Abstract
Plasma cholesteryl ester transfer protein (CETP) activity is high in rabbits, intermediate in humans, and nondetectable in rodents. Human apolipoprotein CI (apoCI) was found to be a potent inhibitor of CETP. The aim of this study was to compare the ability of rabbit and human apoCI to modulate the interaction of CETP with HDLs and to evaluate to which extent apoCI contributes to plasma cholesteryl ester transfer rate in normolipidemic humans and rabbits. Rabbit apoCI gene was cloned and sequenced, rabbit and human apoCI were purified to homogeneity, and their ability to modify the surface charge properties and the CETP inhibitory potential of HDL were compared. It is demonstrated that unlike human apoCI, rabbit apoCI does not modulate cholesteryl ester transfer rate in total plasma. Whereas both human and rabbit apoCI readily associate with HDL, only human apoCI was found to modify the electrostatic charge of HDL. In humans, both CETP and apoCI at normal, physiological levels contribute significantly to the plasma cholesteryl ester transfer rate. In contrast, CETP is the sole major determinant of cholesteryl ester transfer in normolipidemic rabbit plasma as a result of the inability of rabbit apoCI to change HDL electronegativity.
Keywords: lipoprotein, electrostatic charge, normolipidemia
Apolipoprotein CI (apoCI) is a small, basic apolipoprotein that is mainly secreted by the liver as a component of VLDLs (1–3). It is a highly exchangeable protein and can dissociate from the VLDL surface to rapidly associate with HDLs, which are the main carriers of apoCI in normolipidemic plasma (3, 4). In hypertriglyceridemic states, in particular when VLDL secretion by the liver is increased (3), substantial amounts of apoCI are associated with the VLDL fraction. As a result of its dual localization in HDL and VLDL, apoCI has been found to play a significant role in the metabolism of these two classes of lipoproteins. On the one hand, apoCI was found to stimulate hepatic production of VLDL (5), to inhibit the hydrolysis of VLDL by LPL (6–8), and recognition of VLDL by cellular receptors (9–13). In vivo studies in animal models supported the hypothesis that apoCI plays a complex and significant role in both the accumulation of VLDL particles in the blood stream and the reduction in their cholesteryl ester content relative to triglycerides (14–19). This effect of apoCI on VLDL clearance has been shown to be of physiological relevance in humans (20, 21). On the other hand, purified human apoCI is a potent inhibitor of cholesteryl ester transfer protein (CETP) activity when associated with HDL, and as such it has the ability to reduce the net mass transfer of cholesteryl esters from HDL toward VLDL (22, 23). The ability of apoCI to decrease specific CETP activity in vivo has been documented in CETPTg/apoCI-KO and CETPTg/HuapoCITg mice (19, 24). However, the physiological relevance of apoCI in regulating plasma CETP activity in humans is unknown.
Unlike mice, but like humans, the rabbit is an athero-susceptible species that displays substantial levels of plasma CETP activity. When rabbits are fed a cholesterol-enriched diet, the plasma concentration of apoB100-containing VLDL is dramatically increased, and this is associated with a marked redistribution of cholesteryl esters from HDL toward VLDL and LDL fractions (25). More interestingly, earlier studies reported that unlike lipoprotein-deficient plasma from rats, pigs, humans, goats, chickens, and cows, no cholesteryl ester transfer inhibitor activity could be characterized in lipoprotein-deficient plasma of rabbits (26). The molecular basis for the lack of any inhibitory activity on cholesteryl ester transfer in rabbit plasma was not identified.
In this study, a comparative analysis of the role of apoCI in the regulation of CETP activity was undertaken in human and rabbit plasma. We demonstrate here that rabbit apoCI has no CETP inhibitory potential, while in turn, human apoCI is found for the first time to constitute a physiological and important contributor to cholesteryl ester transfer rates in healthy subjects.
MATERIALS AND METHODS
Subjects
Healthy subjects (total cholesterol, 5.67 ± 0.92 mmol/l; triglycerides, 1.04 ± 0.54 mmol/l; CETP, 3.01 ± 0.65 mg/l; apoCI, 72.9 ± 17.8; age, 49.6 ± 13.4 years; male/female, 15/13; n = 28) with no history of coronary artery disease or diabetes were recruited. The study complied with the Declaration of Helsinki, the protocol was approved by the local medical ethics committee (Bocage Hospital, Dijon, France), and all participants gave written informed consent. Fasting blood samples were collected into EDTA-containing glass tubes, plasma was separated by 15 min of centrifugation at 3,000g (4°C), and aliquots were frozen at −80°C for subsequent analysis.
Rabbits
New Zealand White rabbits (n = 30) were fed a standard chow diet, and blood samples were collected into EDTA-containing glass tubes after a 4 h fasting period.
Sequencing of rabbit apoCI cDNA
Total RNA was extracted from rabbit liver using Trizol reagent. cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen, Cergy Pontoise, France) and both random and oligo (dT) primers following the instructions provided by the manufacturer. A full-length apoCI cDNA sequence was obtained in two steps. First, a 118 bp fragment of the apoCI cDNA (162–279) was amplified by PCR using degenerated primers designed from human and mouse apoCI sequences (118 forward, 5′-TGGATAARCTRAARGAGTTTGG-3′; 118 reverse: 5′-AATGTCTCTGAAAACCACTICCG-3′). The 5′and 3′ end sequences were obtained by RACE using the GeneRacer™ kit (Invitrogen) following the instructions provided by the manufacturer. The two rounds of PCR were performed with primers designed from the 118 bp sequence obtained previously (3′ RACE 1, 5′-CCCTGGAAGAAAAGGCCCGGATGG-3′; 3′ RACE 2, 5′-AGGCCCGGATGGCCATTGAACAC-3′; 5′ RACE 1, 5′-CGGTCTTGGTGGTGATTTCGCTCTG-3′; 5′ RACE 2, 5′-TGTTCAATGGCCATCCGGGCCTT-3′). A high-fidelity Taq DNA polymerase (Invitrogen) was used for the PCR experiments. All the PCR products were cloned into a TOPO™ TA vector prior to sequencing (Genome Express, Meylan, France).
MALDI time-of-flight mass spectrometry of full-length and fragmented rabbit and human apoCI
Human and rabbit apoCI were purified from delipidated HDL apolipoproteins by chromatofocusing as previously described (22, 27). Full-length apoCI from rabbits and humans were analyzed on an Ultraflex II MALDI-time-of-flight (TOF)/TOF mass spectrometer (Bruker Daltonique, Strasbourg, France) in the positive 25 kV linear mode (23). Fragments of purified apoCI (5 µg) were obtained by partial hydrolysis with 300 ng of trypsin (Roche Diagnostics, Mannheim, Germany) for 10 to 150 min at 37°C, and they were desalted on ZipTip microcolumns (ZipTip µC18; Millipore, Molsheim, France) prior to MALDI-TOF mass spectrometer analysis. For de novo sequencing experiments, to facilitate y ions, partial hydrolysates were first guanidinated overnight at 65°C with an ammonia solution of methylurea (3.2 mol/l and 0.64 mol/l, respectively). After desalting on ZipTip microcolumns, the peptides were then derivatized with a solution of 0.5% 4-sulfophenyl-isothiocyanate (Sigma-Aldrich, Lyon, France) in ethanol:pyridine:H2O (50:25:25, v:v) for 1 h at 65°C. Doubly derivatized peptides were finally desalted on ZipTip microcolumns. One microliter of zip-tipped peptides were mixed with 6 µl of a solution 1.1 mg/ml of α-cyano-4-hydroxycinnamic acid (Bruker Daltonique ) in an acetonitrile:trifluoroacetic acid 0.1% (2:1, v:v) solution. Finally, 3 µl of the mixture was spotted on a MTP 384 ground steel target plate (Bruker Daltonique) and analyzed on an Ultraflex II MALDI-TOF/TOF mass spectrometer (Bruker Daltonique) in the reflectron positive mode. Angiotensin II [molecular mass (MM) = 1046.5420)], Angiotensin I (MM = 1296.5863), substance P (MM = 1347.7361), Bombesin (MM = 1619.8230), ACTH Clip 1-17 (MM = 2093.0868), ACTH Clip 18-38 (MM = 2465.1990), and Somatostatin 28 (MM = 3147.4714) were used as external calibration standards (PeptideMix Calibration standard; Bruker Daltonique).
LPL activity assay
The effect of apoCI enrichment on the lipolysis of VLDL by LPL was determined according to the general procedure previously described (21, 28). Briefly, ultracentrifugally isolated human VLDL (60 nmol triglyceride per assay) was preincubated for 15 min at 37°C in the presence of increasing amounts of purified rabbit apoCI or purified human apoCI in 0.1 M Tris buffer (pH 8.5) containing 10 g/l of fatty-acid-free BSA. Final concentrations of rabbit and human apoCI in the incubation mixtures ranged from 0 up to 7.7 μM. Triglyceride hydrolysis was conducted at 37°C by adding purified bovine milk lipoprotein lipase. Triton X-100 was added after 6 min, and triglyceride hydrolysis was quantitated as the amount of released nonesterified fatty acids that were assayed by using an enzymatic assay (NEFA-C; Wako, Osaka, Japan).
Fluorescent cholesteryl ester transfer assay
In this assay, transfer activity was determined as the rate of transfer of fluorescent nitrobenzoxadiazol (NBD)-labeled cholesteryl esters from labeled liposome donors to lipoprotein acceptors (Roar Biomedical, New York, NY). Either total plasma or purified CETP was used as a source of cholesteryl ester transfer activity, with the interference or not of endogenous lipoproteins, as previously described (19, 23, 24, 29). In the lipoprotein-independent assay, a source of CETP was incubated with labeled liposome donors and exogenous lipoprotein acceptors in excess. In the lipoprotein-dependent assay, incubation mixtures contained labeled liposome donors that were added to total plasma, and cholesteryl ester transfer rate was measured from the liposomes to the endogenous plasma lipoprotein acceptors. In all cases, the CETP-mediated transfer of NBD-cholesteryl esters was monitored by the increase in fluorescence intensity (excitation, 465 nm; emission, 535 nm) and expressed as fluorescence light units per min. Transfer rates were determined from the linear, initial portion of the time course transfer curves. Variation of CETP activity was calculated as the ratio of transfer rates in the presence of apoCI to corresponding transfer rates with no added apoCI.
Radiolabeled cholesteryl ester transfer assay
In this assay, HDLs were biosynthetically labeled with [3H] cholesteryl esters, and transfer activity was determined by measuring the rate of transfer of radiolabeled cholesteryl esters from HDL (cholesterol, 10 nmol) to unlabeled LDL (cholesterol, 32 nmol) in the presence of a source of CETP and in a total volume of 50 μl as previously described (22, 29). Transfer rates were calculated from the known specific radioactivity of the HDL donors and the accumulation of radiolabeled cholesteryl esters in the LDL acceptors after deduction of blank values from control mixtures that were incubated at 4°C. As for the fluorescent assay, variation of CETP activity in the radiolabeled assay was calculated as the ratio of transfer rates in the presence of apoCI to corresponding transfer rates with no added apoCI.
Electrophoretic analyses
Ultracentrifugally isolated HDLs were analyzed for their apolipoprotein composition and electrostatic charge properties by SDS-PAGE and nondenaturing agarose gel electrophoresis, respectively. For apolipoprotein analysis, delipidated HDL proteins were applied on 4–12% SDS-polyacrylamide gels, and the apparent molecular weights of protein bands were determined by reference to protein standards (23). For the determination of electrophoretic mobility, native HDLs were applied on 0.5% agarose gels, and surface potentials (mV) were calculated from electrophoretic velocity as previously described (23, 30).
Lipid and protein analyses
Human CETP, rabbit CETP, human apoCI, and rabbit apoCI were assayed by competitive ELISAs with anti-CETP and anti-apoCI antisera as previously described (21, 31). TP1 anti-CETP monoclonal antibodies (Heart Institute, Ottawa, Canada) were used for the human and rabbit CETP assays; homemade rabbit anti-human apoCI polyclonal antibodies were used for the human apoCI assay; and goat anti-human apoCI polyclonal antibodies (US Biological, Swampscott, MA) were used for rabbit apoCI assay. Serum total cholesterol, triglyceride, and HDL cholesterol concentrations were determined on a dimension Xpand (Dade Behring, Paris, France) using enzymatic methods.
Statistical analysis
Data are shown as mean ± SD. Relationships between CETP and apoCI concentrations and other parameters were analyzed by univariate linear regression analysis. Multiple linear regression was performed to search for independent contributions of CETP, apoCI, and triglycerides to CETP activity in human and rabbit plasmas. A Mann-Whitney test was used for comparison of the effect of apoCI in vitro. P values < 0.05 were considered statistically significant.
RESULTS
Cloning and sequencing of rabbit apoCI gene and characterization of the plasma protein
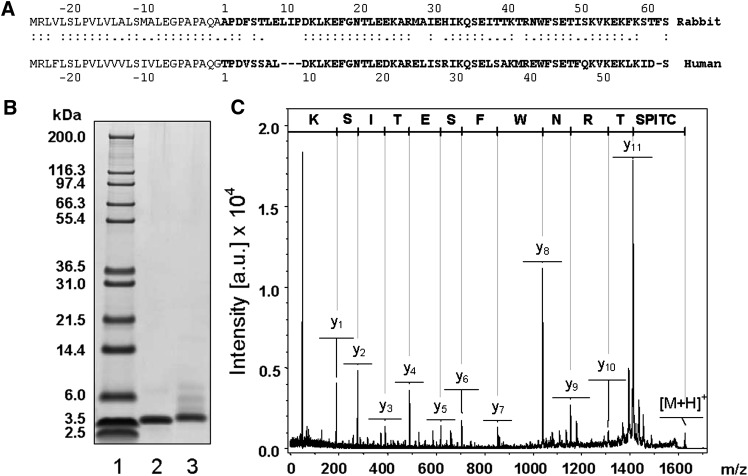
A full-length cDNA sequence of rabbit apolipoprotein CI was obtained from rabbit liver in two steps as described in Materials and Methods. The cDNA encompasses 441 bp with a 52 bp 5′ untranslated region, a 261 bp coding region, and a 128 bp 3′ untranslated region (GenBank accession number EU673468). The 87 amino acid deduced sequence is composed of a 26 amino acid signal peptide and a 61 amino acid mature protein (Fig. 1A). In a second step, the rabbit apoCI protein was purified from rabbit HDL (Fig. 1B). The MS analysis of partially trypsinized rabbit apoCI by peptide mass finger printing revealed a sequence coverage of 87% of the mature deduced sequence (data not shown). The first 28 amino acids of deduced mature rabbit apoCI sequence matches the sole fragment sequence that is found in GenBank, and direct analysis of the 41-TRNWFSETISK-51 C-terminal peptide of purified rabbit apoCI by MALDI-TOF/TOF matched the deduced sequence exactly (Fig. 1A–C). The complete sequence of rabbit apoCI is reported here for the first time, and it was ascertained through two determinations with total liver RNAs extracted from two distinct rabbits. In addition, the molecular weight of apoCI purified from rabbit plasma was further determined independently by mass spectrometry analysis. It was found to be 7107.05, which fits closely to the calculated molecular weight of 7106.12, which can be deduced from the amino acid sequence of mature apoCI. Rabbit apoCI shows a 74.3% nucleotide homology and a 65.5% amino acid homology with human apoCI.
Fig. 1.
Characterization of rabbit apolipoprotein CI. A: The 61 amino acid sequence of rabbit apoCI (1_61; bold) and the signal peptide sequence (plain; −1_−26) was deduced from the cDNA sequence (GenBank accession number EU673468). Alignment with the 57 amino acid sequence of mature human apoCI (1_57) and human signal peptide (−1_−26) was conducted by using the Needleman-Wunsch global alignment method (EMBOSS software). B: SDS-PAGE of Mark12 calibration kit (lane 1), purified human apoCI (lane 2), and purified rabbit apoCI (lane 3). Human and rabbit apoCI were purified by chromatofocusing (see Methods). C: The MALDI-TOF/TOF spectrum of the 41TRNWFSETISK51 doubly derivatized rabbit apoCI peptide matches exactly the deduced amino acid sequence in A.
Like human apoCI, purified rabbit apoCI also inhibits LPL activity
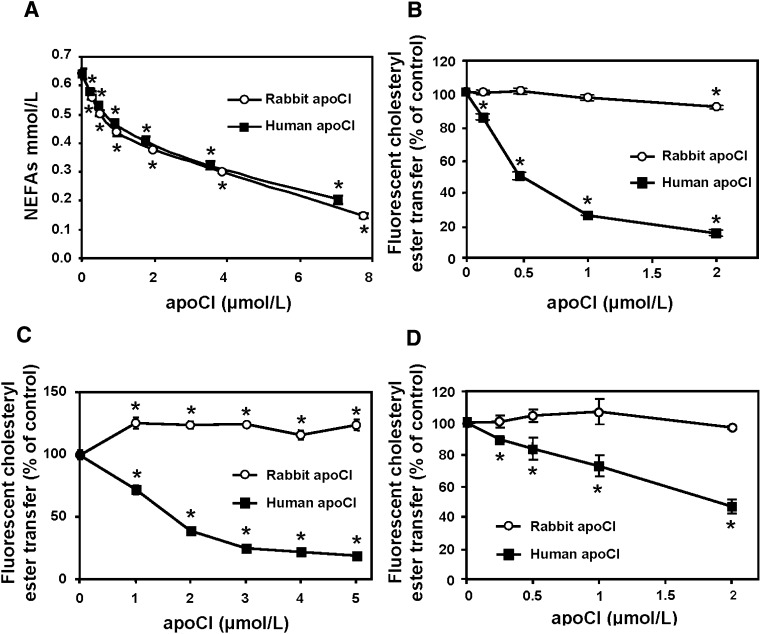
Previous studies demonstrated that human apoC1 is a significant inhibitor of LPL activity (8). As shown in Fig. 2A, in vitro enrichment of ultracentrifugally isolated human VLDL with either rabbit apoCI or human apoCI produced a concentration-dependent inhibition of the release of nonesterified fatty acids induced by purified LPL in the presence of fatty-acid-free BSA. Similar inhibition curves of LPL activity were obtained with human apoCI and rabbit apoCI, in both cases with up to approximately 70% inhibition with the highest doses of apoCI studied (Fig. 2A).
Fig. 2.
Comparison of the concentration-dependent effects of human and rabbit apoCI on LPL activity and CETP activity as measured with a lipoprotein-independent (B and C) or a lipoprotein-dependent (D) fluorescent assay. A: Ultracentrifugally isolated human VLDLs were preincubated with increasing concentrations of either purified human apoCI (squares in A) or purified rabbit apoCI (open circles in A), and triglyceride hydrolysis was subsequently induced by the addition of purified bovine milk lipoprotein lipase. Triglyceride hydrolysis activity is expressed as the amount of released nonesterified fatty acids (NEFAs) over a 6 min period. B: Transfer of fluorescent NBD-labeled cholesteryl esters from liposome donors to lipoprotein acceptors was measured in the presence of partially purified human CETP and in the presence of increasing concentrations of either human apoCI (squares in B) or rabbit apoCI (circles in B). C: Transfer of fluorescent NBD-labeled cholesteryl esters from liposome donors to lipoprotein acceptors was measured in the rabbit d 1.21 g/ml infranatant, which was supplemented with increasing concentrations of either human apoCI (squares in C) or rabbit apoCI (circles in C). D: Transfer of fluorescent NBD-labeled cholesteryl esters from liposome donors to endogenous lipoprotein acceptors in total human plasma, which was supplemented with increasing concentrations of either human apoCI (squares in D) or rabbit apoCI (circles in D). Data are mean ± SD of three determinations. *P < 0.05 versus homologous control with no apoCI added (Mann-Whitney).
Purified rabbit apoCI, unlike human apoCI, does not inhibit CETP activity
Previous studies demonstrated that human apoC1 is a potent inhibitor of human CETP activity (22–24). Again, as shown in Fig. 2B, human apoCI inhibited the rate of transfer of fluorescent cholesteryl esters from labeled liposome donors to lipoprotein acceptors in a concentration-dependent manner, with an 82% reduction in human CETP activity with the highest concentration studied (2 μM). In contrast, rabbit apoCI did not inhibit CETP in the fluorescent activity assay (Fig. 2B). As shown in Fig. 2C, a potent blockade of CETP activity was obtained in the d > 1.21 g/ml fraction of rabbit plasma that was supplemented with fluorescent liposome donors and human apoCI, again with no marked inhibitory effect when the d > 1.21 g/ml plasma fraction was supplemented with fluorescent liposome donors and rabbit apoCI. Again, and in contrast to human apoCI, rabbit apoCI was unable to inhibit CETP activity in total human plasma (Fig. 2D).
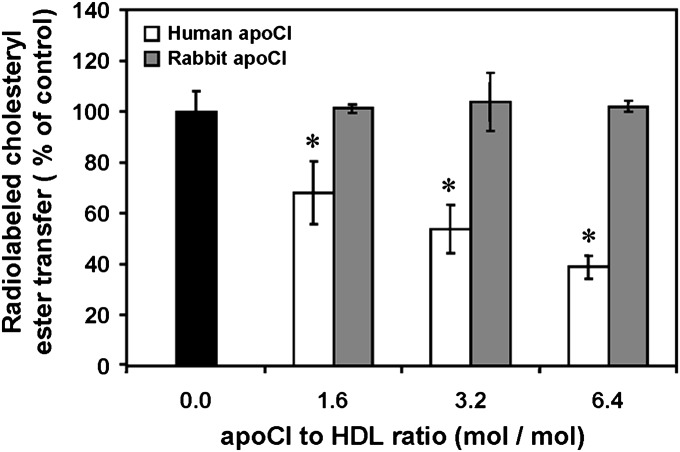
As observed with the fluorescent activity assay using labeled liposomes, only human apoCI, not rabbit apoCI, was able to inhibit the CETP-mediated transfer of radiolabeled cholesteryl esters from HDL donors to unlabeled LDL acceptors in the presence of purified CETP (Fig. 3).
Fig. 3.
Comparison of the concentration-dependent effects of human and rabbit apoCI on CETP activity as measured from radiolabeled HDL to unlabeled LDL. Transfer activity was determined as the rate of transfer of radiolabeled cholesteryl esters from [3H] cholesteryl ester-HDL to unlabeled LDL in the presence of partially purified human CETP and in the presence of increasing concentrations of either human apoCI (white bars) or rabbit apoCI (gray bars). Data are mean ± SD of three determinations. *P < 0.05 versus homologous control with no apoCI added (black bars; Mann-Whitney).
Circulating apoCI modulates the cholesteryl ester transfer rate in human plasma but not in rabbit plasma
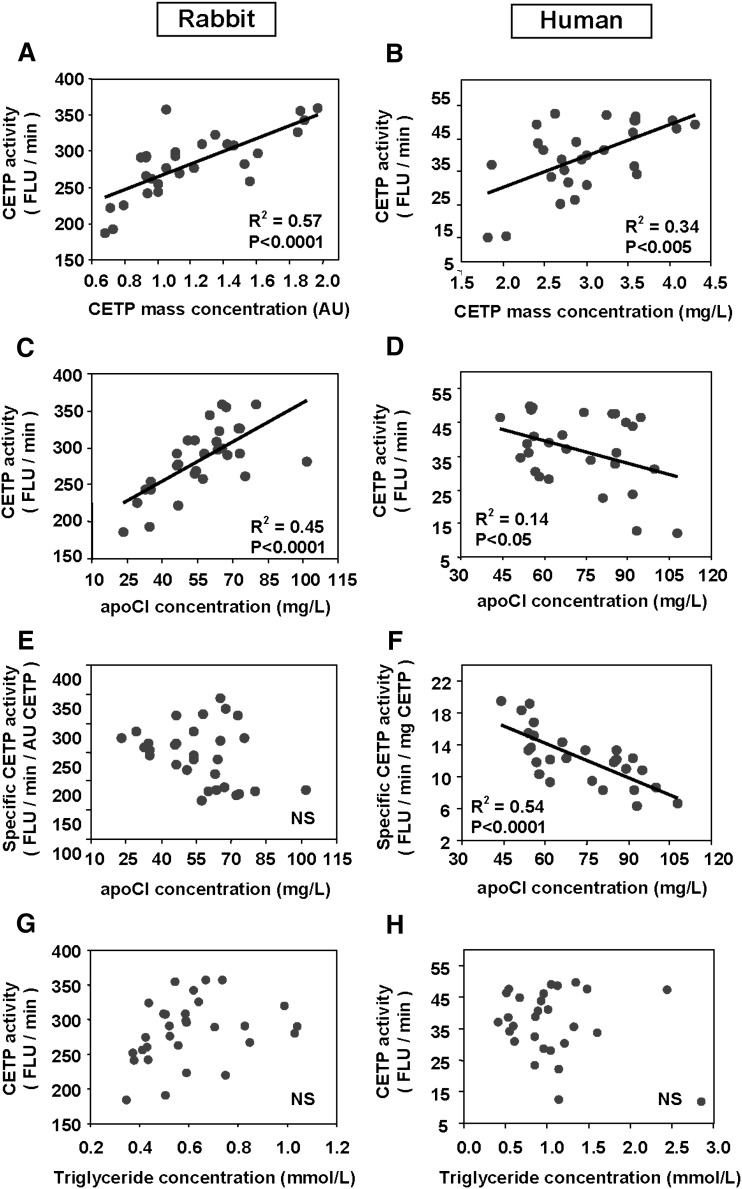
To evaluate the relative contribution of apoCI to cholesteryl ester transfer activity in human and rabbit plasmas, apoCI was quantitated in normolipidemic subjects and rabbits by using specific ELISAs. As shown in Fig. 4, plasma concentration of CETP was found to constitute a significant determinant of cholesteryl ester transfers in both rabbits and humans, as assessed by positive, highly significant correlations between CETP concentration (as determined by ELISA) and cholesteryl ester transfer activity (as determined by a fluorimetric assay; Fig. 4A, B). Plasma concentration of apoCI correlated positively with cholesteryl ester transfer activity in rabbits (Fig. 4C) but negatively with cholesteryl ester transfer activity in humans (Fig. 4D). In humans, a strong, negative correlation was found between plasma concentrations of apoCI and specific CETP activity, which was calculated as the cholesteryl ester transfer rate to CETP mass ratio (Fig. 4F). In contrast to human plasma, no significant correlation between apoCI concentration and specific CETP activity was observed in rabbit plasma (Fig. 4E). Finally, in univariate analysis, triglycerides did not correlate significantly with cholesteryl ester transfer activity, neither among the normolipidemic human subjects nor among the normolipidemic rabbits (Fig. 4G, H). However, triglycerides correlated positively with both rabbit and human apoCI (R2 = 0.2755, P = 0.029, and R2 = 0.1755, P = 0.0271, respectively). Multivariate analysis was performed by including the concentration of the CETP catalyst, the concentration of triglycerides, and the amount of the apoCI inhibitor (Table 1). Multivariate analysis for the association between the rate of plasma cholesteryl ester transfer, as a dependent variable, and CETP, triglyceride, and apoCI concentrations revealed that only CETP mass contributed significantly to the rate of plasma cholesteryl ester transfer in rabbits (Table 1). In contrast, multiple regression analysis revealed that both CETP concentration and apoCI were significant and independent contributors to the rate of cholesteryl ester transfer in human plasma (Table 1). It is noteworthy that specific CETP activity in normolipidemic subjects in the upper range of apoCI values (approximately 100 mg/l of apoCI) was half that in normolipidemic subjects with the lowest apoCI concentration (approximately 50 mg/l) (Fig. 4).
Fig. 4.
Relationship between apoCI, triglycerides, CETP mass concentration, and cholesteryl ester transfer activity in plasma of normolipidemic rabbits (left) and humans (right). Triglycerides were assayed by an enzymatic method. ApoCI and CETP concentrations were measured by specific ELISAs, and CETP activity was quantitated using a fluorescent assay measuring the transfer of fluorescent NBD-labeled cholesteryl esters from liposome donors to endogenous lipoprotein acceptors in total human plasma (see Materials and Methods). Specific CETP activity was calculated as the ratio of cholesteryl ester transfer activity to CETP mass concentration. Correlation coefficients were calculated by linear regression analysis.
TABLE 1.
Multivariate analysis for the association between CETP activity, as a dependent variable, and CETP, apoCI, and triglyceride concentrations
| Rabbit Model |
Human Model |
|||||
|---|---|---|---|---|---|---|
| Variable | Regression Coefficient | SE | P | Regression Coefficient | SE | P |
| CETP | 69.003 | 17.707 | 0.0006 | 12.639 | 2.015 | <0.0001 |
| apoCI | 0.661 | 0.469 | 0.1702 | −0.380 | 0.081 | <0.0001 |
| TG | 28.982 | 33.961 | 0.4012 | 1.517 | 2.512 | 0.5515 |
| R2 | 0.650 | 0.680 | ||||
The two models were built including CETP, apoCI, and triglycerides (TG). CETP mass concentration was the only significant predictor of plasma CETP activity in rabbits (n = 30), while both CETP mass and apoCI concentrations were independent predictors of plasma cholesteryl ester transfer rate in humans (n = 28). Included parameters accounted for 65% and 68% of the variation in plasma CETP activity values in the rabbit and human models, respectively.
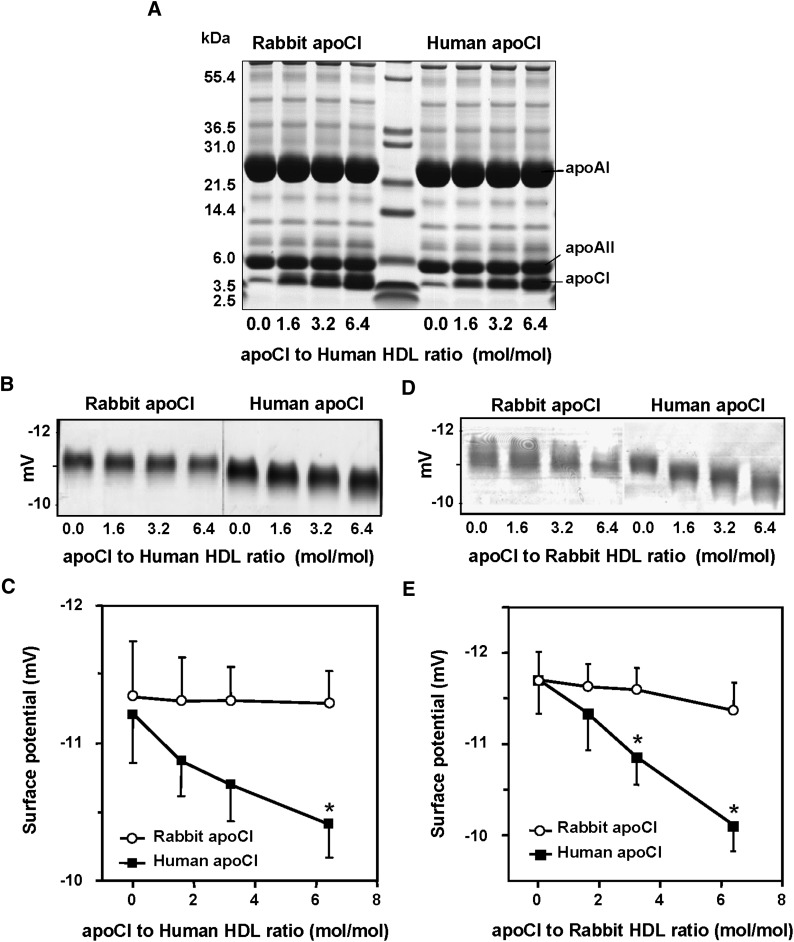
Unlike binding of human apoCI to HDL, binding of rabbit apoCI to HDL does not modify the surface charge properties
Human apolipoprotein CI acts as a physiological inhibitor of CETP through its ability to modify the electrostatic charge properties of plasma HDLs (23). As shown in Fig. 5A, both human and rabbit apoCI were found to readily associate with isolated human HDL. In both cases, it occurred in a concentration-dependent manner and without concomitant changes in the overall apolipoprotein composition of HDL, which contained apoAI and apoAII as the two major apolipoproteins (Fig. 5A).
Fig. 5.
Comparative effect of the association of human and rabbit apoCI with human or rabbit HDLs on their electrostatic charge. A: Human HDLs in TBS were incubated for 1 h at room temperature with increasing concentrations of purified human apoCI (left) or purified rabbit apoCI (right). Final protein to HDL molar ratio in incubation mixtures ranged from 0 up to 6.4. The HDL complexes were subsequently recovered by one single ultracentrifugation run at d = 1.21 g/ml, and protein profiles were analyzed by SDS-PAGE. The Mark-12 molecular weight calibration kit was applied to the middle track. B, C: Human HDL were incubated for 1 h at room temperature with increasing concentrations of purified human apoCI or purified rabbit apoCI. D, E: Rabbit HDL were incubated for 1 h at room temperature with increasing concentrations of purified human apoCI or purified rabbit apoCI. The final protein to HDL molar ratio in incubation mixtures ranged from 0 up to 6.4. The HDL complexes were subsequently recovered by one single ultracentrifugation run at d = 1.21 g/ml, and electrophoretic mobility of HDL complexes was analyzed on 0.5% agarose gels (B), and surface potential values (mV; C) were calculated as described in Materials and Methods. Data are mean ± SD of three determinations. *P < 0.05 versus homologous control with no apoCI added (Mann-Whitney).
The electrostatic charge properties of human and rabbit apoCI at the HDL surface were compared by agarose gel elecrophoresis. As shown in Fig. 5B and C, human apoCI produced a significant, concentration-dependent increase in the mean surface potential of native human HDL, which ranged from −11.2 mV with no addition to −10.5 mV with the highest apoCI concentration studied (P < 0.05). In contrast, association of rabbit apoCI with human HDL was unable to produce a significant change in mean surface potential along the apoCI to HDL molar ratio range studied (Fig. 5B, C). In analogous experiments with native rabbit HDL, human apoCI was still able to produce a concentration-dependent increase in HDL surface potential, again in this case with no significant effect of rabbit apoCI on the electrostatic charge of rabbit HDL (Fig. 5D, E).
DISCUSSION
Rabbits display a number of pathophysiological features in terms of both lipoprotein metabolism and atherogenesis that make them more suitable as an experimental model for humans than are rats or mice. These features include in particular the production of apoB100-containing VLDL by the liver, substantial circulating levels of potentially atherogenic VLDL and LDL, and a high susceptibility to diet-induced hypercholesterolemia and atherosclerosis (32–34). Interestingly, the rabbit has been shown to display elevated CETP activity (35, 36) and no detectable lipid transfer inhibitory activity (26), suggesting that high levels of plasma cholesteryl ester transfer might contribute significantly to the high susceptibility to atherosclerosis in this species. In support of the latter view, rabbits have been found to respond strongly to treatment with pharmacological CETP inhibitors with concomitant increases in plasma HDL cholesterol and decreases in plasma LDL cholesterol and with a significant decrease in atherosclerotic lesions in treated cholesterol-fed animals (37).
The rabbit apoCI gene was cloned and sequenced for the first time in this study. Although it shows a high homology in cDNA and amino acid sequence to human and mouse apoCI, it was found to be totally dysfunctional in terms of CETP inhibition, which constitutes a new and potentially important feature of the rabbit model. Indeed, and unlike purified mouse and human apoCI, purified rabbit apoCI was unable to block the CETP-mediated transfer of labeled cholesteryl esters in vitro, and concordant observations were made in transfer assays using either fluorescent liposomes or radiolabeled HDL as donor substrates. We took advantage of this observation to demonstrate that variations in circulating plasma levels of apoCI do not influence the CETP-mediated lipid transfer reaction when it does not have the ability to change the surface charge properties of lipoproteins. Similarly to native rabbit apoCI, but unlike full-length human apoCI and its C-terminal α-helix with significant electrostatic properties, the N-terminal α-helix of human apoCI was found to have no effect on HDL electronegativity and no CETP inhibitory property (23). In addition, this study extends earlier observations that demonstrated that the surface potential constitutes a key determinant of the interaction of CETP with the lipoprotein surface, and changes in the surface potential of HDL by the way of chemical modifications, insertion of negatively charged lipid residues, or substitution of apolipoprotein molecules were all found to result in alterations of the CETP-mediated lipid transfer reaction (23, 38–40). In support of the physiological relevance of human apoCI in modifying the electrostatic charge and the lipid transfer properties of HDL, significant changes in both parameters were previously observed in vitro with apoCI to HDL molar ratio as low as 1.8 (23). It is close to the molar ratio of approximately 1 in normolipidemic human plasma with the bulk of apoCI bound to HDL (3, 4). The addition of exogenous human apoCI to rabbit plasmas inhibited CETP activity, indicating that the lack of apoCI-mediated inhibition of CETP in rabbit plasma is effectively due to apoCI per se and not to putative specificities of the lipoprotein environment. This may account, at least in part, for the lack of any inhibition of lipid transfer that has already been reported in rabbit plasma (26). Specific behavior of rabbit apoCI seems to be restricted to CETP inhibition since its capacity to inhibit lipoprotein lipase was maintained as judged in vitro by comparison with human apoCI in this study.
In contrast to rabbit apoCI, mouse apoCI was found in earlier studies to be a potent inhibitor of CETP in human CETP transgenic mice (22, 24). In addition, overexpression of human apoAI in transgenic rats expressing simian CETP was found to result in both the displacement of apoCI from the HDL surface and the concomitant disappearance of CETP inhibitory potential (41). Concentration-dependent inhibition of CETP was found to constitute a unique property of HDL in earlier studies, and kinetic analyses demonstrated that, unlike HDL, isolated LDL has no CETP inhibitory properties (42, 43). However, recent studies reported that LDL could also contribute to modulation of CETP activity, which might then be regulated by two distinct/independent factors: the LDL-associated apoF and the HDL-associated apoCI (44). Although the consequences of the HDL/apoCI-mediated inhibition of CETP in terms of plasma cholesteryl ester transfer rates and cholesterol distribution have been documented in mouse and rat models (two species in which HDL predominate in plasma), extrapolation to humans still suffers from a number of limitations. First, the mouse and rat are CETP-deficient species (35, 36), and as a consequence, the CETP inhibitory property of apoCI could only be demonstrated in vivo in compound models in which up- or downregulation of apoCI was achieved in animals with a human CETP or a simian CETP transgenic background (24, 41). Second, endogenous mouse apoCI, just like human apoCI, has a remarkable ability to block the cholesteryl ester transfer activity of CETP, and mouse apoCI deficiency in a compound mouse apoCI-KO/human apoCITg model would be a prerequisite to explore the entire spectrum of apoCI concentration in human plasma, from low to high values. Third, overexpression of apoCI in the mouse model produces concomitant decreases in LPL activity and clearance of plasma VLDL, both of which play an important role and can affect the CETP-mediated lipid transfer reaction independently (8). In fact, previous studies have demonstrated that overexpression of apoCI in the CETPTg mouse model produces two opposite effects with regard to levels of plasma CETP activity: i) a blockade of CETP as assessed by a significant decrease in specific CETP activity, but ii) an increase in VLDL acceptors and CETP concentration producing an opposite tendency toward a rise in the total plasma CET rate (19). The latter effect even seemed to predominate in the HuCITg/CETPTg mouse model in terms of cholesteryl ester transfer rates since overexpression of apoCI resulted in a rise, not a decrease, in the total plasma CET rate (19).
Beyond previous studies in genetically engineered mice, this study brings the first evidence of the inhibition of CETP by apoCI in normolipidemic human plasma, in particular through a strong, negative correlation between the plasma concentration of human apoCI and specific CETP activity. These results come in new support of previous studies that demonstrated that the immunospecific removal of apoCI from the HDL surface was indeed associated with a significant decrease in their ability to block the CETP-mediated lipid transfer reaction in humans (22). The relevance of observations in humans was further sustained by the comparison with observations in rabbits. In the latter species, the positive association of apoCI with CETP activity in univariate analysis might actually reflect coordinated variations in CETP and apoCI expression, and increasing apoCI concentration did not translate into a significant decrease in specific CETP activity in rabbits. Multivariate analysis confirmed that CETP was the only significant determinant of the rate of plasma cholesteryl ester transfer in normal rabbits fed a chow diet, indicating that in normal rabbits, interindividual variations in either triglycerides or apoCI did not modulate plasma cholesteryl ester transfer rates. In contrast, both CETP and apoCI concentrations in normolipidemic humans were found to contribute significantly to plasma CETP in multivariate analysis, again with no significant effect of triglycerides in the population studied. In other words, CETP activity in normolipidemic human plasma is largely influenced by the endogenous apoCI inhibitor, and, in contrast to rabbits, CETP concentration should not be considered a reliable marker of plasma cholesteryl ester transfer rates in humans. It should be emphasized that mean plasma triglyceride levels in both the normolipidemic subjects and rabbits were below the elevated values at which cholesteryl ester transfer activity is known to be magnified (45, 46). The degree of hyperytriglyceridemia in humans is known to influence the CETP-mediated accumulation of cholesteryl esters in VLDLs (47, 48), indicating that the low triglyceride levels in this study might account in part for the lack of a significant effect of triglycerides on CETP activity. In normolipidemic humans with low plasma triglycerides, most of plasma apoCI (>90% of total) is known to localize in HDL (3). Thus, it is plausible that in this case the CETP inhibitory potential of HDL-bound apoCI predominates over the concomitant and potentially harmful ability of VLDL-bound apoCI to produce an accumulation of triglycerides in the bloodstream. In turn, the hypertriglyceridemic potential of apoCI resulting from impaired triglyceride clearance might well predominate when apoCI-rich VLDLs tend to accumulate [i.e., as in transgenic mice overexpressing human apoCI (19) and in dyslipidemic patients (20, 21)]. In the latter case, and in contrast to normolipidemia in which the contribution of triglyceridemia to total plasma cholestryl ester transfer activity is minimal, high triglyceride-rich lipoprotein acceptors might also contribute significantly to the plasma cholesteryl ester transfer rate in addition to CETP alone or both CETP and apoCI in rabbits and humans, respectively.
It is concluded that apoCI at normal, physiological levels contributes significantly to the reduction in the plasma CET rate in humans. Specific CETP activity in normolipidemic plasma with low apoCI concentration was up to twice that in plasma with a high apoCI concentration, indicating that apoCI should be considered a major determinant of CETP activity in humans. Whether the apoCI level and the degree of endogenous CETP inhibition might account for differences in the outcomes of pharmacological CETP inhibition in rabbits and humans will deserve further attention.
Acknowledgments
The authors thank Philip Bastable for manuscript editing.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- CETP
- cholesteryl ester transfer protein
- NBD
- nitrobenzoxadiazol
- TOF
- time of flight
This work was supported by INSERM, the Université de Bourgogne, the Centre Hospitalier Universitaire de Dijon, the Agence Nationale de la Recherche, the Fondation de France, and by an International HDL Research Awards Program grant.
REFERENCES
- 1.Polz E., Kotite L., Havel R. J., Kane J. P., Sata T. 1980. Human apolipoprotein CI: concentration in blood serum and lipoproteins. Biochem. Med. 24: 229–237. [DOI] [PubMed] [Google Scholar]
- 2.Malmendier C. L., Lontie J. F., Grutman G. A., Delcroix C. 1986. Metabolism of apolipoprotein CI in normolipoproteinemic human subjects. Atherosclerosis. 62: 167–172. [DOI] [PubMed] [Google Scholar]
- 3.Cohn J. S., Tremblay M., Batal R., Jacques H., Veilleux L., Rodriguez C., Bernier L., Mamer O., Davignon J. 2002. Plasma kinetics of VLDL and HDL apoC-I in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 43: 1680–1687. [DOI] [PubMed] [Google Scholar]
- 4.Cohn J. S., Tremblay M., Boulet L., Jacques H., Davignon J., Roy M., Bernier L. 2003. Plasma concentration and lipoprotein distribution of ApoC-I is dependent on ApoE genotype rather than the Hpa I ApoC-I promoter polymorphism. Atherosclerosis. 169: 63–70. [DOI] [PubMed] [Google Scholar]
- 5.Westerterp M., de Haan W., Berbée J. F., Havekes L. M., Rensen P. C. 2006. Endogenous apoC-I increases hyperlipidemia in apoE-knockout mice by stimulating VLDL production and inhibiting LPL. J. Lipid Res. 47: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 6.Havel R. J., Fielding C. J., Olivecrona T., Shore V. G., Fielding P. E., Egelrud T. 1973. Cofactor activity of protein components of human very low density lipoproteins in the hydrolysis of triglycerides by lipoprotein lipase from different sources. Biochemistry. 12: 1828–1833. [DOI] [PubMed] [Google Scholar]
- 7.Conde-Knape K., Bensadoun A., Sobel J. H., Cohn J. S., Shachter N. S. 2002. Overexpression of apoC-I in apoE-null mice: severe hypertriglyceridemia due to inhibition of hepatic lipase. J. Lipid Res. 43: 2136–2145. [DOI] [PubMed] [Google Scholar]
- 8.Berbée J. F., van der Hoogt C. C., Sundararaman D., Havekes L. M., Rensen P. C. 2005. Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL. J. Lipid Res. 46: 297–306. [DOI] [PubMed] [Google Scholar]
- 9.Kowal R. C., Herz J., Weisgraber K. H., Mahley R. W., Brown M. S., Goldstein J. L. 1990. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 265: 10771–10779. [PubMed] [Google Scholar]
- 10.Weisgraber K. H., Mahley R. W., Kowal R. C., Herz J., Goldstein J. L., Brown M. S. 1990. Apolipoprotein C–I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein. J. Biol. Chem. 265: 22453–22459. [PubMed] [Google Scholar]
- 11.Windler E., Chao Y., Havel R. J. 1980. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J. Biol. Chem. 255: 8303–8307. [PubMed] [Google Scholar]
- 12.Sehayek E., Eisenberg S. 1991. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 266: 18259–18267. [PubMed] [Google Scholar]
- 13.Swaney J. B., Weisgraber K. H. 1994. Effect of apolipoprotein C–I peptides on the apolipoprotein E content and receptor-binding properties of beta-migrating very low density lipoproteins. J. Lipid Res. 35: 134–142. [PubMed] [Google Scholar]
- 14.Simonet W. S., Bucay N., Pitas R. E., Lauer S. J., Taylor J. M. 1991. Multiple tissue-specific elements control the apolipoprotein E/C-I gene locus in transgenic mice. J. Biol. Chem. 266: 8651–8654. [PubMed] [Google Scholar]
- 15.Shachter N. S., Ebara T., Ramakrishnan R., Steiner G., Breslow J. L., Ginsberg H. N., Smith J. D. 1996. Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein Cl. J. Clin. Invest. 98: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jong M. C., Dahlmans V. E., van Gorp P. J., van Dijk K. W., Breuer M. L., Hofker M. H., Havekes L. M. 1996. In the absence of the low density lipoprotein receptor, human apolipoprotein C1 overexpression in transgenic mice inhibits the hepatic uptake of very low density lipoproteins via a receptor-associated protein-sensitive pathway. J. Clin. Invest. 98: 2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jong M. C., Gijbels M. J., Dahlmans V. E., Gorp P. J., Koopman S. J., Ponec M., Hofker M. H., Havekes L. M. 1998. Hyperlipidemia and cutaneous abnormalities in transgenic mice overexpressing human apolipoprotein C1. J. Clin. Invest. 101: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jong M. C., van Dijk K. W., Dahlmans V. E., Van der Boom H., Kobayashi K., Oka K., Siest G., Chan L., Hofker M. H., Havekes L. M. 1999. Reversal of hyperlipidaemia in apolipoprotein C1 transgenic mice by adenovirus-mediated gene delivery of the low-density-lipoprotein receptor, but not by the very-low-density-lipoprotein receptor. Biochem. J. 338: 281–287. [PMC free article] [PubMed] [Google Scholar]
- 19.Gautier T., Masson D., Jong M. C., Pais de Barros J. P., Duverneuil L., Le Guern N., Deckert V., Dumont L., Bataille A., Zak Z., et al. 2005. Apolipoprotein CI overexpression is not a relevant strategy to block cholesteryl ester transfer protein (CETP) activity in CETP transgenic mice. Biochem. J. 385: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björkegren J., Boquist S., Samnegârd A., Lundman P., Tornvall P., Ericsson C. G., Hamsten A. 2000. Accumulation of apolipoprotein C–I-rich and cholesterol-rich VLDL remnants during exaggerated postprandial triglyceridemia in normolipidemic patients with coronary artery disease. Circulation. 101: 227–230. [DOI] [PubMed] [Google Scholar]
- 21.Dautin G., Soltani Z., Ducloux D., Gautier T., Pais de Barros J. P., Gambert P., Lagrost L., Masson D. 2007. Hemodialysis reduces plasma apolipoprotein C–I concentration making VLDL a better substrate for lipoprotein lipase. Kidney Int. 72: 871–878. [DOI] [PubMed] [Google Scholar]
- 22.Gautier T., Masson D., Pais de Barros J. P., Athias A., Gambert P., Aunis D., Metz-Boutigue M. H., Lagrost L. 2000. Human apolipoprotein C–I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J. Biol. Chem. 275: 37504–37509. [DOI] [PubMed] [Google Scholar]
- 23.Dumont L., Gautier T., Pais de Barros J. P., Laplanche H., Blache D., Ducoroy P., Fruchart J., Fruchart J. C., Gambert P., Masson D., et al. 2005. Molecular mechanism of the blockade of plasma cholesteryl ester transfer protein by its physiological inhibitor apolipoprotein CI. J. Biol. Chem. 280: 38108–38116. [DOI] [PubMed] [Google Scholar]
- 24.Gautier T., Masson D., Jong M. C., Duverneuil L., Le Guern N., Deckert V., Pais de Barros J. P., Dumont L., Bataille A., Zak Z., et al. 2002. Apolipoprotein CI deficiency markedly augments plasma lipoprotein changes mediated by human cholesteryl ester transfer protein (CETP) in CETP transgenic/ApoCI-knocked out mice. J. Biol. Chem. 277: 31354–31363. [DOI] [PubMed] [Google Scholar]
- 25.Roth R. I., Gaubatz J. W., Gotto A. M., Jr., Patsch J. R. 1983. Effect of cholesterol feeding on the distribution of plasma lipoproteins and on the metabolism of apolipoprotein E in the rabbit. J. Lipid Res. 24: 1–11. [PubMed] [Google Scholar]
- 26.Morton R. E., Zilversmit D. B. 1981. A plasma inhibitor of triglyceride and cholesteryl ester transfer activities. J. Biol. Chem. 256: 11992–11995. [PubMed] [Google Scholar]
- 27.Tournier J. F., Bayard F., Tauber J. P. 1984. Rapid purification and activity of apolipoprotein C1 on the proliferation of bovine vascular endothelial cells in vitro. Biochim. Biophys. Acta. 804: 216–220. [DOI] [PubMed] [Google Scholar]
- 28.de Man F. H., de Beer F., van de Laarse A., Smelt A. H., Leuven J. A. 1998. Havekes, L.M. Effect of apolipoprotein E variants on lipolysis of very low density lipoproteins by heparan sulphate proteoglycan-bound lipoprotein lipase. Atherosclerosis. 136: 255–262. [DOI] [PubMed] [Google Scholar]
- 29.Lagrost L., Athias A., Gambert P., Lallemant C. 1994. Comparative study of phospholipid transfer activities mediated by cholesteryl ester transfer protein and phospholipid transfer protein. J. Lipid Res. 35: 825–835. [PubMed] [Google Scholar]
- 30.Sparks D. L., Phillips M. C. 1992. Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J. Lipid Res. 33: 123–130. [PubMed] [Google Scholar]
- 31.Guyard-Dangremont V., Lagrost L., Gambert P., Lallemant C. 1994. Competitive enzyme-linked immunosorbent assay of the human cholesteryl ester transfer protein (CETP). Clin. Chim. Acta. 231: 147–160. [DOI] [PubMed] [Google Scholar]
- 32.Taylor J. M., Fan J. 1997. Transgenic rabbit models for the study of atherosclerosis. Front. Biosci. 2: d298–d308. [DOI] [PubMed] [Google Scholar]
- 33.Brousseau M. E., Hoeg J. M. 1999. Transgenic rabbits as models for atherosclerosis research. J. Lipid Res. 40: 365–375. [PubMed] [Google Scholar]
- 34.Fan J., Watanabe T. 2003. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol. Ther. 99: 261–282. [DOI] [PubMed] [Google Scholar]
- 35.Ha Y. C., Barter P. J. 1982. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp. Biochem. Physiol. B. 71: 265–269. [DOI] [PubMed] [Google Scholar]
- 36.Guyard-Dangremont V., Desrumaux C., Gambert P., Lallemant C., Lagrost L. 1998. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp. Biochem. Physiol. B. 120: 517–525. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto H., Yonemori F., Wakitani K., Minowa T., Maeda K., Shinkai H. 2000. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 406: 203–207. [DOI] [PubMed] [Google Scholar]
- 38.Nishida H. I., Arai H., Nishida T. 1993. Cholesterol ester transfer mediated by lipid transfer protein as influenced by changes in the charge characteristics of plasma lipoproteins. J. Biol. Chem. 268: 16352–16360. [PubMed] [Google Scholar]
- 39.Masson D., Athias A., Lagrost L. 1996. Evidence for electronegativity of plasma high density lipoprotein-3 as one major determinant of human cholesteryl ester transfer protein activity. J. Lipid Res. 37: 1579–1590. [PubMed] [Google Scholar]
- 40.Desrumaux C., Athias A., Masson D., Gambert P., Lallemant C., Lagrost L. 1998. Influence of the electrostatic charge of lipoprotein particles on the activity of the human plasma phospholipid transfer protein. J. Lipid Res. 39: 131–142. [PubMed] [Google Scholar]
- 41.Masson D., Pais de Barros J. P., Zak Z., Gautier T., Le Guern N., Assem M., Chisholm J. W., Paterniti J. R., Jr., Lagrost L. 2006. Human apoA-I expression in CETP transgenic rats leads to lower levels of apoC-I in HDL and to magnification of CETP-mediated lipoprotein changes. J. Lipid Res. 47: 356–365. [DOI] [PubMed] [Google Scholar]
- 42.Barter P. J., Jones M. E. 1980. Kinetic studies of the transfer of esterified cholesterol between human plasma low and high density lipoproteins. J. Lipid Res. 21: 238–249. [PubMed] [Google Scholar]
- 43.Ihm J., Quinn D. M., Busch S. J., Chataing B., Harmony J. A. 1982. Kinetics of plasma protein-catalyzed exchange of phosphatidylcholine and cholesteryl ester between plasma lipoproteins. J. Lipid Res. 23: 1328–1341. [PubMed] [Google Scholar]
- 44.He Y., Greene D. J., Kinter M., Morton R. E. 2008. Control of cholesteryl ester transfer protein activity by sequestration of lipid transfer inhibitor protein in an inactive complex. J. Lipid Res. 49: 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries R., Perton F. G., Dallinga-Thie G. M., van Roon A. M., Wolffenbuttel B. H., van Tol A., Dullaart R. P. 2005. Plasma cholesteryl ester transfer is a determinant of intima-media thickness in type 2 diabetic and nondiabetic subjects: role of CETP and triglycerides. Diabetes. 54: 3554–3559. [DOI] [PubMed] [Google Scholar]
- 46.Boekholdt S. M., Kuivenhoven J. A., Wareham N. J., Peters R. J., Jukema J. W., Luben R., Bingham S. A., Day N. E., Kastelein J. J., Khaw K. T. 2004. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation. 110: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 47.Mann C. J., Yen F. T., Grant A. M., Bihain B. E. 1991. Mechanism of plasma cholesteryl ester transfer in hypertriglyceridemia. J. Clin. Invest. 88: 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guérin M., Le Goff W., Lassel T. S., Van Tol A., Steiner G., Chapman M. J. 2001. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes. Impact of the degree of triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 21: 282–288. [DOI] [PubMed] [Google Scholar]