Abstract
Data is limited on measures influencing cholesterol homeostasis in subjects at high risk of developing cardiovascular disease (CVD) relative to established risk factors. To address this, we quantified circulating indicators of cholesterol homeostasis (plasma phytosterols and cholesterol precursor concentrations as surrogate measures of cholesterol absorption and synthesis, respectively) in Framingham Offspring Study Cycle-6 participants diagnosed with established CVD and/or ≥50% carotid stenosis not taking lipid lowering medication (cases, N = 155) and matched controls (N = 414). Cases and controls had similar plasma LDL-cholesterol; HDL-cholesterol was significantly lower in males, while triglyceride concentrations were significantly higher in female cases relative to their respective controls. Cholesterol absorption markers were significantly higher (229 ± 7 vs. 196 ± 4, 169 ± 6 vs. 149 ± 3 and 144 ± 5 vs. 135 ± 3 for campesterol, sitosterol, and cholestanol, respectively), whereas cholesterol synthesis markers were significantly lower (116 ± 4 vs. 138 ± 3, 73 ± 3 vs. 75 ± 2 for lathosterol and desmosterol, respectively) in cases compared with controls, irrespective of sex. After controlling for standard risk factors, campesterol (2.47 [1.71-3.56]; P < 0.0001), sitosterol (1.86 [1.38-2.50]; P < 0.0001), cholestanol (1.57 [1.09-2.27]; P = 0.02), desmosterol (0.59 [0.42-0.84]; P = 0.003), and lathosterol (0.58 [0.43-0.77]; P = 0.0002) were significantly associated with CVD (odds ratio [95% confidence interval]). These data suggest that impaired cholesterol homeostasis, reflected by lower synthesis and higher absorption marker concentrations, are highly significant independent predictors of prevalent CVD in this study population.
Keywords: lipids, lipoproteins, lathosterol, desmosterol, phytosterols, campesterol, sitosterol, coronary heart disease
An elevated LDL-cholesterol concentration is a well-established independent risk factor for cardiovascular disease (CVD) (1). Large-scale primary and secondary prevention trials aimed at lowering plasma total and LDL-cholesterol concentrations have resulted in marked reductions in CVD events and mortality (2–9). However, CVD events still occur in a substantial proportion of individuals not presenting with elevated LDL-cholesterol concentrations. Using the 90th percentile for age and sex according to the Lipid Research Clinics data for LDL-cholesterol and triglyceride concentrations and the 10th percentile for HDL-cholesterol, in patients with premature coronary artery disease (CAD), relative to matched control subjects, only 12% versus 9% had elevated LDL-cholesterol concentrations, 19% versus 4% had low HDL-cholesterol concentrations, and 10% versus 9% had elevated triglyceride concentrations (10). Based on the aforementioned criteria, 39% of patients with premature CAD had no conventional lipid abnormality. Similarly, data from several clinical trials suggest that although LDL-cholesterol lowering was highly efficacious, the reduction in the relative risk of major coronary events was poorly associated with baseline serum lipid concentrations (2–6, 8, 9, 11).
Cholesterol homeostasis plays an important role in the regulation of whole-body cholesterol content by balancing input (intestinal absorption of dietary and biliary cholesterol) and output (hepatic and extra-hepatic synthesis), thereby preventing the net accumulation of cholesterol in circulation and tissues (12). A disruption in any one of these processes can alter this balance with resultant disease progression (13–15). Only limited data are available on measures influencing cholesterol homeostasis in subjects at high risk of developing CVD relative to established risk factors. The objective of the present study was to determine the association between CVD status and circulating indicators of cholesterol homeostasis, plasma concentrations of phytosterols and cholestanol, which are surrogate markers of cholesterol absorption (16–18), and cholesterol precursors, which are surrogate markers of cholesterol synthesis (19, 20), in Framingham Offspring Study (FOS) Cycle-6 participants with documented CVD and/or ≥50% carotid stenosis not taking lipid lowering medication and matched control subjects.
METHODS
Study population
The rationale, design, and methods for the FOS have been described elsewhere in detail (21). Briefly, this is a longitudinal community-based study initiated in 1971 with a sample of 5,135 men and women consisting of the offspring of the original Framingham Heart Study (22) and their spouses. Of the 3,532 participants who underwent a standardized medical history and physical examination at the sixth examination cycle (1996–1997), we identified 155 cases (n = 51 female and n = 104 male), defined as subjects with documented CVD (n = 117) and/or ≥50% carotid stenosis (n = 38) and who were not taking lipid lowering medications (statins, cholestyramine, niacin, or fibrates). Using a 3:1 ratio, 465 control subjects matched on the basis of age, sex, body mass index, systolic blood pressure, and smoking status were identified. Plasma samples were available for all the cases and for 414 of the control subjects (n = 140 female and 274 male). This study was approved by the Institutional Review Boards for Human Research at Tufts University-Tufts Medical Center and Boston University. Informed consent was obtained from study subjects.
Definition and assessment of CVD status
CVD was defined as occurrence of myocardial infarction (MI), ≥50% carotid stenosis (verified using Doppler), coronary insufficiency, angina pectoris, cerebrovascular accident, or transient ischemic attack prior to the sixth examination cycle. Information about CVD events was confirmed with the aid of medical histories, physical examinations at the study clinic, hospitalization records, and communication with personal physicians as previously described (22, 23). We identified 155 cases, 117 had prevalent CVD at the sixth examination cycle and 63 had stenosis ≥50%. Of those 117 with CVD, 59 were MI cases.
Covariate information
Height and weight were measured with the subject standing. Body mass index was calculated (kg/m2). Subjects were classified as hypertensive if their diastolic blood pressure was ≥90 mm Hg or systolic blood pressure was ≥140 mm Hg, or if use of antihypertensive medications was reported. Diabetes was defined as fasting glucose ≥126 mg/dl or use of insulin or oral hypoglycemic medications. Current smokers were defined as those who reported smoking at least one cigarette per day during the previous year. Estrogen use was classified as current or no use at the time of the examination.
Assessment of dietary intake
Usual dietary intake was assessed using a validated semiquantitative 126-item food frequency questionnaire (FFQ) (24, 25). Nutrient intakes were calculated by multiplying the frequency of consumption of each unit of food from the FFQ by the nutrient content of the specified portion. Dietary information was judged as unreliable and excluded from further analysis if reported energy intakes were <2.51 MJ/d (600 kcal) or >16.74 MJ/d (4,000 kcal/d) for women and >17.57 MJ/d (4,200 kcal/d) for men, respectively, or if ≥12 food items were left blank. Complete FFQ data was available for 506 of the 569 participants.
Lipid and lipoprotein analyses
Fasting plasma total cholesterol, triglycerides, HDL-cholesterol, and glucose concentrations were measured using standard enzymatic methods, as previously described (26, 27). LDL-cholesterol concentrations were calculated according to the formula of Friedewald et al. (28). The assays were standardized through the Lipid Standardization Program of the CDC (Atlanta, GA).
Cholesterol homeostasis analyses
Plasma noncholesterol sterol concentrations were quantified using gas chromatography as previously described (20, 29). Peaks of interest were identified by comparison with authentic standards (Supelco, Bellefonte, PA) and expressed relative to the internal standard. Interassay CVs were on average ≤5.5% for most of the sterols. The investigators and laboratory personnel were blinded as to case-control status. Each case-control set was analyzed in the same run by the same technician in a random sequence under identical conditions. External quality control samples were routinely interspersed per 12 samples and analyzed with study samples. The noncholesterol sterols measured included the cholesterol precursors -squalene, desmosterol, and lathosterol, and the absorption markers -cholestanol, campesterol, and sitosterol. Because the noncholesterol sterols are transported in plasma by lipoproteins, their concentration has been expressed relative to the concentration of plasma total cholesterol (μmol/mmol of cholesterol) to correct for the different number of lipoprotein acceptor particles.
Statistical analyses
All continuous variables were summarized using means and SD or SE. Two-sample t-tests were used to compare baseline variables and intake of selected nutrients. Plasma lipid, phytosterol, cholestanol, and cholesterol precursor concentrations were compared between cases and control subjects using conditional logistic regression, which accounts for the matched nature of the sample. Similarly, multivariable conditional logistic regression adjusted for diastolic blood pressure, LDL- and HDL-cholesterol, triglyceride concentrations, and diabetes and hypertension medication (in addition to matching variables: age, sex, systolic blood pressure, body mass index, and smoking) was used to determine the association between each cholesterol homeostasis marker and CVD status. Triglyceride concentrations and all cholesterol homeostasis markers were log-transformed in the models to correct for their skewed distributions and standardized to express the results on a comparable scale. All analyses were performed using SAS version 8.2 or higher and two-sided P-values < 0.05 were considered statistically significant. Conditional logistic regression was performed using SAS survival routine “PHREG” with case-control status as the stratifying variable and odds ratios (OR) as the risk metric.
RESULTS
Baseline characteristics
The baseline characteristics of the cases and control subjects are depicted in Table 1. Matching ensured that age, sex, body mass index, systolic blood pressure, and smoking status were comparable between cases and controls. No significant differences were observed in body weight, waist circumference, and exogenous hormone use (females; data not shown). Cases, irrespective of sex, had significantly higher plasma glucose concentrations, diastolic blood pressure, and percentage of diabetics, as well as β-blocker and diabetic medication users, relative to the controls. The percentage of hypertensive subjects and those treated for high blood pressure was higher among the male cases relative to controls.
TABLE 1.
Baseline characteristicsa
| Variables | Cases N = 155 [51F/104M] | Controls N = 414 [140F/274M] | Pb | |
|---|---|---|---|---|
| Age (years) | Females | 68.6 ± 1.1 | 67.7 ± 0.6 | Matched |
| Males | 67.0 ± 0.8 | 65.7 ± 0.5 | ||
| All | 67.5 ± 0.7 | 66.4 ± 0.4 | ||
| Body weight (kg) | Females | 73.1 ± 2.2 | 74.8 ± 1.5 | 0.81 |
| Males | 86.1 ± 1.4 | 87.6 ± 0.8 | 0.63 | |
| All | 81.8 ± 1.3 | 83.3 ± 0.8 | 0.47 | |
| Body mass index (kg/m2) | Females | 29.3 ± 0.8 | 29.6 ± 0.6 | Matched |
| Males | 28.7 ± 0.4 | 28.7 ± 0.2 | ||
| All | 28.9 ± 0.4 | 29.0 ± 0.2 | ||
| Waist circumference (cm) | Females | 100.2 ± 2.2 | 100.5 ± 1.4 | 0.91 |
| Males | 102.6 ± 1.0 | 102.2 ± 0.6 | 0.75 | |
| All | 101.8 ± 1.0 | 101.6 ± 0.6 | 0.89 | |
| Glucose (mg/dl) | Females | 120.2 ± 42.3 | 105.5 ± 26.1 | 0.02 |
| Males | 119.9 ± 41.0 | 107.1 ± 28.1 | 0.005 | |
| All | 120.1 ± 41.4 | 106.6 ± 27.4 | 0.0003 | |
| Systolic blood pressure(mm Hg) | Females | 141.8 ± 2.67 | 141.2 ± 1.8 | Matched |
| Males | 132.3 ± 1.8 | 133.6 ± 1.1 | ||
| All | 135.4 ± 1.5 | 136.2 ± 1.0 | ||
| Diastolic blood pressure(mm Hg) | Females | 72.4 ± 1.5 | 75.7 ± 0.8 | 0.06 |
| Males | 72.2 ± 0.8 | 76.7 ± 0.6 | <0.001 | |
| All | 72.3 ± 0.7 | 76.4 ± 0.5 | <0.001 | |
| High diastolic blood pressurec (%) [n] | Females | 8 [4] | 6 [8] | 0.74 |
| Males | 0 [0] | 6 [17] | 0.005 | |
| All | 3 [4] | 6 [25] | 0.09 | |
| Hypertension (%) [n] | Females | 73 [37] | 61 [86] | 0.16 |
| Males | 61 [63] | 51 [139] | 0.07 | |
| All | 65 [100] | 54 [225] | 0.02 | |
| History of blood pressure (%) [n] | Females | 47 [24] | 45 [63] | 0.80 |
| Males | 29 [30] | 33 [90] | 0.45 | |
| All | 35 [54] | 37 [153] | 0.64 | |
| Treated blood pressure (%) [n] | Females | 51 [26] | 39 [55] | 0.15 |
| Males | 52 [54] | 34 [94] | 0.001 | |
| All | 52 [80] | 36 [149] | 0.001 | |
| Beta-blocker users (%) [n] | Females | 22 [11] | 10 [14] | 0.04 |
| Males | 43 [45] | 10 [27] | <0.0001 | |
| All | 36 [56] | 10 [41] | <0.0001 | |
| Diabetics (%) [n] | Females | 32 [16] | 10 [14] | 0.0003 |
| Males | 29 [30] | 12 [33] | <0.0001 | |
| All | 30 [46] | 11 [47] | <0.0001 | |
| Diabetes medications (%) [n] | Females | 16 [8] | 5 [7] | 0.03 |
| Males | 16 [17] | 6 [17] | 0.002 | |
| All | 16 [25] | 6 [24] | <0.0001 | |
| High glucosed (%) [n] | Females | 29 [15] | 8 [11] | 0.0001 |
| Males | 24 [25] | 10 [27] | 0.0003 | |
| All | 26 [40] | 9 [38] | <0.0001 | |
| Smokers (%) [n] | Females | 24 [12] | 22 [31] | Matched |
| Males | 12 [12] | 12 [32] | ||
| All | 15 [24] | 15 [63] |
Values are mean (SE).
P-value based on two sample t-test.
≥90 mm Hg.
≥126 mg/dl.
ApoE genotyping data was available for 489 subjects (N = 138 cases and N = 351 controls). Subjects were grouped as follows: “E2” combined E2/E2 and E3/2 carriers, “E3” included E3/E3 carriers, and “E4” combined E4/E3 and E4/E4 carriers. The genotype distribution for cases was 20/118/0 (% distribution = 14/86/0) and for the controls was 50/398/3 (% distribution = 14/85/1) (apo E2/E3/E4, respectively). These distributions were not significantly different (Fisher's Exact test). Data on the distribution of polymorphisms in genes related to cholesterol metabolism such as ATP-binding cassette (ABC)G8, ABCG5, and NPC1L1 were available in ∼258–314 subjects (supplementary Table I). No significant differences in distribution of these polymorphisms were observed between cases and controls. It must be noted that this study was not designed or powered to examine the association between polymorphisms in these genes and variations in plasma concentrations of plant sterols.
Dietary profile
No significant difference was observed in macronutrient, fatty acid, cholesterol, fiber, alcohol, or vitamin E intakes between the case and control subjects (Table 2). The phytosterol content of the diets could not be calculated from the data available; however, the polyunsaturated-saturated fat ratio, which has been shown to correlate with the phytosterol content of the diet (17), was similar between both groups. It should be noted that the FFQ data were collected prior to the introduction of phytosterol-enriched products into the marketplace.
TABLE 2.
Diet composition determined using FFQa
| Variable | Cases N = 130 | Controls N = 376 | Pb |
|---|---|---|---|
| Energy (kcal) | 1927 ± 617 | 1866 ± 627 | 0.34 |
| Carbohydrate (%E)c | 51.9 ± 9.5 | 51.6 ± 8.9 | 0.76 |
| Protein (%E)c | 16.8 ± 3.4 | 16.7 ± 3.3 | 0.64 |
| Total fat (%E)c | 28.6 ± 6.9 | 29.6 ± 6.4 | 0.15 |
| Saturated fatty acids | 9.9 ± 2.9 | 10.3 ± 2.8 | 0.22 |
| 12:0 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.32 |
| 14:0 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.45 |
| 16:0 | 5.4 ± 1.5 | 5.6 ± 1.4 | 0.16 |
| 18:0 | 2.6 ± 0.8 | 2.7 ± 0.8 | 0.25 |
| MUFA | 10.7 ± 2.6 | 11.2 ± 2.7 | 0.09 |
| 16:1n-6 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.17 |
| 18:1n-6 | 9.8 ± 2.5 | 10.3 ± 2.5 | 0.09 |
| PUFA | 5.5 ± 1.8 | 5.5 ± 1.6 | 0.67 |
| 18:2n-6 | 4.6 ± 1.6 | 4.7 ± 1.4 | 0.54 |
| 18:3 n-3 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.74 |
| 20:4n-6 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.84 |
| 20:5n-3 | 0.04 ± 0.04 | 0.04 ± 0.04 | 0.78 |
| 22:6n-3 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.82 |
| Polyunsaturated-saturated fat ratio | 0.58 ± 0.02 | 0.55 ± 0.01 | 0.74 |
| Cholesterol (mg/d) | 243 ± 127 | 236 ± 118 | 0.59 |
| Fiber (g/d) | 19.7 ± 8.2 | 18.8 ± 7.7 | 0.24 |
| Alcohol (%E)c | 6.7 ± 10.5 | 6.1 ± 11.3 | 0.60 |
| Vitamin E (mg/d)d | 95 ± 181 | 116 ± 213 | 0.28 |
Values are mean (SE).
P-value based on two sample t-test.
Percentage of energy.
Includes supplements.
Plasma lipoprotein profile and cholesterol homeostasis markers
Total and LDL-cholesterol concentrations were similar between the cases and controls (Table 3). Female cases were characterized by higher triglyceride concentrations and male cases by lower HDL-cholesterol concentrations compared with their respective controls.
TABLE 3.
Plasma lipoprotein profile and cholesterol homeostasis markersa
| Variable | Cases (N = 155) | Controls (N = 414) | Pb | |
|---|---|---|---|---|
| Plasma lipids and lipoproteins (mg/dl)c | ||||
| Total cholesterol | Females | 227.8 ± 6.2 | 219.4 ± 2.8 | 0.14 |
| Males | 193.4 ± 3.6 | 198.8 ± 2.2 | 0.28 | |
| All | 204.7 ± 3.4 | 205.7 ± 1.8 | 0.97 | |
| LDL-cholesterol | Females | 138.0 ± 5.2 | 133.9 ± 2.4 | 0.52 |
| Males | 123.5 ± 3.0 | 126.1 ± 2.0 | 0.60 | |
| All | 128.1 ± 2.7 | 128.8 ± 1.5 | 0.94 | |
| HDL-cholesterol | Females | 54.7 ± 2.1 | 57.4 ± 1.3 | 0.31 |
| Males | 40.8 ± 1.1 | 44.3 ± 0.8 | 0.02 | |
| All | 45.3 ± 1.1 | 48.7 ± 0.8 | 0.01 | |
| Triglycerides | Females | 213.0 ± 40.2 | 139.9 ± 5.2 | 0.04 |
| Males | 145.5 ± 8.9 | 147.3 ± 6.2 | 0.72 | |
| All | 167.7 ± 14.6 | 144.8 ± 4.5 | 0.15 | |
| Cholesterol synthesis markers (102 mmol/mol of cholesterol) | ||||
| Squalene | Females | 36.0 ± 2.6 | 34.8 ± 1.6 | 0.31 |
| Males | 34.0 ± 1.9 | 35.0 ± 1.3 | 0.76 | |
| All | 34.7 ± 1.5 | 35.0 ± 1.0 | 0.40 | |
| Desmosterol | Females | 75.2 ± 5.8 | 77.2 ± 3.4 | 0.15 |
| Males | 72.1 ± 3.5 | 74.3 ± 1.9 | 0.05 | |
| All | 73.2 ± 3.0 | 75.3 ± 1.7 | 0.02 | |
| Lathosterol | Females | 128.1 ± 7.0 | 149.5 ± 5.5 | 0.01 |
| Males | 109.9 ± 4.4 | 132.2 ± 3.6 | <0.0001 | |
| All | 115.9 ± 3.8 | 138.0 ± 3.1 | <0.0001 | |
| Cholesterol absorption markers (102 mmol/mol of cholesterol) | ||||
| Campesterol | Females | 220.9 ± 11.4 | 183.8 ± 6.2 | 0.001 |
| Males | 232.8 ± 8.8 | 201.5 ± 4.3 | <0.0001 | |
| All | 228.9 ± 7.0 | 195.5 ± 3.6 | <0.0001 | |
| Sitosterol | Females | 167.7 ± 11.4 | 131.8 ± 5.0 | 0.001 |
| Males | 169.0 ± 7.1 | 157.7 ± 4.0 | 0.008 | |
| All | 168.6 ± 6.0 | 149.0 ± 3.2 | <0.0001 | |
| Cholestanol | Females | 143.2 ± 8.5 | 134.0 ± 5.0 | 0.11 |
| Males | 144.3 ± 6.3 | 135.5 ± 3.4 | 0.02 | |
| All | 144.0 ± 5.0 | 135.0 ± 2.8 | 0.006 | |
| Cholesterol synthesis/absorption ratios | ||||
| Lathosterol/campesterol | Females | 0.64 ± 0.05 | 0.93 ± 0.04 | 0.0001 |
| Males | 0.54 ± 0.03 | 0.73 ± 0.02 | <0.0001 | |
| All | 0.57 ± 0.02 | 0.80 ± 0.02 | <0.0001 | |
| Lathosterol/sitosterol | Females | 0.97 ± 0.10 | 1.38 ± 0.08 | 0.0002 |
| Males | 0.77 ± 0.04 | 1.01 ± 0.04 | <0.0001 | |
| All | 0.83 ± 0.04 | 1.14 ± 0.04 | <0.0001 |
Values are mean (SE).
P-value based on conditional logistic regression; triglycerides and synthesis and absorption markers were log-transformed in the analysis to correct for their skewed distributions.
To convert values for cholesterol and triglycerides to mmol/l, divide by 38.67 and 88.54, respectively.
Concentrations of all the cholesterol absorption markers assessed were significantly higher (15%, 12%, and 6% for campesterol, sitosterol, and cholestanol, respectively) in the cases compared with the controls. Conversely, concentrations of the cholesterol synthesis markers demosterol and lathosterol were significantly lower (3% and 16%, respectively) in the cases compared with the controls. However, the early precursor in the cholesterol biosynthetic pathway, squalene, was not significantly different between groups. Taken together, these alterations resulted in a 29% and 27% lower lathosterol-campesterol and lathosterol-sitosterol ratio, respectively, in cases compared with controls (both P < 0.0001). These ratios provide an overall assessment of cholesterol homeostasis, as they take into account the relative contributions of cholesterol synthesis as well as absorption (30).
To better understand the relationship between the cholesterol homeostasis markers and CVD risk factors, correlation coefficients were calculated (Table 4). In both cases and controls, squalene, an early precursor in the cholesterol synthetic pathway, was positively associated with plasma HDL-cholesterol concentrations and negatively associated with plasma triglyceride concentrations. In the controls but not in the cases, desmosterol was negatively associated with total and LDL-cholesterol concentrations, while lathosterol was negatively associated with triglyceride concentrations. While these findings are consistent with those reported by other investigators (31–33), the correlations are relatively modest and the clinical relevance is unclear.
TABLE 4.
Correlation between lipid risk factors and cholesterol homeostasis markersa
| Cases |
Controls |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | TC | LDL-C | HDL-C | TG | TC | LDL-C | HDL-C | TG |
| Cholesterol synthesis markers | ||||||||
| Squalene | −0.06 | −0.06 | −0.17b | 0.35d | −0.06 | −0.16c | −0.17d | 0.29d |
| Desmosterol | −0.03 | −0.05 | −0.11 | 0.08 | -0.12b | −0.10b | −0.04 | −0.04 |
| Lathosterol | 0.08 | 0.06 | 0.04 | 0.09 | 0.02 | −0.07 | 0.05 | 0.16d |
| Cholesterol absorption markers | ||||||||
| Campesterol | −0.01 | −0.03 | 0.01 | −0.08 | −0.09 | −0.06 | −0.06 | −0.07 |
| Sitosterol | −0.10 | −0.07 | 0.08 | −0.16b | −0.07 | −0.01 | −0.05 | −0.08 |
| Cholestanol | −0.39d | −0.36d | −0.13 | −0.15 | −0.27d | −0.26d | 0.02 | −0.07 |
| Cholesterol synthesis-absorption ratio | ||||||||
| Lathosterol-campesterol | 0.08 | 0.03 | 0.03 | 0.13 | 0.08 | −0.02 | 0.08 | 0.18d |
| Lathosterol-sitosterol | 0.12 | 0.09 | −0.03 | 0.18b | 0.06 | −0.04 | 0.07 | 0.17d |
Pearson product moment correlation coefficient; triglycerides and cholesterol synthesis and absorption markers were log-transformed during analysis to correct for their skewed distributions.
P < 0.05.
P < 0.01.
P < 0.001.
In terms of the cholesterol absorption markers, correlation coefficients were slightly stronger, with an inverse relation being observed between cholestanol and plasma total and LDL-cholesterol concentrations in both cases and controls. Likewise, in both groups, the cholesterol synthesis: absorption ratios were positively associated with plasma triglyceride concentrations. These data suggest that the cholesterol homeostasis markers are more closely associated with plasma triglyceride concentrations than LDL-cholesterol concentrations, which is consistent with the plasma lipid profile for this cohort.
In univariate analysis, no significant association was observed between plasma total and LDL-cholesterol concentrations and prevalent CVD or stenosis (data not shown). Higher plasma HDL-cholesterol concentrations were associated with a lower CVD risk in males (OR per 1 SD [12.8 mg/dl] of HDL = 0.73, 95% confidence interval (CI) [0.56–0.94]), whereas higher plasma triglyceride concentrations were associated with a higher CVD prevalence in females (as triglyceride level increases by 66%, i.e., from 100 to 166 mg/dl, likelihood of CVD increases by 42%: OR = 1.42, 95% CI [1.02-1.99]).
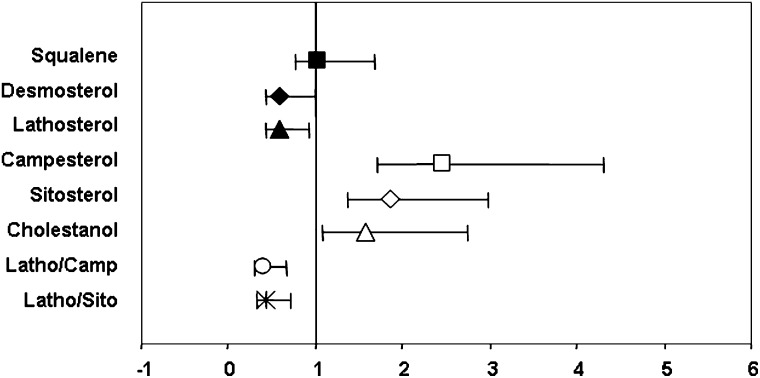
In multiple conditional logistic regression, after controlling for standard lipid and nonlipid risk factors, diabetes, and medications (Fig. 1), the cholesterol absorption markers campesterol, sitosterol, and cholestanol were associated with a 2.47-, 1.86-, and 1.57-fold increase in CVD risk, respectively. In contrast, the ORs for the cholesterol synthesis markers desmosterol and lathosterol were 0.59 and 0.58, which translates into a 0.41 and 0.42 reduction in CVD risk, respectively (all values are OR per 1 SD in log marker concentration, corresponding to a 47% increase in campesterol, 56% increase for both sitosterol and cholestanol, 62% increase in desmosterol, and 54% increase in lathosterol; P < 0.05). The cholesterol synthesis-absorption ratios were associated with an ∼30% lower CVD risk (per 1 SD of log ratio, corresponding to 72% and 88% increases in lathosterol-campesterol and lathosterol-sitosterol ratios, respectively).
Fig. 1.
Multivariable-adjusted odd ratios for cholesterol homeostasis markers and CVD risk based on conditional logistic regression. Error bars represent 95% CI.
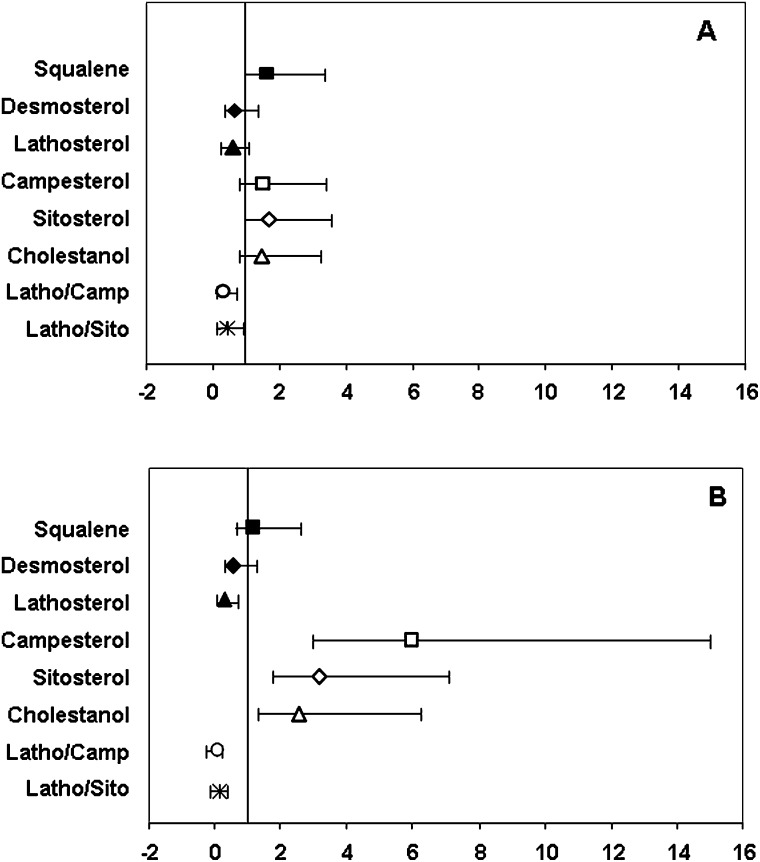
To further explore these associations, the distribution of each marker was divided into tertiles and the OR was calculated using the lowest tertile as referent (Fig. 2). The results were similar to those reported above, although a more pronounced difference was observed in the magnitude of the risk when the highest tertile was compared with the referent (Fig. 2B). Specifically, the cholesterol absorption markers campesterol, sitosterol, and cholestanol were associated with a 5.98-, 3.16-, and 2.57-fold increase in CVD risk (highest vs. lowest tertile), respectively. In contrast, the cholesterol synthesis marker lathosterol was associated with a 0.29-fold decrease in CVD risk, respectively highest vs. lowest tertile (all P-values <0.05).
Fig. 2.
Multivariable-adjusted odd ratios for cholesterol homeostasis markers and CVD risk based on conditional logistic regression. Error bars represent 95% CI per middle tertile (A) and highest tertile 2 (B) relative to the lowest tertile used as referent.
DISCUSSION
Present study results demonstrate that individuals with CVD or ≥50% carotid stenosis have an altered cholesterol homeostasis profile, characterized by higher cholesterol absorption (plasma campesterol, sitosterol, and cholestanol concentrations) and lower cholesterol synthesis (desmosterol and lathosterol concentrations) relative to matched control subjects with comparable plasma cholesterol concentrations. Furthermore, the cholesterol homeostasis markers were highly significant predictors of CVD risk relative to established risk factors in this study population.
A few small-scale studies (32–35) and five population-based studies (31, 36–39) have assessed the relationship between cholesterol homeostasis and CVD risk. Consistent with the present findings, all the small-scale studies (32–35) reported significantly higher cholesterol absorption marker concentrations relative to control subjects. In the two studies (32, 35) that also measured cholesterol synthesis marker concentrations, one showed a significantly lower plasma lathosterol concentration, but the other did not find any difference between groups.
Among the population-based studies, results have been contradictory. The Dallas Heart Study (37) reported no significant difference in cholesterol absorption markers in subjects with and without a family history of CAD. A subset of subjects from each group also underwent coronary artery calcification scoring, but again, no difference was found in the concentration of the absorption markers. Cholesterol synthesis markers were not evaluated in this study. It must be noted that subjects were classified on the basis of family history of CAD, not personal history of disease, and only a subset of subjects underwent coronary artery calcification scoring, thus reducing the discriminating power of the data. Additionally, the average age of the study participants was lower than in previous studies; consequently, one would expect the extent and severity of disease in these subjects to be lower. Given that alterations in cholesterol homeostasis have been shown to be related to the severity of CAD (33), this could potentially explain the lack of association observed in the above study.
The Prospective Cardiovascular Munster study (31) and a preliminary report from the MONICA/KORA study (39) found significant positive associations between cholesterol absorption markers and risk of incident MI in men. The increase in risk was 1.8 for sitosterol in the Prospective Cardiovascular Munster study and 3.0 for campesterol in MONICA/KORA. This is consistent with what we observed in the Framingham Offspring Cohort. In contrast, the EPIC-Norfolk study (36) and the LASA study (38) both reported that increased concentrations of the absorption markers were associated with decreased CVD risk. No associations were observed between the cholesterol synthesis markers and CVD risk.
However, data on diet composition and distribution of genes related to cholesterol homeostasis were not always reported in prior studies, making it difficult to exclude these factors as potential confounders. Additionally, cohorts differed in baseline characteristics such as mean age and plasma lipoprotein profile, which could affect the stage of the disease and thus the magnitude or direction of the associations observed.
The FOS cohort studied provided a unique opportunity to address the relationship between cholesterol homeostasis and CVD risk independent of the potential confounding factor of hypercholesterolemia. CVD was predominantly associated with lower HDL-cholesterol and higher triglyceride concentrations. Cases also exhibited some of the features of metabolic syndrome (40) and had a higher proportion of diabetics. While there are no studies of cholesterol homeostasis in subjects with metabolic syndrome and CVD, it has been reported that individuals with diabetes and CHD have a high cholesterol absorption and low cholesterol synthesis profile (41). Interestingly, in the absence of CHD, individuals with diabetes (42–44) or metabolic syndrome (45, 46) exhibit a low cholesterol absorption and high cholesterol synthesis profile. These findings suggest that shifts in the balance of whole-body cholesterol homeostasis may predispose individuals to the development of CVD. The exact mechanism is unclear, but it may involve the need to increase the influx of cholesterol to the liver, which together with triglyceride is required for the formation and secretion of triglyceride-rich VLDL particles. This greater influx of cholesterol via the LDL receptors would lead to a downregulation of cholesterol synthesis without any appreciable change in circulating LDL-cholesterol concentrations, which is consistent with the lipoprotein profile observed in the cases. Additionally, a decrease in biliary cholesterol secretion could also account for the increased cholesterol influx. Rajaratnam et al. (47) have shown that diminished biliary cholesterol secretion and fecal elimination of cholesterol as bile acids and neutral steroids, as well as reduced cholesterol synthesis, were associated with increased risk of CAD in women with normal LDL-cholesterol concentrations but low HDL-cholesterol concentrations.
In addition to using plasma phytosterol concentrations as indicators of cholesterol absorption, it has been suggested that these sterols themselves are atherogenic. The observation of premature heart disease in patients with sitosterolemia, caused by mutations in either of two ABC half transporters, ABCG5 or ABCG8 and characterized by >50-fold elevation in plasma phytosterol concentrations, has stimulated this hypothesis (48). The cross-sectional nature of this study precludes directly testing this hypothesis.
A limitation of this study is that the observational design does not rule out the possibility that the associations observed could have been influenced by potential confounders. While we controlled for some of the confounders (medication use, diabetes, diastolic blood pressure) and showed that factors known to be associated with cholesterol homeostasis, including dietary intake and distribution of specific genotypes and genetic polymorphisms, were not different between cases and controls, we cannot exclude the influence of unmeasured factors. Second, the phytosterol content of the diets could not be assessed. However, as stated earlier, the FFQ data were collected prior to the introduction of phytosterol-enriched products into the marketplace, which eliminated confounding from this source. While we acknowledge the limitations associated with the FFQ, there is no reason to suspect that the bias was more predominant in one group over the other. It should also be noted that in addition to measuring campesterol and sitosterol concentrations, we measured cholestanol concentrations as a marker of cholesterol absorption and the results were in agreement. Cholestanol is not a phytosterol and is therefore independent of dietary influences. Third, only a single determination of cholesterol absorption and synthesis markers was made during Cycle 6, which could have introduced some variability in the results due to diurnal and time variations. To minimize this variation, only fasted samples collected according to a strict protocol within a specified timeframe were used. Finally, the FOS population studied was predominantly Caucasian and had a unique lipoprotein profile, which helped address the question of whether there was an independent effect of cholesterol homeostasis on CVD, but care must be taken in extrapolating the results to other populations, ethnicities, and disease states.
In conclusion, present results indicate that alterations in cholesterol homeostasis, namely high cholesterol absorption and low cholesterol synthesis, are associated with increased CVD risk in a subset of men and women with similar plasma LDL-cholesterol concentrations. Additionally, the cholesterol homeostasis markers appear to be better predictors of disease than traditional lipid risk factors in this study population.
Supplementary Material
Acknowledgments
The authors are grateful to the FOS participants and staff, Peter Wilson for support and encouragement, Dr. Jose Ordovas for the apoE genotyping, Dr. Michael Dean for the data on ABCG8 (A632V), ABCG8 (C54Y), ABCG8 (K400K), and ABCG5 (Q604E), Dr. Eliana Polisecki for the NPCILI and ABCG8 (D19H) data, and Gail Rogers for programming assistance with the FFQ data.
Footnotes
Abbreviations:
- ABC
- ATP-binding cassette
- CAD
- coronary artery disease
- CI
- confidence interval
- CVD
- cardiovascular disease
- E
- energy
- FFQ
- food frequency questionnaire
- FOS
- Framingham Offspring Study
- OR
- odds ratio
- MI
- myocardial infarction
This work was supported by grant HL-074388 (N.R.M., J.M.L., P.F.J., E.J.S., A.H.L.) from the National Institutes of Health, NO1-HC-25195 (M.P., R.B.D.), and the US Department of Agriculture, under agreement no. 58-1950-4-401. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of authors and do not necessarily reflect the view of the US Department of Agriculture.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of a table.
REFERENCES
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421. [PubMed] [Google Scholar]
- 2.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. for Cholesterol Treatment Trialists' (CTT) Collaborators. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group. 2002. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 360: 23–33.12114037 [Google Scholar]
- 4.Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. 1998. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 339: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H., Arakawa K., Itakura H., Kitabatake A., Goto Y., Toyota T., Nakaya N., Nishimoto S., Muranaka M., Yamamoto A., et al. MEGA Study Group. 2006. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 368: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 6.Sacks F. M., Pfeffer M. A., Moye L. A., Rouleau J. L., Rutherford J. D., Cole T. G., Brown L., Warnica J. W., Arnold J. M., Wun C. C., et al. 1996. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N. Engl. J. Med. 335: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 7.Scandinavian Simvastatin Survival Study Group. 1994. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 344: 1383–1389. [PubMed] [Google Scholar]
- 8.Thavendiranathan P., Bagai A., Brookhart M. A., Choudhry N. K. 2006. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 166: 2307–2313. [DOI] [PubMed] [Google Scholar]
- 9.West of Scotland Coronary Prevention Study Group. 1995. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Engl. J. Med. 333: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 10.Genest J., Jr., McNamara J. R., Ordovas J. M., Jenner J. L., Silberman S. R., Anderson K. M., Wilson P. W., Salem D. N., Schaefer E. J. 1992. Lipoprotein cholesterol, apolipoprotein A-I and B and lipoprotein (a) abnormalities in men with premature coronary artery disease. J. Am. Coll. Cardiol. 19: 792–802. [DOI] [PubMed] [Google Scholar]
- 11.Scandinavian Simvastatin Survival Study Writing Group. 1995. Baseline serum cholesterol and treatment effect in the Scandinavian Simvastatin Survival Study (4S). Lancet. 345: 1274–1275. [PubMed] [Google Scholar]
- 12.Kruit J. K., Groen A. K., van Berkel T. J., Kuipers F. 2006. Emerging roles of the intestine in control of cholesterol metabolism. World J. Gastroenterol. 12: 6429–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson P. A., Rudel L. L. 1999. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 10: 315–320. [DOI] [PubMed] [Google Scholar]
- 14.Matthan N. R., Lichtenstein A. H. 2004. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 174: 197–205. [DOI] [PubMed] [Google Scholar]
- 15.Santosa S., Varady K. A., AbuMweis S., Jones P. J. H. 2007. Physiological and therapeutic factors affecting cholesterol metabolism: does a reciprocal relationship between cholesterol absorption and synthesis really exist? Life Sci. 80: 505–514. [DOI] [PubMed] [Google Scholar]
- 16.Kesaniemi Y. A., Miettinen T. A. 1987. Cholesterol absorption efficiency regulates plasma cholesterol level in the Finnish population. Eur. J. Clin. Invest. 17: 391–395. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen T. A., Tilvis R. S., Kesaniemi Y. A. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131: 20–31. [DOI] [PubMed] [Google Scholar]
- 18.Kuksis A. 2001. Plasma non-cholestrol sterols. J. Chromatogr. A. 935: 203–236. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkhem I., Miettinen T., Reihner E., Ewerth S., Angelin B., Einarsson K. 1987. Correlation between serum levels of some cholesterol precursors and activity of HMG-CoA reductase in human liver. J. Lipid Res. 28: 1137–1143. [PubMed] [Google Scholar]
- 20.Matthan N. R., Raeini-Sarjaz M., Lichtenstein A. H., Ausman L. M., Jones P. J. H. 2000. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 35: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 21.Feinleib M., Kannel W. B., Garrison R. J., McNamara P. M., Castelli W. P. 1975. The Framingham Offspring Study: design and preliminary data. Prev. Med. 4: 518–525. [DOI] [PubMed] [Google Scholar]
- 22.Dawber T. R., Meadors G. F., Moore F. 1951. Epidemiological approaches to heart disease: the Framingham Study. Am. J. Public Health. 41: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel W. B., Feinleib M., McNamara P. M., Garrison R. J., Castelli W. P. 1979. An investigation of coronary heart disease in families. The Framingham offspring study. Am. J. Epidemiol. 110: 281–290. [DOI] [PubMed] [Google Scholar]
- 24.Rimm E. B., Giovannucci E. L., Stampfer M. J., Colditz G. A., Litin L. B., Willett W. C. 1992. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 135: 1114–1126. [DOI] [PubMed] [Google Scholar]
- 25.Willett W. C., Sampson L., Stampfer M. J., Rosner B., Bain C., Witschi J., Hennekens C. H., Speizer F. E. 1985. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 122: 51–65. [DOI] [PubMed] [Google Scholar]
- 26.McNamara J. R., Schaefer E. J. 1987. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin. Chim. Acta. 166: 1–8. [DOI] [PubMed] [Google Scholar]
- 27.Warnick G. R., Benderson J., Albers J. J. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 28: 1379–1388. [PubMed] [Google Scholar]
- 28.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 29.Matthan N. R., Giovanni A., Schaefer E. J., Brown B. G., Lichtenstein A. H. 2003. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J. Lipid Res. 44: 800–806. [DOI] [PubMed] [Google Scholar]
- 30.Nissinen M. J., Gylling H., Miettinen T. A. 2008. Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. Br. J. Nutr. 99: 370–378. [DOI] [PubMed] [Google Scholar]
- 31.Assmann G., Cullen P., Erbey J., Ramey D. R., Kannenberg F., Schulte H. 2005. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr. Metab. Cardiovasc. Dis. 16: 13–21. [DOI] [PubMed] [Google Scholar]
- 32.Rajaratnam R. A., Gylling H., Miettinen T. A. 2000. Independent association of serum squalene and noncholesterol sterols with coronary artery disease in postmenopausal women. J. Am. Coll. Cardiol. 35: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland W. H. F., Willaims M. J. A., Nye E. R., Restieaux N. J., de Jong S. A., Walker H. L. 1998. Association of plasma noncholesterol sterol levels with severity of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 8: 386–391. [Google Scholar]
- 34.Glueck C. J., Speirs J., Tracy T., Streicher P., Illig E., Vandegrift J. 1991. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism. 40: 842–848. [DOI] [PubMed] [Google Scholar]
- 35.Sudhop T., Lutjohann D., Agna M., von Ameln C., Prange W., von Bergmann K. 2003. Comparison of the effects of sitostanol, sitostanol acetate, and sitostanol oleate on the inhibition of cholesterol absorption in normolipemic healthy male volunteers. A placebo controlled randomized cross-over study. Arzneimittelforschung. 53: 708–713. [DOI] [PubMed] [Google Scholar]
- 36.Pinedo S., Vissers M. N., von Bergmann K., Elharchaoui K., Lutjohann D., Luben R., Wareham N. J., Kastelein J. J. P., Khaw K-T., Boekholdt S. M. 2007. Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk population study. J. Lipid Res. 48: 139–144. [DOI] [PubMed] [Google Scholar]
- 37.Wilund K. R., Yu L., Xu F., Vega G. L., Grundy S. M., Cohen J. C., Hobbs H. H. 2004. No association between plasma levels of plant sterols and atherosclerosis in mice and men. Arterioscler. Thromb. Vasc. Biol. 24: 2326–2332. [DOI] [PubMed] [Google Scholar]
- 38.Fassbender K., Lutjohann D., Dik M. G., Bremmer M., Konig J., Walter S., Liu Y., Letiembre M., Von Bergmann K., Jonker C. 2008. Moderately elevated plant sterol levels are associated with reduced cardiovascular risk–the LASA study. Atherosclerosis. 196: 283–288. [DOI] [PubMed] [Google Scholar]
- 39.Thiery J., Ceglarek U., Fiedler G. M., Leichtle A., Baumann S., Teupser D., Lang O., Baumert J., Meisinger C., Loewel H., et al. 2006. Elevated campesterol serum levels: a significant predictor of incident myocardial infarction: results of the population-based MONICA/KORA follow-up study 1994 to 2005. Circ. Suppl. 114: Abs. 4099. [Google Scholar]
- 40.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2001. Executive summary of the third report of the National Cholesterol Education Program (NCEP). JAMA. 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 41.Gylling H., Miettinen T. A. 1996. Cholesterol absorption and lipoprotein metabolism in type II diabetes mellitus with and without coronary artery disease. Atherosclerosis. 126: 325–332. [DOI] [PubMed] [Google Scholar]
- 42.Gylling H., Miettinen T. A. 1997. Cholesterol absorption, synthesis and LDL metabolism in NIDDM. Diabetes Care. 20: 90–95. [DOI] [PubMed] [Google Scholar]
- 43.Briones E. R., Steiger D. L., Palumbo P. J., O'Fallon W. M., Langworthy A. L., Zimmerman B. R., Kottke B. A. 1986. Sterol excretion and cholesterol absorption in diabetics and nondiabetics with and without hyperlipidemia. Am. J. Clin. Nutr. 44: 353–361. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland W. H., Scott R. S., Lintott C. J., Robertson M. C., Stapely S. A., Cox C. 1992. Plasma non-cholestrol sterols in patients with non-insulin dependent diabetes mellitus. Horm. Metab. Res. 24: 172–175. [DOI] [PubMed] [Google Scholar]
- 45.Simonen P., Gylling H., Howard A. N., Miettinen T. 2000. Introducing a new component of the metabolic syndrome: low cholesterol absorption. Am. J. Clin. Nutr. 72: 82–88. [DOI] [PubMed] [Google Scholar]
- 46.Anderson G. H., Woodend D. 2003. Effect of glycemic carbohydrates on short-term satiety and food intake. Nutr. Rev. 61: S17–S26. [DOI] [PubMed] [Google Scholar]
- 47.Rajaratnam R. A., Gylling H., Miettinen T. A. 2001. Cholesterol absorption, synthesis, and fecal output in postmenopausal women with and without coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 21: 1650–1655. [DOI] [PubMed] [Google Scholar]
- 48.Berge K. E., von Bergmann K., Lutjohann D., Guerra R., Grundy S. M., Hobbs H. H., Cohen J. C. 2002. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 43: 486–494. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.