Abstract
Individuals with type 2 diabetes mellitus (DM) characteristically have elevated fasting and postprandial (PP) plasma triglycerides (TG). Previous case-control studies indicated that PPTG levels predict the presence of coronary artery disease (CAD) in people without DM; however, the data for patients with DM are conflicting. Therefore, we conducted a case-control study in DM individuals, 84 with (+) and 80 without (−) CAD. Our hypothesis was that DM individuals with or without CAD would have similar PPTG levels, but CAD+ individuals would have more small d<1.006 g/L lipoprotein particles. Several markers of PP lipid metabolism were measured over 10 h after a fat load. PP lipoprotein size and particle number were also determined. There was no significant difference in any measure of PP lipid metabolism between CAD+ and CAD−, except for apoB48, which was actually higher in CAD−. We followed 69 CAD− participants for a mean 8.7 years; 33 remained free of any cardiovascular event. There were no PP differences at baseline between these 33 who remained CAD− and either the 36 original CAD− who subsequently developed CAD or the original CAD+ group.PP measurements of TG-rich lipoproteins do not predict the presence of CAD in individuals with DM.
Keywords: triglycerides, apolipoprotein B48, remnant lipoproteins
Type 2 diabetes mellitus (DM) is associated with 2- to 4-fold increases in atherosclerotic cardiovascular disease (ASCVD), particularly coronary artery disease (CAD) (1), and a dyslipidemia characterized by high levels of plasma triglycerides (TG) and low levels of HDL cholesterol (C) (2, 3). Although low HDL-C is an acknowledged risk factor for CAD, high TG levels have not been demonstrated consistently to be a risk factor, usually due to loss of significance when other known risk factors, particularly glucose and HDL-C, are taken into account (4, 5). However, large meta-analyses have identified plasma level of fasting TG as an independent predictor of future CVD, particularly in women (6, 7). Importantly, two recent studies indicated the importance of increased nonfasting or postprandial (PP) TG levels (8, 9) as predictors of ASCVD. In those studies, nonfasting TG concentrations remained independent predictors of ASCVD events, even after adjusting for other risk factors including HDL-C.
The importance of these recent findings is highlighted by the fact that most individuals are usually within a few hours of eating food during much of the day. Indeed, studies from several laboratories, including our own, have demonstrated that the excursion of plasma TG after ingestion of a high-fat meal is predictive of the presence of CAD (10–13) or carotid atherosclerosis (14) in people without DM. In two of those studies (13, 14), the predictive power of PPTG was independent of fasting TG concentrations. On the other hand, there are only a few studies that have examined PP lipemia (PPL) in populations with DM, and the results have been conflicting. One small study in DM patients did not show an association between PPL and CAD (15). A later study by the same group of investigators confirmed the initial overall finding but did observe that PP excursions of apolipoprotein (apo) B48 and apoB100 intermediate density lipoproteins (IDL) were greater in the presence of more severe CAD (16). A more recent study demonstrated that participants with myocardial infarction (MI) and DM had a higher response of plasma TG and retinyl palmitate (RP; used as a marker of intestinal remnant lipoproteins) compared with individuals with DM who had not had an MI (17).
In an attempt to clarify the role of PPL in patients with DM, we conducted a case-control study in people with DM, with or without evidence of clinical CAD. Our hypothesis was that, although people with DM and documented CAD would not differ by usual measures of PPL, they would have a greater number of small PP lipoprotein particles in the d<1.006 g/L density range (a mixture of chylomicrons, chylomicron remnants, and VLDL) compared with a group of people with DM but no CAD. These smaller PP d<1.006 lipoproteins might be more atherogenic.
METHODS
We performed a cross-sectional, case-control study in DM individuals. Participants were first identified by querying a large clinical database maintained by the New York Presbyterian Hospital and Columbia University Medical Center for patients with DM who had a MI any time in the past, a coronary angiogram within the previous year, or an exercise perfusion scan (stress thallium study) within one year. The physicians caring for those patients then obtained permission for the investigators to contact them. The presence of DM was defined by medical record diagnosis, fasting glucose >140 mg/dl, or the use of diabetes medications. The CAD+ (N = 84) group was defined by the following criteria: documented prior MI, PTCA/stent, CABG, or >75% stenosis in any vessel by coronary angiography. The CAD− (N = 80) group was defined as: the absence of a prior MI and <50% stenosis in all vessels by coronary angiography within the past year, or the combination of a normal exercise perfusion scan and the absence of a prior MI or any interventional cardiac procedures. In the CAD+, 62 of the 84 participants had a previous MI, 41 had a CABG, 38 had an angioplasty or stent placement, and 70 had >75% stenosis in at least one vessel on angiography. Among the CAD−, 21 of the 80 participants had no history of an MI and <50% stenosis in all vessels on angiography, while 59 had normal perfusion scans and no prior CAD event.
Because of the untimely death of the lead investigator (C.T.), analysis of the data and generation of the manuscript were delayed for several years. However, this allowed us to determine outcome data in our study populations. Thus, we were able to ascertain the health status of 69 of the 80 CAD− participants (11 participants were lost to follow-up) 7 years after the original study was completed (mean follow-up of 8.7 years). In those 69 participants, we determined if they had had a MI, developed an abnormal ECG consistent with a MI, or required any interventional treatment (PTCA or CABG).
Participants
The study was approved by the Columbia University Medical Center Institutional Review Board and all participants signed informed consent. Exclusion criteria included: MI or cardiac procedures within the past 3 months, cancer, severe congestive heart failure (ejection fraction < 20%), kidney or liver disease, pancreatitis, other gastrointestinal conditions, creatinine levels > 1.3, abnormal liver function tests, abnormal CBC, fasting TG > 400 mg/dl, urine protein > 2+ on urinalysis or > 1000 mg/24 h, abnormal thyroid function tests, body mass index (BMI) > 34 for men and > 37 for women or BMI < 20 for both sexes, and age < 35 or > 75 years. All participants completed the study.
Study procedures
The protocol we used has been described previously by our group (13). Briefly, participants were admitted to the Clinical Research Center after fasting for 12 h. Participants had not ingested any alcoholic beverages for at least 5 days prior to study. Lipid-altering medications had been stopped 4 weeks prior to the study. Participants had a fasting blood glucose measured by a bedside device and, based on the results, were given some or all of their diabetes medications. This approach was introduced because of the relatively low carbohydrate and caloric intake during the 10-h test. Participants were weighed and their height, waist, and hip circumferences were measured. After fasting bloods were obtained, participants ingested a high-fat beverage that provided 1,237 kcal/2 m2 body surface area. The beverage consisted of 75% fat (40% saturated, 20% monounsaturated, and 15% polyunsaturated), 10% protein, and 15% carbohydrate. Aqueous vitamin A (Vitadral Trophen, Pharma Wenigerode) was added to provide 100,000 U/2 m2 body surface area. The high-fat beverage was consumed within 15 min. Sequential blood samples were obtained at 3, 5, 7, and 10 h after ingestion of the beverage. Except for use of the bathroom, participants remained in a semirecumbent position for the complete study.
Nutrition
Standardized Block 98.2 food frequency questionnaires were obtained on all participants by a registered dietitian and tabulated.
Laboratory
Total C, TG, HDL-C, and glucose were measured using standard enzymatic techniques on a Roche Hitachi 912 chemistry analyzer. LDL-C levels were calculated by the Friedewald method (18). Lipoproteins were isolated from the fasting and PP samples by sequential ultracentrifugation of 2 ml of plasma, beginning with d.1.006 (19), in a 50.3ti rotor using a Beckman TL100 ultracentrifuge. The lipoproteins were removed in volumes of 2.0 ml and used for determination of TG and C. Lipoprotein particle number and size were determined at Liposcience Inc.. Raleigh, NC (20). Total apoB and apoA-I were measured in serum using an immunoturbidometric method (Roche Diagnostics Systems) on a Hitachi 912 chemistry analyzer. ApoB48 levels were measured by an ELISA kit from BioVendor USA/Canada (21). Remnant lipoprotein (RLP)-C was determined using reagents obtained from Otsuka Pharmaceutical Co. (22) RP levels were determined using reverse phase HPLC (23). Additionally, CBC, metabolic panel, and HbA1c were measured on fasting samples by the hospital clinical laboratory.
Statistics
PP incremental area under the curve (IAUC) for TG, d<1.006 TG, RP, RLP-C, and other analytes were calculated as previously described (13). TG and RP levels, as well as IAUCs for TG and RP, were log-transformed to achieve normal distributions prior to statistical analysis; results for these measures are reported as medians and interquartile ranges. All comparisons between cases and controls were done by unpaired t-tests. All results are reported as means and SD; error bars in figures are of SEM. With 84 cases and 80 controls, we had 80% power, testing at P = 0.05, to detect any difference that exceeded 45% of the within-group SD. Thus, if an outcome measure had a 20% within-group SD, we had the power to find significance if the case-control difference exceeded 9%. Confidence limits were calculated on case-control differences as part of the unpaired t-tests using SAS (version 9.1, SAS Software, Inc.).
RESULTS
Cases and controls had similar demographic characteristics except for sex and ethnicity
Our study enrolled 164 individuals with DM (66 females and 98 males), 84 of whom were CAD+ and 80 were CAD−. The clinical characteristics of each group are presented in Table 1. The groups were similar regarding age, duration of diabetes, HbA1c, BMI, and waist-hip ratio. There were no significant differences in dietary intake between the CAD− (percent of calories: 17.8% protein, 34.7% fat, 46.9% carbohydrate, 10.1% saturated fat) and CAD+ percent of calories: 18.1% protein, 33.4% fat, 47.6% carbohydrate, 10.1% saturated fat) group. Total calories (CAD+, 1739.4; CAD−,1754.1 kcal/d) and dietary C (CAD−, 261.7; CAD+, 247.2 mg/d) were also similar in the groups, although it is likely that there was underreporting of caloric intake in both groups. There were significantly more Hispanics (68% vs. 52%) in the CAD− group, and more whites/others among the CAD+ (41% vs. 21%). However, we did not find case-control differences in PPL by ethnicity (data not shown). There were also more women than men (50% vs. 31%) in the study population (see below).
TABLE 1.
Baseline characteristics
| Controls (n = 80) | Cases (n = 84) | P | |
|---|---|---|---|
| Age (years) | 61.1 ± 8.9 | 61.4 ± 7.4 | n.s. |
| Duration of DM (years) | 9.1 ± 7.2 | 8.5 ± 7.5 | n.s. |
| HbA1c (%) | 9.8 ± 2.2 | 10.4 ± 2.9 | n.s. |
| BMI (kg/m2) | 31.4 ± 5.6 | 30.2 ± 5.3 | n.s. |
| Waist-hip ratio | 0.98± .09 | 0.99 ±.07 | n.s. |
| Females n (%) | 40 (50%) | 26 (31%) | 0.01 |
| Ethnicity n (%) | |||
| Hispanics | 54 (68%) | 44 (52%) | 0.05 |
| African American | 9 (11%) | 6 (7%) | n.s. |
| White + other | 17 (21%) | 34 (41%) | 0.01 |
n.s, nonsignificant.
HbA1c levels were elevated in both CAD− and CAD+ groups (Table 1); they were not different by gender but tended to be higher in participants receiving insulin (10.9 ± 3 vs. 9.9 ± 3; P = 0.07). There was a trend to differences by ethnicity: 10.0 ± 2.1 for Blacks, 10.5 ± 2.7 for Hispanics, and 9.5 ± 2.4 for others (mostly Whites) (ANOVA P = 0.09).
Cases and controls received similar treatment for diabetes except for the use of metformin
Participants were on different regimens for the treatment of their DM. A small number of participants were only on diet treatment at the time of the study (10 cases and 6 controls). Of those receiving medications, use of sulfonylurea or repaglinide was reported in 62% of CAD+ and 69% of CAD−; metformin in 32% of CAD+ and 50% of CAD−; thiazolidinediones in 5% of CAD+ and 9% of CAD−; and insulin in 19% of CAD+ and 21% of CAD−. We performed separate analyses in cases and controls to see if the TG and VLDL-TG PP areas were different between participants who were on metformin and those who were not; we found no significant differences in either group (data not shown).
Fasting lipid and lipoprotein levels were similar in cases and controls
The lipid and lipoprotein results for females and males were similar and were combined for analyses. Fasting levels of total C, TG, VLDL TG, HDL-C, LDL-C, and apoA-1 were similar in both groups. Fasting levels of total apoB were higher in the CAD+ (117 ± 27 mg/dl) than in the CAD− group (108 ± 27 mg/dl); P < 0.04) (Table 2).
TABLE 2.
Fasting lipids and lipoproteins
| Controls (n = 80) | Cases (n = 84) | P | |
|---|---|---|---|
| Total C (mg/dl) | 188 ± 36 | 198 ± 400 | n.s. |
| TG (mg/dl) | 147 (113–207) | 143 (104–225) | n.s. |
| VLDL TG (mg/dl) | 89 (60–151) | 91 (55–159) | n.s. |
| HDL-C (mg/dl) | 35 ± 100 | 32 ± 9 | n.s. |
| LDL-C (mg/dl) | 119 ± 34 | 127 ± 34 | n.s. |
| ApoA1 (mg/dl) | 124 ± 19 | 120 ± 22 | n.s. |
| ApoB (mg/dl) | 108 ± 27 | 117 ± 27 | 0.04 |
| ApoB48 (mg/l) | 7.6 ± 5 | 9.0 ± 7 | n.s. |
Values for total C, HDL-C, LDL-C, ApoA1, ApoB, and ApoB48 are expressed as mean ± SD. Values for TG and VLDL TG are expressed as median and interquartile range. These measurements were obtained on the day of the PP test, one month after discontinuation of any lipid-altering medications. n.s, nonsignificant.
Cases and controls had similar levels of PPL
The excursions of plasma TG and d<1.006 TG in cases and controls after the high-fat load were nearly identical (Fig. 1A, B, respectively), confirming our hypothesis that there were no differences in the CAD+ vs. the CAD− groups. The actual IAUCs for TG and d<1.006 TG are presented in Table 3. Because gender has been shown to affect PP responses and we had more women in this study, we analyzed the data separately by gender. Although women in both groups had lower TG and d<1.006 TG areas than did men, there were no case-control differences within gender (Fig. 1C, D; Table 3). Additionally, because administration of insulin just prior to the study (see “Methods”) might have had an acute effect on TG or VLDL-TG PP areas, we analyzed our data separately for those patients taking and those not taking insulin (Table 3). Although insulin use was associated with lower PPTG and PP d<1.006 TG, there were no case-control differences in either group. Additionally, there was no difference in HbA1c levels between cases and controls, nor did HbA1c affect case-control differences in PPTG areas.
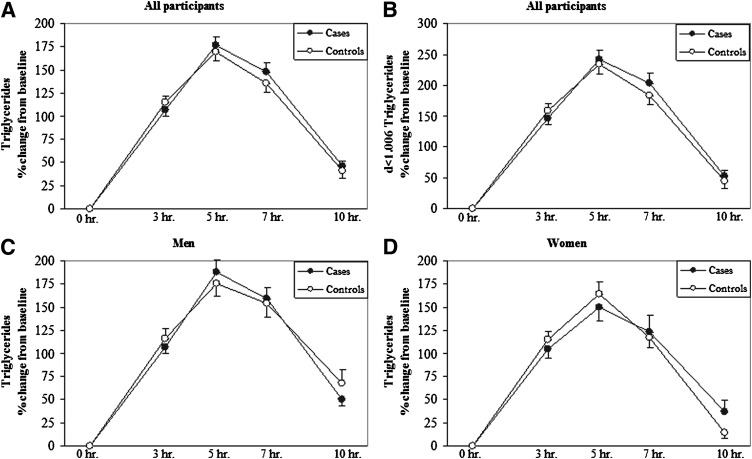
Fig. 1.
Levels of plasma and d<1.006 TG during the PP period. Percent change from baseline of PPTG (A) and PP d<1.006 lipoprotein TG (B) for all participants, and PPTG for men (C) and women (D) over a 10 h period after a high-fat formula feeding. There were no differences between the CAD+ and CAD− groups.
TABLE 3.
IAUC for PPTG and PP d<1.006 TG in cases versus control, adjusting for gender and insulin used
|
n |
TG IAUC |
d<1.006 TG IAUC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Cases | Control | Cases | P | Control | Cases | P | ||
| All subjects | 80 | 84 | 1450 (908–2230) | 1441 (979–2436) | n.s. | 1209 (716–1884) | 1170 (767–1869) | n.s. | |
| Men | 40 | 58 | 1656 (976–2619) | 1562 (1057–2501) | n.s. | 1456 (797–2229) | 1328 (790–1953) | n.s. | |
| Women | 40 | 26 | 1223a (777–1901) | 1126a (821–2371) | n.s. | 968a (621–1711) | 873a (469–1738) | n.s. | |
| On insulin | No | 63 | 68 | 1497 (938–2395) | 1582 (1034–2575) | n.s. | 1277 (796–1909) | 1379 (819–1994) | n.s. |
| Yes | 17 | 16 | 1126b (631–1829) | 1023b (885–1586) | n.s. | 877b (396–1577) | 789b (493–1206) | n.s. | |
Women had lower TG IAUC and d<1.006 IAUC than men (P < 0.01).
Insulin use was associated with lower TG IAUC and d<1,006 iAUC (P < 0.05).
n.s, nonsignificant.
Cases did not have greater PP responses of apoB48 and total apoB than controls, and there were no differences in the size distribution or number of d<1.006 particles during PPL
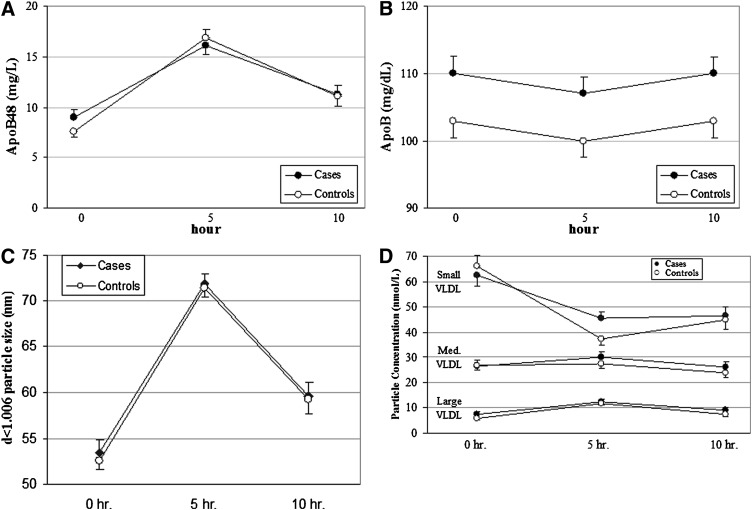
Our main hypothesis was that, despite the absence of differences in PP lipids in CAD+ versus CAD−, there would be a greater number of smaller lipoprotein particles within the d<1.006 range in the cases after the fat load. We examined this specific hypothesis with several approaches. First, we determined the number of intestinal particles, as reflected by levels of apoB48. Plasma apoB48 levels at 0, 5, and 10 h were similar between cases and controls (Fig. 2A). However, the apoB48 IAUC (mg/L/hour) was slightly, but significantly, higher in CAD− compared with CAD+ (55.2 ± 44 vs. 41.3 ± 27, respectively; P < 0.02). Total apoB, which even in the PP state is >95% apoB100, was (as noted above) higher in CAD+ at time 0 and remained higher at 5 and 10 h (Fig. 2B). However, apoB levels changed very little during the PP period, and the apoB IAUCs (mg/dl-hr) were very similar between CAD− and CAD+ (−13.5 ± 40.4 vs. −17.7 ± 66.8, respectively).
Fig. 2.
Levels of apoB48, apoB, d<1.006 particle size, and particle concentration during the PP period. Concentrations of apoB48 (A), total apoB (B), size of density <1.006 lipoproteins in nm (C), and number of d<1.006 particles in nmol/l across three size ranges (D) at baseline, 5 h, and 10 h after ingestion of a high-fat formula feeding. The IAUC for apoB48 was greater in CAD− than in CAD+ (P < 0.02). There were no differences between the CAD+ and CAD− groups for the other variables.
The NMR method of Otvos J. et al. (20) was used to determine the mean size of all d<1.006 particles at those same times. As expected, we observed an increase in the size of d<1.006 lipoproteins at 5 h after ingestion of the fat load, and that increase in size disappeared by the 10 h sample in both groups (Fig. 2C). However, there were no differences in the mean d<1.006 lipoprotein size between CAD+ and CAD− at any time during the PP period (0 h, 53.5 ± 12.2 nm vs. 52.6 ± 8.1 nm; 5 h, 71.9 ± 9.9 nm vs. 71.5 ± 9.3 nm; 10 h, 59.6 ± 13.7 nm vs. 59.3 ± 14.2 nm, respectively). Examination of the number of d<1.006 lipoproteins during the PP period revealed a decrease in small particles over the course of 10 h and modest increases in medium and large particle numbers at 5 h that returned to baseline by 10 h (Fig. 2D). Importantly, the IAUCs for the number of d<1.006 particles in small (CAD+, −128 ± 270; CAD−, −189.3 ± 249), medium (CAD+, 14.9 ± 103; CAD−, −4.0 ± 85), and large (CAD+, 29.2 ± 36; CAD−, 34.2 ± 34) particles did not differ between the CAD+ and CAD− groups. In addition, sizes of LDL (0 h, 20.3 ± 0.8 nm CAD+ vs. 20.4 ± 0.9 nm CAD−; 5 h, 20.3 ± 0.8 nm CAD+ vs. 20.4 ± 0.9 nm CAD−; 10 h, 20.4 ± 0.9 nm CAD+ vs. 20.5 ± 1 nm CAD−) and of HDL (0 h, 8.8 ± 0.3 nm CAD+ vs. 8.8 ± 0.4 nm CAD−; 5 h, 8.92 ± 0.3 nm CAD+ vs. 8.98 ± 0.3 nm CAD−; 10 h, 8.81 ± 0.3 nm CAD+ vs. 8.89 ± 0.3 nm CAD−) did not differ between the two groups at any of the PP times.
Cases and controls had similar levels of remnant lipoproteins during PPL
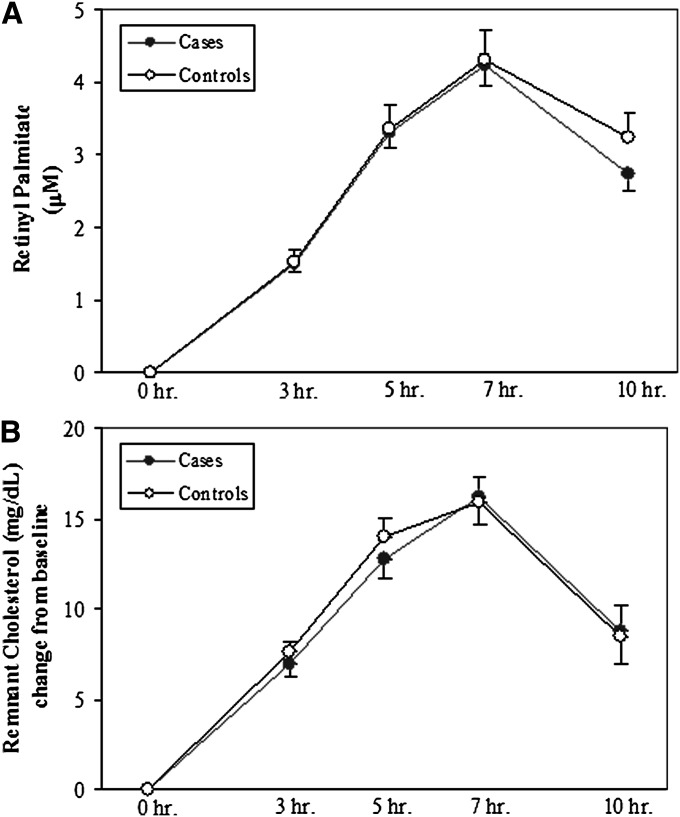
We next looked for differences in PP remnant lipoproteins between CAD+ and CAD−. First, we incorporated retinol (vitamin A) into the fat load as a means of following the removal of intestinal particles, particularly remnant particles, during the PP period (24, 13); There were no differences in the levels of RP between CAD+ and CAD− participants (Fig. 3A). Second, we determined the levels of RLP-C as a second measure of remnant lipoproteins during PPL, although this measurement includes both apoB48 and some apoB100 lipoproteins (22). Again, there were no case-control differences (Fig. 3B).
Fig. 3.
Levels of remnant lipoproteins during the PP period. PP RP concentrations (A) and changes from baseline of PP RLP-C concentrations (B) during 10 h after a high-fat formula feeding. There were no differences between CAD+ and CAD− groups.
There was no association between severity of disease and PPL in CAD+ participants
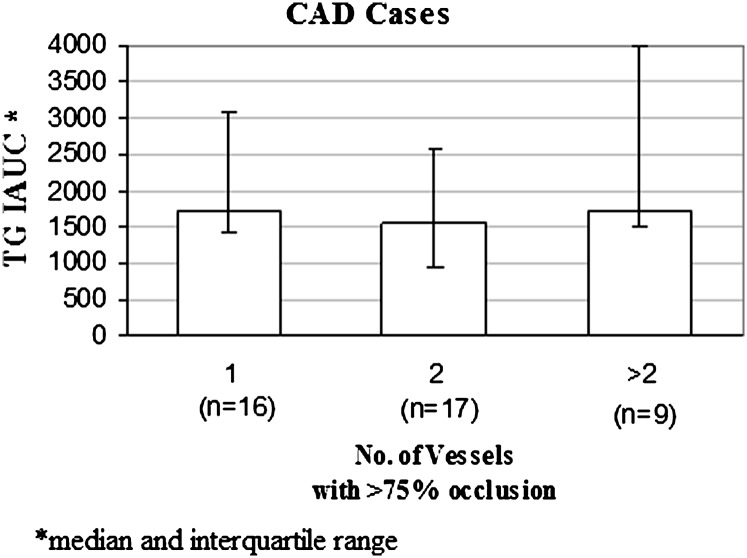
We conducted a post hoc analysis of the participants who had been enrolled based on the finding of >75% stenosis in a major coronary artery by angiography. We could ascertain the number of vessels affected with >75% stenosis in 42 of the participants who had CAD+ by angiography; there was no association between the number of vessels involved and PPTG levels (Fig. 4). The same was true for d<1.006 TG IAUC (data not shown).
Fig. 4.
Association between severity of CAD and PPTG. Subgroup analysis in 42 participants enrolled as CAD+ based on coronary angiographic findings of at least one vessel with >75% stenosis. We found no association between the number of vessels with >75% stenosis and TG IAUC.
There was no difference in PPL between CAD− participants who remained free of CAD and those who developed CAD during the subsequent 7–10 years
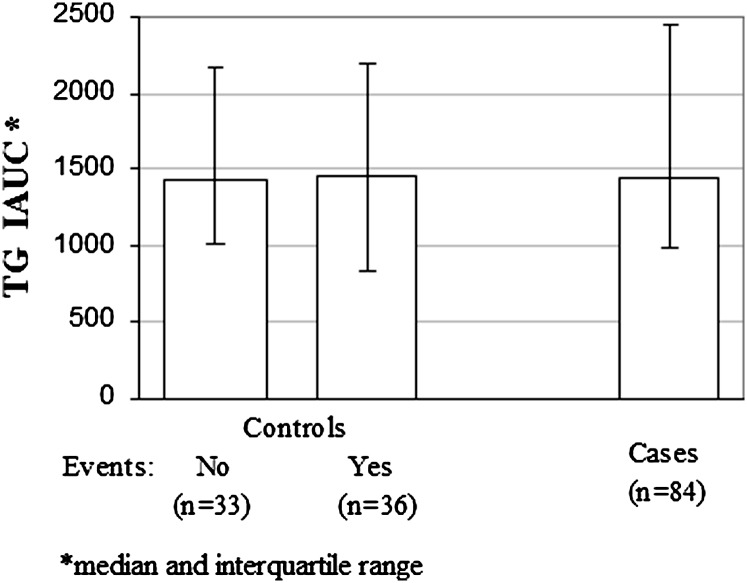
Because all of our control participants had been referred for either coronary angiography or a thallium stress test, selection bias may have played a role in our inability to differentiate them from our cases in terms of PPL. In an effort to better categorize our control population, we collected follow-up data in the CAD− group between the time they were studied (1997–2000) and early 2008. We were able to ascertain the present health status of 69 of the 80 control participants (11 participants were lost to follow-up), with a mean follow-up duration of 8.7 years. Thirty-three of the 69 participants remained free of CAD, defined as the absence of ECG abnormalities, MI, or PTCA/stent during the follow-up. When we compared the original PPTG responses in these 33 participants to the original PPTG responses in the participants who later converted from CAD− to CAD+, there were no differences between the groups (Fig. 5). We have included the PPTG IAUC for the originally defined 84 CAD+ participants for comparison. There was also no difference in baseline lipids between CAD− participants who remained CAD− and those who converted to CAD+.
Fig. 5.
PPTG in control participants followed for 7–10 years. Subgroup analysis comparing 33 original CAD− participants who continued to be free of clinical CAD 7–10 years after their original high-fat formula feeding test with 36 original CAD− participants who converted to CAD+ over that period. There were no differences in TG IAUC between those two groups. Additionally, TG IAUC in the original CAD+ group did not differ from the TG IAUCs in either of the other two subgroups.
DISCUSSION
Although recent studies have provided additional support for the view that nonfasting or PPTG levels are independent risk factors for the development of ASCVD (8, 9), there remains uncertainty regarding the importance of TG as a risk factor. This difficulty could derive from several factors. First, plasma TG levels might only be a weak surrogate for the number of atherogenic d<1.006 particles in the circulation; these lipoproteins vary greatly in size and composition, and so two individuals with the same fasting TG concentration might differ significantly in the number of circulating d<1.006 particles. Differences in the size and number of d<1.006 particles in the circulation could be critical, as it is likely that smaller, cholesteryl ester-enriched d<1.006 particles are more atherogenic than larger, more TG-enriched d<1.006 particles (25–27). Second, fasting TG, the commonly used measure in epidemiologic studies, might not be as useful for risk assessment as PPTG levels. Several case-control studies in nondiabetics have linked PPTG excursion after a high-fat load to the presence of ASCVD (10–12), including two studies in which PPTG levels were predictive of the presence of CAD (13) or carotid intima medial thickening (14) even after adjusting for both HDL-C and fasting TG levels. Meyer et al. (28) found differences in Sf>1000 TG and IDL apoB48 PP levels in nondiabetic women with CAD versus those without CAD. However, those authors did not observe differences between the two groups in plasma PPTG or d<1.006 apoB48 levels.
The few reports of PPL in individuals with DM have provided varied results (15–17, 29). Syvanne et al. (15) studied 30 participants with and 30 participants without CAD; one-half of each group had DM. In that study, which had a younger population than our study (54.3 vs. 61.4 years), the investigators did not find differences in PPTG between DM patients with and without CAD. In a second study from the same group, 43 participants with DM were studied, and the lack of differences between the groups with severe CAD (>75% stenosis) and mild CAD (<50% stenosis) in the excursions of plasma TG, apoB48, or apoB100, in large or small VLDL, or in IDL during the several hours after a fat load, was confirmed (16). Furthermore, in a subset of these participants, Mero et al. (16) also found no significant associations between CAD severity (determined by angiography) and PP excursions of either apoB48 or apoB100 in the d<1.006 range of lipoproteins. However, they did observe a positive association between CAD severity and the PP excursions of TG, apoB48, and apoB100 in IDL. In our study, we also did not find any associations between severity of disease and either PPTG or d<1.006 TG levels. In addition, the IAUC for plasma apoB48 was actually greater in the CAD− group, while the IAUC for total apoB was not different between the CAD+ and CAD− groups in our study. Although we did not study IDL directly, we found no case-control difference in either the mean size of d<1.006 lipoproteins or the excursions of large, medium, or small d<1.006 particles during the PP period. Furthermore, levels of RP and RLP-C, two accepted measures of remnant lipoproteins, did not differ between the CAD+ and CAD− groups.
Recently, Carstensen et al. (17) examined DM males and found that individuals with a prior MI (N = 17) had greater PPTG-rich lipoprotein responses than those without (N = 15). A significant difference between that study and our study was a difference in fasting TG level between their cases and controls. Their cases had a fasting TG that was about 30% greater than the level in their control group; in fact, the TG concentration in their control group was similar to that in both the cases and controls in our study. Because fasting TG is the major determinant of PPTG levels (30, 31), the differences in fasting TG levels between cases and controls in the study by Carstensen et al. (17) could explain the differences in PPTG excursions they observed. Other differences between their study and ours include a longer duration of diabetes and higher HbA1c levels in our patients. It should be noted, however, that fasting TG levels are similar in diabetics with or without CAD (32). In our study, as expected, fasting TG predicted PPTG IAUC in both the CAD+ and CAD− groups, but there was no difference in that relationship between the two groups (data not shown).
Because abnormal PPL is common in DM, we hypothesized that more subtle differences, such as size of TG-rich lipoproteins during the several hours after ingestion of a fat load, might be a better discriminator of case-control status. Several large cohort studies have indicated that abnormalities in the distribution of fasting TG-rich lipoproteins (13) and C-enriched LDL particles (33–35) are associated with ASCVD, although the independent predictive power of lipoprotein subclasses remains in doubt (36–38). Lipoprotein size was not measured in the previous studies of PPTG in patients with DM (15–17). We used NMR (20) to assess the effects of a fat load on the size and number of d<1.006 lipoproteins in our cases and controls. Suter et al. (39) demonstrated increases in d<1.006 particle size after fat loads in healthy participants on Orlistat or placebo that were similar to the changes we have observed. There have been no prior studies of NMR-measured changes in lipoprotein size or number during the PP period in diabetics with or without CAD. The NMR results, together with the other several parameters we measured, do not support our initial hypothesis about qualitative differences in PP d<1.006 lipoproteins in DM participants with CAD. Our data, which demonstrate a greater rise in apoB48 lipoproteins during the first 5 h of the PP period in the CAD− group while the increase in TG levels during the same time period was essentially identical in both groups, suggest that the CAD− group actually had smaller particles. Overall, we have no evidence to support our main hypothesis that CAD+ would have more small d<1.006 lipoproteins during PPL.
A difference may be found to be statistically nonsignificant for one or both of two reasons: the true difference may be too small to be of biological or practical interest, or the study may have been inadequately powered to detect a meaningful difference. To assess the latter, we carried out a post hoc power analysis by constructing confidence limits on case-control differences (Table 4). The results indicate that we can confidently rule out even fairly small (20–30%) differences between CAD+ and CAD− groups. Importantly, because the upper limit of confidence for the difference in apoB48 IAUCs between CAD+ and CAD− is less than zero, we can rule out any greater PP increase in apoB48 in the CAD+ group.
TABLE 4.
Confidence limits on case-control differences in IAUCs
| CAD− | CAD+ | Lower Confidence Limit on Difference | Upper Confidence Limit on Difference | |
|---|---|---|---|---|
| log TG | 7.22 | 7.31 | −0.11 | 0.30 |
| log d<1.006 TG | 7.03 | 7.09 | −0.18 | 0.29 |
| ApoB100 | −13.5 | −17.7 | −21 | 13 |
| ApoB48 | 55.4 | 41.3 | −25 | −3 |
| d<1.006 particle size | 110 | 106 | −23 | 14 |
| log RP | 3.00 | 3.06 | −0.14 | 0.27 |
| RLP-C | 99.6 | 96.4 | −27.2 | 20.8 |
We realize that potential shortcomings in our recruitment strategy may have introduced a bias against finding differences between cases and controls. For example, all of the CAD− individuals had been referred for diagnostic tests to determine whether they had clinically significant CAD. Thus, our “non-CAD” population was deemed to be at high risk for CVD by their physicians, possibly at a risk that was very close to that of our CAD+ population. However, we were able to follow-up on the present health status of a large percentage of our control group, and those CAD− participants remaining free of disease 8.7 years after the study ended had the same PP IAUCs as control participants who had their first clinical CAD event during that same follow-up period. If we use the 33 participants who remained free of CAD for 7–10 years after they participated in our study as “true controls,” they have the same PPTG IAUC as our original CAD+ group (Fig. 5).
Our participants had relatively high levels of HbA1c, indicating poor control of their DM. Although there was a positive correlation between baseline HbA1c and PPTG area, this did not affect the lack of association between PPTG area and case-control status. HbA1c levels were considerably lower in the three prior published studies that examined the association of PPL and case-control status in DM (15–17). However, two of those studies (15, 16) found essentially the same results that we are reporting.
Our study supports and extends the results from earlier studies that did not find utility in the use of PPL markers to predict the presence of CAD in patients with DM. Thus, unlike the situation in people without DM, where measures of PPL may be useful in identifying individuals at high risk for the presence of ASCVD (13, 14), PPL is not useful as a predictor of the presence of CAD in people with DM. The reason for the difference between studies of nondiabetics and studies of diabetics relative to PPL as a predictor of CAD status may derive from the fact that dyslipidemia is a central component of the insulin resistance that is characteristic of DM. Our results do not, in any manner, diminish the very convincing compilation of data indicating that PPL is an atherogenic state, nor do our results indicate that our CAD− group was free of atherosclerosis at the time of the study. Indeed, almost one-half of our CAD− group converted to CAD+ during a 7–10 year follow-up period. However, PPL was not different between the group that had an event and the group that remained CAD− throughout the long follow-up period. Thus, our results do indicate that specifically testing for PPL will not provide valuable information regarding whether someone with DM has or does not have CAD.
Acknowledgments
The authors would like to acknowledge the contributions of Dr. James E. Otvos for determining lipoprotein size by NMR. The authors also thank the Columbia University CRC inpatient unit, outpatient units, the Irving Institute for Clinical and Translational Research (IICTR) Biomarkers Laboratory staff, and the staff of the IICTR Bionutrition Unit for their clinical and technical support.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- ASCVD
- atherosclerotic cardiovascular disease
- BMI
- body mass index
- C
- cholesterol
- IAUC
- incremental area under the curve
- CAD
- coronary artery disease
- DM
- diabetes mellitus
- IDL
- intermediate density lipoprotein
- MI
- myocardial infarction
- PP
- postprandial
- PPL
- postprandial lipemia
- RP
- retinyl palmitate
- TG
- triglyceride
This work was supported by funds from the grants: National Institutes of Health, NHLBI-JDF: P01 HL 57217; National Institutes of Health, NCRR: M01 RR00645-25; and T32HL07343.
REFERENCES
- 1.Stamler J., Vaccaro O., Neaton J. D., Wentworth D. 1993. Diabetes, other risk factors, and 12 yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 16: 434–444. [DOI] [PubMed] [Google Scholar]
- 2.Krauss R. M., Siri P. W. 2004. Metabolic abnormalities: triglyceride and low-density lipoprotein. Endocrinol. Metab. Clin. North Am. 33: 405–415. [DOI] [PubMed] [Google Scholar]
- 3.Chahil T. J., Ginsberg G. N. 2006. Diabetic dyslipidemia. Endocrinol. Metab. Clin. North Am. 35: 491–510. [DOI] [PubMed] [Google Scholar]
- 4.Hulley S. B., Rosenman R. H., Bawol R. D., Brand R. J. 1980. Epidemiology as a guide to clinical decisions: The association between triglyceride and coronary heart disease. N. Engl. J. Med. 302: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg H. N. 2002. New perspectives on atherogenesis/role of abnormal triglyceride rich lipoprotein metabolism. Circulation. 106: 2137–2142. [DOI] [PubMed] [Google Scholar]
- 6.Hokanson J. E., Austin M. A. 1996. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J. Cardiovasc. Risk. 3: 213–219. [PubMed] [Google Scholar]
- 7.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S. M., Khaw K. T., Gudnason V. 2007. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 subjects in 29 Western prospective studies. Circulation. 115: 450–458. [DOI] [PubMed] [Google Scholar]
- 8.Bansal S., Buring J. E., Rifai N., Mora S., Sacks F. M., Ridker P. M. 2007. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 298: 309–316. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard B. G., Benn M., Schnohr P., Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298: 299–308. [DOI] [PubMed] [Google Scholar]
- 10.Simons L. A., Dwyer T., Simons J., Bernstein L., Mock P., Poonia N. S., Balasubramaniam S., Baron D., Branson J., Morgan J., et al. 1987. Chylomicrons and chylomicron remnants in coronary artery disease: a case-control study. Atherosclerosis. 65: 181–189. [DOI] [PubMed] [Google Scholar]
- 11.Simpson H. S., Williamson C. M., Olivecrona T., Pringle S., Maclean J., Lorimer A. R., Bonnefous F., Bogaievsky Y., Packard C. J., Sheperd J. 1990. Postprandial lipemia, fenofibrate and coronary artery disease. Atherosclerosis. 85: 193–202. [DOI] [PubMed] [Google Scholar]
- 12.Groot P. H. E., van Stiphout W. A. H. J., Krauss X. H., Jansen H., van Tol A., van Ramshorst E., Chin-on S., Hofman A., Cresswell S. R., Havekes L. 1991. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler. Thromb. 11: 653–662. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg H. N., Jones J., Blaner W. S., Thomas A., Karmally W., Fields L., Blood D., Begg M. D. 1995. Association of postprandial triglyceride and retinyl palmitate responses with newly diagnosed exercise-induced myocardial ischemia in middle-aged men and women. Arterioscler. Thromb. Vasc. Biol. 15: 1829–1838. [DOI] [PubMed] [Google Scholar]
- 14.Sharret A. R., Chambless L. E., Heiss G., Paton C. C., Patsch W. 1995. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. Arterioscler. Thromb. Vasc. Biol. 15: 2122–2129. [DOI] [PubMed] [Google Scholar]
- 15.Syvanne M., Hilden H., Taskinen M-R. 1994. Abnormal metabolism of postprandial lipoproteins in patients with non-insulin-dependent diabetes mellitus is not related to coronary artery disease. J. Lipid Res. 35: 15–26. [PubMed] [Google Scholar]
- 16.Mero N., Malmstrom R., Steiner G., Taskinen M-R., Syvann M. 2000. Postprandial metabolism of apolipoprotein B-48-and B-100-containing particles in type 2 diabetes mellitus: relations to angiographically verified severity of coronary artery disease. Atherosclerosis. 150: 167–177. [DOI] [PubMed] [Google Scholar]
- 17.Carstensen M., Thomsen C., Gotzsche O., Holst J. J., Schrezenmeir J., Hermansen K. 2004. Differential postprandial lipoprotein responses in type 2 diabetic men with and without clinical evidence of a former myocardial infarction. Rev. Diabet. Stud. 1: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald W. T., Levy R. I., Drederickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative centrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 19.Nagashima K., Lopez C., Donovan D., Ngai C., Fontanez N., Bensadoun A., Fruchart-Najib J., Holleran S., Cohn J.S., Ramakrishnan R., et al. Effects of the PPAR agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. JCI 1323–1332, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otvos J. 1999. Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy. Clin. Cardiol. 22 (Suppl. 6): II21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita M., Kojima M., Matsushima T., Teramoto T. 2005. Determination of apolipoprotein B-48 in serum by a sandwich ELISA. Clin. Chim. Acta. 351: 115–120. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima K., Saito T., Tamura A., Suzuki M., Nakano T., Adachi M., Tanaka A., Tada N., Nakamura H., Campos E. 1993. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apoB-100 and anti apo A-I immunoaffinity mixed gels. Clin. Chim. Acta. 223: 53–71. [DOI] [PubMed] [Google Scholar]
- 23.Bieri J. G., Tolliver T. J., Catignani G. L. 1979. Simultaneous determination of a-tocopherol and retinol in plasma or red cells by high-pressure liquid chromatography. Am. J. Clin. Nutr. 32: 2143–2149. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub M. S., Eisenberg S., Breslow J. L. 1987. Dietary fat clearance in normal subjects is regulated by genetic variation in apolipoprotein E. J. Clin. Invest. 80: 1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordestgaard B. G., Wootton R., Lewis B. 1995. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler. Thromb. Vasc. Biol. 15: 534–542. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard B. G., Tybjaerg-Hansen A., Lewis B. 1992. Influx in vivo of low density, intermediate density, and very low density lipoproteins into aortic intimas of genetically hyperlipidemic rabbits. Roles of plasma concentrations, extent of aortic lesion, and lipoprotein particle size as determinants. Arterioscler. Thromb. 12: 6–18. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg H. N. 2001. Hypertriglyceridemia: new insights and new approaches to pharmacologic therapy. Am. J. Cardiol. 87: 1174–1180. [DOI] [PubMed] [Google Scholar]
- 28.Meyer E., Westerveld H. T., Ruyter-Meijstek F. C., Van Greevebroek M. M. J., Rienks R., Rijn H. J. M., Erkelens D. W., Bruin T. W. A. 1996. Abnormal postprandial apolipoprotein B-48 and triglyceride responses in normolipidemic women with greater than 70% stenotic coronary artery disease: a case-control study. Atherosclerosis. 124: 221–235. [DOI] [PubMed] [Google Scholar]
- 29.Mero N., Syvanne M., Taskinen M. R. 1998. Postprandial lipid metabolism in diabetes. Atherosclerosis. 141: S53–55. [DOI] [PubMed] [Google Scholar]
- 30.Syvanne M., Talmud P. J., Humphries S. E., Fisher R. M., Rosseneu M., Hilden H., Taskinen M. R. 1997. Determinants of postprandial lipemia in men with coronary artery disease and low levels of HDL cholesterol. J. Lipid Res. 38: 1463–1472. [PubMed] [Google Scholar]
- 31.Cohn J. S., McNamara J. R., Cohn S. D., Ordovas J. M., Schaefer E. J. 1988. Plasma apolipoprotein changes in the triglyceride-rich lipoprotein fraction of human participant subjects fed a fat-rich meal. J. Lipid Res. 29: 925–936. [PubMed] [Google Scholar]
- 32.Knopp R. H., D'Emden M., Smilde J. G., Pocock S. J. 2006. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in participant subjects with type 2 diabetes. Diabetes Care. 29: 1478–1485. [DOI] [PubMed] [Google Scholar]
- 33.Austin M. A., Breslow J. L., Hennekens C. H., Buring J. E., Krauss R. M. 1988. Low density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 260: 1917–1921. [PubMed] [Google Scholar]
- 34.Lamarche B., Tchernof A., Moorjani S., Cantin B., Dagenais G. R., Lupien P. J., Després J. P. 1997. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation. 95: 69–75. [DOI] [PubMed] [Google Scholar]
- 35.Mora S., Szkio M., Otvos J. D., Greenland P., Psaty B. M., Goff D. C., Jr., O'Leary D.H., Saad M.F., Tsai M.Y., Sharrett A. 2007. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 192: 211–217. [DOI] [PubMed] [Google Scholar]
- 36.Stampfer M. J., Krauss R. M., Ma J., Blanche P. J., Holl L. G., Sacks F. M., Hennekens C. H. 1996. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 276: 882–888. [PubMed] [Google Scholar]
- 37.Sacks F. M., Campos H. 2003. Clinical review 163: cardiovascular endocrinology: low-density lipoprotein size and cardiovascular disease: a reappraisal. J. Clin. Endocrinol. Metab. 88: 4525–4532. [DOI] [PubMed] [Google Scholar]
- 38.El Harchaoui K., van der Steeg W. A., Stroes E. S., Kuivenhoven J. A., Otvos J. D., Wareham N. J., Hutten B. A., Kastelein J. J., Khaw K. T., Boekholdt S. M. 2007. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 49: 547–553. [DOI] [PubMed] [Google Scholar]
- 39.Suter P. M., Marmier G., Veya-Linder C., Hanseler E., Lentz J., Vetter W., Otvos J. D. 2005. Effect of orlistat on postprandial lipemia, NMR lipoprotein subclass profiles and particle size. Atherosclerosis. 180: 127–135. [DOI] [PubMed] [Google Scholar]