Abstract
We propose a translational approach to the study of anorexia nervosa (AN) based on our human subject studies where there are characteristic elevations in 5-HT1A receptor binding, associated harm avoidance behaviors, reduced impulsivity, and comorbid anxiety disorders. Towards this goal, the hyponeophagia assay was implemented whereby food-deprived mice show increased latency to begin feeding in a novel, anxiogenic environment. The non-selective serotonin agonist, 5-MeODMT, potentiates feeding inhibition compared to the inhibition generated by the anxiogenic environment in a drug-by-environment interaction. Thus, using hyponeophagia in mice, it was possible to study the following key components of AN: anxiety; feeding inhibition; and a modulatory role of the serotonergic system. A major prediction of the proposed AN model is that 5-HT1A receptor activation is necessary for feeding inhibition. In support of this model, the 5-HT1A receptor antagonist, WAY100635, reverses the 5-MeODMT-dependent potentiation of feeding inhibition. Our findings hint at a mechanistic role for increased 5-HT1A receptor activation in restricting-type AN. Further implications for the interplay between anxiety and feeding inhibition in AN are discussed.
Keywords: Serotonin, Anorexia, Feeding, Anxiety, Impulsivity, Novelty
Introduction
Serotonin (5-HT) release has been reported consistently to modulate impulsive behaviors (Carli et al., 2006). Serotonergic neurons originate in the raphe nuclei and project to various cortical regions, including the frontal lobes (Ago et al., 2003; Varnas et al., 2004). Abnormal frontal inhibition and altered serotonergic signaling likely play important roles in anorexia nervosa (AN), whereby patients show marked harm avoidance and have significantly altered 5-HT receptor binding profiles in cortical regions (Frank and Kaye 2005). AN is characterized by a highly distorted body image and a relentless pursuit of weight loss leading to emaciation and even death. This disorder involves an abnormally high level of “impulse” control, in which normal food seeking behavior is actively inhibited, even when in a state of hunger and malnutrition (American Psychiatric Association, 2000). Diagnostically, the onset of AN is often preceded by anxiety disorders and these anxiety disorders persist as significant comorbid illnesses (Kaye et al., 2004). Since there are no proven treatments, new insights into neurobiological mechanisms are needed to identify potential drug targets.
To empirically test the relationship between serotonergic signaling and the impulse to feed in an anxiogenic setting, we used a translational approach termed hyponeophagia, where exposure of mice to novelty inhibits the initiation of feeding (Trullas and Skolnick, 1993). We chose hyponeophagia to explore treatment strategies that might be specific to anxiety assayed via feeding inhibition, because anxiety might involve multiple neuronal circuits that show selective activation depending on context, stressor, or test condition (Lang et al., 2000). Most pharmacological studies of hyponeophagia have been performed in rats, with fewer studies using mice (Dulawa and Hen, 2005). Rodents are typically exposed to a novel environment which is anxiogenic, such as an unfamiliar cage or an open field, and the latency to begin feeding and/ or the amount consumed is measured. Using latency to feed as a measure has been shown to have the advantages of reliability and magnitude of response (Merali et al., 2003). Prior to testing, the rodents are either food deprived or trained to consume a palatable food that is provided during the assay. Anxiolytic treatment with benzodiazepines, 5HT1A agonists. or 5-HT re-uptake inhibitors, reduces the latency to feed, while anxiogenic treatment with the nonselective 5-HT agonist, 5-methoxy-N,N-dimethyltryptamine (5-MeODMT), increases the latency to feed in the novel environment. We optimized our assay to limit the need for training or individual housing and to limit interference from sensorimotor deficits by providing ready access to food.
Biochemically, PET imaging in AN reveals an increased ratio of 5HT1A/5-HT2A receptor binding potentials (Frank and Kaye 2005). Moreover, harm avoidance, a measure of anxiety and behavioral inhibition, is positively correlated with 5-HT1A receptor binding in mesial temporal cortex and subgenual cingulate cortex, and with 5-HT2A receptor binding in medial orbitofrontal cortex (Bailer et al., 2004; Bailer et al., 2007; Bailer et al., 2005; Frank et al., 2002). A better understanding of the relationship between 5-HT, anxiety, and feeding in AN might lead to better treatments. Feeding is anxiogenic for AN, which is paradoxical, as most people feel relief and comfort after eating (Frank and Kaye 2005). It is likely that 5-HT plays a role in this phenomenon, since serotonergic manipulations that are anxiogenic in healthy human subjects, such as acute depletion of tryptophan, the precursor of 5-HT, and treatment with meta-chlorophenylpiperazine (mCPP), paradoxically show clinical efficacy for anxiolysis in AN (Frank et al., 2001; Kaye et al., 2003). Considerable evidence from studies of animals and healthy humans support the likelihood that 5-HT1A and 5-HT2A receptors plays a role in anxiety (File et al., 2000; Moresco et al., 2002; Tauscher et al., 2001; Weisstaub et al., 2006). It is important to note that 5-HT2A and 5-HT1A postsynaptic receptors are highly co-localized (80%) in rodent frontal cortex (Amargos-Bosch et al., 2004), and other cortical regions (Varnas et al., 2004). Postsynaptic 5-HT1A and 5-HT2A receptors mediate, respectively, the direct hyperpolarizing and depolarizing actions of 5-HT on prefrontal neurons (Santana et al., 2004), which in turn project to numerous cortical and subcortical areas. Interactions between 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex (mPFC) and related regions appear to modulate anxiety, attentional functioning (Winstanley et al., 2003), impulsivity, compulsive perseveration (Carli et al., 2006), and exploration of novel environments (Krebs-Thomson and Geyer, 1998). Mechanistic studies are needed to further develop a model of AN, whereby an imbalance between enhanced 5-HT1A and diminished 5-HT2A receptor binding potentials contributes to feeding and behavioral symptoms.
The purpose of this study is to explore the role of 5-HT1A receptors in serotonergic potentiation of hyponeophagia. We first validated the experimental conditions of our assay by showing that mice are inhibited in beginning to feed in a novel anxiogenic environment and anxiolytic treatment (i.e. diazepam) can reverse this inhibition. Then, we confirm a role for the serotonergic system under our assay conditions by showing that mice treated with the nonselective 5-HT agonist, 5-MeODMT, show an exaggerated inhibition to begin feeding in a novel environment compared to placebo, as described previously in rats (Shephard and Broadhurst, 1982). Second, we hypothesize that 5-HT1A receptor activity is necessary for the 5-MeODMT-driven inhibition to begin feeding in a novel environment. We tested this hypothesis by combining the nonselective 5-HT agonist 5-MeODMT with the 5-HT1A receptor antagonist WAY100635 and asked if this combination reversed the 5-MeODMT-driven inhibition to begin feeding. WAY100635 reversed this inhibition driven by 5-MeODMT, thus supporting our hypothesis and hinting at a mechanistic role for increased 5-HT1A receptor activity in the inhibition to feed seen in AN.
Methods
Subjects
Briefly, wild-type C57BL/6N male mice, were acquired at 6–8 weeks of age (Charles River, Wilmington, MA) and acclimated to the vivarium. Mice were group housed under a 12 hour light/ dark reverse cycle in a temperature-controlled environment. Behavior was assayed between noon and 5 pm. All animal testing was conducted within NIH laboratory animal care guidelines and under IACUC approval.
Hyponeophagia Procedure
We implemented the hyponeophagia assay as previously described (Jennings et al., 2006; Shephard and Broadhurst, 1983) with the following optimizations to address the corresponding methodological considerations. Wild-type C57BL/6N male mice were food deprived overnight for 18 hours before testing. Feeding latency was assayed by placing the mice in a novel plastic cage, without bedding and under bright light. On the floor of the cage were 15 evenly spaced food pellets. This procedure ensures that mouse is immediately aware of the food and minimizes sensorimotor confounds in the assay. Latency to feed was measured from the time the mouse was placed in the novel environment until the mice persistently fed for greater than 3 seconds while holding the food with their front paws. Latencies were scored up to a cutoff time of 300 seconds. After testing, all mice were returned to their home cages and given free access to food. To assess feeding in the home cage environment, mice were presented with 15 pellets that were evenly distributed across the home cage in a darkened room and latency to begin feeding was assayed from the time of exposure to food as above. Latency was scored by a rater blind to treatment group. As previous hyponeophagia studies have been established predominantly using male mice, we followed this path to establish our assay. Since AN predominantly affects women (American Psychiatric Association, 2000), we hope to extend our studies with female mice in the future.
Behavioral testing
In experiment 1 and 2, we conducted an initial validation of the hyponeophagia paradigm, comparing feeding latency across novel and home cage environments. In addition, we compared the effects of the anxiolytic treatment diazepam and the anxiogenic treatment 5-MeODMT in the novel environment. Mice were assayed for feeding latency 25 minutes after treatment with vehicle, diazepam, or 5-MeODMT. In experiment 3, we compared the effects of the anxiogenic treatment 5-MeODMT with and without pretreatment with the 5-HT1A receptor antagonist WAY100635 in both home and novel environments. Mice were pretreated with WAY100635 ten minutes before treatment with vehicle or 5-MeODMT.
Statistics
Data are depicted as means/ standard errors and tests of significance were performed as described below. For experiment 1, an analysis of variance (ANOVA) was performed with environment as a between-subject factor. For experiment 2, two separate two-tailed t-tests were performed comparing diazepam treatment to vehicle, and 5-MeODMT treatment to vehicle. For experiment 3, a two-way ANOVA using 5-MeODMT treatment and environment were used as between-subject factors. For experiment 4, a two-way ANOVA using 5-MeODMT treatment and WAY100635 treatment were used as between-subject factors. Statistical analysis was performed using the BMDP statistical package (Statistical Solutions, Sangus, MA). Post hoc analyses were also completed when appropriate using Tukey’s post hoc test.
Drugs
Mice were treated subcutaneously with either drug or vehicle as described. Vehicle was either saline (0.9% NaCl) or 0.3% Tween-80/ 0.9 % NaCl (for diazepam experiments only). In experiment 2, when 5-MeODMT and diazepam groups shared the vehicle group, half of the vehicle animals were treated with saline vehicle while half of the group was treated with 0.3% Tween-80 vehicle. Because statistical analyses indicated no difference between the vehicle groups, data were pooled across them. Diazepam (Abbott, Abbott Park, IL) was administered at a dose of 1 mg/kg and 5-methoxy-N,N-dimethyltryptamine oxalate (RBI-Sigma, St. Louis, MO) was administered at a dose of 0.625 mg/kg to maximize efficacy, while limiting sensorimotor side-effects based on data from ongoing motor assays in the laboratory. WAY100635 (Sigma, St. Louis, MO) was administered at a dose of 0.3 mg/kg and injected 10 min prior to the 5-MeODMT or vehicle treatment.
Results
Mice are inhibited from beginning to feed in a novel environment
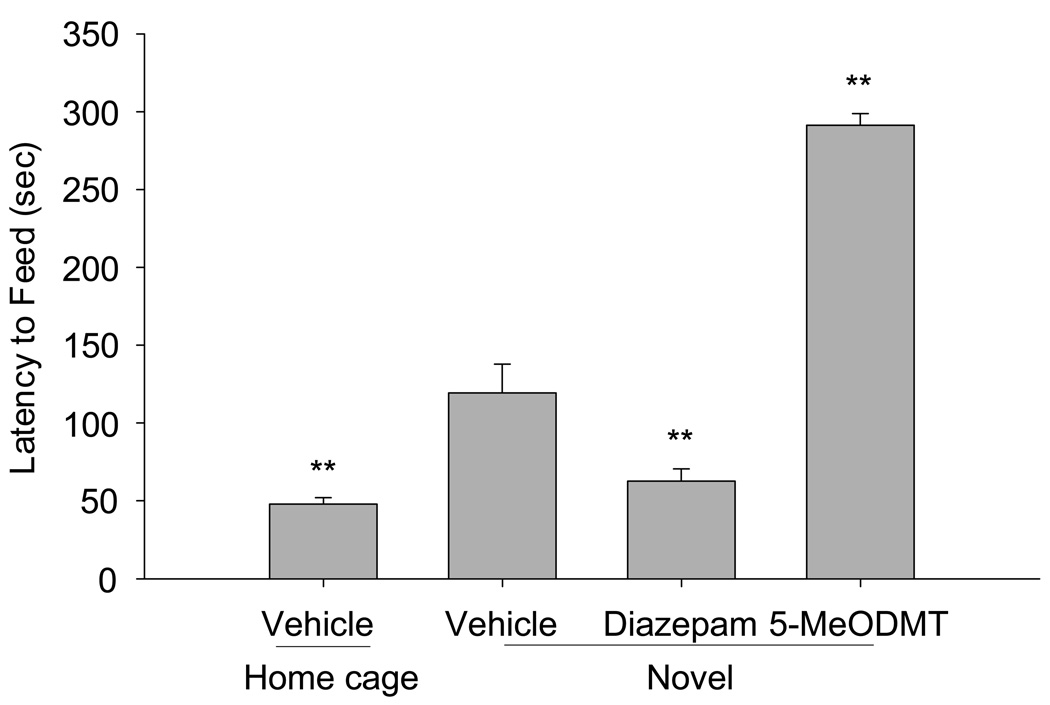
Under our experimental conditions, mice subcutaneously treated with vehicle alone show a significantly greater latency to begin feeding in the novel environment compared to feeding in the home cage (Figure 1; t(1, 14) = 16.07, p<0.01). This inhibition to begin feeding was significantly reversed when mice in the novel environment were treated with 1 mg/kg diazepam (Figure 1; t(1, 14) = 9.03, p< 0.01).
Figure 1.
Wild-type C57BL/6N mice were assayed for latency to feed in the novel, anxiogenic environment as well as in their home cage, as described. Each condition represents data from 6–8 mice. Diazepam was injected subcutaneously at a dose of 1 mg/ kg and 5-MeODMT at 0.625 mg/kg. Vehicle for the 5-MeODMT condition was saline (0.9% NaCl) and for the diazepam condition, it was 0.3% Tween-80/ 0.9 % NaCl. The anxiogenic novel environment increased feeding latency and diazepam reversed this increase. 5-MeODMT further increased feeding latency in the novel environment compared to vehicle. (**p < 0.01 versus vehicle in novel environment by T-test).
Non-selective 5-HT agonist 5-MeODMT potentiates inhibition to begin feeding in a novel environment-dependent manner
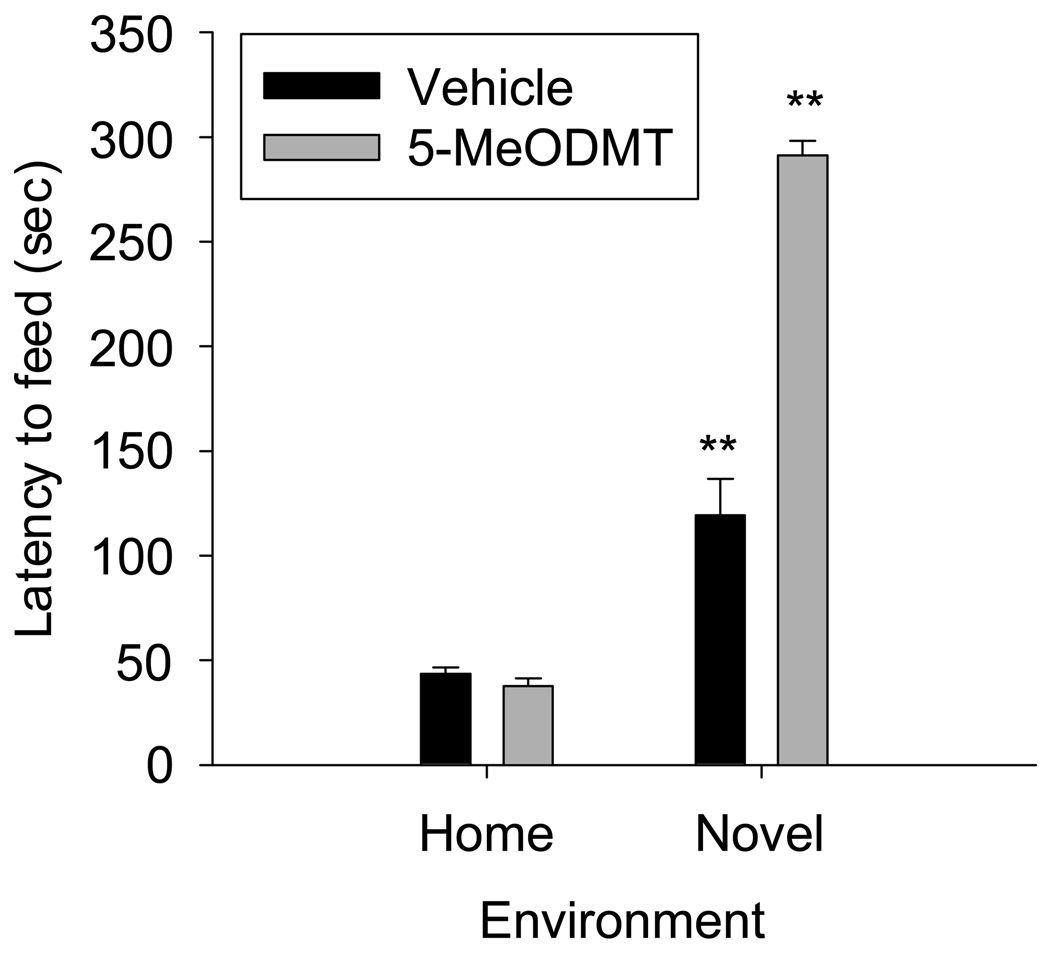
We next treated mice with 0.625 mg/kg 5-MeODMT and assayed for latency to begin feeding in a novel vs. familiar environment. 5-MeODMT significantly increased the latency to begin feeding in a novel environment compared to vehicle (Figure 2; Drug X Environment interaction: F(1,34)=108.62, p<0.001, 5-MeODMT vs. vehicle in novel environment, p<0.01 Tukey’s post hoc test). 5-MeODMT did not increase latency to feed in the home cage (Figure 2, 5-MeODMT vs. vehicle in home cage, n.s., Tukey’s post hoc test).
Figure 2.
5-MeODMT was injected subcutaneously at a dose of 0.625 mg/kg and latency to feed was assayed in both the anxiogenic, novel environment as well as in the home cage. 5-MeODMT inhibited feeding compared to vehicle in the novel environment (this data is depicted from Figure 1 for comparison) but was not significantly different than vehicle in the home cage. (**p < 0.01 versus vehicle in novel environment, Tukey’s test).
5-HT1A receptor antagonist WAY100635 reverses 5-MeODMT-dependent potentiation of feeding inhibition
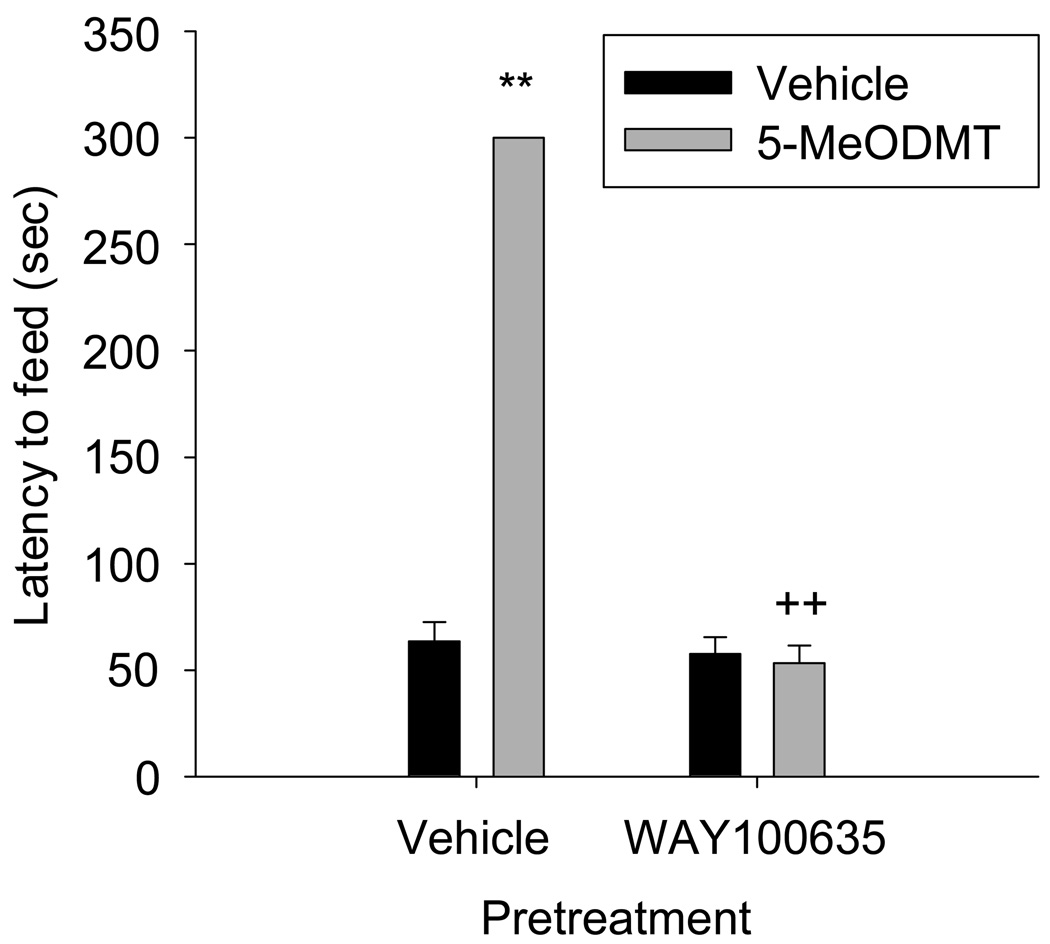
We hypothesized that 5-HT1A receptor activation is necessary for 5-MeODMT to increase the latency to begin feeding in the novel environment. To test this hypothesis, we pretreated mice with the 5-HT1A receptor antagonist WAY100635 at a dose of 0.3 mg/kg or vehicle 10 min prior to injecting 5-MeODMT or vehicle and assayed mice for latency to begin feeding in a novel environment. Pretreatment with WAY100635 reversed the 5-MeODMT-dependent increase in latency to begin feeding, but did not affect feeding when administered alone (Figure 3, WAY100635 × 5-MeODMT interaction: F(1,25)=313, p<0.001).
Figure 3.
Mice were pretreated with subcutaneous injections of either vehicle or 0.3 mg/kg WAY100635, then treated with a second subcutaneous injection of vehicle or 5-MeODMT and assayed for latency to begin feeding in the novel environment. WAY100635 reversed the inhibition to begin feeding seen in the presence of 5-MeODMT alone. (**p < 0.001 vs. vehicle/ vehicle, ++p < 0.001 vs. vehicle/ 5-MeODMT, Tukey’s post test).
Discussion
While hyponeophagia studies, especially with pharmacological interventions, have been performed largely in rats over the years, we wanted to establish such an assay in mice given the availability of genetic interventions (Dulawa and Hen, 2005; Jennings et al., 2006). We modified previous methods to limit sensorimotor confounds as well as to represent the components relevant for the translational study of AN: anxiety-induced inhibition to feed while hungry and manipulation of the serotonergic pathway. As a role for 5-MeODMT in hyponeophagia has been described previously only in rats, we confirmed this role in mice under our experimental conditions. We next tested the hypothesis that this effect of 5-MeODMT in hyponeophagia was a drug-by-environment interaction. Our data supported this hypothesis as well (Figure 2). By doing so, we demonstrated a role for the serotonergic system in potentiating feeding inhibition in a novel, anxiogenic environment. In sum, we find that our hyponeophagia assay combines the attributes we seek to model in a translational approach to AN, i.e. anxiety, feeding inhibition in a hungry state, and regulation of feeding behaviors by changes in serotonergic tone.
Using this model, we are able to test a major prediction of our AN model: when the non-selective 5-HT agonist 5-MeODMT potentiates feeding inhibition in an anxiogenic environment, is it doing so via activation of 5-HT1A receptors? First, our experiments using the 5-HT1A receptor antagonist WAY100635 (Figure 3) support our hypothesis that 5-HT1A receptor activation is necessary for the 5-MeODMT-dependent potentiation of feeding inhibition in an anxiogenic environment. Second, our data suggest that 5-HT1A receptor activation is not necessary for the inhibition of feeding induced by the anxiogenic environment alone. Consistent with previous studies (Rochford et al., 1997), as a validation of both the anxiogenic quality of the novel environment and our assay conditions, the benzodiazepine diazepam is able to inhibit the underlying neurotransmitter pathway(s), presumably by activating γ-amino butyric acid (GABA) receptors. We next consider whether the 5-MeODMT-dependent potentiation of feeding inhibition is related to anxiety. This potentiation of feeding inhibition is likely anxiety-related since the 5-MeODMT effect shows a drug-by-environment interaction with the anxiogenic environment. Furthermore, based on our methods designed to minimize sensorimotor influences and the lack of an effect of 5-MeODMT in the home cage, it is unlikely that 5-MeODMT causes its effect via generalized sensorimotor distortions.
An intriguing implication of our studies with WAY100635 arises when we consider previous studies that have shown that activating 5-HT1A receptors via agonists, such as buspirone, reverse the feeding inhibition derived from the novel anxiogenic environment (Merali et al., 2003). Studies using a variant of hyponeophagia, called novelty suppressed feeding, in knockout mice lacking the 5-HT1A receptor, show that expression in the forebrain but not in the raphe nuclei during early postnatal development, is necessary for normal anxiety behavior in the adult (Gross et al., 2002). This study defines a temporal and spatial parameter for the role of 5-HT1A receptors in anxiety and confirms prior data (Ramboz et al., 1998) that a loss of 5-HT1A receptor function specifically during development leads to an increase in anxiety behaviors. In the studies using novelty suppressed feeding (Gross et al., 2002), loss of 5-HT1A receptors only during postnatal development led to potentiated feeding inhibition and re-expression of 5-HT1A receptors during this period but not adulthood, led to reversal of this potentiation. In our hyponeophagia assay, antagonizing 5-HT1A receptors in the presence of a nonselective 5-HT agonist causes reversal of the 5-MeODMT-derived feeding inhibition but not of the novel environment-derived component. As discussed above, our results support the AN model where increased 5HT1A/5-HT2A receptor activation promotes the ill anorexic state, whereas knocking out 5HT1A receptors in an assay of novelty suppressed feeding also inhibits feeding, instead of reducing feeding inhibition. This difference in outcome is likely due to the transience of the pharmacological blockade of the 5HT1A receptor in healthy mice (our study) versus the developmental effects of 5-HT1A gene deletion on novelty suppressed feeding. Another genetic knockout study using the novelty suppressed feeding assay that also contradicts our AN model (Weisstaub et al., 2006), finds that knocking out 5HT2A receptors diminishes feeding inhibition compared to that seen in wild-type mice and that 5HT2A receptor expression in the frontal cortex is necessary to reverse this effect. In the studies that disrupt 5HT2A receptor activity, methodological differences with their corresponding time course of actions, i.e. acute pharmacological (Griebel et al., 1997) versus genetic or antisense approaches (Cohen, 2005), do lead to distinct outcomes. Therefore, to accurately reflect the biological actions of an elevated 5HT1A/5-HT2A receptor profile, future iterations of our AN model will need to factor in the effect of downstream signaling from these receptors and the neuroplasticity that likely follows.
Several caveats apply to translational approaches, including those described above, whereby experiments in rodents are used to test our model of AN derived from data that were generated in human studies. As the neurobiology of AN is largely unknown, our approach cannot have strict construct and etiological validity at this time (Geyer and Markou, 2002). However, we argue that assaying putative anxiety-induced inhibition of feeding in mice while hungry, provides a compelling approach to test our model of serotonergic modulation of feeding and emotional behavior. Several of the commonly used paradigms of anxiety, such as elevated plus maze, open-field activity and social interaction test (Belzung and Griebel, 2001), also involve the use of psychogenic stressors although they lack a measure of feeding behavior. Hyponeophagia provides an ethologically relevant paradigm to assess inhibition of approach to food, a non-defensive behavior, when mice are placed in an unfamiliar environment (Merali et al., 2003). Ethologically relevant paradigms characteristically have substantial face validity, as in the case of mouse defensive behaviors, which have been proposed as models of defense-related psychopathology in humans (Blanchard and Blanchard, 2001). Similarly, hyponeophagia provides a useful model for AN, whereby the majority of a large, well characterized patient sample met diagnostic criteria for anxiety disorders (Kaye et al., 2004). The fact that the anxiety diagnoses had onset in childhood and preceded the onset of AN, underscores the need to investigate the mechanisms of anxiety symptoms in individuals ill with AN to provide mechanistic insight for treatment.
Another aspect of the psychogenic-stressor based tests mentioned above is that they measure “normal” or “state” anxiety by introducing the rodent to a predator or novel situations and typically assaying spatiotemporal parameters. A problem with tests of “state” anxiety has been that they do not discriminate between anxiolysis from various classes of anxiolytics, such as benzodiazepines, 5HT1A agonists, and 5-HT re-uptake inhibitors. On the other hand, models of “pathological” or “trait” anxiety do respond specifically to a particular mechanism of anxiolysis and begin with a higher baseline of anxiety-like behaviors without the need for anxiogenic stimuli. However, genetic models of “trait” anxiety in mice missing a gene for a given neuropeptide, enzyme, or receptor most accurately model the behavioral manifestations of a single gene deletion (Belzung and Griebel, 2001), rather than modeling the genetic complexity of anxiety (Hettema et al., 2001). One solution that mitigates the limitations of both “state” and “trait” models is to establish a baseline with a combination of pharmacological and novel stimuli, as described above, where hyponeophagia was assessed upon treatment with 5-MeODMT in a novel environment. Such an approach may facilitate the delineation of anxiety responsive to serotonergic agonists/ antagonists versus that responsive to benzodiazepines.
To predict what drugs might prove effective in AN, it may be useful to model the feeding inhibition data in terms of the neurochemical responses of anxiety assayed in hyponeophagia. Anxiety induced by the novel environment alone in hyponeophagia responds to anxiolysis by benzodiazepines, as does anxiety in other psychogenic paradigms mentioned above (Merali et al., 2003). While symptoms of anxiety disorders do respond to benzodiazepines (Davidson, 2009), to our knowledge there are no published reports supporting clinical efficacy in treating anxiety symptoms in individuals ill with AN. Anxiety induced by 5-MeODMT in a novel environment is responsive to anxiolysis by 5-HT1A antagonism, and serotonergic manipulations that are anxiogenic in healthy human subjects, such as acute tryptophan depletion from the diet and treatment with meta-chlorophenylpiperazine (mCPP), paradoxically show clinical efficacy for anxiolysis in AN (Frank et al., 2001; Kaye et al., 2003). A “two pathway” anxiety circuit that inhibits feeding may be used to describe these findings: “pathway one” is engaged by the novel environment in hyponeophagia and is responsive to anxiolytic drugs such as benzodiazepines and models anxiety disorders; “pathway two” is engaged by 5-MeODMT in hyponeophagia and is responsive to paradoxical serotonergic manipulations, but not to anxiolytics such as benzodiazepines, and models the anxiety in AN. Further translational work is necessary to understand the neurochemical basis of anxiety in AN, so that novel pharmacotherapeutic strategies can be developed for this fatal illness.
Acknowledgments
These studies were supported by grants from the National Institute on Drug Abuse (DA02925) and the National Institute of Mental Health (MH074697) and by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. The authors thank Dr. Stephanie Dulawa for her advice in establishing hyponeophagia and Dr. Adam Halberstadt for his helpful discussions during the design of these studies.
References
- Ago Y, Koyama Y, Baba A, Matsuda T. Regulation by 5-HT1A receptors of the in vivo release of 5-HT and DA in mouse frontal cortex. Neuropharmacology. 2003;45:1050–1056. doi: 10.1016/s0028-3908(03)00304-6. [DOI] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MJS, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition - Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bailer U, Frank G, Price J, Meltzer CC, Mathis C, Henry S, McConaha C, Wagner A. Altered 5-HT1A receptor binding after recovery from anorexia nervosa. International Journal of Neuropsychopharmacology. 2004;7 Suppl 1:S474. [Google Scholar]
- Bailer UF, Frank G, Henry S, Price J, Meltzer C, Mathis C, Wagner A, Thornton L, Hoge J, Ziolko SK, Becker C, McConaha C, Kaye WH. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol. Psychiatry. 2007;61:1090–1099. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, Mathis CA, Drevets WC, Wagner A, Hoge J, Ziolko SK, McConana CW, Kaye WH. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]WAY100635. Arch Gen Psychiatry. 2005;62:1032. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Blanchard DGG, Blanchard R. Mouse defensive behaviors: Pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi R, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharm. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Cohen H. Anxiolytic effect and memory improvement in rats by antisense oligodeoxynucleotide to 5-hydroxytryptamine-2A precursor protein. Depress Anxiety. 2005;22:84–93. doi: 10.1002/da.20087. [DOI] [PubMed] [Google Scholar]
- Davidson J. First-line pharmacotherapy approaches for generalized anxiety disorder. J Clin Psychiatry. 2009;70:25–31. doi: 10.4088/jcp.s.7002.05. [DOI] [PubMed] [Google Scholar]
- Dulawa C, Hen R. Recent advances in animal models of chronic antidepressant effects: the noventy-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66:65. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Frank G, Kaye WH. Positron emission tomography studies in eating disorders: multireceptor brain imaging, correlates with behavior and implications for pharmacotherapy. Nuc Med & Biol. 2005;32:755–761. doi: 10.1016/j.nucmedbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Frank G, Meltzer CC, Price J, Drevets WC, Greer P, Mathis C, Kaye WH. Society of Biological Psychiatry. Philadelphia, PA: Biological Psychiatry; 2002. Alterations of 5-HT1A and 2A receptors persist after recovery from anorexia and bulimia nervosa; p. 11S. [Google Scholar]
- Frank GK, Kaye WH, Weltzin TE, Perel J, Moss H, McConaha C, Pollice C. Altered response to meta-chlorophenylpiperazine in anorexia nervosa: support for a persistent alteration of serotonin activity after short-term weight restoration. Int J Eat Disord. 2001;30:57–68. doi: 10.1002/eat.1054. [DOI] [PubMed] [Google Scholar]
- Geyer M, Markou A, editors. The role of preclinical models in the development of psycotropic drugs. Philadelphia: Lippincott, Williams & Wilkins; 2002. pp. 445–455. [Google Scholar]
- Griebel G, Perrault G, Sanger D. A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropsychopharm. 1997;36:793–802. doi: 10.1016/s0028-3908(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hettema J, Neale M, Kendler K. A review and metaanalysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Jennings K, Loder M, Sheward W, Pei Q, Deacon R, Benson M, Olverman J, Hastie N, Harmar A, Shen S, Sharp T. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neuroscience. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W, Bulik C, Thornton L, Barbarich N, Masters K, Fichter M, Halmi K, Kaplan A, Strober M, Woodside DB, Bergen A, Crow S, Mitchell J, Rotondo A, Mauri M, Cassano G, Keel PK, Plotnicov K, Pollice C, Klump K, Lilenfeld LR, Devlin B, Quadflieg R, Berrettini WH. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, McConaha CW, Kishore A. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord. 2003;33:257–267. doi: 10.1002/eat.10135. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- Lang P, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psych. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, Rizzo G, Panzacchi A, De Peri L, Ivernizzi G, Fazio F. In vivo serotonin 5HT2A receptor binding and personality traits in healthy subjects: A positron emission tomography study. NeuroImage. 2002;17:1470–1478. doi: 10.1006/nimg.2002.1239. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford J, Beaulieu S, Rousse I, Glowa J, Barden N. Behavioral reactivity to aversive stimuli in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: effects of diazepam and FG-7142. Psychopharm (Berl) 1997;132:145–152. doi: 10.1007/s002130050330. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotoinin1A and serotonin2A receptor in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004:1. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Shephard R, Broadhurst P. Hyponeophagia and arousal in rats: effects of diazepam, 5-methoxy=N, N-dimethyltryptamine, d-amphetamine and food deprivation. Psychopharm (Berl) 1982;78:368–372. doi: 10.1007/BF00433744. [DOI] [PubMed] [Google Scholar]
- Shephard R, Broadhurst PBPL. Hyponeophagia in the Roman rat strains: effects of 5-methoxy-N, N-dimethyltryptamine, diazepam, methysergide and the steroisomers of propranolol. Eur J Pharmacol. 1983;95:177–184. doi: 10.1016/0014-2999(83)90632-5. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT1A receptor binding and anxiety: a [11C]WAY-100635 PET investigation in healthy volunteers. American Journal of Psychiatry. 2001;158:1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharm (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub N, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung J, Sibille E, Underwood M, Itohara S, Dauer W, Ansorge M, Morelli E, Mann J, Toth M, Aghajanian G, Sealfon S, Hen R, Gingrich J. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley J, Theobald D, Glennon J, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharm (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]