Abstract
Electronegative LDL, a charge-modified LDL (cm-LDL) subfraction that is more negatively charged than normal LDL, has been shown to be inflammatory. We previously showed that pravastatin and simvastatin reduced the electronegative LDL subfraction, fast-migrating LDL (fLDL), as analyzed by capillary isotachophoresis (cITP). The present study examined the effects of rosuvastatin on the more electronegative LDL subfraction, very-fast-migrating LDL (vfLDL), and small, dense charge-modified LDL (sd-cm-LDL) subfractions. Patients with hypercholesterolemia or those who were being treated with statins (n = 81) were treated with or switched to 2.5 mg/d rosuvastatin for 3 months. Rosuvastatin treatment effectively reduced cITP cm-LDL subfractions of LDL (vfLDL and fLDL) or sdLDL (sd-vfLDL and sd-fLDL), which were closely related to each other but were different from the normal subfraction of LDL [slow-migrating LDL (sLDL)] or sdLDL (sd-sLDL) in their relation to the levels of remnant-like particle cholesterol (RLP-C), apolipoprotein (apo) C-II, and apoE. The percent changes in cm-LDL or sd-cm-LDL caused by rosuvastatin were correlated with those in the particle concentrations of LDL or sdLDL measured as LDL-apoB or sdLDL-apoB and the levels of HDL-C, RLP-C, apoC-II, and apoE. In conclusion, rosuvastatin effectively reduced both the vfLDL subfraction and sd-cm-LDL subfractions as analyzed by cITP.
Keywords: LDL particle concentrations, hypercholesterolemia, small dense LDL, statins
An increased low-density lipoprotein cholesterol (LDL-C) level is an established risk factor for cardiovascular disease. Statins (3-hydroxy-3-methylglutaryl CoA reductase inhibitors) effectively reduce LDL-C levels and the risk of cardiovascular events (1). Statins also diminish inflammation as indicated by a reduction of C-reactive protein (CRP) (2, 3). However, the mechanism by which statins exert their antiinflammatory action is not fully understood.
Modified forms of LDL that exist in human plasma, including oxidized LDL (OxLDL), small, dense LDL (sdLDL), and glycated LDL, are considered to be atherogenic. Increased OxLDL and sdLDL levels are associated with an increased risk of coronary heart disease (CHD) (4, 5). Various forms of modified LDL share a common characteristic in that they show increased electrophoretic mobility compared with normal LDL. An LDL subfraction that is more negatively charged than normal LDL has been separated by anion-exchange chromatography and is defined as electronegative LDL [LDL(-)] (6). The proportion of LDL(-) in total LDL has been shown to be increased in patients with hypercholesterolemia (HC), hypertriglyceridemia (HTG), diabetes mellitus (DM), and CHD (7–10).
LDL(-) separated from either normal subjects or patients with HC has been consistently shown to be inflammatory (8, 11–13). LDL(-) in human plasma is formed from multiple origins (6). The inflammatory marker lipoprotein-associated phospholipase A2 [also called platelet-activating factor acetylhydrolase (PAF-AH)] has been shown to be associated with LDL(-) (14). The clinical importance of LDL(-) is still unknown, as the chromatography technique requires the isolation of LDL from plasma for LDL(-) analysis and thus is labor intensive and time-consuming (7, 8, 13), which limits its use as a routine analytical technique. However, it may be important to reduce plasma levels of LDL(-), an atherogenic lipoprotein that resides in plasma longer than normal LDL due to its lower affinity for LDL receptors (15).
Capillary isotachophoresis (cITP) is an analytical technique for analyzing charge-based lipoprotein subfractions directly in plasma (16–18). It is advantageous to analyze LDL subfractions in the presence of HDL and plasma proteins because they protect LDL from artificial modification. The cITP technique separates plasma lipoproteins into three high-density lipoprotein (HDL) subfractions [fast-, intermediate-, and slow-migrating HDL (fHDL, iHDL, and sHDL)], two triglyceride (TG)-rich lipoprotein (TRL) subfractions [fast- and slow-migrating TRL (fTRL and sTRL)], and two major LDL subfractions [fast- and slow-migrating LDL (fLDL and sLDL)] according to their electrophoretic mobilities (18, 19). The levels of cITP LDL subfractions can be determined as peak areas relative to that of an internal marker (18–22).
We have previously shown that cITP fLDL is an electronegative LDL subfraction because fLDL is increased and sLDL is decreased when LDL is mildly oxidized (19). Our recent study (23) showed that cITP fLDL levels were elevated in patients with HC and risk factors of CHD and were effectively reduced by low-dose pravastatin and simvastatin (The SPECIAL study).
We previously found that a new electronegative LDL subfraction that has faster electrophoretic mobility than cITP fLDL was increased when LDL was moderately oxidized, and defined this more electronegative LDL subfraction as very-fast-migrating LDL (vfLDL) (19). We observed drastically increased cITP vfLDL in patients with extremely low levels of HDL-C and low levels of LDL-C (19). The cITP vfLDL comigrates with cITP sTRL in human plasma and therefore needs to be analyzed in plasma depleted of TRL. It has not yet been determined whether the more electronegative LDL subfraction (cITP vfLDL) is increased in patients with HC and could be effectively reduced by statin treatment.
In the present study, we examined the effects of rosuvastatin on cm-LDL subfractions of LDL (vfLDL and fLDL) and sdLDL (sd-vfLDL and sd-fLDL) as analyzed by cITP and the relation of the effects of rosuvastatin on LDL particle concentrations and HDL-C levels in HC patients with CHD or risk factors for CHD.
METHODS
Patients
Eighty-one outpatients (32 men and 49 women) with CHD or risk factors for CHD and HC or who were being treated with statins were enrolled in the study. The inclusion criteria were patients who had LDL-C levels greater than 140 mg/dl and more than one CHD risk factor, had LDL-C levels greater than 120 mg/dl and more than three CHD risk factors, or had received pravastatin (5–10 mg/d), simvastatin (5 mg/d), fluvastatin (20 mg/d), atorvastatin (5–20 mg/d), or pitavastatin (1 mg/d) for more than 3 months. Age (men ≥45 years; women ≥55 years), hypertension (HT), history of CHD, DM, and smoking were considered as CHD risk factors. Patients with a history of myocardial infarction or stable angina were considered to have CHD. Patients who were receiving antihypertensive therapy or who had systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg were considered to have hypertension. Patients who were receiving antidiabetic therapy or who had fasting blood glucose concentrations ≥126 mg/dl and/or 2-h glucose concentration ≥200 mg/dl after an oral glucose tolerance test were considered to have DM. Current smokers were considered to have smoking as a CHD risk factor. Men had a higher prevalence of CHD (59.4% vs. 26.5%) and a higher percentage of patients with ≥3 risk factors of CHD (46.9% vs. 22.4%) than women. Exclusion criteria included: a history of allergy with statin treatment; liver diseases, including cirrhosis; severe renal disease complications, including diabetic nephropathy and renal failure; acute myocardial infarction; unstable angina; LDL apheresis; receiving nicotinic acid; and immunosuppressant and hormone therapy. The study protocol was approved by the Ethics Committee of Fukuoka University Hospital, and written informed consent was obtained from all patients.
All of the patients were treated with low-dose (2.5 mg/d) rosuvastatin for 3 months, and patients who were receiving other statins (75.0% of the men and 87.8% of the women) were switched to treatment with 2.5 mg rosuvastatin. Overnight fasting blood was drawn at baseline and after treatment with rosuvastatin for 3 months. Plasma containing 2 mM EDTA was separated for the measurement of lipid and lipoproteins.
Separation of plasma d>1.006 g/ml and d>1.040 g/ml fractions by quantitative ultracentrifugation
Plasma d>1.006 g/ml fraction, which was depleted of very-low-density lipoprotein (VLDL) and contained classically defined LDL particles, i.e., intermediate-density lipoprotein (d 1.006–1.019 g/ml) and the bulk of LDL (d 1.019–1.063 g/ml) (24), and d>1.040 g/ml fraction (depleted of TRL and large, buoyant LDL) were separated by quantitative ultracentrifugation for measurement of the LDL and sdLDL compositions and cITP LDL and sdLDL subfractions, as described previously (19).
Measurement of charge-based LDL subfractions by cITP
Lipoprotein subfractions were analyzed in EDTA plasma by cITP on a Beckman P/ACE MDQ system (Beckman-Coulter Inc., Tokyo, Japan) as described previously (18, 19, 22). cITP LDL and sdLDL subfractions were analyzed in plasma d>1.006 g/ml and d>1.040 g/ml fractions, respectively (19). Six μl of plasma or plasma fraction was used for routine analysis. Plasma lipoproteins were prestained with a lipophilic dye, NBD C6-ceramide (Molecular Probes, Inc., Eugene, OR), before separation. Levels of cITP lipoprotein subfractions were expressed as the peak area relative to that of an internal marker, 5-carboxy-fluorescein (18, 20–22).
Measurement of plasma levels of lipids, lipoproteins, and apolipoproteins, activities of lipoprotein-associated enzymes, the sizes of HDL and LDL, and CRP concentrations
Plasma levels of free cholesterol (FC), TG, phospholipids (PL), HDL-C, and LDL-C were measured by enzymatic methods as described previously (19). Plasma remnant-like particle cholesterol (RLP-C) levels were measured using a MetaboLead RemL-C reagent kit for an autoanalyzer, kindly provided by Kyowa Medex, Tokyo, Japan. Plasma levels of apolipoprotein (apo)A-I, apoB, apoC-II, and apoE were measured by turbidimetric immunoassay methods as described previously (22).
Serum basal paraoxonase (PON1) activity was measured on an autoanalyzer (Hitachi 7600-020S; Hitachi High-Technologies, Tokyo, Japan) with paraoxon as a substrate, as described previously (25). Plasma PAF-AH activity was measured using an Azwell Auto PAF-AH reagent kit (Alfresa, Osaka, Japan) as described previously (26).
ApoB-depleted plasma was separated using the phosphotungstate-Mg2+ precipitation method, as described previously (27), for measurement of HDL-FC, HDL-PL, and HDL-associated PAF-AH (HDL-PAF-AH) activity. LDL-associated PAF-AH (LDL-PAF-AH) activity was calculated from PAF-AH activities in whole plasma and apoB-depleted plasma (26).
Particle concentrations of LDL and sdLDL were measured as apoB levels in plasma d>1.006 g/ml and d>1.040 g/ml fractions. sdLDL-C levels were measured as LDL-C levels in plasma d>1.040 g/ml fraction. Levels of LDL-FC and LDL-PL or levels of sdLDL-FC and sdLDL-PL were calculated from FC and PL levels measured in plasma d>1.006 g/ml fraction or d>1.040 g/ml fraction and apoB-depleted plasma.
HDL and LDL particle sizes were measured by composite gradient gel electrophoresis as described previously (19, 28–30).
Serum CRP concentrations were measured in a ‘high-sensitivity’ assay using particle-enhanced immunonephelometry with N-latex CRPII (Dade Behring Marburg GmbH, Marburg, Germany).
Statistical analysis
All of the data analyses were performed using the SAS (Statistical Analysis System) Software Package (Ver. 9.1.3, SAS Institute Inc.., Cary, NC) at Fukuoka University (Fukuoka, Japan). The normality of the distribution of continuous variables was examined by the Shapiro-Wilk test. Changes in continuous variables during the study period were examined by the Wilcoxon signed-rank test. Data are presented as mean ± standard error (SE) and median values are given for percentage changes. The Spearman correlation was used to examine the correlation among continuous variables. Significance was considered to be a P value of less than 0.05 unless indicated otherwise.
RESULTS
Effects of rosuvastatin on the compositions of HDL, LDL, and sdLDL and plasma levels of TG, RLP-C, apolipoproteins, and inflammatory markers
As shown in Table 1, treatment with 2.5 mg rosuvastatin for 3 months increased plasma levels of HDL-C, HDL-FC, HDL-PL, apoA-I, and HDL-PAF-AH activity in all of the patients without significantly affecting serum PON1 activity or the size of HDL. Rosuvastatin decreased plasma levels of LDL-C, LDL-FC, LDL-PL, and LDL-apoB, LDL-PAF-AH activity, and levels of sdLDL-C, sdLDL-FC, sdLDL-PL, and sdLDL-apoB without affecting the size of LDL. Rosuvastatin also significantly decreased the ratio of LDL-C to HDL-C (LDL-C/HDL-C), plasma levels of TG, RLP-C, apoB, apoC-II, and apoE, and plasma PAF-AH activity without significantly affecting serum CRP concentrations in all of the patients (Table 1). Therefore, rosuvastatin treatment increased lipid and protein contents in HDL and decreased those in LDL and sdLDL. Particle concentrations of LDL and sdLDL, measured as LDL-apoB and sdLDL-apoB concentrations, were reduced similarly in men and women (LDL-apoB, −17% and −16%; sdLDL-apoB, −21% and −19%).
TABLE 1.
Changes in the compositions of HDL, LDL, and sdLDL, plasma levels of TG, RLP-C, and apolipoproteins, serum CRP concentrations, and activities of plasma and lipoprotein-associated PAF-AH activity
| Period of treatment | Overall |
Women |
Men |
||||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Median % Δ | Mean ± SE | Median % Δ | Mean ± SE | Median % Δ | ||
| HDL-C (mg/dl) | Baseline | 59 ± 1 | 62 ± 2 | 54 ± 2 | |||
| 3 Mo. | 62 ± 2 | 5%* | 64 ± 2 | 2% | 58 ± 2 | 9%* | |
| HDL-FC (mg/dl) | Baseline | 13 ± 0 | 14 ± 1 | 12 ± 1 | |||
| 3 Mo. | 26 ± 1 | 94%* | 26 ± 1 | 81%* | 26 ± 2 | 111%* | |
| HDL-PL (mg/dl) | Baseline | 103 ± 2 | 108 ± 3 | 96 ± 4 | |||
| 3 Mo. | 113 ± 2 | 8%* | 116 ± 3 | 7%* | 107 ± 4 | 12%* | |
| ApoA-I (mg/dl) | Baseline | 144 ± 2 | 150 ± 3 | 135 ± 4 | |||
| 3 Mo. | 149 ± 2 | 3%* | 153 ± 3 | 3% | 142 ± 4 | 7%* | |
| PON1 activity (IU/L) | Baseline | 329 ± 12 | 331 ± 15 | 326 ± 20 | |||
| 3 Mo. | 331 ± 12 | 2% | 333 ± 16 | 0.5% | 328 ± 19 | 2% | |
| HDL-PAF-AH activity (IU/L) | Baseline | 35 ± 1 | 35 ± 2 | 34 ± 2 | |||
| 3 Mo. | 38 ± 1 | 8%* | 39 ± 2 | 8%* | 38 ± 2 | 9%* | |
| HDL size (nm) | Baseline | 9.20 ± 0.06 | 9.28 ± 0.07 | 9.08 ± 0.10 | |||
| 3 Mo. | 9.24 ± 0.05 | 0.3% | 9.29 ± 0.07 | 0.0% | 9.15 ± 0.09 | 0.8% | |
| LDL-C (mg/dl) | Baseline | 132 ± 4 | 134 ± 5 | 129 ± 6 | |||
| 3 Mo. | 105 ± 3 | −18%* | 109 ± 4 | −18%* | 99 ± 5 | −20%* | |
| LDL-FC (mg/dl) | Baseline | 90 ± 2 | 92 ± 2 | 88 ± 3 | |||
| 3 Mo. | 66 ± 2 | −28%* | 69 ± 2 | −25%* | 61 ± 3 | −32%* | |
| LDL-PL (mg/dl) | Baseline | 95 ± 2 | 98 ± 3 | 92 ± 3 | |||
| 3 Mo. | 76 ± 2 | −20%* | 80 ± 3 | −19%* | 71 ± 3 | −22%* | |
| LDL-apoB (mg/dl) | Baseline | 94 ± 2 | 95 ± 3 | 92 ± 3 | |||
| 3 Mo. | 78 ± 2 | −16%* | 81 ± 3 | −16%* | 73 ± 3 | −17%* | |
| LDL-PAF-AH activity (IU/L) | Baseline | 335 ± 17 | 327 ± 21 | 347 ± 27 | |||
| 3 Mo. | 265 ± 14 | −22%* | 267 ± 19 | −19%* | 263 ± 20 | −24%* | |
| LDL size (nm) | Baseline | 26.32 ± 0.06 | 26.38 ± 0.07 | 26.22 ± 0.10 | |||
| 3 Mo. | 26.29 ± 0.05 | −0.2% | 26.36 ± 0.07 | −0.2% | 26.18 ± 0.08 | −0.1% | |
| sdLDL-C (mg/dl) | Baseline | 61 ± 2 | 60 ± 3 | 62 ± 3 | |||
| 3 Mo. | 46 ± 2 | −26%* | 47 ± 3 | −25%* | 45 ± 2 | −29%* | |
| sdLDL-FC (mg/dl) | Baseline | 23 ± 1 | 23 ± 1 | 23 ± 1 | |||
| 3 Mo. | 6 ± 1 | −70%* | 7 ± 1 | −68%* | 5 ± 2 | −77%* | |
| sdLDL-PL (mg/dl) | Baseline | 52 ± 2 | 52 ± 2 | 51 ± 2 | |||
| 3 Mo. | 39 ± 1 | −23%* | 41 ± 2 | −22%* | 37 ± 2 | −24%* | |
| sdLDL-apoB (mg/dl) | Baseline | 53 ± 2 | 53 ± 3 | 54 ± 2 | |||
| 3 Mo. | 43 ± 2 | −20%* | 44 ± 2 | −19%* | 41 ± 2 | −21%* | |
| LDL-C/HDL-C | Baseline | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.6 ± 0.2 | |||
| 3 Mo. | 1.8 ± 0.1 | −21%* | 1.8 ± 0.1 | −18%* | 1.8 ± 0.2 | −22%* | |
| TG (mg/dl) | Baseline | 121 ± 7 | 129 ± 10 | 109 ± 9 | |||
| 3 Mo. | 113 ± 7 | −10%* | 114 ± 8 | −13%* | 112 ± 10 | −5% | |
| RLP-C (mg/dl) | Baseline | 6.8 ± 0.5 | 7.3 ± 0.7 | 6.0 ± 0.5 | |||
| 3 Mo. | 5.4 ± 0.4 | −20%* | 5.6 ± 0.5 | −26%* | 5.2 ± 0.6 | −20%* | |
| ApoB (mg/dl) | Baseline | 99 ± 2 | 101 ± 4 | 96 ± 3 | |||
| 3 Mo. | 83 ± 2 | −15%* | 86 ± 3 | −15%* | 78 ± 3 | −16%* | |
| ApoC-II (mg/dl) | Baseline | 5.2 ± 0.2 | 5.6 ± 0.3 | 4.7 ± 0.2 | |||
| 3 Mo. | 4.8 ± 0.2 | −6%* | 5.0 ± 0.3 | −5%* | 4.5 ± 0.2 | −9% | |
| ApoE (mg/dl) | Baseline | 4.5 ± 0.1 | 4.8 ± 0.2 | 4.1 ± 0.1 | |||
| 3 Mo. | 4.3 ± 0.1 | −7%* | 4.5 ± 0.1 | −7%* | 3.9 ± 0.1 | −9%* | |
| CRP (mg/dl) | Baseline | 0.12 ± 0.03 | 0.08 ± 0.01 | 0.19 ± 0.08 | |||
| 3 Mo. | 0.09 ± 0.01 | −3% | 0.08 ± 0.01 | 2% | 0.09 ± 0.02 | −21%* | |
| Plasma PAF-AH activity (IU/L) | Baseline | 366 ± 18 | 354 ± 23 | 382 ± 28 | |||
| 3 Mo. | 300 ± 15 | −19%* | 300 ± 21 | −17%* | 300 ± 21 | −19%* | |
* P < 0.05, significant changes during the study as assessed by the Wilcoxon signed-rank test.
Distribution of charge-based LDL and sdLDL subfractions as analyzed by cITP in HC patients with similar LDL-C and sdLDL-C levels
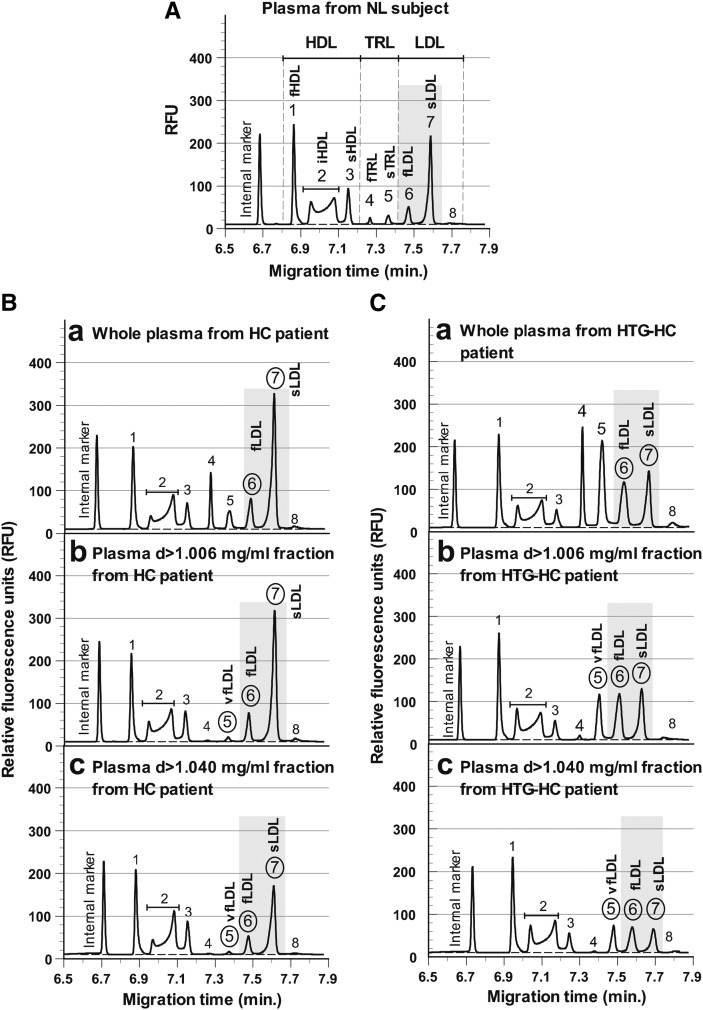
Figure 1 shows typical lipoprotein subfractions in plasma from a normolipidemic (NL) volunteer subject (TG, HDL-C, and LDL-C, 49, 60, and 91 mg/dl, respectively), a patient with HC (139, 51, and 146 mg/dl), and a diabetic patient with HTG and HC (355, 54, and 147 mg/dl), as analyzed by cITP. As shown, the HC patient (Fig. 1B.a) and the HTG-HC patient (Fig. 1C.a) had increased cITP fLDL (peak 6) in plasma compared with the NL subject (Fig. 1A), and the HTG patient had apparently increased cITP fTRL and sTRL (peaks 4 and 5) compared with the HC patient, as expected. However, although the HC and HTG-HC patients had similar LDL-C levels (146 and 147 mg/dl, respectively), they had apparently different distributions of cITP LDL subfractions (Figs. 1B.b, C.b). The cITP vfLDL (peak 5) was the minor LDL subfraction and sLDL (peak 7) was the major LDL subfraction in plasma d>1.006 g/ml fraction from the HC patient (Fig. 1B.b), but cITP vfLDL was drastically increased and the three cITP LDL subfractions, vfLDL, fLDL, and sLDL, were evenly distributed in plasma d>1.006 g/ml fraction from the HTG-HC patient (Fig. 1C.b).
Fig. 1.
A: Typical plasma lipoprotein subfractions as characterized by capillary isotachophoresis (cITP) in a normolipidemic subject (TG, HDL-C, and LDL-C: 49, 60, and 91 mg/dl, respectively). B and C: cITP lipoprotein subfractions in a patient with hypercholesterolemia (HC) (TG, HDL-C, and LDL-C: 139, 51, and 146 mg/dl, respectively) and a patient with both hypertriglyceridemia (HTG) and HC (TG, HDL-C, and LDL-C: 355, 54, and 147 mg/dl, respectively) in whole plasma (a) and plasma d>1.006 g/ml (b) and d>1.040 g/ml (c) fractions. Peaks 1-3, fast-, intermediate-, and slow-migrating HDL (fHDL, iHDL, and sHDL) subfractions ; peaks 4 and 5, fast- and slow-migrating TRL (fTRL and sTRL) subfractions; peak 5 in b and c, very-fast-migrating LDL (vfLDL) subfraction; peaks 6 and 7, fast- and slow-migrating LDL (fLDL and sLDL) subfractions; peak 8, a minor LDL subfraction.
The distributions of cITP sdLDL subfractions as analyzed in plasma d>1.040 g/ml fraction were similar to those of LDL subfractions in the same patients except that the proportion of sd-fLDL was greater than that of fLDL ( ). However, the distributions of cITP sdLDL subfractions in the HC and HTG-HC patients (Figs. 1B.c, C.c) were drastically different despite similar sdLDL-C levels (67 and 74 mg/dl, respectively).
Therefore, the levels of cITP vfLDL and fLDL subfractions and cITP sd-vfLDL and sd-fLDL could not be predicted based on LDL-C and sdLDL-C levels.
Effects of rosuvastatin on charge-based LDL and sdLDL subfractions as analyzed by cITP
In all of the patients, the proportions of cITP vfLDL and fLDL in total LDL as measured in plasma d>1.006 g/ml fraction were 8.4 ± 0.6% and 25.0 ± 0.8%, respectively. As shown in Table 2, rosuvastatin treatment significantly decreased cITP fLDL and sLDL subfractions in whole plasma, vfLDL, fLDL, and sLDL subfractions in plasma d>1.006 g/ml fraction, and sd-vfLDL, sd-fLDL, and small, dense, slow-migrating LDL (sd-sLDL) subfractions in both men and women. The proportion of fLDL or sd-fLDL was not significantly affected (data not shown). Rosuvastatin caused similar reductions in vfLDL and fLDL subfractions (−18% and −22%) and reductions in sd-vfLDL and sd-fLDL subfractions (−24% and −20%) in all of the patients.
TABLE 2.
Changes in cITP LDL subfractions measured in whole plasma and plasma d>1.006 g/ml fraction and cITP sdLDL subfraction measured in d>1.040 g/ml fraction
| Period of treatment | Overall |
Women |
Men |
||||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Median %Δ | Mean ± SE | Median %Δ | Mean ± SE | Median %Δ | ||
| Whole plasma | |||||||
| cITP sTRL/vfLDL | Baseline | 0.52 ± 0.04 | 0.57 ± 0.05 | 0.44 ± 0.04 | |||
| 3 Mo. | 0.44 ± 0.03 | −11%* | 0.45 ± 0.04 | −21%* | 0.41 ± 0.04 | −8% | |
| cITP fLDL | Baseline | 0.80 ± 0.04 | 0.84 ± 0.05 | 0.75 ± 0.05 | |||
| 3 Mo. | 0.67 ± 0.03 | −17%* | 0.70 ± 0.04 | −14%* | 0.63 ± 0.05 | −17%* | |
| cITP sLDL | Baseline | 1.85 ± 0.06 | 1.80 ± 0.06 | 1.93 ± 0.11 | |||
| 3 Mo. | 1.58 ± 0.05 | −16%* | 1.58 ± 0.05 | −14%* | 1.57 ± 0.09 | −17%* | |
| Plasma d>1.006 g/ml fractioncITP vfLDL | |||||||
| cITP vfLDL | Baseline | 0.25 ± 0.02 | 0.28 ± 0.03 | 0.20 ± 0.03 | |||
| 3 Mo. | 0.18 ± 0.01 | −18%* | 0.20 ± 0.02 | −17%* | 0.15 ± 0.02 | −23%* | |
| cITP fLDL | Baseline | 0.72 ± 0.03 | 0.75 ± 0.05 | 0.66 ± 0.04 | |||
| 3 Mo. | 0.56 ± 0.03 | −22%* | 0.59 ± 0.04 | −19%* | 0.50 ± 0.04 | −25%* | |
| cITP sLDL | Baseline | 1.87 ± 0.06 | 1.80 ± 0.06 | 1.97 ± 0.11 | |||
| 3 Mo. | 1.58 ± 0.05 | −17%* | 1.58 ± 0.05 | −15%* | 1.57 ± 0.09 | −20%* | |
| Plasma d>1.040 g/ml fractioncITP sd-vfLDL | |||||||
| cITP sd-vfLDL | Baseline | 0.17 ± 0.02 | 0.18 ± 0.02 | 0.16 ± 0.02 | |||
| 3 Mo. | 0.12 ± 0.01 | −24%* | 0.13 ± 0.02 | −22%* | 0.11 ± 0.01 | −26%* | |
| cITP sd-fLDL | Baseline | 0.47 ± 0.03 | 0.46 ± 0.04 | 0.47 ± 0.04 | |||
| 3 Mo. | 0.36 ± 0.02 | −20%* | 0.36 ± 0.03 | −22%* | 0.34 ± 0.03 | −20%* | |
| cITP sd-sLDL | Baseline | 1.13 ± 0.04 | 1.04 ± 0.06 | 1.26 ± 0.07 | |||
| 3 Mo. | 0.90 ± 0.04 | −20%* | 0.88 ± 0.04 | −15%* | 0.95 ± 0.06 | −25%* | |
* P < 0.05, significant changes during the study as assessed by the Wilcoxon signed-rank test.
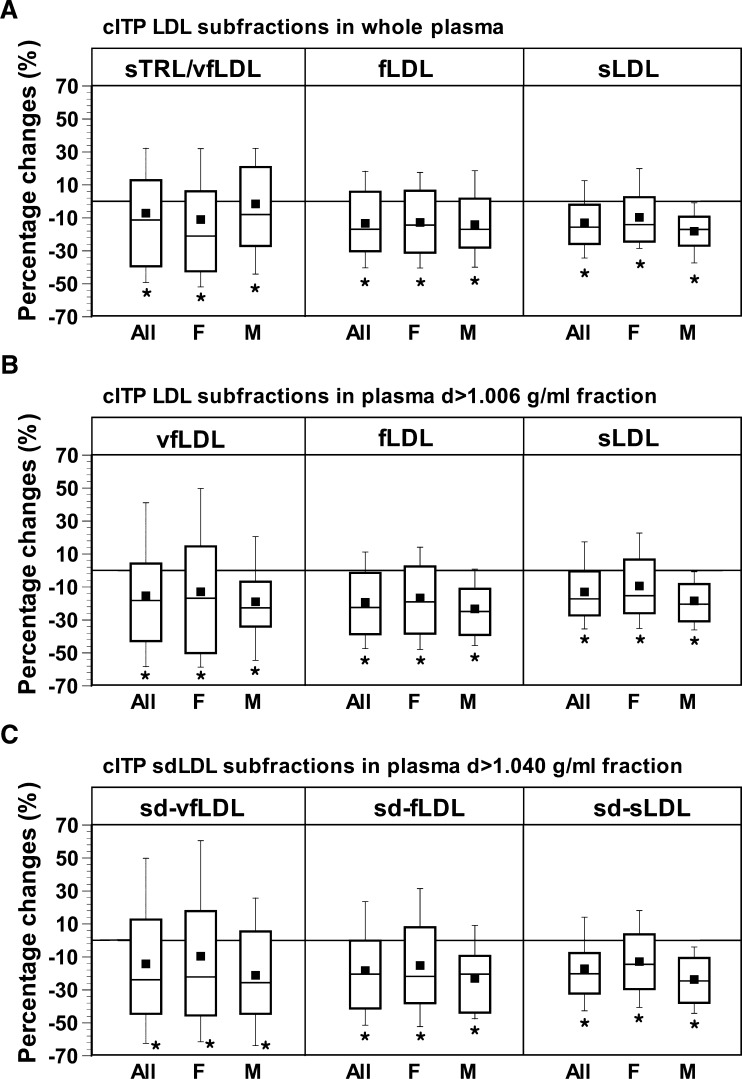
Figure 2 shows box-and-whisker plots of the percentage changes in the cITP LDL subfraction in whole plasma and the plasma d>1.006 g/ml fraction and cITP sdLDL subfraction in the d>1.040 g/ml fraction caused by rosuvastatin. As shown, the median percentage changes in the three subfractions of LDL (vfLDL, fLDL, and sLDL) or sdLDL (sd-vfLDL, sd-fLDL, and sd-sLDL) were similar in all of the patients and in female and male patients, respectively, but the percentage changes in cITP vfLDL or sd-vfLDL for all of the patients varied to a greater extent compared with fLDL and sLDL or sd-fLDL and sd-sLDL due to the relatively low levels of cITP vfLDL and sd-vfLDL (Table 2).
Fig. 2.
Box-and-whisker plots showing the mean (▪), median (middle bar in the rectangle), and 10th (bottom bar), 25th (bottom of rectangle), 75th (top of rectangle), and 90th (top bar) percentiles of percentage changes in LDL subfractions measured in whole plasma (A) and the plasma d>1.006 g/ml fraction (B) and the small-dense (sd)LDL subfraction measured in the plasma d>1.040 g/ml fraction (C) by cITP in all of the patients and in female and male patients during the period of rosuvastatin treatment. F, female; M, male; sTRL, slow-migrating triglyceride-rich lipoprotein; vfLDL, very-fast-migrating LDL; fLDL, fast-migrating LDL; sLDL, slow-migrating LDL as measured by cITP. * P < 0.05, significant changes caused by rosuvastatin treatment as assessed by the Wilcoxon signed-rank test.
Pathophysiological relevance of the different LDL and sdLDL subfractions as analyzed by cITP
Table 3 shows the relation between levels of cITP LDL and sdLDL subfractions and the compositions of HDL, LDL, and sdLDL or plasma levels of TG, RLP-C, apolipoproteins, and inflammatory markers in all of the patients at baseline. Levels of cITP fLDL and sLDL analyzed in whole plasma and in plasma d>1.006 g/ml fraction were strongly correlated (r = 0.95 and 0.95, respectively), as expected (Table 3). cITP fLDL and vfLDL subfractions of LDL or sdLDL, as measured in plasma d>1.006 g/ml or d>1.040 g/ml fractions, were strongly correlated with each other (r = 0.93 or 0.93), but were not correlated with the sLDL subfraction of LDL (r = −0.03) or sdLDL (r = 0.22). Therefore, the two cm-LDL subfractions of LDL and sdLDL were closely related to each other but were not related to the normal subfraction (sLDL) of LDL or sdLDL.
TABLE 3.
Relations between cITP LDL and sdLDL subfractions and plasma lipid variables at baseline
| Whole plasma |
Plasma d>1.006 g/ml fraction |
Plasma d>1.040 g/ml fraction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| cITP sTRL/vfLDL | cITP fLDL | cITP sLDL | cITP vfLDL | cITP fLDL | cITP sLDL | cITP sd-vfLDL | cITP sd-fLDL | cITP sd-sLDL | |
| Whole plasma | |||||||||
| fLDL | 0.88* | ||||||||
| sLDL | −0.07 | 0.03 | |||||||
| Plasma d>1.006 g/ml fraction | |||||||||
| vfLDL | 0.79* | 0.86* | −0.21 | ||||||
| fLDL | 0.78* | 0.95* | −0.05 | 0.93* | |||||
| sLDL | −0.14 | 0.02 | 0.95* | −0.21 | −0.03 | ||||
| Plasma d>1.040 g/ml fraction | |||||||||
| sd-vfLDL | 0.82* | 0.87* | −0.11 | 0.89* | 0.87* | −0.11 | |||
| sd-fLDL | 0.78* | 0.88* | −0.01 | 0.80* | 0.86* | 0.01 | 0.93* | ||
| sd-sLDL | 0.00 | 0.03 | 0.78* | −0.17 | −0.06 | 0.78* | 0.06 | 0.22 | |
| HDL-C | −0.35* | −0.32* | −0.27* | −0.12 | −0.19 | −0.26* | −0.31* | −0.36* | −0.33* |
| HDL-FC | 0.38* | −0.34* | −0.32* | −0.13 | −0.20 | −0.31* | −0.32* | −0.38* | −0.40* |
| HDL-PL | −0.23* | −0.25* | −0.35* | −0.07 | −0.14 | −0.37* | −0.25* | −0.30* | −0.41* |
| ApoA-I | −0.18 | −0.22 | −0.33* | −0.02 | −0.12 | −0.34* | −0.19 | −0.27* | −0.36* |
| PON1 activity | −0.01 | −0.06 | −0.05 | −0.02 | −0.06 | −0.09 | −0.02 | 0.02 | 0.09 |
| HDL-PAF-AH activity | −0.34* | −0.26* | 0.13 | −0.28* | −0.23* | 0.07 | −0.29* | −0.31* | −0.07 |
| HDL size | −0.41* | −0.29* | −0.14 | −0.12 | −0.16 | −0.12 | −0.30* | −0.34* | −0.28* |
| LDL-C | 0.32* | 0.49* | 0.69* | 0.31* | 0.47* | 0.70* | 0.33* | 0.44* | 0.64* |
| LDL-FC | 0.13 | 0.32* | 0.66* | 0.21 | 0.36* | 0.65* | 0.15 | 0.25* | 0.54* |
| LDL-PL | 0.22* | 0.41* | 0.57* | 0.31* | 0.44* | 0.57* | 0.23* | 0.31* | 0.45* |
| LDL-apoB | 0.38* | 0.54* | 0.60* | 0.41* | 0.55* | 0.61* | 0.43* | 0.52* | 0.59* |
| LDL-PAF-AH activity | 0.32* | 0.39* | 0.50* | 0.20 | 0.32* | 0.40* | 0.33* | 0.35* | 0.38* |
| LDL size | −0.51* | −0.37* | 0.24* | −0.34* | −0.29* | 0.27* | −0.51* | −0.51* | −0.02 |
| sdLDL-C | 0.42* | 0.46* | 0.57* | 0.30* | 0.40* | 0.56* | 0.48* | 0.60* | 0.79* |
| sdLDL-FC | 0.27* | 0.32* | 0.61* | 0.20 | 0.29* | 0.60* | 0.35* | 0.45* | 0.80* |
| sdLDL-PL | 0.43* | 0.46* | 0.44* | 0.36* | 0.42* | 0.44* | 0.45* | 0.52* | 0.62* |
| sdLDL-apoB | 0.51* | 0.55* | 0.46* | 0.42* | 0.49* | 0.44* | 0.59* | 0.68* | 0.69* |
| LDL-C/HDL-C | 0.46* | 0.55* | 0.59* | 0.33* | 0.47* | 0.60* | 0.44* | 0.54* | 0.59* |
| TG | 0.85* | 0.67* | 0.07 | 0.48* | 0.49* | −0.03 | 0.56* | 0.57* | 0.14 |
| RLP-C | 0.85* | 0.73* | 0.19 | 0.56* | 0.59* | 0.09 | 0.61* | 0.62* | 0.21 |
| ApoB | 0.49* | 0.62* | 0.55* | 0.48* | 0.60* | 0.54* | 0.51* | 0.58* | 0.55* |
| ApoC-II | 0.60* | 0.47* | −0.01 | 0.36* | 0.36* | −0.06 | 0.36* | 0.39* | 0.04 |
| ApoE | 0.50* | 0.55* | −0.03 | 0.58* | 0.60* | −0.07 | 0.48* | 0.46* | −0.12 |
| CRP | 0.00 | 0.03 | 0.15 | −0.05 | −0.02 | 0.15 | 0.00 | −0.05 | 0.06 |
| Plasma PAF-AH activity | 0.30* | 0.37* | 0.44* | 0.19 | 0.30* | 0.35* | 0.32* | 0.34* | 0.32 |
Data are presented as correlation coefficient.* P < 0.05, as assessed by the Spearman correlation.
cITP cm-LDL subfractions of LDL (vfLDL, fLDL) and sdLDL (sd-vfLDL, sd-fLDL) were negatively correlated with HDL-PAF-AH activity, and all of the three cITP sdLDL subfractions (sd-vfLDL, sd-fLDL, and sd-sLDL) were negatively correlated with HDL-C, HDL-FC, and HDL-PL levels and HDL size (Table 3).
Both cITP fLDL and sLDL subfractions of LDL or sdLDL were positively correlated with cholesterol, FC, PL, and apoB concentrations of LDL or sdLDL (Table 3). However, whereas cITP cm-LDL subfractions (vfLDL and fLDL) and sd-cm-LDL subfractions (sd-vfLDL and sd-fLDL) were negatively correlated with the size of LDL (r = −0.34, −0.29, −0.51, and −0.51), cITP sLDL was positively correlated with LDL size (r = 0.27) and sd-sLDL was not correlated with LDL size (r = −0.02) (Table 3).
Also, cITP vfLDL and fLDL subfractions and sd-vfLDL and sd-fLDL subfractions were positively correlated with levels of TG, RLP-C, apoC-II, and apoE, whereas sLDL and sd-sLDL were not related to these TRL-related variables (Table 3).
Therefore, cITP cm-LDL subfractions of LDL (vfLDL and fLDL) and sdLDL (sd-vfLDL and sd-fLDL) were closely related to each other, but were different from the normal LDL subfraction of LDL (sLDL) and sdLDL (sd-sLDL) with regard to LDL size and TG-related variables. Accordingly, the vfLDL and fLDL subfractions were combined in the following statistical analyses.
Relation between the effects of rosuvastatin on cITP cm-LDL and sd-cm-LDL subfractions and HDL-C levels, LDL particle concentrations, and TRL-related variables
Table 4 shows the relation between the percentage changes in cITP cm-LDL (vfLDL + fLDL) and sLDL subfractions of LDL and sdLDL and those in the compositions of HDL, LDL, and sdLDL and plasma levels of TG, RLP-C, and apolipoproteins in all of the patients during the study. The percentage changes in cm-LDL and sd-cm-LDL were negatively correlated with those in HDL-C (r = −0.25 and −0.28), and the percentage changes in cITP sd-cm-LDL were also negatively correlated with those in HDL-PL and apoA-I (Table 4).
TABLE 4.
Relation between percentage changes in cITP LDL and sdLDL subfractions and percentage changes in plasma lipid variables that were significantly affected by rosuvastatin treatment
| Plasma d>1.006 g/ml fraction |
Plasma d>1.040 g/ml fraction |
|||
|---|---|---|---|---|
| % changes in cm-LDL | % changes in sLDL | % changes in sd-cm-LDL | % changes in sd-sLDL | |
| % changes in HDL-C | −0.25* | −0.01 | −0.28* | −0.02 |
| % changes in HDL-FC | −0.06 | 0.16 | −0.12 | 0.09 |
| % changes in HDL-PL | −0.13 | −0.08 | −0.22* | −0.11 |
| % changes in apoA-I | −0.18 | −0.10 | −0.25* | −0.07 |
| % changes in HDL-PAF-AH | −0.01 | 0.15 | −0.14 | 0.03 |
| % changes in LDL-C | 0.61* | 0.78* | 0.50* | 0.60* |
| % changes in LDL-FC | 0.33* | 0.46* | 0.26* | 0.37* |
| % changes in LDL-PL | 0.47* | 0.61* | 0.30* | 0.41* |
| % changes in LDL-apoB | 0.66* | 0.69* | 0.55* | 0.58* |
| % changes in LDL-PAF-AH | 0.71* | 0.60* | 0.62* | 0.48* |
| % changes in sdLDL-C | 0.48* | 0.60* | 0.59* | 0.82* |
| % changes in sdLDL-FC | −0.03 | −0.01 | 0.02 | 0.10 |
| % changes in sdLDL-PL | 0.43* | 0.43* | 0.45* | 0.47* |
| % changes in sdLDL-apoB | 0.52* | 0.46* | 0.64* | 0.70* |
| % changes in LDL-C/HDL-C | 0.69* | 0.63* | 0.62* | 0.50* |
| % changes in TG | 0.55* | −0.03 | 0.46* | 0.02 |
| % changes in RLP-C | 0.64* | 0.10 | 0.51* | 0.09 |
| % changes in apoB | 0.71* | 0.67* | 0.61* | 0.58* |
| % changes in apoC-II | 0.41* | 0.17 | 0.33* | 0.13 |
| % changes in apoE | 0.69* | 0.17 | 0.58* | 0.10 |
Data are presented as correlation coefficient. * P < 0.05, as assessed by the Spearman correlation.
The percentage changes in both cm-LDL and sLDL were positively correlated with those in LDL-related variables (LDL-C, LDL-FC, LDL-PL, LDL-apoB, and LDL-PAF-AH) and LDL-C/HDL-C, and the percentage changes in both sd-cm-LDL and sd-sLDL were positively correlated with those in sdLDL-C, sdLDL-PL, and sdLDL-apoB (Table 4).
Although the percentage changes in cm-LDL and sd-cm-LDL were also positively correlated with those in TG, RLP-C, apoC-II, and apoE, the percentage changes in sLDL and sd-sLDL were not correlated with those in TRL-related variables (Table 4).
Therefore, the reducing effects of rosuvastatin on both cm-LDL and normal LDL subfractions were related to the reduction in LDL particle concentrations. However, the reducing effects of rosuvastatin on cm-LDL were also related to changes in HDL-C and TRL-related variables.
DISCUSSION
An increased LDL-C level is an established risk factor for CHD. Although the ability of LDL-C to predict the risk for CHD is moderate, the reduction of LDL-C levels by lipid-lowering drugs (statins) has consistently been shown to reduce cardiovascular events in primary and secondary prevention. Inflammatory processes play a central role in the progression of atherosclerosis and plaque rupture (31). Statins have been shown to decrease CRP (2, 3) and improve the antiinflammatory potential of HDL (32, 33).
LDL(-), a cm-LDL subfraction in human plasma, has been shown to be inflammatory due to its increased content of lysophosphatidylcholine and nonesterified fatty acid (34). This atherogenic lipoprotein is formed from multiple origins (6) and resides in plasma longer than normal LDL due to its lower affinity for LDL receptors (15). Although the clinical significance of LDL(-) is still not clear, it may be important to examine the effects of statins on the levels of LDL(-), an integrated index for various forms of atherogenic modified LDL in plasma including OxLDL, sdLDL, and glycated LDL.
Normally, fLDL subfraction in human plasma is analyzed by cITP as LDL(-) (18, 19, 22). Our previous study (23) showed that two commonly used statins, pravastatin and simvastatin, effectively reduced cITP fLDL in patients with HC and risk factors of CHD (the SPECIAL study). However, when plasma d>1.006 g/ml fraction (depleted of TRL) and d>1.040 g/ml fraction (contains sdLDL) from a patient with HDL deficiency were analyzed, we found that a new LDL(-) subfraction that had faster electrophoretic mobility than fLDL, i.e., vfLDL, was drastically increased (19). This more electronegative LDL subfraction, vfLDL, was also observed in patients with HC (Fig. 1). Since the cITP vfLDL subfraction had the same electrophoretic mobility as the sTRL subfraction in plasma, the vfLDL subfraction cannot be measured in whole plasma but needs to be analyzed in plasma depleted of TRL (Fig. 1). The present study examined the effects of rosuvastatin on the levels of cITP vfLDL subfraction and sd-cm-LDL subfractions, which are the presumably most atherogenic modified LDL, and examined the relation among the three charge-based subfractions of LDL (vfLDL, fLDL, and sLDL) and sdLDL (sd-vfLDL, sd-fLDL, and sd-sLDL).
We demonstrated that the distribution of charge-based LDL subfractions could not be predicted from the LDL-C level because the two HC patients who had similar LDL-C levels had drastically different distributions of charge-based subfractions of LDL (Fig. 1). We showed that sdLDL, similar to plasma LDL, also contained LDL subfractions that had different electrophoretic mobilities (cITP sd-vfLDL, sd-fLDL, and sd-sLDL) (Fig. 1), and that the distribution of charge-based sdLDL subfractions could also not be predicted from the sdLDL-C level.
To clarify whether the cm-LDL and normal LDL subfractions may have different physiological relevance, we examined the relation among the three charge-based subfractions of LDL and sdLDL and their relation to plasma lipid-related variables at baseline (Table 3). All of the three cITP subfractions of LDL (vfLDL, fLDL, and sLDL) or sdLDL (sd-vfLDL, sd-fLDL, and sd-sLDL) were correlated with cholesterol content and particle concentrations of LDL or sdLDL (Table 3), as expected. However, our findings indicates that cm-LDL and normal LDL are discrete LDL subfractions, because charge-modified subfractions of LDL (vfLDL and fLDL) and sdLDL (sd-vfLDL and sd-fLDL) were very closely related to each other but were apparently different from the normal LDL subfraction of LDL (sLDL) or sdLDL (sd-LDL) with regard to their relation to LDL size, TG, RLP-C, apoC-II, and apoE (Table 3).
We observed strong correlations between cITP fLDL measured in whole plasma and plasma d>1.006 g/ml fraction (Table 3, r = 0.95) and between cITP vfLDL and fLDL measured in plasma d>1.006 g/ml fraction (Table 3, r = 0.93). Therefore, the measurement of cITP fLDL in plasma should be sufficient in routine clinical practice, also considering that changes in physicochemical properies may occur during the ultracentrifugation of lipoproteins.
This study is the first to show that low-dose rosuvastatin effectively and similarly reduced both cITP vfLDL and fLDL subfractions of LDL and sdLDL in HC patients with CHD and risk factors of CHD (Table 2). Although the reduction of cm-LDL and sd-cm-LDL may represent a novel pleiotropic effect of rosuvastatin, the mechanism for these effects is still not clear. It is possible that the clearance of cm-LDL via LDL receptor may be increased. It is well known that statins up-regulate the number of LDL receptors due to the inhibition of cellular cholesterol synthesis. Simvastatin has been shown to increase the affinity of LDL(-) for LDL receptors in patients with familial hypercholesterolemia (35). In the present study, we found that the percentage changes in cm-LDL and sd-cm-LDL were correlated with the particle concentrations of LDL and sdLDL, measured as LDL-apoB and sdLDL-apoB, respectively (Table 4).
It is also possible that cm-LDL derived from VLDL remnant particles may be reduced by rosuvastatin. Pitavastatin, another strong statin, has been shown to decrease the secretion of apoB from HepG2 cells (36) and to reduce VLDL secretion in Watanabe heritable hyperlipidemic rabbits (37), an animal model of familial hypercholesterolemia. We showed that both the common statins pravastatin and simvastatin (23), and rosuvastatin (Table 1) effectively reduced RLP-C levels. We consistently found an association between cITP fLDL and RLP-C in patients with HC in our previous studies (22, 23) and in the present study (Table 3). Other authors have shown that LDL(-) had increased contents of TG, apoE, and apoC-III (8). In the present study, we also found that the percent reductions in cm-LDL and sd-cm-LDL subfractions caused by rosuvastatin treatment were correlated with those in TG, RLP-C, apoC-II, and apoE. Therefore, it is clear that a link exists between cm-LDL and TRL.
Another possible mechanism by which rosuvastatin reduces cm-LDL and sd-cm-LDL may be related to its effects on HDL. Simvastatin has been shown to improve the antiinflammatory potential of HDL (32, 33). In the present study, we showed that rosuvastatin not only significantly increased HDL-C levels but also increased plasma levels of apoA-I, the major protein of HDL, by about 3% and HDL-PL by about 8% (Table 1). Our previous in vitro experiments showed that pitavastatin drastically reduces cellular free cholesterol and increases mRNA apoA-I levels in HepG2 cells (38). Therefore, it is possible that rosuvastatin may increase the secretion of nascent HDL, which is lipid poor and contains apoA-I and PL. Our previous in vitro experiment showed that apoA-I/phosphatidylcholine discs reduce cm-LDL as analyzed by cITP (19). Nascent HDL may remodel LDL by causing the movement of lipid, lipid peroxides, enzyme, and/or protein from LDL to HDL (19, 39), thus reducing sd-cm-LDL.
In conclusion, rosuvastatin effectively reduced both the fLDL and vfLDL subfractions of LDL and sdLDL. Further studies are needed to investigate whether increased sd-cm-LDL may improve the ability of LDL-C to predict the risk of CHD and be a potential therapeutic target for treating CHD.
Footnotes
- apo
- apolipoprotein
- cITP
- capillary isotachophoresis
- cm-LDL
- charge-modified LDL
- CHD
- coronary heart disease
- CRP
- C-reactive protein
- DM
- diabetes mellitus
- FC
- free cholesterol
- fHDL
- fast-migrating HDL
- fLDL
- fast-migrating LDL
- fTRL
- fast-migrating TRL
- HC
- hypercholesterolemia
- HDL-C
- HDL cholesterol
- HDL-PAF-AH
- HDL-associated PAF-AH
- HT
- hypertension
- HTG
- hypertriglyceridemia
- iHDL
- intermediate-migrating HDL
- LDL(-)
- electronegative LDL
- LDL-C
- LDL cholesterol
- LDL-PAF-AH
- LDL-associated PAF-AH
- NL
- normolipidemic
- OxLDL
- oxidized LDL
- PAF-AH
- platelet-activating factor acetylhydrolase
- PL
- phospholipids
- RLP-C
- remnant-like particle cholesterol
- sLDL
- slow-migrating LDL
- sdLDL
- small, dense LDL
- sd-cm-LDL
- small, dense charge-modified LDL
- sd-sLDL
- small, dense, slow-migrating LDL
- sHDL
- slow-migrating HDL
- sTRL
- slow-migrating TRL
- TG
- triglyceride
- TRL
- TG-rich lipoprotein
- vfLDL
- very-fast-migrating LDL
This work was supported by grants-in-aid from the Ministry of Education, Science and Culture of Japan (Nos. 15790403, 16590806, 18590826, and 18591009), by research grants (Nos. 996006 and 026001) from the Central Research Institute of Fukuoka University (2005, 2006), and the National Institutes of Health (HL28972 and HL45522).
REFERENCES
- 1.Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr., Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr., Stone N. J. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 110: 227–239. [DOI] [PubMed] [Google Scholar]
- 2.Kinlay S. 2007. Low-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: a meta-analysis. J. Am. Coll. Cardiol. 49: 2003–2009. [DOI] [PubMed] [Google Scholar]
- 3.Mora S., Ridker P. M. 2006. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)—can C-reactive protein be used to target statin therapy in primary prevention? Am. J. Cardiol. 97: 33A–41A. [DOI] [PubMed] [Google Scholar]
- 4.Hirano T., Ito Y., Koba S., Toyoda M., Ikejiri A., Saegusa H., Yamazaki J., Yoshino G. 2004. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler. Thromb. Vasc. Biol. 24: 558–563. [DOI] [PubMed] [Google Scholar]
- 5.Holvoet P., Vanhaecke J., Janssens S., Van de Werf F., Collen D. 1998. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 98: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Quesada J. L., Benitez S., Ordonez-Llanos J. 2004. Electronegative low-density lipoprotein. Curr. Opin. Lipidol. 15: 329–335. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Quesada J. L., Benitez S., Otal C., Franco M., Blanco-Vaca F., Ordonez-Llanos J. 2002. Density distribution of electronegative LDL in normolipemic and hyperlipemic subjects. J. Lipid Res. 43: 699–705. [PubMed] [Google Scholar]
- 8.Sanchez-Quesada J. L., Camacho M., Anton R., Benitez S., Vila L., Ordonez-Llanos J. 2003. Electronegative LDL of FH subjects: chemical characterization and induction of chemokine release from human endothelial cells. Atherosclerosis. 166: 261–270. [DOI] [PubMed] [Google Scholar]
- 9.Benitez S., Perez A., Sanchez-Quesada J. L., Wagner A. M., Rigla M., Arcelus R., Jorba O., Ordonez-Llanos J. 2007. Electronegative low-density lipoprotein subfraction from type 2 diabetic subjects is proatherogenic and unrelated to glycemic control. Diabetes Metab. Res. Rev. 23: 26–34. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira J. A., Sevanian A., Rodrigues R. J., Apolinario E., Abdalla D. S. 2006. Minimally modified electronegative LDL and its autoantibodies in acute and chronic coronary syndromes. Clin. Biochem. 39: 708–714. [DOI] [PubMed] [Google Scholar]
- 11.Benitez S, Camacho M, Bancells C, Vila L, Sanchez-Quesada JL, Ordonez-Llanos J. 2006. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim. Biophys. Acta. 1761: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 12.De Castellarnau C., Bancells C., Benitez S., Reina M., Ordonez-Llanos J., Sanchez-Quesada J. L. 2007. Atherogenic and inflammatory profile of human arterial endothelial cells (HUAEC) in response to LDL subfractions. Clin. Chim. Acta. 376: 233–236. [DOI] [PubMed] [Google Scholar]
- 13.De Castellarnau C., Sanchez-Quesada J. L., Benitez S., Rosa R., Caveda L., Vila L., Ordonez-Llanos J. 2000. Electronegative LDL from normolipemic subjects induces IL-8 and monocyte chemotactic protein secretion by human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20: 2281–2287. [DOI] [PubMed] [Google Scholar]
- 14.Gaubatz J. W., Gillard B. K., Massey J. B., Hoogeveen R. C., Huang M., Lloyd E. E., Raya J. L., Yang C. Y., Pownall H. J. 2007. Dynamics of dense electronegative low density lipoproteins and their preferential association with lipoprotein phospholipase A(2). J. Lipid Res. 48: 348–357. [DOI] [PubMed] [Google Scholar]
- 15.Benitez S., Villegas V., Bancells C., Jorba O., Gonzalez-Sastre F., Ordonez-Llanos J., Sanchez-Quesada J. L. 2004. Impaired binding affinity of electronegative low-density lipoprotein (LDL) to the LDL receptor is related to nonesterified fatty acids and lysophosphatidylcholine content. Biochemistry. 43: 15863–15872. [DOI] [PubMed] [Google Scholar]
- 16.Böttcher A., Schlosser J., Kronenberg F., Dieplinger H., Knipping G., Lackner K. J., Schmitz G. 2000. Preparative free-solution isotachophoresis for separation of human plasma lipoproteins: apolipoprotein and lipid composition of HDL subfractions. J. Lipid Res. 41: 905–915. [PubMed] [Google Scholar]
- 17.Schmitz G., Mollers C., Richter V. 1997. Analytical capillary isotachophoresis of human serum lipoproteins. Electrophoresis. 18: 1807–1813. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B., Kaneshi T., Ohta T., Saku K. 2005. Relation between insulin resistance and fast-migrating LDL subfraction as characterized by capillary isotachophoresis. J. Lipid Res. 46: 2265–2277. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B., Uehara Y., Hida S., Miura S. I., Rainwater D. L., Segawa M., Kumagai K., Rye K. A., Saku K. 2007. Effects of apoA-I/phosphatidylcholine discs on charge-based LDL subfractions as characterized by capillary isotachophoresis. J. Lipid Res. 48: 1175–1189. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B., Maeda N., Okada K., Tatsukawa M., Sawayama Y., Matsunaga A., Kumagai K., Miura S., Nagao T., Hayashi J., et al. 2006. Association between fast-migrating low-density lipoprotein subfraction as characterized by capillary isotachophoresis and intima-media thickness of carotid artery. Atherosclerosis. 187: 205–212. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B., Matsunaga A., Saku K., Nakano S., Yamada T. 2004. Associations among plasma lipoprotein subfractions as characterized by analytical capillary isotachophoresis, apolipoprotein E phenotype, Alzheimer disease, and mild cognitive impairment. Arterioscler. Thromb. Vasc. Biol. 24: e144–e146. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B., Böttcher A., Imaizumi S., Noda K., Schmitz G., Saku K. 2007. Relation between charge-based apolipoprotein B-containing lipoprotein subfractions and remnant-like particle cholesterol levels. Atherosclerosis. 191: 153–161. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B., Miura S. I., Yanagi D., Noda K., Nishikawa H., Matsunaga A., Shirai K., Iwata A., Yoshinaga K., Adachi H., et al. 2008. Reduction of charge-modified LDL by statin therapy in patients with CHD or CHD risk factors and elevated LDL-C levels: the SPECIAL Study. Atherosclerosis. 201: 353–359. [DOI] [PubMed] [Google Scholar]
- 24.Nauck M., Warnick G. R., Rifai N. 2002. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin. Chem. 48: 236–254. [PubMed] [Google Scholar]
- 25.Zhang B., Eto S., Fan P., Bian C., Shimoji E., Saito T., Saku K. 2003. Paraoxonase (Pon1) Q192R polymorphism and serum Pon1 activity in diabetic patients on maintenance hemodialysis. Clin. Nephrol. 60: 257–265. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B., Fan P., Shimoji E., Itabe H., Miura S., Uehara Y., Matsunaga A., Saku K. 2006. Modulating effects of cholesterol feeding and simvastatin treatment on platelet-activating factor acetylhydrolase activity and lysophosphatidylcholine concentration. Atherosclerosis. 186: 291–301. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B., Fan P., Shimoji E., Xu H., Takeuchi K., Bian C., Saku K. 2004. Inhibition of cholesteryl ester transfer protein activity by JTT-705 increases apolipoprotein E-containing high-density lipoprotein and favorably affects the function and enzyme composition of high-density lipoprotein in rabbits. Arterioscler. Thromb. Vasc. Biol. 24: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 28.Rainwater D. L., Martin L. J., Comuzzie A. G. 2001. Genetic control of coordinated changes in HDL and LDL size phenotypes. Arterioscler. Thromb. Vasc. Biol. 21: 1829–1833. [DOI] [PubMed] [Google Scholar]
- 29.Rainwater D. L., Moore P. H., Jr., Gamboa I. O. 2004. Improved method for making nondenaturing composite gradient gels for the electrophoretic separation of lipoproteins. J. Lipid Res. 45: 773–775. [DOI] [PubMed] [Google Scholar]
- 30.Rainwater D. L., Moore P. H., Jr., Shelledy W. R., Dyer T. D., Slifer S. H. 1997. Characterization of a composite gradient gel for the electrophoretic separation of lipoproteins. J. Lipid Res. 38: 1261–1266. [PubMed] [Google Scholar]
- 31.Tsimikas S., Willerson J. T., Ridker P. M. 2006. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J. Am. Coll. Cardiol. 47: C19–C31. [DOI] [PubMed] [Google Scholar]
- 32.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756. [DOI] [PubMed] [Google Scholar]
- 33.Ansell B. J., Watson K. E., Fogelman A. M., Navab M., Fonarow G. C. 2005. High-density lipoprotein function recent advances. J. Am. Coll. Cardiol. 46: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 34.Benitez S., Camacho M., Arcelus R., Vila L., Bancells C., Ordonez-Llanos J., Sanchez-Quesada J. L. 2004. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells. Relationship with electronegative LDL. Atherosclerosis. 177: 299–305. [DOI] [PubMed] [Google Scholar]
- 35.Benitez S., Ordonez-Llanos J., Franco M., Marin C., Paz E., Lopez-Miranda J., Otal C., Perez-Jimenez F., Sanchez-Quesada J. L. 2004. Effect of simvastatin in familial hypercholesterolemia on the affinity of electronegative low-density lipoprotein subfractions to the low-density lipoprotein receptor. Am. J. Cardiol. 93: 414–420. [DOI] [PubMed] [Google Scholar]
- 36.Ooyen C., Zecca A., Bersino A. M., Catapano A. L. 1999. NK-104, a potent 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, decreases apolipoprotein B-100 secretion from Hep G2 cells. Atherosclerosis. 145: 87–95. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H., Yamazaki H., Aoki T., Kojima J., Tamaki T., Sato F., Kitahara M., Saito Y. 2000. Lipid-lowering and antiatherosclerotic effect of NK-104, a potent 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, in Watanabe heritable hyperlipidemic rabbits. Arzneimittelforschung. 50: 995–1003. [DOI] [PubMed] [Google Scholar]
- 38.Fan P., Zhang B., Kuroki S., Saku K. 2004. Pitavastatin, a potent hydroxymethylglutaryl coenzyme a reductase inhibitor, increases cholesterol 7 alpha-hydroxylase gene expression in HepG2 cells. Circ. J. 68: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 39.Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., et al. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41: 1481–1494. [PubMed] [Google Scholar]