Abstract
The relative influence of genetics and the environment on factors associated with cardiovascular disease (CVD) and metabolic syndrome (MetS) remains unclear. We performed model-fitting analyses to quantify genetic, common environmental, and unique environmental variance components of factors associated with CVD and MetS [waist circumference, blood pressure, fasting plasma glucose and insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and fasting plasma lipids] in adult male and female monozygotic twins reared apart or together. We also investigated whether MetS components share common influences. Plasma cholesterol and triglyceride concentrations were highly heritable (56–77%, statistically significant). Waist circumference, plasma glucose and insulin, HOMA-IR, and blood pressure were moderately heritable (43–57%, statistically significant). Unique environmental factors contributed to the variance of all variables (20–38%, perforce statistically significant). Common environmental factors contributed 23, 30, and 42% (statistically significant) of the variance of waist circumference, systolic blood pressure, and plasma glucose, respectively. Two shared factors influenced MetS components; one influenced all components except HDL cholesterol, another influenced only lipid (triglyceride and HDL cholesterol) concentrations. These results suggest that genetic variance has a dominant influence on total variance of factors associated with CVD and MetS and support the proposal of one or more underlying pathologies of MetS.
Keywords: twins, heritability, genetics, risk factors
Cardiovascular disease (CVD) is the leading cause of death in the United States (1). Metabolic syndrome (MetS) is defined as the clustering of factors associated with elevated CVD risk including glucose intolerance, dyslipidaemia, hypertension, and central adiposity (2). Estimated to affect over 34% of American adults (1), MetS is designated as a secondary target of coronary heart disease risk-reduction therapy after the primary target, LDL cholesterol (3). “Cardiometabolic syndrome” is a relatively new term that describes the clustering of factors that influence CVD and type 2 diabetes risk (4). Cardiometabolic syndrome has been established to be a complex condition of multifactorial etiology (5); however, the relative influence of genetic and environmental factors on components of cardiometabolic syndrome remains imprecisely known.
The relative influence of genetic factors is expressed as heritability, which is the proportion of total phenotypic variance in a given trait that is attributable to genetic variation (6). A number of studies have estimated the heritability of components of cardiometabolic syndrome but these estimates vary widely. For example, heritability estimates range from 39–76% for waist circumference (7–13), 8–51% for fasting insulin (12–22), 7–77% for fasting glucose (11–13, 16, 19–24), 8–59% for insulin resistance assessed by the homeostatic model assessment of insulin resistance (HOMA-IR) (12, 13, 19–23, 25–28), 20–66% for diastolic blood pressure (11, 12, 18, 29–38), 8–72% for fasting total cholesterol (12, 29, 30, 38–42), 60% for fasting VLDL (20), 31–68% for fasting LDL (12, 19, 22, 29, 30, 38, 41, 42), 21–79% for fasting HDL (11–13, 18–20, 22, 25, 29, 30, 38–42), and 19–72% for fasting triglycerides (11–13, 18–20, 22, 25, 29, 30, 38, 40, 41). Most of these studies have used the “classical twin study” comparing monozygotic and dizygotic twins (7, 8, 10, 15, 16, 18, 23, 27, 29, 30, 34, 36, 38, 39, 41, 42), which may overestimate the heritability (43). In contrast, the family and adoption designs used in other studies (9, 11–14, 19–22, 24, 26, 28, 31, 32, 35, 37) may underestimate this variance component (43). Only five reports have focused on monozygotic twins reared apart (17, 25, 33, 40, 44), which may provide the least biased estimate of heritability (43). However, all these studies examined only a small subset of cardiometabolic syndrome components and four used the same population of Swedish twins. In addition, only three articles reported heritabilities of all five MetS components (11–13) as defined by the Third Report of the National Cholesterol Education Program's Adult Treatment Panel (ATP III) (3) and only one estimated the heritability of MetS as a syndrome (24%) (11). Furthermore, few studies have investigated whether clustered components of cardiometabolic syndrome are under the influence of shared genetic and environmental factors (17, 23, 25, 45, 46). Comparison of results among these studies is difficult because each study investigated a different combination of cardiometabolic syndrome components. The potential shared influences of the ATP III-defined MetS components have not yet been investigated, and such an investigation would provide insight into the etiology of this commonly-referenced syndrome.
The objectives of this study were to quantify genetic and environmental influences on cardiometabolic syndrome components, and to investigate whether MetS components share common genetic and environmental influences. This work was part of the Tufts Twin Study, a cross-sectional investigation of the heritability of energy regulation measures in a population of monozygotic twins reared apart (MZAs) or reared together (MZTs).
MATERIALS AND METHODS
Subjects
Subjects were 157 adult men and women, aged 18–76 years, who participated in the Tufts Twin Study at the Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging (HNRCA) at Tufts University. They included 78 monozygotic twin pairs who were either reared apart since near birth (29 pairs) or reared together (49 pairs), and one singleton monozygotic twin whose reared-together twin did not participate in the study. The singleton twin was included in data analyses because singleton data may reduce biases due to nonrandom ascertainment (47). Participants were healthy without any major medical condition including diabetes, active cancer, heart disease, cachexia, eating disorders, AIDS, psychiatric disorders, pregnancy, or recent weight change. MZAs were recruited through their participation in the Minnesota Study of Twins Reared Apart at the University of Minnesota (47a) and lived in North America, Europe (United Kingdom, The Netherlands, Germany, and Poland), South Africa, or Australia. MZTs were recruited by advertisements in the New England area. A few MZTs were from other parts of the United States, Canada, and Germany. Subjects were reimbursed for travel expenses and given a stipend for their participation in the study. The protocols were approved by the Institutional Review Board at Tufts University and all subjects gave written and informed consent.
Protocol
Each subject was admitted to the Metabolic Research Unit at the HNRCA for ∼4 days of observation and completed a variety of examinations and questionnaires concerning energy intake, expenditure, and regulation. Subjects from overseas spent a week in Minnesota prior to travel to Boston, which allowed for recovery from jetlag. During the 4-day inpatient study at Tufts, subjects slept and ate all meals in the research center. Assessments were made in the areas of anthropometry, blood pressure, fasting plasma lipids, glucose, and insulin.
Nonlaboratory assessments
Body weight and height were measured to the nearest 0.01 kg and 0.1 cm, respectively, on each of the 4 study days. Mean body weight and height were used to determine body mass index (BMI), calculated as weight/height2 (kg/m2). Waist circumference was measured to the nearest 0.05 cm. Systolic and diastolic blood pressures were measured by aneroid sphygmomanometer under standardized conditions.
Laboratory assessments
Blood samples taken after a 12-h supervised overnight fast were collected and assayed for total cholesterol and triglyceride with an Abbott Spectrum analyzer using enzymatic reagents from Abbott Diagnostics (Dallas, TX) (48). HDL cholesterol was measured in the supernatant fraction after precipitation of apolipoprotein (apo) B-containing lipoproteins using dextran sulfate magnesium (49). LDL cholesterol concentrations were estimated using the Friedwald formula (LDL cholesterol = total cholesterol − HDL cholesterol − triglyceride/5) (50). LDL cholesterol concentrations were determined directly when triglyceride concentrations exceeded 400 mg/dl (Olympus America Inc., Melville, NY). Lipid assays were standardized through the Lipid Standardization Program of the Centers for Disease Control and Prevention, Atlanta, GA. Fasting blood glucose concentrations were determined using a coupled enzyme assay (50) and plasma insulin using a competitive binding radioimmunoassay (51). HOMA-IR was calculated [(mg/dl) × insulin (µU/ml) / 405] to quantify insulin resistance (52).
Statistical analysis
Descriptive statistics were calculated using SAS 9.1 (53). To obtain normal or near-normal distributions, certain variables were transformed using a natural log transformation (weight, BMI, systolic blood pressure, fasting plasma glucose, fasting plasma insulin, HOMA-IR, total cholesterol, VLDL, LDL, and triglycerides) or a Blom transformation (waist circumference). Log transformed variables were then multiplied by 100 to increase the variance, which facilitated model-fitting analyses.
Intrapair correlations.
Intrapair (intraclass) MZA and MZT correlation coefficients were calculated as a measure of within-pair similarity using SPSS 15.0 (54). MZA intrapair correlations provide a simple estimate of heritability, because their covariance consists solely of the genetic component of variance (6). However, this technique of heritability estimation is inferior to model-fitting analyses because it may lead to nonsensical estimates of heritability, it does not easily generalize to multivariate genetic factor models, it is inefficient when there are missing data, and it does not optimally combine data from multiple groups (55).
Model-fitting analyses.
Model-fitting analyses were based on the decomposition of variance into genetic (G), common or shared environmental (C), and unique or nonshared environmental (E) components. Genetic variance (VG) is caused by differences in genes between individuals. Common environmental variance (VC) is due to environmental factors responsible for resemblance between family members, whereas unique environmental variance (VE) is due to environmental factors that contribute to differences between family members (6). Unique environmental variance comprises any variance that is not due to genetic or common environmental factors, including variance due to measurement error. Total phenotypic variance (VP) can be represented as VP = VG + VC + VE. Heritability was defined as the proportion of total phenotypic variance in a given trait that is attributable to genotypic variance (6).
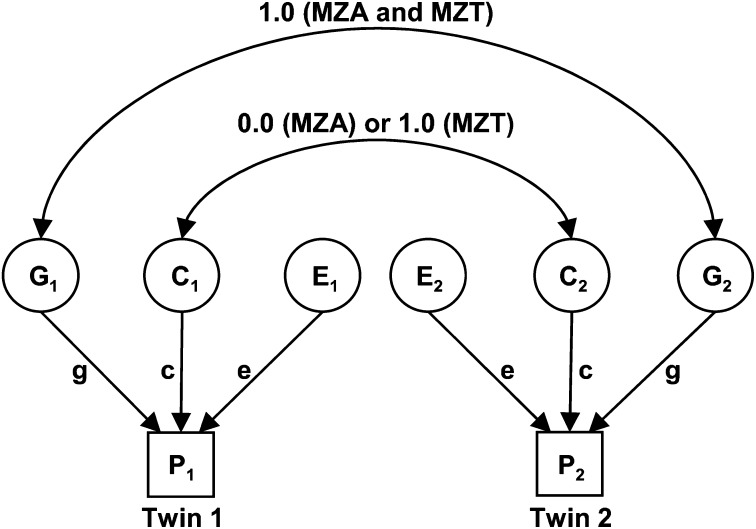
In the present study, variance decomposition was applied in the MZA/MZT design, where it was assumed that all monozygotic twin pairs share 100% of their genetic material. MZT pairs share all common environmental factors (such as parents, siblings, home, and economic factors), and MZAs do not correlate for these common environmental effects. That is, it was assumed that MZAs were placed in homes selected at random from the population. These relationships can be depicted in a path diagram (Fig. 1). The covariance of MZAs (COVMZA) is VG and the covariance of MZTs (COVMZT) is VG + VC.
Fig. 1.
Path diagram of the univariate MZA/MZT GCE twin model. C, common environmental factors; E, unique environmental factors; G, genetic factors; MZA, monozygotic twins reared apart; MZT, monozygotic twins reared together; P1, phenotype of twin 1; P2, phenotype of twin 2; g, c, e are path coefficients. Circles represent latent (unmeasured) variables. Squares represent observed (measured) variables. Single-headed arrows represent hypothesized casual relationships between variables. Double-headed arrows represent correlation or covariance between variables.
The MZA/MZT twin model used here was based on the following assumptions: 1): traits follow polygenic autosomal inheritance; 2) the observed phenotypic variance is a linear additive function of genetic and environmental variances; 3) genetic and environmental effects are uncorrelated and there is no genotype by environmental interaction; 4) there is no selective placement (nonrandom adoption of twins into similar families); and 5) genetic and environmental factors are of the same magnitude in males and females (56). Note also that any genetic effects of assortative mating contribute to VG and that differences in methylation within a twin pair contribute to VE.
Model-fitting analyses were performed using Mx, a structural equation modeling software package (57). Mx fits the MZA/MZT GCE model to the raw observed data. It estimates parameters using maximum likelihood and computes goodness-of-fit statistics based on minus twice the natural logarithm of the likelihood (−2lnL). Likelihood ratio tests (LRT) are used to test hypotheses, because under certain regularity conditions, the difference in −2lnL between nested models (which differ because one or more parameters are constrained to equal each other or specific values) is asymptotically distributed as c2 with degrees of freedom (df) equal to the difference in the number of free parameters in the two models. However, under the null hypothesis that a variance component is zero, the LRT is distributed as a 50:50 mixture of c2 with 1 df and zero (58, 59).
Univariate analyses were performed on 14 variables to estimate the proportion of variance due to G, C, and E. The variables analyzed were: weight, height, BMI, waist circumference, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, fasting plasma insulin, HOMA-IR, total cholesterol, VLDL, LDL, HDL, and triglycerides. Age and gender were included in the analyses as covariates.
Multivariate analysis was used to investigate how genetic and environmental factors influence the components of the ATP-III-defined MetS (3). Several multivariate GCE models were fit to the five components of MetS: waist circumference, fasting plasma glucose, triglycerides, HDL, and blood pressure. Of the two measures of blood pressure, only diastolic blood pressure was included in the analysis to avoid overemphasis on blood pressure. Diastolic blood pressure was chosen because it showed greater correlations with the other MetS components compared with systolic blood pressure (data not shown). Age and gender were included in the analysis as covariates. The following series of standard models was used; each represents a different possible set of relationships between the observed variables and the latent factors: Cholesky decomposition; independent pathway; and one-, two- and three-factor common pathway (47). In addition, a hypothesis-driven model was considered. These models were compared on the basis of likelihood and parsimony to determine the model with the best fit to the data. The difference in likelihood was assessed by calculating the difference in −2lnL between models. Parsimony was assessed by Akaike's Information Criterion (AIC), which may be computed as −2lnL − 2df, where the more negative value indicated the most parsimonious model.
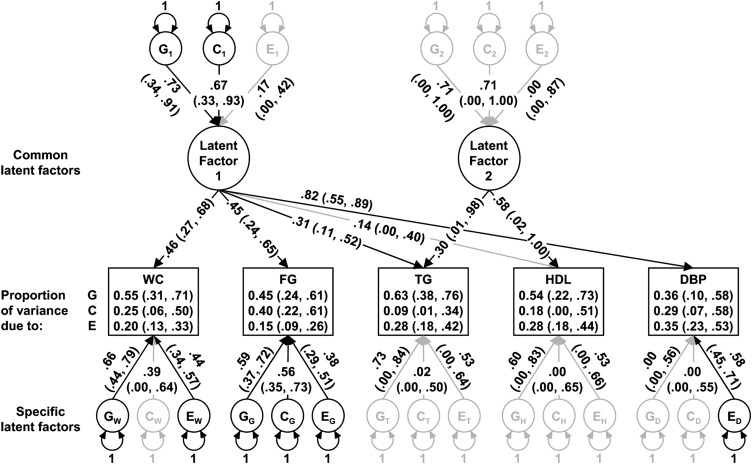
The hypothesis-driven model is based on established correlations between the components of MetS. Visceral adiposity has been shown to be associated with an increase in fasting plasma glucose, as well as an increase in triglycerides and decrease in HDL, and an increase in blood pressure (60). It has also been shown that an increase in triglycerides is associated with a decrease in HDL independent of waist circumference (61). Therefore, in the hypothesis-driven model, a common latent factor (Latent Factor 1, Fig. 3) is depicted as potentially influencing all five observed variables and another common latent factor (Latent Factor 2, Fig. 3) is depicted as potentially influencing only the lipid measurements. Similar to the independent and common pathway models, specific latent G, C, and E factors are modeled for each of the observed variables, to capture any variance that could not be explained by the common latent factors.
Fig. 3.
Hypothesis-driven two-factor common pathway model path diagram of the metabolic syndrome. Rectangles represent observed variables. Circles represent latent or unmeasured variables. Single-headed arrows represent hypothesized casual relationships between variables. Double-headed arrows represent variance. Path coefficients are standardized parameter estimates and 95% confidence intervals are reported in parentheses. Darkened lines indicate significant paths. Subscripts indicate variable or factor under influence. C, common environmental factors; DBP, diastolic blood pressure; E, unique environmental factors; FG, transformed (100 × ln of) fasting plasma glucose; G, genetic factors; HDL, fasting plasma HDL cholesterol; TG, transformed (100 × ln of) fasting plasma triglycerides; WC, Blom transformation of waist circumference.
RESULTS
The majority of the subjects were female (72% of MZAs and 76% of MZTs) and Caucasian (97% of MZAs and 94% of MZTs). MZA twins were statistically significantly older than MZT twins (mean age [range]: 49 [22–76] and 29 [18–47] years, respectively, Table 1). Mean values of body weight, BMI, waist circumference, systolic and diastolic blood pressure, plasma glucose and insulin, HOMA-IR, plasma total cholesterol, plasma VLDL cholesterol, plasma LDL cholesterol, and plasma triglyceride concentrations were statistically significantly higher for MZAs compared with MZTs, but not after the data were adjusted for age (Table 1).
TABLE 1.
Characteristics of study population
| Mean ± s.d. (na) |
|||
|---|---|---|---|
| MZA | MZT | Pb | |
| Age (years) | 49.1 ± 12.0 (58) | 28.7 ± 7.3 (99) | <0.0001 |
| Weight (kg) | 75.3 ± 18.8 (58) | 66.1 ± 11.1 (99) | 0.0047c |
| Height (cm) | 166.3 ± 9.3 (58) | 169.6 ± 7.6 (99) | 0.0794 |
| BMI (kg/m2) | 27.0 ± 5.2 (58) | 23.0 ± 3.2 (99) | <0.0001c |
| WC (cm) | 87.8 ± 14.9 (58) | 75.8 ± 8.8 (99) | <0.0001c |
| SBP (mmHg) | 119.2 ± 14.7 (57) | 109.8 ± 8.9 (99) | 0.0002c |
| DBP (mmHg) | 74.1 ± 8.5 (57) | 68.8 ± 6.5 (99) | 0.0006c |
| FG (mg/dl) | 93.3 ± 21.4 (58) | 83.3 ± 6.3 (99) | 0.0007c |
| FI (μU/ml) | 12.8 ± 6.0 (44) | 9.8 ± 3.4 (90) | 0.0043c |
| HOMA-IR | 3.1 ± 2.1 (44) | 2.0 ± 0.8 (90) | 0.0010c |
| TC (mg/dl) | 210.7 ± 47.1 (52) | 167.6 ± 35.3 (98) | <0.0001c |
| VLDL (mg/dl) | 26.2 ± 15.2 (52) | 19.0 ± 9.6 (98) | 0.0096c |
| LDL (mg/dl) | 134.3 ± 41.8 (52) | 99.4 ± 28.5 (98) | <0.0001c |
| HDL (mg/dl) | 50.5 ± 9.9 (52) | 49.2 ± 10.0 (98) | 0.6300 |
| TG (mg/dl) | 130.8 ± 76.2 (52) | 95.3 ± 48.1 (98) | 0.0097c |
BMI, body mass index; DBP, diastolic blood pressure; FG, fasting plasma glucose; FI, fasting plasma insulin; HDL, fasting plasma HDL; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, fasting plasma LDL; MZA, monozygotic twins reared apart; MZT, monozygotic twins reared together; SBP, systolic blood pressure; TC, fasting plasma total cholesterol; TG, fasting plasma triglycerides; VLDL, fasting plasma VLDL; WC, waist circumference.
n, number of individuals.
P for statistical difference between MZA and MZT twins corrected for sampling among twins.
Differences between MZA and MZT means were not statistically significant when adjusting for age, age2, and age3 (P>0.05).
MZT intrapair (intraclass) correlations were greater than MZA intrapair correlations for body weight, BMI, waist circumference, and systolic and diastolic blood pressure, suggesting that common environmental factors play a role in these phenotypes (Table 2). MZA intrapair correlations are a simple estimate of heritability showing significant estimates for cardiometabolic syndrome components ranging from 46% for plasma insulin to 82% for total cholesterol concentrations. However, as previously noted, this technique of heritability estimation is not as reliable as model-fitting analyses (55).
TABLE 2.
Intrapair MZA and MZT correlations
| MZA |
MZT |
|||
|---|---|---|---|---|
| na | Intrapair Correlation (95% CI) | na | Intrapair Correlation (95% CI) | |
| Weightb | 29 | 0.78 (0.59, 0.89) | 49 | 0.86 (0.76, 0.92) |
| Height | 29 | 0.96 (0.92, 0.98) | 49 | 0.94 (0.90, 0.97) |
| BMIb | 29 | 0.65 (0.38, 0.82) | 49 | 0.80 (0.66, 0.88) |
| WCc | 29 | 0.78 (0.59, 0.89) | 49 | 0.80 (0.67, 0.88) |
| SBPb | 28 | f | 49 | 0.73 (0.56, 0.84) |
| DBP | 28 | 0.59 (0.29, 0.79) | 49 | 0.62 (0.41, 0.76) |
| FGb | 29 | 0.62 (0.34, 0.80) | 49 | 0.57 (0.35, 0.74) |
| FIb | 21 | 0.61 (0.27, 0.82) | 44 | 0.46 (0.20, 0.67) |
| HOMA-IRb | 21 | 0.64 (0.31, 0.84) | 44 | 0.56 (0.32, 0.73) |
| TCb | 26 | 0.82 (0.64, 0.91) | 48 | 0.82 (0.71, 0.90) |
| VLDLb | 26 | 0.71 (0.45, 0.86) | 48 | 0.70 (0.52, 0.82) |
| LDLb | 26 | 0.85 (0.69, 0.93) | 48 | 0.81 (0.68, 0.89) |
| HDL | 26 | 0.59 (0.28, 0.79) | 48 | 0.74 (0.58, 0.84) |
| TGb | 26 | 0.71 (0.45, 0.86) | 48 | 0.70 (0.53, 0.82) |
BMI, body mass index; DBP, diastolic blood pressure; FG, fasting plasma glucose; FI, fasting plasma insulin; HDL, fasting plasma HDL; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, fasting plasma LDL; MZA, monozygotic twins reared apart; MZT, monozygotic twins reared together; SBP, systolic blood pressure; TC, fasting plasma total cholesterol; TG, fasting plasma triglycerides; VLDL, fasting plasma VLDL; WC, waist circumference.
n, number of twin pairs.
Variable was transformed by multiplying the natural log of the variable by 100.
Variable was transformed by a Blom transformation.
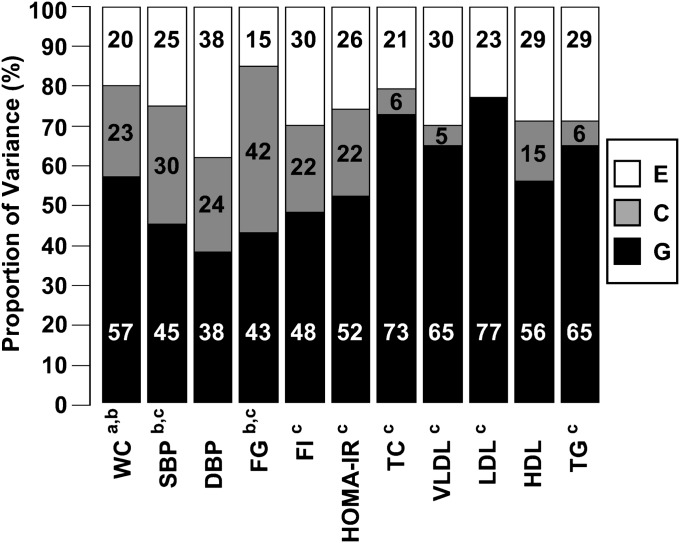
All components of cardiometabolic syndrome were found to have a statistically significant heritability (Fig. 2, confidence intervals reported in Table 3). Heritability estimates of the plasma lipid variables (total, VLDL, LDL and HDL cholesterol, and triglyceride concentration) were higher (56–77%) than heritability estimates of blood pressure (38–45%) and glucose and insulin measures (43–52%). The proportion of variance due to unique environmental influences factors (which is perforce statistically significant because the likelihood of the data approaches zero in the limit as the estimate of E does) ranged from 13% for weight to 38% for diastolic blood pressure. The proportion of variance due to common environmental influences was found to be statistically significant for waist circumference (23%), systolic blood pressure (30%), and plasma glucose (42%).
Fig. 2.
Variance components from univariate GCE model for components of cardiometabolic syndrome. C, common environmental; DBP, diastolic blood pressure; E, unique environmental; FG, fasting plasma glucose; FI, fasting plasma insulin; G, genetic; HDL, fasting plasma HDL; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, fasting plasma LDL; SBP, systolic blood pressure; TC, fasting plasma total cholesterol; TG, fasting plasma triglycerides; VLDL, fasting plasma VLDL; WC, waist circumference. a The variable was transformed by a Blom transformation. b Significant C component. All variables have significant G and E components. c The variable was transformed by multiplying the natural log of the variable by 100.
TABLE 3.
Variance components from univariate GCE model for anthropometrics and cardiometabolic disease components
| Proportion of variance (95% CIs) |
|||
|---|---|---|---|
| Genetic | Common Environmental | Unique Environmental | |
| Anthropometrics | |||
| Weighta | 0.62 (0.40, 0.76) | 0.26 (0.11, 0.48) | 0.13 (0.08, 0.21) |
| Height | 0.91 (0.84, 0.09) | 0.00 (0.00, 0.07) | 0.09 (0.05, 0.13) |
| BMIa | 0.50 (0.24, 0.69) | 0.33 (0.14, 0.60) | 0.17 (0.10, 0.27) |
| WCb | 0.57 (0.32, 0.73) | 0.23 (0.04, 0.48) | 0.20 (0.13, 0.33) |
| Blood pressure | |||
| SBPa | 0.45 (0.14, 0.66) | 0.30 (0.06, 0.62) | 0.25 (0.16, 0.40) |
| DBP | 0.38 (0.07, 0.61) | 0.24 (0.00, 0.58) | 0.38 (0.24, 0.60) |
| Plasma glucose and insulin | |||
| FGa | 0.43 (0.21, 0.61) | 0.42 (0.23, 0.65) | 0.15 (0.09, 0.25) |
| FI | 0.48 (0.21, 0.67) | 0.22 (0.00, 0.52) | 0.30 (0.18, 0.53) |
| HOMA-IRa | 0.52 (0.24, 0.71) | 0.22 (0.00, 0.52) | 0.26 (0.15, 0.45) |
| Plasma lipids | |||
| TCa | 0.73 (0.50, 0.84) | 0.06 (0.00, 0.30) | 0.21 (0.13, 0.33) |
| VLDLa | 0.65 (0.37, 0.78) | 0.05 (0.00, 0.33) | 0.30 (0.19, 0.45) |
| LDLa | 0.77 (0.58, 0.85) | 0.00 (0.00, 0.20) | 0.23 (0.14, 0.34) |
| HDL | 0.56 (0.25, 0.75) | 0.15 (0.00, 0.47) | 0.29 (0.18, 0.45) |
| TGa | 0.65 (0.38, 0.79) | 0.06 (0.00, 0.34) | 0.29 (0.19, 0.45) |
BMI, body mass index; DBP, diastolic blood pressure; FG, fasting plasma glucose; FI, fasting plasma insulin; HDL, fasting plasma HDL; HOMA-IR, homeostatic model assessment of insulin resistance; LDL, fasting plasma LDL; SBP, systolic blood pressure; TC, fasting plasma total cholesterol; TG, fasting plasma triglycerides; VLDL, fasting plasma VLDL; WC, waist circumference.
Variable was transformed by multiplying by the natural log of the variable by 100.
Variable was transformed by a Blom transformation.
Multivariate analyses were conducted to examine the extent to which components of MetS share common genetic and environmental influences. MZA and MZT variances, covariances, and correlations are shown in Table 4 for the five components of MetS. Table 5 shows goodness-of-fit data for the six models tested for the MetS multivariate analysis. The common pathway, the two-factor common pathway, and the hypothesis-driven models were similarly parsimonious (AIC of 3550.4, 3549.3, and 3550.1, respectively). Nevertheless, the hypothesis-driven model was chosen as the best model because it was the most biologically plausible.
TABLE 4.
Variance-covariance and correlation matrices of metabolic syndrome components for MZAs and MZTs
| Monozygotic twins reared apart (MZA) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| blWC1 | trFG1 | trTG1 | HDL1 | DBP1 | blWC2 | trFG2 | trTG2 | HDL2 | DBP2 | |
| blWC1 | 1.01 | 0.30 | 0.46 | −0.40 | 0.68 | 0.80 | 0.34 | 0.52 | −0.30 | 0.68 |
| trFG1 | 6.45 | 472.46 | 0.23 | −0.16 | 0.45 | 0.18 | 0.66 | 0.20 | −0.14 | 0.29 |
| trTG1 | 22.33 | 237.87 | 2092.22 | −0.39 | 0.42 | 0.22 | 0.22 | 0.71 | −0.34 | 0.47 |
| HDL1 | −4.75 | −39.66 | −197.37 | 124.41 | −0.09 | −0.38 | −0.18 | −0.26 | 0.61 | −0.23 |
| DBP1 | 4.96 | 75.30 | 150.40 | −7.94 | 57.50 | 0.41 | 0.40 | 0.29 | 0.08 | 0.61 |
| blWC2 | 1.02 | 4.92 | 13.74 | −5.65 | 3.98 | 1.63 | 0.28 | 0.37 | −0.34 | 0.64 |
| trFG2 | 5.01 | 211.85 | 153.47 | −30.39 | 45.52 | 5.24 | 218.01 | 0.19 | −0.03 | 0.63 |
| trTG2 | 27.78 | 229.51 | 1633.55 | −145.04 | 113.65 | 24.98 | 146.14 | 2535.45 | −0.38 | 0.53 |
| HDL2 | −2.75 | −27.28 | −133.03 | 59.34 | 5.21 | −3.93 | −3.66 | −166.62 | 74.98 | −0.06 |
| DBP2 |
6.42 |
59.41 |
208.39 |
−24.59 |
44.02 |
7.69 |
87.03 |
258.58 |
−4.95 |
87.92 |
| Monozygotic twins reared together (MZT) |
||||||||||
| blWC1 |
trFG1 |
trTG1 |
HDL1 |
DBP1 |
blWC2 |
trFG2 |
trTG2 |
HDL2 |
DBP2 |
|
| blWC1 | 0.59 | 0.24 | 0.19 | −0.30 | 0.46 | 0.79 | 0.28 | 0.07 | −0.15 | 0.32 |
| trFG1 | 1.31 | 49.17 | 0.09 | −0.07 | 0.33 | 0.12 | 0.57 | 0.21 | −0.02 | 0.36 |
| trTG1 | 6.31 | 27.96 | 1755.75 | −0.29 | 0.20 | 0.06 | 0.16 | 0.70 | −0.09 | 0.13 |
| HDL1 | −2.34 | −4.63 | −121.10 | 98.30 | −0.14 | −0.23 | −0.12 | −0.17 | 0.74 | −0.10 |
| DBP1 | 2.22 | 14.53 | 52.52 | −8.80 | 38.71 | 0.28 | 0.30 | 0.23 | −0.05 | 0.62 |
| blWC2 | 0.46 | 0.65 | 1.84 | −1.74 | 1.32 | 0.57 | 0.21 | 0.08 | −0.12 | 0.17 |
| trFG2 | 1.68 | 31.40 | 52.87 | −9.69 | 14.84 | 1.28 | 62.62 | 0.31 | −0.15 | 0.32 |
| trTG2 | 2.43 | 64.90 | 1340.06 | −75.77 | 63.31 | 2.72 | 112.50 | 2052.38 | 0.06 | 0.13 |
| HDL2 | −1.19 | −1.54 | −39.32 | 74.25 | −3.04 | −0.93 | −12.07 | 25.43 | 101.71 | −0.16 |
| DBP2 | 1.66 | 17.04 | 37.55 | −6.98 | 25.70 | 0.88 | 16.92 | 40.70 | −11.16 | 45.60 |
Variances are on the leading diagonal, covariances are below the diagonal, and correlations are above the diagonal. Variables are: blWC, Blom transformation of waist circumference; trFG, 100 x natural log of fasting plasma glucose; trTG, 100 x natural log of fasting plasma triglycerides; HDL, fasting plasma HDL; DBP, diastolic blood pressure. Numbers at the end of the variable name indicate twin 1 or twin 2.
TABLE 5.
Model comparison for multivariate analysis of the metabolic syndrome components
| Model | -2lnL | df | χ2 | Δdf | P | AIC |
|---|---|---|---|---|---|---|
| Cholesky decomposition | 4998 | 710 | 3577.7 | |||
| Independent pathway | 5007 | 725 | 9 | 15 | 0.857 | 3557.1 |
| Common pathway | 5016 | 733 | 19 | 23 | 0.716 | 3550.4 |
| 2-factor common pathway | 5005 | 728 | 8 | 18 | 0.984 | 3549.3 |
| 3-factor common pathway | 5004 | 725 | 6 | 15 | 0.977 | 3553.8 |
| Hypothesis-driven | 5012 | 731 | 14 | 21 | 0.851 | 3550.1 |
AIC, Akaike's Information Criterion; χ2, difference chi-squared compared with Cholesky decomposition; Δdf, difference degrees of freedom compared with Cholesky decomposition; lnL, log-likelihood; df, degrees of freedom; P for statistical difference compared with Cholesky decomposition.
A path diagram for the multivariate analysis of MetS components is shown in Fig. 3. Standardized parameter estimates are indicated along the paths, with 95% confidence intervals reported in parentheses. The paths depicted as bolded lines were statistically significant. A common latent factor that was statistically significantly influenced by genetic and common environmental factors was found to statistically significantly influence waist circumference, plasma glucose, plasma triglycerides, and diastolic blood pressure. Another common latent factor was found to statistically significantly influence plasma triglycerides and HDL cholesterol concentrations. Additionally, waist circumference and plasma glucose were statistically significantly influenced by specific genetic and environmental factors, whereas diastolic blood pressure was statistically significantly influenced only by a specific unique environmental factor, and the plasma lipid concentrations were not statistically significantly influenced by specific factors. As a result, most of the variance of plasma triglyceride and HDL cholesterol concentrations and diastolic blood pressure was explained by the common latent factors, whereas most of the variance of waist circumference and plasma glucose concentrations was explained by specific latent factors. Estimates of the proportion of variance due to genetic, common environmental, and unique environmental factors derived from the multivariate model are also reported in Fig. 2. These values differ slightly from the univariate estimates because multivariate analyses exploit additional data in the form of covariances between variables, both within-person and across-twins.
DISCUSSION
Although the potential contribution of genetic and environmental influences to cardiometabolic syndrome components has received considerable attention (62), current estimates of the heritability vary widely and are only available for a subset of those components. Furthermore, despite attempts to identify an underlying pathology of MetS, few studies have examined whether MetS components share common genetic and environmental influences. Our report uses the powerful MZA/MZT study design to examine the influence of genetic and environmental factors on cardiometabolic syndrome components (adiposity, blood pressure, insulin resistance, and plasma lipid and lipoprotein concentrations) and also to investigate the composition of genetic and environmental influences on components of MetS using a multivariate model-fitting method.
In general, in our MZA/MZT cohort, cardiometabolic syndrome components were found to be more heritable than previously reported. This finding may be due to differences in study design or sampling variation. The high heritability of plasma lipid and lipoprotein concentrations is consistent with prior research findings that have identified genes influencing lipid and lipoprotein concentrations, or their response to dietary change. For example, genetic polymorphisms for proteins involved in lipoprotein metabolism, such as apoE, cholesteryl ester transfer protein, and hepatic lipase, have been either identified as candidate genes for CVD risk or found to modify the lipid response to dietary change (63, 64). The moderate heritability estimates of the other cardiometabolic syndrome components is also consistent with previous reports of genetic polymorphisms for proteins that impact on obesity, blood pressure, or insulin resistance, such as peroxisome proliferator-activated receptor γ, angiotensinogen, and calpain-10, respectively (62, 65, 66).
The low estimates of environmental influence, and specifically common environmental influence, on cardiometabolic syndrome components present a shift from the previously proposed importance of the environment on CVD risk (67). For example, a diet high in saturated fat has been shown to raise plasma LDL cholesterol concentrations (68) and physical inactivity has been associated with lower plasma HDL cholesterol concentrations (69). The results of our study suggest that, despite these established causal relationships between environmental factors and cardiometabolic syndrome components, genetic variation is still the dominant source of phenotypic variation in these components. This does not, however, rule out the importance of lifestyle modification to lower CVD and type 2 diabetes risk. Our data support the hypothesis that changes in our environment could result in modest yet clinically important improvements in lipid and lipoprotein profiles, insulin sensitivity, blood pressure, and body weight, which can lower CVD and type 2 diabetes risk (67).
Results from this study also suggest that environmental influences on cardiometabolic syndrome components appear to be primarily from factors specific to the individual rather than factors shared by family members. Although previous studies have shown that environmental factors such as diet and exercise are shared within families (70, 71), our results suggest that, in adult twins, this early familial environmental influence is overcome by other environmental influences that are specific to the individual. However, for some parameters, common environmental influences may decrease with age as individuals live independently and no longer share a common environment with other household members as they did earlier in life. It is possible that the influence of common environmental factors would be greater in a population of adolescents or young adults compared with an older adult population, such as the twin population used in this study. However, given that cardiometabolic syndrome disproportionately affects older adults, the heritability values reported in this study are most applicable to the population at highest risk.
It is possible that estimates of genetic and environmental effects on cardiometabolic syndrome components in both our study and others could be influenced by the twin intrauterine environment. A shared intrauterine environmental factor could cause a twin pair to be more concordant for a phenotype, but because we made the assumption that MZAs do not share a common environment, this concordance would be attributed to genetic effects and would inflate the heritability estimate. Alternatively, if the intrauterine environment caused a twin pair to be more discordant for a phenotype (for example, if the twins differed in their prenatal nutrient intake), estimates of unique environmental influence would be higher than if the intrauterine environment had no effect. Although this is an important question, it is accepted that knowledge of intrauterine environmental effects is insufficient to invalidate twin studies (72). Correlations between birth weight and adult weight are modest (73, 74), which suggests that intrauterine effects are unlikely to persist to form a substantial part of adult variation in MetS variables.
Findings from our multivariate analyses suggest that components of the ATP-III-defined MetS share common influences although certain components appear to be more closely linked than others. Waist circumference, plasma glucose, plasma triglyceride concentration, and diastolic blood pressure were linked through a common latent factor, which was estimated to be 53% heritable but was statistically significantly influenced by both genetic and common environmental factors. We speculate that this common factor is related to insulin resistance, a previously proposed underlying cause of MetS (75). It has been suggested that insulin resistance leads to hyperglycemia by decreasing glucose uptake in muscle (76), dyslipidaemia (low HDL cholesterol and high triglyceride concentration) caused by an increase of free fatty acids transported to the liver resulting from an increase in adipocyte lipolysis (76), and hypertension possibly caused by endothelial dysfunction, vascular changes, and increased salt sensitivity due to hyperinsulinemia (77).
Waist circumference and plasma glucose were statistically significantly and predominantly influenced by factors specific to each phenotype, indicating that, although they share common influences with the other MetS components, the majority of their influences appear to be distinct. A variety of factors have been suggested to be associated with abdominal obesity independent of total body fatness, including fiber intake, smoking cessation, and television watching (78), which could theoretically not be associated with other MetS components. Although abdominal obesity has been proposed to be an underlying pathology of MetS, the apparently moderate proportion of variance attributed to the common factor may be due to the large number of potential influences on abdominal obesity. Similarly, a variety of influences on plasma glucose, such as menopause and alcohol intake (79, 80), may also have little influence on other MetS components.
As expected, the plasma lipid measures of MetS (triglycerides and HDL) appear to be closely linked through a common latent factor, which was influenced by both genetic and common environmental factors (although the relationships did not reach statistical significance), resulting in a nonstatistically significant heritability estimate of 50%. We speculate that this common factor is related to lipid metabolism, and perhaps specifically to the actions of cholesteryl ester transfer protein, which is involved in the mechanisms linking high plasma triglyceride and low HDL cholesterol concentrations (77).
The results of this study should be interpreted within the context of several limitations. First, although the MZA/MZT study design is statistically powerful for assessing the relative influence of genetic versus environmental factors on traits, the sample size of this study is small for genetic epidemiological purposes. Second, our results may not be generalizable to other cohorts because our subjects were predominantly Caucasians living in Western societies. Third, the inclusion of subjects from different Western countries may also limit conclusions because cultural differences between Western societies may in fact affect results. Fourth, the potential violation of one or more of the MZA/MZT twin model assumptions, which were previously described, could affect results. For example, there could have been selective placement of MZAs into similar families. However, assumptions of the MZA/MZT twin model are standard and can potentially be tested in future studies (56). Finally, although we assessed the relative contributions of genetic and environmental influences on factors associated with cardiovascular disease and metabolic syndrome, our aim in this study was not to identify the specific influences. Further research investigating the impact of specific genes and environmental factors on cardiometabolic syndrome components will continue to provide insight into the most successful CVD and MetS therapies.
In conclusion, this study of the heritability of cardiometabolic syndrome components in a unique population of adult MZAs and MZTs found generally higher heritability estimates than previously reported. Plasma lipid and lipoprotein concentrations were found to be highly heritable and waist circumference, insulin resistance, plasma glucose and insulin, and blood pressure were found to be at least moderately heritable. Multivariate analysis of the components of the ATP-III-defined MetS revealed common influences, which appear to be almost equally affected by differences in genes and the common environment. Waist circumference and plasma glucose were primarily influenced by genetic and environmental factors specific to each phenotype, indicating that these two cardiometabolic syndrome components may be affected by a variety of influences in addition to insulin resistance, a proposed underlying MetS pathology. Combined, these data suggest that genes play a dominant role in the development of cardiometabolic syndrome components and that there are common genetic and environmental influences that affect certain cardiometabolic syndrome components leading to the development of MetS.
Acknowledgments
The authors thank the volunteers who participated in this study, Angela Vinken for her assistance with data collection, Dr. Gerard E. Dallal for his statistical assistance during manuscript preparation, and Brenda Roche for her assistance in data preparation.
Footnotes
Abbreviations:
- AIC
- Akaike's information criterion
- apo
- apolipoprotein
- ATP III
- Third Report of the National Cholesterol Education Program's Adult Treatment Panel
- BMI
- body mass index
- CVD
- cardiovascular disease
- df
- degrees of freedom
- HNRCA
- Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University
- HOMA-IR
- homeostatic model assessment of insulin resistance
- −2lnL
- minus twice the natural logarithm of the likelihood
- LRT
- likelihood ratio tests
- MetS
- metabolic syndrome
- MZA
- monozygotic twins reared apart
- MZT
- monozygotic twins reared together
- G
- genetic influences
- C
- common environmental influences
- E
- unique environmental influences
- VC
- common environmental variance
- VE
- unique environmental variance
- VG
- genetic variance
- VP
- total phenotypic variance
Funding support for this project was provided by the National Institutes of Health (DK046124, DK79003, DK76092, DK78867, MH65322, DA18673, DK73321 and 5T32 HL069772) and the US Department of Agriculture under agreements no. 1950-51000-061-04S, 1950-51000-059-04S. and 1950-51000-061-05S. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture.
REFERENCES
- 1.Lloyd-Jones D., Adams R., Carnethon M., De Simone G., Ferguson T. B., Flegal K., Ford E., Furie K., Go A., Greenlund K., et al. 2009. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 119: e21–e181. [DOI] [PubMed] [Google Scholar]
- 2.Desroches S., Lamarche B. 2007. The evolving definitions and increasing prevalence of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 32: 23–32. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. 2001. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan G., Whaley-Connell A., Mugo M., Stump C., Sowers J. R. 2005. The cardiometabolic syndrome as a cardiovascular risk factor. Am. J. Med. Sci. 330: 311–318. [DOI] [PubMed] [Google Scholar]
- 5.Castro J. P., El-Atat F. A., McFarlane S. I., Aneja A., Sowers J. R. 2003. Cardiometabolic syndrome: pathophysiology and treatment. Curr. Hypertens. Rep. 5: 393–401. [DOI] [PubMed] [Google Scholar]
- 6.Plomin R., DeFries J. C., McClearn G. E., Rutter M. 1997. Behavioral genetics. 3rd ed. W.H. Freeman and Company, New York. [Google Scholar]
- 7.Selby J. V., Newman B., Quesenberry C. P., Jr., Fabsitz R. R., Carmelli D., Meaney F. J., Slemenda C. 1990. Genetic and behavioral influences on body fat distribution. Int. J. Obes. 14: 593–602. [PubMed] [Google Scholar]
- 8.Rose K. M., Newman B., Mayer-Davis E. J., Selby J. V. 1998. Genetic and behavioral determinants of waist-hip ratio and waist circumference in women twins. Obes. Res. 6: 383–392. [DOI] [PubMed] [Google Scholar]
- 9.Hunt M. S., Katzmarzyk P. T., Perusse L., Rice T., Rao D. C., Bouchard C. 2002. Familial resemblance of 7-year changes in body mass and adiposity. Obes. Res. 10: 507–517. [DOI] [PubMed] [Google Scholar]
- 10.Schousboe K., Visscher P. M., Erbas B., Kyvik K. O., Hopper J. L., Henriksen J. E., Heitmann B. L., Sorensen T. I. 2004. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int. J. Obes. Relat. Metab. Disord. 28: 39–48. [DOI] [PubMed] [Google Scholar]
- 11.Lin H. F., Boden-Albala B., Juo S. H., Park N., Rundek T., Sacco R. L. 2005. Heritabilities of the metabolic syndrome and its components in the Northern Manhattan Family Study. Diabetologia. 48: 2006–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayoumi R. A., Al-Yahyaee S. A., Albarwani S. A., Rizvi S. G., Al-Hadabi S., Al-Ubaidi F. F., Al-Hinai A. T., Al-Kindi M. N., Adnan H. T., Al-Barwany H. S., et al. 2007. Heritability of determinants of the metabolic syndrome among healthy Arabs of the Oman family study. Obesity (Silver Spring). 15: 551–556. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen-Torvik L. J., Pankow J. S., Jacobs D. R., Steffen L. M., Moran A. M., Steinberger J., Sinaiko A. R. 2007. Heritability and genetic correlations of insulin sensitivity measured by the euglycaemic clamp. Diabet. Med. 24: 1286–1289. [DOI] [PubMed] [Google Scholar]
- 14.Comuzzie A. G., Blangero J., Mahaney M. C., Haffner S. M., Mitchell B. D., Stern M. P., MacCluer J. W. 1996. Genetic and environmental correlations among hormone levels and measures of body fat accumulation and topography. J. Clin. Endocrinol. Metab. 81: 597–600. [DOI] [PubMed] [Google Scholar]
- 15.Beck-Nielsen H. 1999. General characteristics of the insulin resistance syndrome: prevalence and heritability. European Group for the study of Insulin Resistance (EGIR). Drugs. 58(Suppl 1): 7–10. (discussion 75–82). [DOI] [PubMed] [Google Scholar]
- 16.Poulsen P., Kyvik K. O., Vaag A., Beck-Nielsen H. 1999. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance–a population-based twin study. Diabetologia. 42: 139–145. [DOI] [PubMed] [Google Scholar]
- 17.Nelson T. L., Vogler G. P., Pedersen N. L., Hong Y., Miles T. P. 2000. Genetic and environmental influences on body fat distribution, fasting insulin levels and CVD: are the influences shared? Twin Res. 3: 43–50. [DOI] [PubMed] [Google Scholar]
- 18.Poulsen P., Vaag A., Kyvik K., Beck-Nielsen H. 2001. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 44: 537–543. [DOI] [PubMed] [Google Scholar]
- 19.Freeman M. S., Mansfield M. W., Barrett J. H., Grant P. J. 2002. Heritability of features of the insulin resistance syndrome in a community-based study of healthy families. Diabet. Med. 19: 994–999. [DOI] [PubMed] [Google Scholar]
- 20.Wu K. D., Hsiao C. F., Ho L. T., Sheu W. H., Pei D., Chuang L. M., Curb D., Chen Y. D., Tsai H. J., Dzau V. J., et al. 2002. Clustering and heritability of insulin resistance in Chinese and Japanese hypertensive families: a Stanford-Asian Pacific Program in Hypertension and Insulin Resistance sibling study. Hypertens. Res. 25: 529–536. [DOI] [PubMed] [Google Scholar]
- 21.Henkin L., Bergman R. N., Bowden D. W., Ellsworth D. L., Haffner S. M., Langefeld C. D., Mitchell B. D., Norris J. M., Rewers M., Saad M. F., et al. 2003. Genetic epidemiology of insulin resistance and visceral adiposity. The IRAS Family Study design and methods. Ann. Epidemiol. 13: 211–217. [DOI] [PubMed] [Google Scholar]
- 22.Mills G. W., Avery P. J., McCarthy M. I., Hattersley A. T., Levy J. C., Hitman G. A., Sampson M., Walker M. 2004. Heritability estimates for beta cell function and features of the insulin resistance syndrome in UK families with an increased susceptibility to type 2 diabetes. Diabetologia. 47: 732–738. [DOI] [PubMed] [Google Scholar]
- 23.Samaras K., Nguyen T. V., Jenkins A. B., Eisman J. A., Howard G. M., Kelly P. J., Campbell L. V. 1999. Clustering of insulin resistance, total and central abdominal fat: same genes or same environment? Twin Res. 2: 218–225. [DOI] [PubMed] [Google Scholar]
- 24.Santos R. L., Zillikens M. C., Rivadeneira F. R., Pols H. A., Oostra B. A., van Duijn C. M., Aulchenko Y. S. 2006. Heritability of fasting glucose levels in a young genetically isolated population. Diabetologia. 49: 667–672. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y., Pedersen N. L., Brismar K., de Faire U. 1997. Genetic and environmental architecture of the features of the insulin-resistance syndrome. Am. J. Hum. Genet. 60: 143–152. [PMC free article] [PubMed] [Google Scholar]
- 26.Hong Y., Weisnagel S. J., Rice T., Sun G., Mandel S. A., Gu C., Rankinen T., Gagnon J., Leon A. S., Skinner J. S., et al. 2001. Familial resemblance for glucose and insulin metabolism indices derived from an intravenous glucose tolerance test in blacks and whites of the HERITAGE Family Study. Clin. Genet. 60: 22–30. [DOI] [PubMed] [Google Scholar]
- 27.Poulsen P., Levin K., Petersen I., Christensen K., Beck-Nielsen H., Vaag A. 2005. Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes. 54: 275–283. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan C. M., Futers T. S., Barrett J. H., Hudson B. I., Freeman M. S., Grant P. J. 2005. RAGE polymorphisms and the heritability of insulin resistance: the Leeds family study. Diab Vasc Dis Res. 2: 42–44. [DOI] [PubMed] [Google Scholar]
- 29.Feinleib M., Garrison R. J., Fabsitz R., Christian J. C., Hrubec Z., Borhani N. O., Kannel W. B., Rosenman R., Schwartz J. T., Wagner J. O. 1977. The NHLBI twin study of cardiovascular disease risk factors: methodology and summary of results. Am. J. Epidemiol. 106: 284–285. [DOI] [PubMed] [Google Scholar]
- 30.Bo S., Cavallo-Perin P., Scaglione L., Pagano G. 1997. Heritability of cardiovascular risk parameters in subjects with increased susceptibility to non-insulin-dependent diabetes mellitus. Acta Diabetol. 34: 280–284. [DOI] [PubMed] [Google Scholar]
- 31.Forsblom C. M., Kanninen T., Lehtovirta M., Saloranta C., Groop L. C. 1999. Heritability of albumin excretion rate in families of patients with Type II diabetes. Diabetologia. 42: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 32.Livshits G., Gerber L. M. 2001. Familial factors of blood pressure and adiposity covariation. Hypertension. 37: 928–935. [DOI] [PubMed] [Google Scholar]
- 33.Iliadou A., Lichtenstein P., Morgenstern R., Forsberg L., Svensson R., de Faire U., Martin N. G., Pedersen N. L. 2002. Repeated blood pressure measurements in a sample of Swedish twins: heritabilities and associations with polymorphisms in the renin-angiotensin-aldosterone system. J. Hypertens. 20: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 34.Snieder H., Harshfield G. A., Treiber F. A. 2003. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 41: 1196–1201. [DOI] [PubMed] [Google Scholar]
- 35.Fava C., Burri P., Almgren P., Groop L., Hulthen U. L., Melander O. 2004. Heritability of ambulatory and office blood pressure phenotypes in Swedish families. J. Hypertens. 22: 1717–1721. [DOI] [PubMed] [Google Scholar]
- 36.Kupper N., Willemsen G., Riese H., Posthuma D., Boomsma D. I., de Geus E. J. 2005. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 45: 80–85. [DOI] [PubMed] [Google Scholar]
- 37.van Rijn M. J., Schut A. F., Aulchenko Y. S., Deinum J., Sayed-Tabatabaei F. A., Yazdanpanah M., Isaacs A., Axenovich T. I., Zorkoltseva I. V., Zillikens M. C., et al. 2007. Heritability of blood pressure traits and the genetic contribution to blood pressure variance explained by four blood-pressure-related genes. J. Hypertens. 25: 565–570. [DOI] [PubMed] [Google Scholar]
- 38.Wessel J., Moratorio G., Rao F., Mahata M., Zhang L., Greene W., Rana B. K., Kennedy B. P., Khandrika S., Huang P., et al. 2007. C-reactive protein, an ‘intermediate phenotype’ for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J. Hypertens. 25: 329–343. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell D. L., Heller R. F., Roberts D. C., Allen J. R., Knapp J. C., Steele P. L., Silove D. 1988. Twin study of genetic and environmental effects on lipid levels. Genet. Epidemiol. 5: 323–341. [DOI] [PubMed] [Google Scholar]
- 40.Heller D. A., de Faire U., Pedersen N. L., Dahlen G., McClearn G. E. 1993. Genetic and environmental influences on serum lipid levels in twins. N. Engl. J. Med. 328: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 41.Beekman M., Heijmans B. T., Martin N. G., Pedersen N. L., Whitfield J. B., DeFaire U., van Baal G. C., Snieder H., Vogler G. P., Slagboom P. E., et al. 2002. Heritabilities of apolipoprotein and lipid levels in three countries. Twin Res. 5: 87–97. [DOI] [PubMed] [Google Scholar]
- 42.Goode E. L., Cherny S. S., Christian J. C., Jarvik G. P., de Andrade M. 2007. Heritability of longitudinal measures of body mass index and lipid and lipoprotein levels in aging twins. Twin Res. Hum. Genet. 10: 703–711. [DOI] [PubMed] [Google Scholar]
- 43.Allison D. B., Kaprio J., Korkeila M., Koskenvuo M., Neale M. C., Hayakawa K. 1996. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int. J. Obes. Relat. Metab. Disord. 20: 501–506. [PubMed] [Google Scholar]
- 44.Kervinen K., Kaprio J., Koskenvuo M., Juntunen J., Kesaniemi Y. A. 1998. Serum lipids and apolipoprotein E phenotypes in identical twins reared apart. Clin. Genet. 53: 191–199. [DOI] [PubMed] [Google Scholar]
- 45.Carmelli D., Cardon L. R., Fabsitz R. 1994. Clustering of hypertension, diabetes, and obesity in adult male twins: same genes or same environments? Am. J. Hum. Genet. 55: 566–573. [PMC free article] [PubMed] [Google Scholar]
- 46.McCaffery J. M., Pogue-Geile M. F., Debski T. T., Manuck S. B. 1999. Genetic and environmental causes of covariation among blood pressure, body mass and serum lipids during young adulthood: a twin study. J. Hypertens. 17: 1677–1685. [DOI] [PubMed] [Google Scholar]
- 47.Neale M. C., Cardon L. R. 1992. Methodology for genetic studies of twins and families. Kluwer Academic Publishers, Boston. [Google Scholar]
- 47a.Tellegen A., Lykken D. T., Bouchard T. J., Wilcox K. J., Segal N. L., Rich S. 1988. Personality similarity in twins reared apart and together. J. Pers. Soc. Psychol. 54: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 48.McNamara J. R., Schaefer E. J. 1987. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin. Chim. Acta. 166: 1–8. [DOI] [PubMed] [Google Scholar]
- 49.Warnick G. R., Benderson J., Albers J. J. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 28: 1379–1388. [PubMed] [Google Scholar]
- 50.Henry J. B. 1984. Todd, Sanford, Davidson. Clinical diagnosis and management by laboratory methods, 17th ed. WB Saunders Company, Philadelphia, PA. [Google Scholar]
- 51.Morgan C. R., Lazarow A. 1963. Immunoassay of insulin: two antibody system. Plasma insulin levels in normal, subdiabetic and diabetic rats. In Diabetes 115–126. [Google Scholar]
- 52.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 53.SAS Institute Inc. 2004. SAS/STAT 9.1 User's Guide SAS Institute Inc, Cary, NC. [Google Scholar]
- 54.SPSS Inc. 2006. SPSS 15.0 Brief Guide SPSS, Inc., Chicago, IL. [Google Scholar]
- 55.Neale M. C. 2003. Twin studies: software and algorithms. In Encyclopedia of the human genome Cooper D. N., editor Macmillan Publishers Ltd, Nature Publishing Group. [Google Scholar]
- 56.Neale M. C. 1998. Twin analysis. In Encyclopedia of biostatistics Armitage P., Colton T., John Wiley, New York. [Google Scholar]
- 57.Neale M. C., Boker S. M., Xie G., Maes H. H. 2003. Mx: Statistical modeling, 6th edition Virginia Commonwealth University Department of Psychiatry, VCU, Richmond, VA. [Google Scholar]
- 58.Sham P. C. 1997. Statistics in human genetics. John Wiley and Sons, New York, NY. [Google Scholar]
- 59.Dominicus A., Skrondal A., Gjessing H. K., Pedersen N. L., Palmgren J. 2006. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav. Genet. 36: 331–340. [DOI] [PubMed] [Google Scholar]
- 60.Despres J. P. 2007. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 6: 51–59. [DOI] [PubMed] [Google Scholar]
- 61.Brewer H. B., Jr. 1999. Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am. J. Cardiol. 83: 3F–12F. [DOI] [PubMed] [Google Scholar]
- 62.Arnett D. K., Baird A. E., Barkley R. A., Basson C. T., Boerwinkle E., Ganesh S. K., Herrington D. M., Hong Y., Jaquish C., McDermott D. A., O'Donnell C. J., American Heart Association Council on Epidemiology and Prevention, American Heart Association Stroke Council, and Functional Genomics and Translational Biology Interdisciplinary Working Group. 2007. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 115: 2878–2901. [DOI] [PubMed] [Google Scholar]
- 63.Ordovas J. M. 2003. Cardiovascular disease genetics: a long and winding road. Curr. Opin. Lipidol. 14: 47–54. [DOI] [PubMed] [Google Scholar]
- 64.Masson L. F., McNeill G. 2005. The effect of genetic variation on the lipid response to dietary change: recent findings. Curr. Opin. Lipidol. 16: 61–67. [DOI] [PubMed] [Google Scholar]
- 65.Ichihara S., Yamada Y. 2008. Genetic factors for human obesity. Cell. Mol. Life Sci. 65: 1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McIntyre E. A., Walker M. 2002. Genetics of type 2 diabetes and insulin resistance: knowledge from human studies. Clin. Endocrinol. (Oxf.). 57: 303–311. [DOI] [PubMed] [Google Scholar]
- 67.Cannon C. P. 2007. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone. 8: 11–28. [DOI] [PubMed] [Google Scholar]
- 68.Grundy S. M., Denke M. A. 1990. Dietary influences on serum lipids and lipoproteins. J. Lipid Res. 31: 1149–1172. [PubMed] [Google Scholar]
- 69.Gulanick M., Cofer L. A. 2000. Coronary risk factors: influences on the lipid profile. J. Cardiovasc. Nurs. 14: 16–28. [DOI] [PubMed] [Google Scholar]
- 70.Birch L. L., Davison K. K. 2001. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr. Clin. North Am. 48: 893–907. [DOI] [PubMed] [Google Scholar]
- 71.Simonen R. L., Perusse L., Rankinen T., Rice T., Rao D. C., Bouchard C. 2002. Familial aggregation of physical activity levels in the Quebec Family Study. Med. Sci. Sports Exerc. 34: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 72.Poulsen P., Vaag A. 2003. The impact of genes and pre- and postnatal environment on the metabolic syndrome. Evidence from twin studies. Panminerva Med. 45: 109–115. [PubMed] [Google Scholar]
- 73.Allison D. B., Paultre F., Heymsfield S. B., Pi-Sunyer F. X. 1995. Is the intra-uterine period really a critical period for the development of adiposity? Int. J. Obes. Relat. Metab. Disord. 19: 397–402. [PubMed] [Google Scholar]
- 74.Loos R. J., Beunen G., Fagard R., Derom C., Vlietinck R. 2002. Birth weight and body composition in young women: a prospective twin study. Am. J. Clin. Nutr. 75: 676–682. [DOI] [PubMed] [Google Scholar]
- 75.Qiao Q., Gao W., Zhang L., Nyamdorj R., Tuomilehto J. 2007. Metabolic syndrome and cardiovascular disease. Ann. Clin. Biochem. 44: 232–263. [DOI] [PubMed] [Google Scholar]
- 76.Timar O., Sestier F., Levy E. 2000. Metabolic syndrome X: a review. Can. J. Cardiol. 16: 779–789. [PubMed] [Google Scholar]
- 77.Reaven G. M. 2005. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 47: 201–210. [PubMed] [Google Scholar]
- 78.Koh-Banerjee P., Chu N. F., Spiegelman D., Rosner B., Colditz G., Willett W., Rimm E. 2003. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am. J. Clin. Nutr. 78: 719–727. [DOI] [PubMed] [Google Scholar]
- 79.Sakai Y., Yamaji T., Tabata S., Ogawa S., Yamaguchi K., Mineshita M., Mizoue T., Kono S. 2006. Relation of alcohol use and smoking to glucose tolerance status in Japanese men. Diabetes Res. Clin. Pract. 73: 83–88. [DOI] [PubMed] [Google Scholar]
- 80.Otsuki M., Kasayama S., Morita S., Asanuma N., Saito H., Mukai M., Koga M. 2007. Menopause, but not age, is an independent risk factor for fasting plasma glucose levels in nondiabetic women. Menopause. 14: 404–407. [DOI] [PubMed] [Google Scholar]