Abstract
Carnitine palmitoyltransferase 1 (CPT1) catalyzes the first step in long-chain fatty acid import into mitochondria, and it is believed to be rate limiting for β-oxidation of fatty acids. However, in muscle, other proteins may collaborate with CPT1. Fatty acid translocase/CD36 (FAT/CD36) may interact with CPT1 and contribute to fatty acid import into mitochondria in muscle. Here, we demonstrate that another membrane-bound fatty acid binding protein, fatty acid transport protein 1 (FATP1), collaborates with CPT1 for fatty acid import into mitochondria. Overexpression of FATP1 using adenovirus in L6E9 myotubes increased both fatty acid oxidation and palmitate esterification into triacylglycerides. Moreover, immunocytochemistry assays in transfected L6E9 myotubes showed that FATP1 was present in mitochondria and coimmunoprecipitated with CPT1 in L6E9 myotubes and rat skeletal muscle in vivo. The cooverexpression of FATP1 and CPT1 also enhanced mitochondrial fatty acid oxidation, similar to the cooverexpression of FAT/CD36 and CPT1. However, etomoxir, an irreversible inhibitor of CPT1, blocked all these effects. These data reveal that FATP1, like FAT/CD36, is associated with mitochondria and has a role in mitochondrial oxidation of fatty acids.
Keywords: carnitine palmitoyltransferase 1, malonyl-CoA, mitochondria, FAT/CD36
Fatty acids are a major energy source for skeletal muscle. They are transported into the cell via a protein-mediated mechanism, involving fatty acid translocase/CD36 (FAT/CD36), plasma-membrane-associated fatty acid binding protein, and fatty acid transport protein (FATP1-6) (1). Once inside the cell, fatty acids can be esterified, metabolized to lipid second messengers, such as leukotrienes and eicosanoids, or β-oxidized in the mitochondrion. The first step of the oxidative pathway is the transport of fatty acids into the mitochondrial matrix. This step is controlled by the carnitine palmitoyltransferase (CPT) system, consisting of CPT1, acylcarnitine translocase, and CPT2 (2).
CPT1 catalyzes the conversion of long-chain fatty acyl-CoAs to acylcarnitines in the presence of l-carnitine and is the rate-limiting step in the transport of long-chain fatty acids (LCFAs) from the cytoplasm to the mitochondrial matrix, where they undergo β-oxidation. CPT1 is tightly regulated by its physiological inhibitor malonyl-CoA. This regulation allows CPT1 to signal the availability of lipid and carbohydrate fuels to the cell (2). Mammalian tissues express three isoforms: CPT1A (liver), CPT1B (muscle and heart), and CPT1C (brain) (3–5).
Owing to the metabolic peculiarities of muscle, the regulation of CPT1B by malonyl-CoA is more complex than that of CPT1A in liver. The regulation of muscle CPT1 by malonyl-CoA cannot fully explain changes in mitochondrial fatty acid import. Thus, malonyl-CoA is not responsible for the increase in fatty acid oxidation during the transition from low to moderate aerobic exercise or for the decrease in fatty acid oxidation during more strenuous exercise (6–8). Moreover, concentrations of malonyl-CoA measured in muscle (1–4 µM) (9, 10) should inhibit CPT1 activity at any time, since the IC50 of CPT1B for malonyl-CoA is much lower than this concentration (∼0.03 µM). Several hypotheses have been advanced to explain this discordance between malonyl-CoA levels, CPT1 regulation, and LCFA oxidation in muscle. Thus, alteration in compartmentalization/cellular distribution of malonyl-CoA may be involved in the control of CPT1 activity. It has been proposed that the malonyl-CoA involved in CPT1 regulation is that synthesized by acetyl-CoA carboxylase 2, which resides in the vicinity of CPT1 in the outer mitochondrial membrane and modulates CPT1 activity (11). Moreover, other proteins could also be involved in the transport of fatty acids into mitochondria. Thus, Bonen and coworkers demonstrated that exercise induces the translocation of FAT/CD36 to mitochondria, thus increasing muscle fatty acid oxidation (12).
FATPs are a family of membrane-bound proteins that catalyze the ATP-dependent esterification of LCFA and very-LCFA to their acyl-CoA derivatives (13–15). Mammals express six different forms of FATP (FATP1-6) with tissue-specific expression patterns (14). FATP1 is a 63 kDa protein that is expressed in cells and tissues with high levels of fatty acid uptake for metabolism or storage, such as skeletal muscle and adipose tissue (15). The regulatory role of FATP1 in metabolism has been partially elucidated by gene manipulation. Overexpression of FATP1 in cultured fibroblasts increases fatty acid uptake (16). Cardiac-specific overexpression of FATP1 in transgenic mice increases myocardial fatty acid uptake and free FA accumulation, but not triacylglyceride (TG) levels, and enhances palmitate oxidation leading to a lipotoxic cardiomyopathy (17). In contrast, when overexpressed in cultured human skeletal myotubes, FATP1 stimulates FA uptake and storage as TG, but not oxidation (18). Deletion of FATP1 protects mice from fatty-acid-induced insulin resistance and intramuscular accumulation of fatty acyl-CoAs (19). Furthermore, FATP1 is involved in hormonal regulation of fatty acid uptake, translocating to the plasma membrane in response to insulin in adipocytes and primary skeletal muscle cells (20, 21).
We have recently shown that overexpression of CPT1A in L6E9 myotubes enhanced CPT1 activity up to 15-fold, whereas fatty acid oxidation did not increase more than 2-fold, even when a mutant form of CPT1 insensitive to malonyl-CoA was overexpressed (22). These data indicate that other factors independent of CPT1/malonyl-CoA interaction could be involved in the control of fatty acid oxidation in these cells. In the search for a novel regulator of fatty acid oxidation in skeletal muscle, we examined the effect of FATP1 overexpression on fatty acid oxidation in L6E9 muscle cells. We show that FATP1 localized in mitochondria and coimmunoprecipitated with CPT1. Moreover, FATP1 overexpression enhanced mitochondrial oxidation of fatty acids. We also observed all these effects in cells overexpressing FAT/CD36, which may have a role in this process in muscle (12). These data reveal a new protein involved in β-oxidation and could help us to understand its complex regulation in skeletal muscle.
EXPERIMENTAL PROCEDURES
Materials and reagents
Protran® nitrocellulose membranes for protein analysis were from Scheicher and Schuell (Keene, NH). The enhanced chemifluorescence detection kit from Amersham Biosciences was used for Western blot analysis. The Bradford solution for protein assay was from Bio-Rad Laboratories (Hercules, CA). FBS, DMEM, and antibiotics were from Gibco-Invitrogen. Defatted BSA (BSA), palmitate, and other chemicals were purchased from Sigma-Aldrich. [1-14C]Palmitic acid and [1-14C]palmitoyl-CoA were from GE Healthcare. Antibodies used included rabbit polyclonal anti-FATP1 (sc-14497) and rabbit polyclonal anti-FAT/CD36 (sc-9154) from Santa Cruz Biotechnologies, anti-green fluorescent protein (GFP) (ref. 8360-1) from Clontech, anti-COI of complex IV from Molecular Probes, and anti-porin from Calbiochem. Silica gel 60 TLC plates were from Merck (Rahway, NJ). Etomoxir was provided by H. P. O. Wolf (GMBH, Allensbach, Germany).
Animals
Samples of gastrocnemius and soleus muscle were taken from male Sprague-Dawley rats (180–200 g) bred in our laboratory. Animals were maintained under a 12 h dark/light cycle at 23°C with free access to food and water. All experimental protocols were approved by the Animal Ethics Committee at the University of Barcelona.
Cell culture
The L6E9 rat skeletal muscle cell line was cultured in a humidified atmosphere containing 5% CO2 in DMEM medium containing 10% FBS, 100 units/ml penicillin G, and 100 μg/ml streptomycin and 25 mmol/l HEPES (pH 7.4) (growth medium). Preconfluent myoblasts (80–90%) were induced to differentiate by lowering FBS to a final concentration of 2% (v/v) (differentiation medium). Cells were completely differentiated after 4 days in this medium.
Adenoviral preparation and transduction of L6E9 cells
Ad-CPT1A encoding rat CPT1A, Ad-FATP1 encoding mouse FATP1, Ad-FAT/CD36 encoding rat FAT/CD36, and Ad-LacZ, which expresses bacterial β-galactosidase, were constructed as previously described (18, 23). Adenoviruses were amplified using the human embryonic kidney cell line (HEK 293) as host. Lysates obtained were titrated using the Adeno-XTM Rapid Titer kit (Clontech) and used directly for cell transduction. The titers of the lysates were 2.3 × 109 pfu/ml for Ad-LacZ, 1 × 1010 pfu/ml for Ad-CPT1A, 1 × 1010 pfu/ml for Ad-FAT/CD36, and 3 × 1010 pfu/ml for Ad-FATP1. Myotubes were transduced at day 4 of differentiation with 20 pfu/cell of Ad-LacZ, Ad-FATP1, or Ad-CPT1A and 5 pfu/cell of Ad-FAT/CD36 in serum-free medium for 30 h. After this time, the infection medium was removed and cells were incubated with serum-free medium for a further 16 h.
Transfection of L6E9 cells
The plasmids pFATP1-GFP and pFAT/CD36-GFP, which include an NH2-terminal fusion protein construct with the GFP (20), were transfected in L6E9 myoblasts using FuGENE 6 (Roche) following the manufacturer's guidelines. After transfection, cells were differentiated and used for the experiments.
Measurement of fatty acid oxidation in intact cells
Palmitate oxidation to CO2 and acid-soluble products (ASPs), essentially acyl-carnitine, Krebs cycle intermediates, and acetyl-CoA (24), were measured in L6E9 cells grown in 6-well plates, differentiated, and transduced as described above. On the day of the assay, cells were washed in Krebs-Ringer bicarbonate HEPES buffer (KRBH buffer: 135 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 2 mM NaHCO3, and 10 mM HEPES, pH 7.4) that contained 0.1% BSA, preincubated at 37°C for 30 min in KRBH 1% BSA and washed again in KRBH 0.1% BSA. Cells were then incubated for 3 h at 37°C with fresh KRBH containing 2.5 mM glucose and 0.8 mM carnitine plus 0.25 mM palmitate and 1 μCi/ml [1-14C]palmitate bound to 1% BSA. Oxidation measurements were performed by trapping the radioactive CO2 in a parafilm-sealed system. The reaction was stopped by the addition of 40% perchloric acid through a syringe that pierced the parafilm.
Isolation of mitochondria from L6E9 cells and rat skeletal muscle
Mitochondria-enriched cell fractions from L6E9 myotubes were obtained as previously described (23) with some modifications. Briefly, L6E9 cells were grown in 150 mm dishes, left to differentiate, and transduced as described above. Cells were scraped out and resuspended in 1 ml of solution A (100 mM KCl, 5 mM MgSO4, 5 mM EDTA, 1 mM ATP, and 50 mM Tris-HCl, pH 7.4). Cells were homogenized using a glass homogenizer (20 strokes with both the loose and the tight pestle) and centrifuged at 2,000 g for 3 min at 4°C. The supernatant was centrifuged again at 16,000 g for 30 min at 4°C. The mitochondria-enriched pellet was resuspended in 50–100 µl of solution B (220 mM sucrose, 70 mM mannitol, 1 mM EDTA, and 10 mM Tris-HCl, pH 7.4) and used for immunoprecipitation or palmitate oxidation assays. The quality of mitochondria was assessed measuring the malonyl-CoA-resistant CPT1 activity, attributable basically to CPT2 activity inside the mitochondrion, allowing us to quantify broken mitochondria. According to this method, the integrity of mitochondria was higher than 80% (data not shown).
Mitochondria-enriched fractions from rat muscle were obtained by differential centrifugation. Two soleus muscle samples or one gastrocnemius muscle sample from each animal were homogenized separately in 9 volumes of solution A using an omnimixer and then centrifuged at 1,000 g for 15 min. The pellet was homogenized and centrifuged at 600 g for 10 min. The resulting supernatant was centrifuged at 15,000 g for 15 min, and the pellet was washed twice in solution A and resuspended at 1 µl/mg tissue in solution B.
Measurement of palmitate and palmitoyl-CoA oxidation in isolated mitochondria
L6E9 cells were cultured in 150 mm dishes, differentiated, and transduced as described above. Mitochondria-enriched fractions were obtained and resuspended in solution B. Fatty acid oxidation was measured as described elsewhere (12) with minor modifications. For palmitate oxidation assays, 50 µl (150 µg) of mitochondria and 50 µl of a 2.5 mM 5:1 palmitate-BSA complex containing 10 µCi/ml [1-14C]palmitate (final concentration: 0.25 mM palmitate and 1 µCi/ml [1-14C]palmitate) were incubated for 1 h with agitation in 400 µl of pregassed (37°C for 15 min with 5% CO2-95% O2) modified Krebs Ringer HEPES (MKRH) buffer (115 mM NaCl, 2.6 mM KCl, 1.2 mM KH2PO4, 10 mM NaHCO3, and 10 mM HEPES, pH 7.4) supplemented with 5 mM ATP, 1 mM NAD+, 0.5 mM l-carnitine, 0.1 mM CoA, 0.5 mM malate, and 25 μM cytochrome C (complete MKRH buffer). For palmitoyl-CoA oxidation assays 50 µl (400 µg) of mitochondria and 50 µl of a 2.5 mM 5:1 palmitoyl-CoA-BSA complex containing 1 µCi/ml [1-14C]palmitoyl-CoA was added to the reaction mixture (final concentration: 0.25 mM palmitoyl-CoA and 0.1 µCi/ml [1-14C]palmitoyl-CoA) were incubated for 0.5 h with agitation in 400 µl of pregassed complete MKRH buffer. Oxidation measurements were performed by trapping the radioactive CO2 and ASPs in a parafilm-sealed system in a 6-well plate with agitation. The reaction was stopped by the addition of 40% perchloric acid through a syringe that pierced the parafilm.
Measurement of palmitate incorporation into cellular lipids
Palmitate incorporation into complex lipids was measured in L6E9 cells that were cultured on 6-well plates and pretreated as described above. Cells were incubated for 16 h at 37°C in serum-free medium containing 0.25 mM palmitate and 1 μCi/ml [1-14C]palmitate bound to 1% BSA. On the day of the assay cells were washed in PBS, and lipids were extracted as described previously (23). Total lipids were dissolved in 30 μl of chloroform and separated by TLC to measure the incorporation of labeled fatty acid into phospholipids (PLs), diacylglycerol (DAG), TGs, and nonesterified labeled palmitate (NE palm), as described (22).
Measurement of acyl-CoA synthetase activity
Samples were assayed for acyl-CoA synthetase activity by the conversion of [1-14C]palmitate to its CoA derivative (13). The assay mixture contained, in a total volume of 200 µl: 100 mM Tris-HCl buffer, pH 7.5, 50 µM [1-14C]palmitate (0.2 µCi/ml), 10 mM ATP, 5 mM MgCl2, 200 µM CoA, 200 µM DTT, and 0.4% Triton X-100. The assay was initiated by addition of 5–10 µl of enzyme suspension (30–50 µg) from total or mitochondrial extracts from L6E9 myotubes. The reaction was carried out at 30°C for 5 min. Reactions were terminated with the addition of 1.25 ml of isopropyl alcohol:heptane:H2SO4 (40:10:1, v/v/v), 0.5 ml of water, and 0.75 ml of heptane to facilitate organic phase separation. The aqueous phase was extracted three times with 0.75 ml of heptane to remove unreacted fatty acids, and the radioactivity was determined by liquid phase scintillation counting.
Immunoprecipitation
Immunoprecipitation studies were performed in L6E9 myotubes and rat skeletal muscle. L6E9 cells were grown on 150 mm plates, differentiated, and transduced with the adenoviruses. After transduction, cells were collected in PBS and homogenized with a douncer in 500 μl of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM EGTA, 1 μM PMSF, 25 μg/ml leupeptin, and 25 μg/ml aprotinin) and centrifuged at 700 g for 10 min to remove nuclei, cell debris, and floating cells. For muscle studies, two soleus samples or one gastrocnemius sample were homogenized in 9 volumes of lysis buffer using a polytron. Homogenates were rotated for 1 h at 4°C in an orbital shaker and centrifuged at 5,000 g for 10 min at 4°C. After that, 1,000 or 2,000 μg of protein was immunoprecipitated with 5 μg/ml of either anti-FATP1 or anti-FAT/CD36 antibody. For immunoprecipitation studies in mitochondria, mitochondrial fractions were obtained as described above, 1% Triton X-100 was added to each preparation, and 150 or 300 μg of each fraction was used. Immunoprecipitates were collected on protein A-Sepharose G beads, washed five times in lysis buffer, resuspended, and incubated for 5 min at 95°C in 1× SDS-PAGE sample buffer. Samples were resolved in 8% SDS-PAGE. A CPT1A-specific polyclonal antibody against amino acids 317–430 of the rat CPT1A (25) (1/6,000 dilution) was used for the detection of CPT1A protein in the immunoprecipitates from L6E9 myotubes. A CPT1B-specific antibody against amino acids 259–760 of the rat CPT1B (26) was used (1/1,000 dilution) for detection of CPT1B protein in the immunoprecipitates from rat skeletal muscle.
Immunofluorescence
Cells grown on cover slips were fixed for 15 min with 3% paraformaldehyde in PBS and then rinsed three times with PBS and subsequently incubated for 10 min in PBS containing 50 mM NH4Cl, for 10 min with PBS containing 20 mM glycine, and for 10 min in PBS containing 0.1% triton X-100 and 0.05% colic acid. Cells were then washed three times in PBS 1× and for 30 min in PBS containing 10% goat serum. Thereafter, cover slips were incubated with primary antibodies at 0.5–1.0 µg/ml anti-COI of complex IV (Molecular Probes) and revealed with fluorochrom-conjugated goat secondary antibodies (Texas Red). Cells were rinsed three times with PBS prior to mounting in medium for fluorescence microscopy (Vectashield). Fluorescence images were obtained with a Leica TCS 4D confocal scanning laser microscope adapted to an inverted Leitz DMIRBE microscope and 63× (numerical aperture 1.4 oil) Leitz Plan-Apo objectives. The light source was an argon/krypton laser (75 mW), with which optical sections (0.1 µm) were obtained. Qualitative analyses of colocalization were assessed using the plugin RGB Profiler from the WCIF ImageJ software. A line was drawn arbitrarily over an area of interest and the plot presented the overlap of the staining of each laser (from distinct labeled proteins). Quantitative analyses were performed by calculating Pearson's correlation coefficient and Mander's overlap coefficient, which are both described in the online manual for the WCIF ImageJ collection. In many forms of correlation analysis, the value for Pearson's range from 1 to −1. A value close to 1 does indicates reliable colocalization. The Mander's overlap coefficient ranges from 1 to 0, with 1 being high colocalization and 0 being low. Having defined and eliminated the background noise from the original picture, we used the plugin Intensity Correlation Analysis from WCIF ImageJ software.
Western blotting
Mitochondrial fractions were obtained by differential centrifugation of muscle cell homogenates. Extracts were prepared by scraping L6E9 cell monolayers from 6 cm dishes into 700 µl of homogenization buffer consisting of 250 mM sucrose, 10 mM HEPES (pH 7.4), 1 mM EDTA, 1 μM PMSF, 25 μg/ml leupeptin, 25 μg/ml pepstatin A, and 25 μg/ml aprotinin. Cells were homogenized with the aid of a 22G-syringe (15 times). The homogenates were centrifuged at 1,500 g for 10 min, and the supernatant was then centrifuged at 10,000 g for 10 min to obtain a pellet enriched in mitochondria and other organelles (27). All fractions were stored at −80°C, and an aliquot of the cell extracts was used for the measurement of protein concentration. Fifty micrograms of protein was resolved in 10% SDS-PAGE, and immunoblotting was performed with the corresponding antibody.
Statistical Analysis
Data are expressed as means ± SE. The significance of differences was assessed by a one-way or two-way ANOVA followed by Newman-Keuls test for comparisons post hoc. Differences were considered significant when P < 0.05.
RESULTS
Effect of overexpression of FATP1 on lipid partitioning in L6E9 myotubes
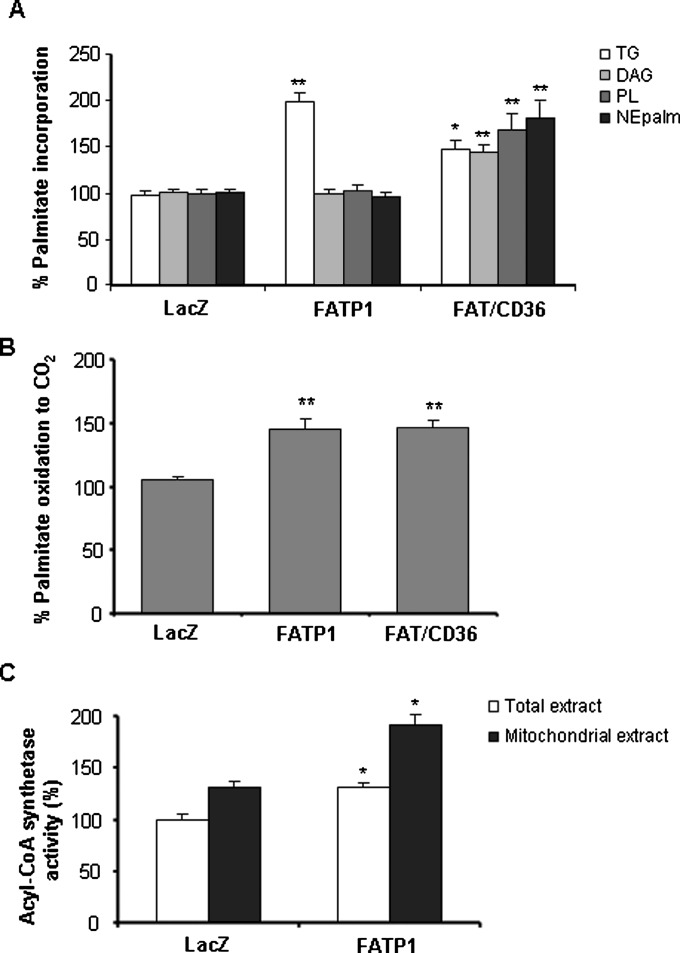
Mouse FATP1 cDNA was delivered into L6E9 myotubes by adenovirus. FATP1 protein levels increased 2-fold in Ad-FATP1-transduced cells compared with control Ad-LacZ-transduced cells (Fig. 1A). To assess the functionality of the overexpressed FATP1, we measured the incorporation of palmitate into lipids and oxidation in intact cells. FATP1 overexpression produced a 2-fold increase in the incorporation of palmitate into TGs (LacZ control value: 0.36 ± 0.04 nmol palmitate/mg protein × h), and no increase was seen for PLs, DAG, or nonesterified palmitate (NEPalm) (LacZ control values in nmol palmitate/mg protein × h: 0.14 ± 0.01, 0.08 ± 0.01, and 0.033 ± 0.003 for PL, DAG, and NEPalm, respectively; Fig. 2A). L6E9 myotubes transduced with Ad-FATP1 showed a 1.5-fold increase in palmitate oxidation to CO2 compared with Ad-LacZ-transduced myotubes (6.5 ± 0.27 nmol palmitate/mg protein × 3 h; Fig. 2B). We also examined the effect of FAT/CD36 overexpression in L6E9 myotubes, a protein that has already been shown to have a role in fatty acid oxidation in muscle (12). Overexpression of FAT/CD36 (2-fold increase in protein levels; Fig. 1B) produced a 1.5- to 1.8-fold increase in palmitate esterification to TG, PL, DAG, and NEPalm (Fig. 2A) and a 1.5-fold increase in palmitate oxidation (Fig. 2B).
Fig. 1.
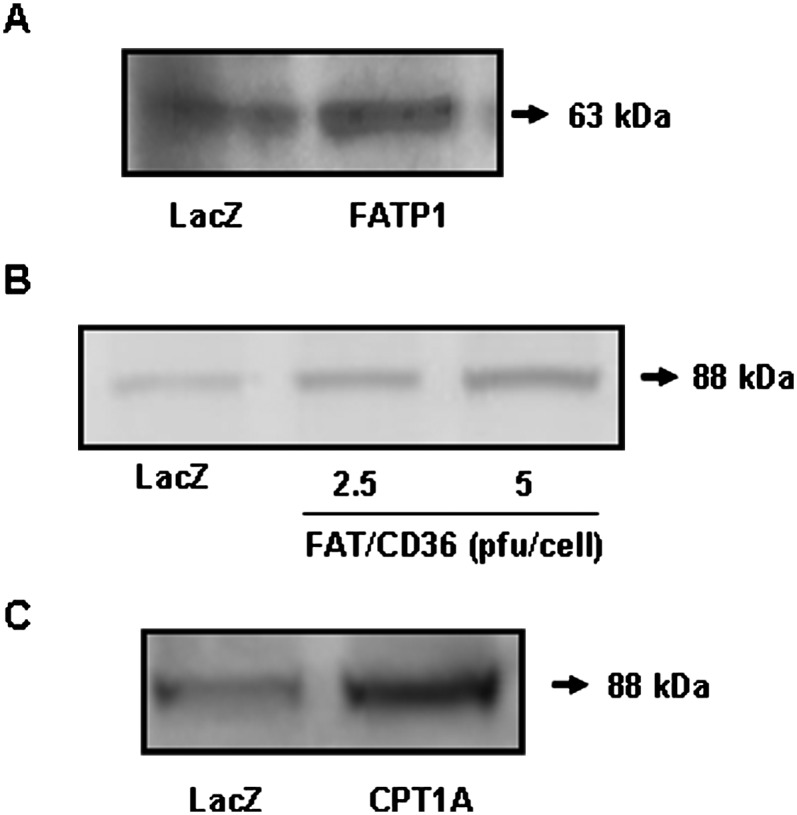
Overexpression of FATP1, FAT/CD36, and CPT1A in L6E9 myotubes. L6E9 myotubes were transduced with Ad-FATP1, Ad-FAT/CD36, Ad-CPT1A, or Ad-LacZ as a control, and a Western blot using a specific antibody against FATP1 (A), FAT/CD36 (B), or CPT1A (C) was performed in 50 μg of total extract.
Fig. 2.
Effect of FATP1 overexpression on palmitate metabolism and acyl-CoA synthetase activity. A: Palmitate incorporation into complex lipids. L6E9 cells that were infected with Ad-LacZ, Ad-FATP1, and Ad-FAT/CD36 were incubated for 16 h in serum-free medium containing 1 μCi/ml [1-14C]palmitic acid and 0.25 mM unlabeled palmitate complexed to 1% (w/v) BSA. Fatty acid incorporation into TGs, DAG, PLs, and NEPalm was assessed using TLC after lipid extraction. B: Fatty acid oxidation. Cells were infected with Ad-LacZ, Ad-FATP1, or Ad-FAT/CD36, and palmitate oxidation to CO2 was measured for 3 h. C: Acyl-CoA synthetase activity was measured in both total and mitochondrial extracts coming from L6E9 myotubes transduced with Ad-LacZ or Ad-FATP1. Data are the mean SE of five experiments performed in triplicate. *P 0.01 versus Ad-LacZ; **P 0.001 versus Ad-LacZ.
Effect of FATP1 overexpression on acyl-CoA synthetase activity
As FATP1 has acyl-CoA synthetase activity (13), we evaluated whether the overexpression of FATP1 in L6E9 myotubes was followed by an increase in this activity. We determined the conversion of palmitate into palmitoyl-CoA in both total and mitochondria-enriched extracts. Acyl-CoA synthetase activity increased by 30% and 70% in total and mitochondrial extracts, respectively, in FATP1-overexpressing L6E9 myotubes compared with LacZ control cells (control values: 2.99 ± 0.16 and 3.91 ± 0.37 nmol palmitoyl-CoA/mg protein × min in total and mitochondrial extracts, respectively; Fig. 2C).
Effect of FATP1 on fatty acid oxidation in isolated mitochondria
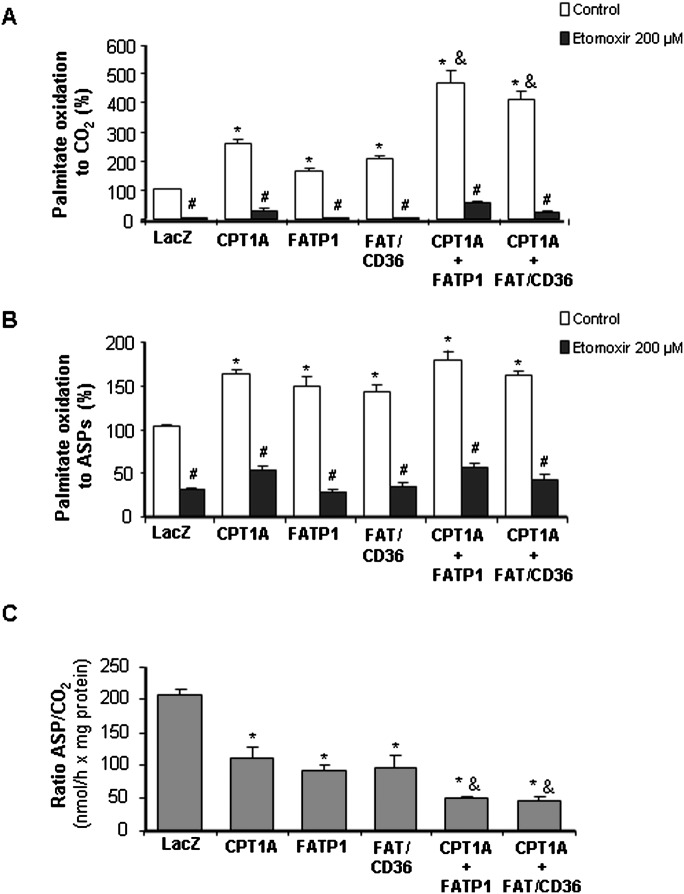
To assess the role of FATP1 in fatty acid oxidation, we measured palmitate oxidation in mitochondria-enriched fractions. Palmitate oxidation to CO2 increased 1.7-fold in mitochondria isolated from FATP1-overexpressing cells compared with Ad-LacZ infected cells (0.16 ± 0.009 nmol palmitate/mg protein × h; Fig. 3A). As a positive control, we also evaluated palmitate oxidation in CPT1-overexpressing cells. Overexpression of CPT1A (3-fold increase in protein levels; Fig. 1C), the endogenous CPT1 isoform expressed in these cells (22), produced a 2.6-fold increase in mitochondrial palmitate oxidation to CO2. Moreover, overexpression of FAT/CD36 also increased palmitate oxidation to CO2 by 2.0-fold. To further evaluate the role of FATP1 in fatty acid oxidation, we overexpressed both FATP1 and CPT1 proteins together. The overexpression of CPT1A and FATP1 enhanced fatty acid oxidation to CO2 by 4.7-fold, showing an additive effect (Fig. 3A). Similarly, cooverexpression of CPT1A and FAT/CD36 also produced an additive effect on palmitate oxidation to CO2 (4.1-fold increase). Palmitate oxidation to ASPs, essentially consisting of acyl-carnitines, Krebs cycle intermediates, and acetyl-CoA, was increased from 1.4- to 1.8-fold in CPT1A-, FAT/CD36-, FATP1-, CPT1A+FAT/CD36-, and CPT1A+FATP1-overexpessing cells compared with Ad-LacZ control cells (36.1 ± 1.5 nmol palmitate/mg protein × h; Fig. 3B). When mitochondria were incubated with etomoxir, an irreversible inhibitor of CPT1, the increments in fatty acid oxidation in FATP1- and FAT/CD36-overexpressing cells were blunted, confirming that CPT1 activity is necessary for fatty acid import into mitochondria (Fig. 3A, B). We also determined the ratio of incomplete (ASPs) versus complete (CO2) oxidation as a measure of mitochondrial fatty acid oxidation efficiency. The overexpression of CPT1A, FATP1, and FAT/CD36 produced a 30–40% decrease in the ratio ASP/CO2. Importantly, the cooverexpression of CPT1A together with FATP1 or FAT/CD36 produced a 50–60% decrease in this ratio (Fig. 3C), indicating that the overexpression of these proteins increased fatty acid oxidation efficiency.
Fig. 3.
Palmitate oxidation in mitochondrial fractions. Mitochondrial fractions that were obtained from cells infected with Ad-LacZ, Ad-FATP1, Ad-CPT1A, or Ad-FAT/CD36 were incubated for 1 h with agitation in 400 μl of pregassed complete MKRH buffer and 50 μl of a 2.5 mM 5:1 palmitate-BSA complex containing 10 μCi/ml [1-14C]palmitic acid, and palmitate oxidation to CO2 (A) and to ASPs (B) was measured. The ratio of ASPs/CO2 was also calculated (C). Data are the mean SE of five experiments performed in duplicate. *P < 0.01 versus Ad-LacZ, **P < 0.001 versus Ad-LacZ, &P < 0.001 versus the overexpression of only one protein (CPT1A, FATP1, or FAT/CD36), and #P < 0.01 versus control without etomoxir.
To assess whether the role of FATP1 in the increase in mitochondrial fatty acid oxidation was due to its acyl-CoA synthetase activity, providing the substrate for CPT1, we measured palmitoyl-CoA oxidation to CO2 and ASPs in isolated mitochondria, where palmitoyl-CoA is oxidized in the absence of acyl-CoA synthetase activity. The overexpression of either CPT1 or FATP1 alone produced an increase in fatty acid oxidation to CO2 compared with LacZ control cells (2.20 ± 0.11 nmol palmitoyl-CoA/mg protein × h), which was additive when both proteins were overexpressed together (Fig. 4A). However, no significant difference was observed in palmitoyl-CoA oxidation to ASPs in FATP1-overexpressing cells compared with LacZ control cells (27.6 ± 0.37 nmol palmitoyl-CoA/mg protein × h; Fig. 4B).
Fig. 4.
Palmitoyl-CoA oxidation in mitochondrial fractions. Mitochondrial fractions that were obtained from cells infected with Ad-LacZ, Ad-FATP1, or Ad-CPT1A were incubated for 0.5 h with agitation in 400 μl of pregassed complete MKRH buffer and 50 μl of a 2.5 mM 5:1 palmitoyl-CoA-BSA complex containing 1 μCi/ml [1-14C]palmitoyl-CoA and palmitoyl-CoA, and oxidation to CO2 (A) and to ASPs (B) was measured. Data are the mean ± SE of three experiments performed in duplicate. *P < 0.01 versus Ad-LacZ; &P < 0.01 versus the overexpression of only one protein (CPT1A or FATP1).
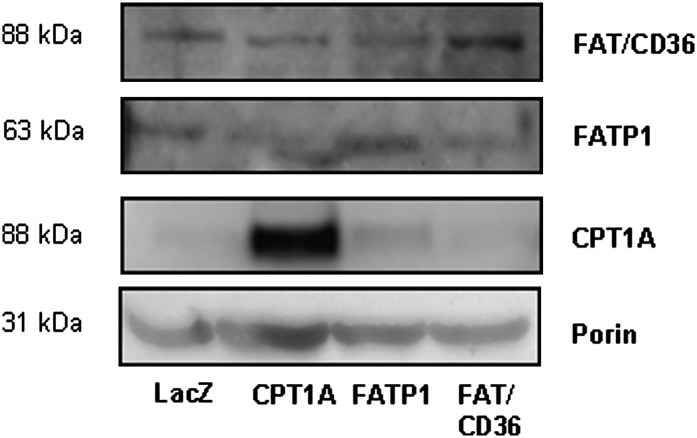
Increased fatty acid uptake has been shown to increase transcription of CPT1 via a peroxisome proliferator-activated receptor (PPAR)-dependent pathway (28). To assess the possibility that the increase in fatty acid oxidation was due to increased expression of CPT1 in cells overexpressing FATP1 as a consequence of an increased influx of fatty acids, we performed a Western blot for CPT1 in L6E9 myotubes. CPT1 protein levels were increased in cells overexpressing CPT1 but not in those cells overexpressing FATP1 or FAT/CD36 (Fig. 5). Indeed, the overexpression of FATP1, FAT/CD36, or CPT1 only affected the expression of the protein that had been overexpressed but not the other proteins studied (Fig. 5).
Fig. 5.
Protein expression levels of CPT1A, FATP1, and FAT/CD36 in L6E9 myotubes. Western blot for CPT1A, FATP1, and FAT/CD36 proteins was performed in 50 μg of mitochondrial fractions obtained from cells infected with Ad-LacZ, Ad-CPT1A, Ad-FATP1, and Ad-FAT/CD36. Western blot against porin was used as a mitochondrial loading control.
Cellular localization of FATP1
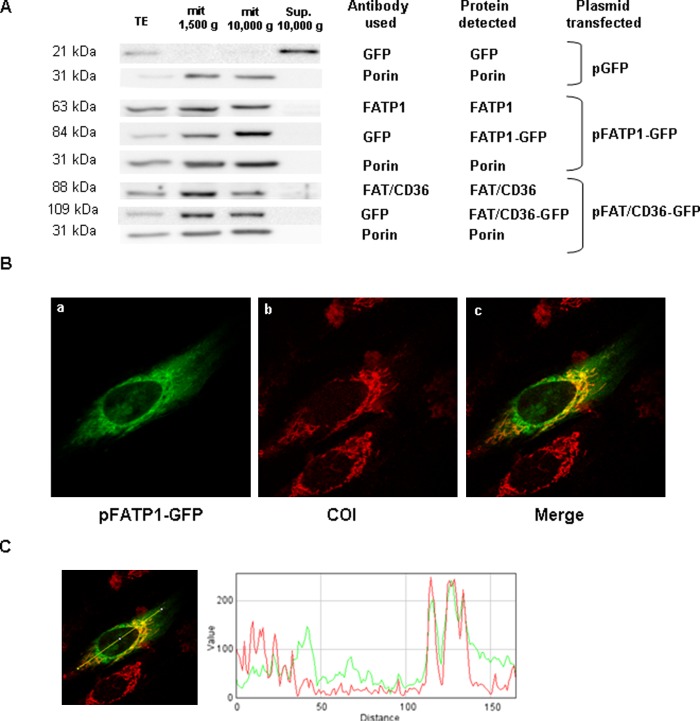
The increase in fatty acid oxidation in isolated mitochondria from FATP1-overexpressing cells suggested that FATP1 could be localized in the mitochondrion. Therefore, L6E9 myotubes were transfected with a plasmid that expressed an N-terminal FATP1-GFP fusion protein, and a Western blot against GFP or FATP1 was performed. We detected a band corresponding to the FATP1-GFP fusion protein (using the GFP antibody) and the endogenous FATP1 protein (using FATP1 antibody) in the 10,000 g mitochondrial pellet but not in the 10,000 g supernatant (Fig. 6A). Indeed, this distribution was similar to that of the mitochondrial marker protein porin. The same result was obtained for endogenous FAT/CD36 protein and overexpressed FAT/CD36-GFP fusion protein, which is consistent with the mitochondrial localization of FAT/CD36 protein in muscle (Fig. 6A).
Fig. 6.
FATP1 localizes in mitochondria in L6E9 myotubes. A: L6E9 myoblasts were transfected with pFATP1-GFP or pFAT/CD36-GFP and induced to differentiate. Western blot analyses were performed 48 h posttransfection on total cell extracts (TE) (35 μg protein), mitochondrial enriched fractions (mit) (35 g protein) after centrifugation at 1,500 g and 10,000 g, or the supernatant (Sup.) of the 10,000 g pellet (35 μg) and membranes hybridized with antibodies against FAT/CD36, FATP1, GFP, or porin as stated. B: Colocalization analysis of the mitochondrial marker COI and FATP1-GFP. L6E9 cells were transfected with pFATP1-GFP and studied 48 h posttransfection. a: Image of the FATP1-GFP protein observed under a confocal microscope. b: Image of mitochondrial network by staining with antibodies against COI. c: Colocalization of the signal of GFP and COI. C: Qualitative analyses of colocalization were assessed using the plugin RGB Profiler from the WCIF ImageJ software. A line was drawn arbitrarily over an area of interest, and the plot presents the overlap of the intensity of each laser (from distinct labeled proteins).
To confirm that FATP1 is localized in mitochondria, we used immunocytochemistry in L6E9 myotubes transfected with the FATP1-GFP construct. As a marker of the mitochondrial network, we used subunit I of cytochrome c oxidase (COI), an enzyme located in the mitochondrial inner membrane that forms part of Complex IV of the respiratory chain (29). We showed that COI protein has a tubular mitochondrial pattern in L6E9 myotubes. The merging of FATP1-GFP with the mitochondrial marker COI revealed a partial overlap of these two proteins, and imaging analysis indicated a substantial colocalization of both proteins (Pearson coefficient of colocalization was 0.537, and Mander's Overlap coefficient was 0.723), confirming the mitochondrial localization of FATP1-GFP protein (Fig. 6B). Qualitative analyses of colocalization were assessed using the plugin RGB Profiler from the WCIF ImageJ software. An arbitrary line was drawn over an area of interest, and the plot presents the overlap of staining of each laser (from distinct labeled proteins; Fig. 6C).
FAPT1 and CPT1 interact in mitochondria
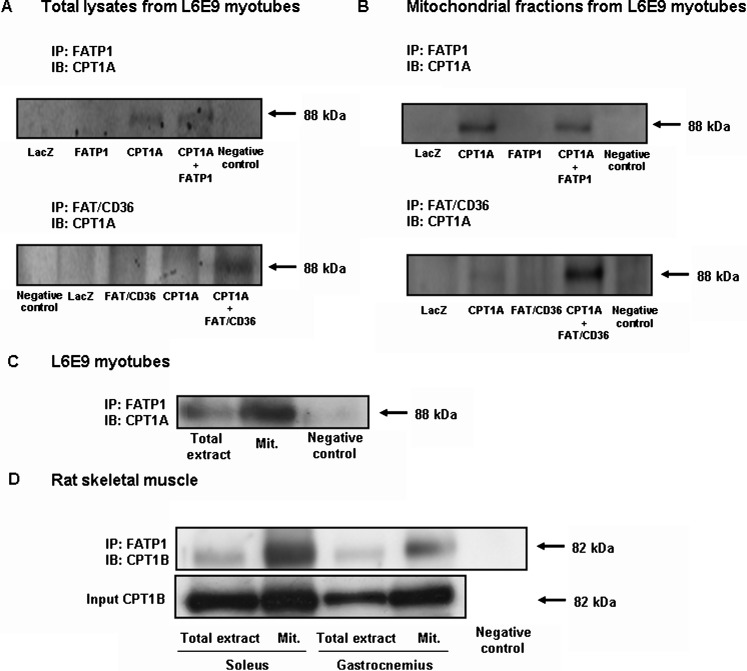
If FATP1 is localized in mitochondria and enhances fatty acid oxidation, FATP1 and CPT1 could interact in the mitochondria. To assess this possibility, we performed coimmunoprecipitation assays. FATP1 was immunoprecipitated from total lysates of cells infected with Ad-LacZ, Ad-FAPT1, Ad-CPT1A, or Ad-FATP1 + Ad-CPT1A, and these immunoprecipitates were then subjected to a Western blot analysis using a CPT1A-specific antibody. We detected a band corresponding to CPT1 in immunoprecipitates obtained from CPT1A-overexpressing cells, showing an interaction between FATP1 and CPT1 (Fig. 7A). To confirm that the two proteins interact in mitochondria, we also performed immunoprecipitation assays using mitochondrial fractions from these cells. As shown for the case of total lysates, FATP1 and CPT1A coimmunoprecipitated in these fractions (Fig. 7B). Since we did not see coimmunoprecipitation of FATP1 and CPT1A in control LacZ cells, to rule out the possibility that the coimmunoprecipitation could be an artifact of the overexpression system, we performed immunoprecipitation assays using a greater amount of either total extracts or mitochondrial extracts. Using this approach, we showed that endogenous FATP1 and CPT1A coimmunoprecipitated in both total and mitochondria-enriched extracts in control L6E9 myotubes (Fig. 7C). FAT/CD36 has been shown to coimmunoprecipitate with CPT1 in human skeletal muscle (30). We performed immunoprecipitation assays in our system, which overexpresses FAT/CD36, and found that the two proteins also coimmunoprecipitated in both total and mitochondrial extracts when both proteins were overexpressed (Fig. 7A, B). Importantly, to confirm that FATP1 and CPT1 colocalize in mitochondria, we performed immunoprecipitation assays in vivo using rat skeletal muscle. FATP1 and CPT1 coimmunoprecipitated in both total extracts and in isolated mitochondrial fractions coming from gastrocnemius and soleus muscles (Fig. 7D).
Fig. 7.
Coimmunoprecipitation of FATP1 and CPT1. L6E9 myotubes were transduced with Ad-LacZ, Ad-CPT1A, Ad-FATP1, and Ad-FAT/CD36, and FATP1 or FAT/CD36 proteins were immunoprecipitated using specific antibodies in either total extracts (1,000 μg) (A) or mitochondrial extracts (150 μg) (B), and CPT1A protein was detected in these immunoprecipitates by Western blot. C: FATP1 protein was immunoprecipitated in either total extracts (2,000 μg) or mitochondrial-enriched extracts (300 μg) from L6E9 myotubes, and CPT1A protein was detected by Western blot. D: FATP1 protein was immunoprecipitated in either total extracts (1,000 μg) or mitochondria-enriched extracts (150 μg) from both gastrocnemius and soleus muscles, and CPT1B protein was detected by Western blot using a specific antibody. Total expression of CPT1B in 50 μg of total and mitochondrial extracts is also shown (Input). A negative control with an unspecific antibody is also shown. IP, immunoprecipitation; IB, immunoblot.
DISCUSSION
Regulation of fatty acid oxidation in skeletal muscle has been the object of many studies attempting to explain the disconnection between malonyl-CoA levels measured in muscle and the IC50 for the muscle isoform of CPT1. In a recent study, we showed that overexpression of CPT1A in L6E9 myotubes enhanced CPT1 activity up to 15-fold, whereas fatty acid oxidation did not increase more than 2-fold, even when a mutant form of CPT1A insensitive to malonyl-CoA was overexpressed (22). These data indicate that other factors independent of the CPT1/malonyl-CoA interaction could be involved in the control of fatty acid oxidation in muscle. Furthermore, proteins other than CPT1 have recently been implicated in fatty acid import into mitochondria in muscle, such as FAT/CD36 (12) and UCP3 (31). FAT/CD36 translocates to the mitochondria during muscle contraction, increasing fatty acid oxidation. Furthermore, a null mutation in skeletal muscle FAT/CD36 reveals that palmitate metabolism is markedly downregulated under insulin- and AICAR-stimulated conditions. This indicates its critical role in fatty acid esterification and oxidation and points to another level of regulation in fatty acid oxidation (32). In these FAT/CD36 KO mice, there was an upregulation of FATP1 and FATP4 in the soleus muscle. This upregulation failed to normalize fatty acid oxidation, but it indicates that other proteins like FATP1 could collaborate in the maintenance of fatty acid metabolism. In search for a novel regulator of fatty acid metabolism in muscle, in this study, we demonstrate for the first time that FATP1, a fatty acid transport protein, is involved in mitochondrial fatty acid oxidation.
Overexpression of FATP1 in L6E9 myotubes using adenovirus increased acyl-CoA synthetase activity and fatty acid incorporation into TGs, but not into other lipid species such as PL, DAG, or NEPalm. These results are consistent with other in vitro studies in 293 cells showing that fatty acids taken up by FATP1 are preferentially channeled into TG synthesis (33). Moreover, FATP1 overexpression increased fatty acid oxidation in intact myotubes. This result contrasts with the effect of FATP1 overexpression in human skeletal muscle cells, where palmitate incorporation into TG, but not fatty acid oxidation, was also increased (18). This discrepancy could be due to differences in the model studied and the method used: in this study, palmitate oxidation was measured in the presence of 2.5 mM glucose, whereas in the previous study, palmitate oxidation was measured in human skeletal muscle cells and in the absence of glucose. The increase in fatty acid oxidation in FATP1-overexpressing L6E9 myotubes in our study is consistent with the increase shown for transgenic mice overexpressing FATP1 in heart (17). However, the TG levels were not increased in the heart of transgenic mice.
To assess whether the increase in fatty acid oxidation is a result of an increase in fatty acid uptake at the plasma membrane or due to a mitochondrial role of FATP1, we isolated mitochondrial-enriched fractions and measured palmitate oxidation. We found that FATP1 overexpression increased mitochondrial fatty acid oxidation, as did the overexpression of CPT1A, the endogenous CPT1 isoform expressed in these cells (22). Importantly, the overexpression of both proteins together produced an additive effect, indicating that FATP1 and CPT1 collaborate in these cells for mitochondrial fatty acid oxidation. It has been suggested than when the rate of mitochondrial β-oxidation is increased and exceeds flux through the tricarboxylic acid cycle and electron transport chain, incomplete fatty acid oxidation occurs, leading to intramitochondrial metabolite accumulation, mitochondrial stress, and cellular insulin resistance (34). In our study, FATP1 overexpression produced a higher increase in complete than in incomplete fatty acid oxidation, indicating that the effect of this protein on fatty acid oxidation does not involve the accumulation of intermediary species of fatty acid oxidation. Hence, FATP1 overexpression increased the efficiency of the mitochondria to oxidize fatty acids, as indicated by the diminished ratio incomplete/complete fatty acid oxidation. This effect was also seen in cells overexpressing CPT1A, especially when both FATP1 and CPT1A were cooverexpressed, showing a higher coupling of mitochondrial fatty acid uptake and oxidation. Interestingly, the use of etomoxir showed that the control of mitochondrial fatty acid uptake was dominated by CPT1, and the overexpression of FATP1 had no effect when CPT1 was inhibited. This indicates that FATP1 contributes to fatty acid oxidation by collaborating with CPT1, but not by controlling the entry of fatty acids to the mitochondria.
If FATP1 has a role in mitochondrial fatty acid oxidation, it might be localized in mitochondria. To examine this possibility, we used different techniques. Western blot analysis demonstrated that the protein was present in mitochondrial fractions but not in cytosolic fractions. Moreover, using immunocytochemistry, we showed both qualitatively and quantitatively that FATP1-GFP protein localized partially in structures that were also positive for the mitochondrial protein COI, indicating that FATP1 is partially localized in mitochondria in L6E9 myotubes. We used GFP fluorescence since the FATP1 antibodies were unsuitable for immunocytochemistry studies. Although the fusion with GFP could affect the localization of the protein, we do not believe this is the case. In the Western blot experiments, the FATP1-GFP and the endogenous protein showed the same distribution pattern of localization after the cellular fractionation, and another report (35) showed that the N-terminal GFP-tagged FATP1 protein retained the ability to bind to membranes. In our study, not all the FATP1 colocalized with the mitochondrial marker COI. It has been described that FATP1 can also localize in the endoplasmic reticulum (ER) (35). Given the reticular pattern of GFP fluorescence that we observed in L6E9 cells, we cannot rule out the possibility that a portion of FATP1-GFP could also localize in the ER. Furthermore, in a previous study in human myotubes, overexpressed FATP1-GFP also showed a cytosolic reticular pattern, but colocalization studies with a mitochondrial or ER marker were not performed (18). Moreover, recent data from our group demonstrated that FATP1 also localizes in mitochondria in human and C2C12 muscle cells (36), and in adipocytes, 70% of FATP1 localizes in cell membrane fraction and approximately 10% in the nuclear-mitochondrial fraction (16).
The additive effect of FATP1 and CPT1 overexpression in fatty acid oxidation and the mitochondrial localization of both proteins indicate that these two proteins may interact in the mitochondrion. We show that both proteins coimmunoprecipitated both in total extracts and in mitochondrial extracts, strongly supporting the hypothesis that FATP1 and CPT1 interact in mitochondria. Importantly, using rat skeletal muscle, we also showed that FATP1 and CPT1 coimmunoprecipitated both in total extracts and in mitochondria-enriched fractions, indicating that the interaction between FATP1 and CPT1 also occurs in skeletal muscle in vivo. Moreover, this coimmunoprecipitation was higher in soleus than in gastrocnemius muscle. This indicates that in oxidative muscle, which uses primary fatty acids as an energy source, the interaction between FATP1 and CPT1 could be higher than in glycolytic muscles. Furthermore, FATP1 expression in rat oxidative muscle (soleus) is approximately twice that in glycolytic (gastrocnemius) muscle (37). Our immunoprecipitation assays in L6E9 myotubes and rat skeletal muscle show that FATP1 and CPT1 interact in basal conditions, suggesting that FATP1 could be constitutively present in mitochondria. However, some hormonal or physiological factors that induce fatty acid oxidation, such as exercise, could also induce the presence of FATP1 in mitochondria. Future studies are needed to examine this possibility.
FATP1 is an integral membrane protein with at least one transmembrane and multiple membrane-associated domains (35). It contains an AMP-binding motif that is essential for transport function (38). FATP1 has acyl-CoA synthetase activity, suggesting that fatty acid uptake catalyzed by FATP1 is linked to the esterification of these fatty acids with CoA (13). Moreover, FATP1 was shown to heterooligomerize with the LCFA-CoA syntethase ACSL1 in differentiated 3T3-L1 adipocytes (39). Given all the above observations, it could be hypothesized that FATP1 could act as a long-chain acyl-CoA synthetase in mitochondria to activate LCFAs, which CPT1 can then use as a substrate. However, interestingly, although acyl-CoA synthetase activity is increased in mitochondrial fractions from FATP1-overexpressing L6E9 myotubes, we show that mitochondrial palmitoyl-CoA oxidation to CO2 is also increased in these fractions, as is the oxidation of palmitate, suggesting a role for FATP1 in mitochondrial fatty acid oxidation independent of its acyl-CoA synthetase activity.
In this study, we have also analyzed the effect of overexpression of FAT/CD36 in L6E9 myotubes. This has been described as a new protein involved in mitochondrial fatty acid oxidation under stimulated conditions (12). Thus, the presence of FAT/CD36 in mitochondria is induced by exercise or contraction (12). However, FAT/CD36 KO mice have normal basal mitochondrial fatty acid oxidation despite the absence of FAT/CD36 protein (40). These results suggest that FAT/CD36 could have a role in mitochondrial fatty acid oxidation only under certain conditions of increased requirements for fatty acid oxidation, such as during exercise or muscle contraction. Moreover, FAT/CD36 has been shown to translocate from intracellular vesicles to the plasma membrane in response to insulin (41). In this study, we have demonstrated that overexpression of FAT/CD36 enhances fatty acid oxidation, in agreement with the increase in fatty acid oxidation shown in contracting muscle in transgenic mice overexpressing FAT/CD36 (42). Furthermore, FAT/CD36 coimmunoprecipitated with CPT1 in L6E9 myotubes, and its overexpression increased mitochondrial fatty acid oxidation and mitochondrial fatty acid oxidation efficiency, showing an additive effect when cooverexpressed with CPT1. It has already been shown that FAT/CD36 translocates to the mitochondrial membrane in response to exercise, increasing the ability of mitochondria to oxidize palmitate, both in rats and in humans (12, 43). Moreover, FAT/CD36 coimmunoprecipitates with CPT1, and this correlates with the level of fat oxidation in skeletal muscle (30). Thus, our results in L6E9 myotubes are in agreement with these previous data and validate our model of study. Therefore, according to our data, FATP1 enhances fatty acid oxidation similar to FAT/CD36, suggesting that such effect may be due to fatty acid recruitment at mitochondria by either fatty acid binding protein. However, in a recent study, we have shown that FATP1, unlike FAT/CD36, stimulates the pyruvate dehydrogenase complex activity and glucose oxidation in cultured myotubes (36), indicating that FATP1 exerts differential effects on mitochondrial metabolism.
In summary, we demonstrate for the first time that FATP1 has a role in mitochondrial fatty acid oxidation in collaboration with CPT1. The exact mechanism of this interaction and its regulation remain to be resolved. However, the data presented here reveal a new protein involved in mitochondrial oxidation of fatty acids and could help us to understand the complex regulation of fatty acid oxidation in skeletal muscle.
Acknowledgments
We thank Dr. Prip-Buus and Dr. Zammit for supplying anti-rat liver CPT1 antibody and anti-rat muscle CPT1 antibody respectively. The L6E9 rat skeletal muscle cell line was kindly provided by Dr. A. Zorzano (University of Barcelona). We are also grateful to Robin Rycroft of the Language Service for valuable assistance in the preparation of the manuscript.
Footnotes
Abbreviations:
- ASP
- acid-soluble product
- COI
- subunit I of cytochrome c oxidase
- CPT1
- carnitine palmitoyltransferase 1
- DAG
- diacylglycerol
- ER
- endoplasmic reticulum
- FATP1
- fatty acid transport protein 1
- FAT/CD36
- fatty acid translocase/CD36
- GFP
- green fluorescent protein
- KRBH
- Krebs-Ringer bicarbonate HEPES buffer
- LCFA
- long-chain fatty acid
- MKRH
- modified Krebs Ringer HEPES
- NEPalm
- nonesterified palmitate
- PL
- phospholipid
- PPAR
- peroxisome proliferator-activated receptor
- TG
- triacylglyceride
This study was supported by Grant SAF2007-61926 from the Ministerio de Educación y Ciencia; by Grant C3/08 from the Fondo de Investigación Sanitaria of the Instituto de Salud Carlos III; by the Ajut de Suport als Grups de Recerca de Catalunya (2005SGR-00733), Spain (to F.G.H.), and by Grants SAF2006-07228 from the Ministerio de Ciencia y Tecnología and REDIMET RD06/0015/0018 (to A.M.G-F.). CIBER Fisiopatología de la Obesidad y Nutrición and CIBER Diabetes y Enfermedades Metabólicas Asociadas are an initiative of Instituto de Salud Carlos III.
REFERENCES
- 1.Bonen A., Chabowski A., Luiken J. J., Glatz J. F. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda). 22: 15–29. [DOI] [PubMed] [Google Scholar]
- 2.McGarry J. D., Brown N. F. 1997. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 244: 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. 1993. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J. Biol. Chem. 268: 5817–5822. [PubMed] [Google Scholar]
- 4.Yamazaki N., Shinohara Y., Shima A., Terada H. 1995. High expression of a novel carnitine palmitoyltransferase I like protein in rat brown adipose tissue and heart: isolation and characterization of its cDNA clone. FEBS Lett. 363: 41–45. [DOI] [PubMed] [Google Scholar]
- 5.Price N., van der Leij F., Jackson V., Corstorphine C., Thomson R., Sorensen A., Zammit V. 2002. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 80: 433–442. [DOI] [PubMed] [Google Scholar]
- 6.Odland L. M., Heigenhauser G. J., Lopaschuk G. D., Spriet L. L. 1996. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am. J. Physiol. 270: E541–E544. [DOI] [PubMed] [Google Scholar]
- 7.Odland L. M., Heigenhauser G. J., Wong D., Hollidge-Horvat M. G., Spriet L. L. 1998. Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. Am. J. Physiol. 274: R894–R902. [DOI] [PubMed] [Google Scholar]
- 8.Dean D., Daugaard J. R., Young M. E., Saha A., Vavvas D., Asp S., Kiens B., Kim K. H., Witters L., Richter E. A., et al. 2000. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 49: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 9.Chien D., Dean D., Saha A. K., Flatt J. P., Ruderman N. B. 2000. Malonyl-CoA content and fatty acid oxidation in rat muscle and liver in vivo. Am. J. Physiol. Endocrinol. Metab. 279: E259–E265. [DOI] [PubMed] [Google Scholar]
- 10.McGarry J. D., Mills S. E., Long C. S., Foster D. W. 1983. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem. J. 214: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGarry J. D. 2002. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 51: 7–18. [DOI] [PubMed] [Google Scholar]
- 12.Campbell S. E., Tandon N. N., Woldegiorgis G., Luiken J. J., Glatz J. F., Bonen A. 2004. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J. Biol. Chem. 279: 36235–36241. [DOI] [PubMed] [Google Scholar]
- 13.Hall A. M., Smith A. J., Bernlohr D. A. 2003. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. Biol. Chem. 278: 43008–43013. [DOI] [PubMed] [Google Scholar]
- 14.Stahl A. 2004. A current review of fatty acid transport proteins (SLC27). Pflugers Arch. 447: 722–727. [DOI] [PubMed] [Google Scholar]
- 15.Bonen A., Miskovic D., Kiens B. 1999. Fatty acid transporters (FABPpm, FAT, FATP) in human muscle. Can. J. Appl. Physiol. 24: 515–523. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer J. E., Lodish H. F. 1994. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 79: 427–436. [DOI] [PubMed] [Google Scholar]
- 17.Chiu H. C., Kovacs A., Blanton R. M., Han X., Courtois M., Weinheimer C. J., Yamada K. A., Brunet S., Xu H., Nerbonne J. M., et al. 2005. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 96: 225–233. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Martinez C., Marotta M., Moore-Carrasco R., Guitart M., Camps M., Busquets S., Montell E., Gomez-Foix A. M. 2005. Impact on fatty acid metabolism and differential localization of FATP1 and FAT/CD36 proteins delivered in cultured human muscle cells. Am. J. Physiol. Cell Physiol. 288: C1264–C1272. [DOI] [PubMed] [Google Scholar]
- 19.Kim J. K., Gimeno R. E., Higashimori T., Kim H. J., Choi H., Punreddy S., Mozell R. L., Tan G., Stricker-Krongrad A., Hirsch D. J., et al. 2004. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J. Clin. Invest. 113: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl A., Evans J. G., Pattel S., Hirsch D., Lodish H. F. 2002. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev. Cell. 2: 477–488. [DOI] [PubMed] [Google Scholar]
- 21.Wu Q., Ortegon A. M., Tsang B., Doege H., Feingold K. R., Stahl A. 2006. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol. Cell. Biol. 26: 3455–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian D., Herrero L., Serra D., Asins G., Hegardt F. G. 2007. CPT I overexpression protects L6E9 muscle cells from fatty acid-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 292: E677–E686. [DOI] [PubMed] [Google Scholar]
- 23.Rubi B., Antinozzi P. A., Herrero L., Ishihara H., Asins G., Serra D., Wollheim C. B., Maechler P., Hegardt F. G. 2002. Adenovirus-mediated overexpression of liver carnitine palmitoyltransferase I in INS1E cells: effects on cell metabolism and insulin secretion. Biochem. J. 364: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veerkamp J. H., van Moerkerk T. B., Glatz J. F., Zuurveld J. G., Jacobs A. E., Wagenmakers A. J. 1986. 14CO2 production is no adequate measure of [14C]fatty acid oxidation. Biochem. Med. Metab. Biol. 35: 248–259. [DOI] [PubMed] [Google Scholar]
- 25.Prip-Buus C., Cohen I., Kohl C., Esser V., McGarry J. D., Girard J. 1998. Topological and functional analysis of the rat liver carnitine palmitoyltransferase 1 expressed in Saccharomyces cerevisiae. FEBS Lett. 429: 173–178. [DOI] [PubMed] [Google Scholar]
- 26.van der Leij F. R., Cox K. B., Jackson V. N., Huijkman N. C., Bartelds B., Kuipers J. R., Dijkhuizen T., Terpstra P., Wood P. A., Zammit V. A., et al. 2002. Structural and functional genomics of the CPT1B gene for muscle-type carnitine palmitoyltransferase I in mammals. J. Biol. Chem. 277: 26994–27005. [DOI] [PubMed] [Google Scholar]
- 27.Lanni A., Moreno M., Lombardi A., Goglia F. 1996. Biochemical and functional differences in rat liver mitochondrial subpopulations obtained at different gravitational forces. Int. J. Biochem. Cell Biol. 28: 337–343. [DOI] [PubMed] [Google Scholar]
- 28.Mascaro C., Acosta E., Ortiz J. A., Marrero P. F., Hegardt F. G., Haro D. 1998. Control of human muscle-type carnitine palmitoyltransferase I gene transcription by peroxisome proliferator-activated receptor. J. Biol. Chem. 273: 8560–8563. [DOI] [PubMed] [Google Scholar]
- 29.Pich S., Bach D., Briones P., Liesa M., Camps M., Testar X., Palacin M., Zorzano A. 2005. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum. Mol. Genet. 14: 1405–1415. [DOI] [PubMed] [Google Scholar]
- 30.Schenk S., Horowitz J. F. 2006. Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am. J. Physiol. Endocrinol. Metab. 291: E254–E260. [DOI] [PubMed] [Google Scholar]
- 31.Schrauwen P., Hesselink M. K. 2004. The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc. Nutr. Soc. 63: 287–292. [DOI] [PubMed] [Google Scholar]
- 32.Bonen A., Han X. X., Habets D. D., Febbraio M., Glatz J. F., Luiken J. J. 2007. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 292: E1740–E1749. [DOI] [PubMed] [Google Scholar]
- 33.Hatch G. M., Smith A. J., Xu F. Y., Hall A. M., Bernlohr D. A. 2002. FATP1 channels exogenous FA into 1,2,3-triacyl-sn-glycerol and down-regulates sphingomyelin and cholesterol metabolism in growing 293 cells. J. Lipid Res. 43: 1380–1389. [DOI] [PubMed] [Google Scholar]
- 34.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56. [DOI] [PubMed] [Google Scholar]
- 35.Lewis S. E., Listenberger L. L., Ory D. S., Schaffer J. E. 2001. Membrane topology of the murine fatty acid transport protein 1. J. Biol. Chem. 276: 37042–37050. [DOI] [PubMed] [Google Scholar]
- 36.Guitart M., Andreu A. L., García-Arumi E., Briones P., Quintana E., Gómez-Foix A. M., García-Martínez C. 2009. FATP1 localizes to mitochondria and enhances pyruvate dehydrogenase activity in skeletal myotubes. Mitochondrion. 9: 266–272. [DOI] [PubMed] [Google Scholar]
- 37.Marotta M., Ferrer-Martnez A., Parnau J., Turini M., Mace K., Gomez Foix A. M. 2004. Fiber type- and fatty acid composition-dependent effects of high-fat diets on rat muscle triacylglyceride and fatty acid transporter protein-1 content. Metabolism. 53: 1032–1036. [DOI] [PubMed] [Google Scholar]
- 38.Stuhlsatz-Krouper S. M., Bennett N. E., Schaffer J. E. 1998. Substitution of alanine for serine 250 in the murine fatty acid transport protein inhibits long chain fatty acid transport. J. Biol. Chem. 273: 28642–28650. [DOI] [PubMed] [Google Scholar]
- 39.Richards M. R., Harp J. D., Ory D. S., Schaffer J. E. 2006. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J. Lipid Res. 47: 665–672. [DOI] [PubMed] [Google Scholar]
- 40.King K. L., Stanley W. C., Rosca M., Kerner J., Hoppel C. L., Febbraio M. 2007. Fatty acid oxidation in cardiac and skeletal muscle mitochondria is unaffected by deletion of CD36. Arch. Biochem. Biophys. 467: 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luiken J. J., Dyck D. J., Han X. X., Tandon N. N., Arumugam Y., Glatz J. F., Bonen A. 2002. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 282: E491–E495. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahimi A., Bonen A., Blinn W. D., Hajri T., Li X., Zhong K., Cameron R., Abumrad N. A. 1999. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 274: 26761–26766. [DOI] [PubMed] [Google Scholar]
- 43.Bezaire V., Bruce C. R., Heigenhauser G. J., Tandon N. N., Glatz J. F., Luiken J. J., Bonen A., Spriet L. L. 2006. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 290: E509–E515. [DOI] [PubMed] [Google Scholar]