Abstract
We used functional magnetic resonance imaging (fMRI) and cytoarchitectonic probability maps to investigate the responsiveness of individual areas in the human primary and secondary somatosensory cortices to hand, face, or trunk stimulation of either body-side. A Bayesian modeling approach to quantify the probability of ipsilateral activations revealed that areas OP 1, OP 4, and OP 3 of the SII cortex as well as the trunk and face representations within all SI subareas (areas 3b, 1, and 2) show robust bilateral responses to unilateral stimulation. Such bilateral response properties are in good agreement with the transcallosal projections demonstrated for these areas in nonhuman primates and other mammals. In contrast, the SI hand region showed a different pattern. Whereas ipsilateral areas 3b and 1 were deactivated by tactile hand stimulation, particularly on the left, there was strong evidence for ipsilateral processing of information from the right hand in area 2. These results demonstrate not only the behavioral importance of the hand representation, but also suggest that area 2 may have particularly evolved to form the cortical substrate of these specialized demands, in line with recent studies on cortical evolution hypothesizing that area 2 has developed with increasing manual abilities in anthropoid primates featuring opposable thumbs.
Keywords: homology, ipsilateral, SI, SII
Introduction
The human brain receives tactile information from the mechanoreceptors of the skin via the spino-cortical tracts terminating in the contralateral thalamus, which in turn mainly forwards to the primary somatosensory cortex (SI) on the postcentral gyrus of the same (contralateral) hemisphere (Kaas and Collins 2003; Kaas 2004). This primarily contralateral representation of tactile input hence matches that of all animal species investigated thus far (Krubitzer 1995; Kaas 2004; Karlen and Krubitzer 2006). In spite of this contralateral dominance for somatosensory processing, however, there is ample evidence for an additional ipsilateral representation of tactile stimuli in the human (and primate) cerebral cortex. For example, bilateral responses in the secondary somatosensory cortex (SII) located on the parietal operculum were described for several body parts including the hands (Disbrow et al. 2000; Ruben et al. 2001; Ferretti et al. 2003; Young et al. 2004; Naito et al. 2005; Blatow et al. 2007) though some variability in the consistency of ipsilateral activations has been noted. Moreover, although the cortical representation of the hands has been the main focus of research due to their behavioral relevance, responses evoked by tactile stimulation of the face (Boling et al. 2002; Iannetti et al. 2003; Blatow et al. 2007) and trunk (Itomi et al. 2000; Fabri et al. 2005) have also been examined using functional magnetic resonance imaging (fMRI). These studies demonstrated that, in contrast to the preponderance of contralateral representation observed for the hands, the head and in particular the trunk are commonly represented bilaterally in SI. The ipsilateral cortical responses to hand stimulation, however, remain a matter of debate (Hlushchuk and Hari 2006). In particular, although it has been a longstanding concept, that the SI hand region is primarily devoted to processing contralateral input, this model is challenged by the fact that integration of tactile information from both hands is essential for many tasks (e.g., bimanual exploration and manipulation) and should hence occur at a relatively early stage of cortical processing. Subdural recordings following median nerve stimulation suggested this integration to occur in the posterior parietal cortex and possibly also in parts of the postcentral gyrus (Allison et al. 1989; Kanno et al. 2003). These findings were later corroborated using similar paradigms in fMRI and electroencephalography experiments (Nihashi et al. 2005; Sutherland and Tang 2006). In a recent article, Hlushchuk and Hari (2006) provided additional support for the hypothesis of ipsilateral SI responses to tactile hand stimulation by demonstrating activation in the more posterior and deactivation in more anterior parts of the ipsilateral postcentral gyrus using fMRI. The authors then speculated that these observations may represent responses generated within areas 2 and 3b, respectively (Hlushchuk and Hari 2006).
In the present study, we aimed at testing and integrating these various information and hypotheses concerning ipsilateral representation of tactile information in the human cerebral cortex. To this end we investigated the cortical responses to unilateral left or right stimulation of the head, hand and trunk within different anatomically defined areas of the primary (areas 3b, 1, and 2) and secondary (areas OP 1, OP 4, and OP 3) somatosensory cortices on either hemisphere. The motivation for examining head and trunk representations in addition to the hands stemmed from the fact that, in contrast to the hands, both the head and the trunk do not show a prominent behavioral asymmetry or dominance. They also differ with respect to sensitivity and somatosensory precision, wherein the head is comparable to the hands, whereas touch representation of the trunk is far less fine graded (Nolan 1985; Davey et al. 2001).
Materials and Methods
The technical aspects of the paradigm and imaging acquisition parameters have been reported elsewhere in great detail (Eickhoff, Grefkes, et al. 2007). We here thus summarize only its main aspects and focus on the description of the current analysis of lateralization in the somatosensory system.
Subjects and Stimulation
The study was approved by the local ethics committee. Fourteen healthy subjects (7 males, mean age 25.6 ± 3.4 years) with no history of neurological or psychiatric illness gave informed consent. Subjects’ handedness was assessed by the Edinburgh handedness inventory (Oldfield 1971). The mean laterality-coefficient of all subjects was 89.6 (SD 10.8), the lowest laterality-coefficient in any subject was 77.8. Two of the examined subjects fell into the 4th and another 5 in the 5th decile for right-handers as proposed by Oldfield (1971), the remaining had a laterality-quotient of 100 and were thus classified into the 10th decile. We could hence confirm the self-reported right-handedness of the participants by this standard inventory. Each of the 3 examined locations (Fig. 1) was separately stimulated on either the left or the right side of the body resulting in 6 experimental conditions. Stimulation was performed by brushing the subject's skin with a sponge in a back-and-forth manner with a frequency of approximately 2 Hz, a highly robust paradigm for evoking somatosensory activations in monkeys and humans (Krubitzer et al. 1995; Disbrow et al. 2000). Upon debriefing, all subjects reported the stimuli to be well perceivable but not painful. The fMRI experiment consisted of 6 sessions in which the different anatomical sites were stimulated. Each session presented the subjects with 11 cycles made up from18 s stimulation followed by 18 s of rest. The order of the sessions was pseudorandomized across subjects. The original MRI experiment also included 2 additional conditions involving left or right stimulation of the legs (shins) which are not included in the current analysis, as within the primary somatosensory cortex the legs are represented on the medial surface of the cerebral hemispheres and hence in close spatial neighborhood to each other (given the 3D volume based analysis). Due to the spatially blurred hemodynamic response function and the smoothing of the data, this situation will render a separation of responses following left respectively right leg stimulation difficult and may induce spurious ipsilateral activation by “spilling over” of the contralateral hemodynamic response to the ipsilateral hemisphere.
Figure 1.
Schematic overview on the skin surface stimulated for each of the different body part conditions. Face and trunk were stimulated with a sponge rubbed in a back-and-forth motion in a rostrocaudal direction, for stimulation of the hands the sponge was rubbed in a back-and-forth motion from proximal to distal.
Image Acquisition and Processing
Functional MR images were acquired on a Siemens Sonata 1.5-T whole-body scanner (Erlangen, Germany) using blood oxygen level–dependent contrast (gradient-echo echo planar imaging [EPI] pulse sequence, time repetition [TR] = 3 s, time echo [TE] = 66 ms, flip angle = 90°, resolution = 3.1 3 3.1 3 3.1 mm3, 30 axial slices for whole-brain coverage). Each session consisted of 132 EPI images preceded by 4 dummy images to allow the MR scanner to reach a steady state. These were discarded prior to further analysis. Additional high-resolution anatomical images (voxel size 1 × 1 × 1 mm) were acquired using a standard T1-weighted 3-dimensional magnetization prepared rapid gradient-echo sequence (TR = 2.2 s, TE = 3.93 ms, flip angle = 15°, 128 sagittal slices). Images were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). The EPI images were corrected for head movement between scans by an affine registration (Ashburner and Friston 2003a). One subject was removed from further analysis due to excessive head motion (more than 1.5 mm movement between scans). The T1 scan was coregistered to the mean of the realigned EPIs. Subsequently normalization to the Montreal Neurological Institute (MNI) single-subject template (Evans et al. 1992; Collins et al. 1994; Holmes et al. 1998) was performed using the SPM5 normalization algorithm (Ashburner and Friston 2003b). The resulting normalization parameters were then also applied to the EPI volumes. These were hereby transformed into standard stereotaxic space and resampled at 1.5 × 1.5 × 1.5 mm voxel size. Finally, the normalized images were spatially smoothed using a 6 mm full width half maximum Gaussian kernel to meet the statistical requirements of the applied Bayesian modeling approach.
Statistical Analysis
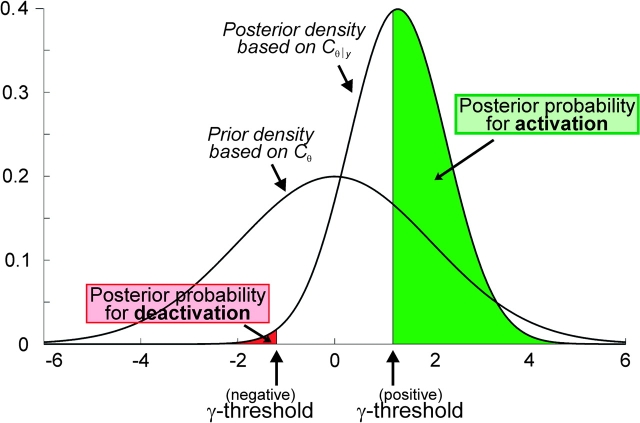
Data was analyzed in the context of the general linear model employed by SPM5. Each experimental condition was modeled using a boxcar input vector convolved with a canonical hemodynamic response function. Low-frequency signal drifts were filtered using a set of discrete cosine functions with a cut-off period of 72 s. No global scaling was applied. The main effects for the 6 stimulation conditions were computed by applying appropriate baseline contrasts, and analyzed across subjects in a 2nd-level Bayesian mixed-effects model (Penny and Holmes 2003). The employed probabilistic empirical Bayes algorithm calculates the conditional distribution for the parameter estimates (across subjects) at each voxel using the variance across voxels as Bayesian prior (Friston, Glaser, et al. 2002; Friston, Penny, et al. 2002; Friston and Penny 2003a, 2003b). The resulting posterior probability maps yield for each voxel (in each condition) the probability for an effect size greater than the prior standard deviation (γ-threshold, cf. Fig. 2). The rationale for using the prior standard deviation as the effect size threshold γ is that it equates to a “background noise level,” that is, to a level of activation that is generic to the brain as a whole. The chosen threshold thus allows directing Bayesian inference to only show those voxels that are almost certainly more active than this generic response (Friston and Penny 2003a, 2003b).
Figure 2.
Inference on the Bayesian model estimated in this study for every voxel of the primary and somatosensory cortex was performed by computing the area under the posterior probability density function. The posterior probability for an activation was given by the portion of the density function which was located to the right of the positive gamma threshold (background noise level as given by the prior standard deviation, i.e., variance across voxels), that for deactivation by the part of the density function which was upper-bounded by the negative gamma threshold.
Lateralization Analysis
The key idea behind the current approach is to 1st identify those parts of the primary (SI) and secondary (SII) somatosensory cortex which respond to the stimulation of a specific body part. Within these defined regions of interest (ROIs) we then analyzed the probabilities for activation following tactile stimulation of the ipsilateral and contralateral side of the body respectively.
As this approach aimed at specifically targeting the degree of hemispheric lateralization within the different architectonically defined subregions of the SI and SII cortices, respectively, anatomical ROIs were 1st generated to represent the a priori anatomical knowledge on their location (Eickhoff et al. 2005; Eickhoff, Heim, et al. 2006). In particular, these ROIs were based on the representation of the architectonically defined areas of the primary somatosensory cortex (SI) (areas 3b, 1, and 2; Geyer et al. 2000; Grefkes et al. 2001) and areas OP 1 (the most likely homologue of primate area SII), OP 4 (the most likely homolog of primate area parietal ventral [PV]) and OP 3 (the most likely homolog of primate area ventral somatosensory [VS]) on the parietal operculum (SII region, Eickhoff, Amunts, et al. 2006; Eickhoff, Schleicher, et al. 2006; Eickhoff, Grefkes, et al. 2007) in the maximum probability map (MPM) of the cortical cytoarchitecture (Eickhoff, Heim, et al. 2006). The MPM in turn describes the most likely anatomical area at each voxel of the MNI reference space based on the observer-independent cytoarchitectonic analysis of 10 human post mortem brains (Zilles et al. 2002; Schleicher et al. 2005; Eickhoff, Paus, et al. 2007), which were normalized to the MNI single-subject template (Eickhoff et al. 2005). Area OP 2, of parietal operculum was not included in the current analysis, as this area most likely is not part of SII but represents the human equivalent of macaque vestibular area parieto-insular vestibular cortex (Eickhoff, Weiss, et al. 2006).
Within each area, somatotopy was established by identifying those voxels, where stimulation of a specific body part was highly likely to evoke brain activation, irrespective of the stimulated side. This was archived by 1st computing for each ROI voxel the union of the posterior probabilities for activation following left- and right-sided stimulation of the particular body part. Only those locations, where the joint posterior probability was greater than 95% were considered responsive. To further increase the specificity of the respective ROIs, these were masked by a conjunction analysis on the differential contrasts between sessions stimulating different body parts. Here the required level of significance (i.e., posterior probability in the Bayesian analysis) was P > 90%. In summary, the final ROI defining the hand region in a particular area of the primary or secondary somatosensory cortex was thus given by those voxels where:
1) The respective area were anatomically located based on the cytoarchitectonic MPM,
2) P(hand response|data) = P(activation by left hand stimulation|data) ∪ P(activation by right hand stimulation|data) > 0.95, and
3) P(hand response > face response|data) ∩ P(hand response > trunk response|data) > 0.9
Within these ROIs the posterior probabilities for activation (greater than γ-threshold, cf. above and Fig. 2) given the fMRI data following stimulation of this body part on either side of the body were extracted and averaged (Eickhoff, Lotze, et al. 2006). These resulting mean posterior probabilities represent an unbiased estimate of the likelihood that the region of a particular area on either hemisphere which represents a given body part reacts to stimulation of the ipsi- and contralateral side of the body. Furthermore, we also analyzed the negative tail of the posterior probability distribution (thresholded at −γ), yielding an estimate for the probability for deactivation, that is, inhibition (Fig. 2). In this context, it is important to mention, that functional magnetic resonance imaging only detects changes in the BOLD signal, an indirect measure of blood flow. However, animal studies combining functional MRI imaging with invasive recording techniques have demonstrated that negative BOLD signals do indeed reflect decreases in neuronal activity and hence deactivation (Harel et al. 2002; Logothetis 2002; Shmuel et al. 2006).
Effect Size Analysis
To confirm that the results from our Bayesian group analysis were not biased by the applied inference model and to provide an additional estimate of the intersubject variability in effect size, we revisited the individual generalized linear model-results of the 13 subjects. For each of them, the maximum effect size estimate (beta-value) for activation following left and right, respectively, stimulation of all body parts was extracted individually for both hemispheres and all areas. These values represent the strength of (de-) activation in the individual cases. Their mean hence provides a representation of the effects of unilateral tactile stimulation within the analyzed architectonic areas which is independently of the computational model used for 2nd-level inference, whereas their standard error quantifies the variability of the cortical response across individuals.
Results
The locations of the ROIs by their responsiveness to tactile stimulation of the hand, face or trunk is shown in Figure 3A and clearly reflects the well known somatotopic organization of the human postcentral gyrus (Kaas and Collins 2003). The somatotopic organization of the human parietal operculum has been subject to a more extensive report (Eickhoff, Grefkes, et al. 2007), and is summarized by the respective ROIs used here as shown in Figure 3B.
Figure 3.
The location of the defined ROIs (given by their anatomical location within the SI respectively SII subareas, a posterior probability for activation following stimulation of the respective body part on either side of > 95% and a probability of > 90% for showing stronger activation than both of the 2 other examined body parts) reveals the somatotopic organization of the human primary (A) and secondary (B) somatosensory cortices. For the display of the somatotopic ROIs in the secondary somatosensory cortex, the temporal lobes have been removed from the template brain in order to allow an unobstructed view on the parietal operculum. Note that the respective ROIs are mutually exclusive in 3-dimensional space but may overlap in the projection of their location to the cortical surface.
Figure 4.

Mean posterior probabilities for activation (green) and deactivation (red) following ipsilateral trunk stimulation as well as those for activation following contralateral trunk stimulation are given for each of the 3 analyzed areas of the primary and secondary somatosensory cortex on either side of the brain. All cortical areas within the SI and SII region show a strong bilateral response to tactile stimulation of the trunk, as the probabilities for ipsilateral activation were no lower than those following contralateral stimulation.
Figure 5.
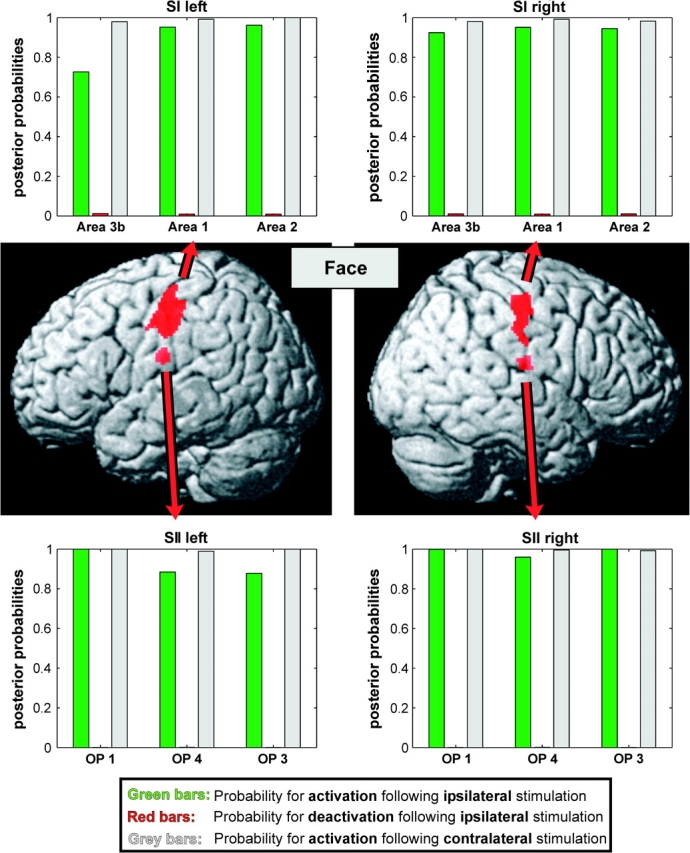
Mean posterior probabilities for activation (green) and deactivation (red) following ipsilateral face stimulation as well as those for activation following contralateral face stimulation are given for each of the 3 analyzed areas of the primary and secondary somatosensory cortex on either side. Similar to the trunk regions within SI and SII (Fig. 4), also the head representations within the analyzed areas showed a pronounced ipsilateral response. However, the posterior probabilities for ipsilateral activation within these regions were slightly lower as compared with those observed within the trunk representations.
Responses to Trunk Stimulation
The mean probability for contralateral activation following tactile stimulation of the trunk by a moving sponge was above 98.3% within all 5 analyzed ROIs (representing areas 3b, 1, and 2 on the postcentral gyrus and areas OP 1, OP 4, and OP 3 on the parietal operculum) of both hemispheres. Indicative of a strongly bilateral representation of proximal body parts, the mean posterior probabilities for activation following ipsilateral stimulation were virtually identical to those observed following contralateral stimulation (mean: 98.2%). The strong ipsilateral responsiveness was furthermore corroborated by the fact that the minimum probability for ipsilateral activation was as high as 96.2%, whereas in none of the analyzed regions, ipsilateral response probabilities were more than 3.7% below that for those following contralateral stimulation (mean difference: 1.2%). Consequently, the probabilities for deactivation were close to zero in all cases. In summary, our data strongly suggest an extensive bilateral representation of the trunk in all areas of the primary and secondary somatosensory cortex.
Responses to Face Stimulation
Like for trunk stimulation, stimulation of the face yielded a high mean probability for contralateral activation in all cytoarchitectonic areas indicating the robustness of the employed stimulation paradigm and a reliable detection of the evoked activity by our fMRI measurements (mean probability 99.3%). Within the 3 analyzed SII subregions on either hemisphere the mean probability for activation following ipsilateral stimulation was 95.4%. These probabilities were hence, on average, only 4.2% lower than those following contralateral stimulation. Within the cytoarchitectonic areas of the primary somatosensory cortex (areas 3b, 1, and 2) the mean posterior probabilities for ipsilateral activation were between 74.7% and 98.2% (mean: 93.0%) and hence on average 5.8% lower as compared with the ones for activation following contralateral stimulation. Accordingly, the posterior probabilities for deactivation were extremely low (max: 0.2%) in all areas. We may summarize our data as showing a consistent bilateral response in the human somatosensory cortex, which was, however, on average 4.0% lower than that observed for the trunk region.
Responses to Hand Stimulation
Tactile stimulation of the hands evoked robust activation in the contralateral primary and secondary somatosensory cortex, where the mean probabilities for activation were above 98.4% for all analyzed areas. The mean posterior probabilities for activation following ipsilateral stimulation, however, were different from those observed for trunk or face stimulation. They furthermore showed a clear differentiation between areas and hemispheres. Within the secondary somatosensory cortex, all probabilities for ipsilateral activations were above 70% (mean: 82.1%) and hence on average 15% lower as compared with those observed within the ipsilateral SII region following stimulation of the trunk or face. In contrast to these secondary somatosensory areas, area 3b and area 1 of the primary somatosensory cortex did not show any tendency for ipsilateral responses to tactile stimulation. Rather, both of these areas showed high probabilities for deactivation most pronounced on the left hemisphere (72.8%/77.7% on the left; 50.2%/54.4% on the right hemisphere). The responses of the most posterior area within the SI complex, area 2, following ipsilateral hand stimulation finally showed a clear hemispheric asymmetry. The hand region within left area 2 had a mean posterior probability for deactivation of 44.9% (along with an 8.3% posterior probability for activation). Right area 2, however, was the only SI area where the posterior probability for activation following ipsilateral hand stimulation was considerably larger than that for deactivation (43.7% vs. 8.0%).
Effect Size Analysis
The pattern described by the mean (across subjects) effect size estimates (Fig. 7) confirmed the results from the Bayesian group analysis. In particular, for face and trunk stimulation, the effect size estimates within all areas were similar for ipsi- and contralateral stimulation, which is in good agreement with the posterior probabilities. The fact, that the standard errors of these estimates are small relative to the mean effect size is also congruent to the high probabilities for activation observed in the Bayesian model. The analysis of single-subject effect size estimates also confirmed our findings regarding differentiated activation pattern within the SI hand representation: areas 3b and 1, which of course showed strong activation to contralateral stimulation, these are deactivated (inhibited) by ipsilateral stimulation, whereas there's an asymmetry in the responsiveness of area 2, such that only right area 2 is activated by ipsilateral as well as contralateral stimulation. For left area 2, finally, the mean effect size was close to zero, whereas the standard error was considerably higher as compared with other regions.
Figure 7.
Mean (±SEM) of the individual effect size estimates (beta-values) for each condition extracted from the single-subject analyses. The close correspondence between the patterns emerging from this mean individual effect size plots and those from the posterior probabilities as derived from the Bayesian group analysis confirmed that the observed effects were indeed stable across subjects.
Discussion
Secondary Somatosensory Cortex
The existence of 3 distinct areas (SII, PV, and VS) within the SII region of nonhuman primates is now widely recognized (Kaas 2004; Kaas and Collins 2003). The present study demonstrates, that the analyzed architectonically defined SII subregions in the human cerebral cortex (OP 1, the likely human equivalent to area SII, OP 4, the presumed homologue to area PV and OP 3, the most likely candidate for human area VS; Eickhoff, Amunts, et al. 2006; Eickhoff, Schleicher, et al. 2006; Eickhoff, Grefkes, et al. 2007) show robust bilateral responses to unilateral stimulation of all examined body part. This confirms previous functional neuroimaging reports of bilateral SII activity to unilateral stimulation (Disbrow et al. 2000; Ruben et al. 2001; Ferretti et al. 2003; Young et al. 2004; Blatow et al. 2007) in an area- and body part–specific fashion allowing for a systematic characterization of ipsilateral response properties. Moreover, these response characteristics match well with the reported high number of neurons in the SII regions showing bilateral receptive fields observed in both nonhuman primates (Cusick et al. 1989; Coq et al. 2004; Fitzgerald et al. 2006) and other mammals (Krubitzer et al. 1986; Catania et al. 1999; Tommerdahl et al. 2005). These congruencies with respect to response characteristics demonstrated here at the level of individual areas in the human brain thus further adds to the notion of a relatively preserved organization of the SII region (at least across primates) as previously suggested by similarities in areal topography and somatotopic organization (Eickhoff, Schleicher, et al. 2006; Eickhoff, Grefkes, et al. 2007).
Primary Somatosensory Cortex
Strepsirrhini (e.g., lemures and galagos) are considered to follow a basic nonprimate plan of SI organization dominated by primary somatosensory area 3b (Wu and Kaas 2003; Kaas 2004). In titi-monkeys, Padberg et al. (2005) confirmed the existence of a separate, well defined area 1 but also provided compelling evidence for an absence of an area 2, both of which is in good correspondence to earlier reports in other New World monkeys (Merzenich et al. 1983; Cusick et al. 1989; Krubitzer and Kaas 1990; Coq et al. 2004; reviewed by Padberg et al. 2007) but contrasts observations in old world monkeys (Pons et al. 1985; Iwamura et al. 2002). In macaques, finally, area 2 has large often bilateral receptive fields with an extensive fine-graded representation of the hand and dense transcallosal connections (Pons et al. 1985; Pons and Kaas 1986; Iwamura 2000; Iwamura et al. 2001, 2002). The emergence of a posterior parietal cortex in primates, the existence of a distinct area 1 in New World monkeys and the presence of area 2 in old world monkeys and humans has repeatedly been linked to increasing manual skills (Kaas 2004; Padberg et al. 2005). This view is supported by the fact, that the relative amount of cortex devoted to the hands is considerably larger within area 2 as compared with, for example, area 3b (Pons et al. 1985; Iwamura et al. 2002). The observation that only area 2 features transcallosal connections within its hand region (Pons and Kaas 1986; Iwamura et al. 2001) also supposed association to manual abilities by enabling the integration of tactile information from both hands for bimanual coordination and exploration (Padberg et al. 2007). Probably the most intriguing evidence linking area 2 to the manual skills comes from a recent study in cebus monkeys, which were shown to be the only species of new word monkeys which features a distinct area 2 similar to that in macaques (Padberg et al. 2007). Strikingly, cebus monkeys are also the only New World monkeys, which, like macaques, possess an opposable thumb, use feeding tools in the wild and are capable of complex object manipulation.
Our data now point toward distinct role for area 2 in the processing hand related information also in humans. Although tactile stimulation of either hand resulted in an inhibition of ipsilateral areas 3b and 1 as previously suggested by (Hlushchuk and Hari 2006), the ipsilateral responses in area 2 showed a strong hemispheric asymmetry (cf. Fig. 6). While left area 2 was deactivated by ipsilateral hand stimulation—albeit to a lesser degree as areas 3b and 1- right area 2 showed a positive response (i.e., activation) to ipsilateral input. As other functional imaging studies also reported a more posterior location of the local maxima during ipsilateral as compared with contralateral hand stimulation (Allison et al. 1989; Nihashi et al. 2005; Hlushchuk and Hari 2006), we conclude that only area 2 shows ipsilateral responses to unilateral hand stimulation. This specific role in the processing of tactile input from the dominant hand in humans strongly supports the notion that area 2 represents an area which has evolved as the cortical substrate of increased manual abilities resulting from opposable thumbs in anthropoid primates (Kaas 2004; Padberg et al. 2005; Padberg et al. 2007).
Figure 6.

Mean posterior probabilities for activation (green) and deactivation (red) following ipsilateral hand stimulation as well as those for activation following contralateral hand stimulation are given for each of the 3 analyzed areas of the primary and secondary somatosensory cortex on either side of the brain. All subareas of the secondary somatosensory cortex responded well to ipsilateral tactile stimulation of the hands, although the mean probabilities for activation were considerably lower as compared with those observed within the trunk and face regions (Figs 4 and 5). In contrast, areas 3b and 1 of the primary somatosensory cortex showed a deactivation following ipsilateral hand stimulation, which was more pronounced in the left hemisphere. Left area 2 finally was also deactivated by ipsilateral stimuli, whereas right area 2 was the only part of the primary somatosensory cortex showing a substantial positive response to ipsilateral tactile stimulation.
As little is known about the functional lateralization of somatosensory cortex in the nonhuman primates (mainly due to the limitation in spatial coverage for electrophysiological recordings), the question remains, whether the observed hemispheric asymmetry may be a new feature that has evolved in humans as a substrate of yet increased fine motor skills. In this case, the newly formed lateralization toward the right hemisphere, which has previously been suggested for kinesthesia (Naito et al. 2005), would parallel the pronounced lateralization of human brain functions such as language (left; Amunts et al. 2004), space-representation (right; Fink et al. 2003) and higher motor functions (left; Vogt et al. 2007). Support for this notion is provided by a comparison of hand preferences between humans and monkeys: In humans, most authors recognize 3 levels of handedness (Annett, 2004; Ellis et al. 1988; Oldfield, 1971; Warren, 1977): 1) choosing always the same hand for a given task, 2) preferring the same hand across tasks and 3) a clear majority of subjects prefers the right hand. Although it is agreed upon, that nonhuman primates may prefer 1 hand for a particular task, there is little evidence for the 2 latter levels of handedness (Deuel and Dunlop 1980; Lehman 1980; Annett and Annett 1991; Mittra et al. 1997; Gardinier et al. 2006). Based on these observations and our own data, we would suggest that the lateralization within area 2 is a specific human feature, which may be regarded as an expression of a more lateralized organization of the human brain per se and is mirrored by a more pronounced handedness. This hypothesis, however, remains to be tested directly, for example, by using monkey-fMRI where task- and imaging specifications can be set up to closely match human studies.
Transcallosal Connections of the Secondary Somatosensory Cortex
Due to the temporal resolution of fMRI, we cannot infer from our data whether the ipsilateral secondary somatosensory cortex is driven by thalamic input or by transcallosal projections. Homotopic transcallosal connections between the different SII areas, however, have repeatedly been demonstrated in many species (Krubitzer et al. 1986; Burton et al. 1995; Catania et al. 1999; Qi et al. 2002; Disbrow et al. 2003; Kaas 2004) and are thus likely to exist in humans too. This view is supported by an fMRI study of a patient undergoing callosotomy, who showed robust bilateral SII activation before the operation which was abolished following surgery (Fabri et al. 2001). Moreover, there is converging evidence from methods providing higher temporal resolution that contralateral SII responses precede ipsilateral ones by about 14 ms, favoring a monosynaptic transcallosal connection (Mima et al. 1997; Frot and Mauguiere 1999; Karhu and Tesche 1999; Ploner et al. 1999). We may hence assume that the observed ipsilateral responses within SII originate predominantly from transcallosal input from the contralateral SII region.
Transcallosal Connections of the Primary Somatosensory Cortex
Face and trunk representations within areas 3b, 1, and 2 feature homotopic transcallosal connections terminating in the same anatomical area (Krubitzer et al. 1986; Krubitzer and Kaas 1990; Kaas and Collins 2003; Wu et al. 2000), which may be considered an important substrate of their bilateral representations, although bilateral thalamic input may contribute as well (Cusick et al. 1985; Krubitzer and Kaas 1987; Iyengar et al. 2007). In contrast, no such connections were observed for the hand region of these 2 areas, that is, there is no direct interhemispheric transfer of somatosensory information from the hands within areas 3b and 1 (Killackey et al. 1983; Krubitzer and Kaas 1990; Padberg et al. 2005). Area 2 in contrast shows dense transcallosal connections also within the hand region (Killackey et al. 1983; Iwamura 2000; Iwamura et al. 2001, 2002) resulting in bilateral receptive fields, which is in line with our results showing bilateral activation to hand stimulation in area 2. The question hence arises how the inhibition of ipsilateral areas 3b and 1 is mediated in the absence of transcallosal connections. Although transcallosal, interareal connections and bilateral thalamic connections may provide possible mechanisms, their existence has mainly been demonstrated in nonprimate mammals (Krubitzer et al. 1998). The most likely pathways for these inhibitory effects thus relies on interhemispheric transfer of information between area 2 on either side (Killackey et al. 1983; Iwamura 2000; Iwamura et al. 2001, 2002) and subsequent cortico-cortical connections from (ipsilateral) area 2 to (ipsilateral) areas 3b and 1 (Pons and Kaas 1986; Burton and Fabri 1995) as discussed by (Hlushchuk and Hari 2006).
The Issue of Handedness
Only right-handed subjects were examined in this experiment to ensure that the examined population was as homogenous as possible with respect to their (behavioral) hand-dominance given that left handed are generally more ambiguous in their preferred hand (Sarma 1989; Meng 2007). Although most right-handed persons have very poor left-hand skills, left-handers usually have a higher proficiency in right hand use, that is, a weaker (behavioral) lateralization (Sarma 1989; Siebner et al. 2002; Meng 2007). The most obvious reasons for this mismatch are the primary specification of many everyday tools for right-handed use and the education of left-handed children toward using their nondominant right hand (Meng 2007). In this context, it is interesting to note, that the observation of weaker lateralization in left-handers dates back to the original publication of the Edinburgh Handedness Inventory (Oldfield 1971), where the author states: “This mode toward the left-handed end may be the result of some adaptation by left-handed people to a world predominantly organized for the right handed.” This view also coincides with the observation of a declining prevalence for left-handedness (and an increasing amount of people who consider themselves “switched”) in older populations (Galobardes et al. 1999), which has been ascribed to a change in the sociocultural environment making left-handedness more acceptable nowadays.
It should thus be noted, that the observed bilateral representation of the dominant hand in ipsilateral area 2 is specific to right-handed persons but does not allow more general inference about dominant hand lateralization per se. To investigate this issue in more depth, experiments with strongly left-handed persons, “retrained” left-handers or ambidexters are needed, potentially using an analysis approach similar to the one demonstrated here.
Conclusions
The present study combined anatomical mapping data, stimulation of multiple locations on either side of the body and a Bayesian modeling approach to quantify the probability of ipsilateral activation within the human somatosensory areas. We could show that all SII areas as well as the trunk and face representations within SI show robust bilateral responses to unilateral stimulation, which is in agreement to their transcallosal projections demonstrated in nonhuman primates. Whereas areas 3b and 1 of the ipsilateral SI region were deactivated by tactile hand stimulation, there was strong evidence for bilateral processing of information from the right hand in area 2. These results demonstrate a distinctive cortical representation pattern of the hand and support the hypothesis that area 2 may have evolved as a substrate of increased manual abilities as concluded from previous observations that this area has up to now only been recognized in primates featuring opposable thumbs (Padberg et al. 2007).
Funding
National Institute of Biomedical Imaging and Bioengineering; the National Institute of Neurological Disorders and Stroke; and the National Institute of Mental Health and the Deutsche Forschungsgemeinschaft (KFO-112) funded the Human Brain Project/Neuroinformatics research.
Acknowledgments
Conflict of Interest: None declared.
References
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol. 1989;62:711–722. doi: 10.1152/jn.1989.62.3.711. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Annett M. Hand preference observed in large healthy samples: classification, norms and interpretations of increased non-right-handedness by the right shift theory. Br J Psychol. 2004;95:339–353. doi: 10.1348/0007126041528130. [DOI] [PubMed] [Google Scholar]
- Annett M, Annett J. Handedness for eating in gorillas. Cortex. 1991;27:269–275. doi: 10.1016/s0010-9452(13)80131-1. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Rigid body registration. In: Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human brain function. 2nd ed. Oxford (United Kingdom): Academic Press; 2003a. pp. 635–655. [Google Scholar]
- Ashburner J, Friston KJ. Spatial normalization using basis functions. In: Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human brain function. 2nd ed. Oxford (United Kingdom): Academic Press; 2003b. pp. 655–673. [Google Scholar]
- Blatow M, Nennig E, Durst A, Sartor K, Stippich C. fMRI reflects functional connectivity of human somatosensory cortex. Neuroimage. 2007;37(3):927–936. doi: 10.1016/j.neuroimage.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Boling W, Reutens DC, Olivier A. Functional topography of the low postcentral area. J Neurosurg. 2002;97:388–395. doi: 10.3171/jns.2002.97.2.0388. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in areas 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. J Comp Neurol. 1995;355:508–538. doi: 10.1002/cne.903550404. [DOI] [PubMed] [Google Scholar]
- Burton H, Fabri M, Alloway K. Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: a revised interpretation of the second somatosensory area in macaque monkeys. J Comp Neurol. 1995;355:539–562. doi: 10.1002/cne.903550405. [DOI] [PubMed] [Google Scholar]
- Catania KC, Lyon DC, Mock OB, Kaas JH. Cortical organization in shrews: evidence from five species. J Comp Neurol. 1999;410:55–72. [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Coq JO, Qi H, Collins CE, Kaas JH. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World Titi monkey (Callicebus moloch) J Comp Neurol. 2004;476:363–387. doi: 10.1002/cne.20237. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Steindler DA, Kaas JH. Corticocortical and collateral thalamocortical connections of postcentral somatosensory cortical areas in squirrel monkeys: a double-labeling study with radiolabeled wheatgerm agglutinin and wheatgerm agglutinin conjugated to horseradish peroxidase. Somatosens Res. 1985;3:1–31. doi: 10.3109/07367228509144574. [DOI] [PubMed] [Google Scholar]
- Cusick CG, Wall JT, Felleman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b S-II, and a ventral somatosensory area. J Comp Neurol. 1989;282:169–190. doi: 10.1002/cne.902820203. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Nowicky AV, Zaman R. Somatopy of perceptual threshold to cutaneous electrical stimulation in man. Exp Physiol. 2001;86:127–130. doi: 10.1113/eph8602086. [DOI] [PubMed] [Google Scholar]
- Deuel RK, Dunlop NL. Hand preferences in the rhesus monkey. Implications for the study of cerebral dominance. Arch Neurol. 1980;37:217–221. doi: 10.1001/archneur.1980.00500530055008. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17:1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Lotze M, Wietek B, Amunts K, Enck P, Zilles K. Segregation of visceral and somatosensory afferents. An fMRI and cytoarchitectonic mapping study. Neuroimage. 2006;31:1004–1014. doi: 10.1016/j.neuroimage.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbas MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The Human Parietal Operculum. I. Cytoarchitectonic Mapping of Subdivisions. Cereb Cortex. 2006;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K. Identifying human parietal-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp. 2006;27:611–621. doi: 10.1002/hbm.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SJ, Ellis PJ, Marshall E. Hand preference in a normal population. Cortex. 1988;24:157–163. doi: 10.1016/s0010-9452(88)80025-x. [DOI] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T. Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci. 2001;13:1071–1079. doi: 10.1162/089892901753294365. [DOI] [PubMed] [Google Scholar]
- Fabri M, Polonara G, Salvolini U, Manzoni T. Bilateral cortical representation of the trunk midline in human first somatic sensory area. Hum Brain Mapp. 2005;25:287–296. doi: 10.1002/hbm.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A, Babiloni C, Gratta CD, Caulo M, Tartaro A, Bonomo L, Rossini PM, Romani GL. Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: an fMRI study. Neuroimage. 2003;20:1625–1638. doi: 10.1016/j.neuroimage.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, Zilles K, Dieterich M. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage. 2003;20:1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field (RF) properties of the macaque second somatosensory cortex: RF size, shape, and somatotopic organization. J Neurosci. 2006;26:6485–6495. doi: 10.1523/JNEUROSCI.5061-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD. Classical and Bayesian inference. In: Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human brain function. 2nd ed. Oxford (United Kingdom): Academic Press; 2003a. pp. 911–970. [Google Scholar]
- Friston KJ, Penny WD. Posterior probability maps and SPMs. Neuroimage. 2003b;19:1240–1249. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguiere F. Timing and spatial distribution of somatosensory responses recorded in the upper bank of the sylvian fissure (SII area) in humans. Cereb Cortex. 1999;9:854–863. doi: 10.1093/cercor/9.8.854. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Bernstein MS, Morabia A. The association between switching hand preference and the declining prevalence of left-handedness with age. Am J Public Health. 1999;89:1873–1875. doi: 10.2105/ajph.89.12.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinier J, Franco V, Schieber MH. Interactions between lateralized choices of hand and target. Exp Brain Res. 2006;170:149–159. doi: 10.1007/s00221-005-0193-9. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11:684–696. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Porro CA, Pantano P, Romanelli PL, Galeotti F, Cruccu G. Representation of different trigeminal divisions within the primary and secondary human somatosensory cortex. Neuroimage. 2003;19:906–912. doi: 10.1016/s1053-8119(03)00139-3. [DOI] [PubMed] [Google Scholar]
- Itomi K, Kakigi R, Maeda K, Hoshiyama M. Dermatome versus homunculus; detailed topography of the primary somatosensory cortex following trunk stimulation. Clin Neurophysiol. 2000;111:405–412. doi: 10.1016/s1388-2457(99)00290-4. [DOI] [PubMed] [Google Scholar]
- Iwamura Y. Bilateral receptive field neurons and callosal connections in the somatosensory cortex. Philos Trans R Soc Lond B Biol Sci. 2000;355:267–273. doi: 10.1098/rstb.2000.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Iriki A, Taoka M, Toda T. Processing of tactile and kinesthetic signals from bilateral sides of the body in the postcentral gyrus of awake monkeys. Behav Brain Res. 2002;135:185–190. doi: 10.1016/s0166-4328(02)00164-x. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Taoka M, Iriki A. Bilateral activity and callosal connections in the somatosensory cortex. Neuroscientist. 2001;7:419–429. doi: 10.1177/107385840100700511. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Qi HX, Jain N, Kaas JH. Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of New World monkeys. J Comp Neurol. 2007;501:95–120. doi: 10.1002/cne.21232. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Collins CE. The organization of somatosensory cortex in anthropoid primates. Adv Neurol. 2003;93:57–67. [PubMed] [Google Scholar]
- Kanno A, Nakasato N, Hatanaka K, Yoshimoto T. Ipsilateral area 3b responses to median nerve somatosensory stimulation. Neuroimage. 2003;18:169–177. doi: 10.1006/nimg.2002.1283. [DOI] [PubMed] [Google Scholar]
- Karhu J, Tesche CD. Simultaneous early processing of sensory input in human primary (SI) and secondary (SII) somatosensory cortices. J Neurophysiol. 1999;81:2017–2025. doi: 10.1152/jn.1999.81.5.2017. [DOI] [PubMed] [Google Scholar]
- Karlen SJ, Krubitzer L. The evolution of the neocortex in mammals: intrinsic and extrinsic contributions to the cortical phenotype. Novartis Found Symp. 2006;270:146–159. [PubMed] [Google Scholar]
- Killackey HP, Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol. 1983;219:384–419. doi: 10.1002/cne.902190403. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. The organization of neocortex in mammals: are species differences really so different? Trends Neurosci. 1995;18:408–417. doi: 10.1016/0166-2236(95)93938-t. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Clarey JC, Tweedale R, Calford MB. Interhemispheric connections of somatosensory cortex in the flying fox. J Comp Neurol. 1998;402:538–559. [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. Thalamic connections of three representations of the body surface in somatosensory cortex of gray squirrels. J Comp Neurol. 1987;265:549–580. doi: 10.1002/cne.902650408. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture, and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. J Comp Neurol. 1986;250:403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- Lehman RA. Distribution and changes in strength of hand preference of cynomolgus monkeys. Brain Behav Evol. 1980;17:209–217. doi: 10.1159/000121800. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng LF. The rate of handedness conversion and related factors in left-handed children. Laterality. 2007;12:131–138. doi: 10.1080/13576500601005727. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Mima T, Ikeda A, Nagamine T, Yazawa S, Kunieda T, Mikuni N, Taki W, Kimura J, Shibasaki H. Human second somatosensory area: subdural and magnetoencephalographic recording of somatosensory evoked responses. J Neurol Neurosurg Psychiatry. 1997;63:501–505. doi: 10.1136/jnnp.63.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra ES, Fuentes A, McGrew WC. Lack of hand preference in wild Hanuman langurs (Presbytis entellus) Am J Phys Anthropol. 1997;103:455–461. doi: 10.1002/(SICI)1096-8644(199708)103:4<455::AID-AJPA3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, Zilles K, Ehrsson HH. Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol. 2005;93:1020–1034. doi: 10.1152/jn.00637.2004. [DOI] [PubMed] [Google Scholar]
- Nihashi T, Naganawa S, Sato C, Kawai H, Nakamura T, Fukatsu H, Ishigaki T, Aoki I. Contralateral and ipsilateral responses in primary somatosensory cortex following electrical median nerve stimulation—an fMRI study. Clin Neurophysiol. 2005;116:842–848. doi: 10.1016/j.clinph.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Nolan MF. Quantitative measure of cutaneous sensation. Two-point discrimination values for the face and trunk. Phys Ther. 1985;65:181–185. doi: 10.1093/ptj/65.2.181. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2? Cereb Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Padberg J, Franca JG, Cooke DF, Soares JG, Rosa MG, Fiorani M, Jr, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. J Neurosci. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Holmes AP. Random effects analysis. In: Frackowiak RS, Friston KJ, Frith CD, Dolan RJ, Price CJ, Ashburner J, Penny WD, Zeki S, editors. Human brain function. 2nd ed. Oxford (United Kingdom): Academic Press; 2003. pp. 843–850. [Google Scholar]
- Ploner M, Schmitz F, Freund HJ, Schnitzler A. Parallel activation of primary and secondary somatosensory cortices in human pain processing. J Neurophysiol. 1999;81:3100–3104. doi: 10.1152/jn.1999.81.6.3100. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Cusick CG, Kaas JH. The somatotopic organization of area 2 in macaque monkeys. J Comp Neurol. 1985;241:445–466. doi: 10.1002/cne.902410405. [DOI] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: a correlative anatomical and electrophysiological study. J Comp Neurol. 1986;248:313–335. doi: 10.1002/cne.902480303. [DOI] [PubMed] [Google Scholar]
- Qi HX, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus) J Comp Neurol. 2002;443:168–182. doi: 10.1002/cne.10113. [DOI] [PubMed] [Google Scholar]
- Ruben J, Schwiemann J, Deuchert M, Meyer R, Krause T, Curio G, Villringer K, Kurth R, Villringer A. Somatotopic organization of human secondary somatosensory cortex. Cereb Cortex. 2001;11:463–473. doi: 10.1093/cercor/11.5.463. [DOI] [PubMed] [Google Scholar]
- Sarma PS. Mixed-handedness and achievement test scores of grade school children. Percept Mot Skills. 1989;68:839–846. doi: 10.2466/pms.1989.68.3.839. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Palomero-Gallagher N, Morosan P, Eickhoff SB, Kowalski T, de Vos K, Amunts K, Zilles K. Quantitative architectectural analysis: a new approach to cortical mapping. Anat Embryol (Berl). 2005;210:373–386. doi: 10.1007/s00429-005-0028-2. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Limmer C, Peinemann A, Drzezga A, Bloem BR, Schwaiger M, Conrad B. Long-term consequences of switching handedness: a positron emission tomography study on handwriting in “converted” left-handers. J Neurosci. 2002;22:2816–2825. doi: 10.1523/JNEUROSCI.22-07-02816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Tang AC. Reliable detection of bilateral activation in human primary somatosensory cortex by unilateral median nerve stimulation. Neuroimage. 2006;33:1042–1054. doi: 10.1016/j.neuroimage.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Simons SB, Chiu JS, Tannan V, Favorov O, Whitsel B. Response of SII cortex to ipsilateral, contralateral and bilateral flutter stimulation in the cat. BMC Neurosci. 2005;6:11. doi: 10.1186/1471-2202-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschlager AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund HJ, Rizzolatti G, Fink GR. Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage. 2007;37:1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Warren JM. Functional lateralization of the brain. Ann N Y Acad Sci. 1977;299:273–280. doi: 10.1111/j.1749-6632.1977.tb41914.x. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Somatosensory cortex of prosimian Galagos: physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457:263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- Young JP, Herath P, Eickhoff S, Choi J, Grefkes C, Zilles K, Roland PE. Somatotopy and attentional modulation of the human parietal and opercular regions. J Neurosci. 2004;24:5391–5399. doi: 10.1523/JNEUROSCI.4030-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Quantitative analysis of cyto- and receptor architecture of the human brain. In: Mazziotta J, Toga A, editors. Brain mapping, the methods. Amsterdam (The Netherlands): Elsevier; 2002. pp. 573–602. [Google Scholar]