Abstract
Plasma levels of lipoprotein-associated phospholipase A2 (Lp-PLA2) and oxidized low density lipoprotein (oxLDL) have been identified as risk factors for cardiovascular disease. Lp-PLA2 is the sole enzyme responsible for the hydrolysis of oxidized phospholipids on LDL particles in atherosclerotic plaques. We have studied the relationship between Lp-PLA2 and oxLDL in carotid endarterectomy (CEA) tissues and in matched plasmas. In extracts from CEA anatomical segments, the levels of oxLDL were significantly associated with the levels of Lp-PLA2 protein (r = 0.497) and activity (r = 0.615). OxLDL and Lp-PLA2 mass/activity were most abundant in the carotid bifurcation and internal segments where plaque was most abundant. In extracts from CEA atheroma, the levels of oxLDL and Lp-PLA2 were significantly correlated (r = 0.634). In matched plasma and atheroma extracts, the levels of Lp-PLA2 were negatively correlated (r = − 0.578). The ratio of Lp-PLA2 to oxLDL was higher in atheromatous tissue (277:1) than in normal tissue (135:1) and plasma (13:1). Immunohistochemical experiments indicated that in plaques, oxLDL and Lp-PLA2 existed in overlapping but distinctly different distribution. Fluorescence microscopy showed both oxLDL and Lp-PLA2 epitopes on the same LDL particle in plasma but not in plaque. These results suggest that the relationship between Lp-PLA2 and oxLDL in the atherosclerotic plaque is different from that in the plasma compartment.
Keywords: carotid endarterectomy, risk factors, atherosclerotic plaque, lysophosphatidylcholine
Atherosclerosis is a multifactoral disease of the arterial wall, initiated by dyslipidemia and exacerbated by inflammation (1, 2). An early event in the progression of the disease is the accumulation of LDL in the subintima of the arterial wall where it may become oxidized (oxLDL) by multiple mechanisms (3, 4). Some resident oxLDL is taken up by scavenger receptors on macrophages, leading to the formation of lipid-laden foam cells in the early atheroma (5, 6). Plasma levels of oxLDL are associated with inflammation and subclinical atherosclerosis development (7) and are also correlated with metabolic syndrome risk factors (8). Hence, circulating levels of oxLDL have become a useful biochemical risk marker for coronary heart disease (9, 10).
The most abundant oxidized phospholipid in oxLDL is oxidized phosphatidylcholine (oxPC), which typically carries a truncated (oxidized) sn-2 acyl chain of approximately nine carbons (11). OxPC is a specific substrate for the enzyme lipoprotein-associated phospholipase A2 (Lp-PLA2), which releases an oxidized short-chain fatty acid and lysophosphatidylcholine (11, 12). Lysophosphatidylcholine a highly atherogenic lipid, which induces multiple deleterious processes in the atherosclerotic plaque (13–15). Lp-PLA2 is a Ca2+-independent serine lipase, also known as platelet-activating factor-acetylhydrolase (16). This enzyme has also been shown to be a prognostic biomarker for cardiovascular disease (CVD) and coronary heart disease (17–20). The oxPC/apolipoprotein B-100 (apoB-100) ratio has been shown to be a significant risk factor for CVD, and when associated with high levels of Lp-PLA2 activity, the prediction of CVD is increased (21). Lp-PLA2 is secreted predominantly by macrophages (22, 23). Its expression and secretion significantly increase as human monocytes differentiate into macrophages and dramatically increase during activation of macrophages in the atherosclerotic lesion (24). Recently, Lp-PLA2 has been localized to necrotic cores and inflammatory areas of coronary vulnerable plaques (25). In plasma, Lp-PLA2 is thought to circulate bound to LDL (∼80%) and HDL (∼20%), although hypercholesterolemia alters this dynamic (26). Lp-PLA2 is reported to bind to the C-terminal segment of apoB-100 on LDL (27), but the relationship between Lp-PLA2 and LDL or oxLDL in atherosclerotic lesions is not well understood.
It is currently unknown if levels of Lp-PLA2 and oxLDL in plasma correlate to levels found in atherosclerotic lesions. Nevertheless, it is reasonable to postulate that the plaque burden in each atherosclerotic artery contributes to the circulating levels of each biomarker. Lavi et al. (28) have demonstrated a net increase of Lp-PLA2 levels in blood that traverses a coronary vascular bed that contains significant atherosclerotic plaque. This observation suggests that a primary source of Lp-PLA2 in blood is macrophage-rich plaque (22–24). Measuring the distribution of Lp-PLA2 and oxLDL in specific areas of the carotid atheroma should help to evaluate this possibility.
Accordingly, the objectives of this study were i) to quantify Lp-PLA2 (mass/activity) and oxLDL in carotid atherosclerotic tissue and plasma; ii) to determine the association of plasma levels of Lp-PLA2 and oxLDL with matched carotid tissue levels; and iii) to determine the macroscopic distribution of Lp-PLA2 and oxLDL as well as their microscopic association in atheroma and plasma.
MATERIALS AND METHODS
Tissues
This study was approved by the Baylor College of Medicine institutional review board for human research, and all subjects participating in this study provided informed consent. Carotid endarterectomy (CEA) tissues were excised from >40 patients undergoing unilateral carotid endarterectomy procedures. Common, bifurcation, internal, and external carotid segments were cut from these CEA tissues. Atheromatous segments were dissected from these tissues based upon gross pathological features. Segments were digitally imaged (Leica DC300) and mounted in OCT blocks for frozen sectioning. The remaining tissue was recovered for total protein extraction. Blood samples were drawn from patients who had undergone CEA to obtain plasma, thereby providing tissue/blood matched pairs. Fasting bloods were generally collected during follow-up visits an average of 4.5 months after surgery.
Determination of Lp-PLA2 protein mass
Plasma levels of Lp-PLA2 protein were measured using the dual monoclonal ELISA diaDexus PLACTM assay. This enzyme immunoassay utilizes two anti-human monoclonal Lp-PLA2 specific antibodies, clones 2C10 and 4B4, and has been previously reported (29, 30). Concentrations of Lp-PLA2 (ng/ml) in tissue extracts and plasma were normalized to total protein concentration (μg/μl) and reported as μg/μg total protein.
Determination of Lp-PLA2 activity
Lp-PLA2 activity was measured by a colorimetric activity method (diaDexus). The assay was performed in a 96-well microtiter plate, using 1-myristoyl-2-(4-nitrophenylsuccinyl)-phosphatidylcholine as substrate. The level of Lp-PLA2 activity in nmol/min/mg was calculated from the slope of the kinetic absorption curve (410 nm), using a standard conversion factor derived from a p-nitrophenol calibration curve. The assay had a coefficient of variation of <4% for duplicate measurements and a sensitivity down to <1.0 nmol/min/mg.
Although there are at least 19 mammalian enzymes that possess PLA2 activity, the Lp-PLA2 (platelet-activating factor-acetylhydrolase) family of phospholipases contains only four enzymes that exhibit unique substrate specificity toward platelet-activating factor and/or oxidized phospholipids (31). Calcium chelators in blood and tissue collection, procurement, and assay were used to assess only calcium-independent lipase activities.
Determination of OxLDL levels
The quantitation of oxLDL was performed with the Mercodia Oxidized LDL ELISA kit (Mercodia, Uppsala, Sweden). This microtiter plate capture ELISA utilizes the monoclonal antibody mAb-4E6, which has been previously described (32). Tissue extract samples were diluted 1:81 compared with 1:6561 used for plasma; otherwise, the protocol was performed as described (32). Copper oxidized LDL carefully prepared by the method of Lopes-Virella et al. (33) was used as standard.
Immunohistochemistry
Detailed protocols are found in the supplemental materials section, including the methods for immunohistochemical analysis of plasma oxLDL, oxLDL controls, and fluorescent microbead standards.
Statistics
The sample set for all experiments reported in Fig. 1 consisted of 16 CEA tissue samples, each cut into four anatomical segments (n = 64). The experiments reported in Figs. 2 and 3 were performed using an additional sample set of 22 CEAs. These CEAs were independent of the first sample set and were matched to homologous plasma from individual subjects. The 20 matched plasma/CEA pairs were obtained over a period of 16 months. Data are expressed as mean ± SD. When comparing two groups, a Mann-Whitney (t-test) was used. Differences between groups (>2) were evaluated by one-way ANOVA, with Newman-Keuls posttest. A value of P < 0.05 was considered significant. Normal probability plots were used to evaluate normality of data and determine appropriate statistical analyses. Correlation analysis (Pearson or Spearman) was followed by linear regression methods with 95% confidence. Statistical analysis was performed using GraphPad Prism Software (San Diego, CA).
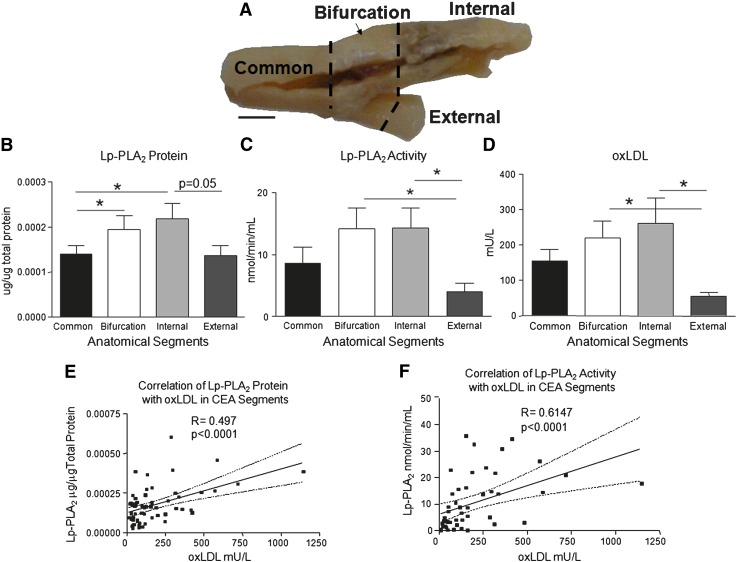
Fig. 1.
Distribution of Lp-PLA2 and oxLDL among anatomical segments of CEA tissues. A: Digital image of CEA tissue showing sites of anatomical segmentation. Distribution among segments of Lp-PLA2 protein (B), Lp-PLA2 activity (C), and oxLDL mass (D). * P 0.05. Correlation of Lp-PLA2 protein, Pearson, (E) and Lp-PLA2 activity with oxLDL mass, Spearman (F). n = 64, bar = 1cm.
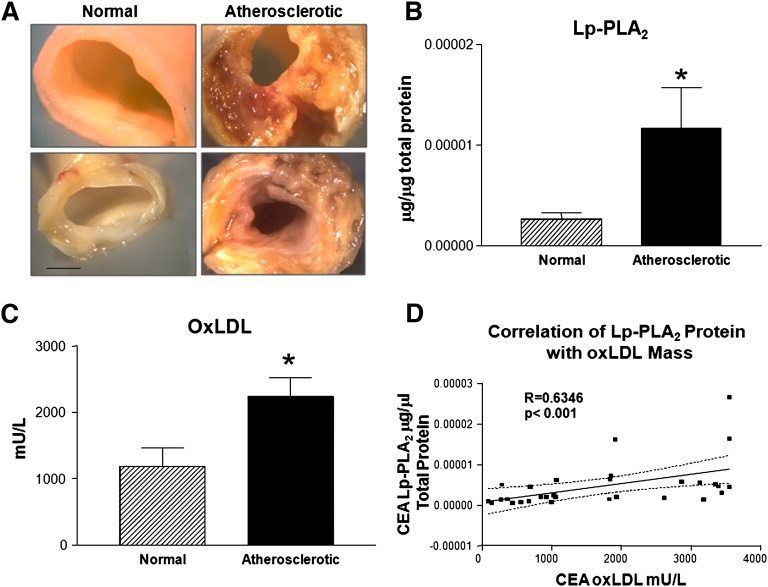
Fig. 2.
Abundance of Lp-PLA2 and oxLDL in normal and atherosclerotic CEA tissue. A: Digital images of normal and atherosclerotic CEA tissues. B: Lp-PLA2 protein abundance in normal (n = 16) and atherosclerotic CEA tissues (n = 15). C: oxLDL abundance in normal (n = 16) and atherosclerotic CEA tissues (n = 15). *P < 0.05. D: Correlation of Lp-PLA2 and oxLDL in CEA atheroma, Spearman. n = 30, bar = 2.5 mm.
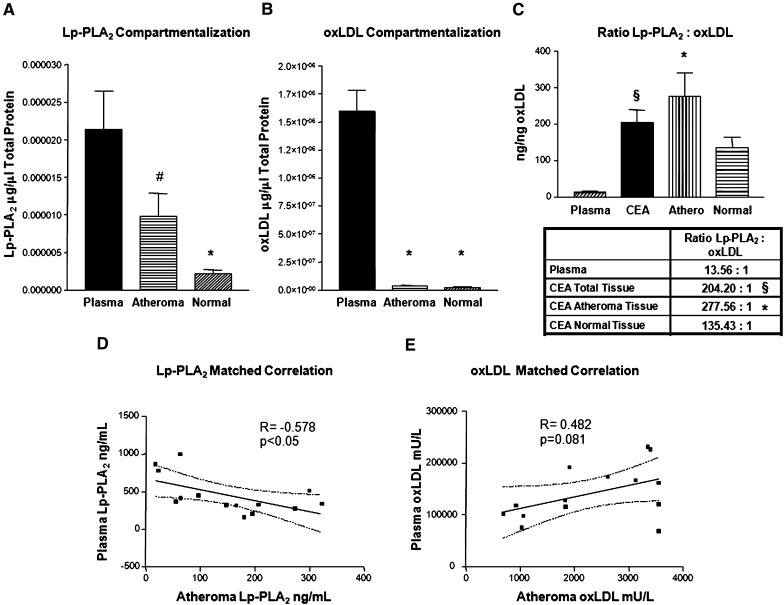
Fig. 3.
Comparison of patient-matched plasma and atheroma levels of LpPLA2 and oxLDL. Abundance of LpPLA2 (A) and ox-LDL (B) in CEA tissue (n = 31) and plasma (n = 15). C: Ratio of Lp-PLA2:oxLDL in plasma and CEA tissue. n = 46. D: Correlation of Lp-PLA2 in patient-matched plasma and CEA atheroma, Pearson. E: Correlation of oxLDL in patient-matched plasma and atheroma, Pearson (n = 28). #P < 0.05, †P < 0.01, *P < 0.001.
RESULTS
Distribution of Lp-PLA2 and oxLDL in anatomic segments of CEA tissue
CEA tissues (n = 16) were collected, imaged, and cut into common, bifurcation, internal, and external segments (Fig. 1A). Segments were halved for frozen sectioning and total protein isolation (n = 64). The internal segments had the greatest abundance of Lp-PLA2 protein, followed by the bifurcation, common, and external segments (Fig. 1B). The bifurcation and internal branches possessed the greatest Lp-PLA2 activities (Fig. 1C). The distribution pattern of oxLDL (Fig. 1D) was similar to that of Lp-PLA2 protein and activity. The internal and bifurcation segments both possessed significantly more oxLDL, as assessed by mAb-4E6, than the common and external branches of the CEA tissue (P < 0.05). We observed that not only is the distribution of both factors similar in CEA tissue segments, but their mass abundances are also correlated (r = 0.497, P < 0.0001, n = 64; Fig. 1E). Consequently, it was not surprising that Lp-PLA2 enzymatic activity was significantly coupled to oxLDL abundance (r = 0.6147, P < 0.0001; Fig. 1F). Thus, the distribution of Lp-PLA2 and oxLDL was greatest in the bifurcation and internal segments where atherosclerotic lesions were most prevalent.
Distribution of Lp-PLA2 and oxLDL in normal and atherosclerotic regions of CEA tissues
CEA tissues (n = 22) were dissected into atherosclerotic and normal regions documented by digital imaging (Fig. 2A). These images were used to categorize these regions by their qualitative features, mainly plaque size and composition. Lp-PLA2 was significantly more abundant in atherosclerotic lesions than in normal tissue (P < 0.05; Fig. 2B). Similarly, oxLDL was also significantly more abundant in atherosclerotic lesions than normal tissue (P < 0.05; Fig. 2C). Consistent with their high abundance in atherosclerotic regions, the levels of Lp-PLA2 and oxLDL in these regions were significantly correlated (r = 0.6346, P < 0.001; Fig. 2D).
Distribution of Lp-PLA2 and oxLDL in the plasma
Lp-PLA2 (ng/ml) and oxLDL (mU/L) were measured in plasma from subjects from whom CEA tissues had been obtained. The lipid profiles and risk factors for 20 subjects are listed in Table 1. One arbitrary unit of oxLDL immunoreactivity is equivalent to 300 ng (32). After unit conversion and normalization (µg/µl total protein), it was possible to compare the abundance of both factors in plasma to levels in atherosclerotic lesions. Matched plasma samples contained significantly more Lp-PLA2 than atherosclerotic (P < 0.05) and normal (P < 0.001) tissues contained (Fig. 3A). Additionally, oxLDL is more abundant in plasma than in either atherosclerotic or normal tissue (P < 0.001; Fig. 3B). During optimization of plasma ELISA assays to measure tissue extracts, it was necessary to modify dilutions of samples to fit into the dynamic range of the assay standard curves. The normal dilution of plasma samples required for analysis with the Mercodia oxLDL kit was 1:6561; for tissue samples, a dilution of 1:81 was optimal. These data were obtained to determine the stoichiometric relationship between oxLDL and Lp-PLA2 in the plasma and tissue compartments. The average protein weight ratio of Lp-PLA2 to oxLDL was ∼13:1 in plasma. This is significantly less than the ratio of 204:1, which was observed in the total CEA tissue (P < 0.01; Fig. 3C). This ratio is substantially increased to 277:1 in atherosclerotic areas of the tissue (P < 0.001; Fig. 3C). The normal tissue areas possessed a ratio of 135:1. Hence, there is more Lp-PLA2 (ng) per oxLDL (ng) in the atherosclerotic lesion than in the plasma. The ratio appears to be appreciably different in each compartment.
TABLE 1.
Characteristics of subjects (n = 20) from whom carotid endarterectomy tissues were obtained (mean ± SD)
| Age (years) | 68.7 ± 7.4 |
| Gender | 14 male/6 female |
| Total cholesterol (mg/dl) | 132.5 ± 31.1 |
| HDL cholesterol (mg/dl) | 33.2 ± 15.7 |
| LDL cholesterol (direct, mg/dl) | 63.4 ± 19.1 |
| LDL cholesterol (calc, mg/dl) | 73.0 ± 20.9 |
| Lp[a] (mg/dl) | 18.1 ± 19.5 |
| Triglycerides (mg/dl) | 112.5 ± 58.0 |
| Smoking | 5 of 20 |
| Diabetes | 2 of 20 |
| Lipid altering therapy (statins) | 6 of 20 |
Relation of levels of Lp-PLA2 and oxLDL in plasma and matched atherosclerotic CEA tissue
It would be very desirable for the plasma levels of Lp-PLA2 and oxLDL to reflect their abundances in atherosclerotic lesions, thereby making them possible tools for noninvasive tracking of atherosclerotic plaque burden. We evaluated this possibility by comparing the levels of Lp-PLA2 and oxLDL in plasma to that in matched CEA tissue extracts. Significantly, in the case of Lp-PLA2, the plasma and tissue levels were negatively correlated (r = − 0.578; Fig. 3D). In the case of oxLDL, plasma and tissue levels were positively borderline correlated (r = 0.482; Fig. 3E).
Localization of Lp-PLA2 and oxLDL in CEA atherosclerotic lesions shows overlapping but distinctly different distribution patterns
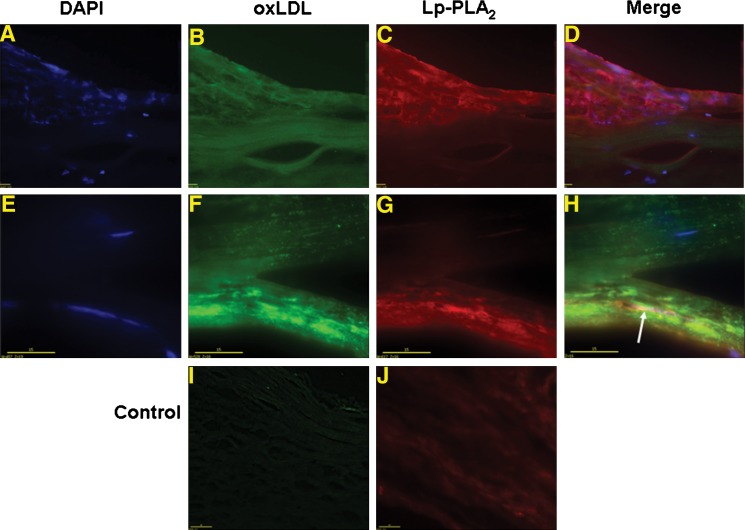
Deconvoluted microscopy and immunochemistry were used to localize Lp-PLA2 and oxLDL in CEA tissues. Three types of localization were observed: distal, proximal, and colocalization. Distal localization is illustrated in Fig. 4B and C in which Lp-PLA2 is highly localized to the lesion shoulder, whereas ox-LDL is broadly spread throughout the entire lesion area. Proximal localization is illustrated in Fig. 4F and G in which both species are localized to an underlying segment. Although Lp-PLA2 and oxLDL had overlapping patterns, they were not coincident. Authentic colocalization is illustrated in Figure 4F–H in which green (oxLDL) combines with red (Lp-PLA2) to give a yellow color (white arrow). This colocalization of these two species was limited to a relatively small area, and the majority of oxLDL and Lp-PLA2 occupied similar areas but did not actually colocalize. The differences between Fig. 4D and H exemplify the variable distributions of oxLDL and Lp-PLA2 in image sets acquired from CEA tissues.
Fig. 4.
Microscopy distribution of Lp-PLA2 and oxLDL in CEA tissues. Deconvoluted microscopy localization of Lp-PLA2 (mAb-2C10 and red Alexa546) and oxLDL (mAb-4E6 and green Alexa488). A–D illustrate inflammation pattern (bar = 20 μm, 20×). E H illustrate oxLDL distribution pattern (bar = 15 μm, 60×). I and J are control experiments with unlabeled antibody prior to conjugated primary antibody incubation (bar = 40 μm, 20×).
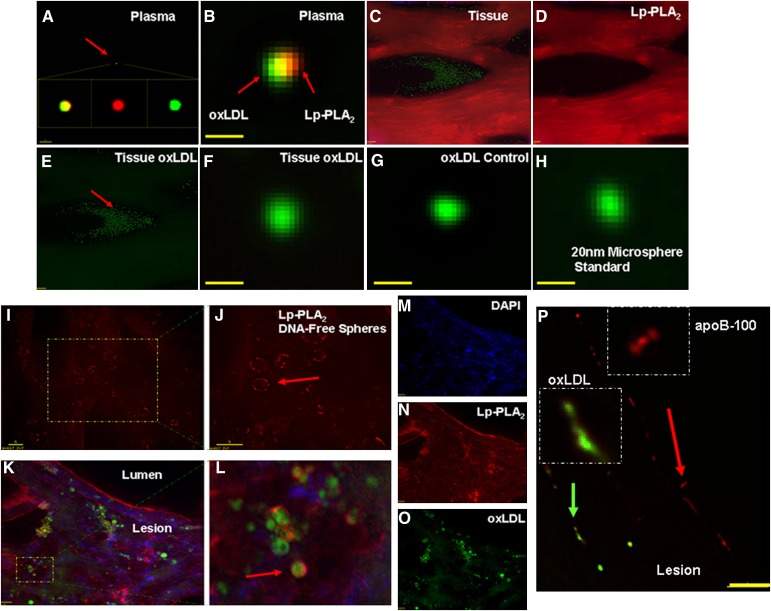
Lp-PLA2 colocalizes to individual oxLDL particles in plasma but not in atheroma
We sought to investigate the interaction between Lp-PLA2 and oxLDL by employing immunohistochemistry. Using high magnification (100×) and fluorescence (deconvolution) microscopy, we observed two distinct interactions between Lp-PLA2 and oxLDL in plasma and in the atheroma (Fig. 5). In plasma, we localized Lp-PLA2 (red) to the surface of individual oxLDL (green) particles (Fig. 5A, B). Figure 5B is an enhanced image of a plasma individual oxLDL particle with Lp-PLA2 colocalized to its surface. Conversely, in CEA tissue sections, actual colocalization of these two factors was not observed. Microscopy analysis revealed that often individual oxLDL particles were localized to lipid pools throughout the plaque as well as the shoulder and cap regions (Fig. 5C–E). In contrast, Lp-PLA2 localized to the edges and adjacent matrix around these lipid pools and oxLDL deposits. We did not find true colocalization of Lp-PLA2 to the surface of individual oxLDL particles within the atherosclerotic tissue sections. Figure 5F illustrates the lack of Lp-PLA2 staining on the surface of a free-standing oxLDL particle in CEA tissue. To our knowledge, this is the first report showing immunohistochemical (fluorescence microscopy) identification of individual oxLDL particles. These measurements were validated by confirming the positive staining of individual oxLDL particles using an oxLDL control (Fig. 5G) and a 20 nm fluorescent microsphere standard (Fig. 5H). Discussion concerning the optical measurements can be found in the supplemental materials section.
Fig. 5.
Colocalization of Lp-PLA2 and oxLDL in CEA tissues by deconvoluted microscopy. Localization of Lp-PLA2 (red, Alexa546), oxLDL (green, Alexa488), and DNA (blue, DAPI). A: Composite of plasma colocalization; red arrow indicates plasma oxLDL; B: enhanced view (100× +, bar = 20 nm). C–E: Microscopy distribution of atheroma Lp-PLA2 and oxLDL (20×, bar = 20 μm). F: Tissue oxLDL (100× + enhanced, bar = 20 nm). G: oxLDL positive control. H: Fluorescent microsphere standard (20 nm). I, J: Lp-PLA2 localized to the surface of DNA-free spheres (4′,6-diamidino-2-phenylindole [DAPI]) (100×, bar = 5 μm). K: Multiplex image of panels M O (20×, bar = 20 μm). L: Enhanced view of K. P: apoB-100 (pAb-qDot625, red arrow) and oxLDL (mAb-qDot525, green arrow) (100×, bar = 5 μm).
Lp-PLA2 colocalizes with large oxLDL-derived particles in carotid atherosclerotic lesions
In CEA tissue sections, immunohistochemistry was used to observe positively stained oxLDL particles of variable sizes. Individual particles (∼20 nm) and particles of much greater size (∼30 nm to 3 µm in diameter) were identified. Whether these larger particles are oxLDL aggregates or oxLDL-derived liposomes is unclear. Positive staining for Lp-PLA2, was observed on large DNA-free spheres (Fig. 5I, J). In Fig. 5J, the enlarged image shows the larger Lp-PLA2-localized spheres (red arrow). It was possible to rotate the image and positively identify Lp-PLA2 on the surface of these spheres (data not shown). Significantly, we were able to determine that these spheres were positive for oxLDL, even though they were larger than authentic oxLDL. Our multiplexed images show the localization of Lp-PLA2 to the surface of oxLDL-positive spheres (Fig. 5K–O). In Fig. 5L, the red arrow points to Lp-PLA2 (red) on the surface of what appears to be a large oxLDL-derived particle (green). We observed that not all apoB-100 particles were positive for oxLDL (4E6), thus illustrating specificity of the 4E6 antibody to recognize only a particular fraction (oxidized) of the LDL pool (Fig. 5P). The red arrow points to apoB-100 particles only, and the green arrow identifies apoB-100-oxLDL positive particles. Taken together, these findings indicate that the relationship between Lp-PLA2 and oxLDL in the atheroma is very different from that in the plasma.
DISCUSSION
The goal of this study was to characterize the association and relationship between Lp-PLA2 and oxLDL in atherosclerotic lesions and plasma. The data presented offer new insights into the association between this centrally important enzyme and its substrate. In this study, we observed that Lp-PLA2 (mass and activity) and oxLDL were distributed to the same anatomical segments of the carotid artery (Fig. 1A–D). The levels of these factors in these segments were significantly correlated (r = 0.497 and 0.615). Lp-PLA2 and oxLDL were found in greatest abundance at the bifurcation and internal segments, regions known for having the most rupture-prone atherosclerotic lesions (34, 35). Accordingly, we measured Lp-PLA2 and oxLDL in atherosclerotic areas of the CEA tissues. Both Lp-PLA2 and oxLDL levels were significantly greater in diseased than nondiseased areas. Again, we observed significant correlation between Lp-PLA2 and oxLDL in this analysis (r = 0.635; Fig. 2D). It is noteworthy that the weight ratio of Lp-PLA2 to oxLDL in plasma (13:1) was significantly different from their ratio in the atheroma (277:1), a 20-fold difference.
Elevated plasma levels of Lp-PLA2 and oxLDL are well documented as prognostic risk markers for cardiovascular disease (7–10, 29, 36, 37). This association suggested that the plasma levels of these analytes might be associated with their levels in atherosclerotic plaques. For example, we previously observed that the plasma level of the cardiovascular risk factor Lp[a] is associated with its levels in lesion-bearing tissues, such as resected bypass vein grafts (38) and aortic aneurysms (39). In this study, however, the plasma levels of oxLDL are only borderline associated with carotid atheroma levels. This observation suggests that the carotid atheroma alone does not make a proportionate contribution of oxLDL to the total level in plasma. A moderate contribution of oxLDL from carotid plaques may be overshadowed by more abundant contributions from larger lesions, such as those in the aorta.
Plasma Lp-PLA2 levels represent risk related to the total burden of vascular inflammation, and not just the burden arising from a particular atheroma. Likewise, the plasma Lp-PLA2 level reflects contributions from not just a single lesion but from multiple atherosclerotic sites. Accordingly, we anticipated that the plasma levels of this biomarker might be greater than the level in a single specific tissue site, such as the carotid plaque. Indeed, we observed that Lp-PLA2 is more abundant in plasma than in CEA tissues when normalized to total protein (Fig. 3A, B). Lavi et al. (28) found that production of Lp-PLA2 in a single vascular bed (i.e., coronary arteries) correlated with coronary atheroma volume determined by intravascular ultrasound by measuring the gradient in Lp-PLA2 concentration from the coronary ostium to the coronary sinus. On the basis of this observation, we postulated that plasma and tissue levels of Lp-PLA2 would be positively associated. Surprisingly, the levels were negatively correlated (Fig. 3D; r = − 0.578). Significantly, Mannheim et al. (40) observed a negative correlation between plaque expression of Lp-PLA2 and plasma HDL levels. Elucidation of how these sources of Lp-PLA2 are inversely related will require further investigation.
We used the 4E6 antibody for detection of an oxidized form of LDL (10). The binding of this antibody to oxidized LDL is not necessarily a measure of binding to oxidized phospholipid epitopes. This antibody targets both oxLDL (in vitro) and/or malondialdehyde-modified LDL (in vivo). These epitopes are reactive aldehyde-modified lysines of apoB-100 that are products of lipid peroxidation. Nonetheless, the 4E6 antibody does not recognize all modified forms of LDL (e.g., acetylated or oxPC). Furthermore, the 4E6 antibody recognizes only one epitope per LDL particle in an environment containing potentially numerous oxidized lipids.
The localization studies and particle histology experiments lend support to the view that the atheroma in the vessel wall is a major site for production of Lp-PLA2 and oxLDL, especially in advanced atherosclerosis where there are lesions of greater number and size. These studies show that Lp-PLA2 and oxLDL are found in greatest abundance at the atheroma shoulder region and lesion cap, as has also been observed by Mannheim et al. (40). Both areas would likely be involved in lipid and protein influx and efflux. However, the mechanism and/or rate of trans-intimal movement may vary among different permeating species. Studies directed toward measuring lipoprotein influx using optical coherence tomography are currently underway (41). Nonetheless, previous studies give added credence to the atheroma-epicenter model for production of Lp-PLA2 and oxLDL. The LDL particle undergoes considerable modification after becoming trapped in the subintimal space (5, 6). It is unlikely that more than minimal amounts of LDL are oxidized in the plasma compartment due to its extensive reducing capacity (e.g., from reduced glutathione and paraoxonase). Additionally, the expression and secretion of Lp-PLA2 dramatically increase during monocyte migration into the vessel wall and subsequent differentiation of monocytes to macrophages (24).
Significant strengths of this study are i) the use of anatomically and pathologically separate carotid tissue segments, ii) tissue extraction and quantitation of oxLDL and Lp-PLA2, iii) direct comparison of tissue and plasma levels of oxLDL and Lp-PLA2, and iv) high-resolution imaging (colocalization) of Lp-PLA2 and oxLDL in the carotid atheroma and plasma. Our results suggest that the relationship between Lp-PLA2 and oxLDL in the carotid atheroma is distinctly different from what is observed in plasma.
Supplementary Material
Footnotes
Abbreviations:
- apoB-100
- apolipoprotein B-100
- CEA
- carotid endarterectomy
- CVD
- cardiovascular disease
- Lp-PLA2
- lipoprotein-associated phospholipase A2
- oxLDL
- oxidized low density lipoprotein
- oxPC
- oxidized phosphatidylcholine
This work was supported in part by grants from the National Institutes of Health (RO1-HL-63090, T32-HL-07812-11, HL-070537, and NIAID AI46773) and by Fonds voor Wetenschappelijk Onderzoek-Vlaanderen Project G027604.
The online version of this article (available at http://www.jlr.org) contains supplementary materials.
REFERENCES
- 1.Wu J. T., Wu L. L. 2006. Linking inflammation and atherogenesis: soluble markers identified for detection of risk factors and for early risk assessment. Clin. Chim. Acta. 366: 74–80. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. 1999. Atherosclerosis: an inflammatory disease. N. Engl. J. Med. 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 3.Hazen S. L. 2008. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 283: 15527–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witztum J. L., Steinberg D. 1991. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88: 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg D. 1997. Low-density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 272: 20963–20966. [DOI] [PubMed] [Google Scholar]
- 6.Heinecke J. W. 1998. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low-density lipoprotein hypothesis. Atherosclerosis. 141: 1–15. [DOI] [PubMed] [Google Scholar]
- 7.Hulthe J., Fagerberg B. 2002. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler. Thromb. Vasc. Biol. 22: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 8.Sigurdardottir V., Fagerberg B., Fagerberg J. 2002. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year-old men (AIR study). J. Intern. Med. 252: 440–447. [DOI] [PubMed] [Google Scholar]
- 9.Toshima S., Hasegawa A., Kurabayashi M., Itabe H., Takano T., Sugano J., Shimamura K., Kimura J., Michishita I., Suzuki T., et al. 2000. Circulating oxidized low density lipoprotein levels: a biochemical risk marker for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 20: 2243–2247. [DOI] [PubMed] [Google Scholar]
- 10.Holvoet P., Collen D. 1998. Oxidation of low density lipoproteins in the pathogenesis of atherosclerosis. Atherosclerosis. 137: S33–S38. [DOI] [PubMed] [Google Scholar]
- 11.Tokumura A., Toujima M., Yoshioka Y., Fukuzawa K. 1996. Lipid peroxidation in low density lipoproteins from human plasma and egg yolk promotes accumulation of 1-acyl analogues of platelet-activating factor-like lipids. Lipids. 31: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 12.Stremler K. E., Stafforini D. M., Prescott S. M., Zimmerman G. A., McIntyre T. M. 1989. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J. Biol. Chem. 264: 5331–5334. [PubMed] [Google Scholar]
- 13.Hsieh C.C., Yen M. H., Liu H. W., Lau Y. T. 2000. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL. Atherosclerosis. 151: 481–491. [DOI] [PubMed] [Google Scholar]
- 14.Kohno M., Yokokawa K., Yasunari K., Minami M., Kano H., Hanehira T., Yoshikawa J. 1998. Induction by lysophosphatidylcholine, a major phospholipid component of atherogenic lipoproteins, of human coronary artery smooth muscle cell migration. Circulation. 98: 353–359. [DOI] [PubMed] [Google Scholar]
- 15.Rong J. X., Berman J. W., Taubman M. B., Fisher E. A. 2002. Lysophosphatidylcholine stimulates monocyte chemoattractant protein-1 gene expression in rat aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 22: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 16.Samanta U., Bahnson B. J. 2008. Crystal structure of human plasma plate-activating factor acetylhydrolase. J. Biol. Chem. 283: 31617–31624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalewski A., Macphee C. 2005. Role of LDL-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 25: 923–931. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne C. M., Hoogeveen R. C., Bang H., Coresh J., Folsom A. R., Chambless L. E., Myerson M., Wu K. K., Sharrett A. R., Boerwinkle E. 2005. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch. Intern. Med. 165: 2479–2484. [DOI] [PubMed] [Google Scholar]
- 19.Garza C. A., Montori V. M., McConnell J. P., Somers V. K., Kullo I. J., Lopez-Jimenez F. 2007. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin. Proc. 82: 159–165. [DOI] [PubMed] [Google Scholar]
- 20.Packard C. J., O’Reilly D. S., Caslake M. J., McMahon A. D., Ford I., Cooney J., Macphee C. H., Suckling K. E., Krishna M., Wilkinson F. E., et al. 2000. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 343: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 21.Kiechl S., Willeit J., Mayr M., Viehweider B., Oberhollenzer M., Kronenberg F., Wiedermann C. J., Oberthaler S., Xu Q., Witztum J. L., et al. 2007. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 27: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 22.Stafforini D. M., Elstad M. R., McIntyre T. M., Zimmerman G. A., Prescott S. M. 1990. Human macrophages secrete platelet-activating factor acetylhydrolase. J. Biol. Chem. 265: 9682–9687. [PubMed] [Google Scholar]
- 23.Hakkinen T., Luoma J. S., Hiltunen M. O., Macphee C. H., Milliner K. J., Patel L., Rice S. Q., Tew D. G., Karkola K., Yla-Herttuala S. 1999. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 19: 2909–2917. [DOI] [PubMed] [Google Scholar]
- 24.Elstad M. R., Stafforini D. M., McIntyre T. M., Prescott S. M., Zimmerman G. A. 1989. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J. Biol. Chem. 264: 8467–8470. [PubMed] [Google Scholar]
- 25.Kolodgie F. D., Burke A. P., Skorija K. S., Ladich E., Kutys R., Makuria A. T., Virmani R. 2006. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26: 2523–2529. [DOI] [PubMed] [Google Scholar]
- 26.Tsimihodimos V., Karabina S. A., Tambaki A. P., Bairaktari E., Miltiadous G., Goudevenos J. A., Cariolou M. A., Chapman M. J., Tselepis A. D., Elisaf M. 2002. Altered distribution of platelet-activating factor- acetylhydrolase activity between LDL and HDL as a function of the severity of hypercholesterolemia. J. Lipid Res. 43: 256–263. [PubMed] [Google Scholar]
- 27.Stafforini D. M., Tjoelker L. W., McCormick S. P., Vaitkus D., McIntyre T. M., Gray P. W., Young S. G., Prescott S. M. 1999. Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J. Biol. Chem. 274: 7018–7024. [DOI] [PubMed] [Google Scholar]
- 28.Lavi S., McConnell J. P., Rihal C. S., Prasad A., Mathew V., Lerman L. O., Lerman A. 2007. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 115: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 29.Caslake M. J., Packard C. J., Suckling K. E., Holmes S. D., Chamberlain P., Macphee C. H. 2000. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis. 150: 413–419. [DOI] [PubMed] [Google Scholar]
- 30.Tselepis A. D., John Chapman M. 2002. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler. Suppl. 3: 57–68. [DOI] [PubMed] [Google Scholar]
- 31.Kudo I., Murakami M. 2002. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 68–69: 3–58. [DOI] [PubMed] [Google Scholar]
- 32.Holvoet P., Macy E., Landeloos M., Jones D., Jenny N. S., Van de Werf F., Tracy R. P. 2006. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin. Chem. 52: 1–4. [DOI] [PubMed] [Google Scholar]
- 33.Lopes-Virella M. F., Koskinen S., Mironova M., Horne D., Klein R., Chassereau C., Enockson C., Virella G. 2000. Preparation of copper oxidized LDL for the measurement of oxidized LDL antibodies by EIA. Atherosclerosis. 152: 107–115. [DOI] [PubMed] [Google Scholar]
- 34.Sui B., Gao P., Lin Y., Gao B., Lui I., An J. 2008. Blood flow patterns and wall shear stress in the internal carotid arteries of healthy subjects. Acta Radiol. 49: 806–814. [DOI] [PubMed] [Google Scholar]
- 35.Harloff A., Albrecht F., Spreer J., Stalder A. F., Bock J., Frydrychowitz A., Shollhorn J., Hetzel A., Schumaker M., Henning J., et al. 2009. 3D blood flow characterstics in the carotid artery bifurcation assessed by flow sensitive 4D MRI at 3T. Magn. Reson. Med. 61: 65–74. [DOI] [PubMed] [Google Scholar]
- 36.Gazi I., Lourida E. S., Filippatos T., Tsimihodimos V., Elisaf M., Tselepis M. D. 2005. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin. Chem. 51: 2264–2273. [DOI] [PubMed] [Google Scholar]
- 37.Blake G. J., Dada N., Fox J. C., Manson J. E., Ridker P. M. 2001. A prospective evaluation of lipoprotein-associated phospholipase A2 levels and the risk of future cardiovascular events in women. J. Am. Coll. Cardiol. 38: 1302–1306. [DOI] [PubMed] [Google Scholar]
- 38.Cushing G. L., Gaubatz J. W., Nava M. L., Burdick B. J., Bocan T. M., Guyton J. R., Weilbaecher D., DeBakey M. E., Lawrie G. M., Morrisett J. D. 1989. Quantitation and localization of apolipoproteins [a] and B in coronary artery bypass vein grafts resected at re-operation. Arteriosclerosis. 9: 593–603. [DOI] [PubMed] [Google Scholar]
- 39.Papagrigorakis E., Iliopoulos D., Asimacopoulos P. J., Safi H. J., Weilbaecher D. J., Ghazzaly K. G., Nava M. L., Gaubatz J. W., Morrisett J. D. 1997. Lipoprotein(a) in plasma, arterial wall, and thrombus from patients with aortic aneurysm. Clin. Genet. 52: 262–271. [DOI] [PubMed] [Google Scholar]
- 40.Mannheim D., Herrmann J., Versari D., Gosyl M., Meyer F. B., McConnell J. P., Lerman L. O., Lerman A. 2008. Enhanced expression of Lp-PLA and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke. 39: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosn M.G., Leba M., Vijayananda A., Rezaee P., Morrisett J. D., Larin K. V. 2009. Effect of temperature on permeation of low density lipoprotein particles through human carotid artery tissues. J. Biophotonics. 2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.