent exposure to oocysts in the contaminated environment. Experimental studies reveal that repeated exposure to Cryptosporidium parvum promotes an IgG response that imparts partial protection against subsequent infection and illness [34]. The extent to which this serological response protects against infection with other Cryptosporidium species is currently unknown.
Impact of HIV infection and malnutrition
Cryptosporidiosis is greatly compounded by HIV infection and malnutrition in sub-Saharan Africa. Among HIV-positive children with diarrhea, prevalence varies between 13.0% in Tanzania [15] and 73.6% in Uganda [18]. The astonishingly high prevalence in Uganda is due partly to the sensitive PCR-based assay used, although notably, the prevalence in HIV-negative Ugandan children is comparable (<6%) to estimates elsewhere in East Africa [15, 21]. In HIV-positive adults, cryptosporidiosis is a marker for low CD4 cell counts independent of diarrhea [35]. Few studies have reported CD4 cell counts in children with cryptosporidiosis. Although low CD4 cell percentage (<25%) was a characteristic of children with persistent diarrhea and cryptosporidiosis in Uganda, this association was insignificant when adjusted for HIV infection [18].
Unlike for individuals with HIV/AIDS, immune defects that increase infection risk in malnourished children are not well defined. There is, however, a clear association between cryptosporidiosis and malnutrition, as shown by studies in Gabon [36], Ghana [30], Niger [32], Tanzania [15], Uganda [6], and Zambia [13] that document significantly higher cryptosporidiosis prevalence among malnourished children. The direction of this association is difficult to ascertain in these cross-sectional studies. Although malnutrition may impair cell-mediated immunity, thus predisposing to infection, it is highly plausible that pathology associated with infection actually impairs nutrient absorption and therefore causes weight loss and growth stunting. This is evidenced by prospective studies in Guinea-Bissau [9] and South America [10, 11, 37] that show that symptomatic and asymptomatic infection—and Cryptosporidium hominis infection in particular—have an adverse and sustained impact on child growth. Although these studies did not control for confounding by HIV infection, the higher documented prevalence of cryptosporidiosis among malnourished children with and without HIV infection (28.9% and 18.9%, respectively [13]), compared with the general pediatric population attending the same hospital in Zambia (HIV-positive children, 16.7%; HIV-negative children, 6.0% [21]), is suggestive of an independent association between cryptosporidiosis and malnutrition and warrants further evaluation.
Community versus hospitalized populations
West Africa has been the site of several community-based studies that confirm the high prevalence of cryptosporidiosis in children with diarrhea. In this setting, prevalence ranges between 7.5% in Liberia [38] and 12.5% in Guinea-Bissau [39]. With the exception of 1 study in the Democratic Republic of the Congo, where the prevalence was 14.8% among children with diarrhea [14], few community-based studies have been conducted outside West Africa. Unsurprisingly, cryptosporidiosis prevalence among children recruited from hospitals was higher; 14.6% in Liberia [38] and 22.2% in the Democratic Republic of the Congo [14]. Although the severity of diarrhea accompanying cryptosporidiosis probably increases the likelihood that parents seek medical care for their child, Hojlyng et al. [38] also note that this assessment could be confounded by young age, because younger children are often overrepresented in health clinics.
Rural versus urban environments
Rural and urban environments might be expected to differentially facilitate Cryptosporidium transmission, because of disparities in animal exposure, access to safe water and sanitation, and population density. On the basis of the limited available evidence, however, it appears that these environments equally favor Cryptosporidium transmission in sub-Saharan Africa. No rural-urban differences in prevalence were observed in Malawi [20], and prevalence was likewise comparable in children residing in towns and periurban and rural areas of Kenya (W. Gatei, personal communication; [5]). Prevalence was also similar in rural and urban areas of Liberia, but, although a positive association existed between cryptosporidiosis and crowding in both areas, rural households with fewer children had a prevalence similar to that in more-crowded urban households [38]. Interestingly, the age distribution of persons with cryptosporidiosis differed between areas in this study, with prevalence peaking at an earlier age in rural areas. Rural-urban variations in age predisposition have also been reported elsewhere for Costa Rica, where the variations were attributed to differences in breast-feeding habits [40]. This does not explain the findings in the Liberian study; bottle-feeding, which was more common in the urban slum, was positively associated with cryptosporidiosis.
Seasonality
Seasonal peaks of cryptosporidiosis have been reported in all regions of sub-Saharan Africa, and, with Kenya [5] and Rwanda [22] as notable exceptions, peaks tend to occur in the wet months. Seasonal patterns probably culminate from several environmental, parasite, and host factors. Storm water runoff during rainy seasons undoubtedly increases environmental transport of feces, and wet, humid conditions favor parasite survival. Morse et al. [20] also note that replenished surface waters serve as both playing areas for children and water holes for animals, so bringing these host populations into close proximity. In West Africa [7, 29] and Zambia [41], human infections peak early in the season, perhaps because susceptible populations develop immunity after repeated exposure with the initial rains. The peak of cryptosporidiosis in the dry seasons in Kenya and Rwanda is intriguing and perhaps relates to the use of alternative, unsafe water sources during those months.
TRANSMISSION
A complex picture of Cryptosporidium transmission has emerged in recent years because of the increasing use of molecular diagnostics. PCR-based techniques have enabled species-specific detection, and sequencing has made it possible to identify parasite subtypes. C. hominis (formerly C. parvum human genotype) and C. parvum (formerly C. parvum bovine genotype) are recognized globally as the most important Cryptosporidium species infecting humans. The former is believed to be transmitted exclusively between humans, whereas C. parvum is transmitted between humans as well as through a zoonotic cycle usually involving ruminants. Although calves are often implicated as the reservoir of C. parvum, the importance of animals in transmission of C. parvum has been brought into question by studies that found that humans are infected with subtypes that perpetuate almost exclusively among humans (reviewed by Xiao and Ryan [42]).
Numerous studies in East and South Africa have demonstrated that the great majority of infections are caused by C. hominis. In Uganda, ~75% of childhood cases are due to C. hominis, whereas only 20% of cases are due to C. parvum [6]. This appears to be a stable phenomenon, because studies in subsequent years yielded similar estimates [18]. Smaller studies of children from Malawi [20, 43], Kenya [5], and South Africa [44] confirm the preponderance of C. hominis. Although these findings have generated speculation that C. hominis is the dominant species in sub-Saharan Africa, no published studies have characterized the Cryptosporidium species and subtypes in Central and West Africa.
The dominance of C. hominis in parts of sub-Saharan Africa is intriguing. Similar observations have been made in the Americas and Australia, whereas C. parvum is the prevailing species in much of Europe (reviewed by Xiao and Ryan [42]). The latter finding is more intuitive, because the broad host range of C. parvum ensures that this species is environmentally ubiquitous. In addition, when hosts are coinfected with both species, C. parvum predominates and rapidly displaces C. hominis [45]. Thus, over time, C. parvum would be expected to become the dominant species in humans, particularly in places where the hygiene level is low, sanitation is limited, and there is close human proximity with animals. Given the evidence to the contrary, it remains a mystery how C. hominis can survive in nature to such an extent that it is the prevailing species in some regions. Indeed, direct human-to-human contact must be critical for C. hominis transmission, because it bypasses the need for a vehicle, such as food or water (liable to contain a mixture of isolates), thus reducing the likelihood of coexposure to C. parvum. Although it is easy to conjecture that high population density might facilitate direct transmission of C. hominis, most epidemiological studies have not provided a contextual link between Cryptosporidium species and environmental exposures.
The study of Cryptosporidium transmission dynamics is made more complex when atypical species are considered. Human infections with Cryptosporidium meleagridis, Cryptosporidium muris, Cryptosporidium felis, Cryptosporidium canis, Cryptosporidium andersoni, and Cryptosporidium suis, though uncommon, have been reported (reviewed by Xiao and Ryan [42]). The natural reservoirs of these species are believed to be poultry, rodents, cats, dogs, cattle, and pigs, respectively; however the broad host ranges are not well characterized. Host factors may also increase the likelihood of infection following exposure to these species, because they are more frequently identified in HIV-positive persons. Two recent studies in East Africa have attempted to correlate species diversity with area of residence. In Malawi, C. meleagridis and C. andersoni were detected only in children from a rural district, although 70% of C. parvum cases were identified in urban areas and C. hominis predominated in both settings [20]. In Kenyan children, 4% of cases were due to C. canis, C. felis, C. meleagridis, and C. muris, and there was no discernible difference by region [5]. Such studies are critical to understanding how these species are transmitted; however, the results are inherently difficult to interpret because of the small number of cases identified.
Although molecular studies have undoubtedly enhanced our understanding of Cryptosporidium transmission in sub-Saharan Africa, there are several shortcomings with the available data. Use of molecular techniques has generally demanded that studies be based in urban referral hospitals—where rural populations are underrepresented and severe, symptomatic infections are overrepresented. Animal exposure in such populations may be limited and infections with C. parvum and atypical species may be consequently underestimated. In addition, there is mounting evidence of species and subtype differences, in terms of diarrhea manifestation [35, 46, 47] and association with growth faltering independent of diarrhea [37]. Estimates derived from diarrheic patients will underestimate the burden of species and subtypes that cause asymptomatic or mild infection, although these may still be important causes of morbidity in children. It is also feasible that each species displays a unique seasonality (as shown in the United Kingdom [48]), conditioned on animal-management activities, for example. Thus, sampling for <12 months may yield biased estimates of the importance of each species.
For species identity to serve as a useful proxy for exposure route, future studies must take a more holistic approach to the epidemiology of cryptosporidiosis. In particular, there needs to be greater emphasis on “contextual typing,” whereby the presence of certain species and/or subtypes can be interpreted in terms of demographic and environmental exposures. The recent study by Ajjampur et al. [46], which incorporated species and/or subtype identification as well as demographic and temporospatial information to describe transmission dynamics in urban India, provides a good example of one such approach. Urban versus rural habitation, sanitation and hygiene practices, animal exposures, and sampling season provide a reasonable framework for interpreting molecular typing results. Such information is critical when making nationwide, cross-continent, and global generalizations. Expansion of studies to include both human and animal subjects as well as asymptomatic hosts will improve our understanding of how each Cryptosporidium species is maintained in nature. The way forward in this regard would be facilitated by development of simplified, species-specific diagnostics that are applicable in resource-poor settings.
PREVENTION AND TREATMENT
Effective control of cryptosporidiosis remains largely elusive in sub-Saharan Africa and other developing regions. Antiretroviral therapy has had a tremendously positive impact on the cryptosporidiosis-related morbidity of patients with HIV/AIDS in developed countries [2, 3], an outcome attributed directly to immune restoration in such individuals. The World Health Organization, however, estimated that only 8% of children in sub-Saharan Africa were receiving antiretroviral therapy in mid-2006 [49]. In addition, and as has been emphasized in this review, young children are particularly vulnerable to cryptosporidiosis, independent of HIV status.
Because host immunity plays a key role in the outcome of infection, the notion of a vaccine to control or reduce the burden of cryptosporidiosis may have merit. However, effective subunit or killed mucosal vaccines targeting enteric pathogens have been difficult to make, more so against protozoa, because of the complexity of these microorganisms. In addition, unlike rotavirus diarrhea, which is confined to the first 18 months of life, cryptosporidiosis is a disease of all ages, and protection would require regular boosting to maintain immunity. In developing countries, where primary exposure to cryptosporidiosis occurs at a young age, vaccination is problematic because of maternal antibody interference. Because exposure occurs regularly after primary infection but with diminished clinical consequence due to acquired immunity, a therapy given at symptom onset to help overcome the primary infection is more practical and cost-effective.
Despite the importance of cryptosporidiosis in childhood health, there has been a lack of serious effort to invest in the development of affordable and effective therapeutics specifically targeting Cryptosporidium species. Existing therapeutics for other apicomplexan diseases are ineffective against Cryptosporidium infection, probably because of the unique intracellular, extracytoplasmic location of Cryptosporidium and the poorly understood host-parasite interface. In addition, difficulties in laboratory propagation, including the absence of an ideal cell culture method, have limited high-throughput drug screening. Hundreds of drugs have been tested in the laboratory, and putative reports suggest that several, including paromomycin, macrolides (e.g., azithromycin and spiramycin), and albendazole, are partially effective. Clinical evaluations of these drugs have been disappointing, largely because they failed to clear the parasite from patients with HIV/AIDS.
To date, the broad-spectrum, anti-infective nitazoxanide (NTZ) has shown the most promise against Cryptosporidium. Trials in Egypt and Zambia found that a 3-day course of NTZ in HIV-seronegative adults and children aged >12 months reduced the duration of diarrhea and parasite excretion and decreased mortality [50–52]. On the basis of these findings, NTZ was approved for treatment of cryptosporidiosis in the United States. The drug has not proven to be the silver bullet, however, for 2 reasons. First, there is a paucity of information about NTZ use in children aged <12 months, despite the clinical vulnerability of this age group. A recent study of NTZ efficacy for rotavirus diarrhea reported that a lower dose was well tolerated in children aged <12 months [53], although the effectiveness of this regimen for cryptosporidiosis was not assessed.
Second, like other drugs tested in clinical trials, NTZ has limited efficacy in immunocompromised individuals. A meta-analysis of randomized, placebo-controlled trials of NTZ (of which there are only 2) among immunocompromised patients concluded that NTZ was no more effective than placebo in resolving diarrhea and achieving parasitological clearance in HIV-positive persons [54]. It has been speculated that HIV-positive persons may benefit from longer-duration regimens or higher doses of NTZ. However, a sustained clinical response was observed in only 59% of patients with HIV/AIDS who received off-label NTZ in a compassionate-use program [55]. Although refinement of the dosing regimen may improve clinical efficacy of NTZ, a prolonged therapeutic course will be impracticable in developing countries because of the expense and likely patient noncompliance.
The failure to develop effective therapy for cryptosporidiosis is largely because of a lack of serious attempts by health agencies and the private sector, mostly because of a perceived limited market for such drugs in developed countries. This is despite the fact that cryptosporidiosis ranks among the most serious causes of global diarrheal illness. There is reason for optimism, however. Recent developments, which include the sequencing of the genomes of C. parvum [56] and C. hominis [57], have led to the identification of new molecular targets for drug development. The availability of a substantive number of chemical libraries for drug discovery should also facilitate screening for effective drugs. Additionally, the Centers for Disease Control and Prevention in the United States has placed cryptosporidiosis prominently among the category B biothreat pathogens. Hopefully, this will counteract a loss of interest by some funding agencies as a result of the success of antiretroviral therapies in developed countries and will provide additional incentive for drug development, which is desperately needed for children with chronic diarrhea and malnutrition, particularly in developing countries.
Acknowledgments
We thank Jeffrey K. Griffiths for his insightful views.
Financial support. National Institutes of Health (R21A1068474) and University of Sydney (traveling scholarship to S.M.M.).
Footnotes
Potential conflicts of interest. S.M.M. and S.T.: no conflicts.
References
- 1.Lake IR, Nichols G, Bentham G, Harrison FC, Hunter PR, Kovats SR. Cryptosporidiosis decline after regulation, England and Wales, 1989–2005. Emerg Infect Dis. 2007;13:623–5. doi: 10.3201/eid1304.060890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1–associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–61. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 3.Maggi P, Larocca AM, Quarto M, et al. Effect of antiretroviral therapy on cryptosporidiosis and microsporidiosis in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis. 2000;19:213–7. doi: 10.1007/s100960050461. [DOI] [PubMed] [Google Scholar]
- 4.Valentiner-Branth P, Steinsland H, Fischer TK, et al. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J Clin Microbiol. 2003;41:4238–45. doi: 10.1128/JCM.41.9.4238-4245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatei W, Wamae CN, Mbae C, et al. Cryptosporidiosis: prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am J Trop Med Hyg. 2006;75:78–82. [PubMed] [Google Scholar]
- 6.Tumwine JK, Kekitiinwa A, Nabukeera N, et al. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg. 2003;68:710–5. [PubMed] [Google Scholar]
- 7.Molbak K, Hojlyng N, Gottschau A, et al. Cryptosporidiosis in infancy and childhood mortality in Guinea Bissau, West Africa. BMJ. 1993;307:417–20. doi: 10.1136/bmj.307.6901.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sodemann M, Jakobsen MS, Molbak K, Martins C, Aaby P. Episode-specific risk factors for progression of acute diarrhoea to persistent diarrhoea in West African children. Trans R Soc Trop Med Hyg. 1999;93:65–8. doi: 10.1016/s0035-9203(99)90183-9. [DOI] [PubMed] [Google Scholar]
- 9.Molbak K, Andersen M, Aaby P, et al. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, West Africa. Am J Clin Nutr. 1997;65:149–52. doi: 10.1093/ajcn/65.1.149. [DOI] [PubMed] [Google Scholar]
- 10.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148:497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 11.Checkley W, Gilman RH, Epstein LD, et al. Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am J Epidemiol. 1997;145:156–63. doi: 10.1093/oxfordjournals.aje.a009086. [DOI] [PubMed] [Google Scholar]
- 12.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61:707–13. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 13.Amadi B, Kelly P, Mwiya M, et al. Intestinal and systemic infection, HIV, and mortality in Zambian children with persistent diarrhea and malnutrition. J Pediatr Gastroenterol Nutr. 2001;32:550–4. doi: 10.1097/00005176-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Henry MC, Alary M, Desmet P, et al. Community survey of diarrhoea in children under 5 years in Kinshasa, Zaire. Ann Soc Belg Med Trop. 1995;75:105–14. [PubMed] [Google Scholar]
- 15.Cegielski JP, Ortega YR, McKee S, et al. Cryptosporidium, Enterocytozoon, and Cyclospora infections in pediatric and adult patients with diarrhea in Tanzania. Clin Infect Dis. 1999;28:314–21. doi: 10.1086/515131. [DOI] [PubMed] [Google Scholar]
- 16.Heron H, Dufillot D, Duong TH, et al. Digestive cryptosporidiosis: prospective study in Gabonese infants with diarrhoea. Ann Pediatr (Paris) 1994;41:225–9. [Google Scholar]
- 17.Assefa T, Mohammed H, Abebe A, Abebe S, Tafesse B. Cryptosporidiosis in children seen at the children’s clinic of Yekatit 12 Hospital, Addis Ababa. Ethiop Med J. 1996;34:43–5. [PubMed] [Google Scholar]
- 18.Tumwine JK, Kekitiinwa A, Bakeera-Kitaka S, et al. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am J Trop Med Hyg. 2005;73:921–5. [PubMed] [Google Scholar]
- 19.Mersha D, Tiruneh M. Frequency of isolation of Cryptosporidium oocysts in Ethiopian children with acute diarrhoeal disease. East Afr Med J. 1992;69:314–5. [PubMed] [Google Scholar]
- 20.Morse TD, Nichols RA, Grimason AM, Campbell BM, Tembo KC, Smith HV. Incidence of cryptosporidiosis species in paediatric patients in Malawi. Epidemiol Infect. 2007;135:1307–15. doi: 10.1017/S0950268806007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chintu C, Luo C, Baboo S, et al. Intestinal parasites in HIV-seropositive Zambian children with diarrhoea. J Trop Pediatr. 1995;41:149–52. doi: 10.1093/tropej/41.3.149. [DOI] [PubMed] [Google Scholar]
- 22.Bogaerts J, Lepage P, Rouvroy D, et al. Cryptosporidiosis in Rwanda: clinical and epidemiological features. Ann Soc Belg Med Trop. 1987;67:157–65. [PubMed] [Google Scholar]
- 23.Simango C, Mutikani S. Cryptosporidiosis in Harare, Zimbabwe. Cent Afr J Med. 2004;50:52–4. [PubMed] [Google Scholar]
- 24.Berkowitz FE, Vallabh W, Buqwana A, Heney C. Cryptosporidiosis in black South African children. S Afr Med J. 1988;74:272–3. [PubMed] [Google Scholar]
- 25.Geyer A, Crewe-Brown HH, Greeff AS, et al. The microbial aetiology of summer paediatric gastroenteritis at Ga-Rankuwa Hospital in South Africa. East Afr Med J. 1993;70:78–81. [PubMed] [Google Scholar]
- 26.Dlamini MS, Nkambule SJ, Grimason AM. First report of cryptosporidiosis in paediatric patients in Swaziland. Int J Environ Health Res. 2005;15:393–6. doi: 10.1080/09603120500156045. [DOI] [PubMed] [Google Scholar]
- 27.Nacro B, Bonkoungou P, Nagalo K, Tall FR, Curtis V. Clinical profile of cryptosporidiosis in a pediatric hospital environment in Burkina Faso [in French] Med Trop (Mars) 1998;58:47–50. [PubMed] [Google Scholar]
- 28.Kassi RR, Kouassi RA, Yavo W, et al. Cryptosporidiosis and isosporiasis in children suffering from diarrhoea in Abidjan. Bull Soc Pathol Exot. 2004;97:280–2. [PubMed] [Google Scholar]
- 29.Adegbola RA, Demba E, De Veer G, Todd J. Cryptosporidium infection in Gambian children less than 5 years of age. J Trop Med Hyg. 1994;97:103–7. [PubMed] [Google Scholar]
- 30.Adjei AA, Armah H, Rodrigues O, et al. Cryptosporidium spp. a frequent cause of diarrhea among children at the Korle-Bu Teaching Hospital, Accra, Ghana. Jpn J Infect Dis. 2004;57:216–9. [PubMed] [Google Scholar]
- 31.Addy PA, Aikins-Bekoe P. Cryptosporidiosis in diarrhoeal children in Kumasi, Ghana. Lancet. 1986;1:735. doi: 10.1016/s0140-6736(86)91119-0. [DOI] [PubMed] [Google Scholar]
- 32.Gay-Andrieu E, Adehossi E, Illa H, Garba Ben A, Kourna H, Boureima H. Prevalence of cryptosporidiosis in pediatric hospital patients in Niamey, Niger. Bull Soc Pathol Exot. 2007;100:193–6. [PubMed] [Google Scholar]
- 33.Reinthaler FF, Hermentin K, Mascher F, Klem G, Sixl W. Cryptosporidiosis in Ogun State, south-west Nigeria. Trop Med Parasitol. 1987;38:51–2. [PubMed] [Google Scholar]
- 34.Chappell CL, Okhuysen PC, Sterling CR, Wang C, Jakubowski W, Dupont HL. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti–C. parvum serum immunoglobulin G. Am J Trop Med Hyg. 1999;60:157–64. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 35.Houpt ER, Bushen OY, Sam NE, et al. Short report: asymptomatic Cryptosporidium hominis infection among human immunodeficiency virus–infected patients in Tanzania. Am J Trop Med Hyg. 2005;73:520–2. [PubMed] [Google Scholar]
- 36.Duong TH, Dufillot D, Koko J, et al. Digestive cryptosporidiosis in young children in an urban area in Gabon. Sante. 1995;5:185–8. [PubMed] [Google Scholar]
- 37.Bushen OY, Kohli A, Pinkerton RC, et al. Heavy cryptosporidial infections in children in northeast Brazil: comparison of Cryptosporidium hominis and Cryptosporidium parvum. Trans R Soc Trop Med Hyg. 2007;101:378–84. doi: 10.1016/j.trstmh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Hojlyng N, Molbak K, Jepsen S. Cryptosporidium spp. a frequent cause of diarrhea in Liberian children. J Clin Microbiol. 1986;23:1109–13. doi: 10.1128/jcm.23.6.1109-1113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carstensen H, Hansen HL, Kristiansen HO, Gomme G. The epidemiology of cryptosporidiosis and other intestinal parasitoses in children in southern Guinea-Bissau. Trans R Soc Trop Med Hyg. 1987;81:860–4. doi: 10.1016/0035-9203(87)90054-x. [DOI] [PubMed] [Google Scholar]
- 40.Mata L, Bolanos H, Pizarro D, Vives M. Cryptosporidiosis in children from some highland Costa Rican rural and urban areas. Am J Trop Med Hyg. 1984;33:24–9. doi: 10.4269/ajtmh.1984.33.24. [DOI] [PubMed] [Google Scholar]
- 41.Nchito M, Kelly P, Sianongo S, et al. Cryptosporidiosis in urban Zambian children: an analysis of risk factors. Am J Trop Med Hyg. 1998;59:435–7. doi: 10.4269/ajtmh.1998.59.435. [DOI] [PubMed] [Google Scholar]
- 42.Xiao L, Ryan UM. Cryptosporidiosis: an update in molecular epidemiology. Curr Opin Infect Dis. 2004;17:483–90. doi: 10.1097/00001432-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Peng MM, Meshnick SR, Cunliffe NA, et al. Molecular epidemiology of cryptosporidiosis in children in Malawi. J Eukaryot Microbiol. 2003;50(Suppl):557–9. doi: 10.1111/j.1550-7408.2003.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 44.Samie A, Bessong PO, Obi CL, et al. Cryptosporidium species: preliminary descriptions of the prevalence and genotype distribution among school children and hospital patients in the Venda region, Limpopo Province, South Africa. Exp Parasitol. 2006;114:314–22. doi: 10.1016/j.exppara.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Akiyoshi DE, Mor S, Tzipori S. Rapid displacement of Cryptosporidium parvum type 1 by type 2 in mixed infections in piglets. Infect Immun. 2003;71:5765–71. doi: 10.1128/IAI.71.10.5765-5771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajjampur SS, Gladstone BP, Selvapandian D, Muliyil JP, Ward H, Kang G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in South India. J Clin Microbiol. 2007;45:915–20. doi: 10.1128/JCM.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cama VA, Bern C, Sulaiman IM, et al. Cryptosporidium species and genotypes in HIV-positive patients in Lima, Peru. J Eukaryot Microbiol. 2003;50(Suppl):531–3. doi: 10.1111/j.1550-7408.2003.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 48.Sopwith W, Osborn K, Chalmers R, Regan M. The changing epidemiology of cryptosporidiosis in North West England. Epidemiol Infect. 2005;133:785–93. doi: 10.1017/S0950268805004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Progress in scaling up access to HIV treatment in low- and middle-income countries. Geneva, Switzerland: World Health Organization; Jun, 2006. [Google Scholar]
- 50.Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:103–6. doi: 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- 51.Rossignol JF, Kabil SM, el-Gohary Y, Younis AM. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin Gastroenterol Hepatol. 2006;4:320–4. doi: 10.1016/j.cgh.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375–80. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- 53.Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368:124–9. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- 54.Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol. 2007;63:387–93. doi: 10.1111/j.1365-2125.2007.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossignol JF. Nitazoxanide in the treatment of acquired immune deficiency syndrome–related cryptosporidiosis: results of the United States compassionate use program in 365 patients. Aliment Pharmacol Ther. 2006;24:887–94. doi: 10.1111/j.1365-2036.2006.03033.x. [DOI] [PubMed] [Google Scholar]
- 56.Abrahamsen MS, Templeton TJ, Enomoto S, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–5. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 57.Xu P, Widmer G, Wang Y, et al. The genome of Cryptosporidium hominis. Nature. 2004;431:1107–12. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]