Abstract
Background
Racial disparities in cancer outcomes have been observed in several malignancies. However, it is unclear if survival differences persist after adjusting for clinical, demographic, and treatment variables. Our objective was to determine whether racial disparities in survival exist among patients enrolled in consecutive trials conducted by the Southwest Oncology Group (SWOG).
Methods
We identified 19 457 adult cancer patients (6676 with breast, 2699 with lung, 1244 with colon, 1429 with ovarian, and 1843 with prostate cancers; 1291 with lymphoma; 2067 with leukemia; and 2208 with multiple myeloma) who were treated on 35 SWOG randomized phase III clinical trials from October 1, 1974, through November 29, 2001. Patients were grouped according to studies of diseases with similar histology and stage. Cox regression was used to evaluate the association between race and overall survival within each disease site grouping, controlling for available prognostic factors plus education and income, which are surrogates for socioeconomic status. Median and ten-year overall survival estimates were derived by the Kaplan–Meier method. All statistical tests were two-sided.
Results
Of 19 457 patients registered, 2308 (11.9%, range = 3.9%–21.6%) were African American. After adjustment for prognostic factors, African American race was associated with increased mortality in patients with early-stage premenopausal breast cancer (hazard ratio [HR] for death = 1.41, 95% confidence interval [CI] = 1.10 to 1.82; P = .007), early-stage postmenopausal breast cancer (HR for death = 1.49, 95% CI = 1.28 to 1.73; P < .001), advanced-stage ovarian cancer (HR for death = 1.61, 95% CI = 1.18 to 2.18; P = .002), and advanced-stage prostate cancer (HR for death = 1.21, 95% CI = 1.08 to 1.37; P = .001). No statistically significant association between race and survival for lung cancer, colon cancer, lymphoma, leukemia, or myeloma was observed. Additional adjustments for socioeconomic status did not substantially change these observations. Ten-year (and median) overall survival rates for African American vs all other patients were 68% (not reached) vs 77% (not reached), respectively, for early-stage, premenopausal breast cancer; 52% (10.2 years) vs 62% (13.5 years) for early-stage, postmenopausal breast cancer; 13% (1.3 years) vs 17% (2.3 years) for advanced ovarian cancer; and 6% (2.2 years) vs 9% (2.7 years) for advanced prostate cancer.
Conclusions
African American patients with sex-specific cancers had worse survival than white patients, despite enrollment on phase III SWOG trials with uniform stage, treatment, and follow-up.
CONTEXT AND CAVEATS
Prior knowledge
Racial disparities have been observed in outcomes for several malignancies, but it is unclear whether these differences remain after adjusting for clinical, demographic, and treatment variables.
Study design
Study subjects were adult cancer patients with lymphoma, leukemia, or multiple myeloma or with breast, lung, colon, ovarian, or prostate cancers who were treated on 35 Southwest Oncology Group randomized phase III clinical trials and who received uniform treatment and follow-up. Patients were grouped according to diseases with similar histology and stage. Associations between race and overall survival within each disease site grouping were studied, controlling for prognostic, treatment, and socioeconomic factors.
Contribution
After adjustment for prognostic factors, African American race was associated with increased mortality from early-stage premenopausal breast cancer, early-stage postmenopausal breast cancer, advanced-stage ovarian cancer, and advanced-stage prostate cancer but not from lung cancer, colon cancer, lymphoma, leukemia, or myeloma. Associations did not change after additional adjustment for socioeconomic status.
Implications
Unrecognized interactions of tumor biological, hormonal, and/or inherited host factors may be contributing to differential survival outcomes by race in sex-specific malignancies.
Limitations
Cancers of non–African American patients may have been more likely detected through screening than those of African American patients for breast cancer and prostate cancer. Some racial disparities in overall survival may be related to disparities in noncancer health and comorbid disease. Racial disparities in adherence to hormonal therapy prescribed in trials may have occurred.
From the Editors
Measurable declines in overall cancer death rates for many major cancers along with improved survival rates have been reported recently (1). However, racial and ethnic disparities in cancer-specific death rates for most cancers indicate that not all segments of the US population have benefited equally from such advances (1,2). The disparity in population-based survival statistics is greatest for patients with cancers of the breast, prostate, colon, ovary, lung, and cervix. However, racial disparities in cancer outcome can be attributed to many factors, including access to quality care resulting in more advanced stage at presentation (3–5), differences in tumor biology resulting in increased aggressiveness or resistance to treatment (6), differences in the quality of care after diagnosis (7,8), and socioeconomic factors influencing treatment options (9,10). There is controversy whether race affects outcome independently of these factors.
The randomized clinical trial setting of cancer cooperative groups presents several advantages in terms of assessing survival outcomes in cancer patients. First, enrollment requirements are standardized to ensure uniform assessment of disease stage, histology, tumor characteristics, and known prognostic factors. Identical treatment and supportive measures during therapy are provided for all participants, thereby controlling for factors that may influence care after diagnosis. Follow-up is specified in the treatment protocol and measured prospectively, so that disease-specific and overall survival can be assessed reliably.
African Americans represent approximately 10% of patients enrolled on cancer treatment clinical trials in the United States (11). However, even in large, individual phase III clinical trials, the small absolute number of African American participants may limit assessment of race-by-outcome interactions. Merging data from several similar trials increases the power to evaluate the African American subgroup for the common cancer types. Our objective was to analyze the prospective phase III clinical trial database of the Southwest Oncology Group (SWOG) to determine whether African American and white patients differ in survival outcomes after controlling for clinical, demographic, and treatment variables.
Patients and Methods
Patients and SWOG Trials
A total of 19 457 eligible adult patients who enrolled on 35 consecutive SWOG phase III trials from October 1, 1974, through November 29, 2001, were analyzed. These studies addressed the following common malignancies, in which patients were grouped according to histology and stage, including 2360 patients with early-stage (adjuvant) premenopausal breast cancer, 4316 with early-stage (adjuvant) postmenopausal breast cancer, 1097 with limited-stage small cell lung cancer, 1602 with advanced-stage non–small cell lung cancer, 1244 with early-stage (adjuvant) colon cancer, 1429 with advanced-stage ovarian cancer, 1843 with advanced-stage prostate cancer, 2208 with multiple myeloma, 1291 with advanced-stage non-Hodgkin lymphoma, and 2067 with acute myelogenous leukemia. The maximum observation time was at least 15 years for all diseases, with all trials reaching their mature follow-up and with their main objectives presented and published (for trial summaries, see Table 1).
Table 1.
Diseases and studies analyzed in the Southwest Oncology Group Database*
| Type of cancer | Trials by study number | Study activation date range | Major eligibility criterion | Clinical adjustment variables | Sample size, No. of patients (No. AA; % AA) |
| Adjuvant premenopausal breast | S7436, S7827, S8313, S8897 | 1975–1993 | No previous hormonal or chemotherapy | Age, No. of positive lymph nodes (≥4 vs <4), tumor size (>5 vs ≤5 cm) | 2360 (249; 10.6) |
| Adjuvant postmenopausal breast | S7436, S7827, S8313, S8814, S8897 | 1975–1993 | No previous hormonal or chemotherapy | Age, No. of positive lymph nodes (≥4 vs <4), tumor size (>5 vs ≤5 cm) | 4316 (413; 9.6) |
| AML | S7823, S8124, S8600, S9031 | 1978–1994 | No previous chemotherapy | Age, sex, performance status (≥2 vs 0–1), WBC, cytogenetics (unfavorable vs favorable) | 2067 (224; 10.8) |
| Limited SCLC | S7924, S8232, S8812 | 1979–1992 | No previous RT or chemotherapy | Age, sex, performance status (≥2 vs 0–1), previous weight loss ≥10 lbs, pleural effusions | 1097 (87; 7.9) |
| Advanced NSCLC | S8241, S8738, S9308, S9509 | 1982–1998 | No previous treatment | Age, sex, performance status (≥2 vs 0–1), previous weight loss ≥5%, LDH (≥ULN vs <ULN) | 1602 (225; 14.0) |
| Multiple myeloma | S7418, S7704, S7927, S8229, S8624 | 1974–1990 | No previous chemotherapy | Age, sex, stage (I–II vs IIIA vs IIIB), serum β-2 (>6 vs ≤6) | 2208 (478; 21.6) |
| Adjuvant colon | S8591 | 1985–1987 | Resectable | Age, sex, Dukes stage (C vs B) | 1244 (70; 5.6) |
| Advanced NHL | S7713, S8516 | 1977–1991 | Int. or high grade with no previous chemotherapy | Sex, IPI risk (high vs low) | 1291 (113; 8.8) |
| Advanced ovarian | S7524, S7925, S8412, S8501, S9701 | 1976–2001 | Stage III or IV | Age, performance status (≥2 vs 0–1), stage (optimal III vs suboptimal III vs IV), histology (M/CC vs other) | 1429 (56; 3.9) |
| Advanced prostate | S8494, S8894 | 1985–1994 | Stage D2 | Age, performance status (≥2 vs 0–1), extent of disease (extensive vs minimal), bone pain, Gleason sum (8–10 vs 2–7), PSA | 1843 (393; 21.3) |
| Total | 35 studies included | 19 457 (2308; 11.9) |
Analyses were stratified by study in all cancers, except for breast, which was also stratified by hormone receptor status, and adjuvant colon in which only one study was included. AA = African American; AML = acute myelogenous leukemia; SCLC = small cell lung cancer; RT = radiation therapy; NSCLC = non–small cell lung cancer; NHL = non-Hodgkin lymphoma; IPI = International Prognostic Index; PSA = prostate-specific antigen; Int. = intermediate; LDH = lactate dehydrogenase; ULN = upper limit of normal; M/CC = mucinous/clear cell; WBC = white blood cell.
Statistical Analysis
The primary outcome of interest was overall survival, measured from the time of registration until date of last contact (censored) or death due to any cause. Ten-year and median survival estimates by race were calculated by the Kaplan–Meier method (12). Covariate adjustment was performed via Cox regression modeling and included the major disease-specific prognostic factors (for the list of these factors and their categories, see Table 2). A visual inspection of the plots by disease group of log[−log(survival)] vs log(survival time) found no evidence of substantial deviation from proportional hazards in all analyses (data not shown). Detailed cause-of-death data were not available. However, estimated cause-specific survival analyses were performed by designating deaths that occurred after documented cancer progression as events and deaths without previous progression as censored observations.
Table 2.
Associations between prognostic factors other than race by disease type and survival*
| Disease | Factor (categories) | HR (95% CI) | P† |
| Premenopausal breast cancer | Age (10-y increase) | 0.89 (0.78 to 1.01) | .06 |
| No. of positive lymph nodes (≥4 vs <4) | 2.36 (1.92 to 2.91) | <.001 | |
| Tumor size (≥5 cm vs <5 cm) | 1.58 (1.24 to 2.03) | <.001 | |
| Postmenopausal breast cancer | Age (10-y increase) | 1.21 (1.14 to 1.29) | <.001 |
| No. of positive lymph nodes (≥4 vs <4) | 2.03 (1.83 to 2.26) | <.001 | |
| Tumor size (≥5 cm vs <5 cm) | 1.34 (1.14 to 1.57) | <.001 | |
| Acute myelogenous leukemia | Age (10-y increase) | 1.25 (1.21 to 1.29) | <.001 |
| Sex (female vs male) | 1.13 (1.03 to 1.24) | .01 | |
| Performance status (≥2 vs 0–1) | 1.33 (1.18 to 1.50) | <.001 | |
| WBC count (10-unit increase) | 1.02 (1.01 to 1.03) | <.001 | |
| Cytogenetics (unfavorable vs favorable) | 2.07 (1.49 to 2.86) | <.001 | |
| Small cell lung cancer | Age (10-y increase) | 1.16 (1.07 to 1.25) | <.001 |
| Sex (female vs male) | 1.30 (1.16 to 1.45) | <.001 | |
| Performance status (≥2 vs 0–1) | 1.18 (0.91 to 1.51) | .20 | |
| Previous weight loss (≥10 lb vs <10 lb) | 1.17 (0.95 to 1.44) | .12 | |
| Pleural effusions (yes vs no) | 1.61 (1.23 to 2.11) | <.001 | |
| Non–small cell lung cancer | Age (10-y increase) | 0.97 (0.92 to 1.03) | .32 |
| Sex (female vs male) | 0.90 (0.81 to 1.01) | .08 | |
| Performance status (≥2 vs 0–1) | 1.89 (1.60 to 2.23) | <.001 | |
| Previous weight loss (≥5% vs <5%) | 1.25 (1.13 to 1.39) | <.001 | |
| LDH (>ULN vs ≤ULN) | 1.56 (1.40 to 1.73) | <.001 | |
| Stage IV (IV vs IIIB) | 1.23 (0.96 to 1.59) | .10 | |
| No. of metastatic sites (>1 vs 1) | 1.34 (1.21 to 1.49) | <.001 | |
| Multiple myeloma | Age (10-y increase) | 1.24 (1.19 to 1.30) | <.001 |
| Sex (female vs male) | 1.09 (1.00 to 1.20) | .05 | |
| Stage IIIB (IIIB vs I–II) | 1.82 (1.60 to 2.07) | <.001 | |
| Stage IIIA (IIIA vs I–II) | 1.42 (1.27 to 1.58) | <.001 | |
| Serum β-2 (>6 vs ≤6) | 1.43 (1.24 to 1.64) | <.001 | |
| Adjuvant colon cancer | Age (10-y increase) | 1.13 (1.06 to 1.21) | <.001 |
| Sex (female vs male) | 1.04 (0.89 to 1.22) | .62 | |
| Dukes stage (C vs B) | 2.04 (1.67 to 2.50) | <.001 | |
| Non-Hodgkin lymphoma | Sex (female vs male) | 1.21 (1.06 to 1.38) | .005 |
| IPI risk group (high vs low) | 1.69 (1.48 to 1.93) | <.001 | |
| Ovarian cancer | Age (10-y increase) | 1.24 (1.17 to 1.32) | <.001 |
| Performance status (≥2 vs 0–1) | 1.12 (0.85 to 1.46) | .42 | |
| Stage IV (IV vs optimal III) | 1.61 (0.77 to 3.37) | .20 | |
| Stage III (suboptimal vs optimal) | 1.37 (0.66 to 2.83) | .40 | |
| Histology (M/CC vs other) | 1.38 (1.02 to 1.86) | .04 | |
| Prostate cancer | Age (10-y increase) | 1.04 (0.97 to 1.10) | .27 |
| Performance status (≥2 vs 0–1) | 1.56 (1.36 to 1.78) | <.001 | |
| Extent of disease (extensive vs minimal) | 1.60 (1.40 to 1.82) | <.001 | |
| Bone pain (yes vs no) | 1.51 (1.35 to 1.70) | <.001 | |
| Gleason sum (8–10 vs 2–7) | 1.46 (1.28 to 1.65) | <.001 | |
| PSA (10-unit increase) | 1.01 (0.96 to 1.05) | .78 |
Race was also included in all models. HR = hazard ratio; CI = confidence interval; WBC = white blood cell; IPI = International Prognostic Index; PSA = prostate-specific antigen; LDH = lactate dehydrogenase; ULN = upper limit of normal; M/CC = mucinous/clear cell.
All statistical tests were two-sided. Modeling was performed by Cox regression.
Each disease-specific Cox regression analysis was stratified by study to account for possible temporal differences that were not captured by histology and stage. For the breast cancer studies, receptor status was also included as a stratification variable. In each analysis, the prognostic factors analyzed included sex (where applicable) and age. For studies of advanced non-Hodgkin lymphoma, because age is a component of the International Prognostic Index (IPI), age was not included as a separate factor (for variables included in each of the multivariable models, see Table 1).
Estimates of socioeconomic status were derived from education category and income level as assessed by the linkage between patient zip code and the US census data (13). The US census data provide median income and education level within each zip code area. Patients from zip code areas in which the median household income was higher than the overall US median household income were coded as having high income; otherwise, patients were coded as having low income. Education category was based on the proportion of residents in a zip code area who completed high school and was handled in a similar fashion as the income level. These variables and the clinical prognostic factors were then entered into the Cox regression model. Routine collection of zip code data began in the late 1980s. Enrollments from earlier studies rarely had available zip code. To account for this discrepancy, indicator variables for missing socioeconomic status data were included in the Cox regression models to retain all patients.
Additional analyses were performed for cancer types in which there was a statistically significant association between race and survival. Patient body mass index, a surrogate for general health status or nutrition level, was entered into the Cox regression model in addition to the clinical prognostic factors and surrogates of socioeconomic status. Stratification by treatment type rather than by study was assessed for qualitative impact on the results. Breast cancer was also analyzed by race within hormone receptor status subgroups. To determine whether the effect of race on overall survival was different between high and low levels of income and education, the interaction of race with these two variables was analyzed separately. All statistical tests were two-sided.
Results
The sample size and the percentage of African Americans for each disease category are shown in Table 1. Of the 19 457 patients analyzed in the 35 SWOG trails, 2308 (11.9%, range = 3.9%–21.6%) were African American. Disease-specific sample sizes ranged from 1097 patients with limited-stage small cell lung cancer to 4316 patients with early-stage (adjuvant) postmenopausal breast cancer.
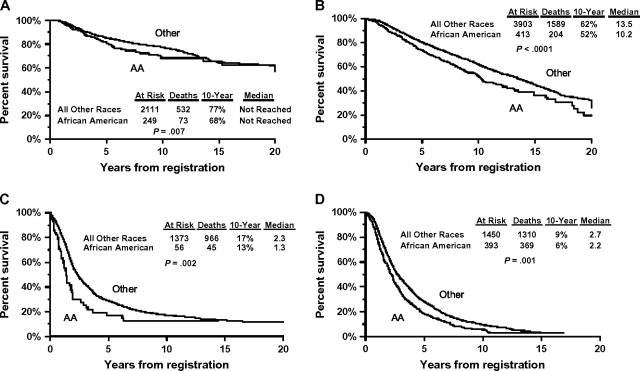
The potential baseline prognostic clinical characteristics included (with race) in the Cox regression models of disease site-specific overall survival are presented in Table 2. The prognostic factor categories, as well as the statistical significance, for each covariate are also shown by disease site. After adjustment for these prognostic factors, African American race was not statistically significantly associated with overall survival among patients with acute myelogenous leukemia (P = .12), limited-stage small cell lung cancer (P = .29), advanced-stage non–small cell lung cancer (P = .20), multiple myeloma (P = .34), early-stage (adjuvant) colon cancer (P = .87), or advanced-stage non-Hodgkin lymphoma (P = .10) (Table 3). However, African American race was statistically significantly associated with worse overall survival among patients with early-stage (adjuvant) premenopausal breast cancer (hazard ratio [HR] for death = 1.41, 95% confidence interval [CI] = 1.10 to 1.82; P = .007), early-stage (adjuvant) postmenopausal breast cancer (HR for death = 1.49, 95% CI = 1.28 to 1.73; P < .001), advanced-stage ovarian cancer (HR for death = 1.61, 95% CI = 1.18 to 2.18; P = .002), and advanced-stage prostate cancer (HR for death = 1.21, 95% CI = 1.08 to 1.37; P = .001). Representative Kaplan–Meier survival plots are shown in Figure 1. Ten-year (and median) Kaplan–Meier overall survival estimates for patients with one of these four types of cancer were uniformly poorer for African American than for all other patients. Specifically, overall survival for African American patients vs all other patients was, respectively, 68% (not reached) vs 77% (not reached) for early-stage premenopausal breast cancer, 52% (10.2 years) vs 62% (13.5 years) for early-stage postmenopausal breast cancer, 13% (1.3 years) vs 17% (2.3 years) for advanced ovarian cancer, and 6% (2.2 years) vs 9% (2.7 years) for advanced prostate cancer. In the multivariable setting, the effect of race on overall survival was little changed when each primary analysis was repeated with race coded as African American vs white only (data not shown). Similar results were obtained from estimated cause-specific survival analyses (Table 3), with increased risks of cancer-specific death from breast cancer, ovarian cancer, and prostate cancer associated with African American race. In this analysis, there was a borderline statistically significant association between African American race and increased risk of death from lymphoma.
Table 3.
Association between race and survival in various cancers*
| Adjusted for clinical prognostic factors† | Adjusted for clinical prognostic factors, income, and education | Estimated cause-specific survival‡ | |||||||
| Disease | HR (95% CI) | P§ | % With available SES data | % Income‖ (AA vs other) | % Education‖ (AA vs other) | HR (95% CI) | P§ | HR (95% CI) | P§ |
| Premenopausal breast cancer | 1.41 (1.10 to 1.82) | .007 | 72 | 45 vs 74 | 41 vs 73 | 1.43 (1.11 to 1.84) | .006 | 1.41 (1.09 to 1.84) | .01 |
| Postmenopausal breast cancer | 1.49 (1.28 to 1.73) | <.001 | 68 | 32 vs 67 | 26 vs 67 | 1.48 (1.27 to 1.72) | <.001 | 1.39 (1.17 to 1.66) | <.001 |
| AML | 1.12 (0.97 to 1.30) | .12 | 44 | 23 vs 60 | 27 vs 62 | 1.10 (0.95 to 1.28) | .20 | 1.05 (0.83 to 1.33) | .66 |
| Limited-stage SCLC | 1.13 (0.90 to 1.42) | .29 | 21 | 21 vs 62 | 7 vs 64 | 1.13 (0.90 to 1.42) | .28 | 1.11 (0.77 to 1.60) | .58 |
| Advanced NSCLC | 0.91 (0.79 to 1.05) | .20 | 63 | 33 vs 64 | 33 vs 65 | 0.88 (0.76 to 1.02) | .08 | 0.89 (0.75 to 1.05) | .17 |
| Multiple myeloma | 0.95 (0.85 to 1.06) | .34 | 29 | 23 vs 56 | 24 vs 61 | 0.94 (0.84 to 1.05) | .25 | 0.85 (0.70 to 1.03) | .10 |
| Early-stage colon cancer | 1.03 (0.73 to 1.46) | .87 | 31 | 44 vs 63 | 44 vs 63 | 0.98 (0.69 to 1.40) | .92 | 0.99 (0.67 to 1.45) | .94 |
| Advanced NHL | 1.20 (0.97 to 1.50) | .10 | 64 | 44 vs 60 | 33 vs 62 | 1.17 (0.94 to 1.45) | .17 | 1.31 (1.00 to 1.71) | .05 |
| Advanced ovarian carcinoma | 1.61 (1.18 to 2.18) | .002 | 73 | 29 vs 69 | 34 vs 69 | 1.65 (1.21 to 2.24) | .002 | 1.48 (1.03 to 2.11) | .03 |
| Advanced-stage prostate cancer | 1.21 (1.08 to 1.37) | .001 | 71 | 25 vs 67 | 21 vs 70 | 1.19 (1.05 to 1.35) | .008 | 1.19 (1.05 to 1.35) | .008 |
HR = hazard ratio; CI = confidence interval; SES = socioeconomic status; AA = African American; AML = acute myelogenous leukemia; NHL = non-Hodgkin lymphoma; SCLC = small cell lung cancer; NSCLC = non–small cell lung cancer.
Clinical prognostic factors are listed in Table 1.
Data were estimated on the basis of deaths after documented progression and adjusted for clinical prognostic factors.
Wald χ2 statistic. All statistical tests were two-sided.
Data are the percentage from high-income areas or from high-education areas.
Figure 1.
Kaplan–Meier overall survival plots for African American patients vs patients of all other races or ethnic groups. A) Premenopausal adjuvant breast cancer. B) Postmenopausal adjuvant breast cancer. C) Advanced ovarian cancer. D) Advanced prostate cancer. The number of patients at risk, the number of deaths, the 10-year survival estimate, and median survival estimate are presented. The Wald χ2 test statistic for the effect of race on survival in the multivariable setting was also included. All statistical tests were two-sided. The 10-year overall survival estimates and the corresponding 95% confidence intervals (CIs) by race (all other patients vs African American patients, respectively) for each disease group are as follows: 77% (95% CI = 76% to 79%) vs 68% (95% CI = 62% to 75%) for premenopausal adjuvant breast cancer, 62% (95% CI = 60% to 64%) vs 52% (95% CI = 47% to 58%) for postmenopausal adjuvant breast cancer, 17% (95% CI = 14% to 19%) vs 13% (95% CI = 3% to 22%) for advanced ovarian cancer, and 9% (95% CI = 8% to 11%) vs 6% (95% CI = 3% to 8%) for advanced prostate cancer. The median overall survival estimates and the corresponding 95% confidence intervals by race (all other patients vs African American patients, respectively) for each disease group are as follows: not reached for premenopausal adjuvant breast cancer, 13.5 years (95% CI = 12.9 to 14.2 years) vs 10.2 years (95% CI = 9.1 to 12.0 years) for postmenopausal adjuvant breast cancer, 2.3 years (95% CI = 2.1 to 2.5 years) vs 1.3 years (95% CI = 1.1 to 1.8 years) for advanced ovarian cancer, and 2.7 years (95% CI = 2.5 to 2.9 years) vs 2.2 years (95% CI = 2.0 to 2.4 years) for advanced prostate cancer. Follow-up survival was truncated at 20 years for consistent presentation between panels. AA = African American.
For every cancer type, fewer African American patients than all other patients were from high-income or high-education areas (Table 3). However, the addition of income and education factors to the clinical characteristics in the multivariable Cox regression analyses had no substantial impact on the associations of race with overall survival.
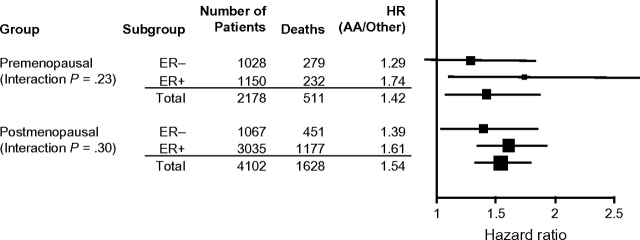
In breast cancer analyses that were stratified by hormone receptor status for both postmenopausal and premenopausal patients (Figure 2), African American patients had a higher mortality than other patients in both hormone receptor–negative and hormone receptor–positive breast cancer cohorts (for premenopausal estrogen receptor [ER]–negative breast cancer, HR = 1.29, 95% CI = 0.91 to 1.83; for premenopausal ER–positive breast cancer, HR = 1.74, 95% CI = 1.11 to 2.71; for postmenopausal ER–negative breast cancer, HR = 1.39, 95% CI = 1.04 to 1.85; and for postmenopausal ER–positive breast cancer, HR = 1.61, 95% CI = 1.35 to 1.93). This association among both premenopausal and postmenopausal patients was in fact stronger for those with hormone receptor–positive breast cancer than those with hormone receptor–negative breast cancer; however, no statistically significant interaction was observed between hormone receptor status and outcome by race among premenopausal patients (P = .23) or postmenopausal patients (P = .30).
Figure 2.
Forest plot of the associations between survival and race among premenopausal and postmenopausal breast cancer patients by estrogen receptor (ER) status. A vertical line representing no effect (HR = 1) is shown. The area of each square is proportional to the sample size for the subgroup, and the length of the whiskers indicate the 95% confidence intervals. P values for interaction are shown. The Wald χ2 test statistic for the effect of race on survival in the multivariate setting is also included. All statistical tests were two-sided. HR = hazard ratio; AA = African American.
Three additional types of analyses were performed for breast, prostate, and ovarian cancers because of the statistically significant association between race and survival outcome in the primary Cox model analyses. First, patient body mass index was added to the multivariable models that included the clinical prognostic factors plus income and education factors. The adverse impact of African American race on overall survival remained statistically significant for early-stage (adjuvant) premenopausal breast cancer (HR = 1.39, 95% CI = 1.08 to 1.80; P = .01), early-stage (adjuvant) postmenopausal breast cancer (HR = 1.46, 95% CI = 1.26 to 1.70; P < .001), advanced-stage ovarian cancer (HR = 1.62, 95% CI = 1.19 to 2.21; P = .002), and advanced-stage prostate cancer (HR = 1.18, 95% CI = 1.04 to 1.34; P = .01). Second, the analyses were stratified by type of treatment rather than by study (or, in the breast cancer analyses, rather than by study and receptor status). This change in stratification had little impact on the strength of the associations by race. Third, to assess whether there was a differential impact of race within level of income (high vs low) and category of education (high vs low), the interaction of race and each of these demographic factors was analyzed. There was no evidence of an interaction between race and income or race and education for early-stage (adjuvant) premenopausal breast cancer (P = .16 and P = .14, respectively), early-stage (adjuvant) postmenopausal breast cancer (P = .16 and P = .11, respectively), advanced-stage ovarian cancer (P = .90 and P = .77, respectively), or advanced-stage prostate cancer (P = .79 and P = .10, respectively). Finally, in the breast cancer analyses, covariate adjustment for tumor size with cut points at 2 and 5 cm (rather than at only 5 cm) resulted in only slight changes to the strength of the associations by race (data not shown).
Discussion
African American patients with breast, prostate, or ovarian cancer who were treated on phase III SWOG trials had statistically significantly worse overall survival than white patients. These results are important given all patients had uniform therapy and follow-up parameters, with adjustment for stage, socioeconomic factors, and known prognostic variables. Of note, these cancers are all sex specific. Racial differences in survival of this magnitude were not observed in patients with the non–sex-specific solid tumors (colon and lung cancer) or in patients with any hematologic malignancy examined.
Worse survival for African American women with breast cancer has been reported by the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry (14), the Department of Defense database (15), large single-institution studies (16), and literature-based meta-analyses (17). However, most of these sources had limited information on treatment, which was usually not standardized and often heterogeneous. Other analyses that evaluated outcomes in cooperative group analyses often did have complete treatment data but yielded conflicting results (18,19). These studies reported that African American and white patients enrolled in selected National Surgical Adjuvant Breast and Bowel Project or Cancer and Leukemia Group B cooperative group trials did not differ in breast cancer mortality after controlling for prognostic factors. However, the small number of African American patients within smaller study samples may have obscured a difference. We found that, after controlling for stage, demographics, socioeconomic variables, tumor characteristics, and treatment factors, this disparity existed among both premenopausal and postmenopausal women who were diagnosed with early-stage breast cancer.
This racial disparity in survival among patients with early-stage breast cancer occurred in patients with both endocrine-responsive and nonresponsive tumors. African American women with breast cancer, especially those who are premenopausal, have a higher incidence of biologically more aggressive cancers with a basal-like subtype or that were triple negative (ie, lacking receptors for estrogen, progesterone, and HER2-neu). Several authors have stated that the predominance of this subtype among African Americans most likely explains previously reported racial disparities in survival outcomes (6,20,21). To address this issue, we analyzed survival by race within hormone receptor status subgroups (Figure 2), although HER2-neu data were not collected in the older trials in our database. Given the statistically significant adverse outcome for African Americans within the hormone receptor–positive group (as well as the hormone receptor–negative group), and the known widening of the disparity in survival over time since the 1980s, the triple-negative biology theory cannot be the sole explanation for the difference in breast cancer outcomes by race. Our results suggest that there are other causes for the disparity when the treatment type and ER status are similar by race (along with the other factors in the breast cancer dataset).
We observed that African American men with advanced prostate cancer who were treated uniformly on phase III clinical trials had a higher mortality rate (HR for death = 1.19, 95% CI = 1.05 to 1.35; P = .008), after adjustment for all other available factors. Age-adjusted mortality rates reported by the SEER national registry consistently demonstrated that African American men have a death rate that is double that of white men (22). Two studies (23,24) reported that even after controlling for the effects of age, preoperative serum prostate-specific antigen level, pathological grade, and stage, the racial disparity in progression-free survival persists among men diagnosed with clinically localized prostate cancer. The disparity in outcome also occurs among men with androgen-independent metastatic prostate cancer. In a single-institution study (25), African American race was the only independent predictor of time to prostate-specific antigen progression, indicating that biological and genetic differences underlie this disparity. Some have speculated that the racial disparity in survival among men with local or regional prostate cancer was explained by socioeconomic factors. For example, Du et al. (10) reported that lower socioeconomic status appeared to be one of the major barriers to achieving comparable outcomes for men with prostate cancer. However, our analysis demonstrated that the disparity in prostate cancer survival persisted despite adjusting for income and education.
We observed an increase in mortality (HR = 1.65, 95% CI = 1.21 to 2.24; P = .002) among African American women with ovarian cancer in our population who were treated uniformly on phase III clinical trials after adjustment for all available factors. A previous study (26) reported that African American patients had more advanced disease and were less likely to undergo cancer-specific surgery than white women, but after adjusting for prognostic factors, African American women still had an increased risk of death from any cause (HR = 1.30, 95% CI = 1.20 to 1.40; P < .001). This disparity in survival was even observed in equal-access care systems, such as Kaiser Permanente (27). In our analysis, which is to our knowledge the only one designed to additionally adjust for treatment-related factors, the disparity persisted.
No deficit in survival was observed for African American patients with nonmetastatic colon cancer in our treatment-controlled dataset. In a previous report (28), African Americans were more likely to die of colon cancer than whites, probably because African Americans were more often diagnosed with advanced-stage disease. However, survival differences were also reported within each stage of disease. These findings may be explained by degree of delivery of adjuvant systemic therapy, in that African American patients are less likely than white patients to be treated with adjuvant chemotherapy (29,30). However, the National Surgical Adjuvant Breast and Bowel Project reported (5) a pooled database analysis in which African Americans experienced a statistically significantly greater risk of death, despite uniform use of adjuvant chemotherapy. It is possible that our smaller colon cancer analysis was underpowered to detect a survival difference or that, with modern treatments for colon cancer, disparities in outcome was reversed.
There are several limitations to our analysis. First, although use of clinical trial data implicitly controls for many treatment and access to care issues, it is possible that cancers of non–African American patients were more likely to be screen detected, especially in the adjuvant breast cancer setting, resulting in possible lead-time bias. Unfortunately, data on patients whose breast cancer was detected by screening and those whose breast cancer was detected clinically were not available. African Americans with pre- or postmenopausal breast cancer were less likely to be diagnosed with tumors smaller than 2 cm in diameter, but incorporation of three categories for tumor size (<2, 2–5, or >5 cm) had little impact on the strength of the survival differences by race. Furthermore, adjustment for the stronger prognostic factor of axillary lymph node status did not lessen the degree of breast cancer survival disparity. Consequently, lead-time bias cannot fully, or even in large part, explain our observations. Second, some of the overall survival differences observed between African American patients and patients of other ethnic or racial groups may be related to noncancer health and comorbid disease disparities, although why this effect would be more dominant in breast, ovarian, and prostate cancers than in other types of cancer is unclear. However, patients who participate in clinical trials may be more compliant and healthier in general than patients who are not eligible or refuse to participate in clinical trials (31). This so-called healthy subject effect might diminish the potential influence of noncancer disparities in survival. Nevertheless, to address this concern, we performed approximate cause-specific survival analyses (Table 3) (32). These analyses found little change in regression estimates by race, indicating that non–cancer-related deaths were similar between the racial groups and thus were not the explanation for the survival disparity. Cause-specific analyses rely on the assumption that the cancer outcome of interest is independent of other outcomes, so our analysis of cancer-specific survival must be viewed with caution, especially because the cause of death was estimated rather than explicitly identified.
Another limitation to our analysis was our inability to assess adherence to the oral adjuvant hormonal therapies prescribed in the protocols in detail. There may be differential rates of adherence to hormonal therapy, with African American women with breast cancer on average being less likely to complete a full 5-year course of treatment (33). This aspect might also explain the prostate cancer findings but most likely not the disparities in hormone receptor–negative breast cancer or in ovarian cancer. An additional limitation pertained to a concern that chemotherapy delivery and dosing may be substandard in African Americans. We conducted an in-depth analysis of two trials from the breast cancer database and found that, after adjustments for baseline white blood cell counts, body surface area, treatment quality, and treatment delivered (relative dose intensity), the disparity in survival outcome by race persisted (34). These factors were not independently associated with increased mortality. We were not, however, able to control for specific comorbid conditions that were not otherwise precluded by study eligibility criteria, are known to be more prevalent in African Americans, and are associated with reduced overall survival. Differential distribution of comorbid conditions may have also influenced the overall survival disparity that we observed, but as discussed above, there did not appear to be a difference in the rate of non–cancer-related deaths by race.
A provocative aspect of our analysis was that African American patients had statistically significantly worse survival in the sex-specific cancers (breast, ovarian, and prostate) but not in the other cancers examined, which include the major tumor types diagnosed in the United States. Although there are other non–sex-specific cancers that were not included in this analysis and that may also show disparities, one explanation for our findings might be that there are hormonal factors that contribute to the aggressiveness of some cancers that differ by race. Another possibility is that inherited genetic differences across races, especially in single-nucleotide polymorphisms, exist in the way resistance to therapy develops in these tumors and/or in how standard doses of drugs are activated and metabolized. Racial and ethnic variations in single-nucleotide polymorphisms for CYP2D6, UGT1A1, and SULT1A have been reported, which may account for differences in both risk and outcome (35–38). Female sex has been found to be independently associated with favorable survival in lung cancer (39,40). It is postulated that hormonal influences including estradiol levels and single-nucleotide polymorphisms that differ by sex, along with pharmacogenetic differences in drug metabolism, explains this observation (40). More research is clearly needed regarding interactions among treatment, sex, race, and survival.
We report the first comprehensive analysis, to our knowledge, from a large, unique cohort of patients who were treated on SWOG clinical trials that demonstrate racial disparities in survival persist for patients with breast, prostate, and ovarian cancer after controlling for prognostic, treatment, and socioeconomic factors. The randomized clinical trial setting used in this analysis ensured similarity in disease stage, eligibility requirements, and treatment plan and allowed adjustment for all other potential prognostic factors. Our findings suggest that unrecognized interactions of tumor biological, hormonal, and/or inherited host factors must be contributing to differential survival outcomes by race in sex-specific malignancies.
Funding
Public Health Service Cooperative Agreement grants awarded by the National Cancer Institute, Department of Health and Human Services: CA32102, CA38926, CA46282, CA22433.
Footnotes
None of the authors have conflicts of interest to disclose regarding this research. The principal investigator (K. S. Albain) and the study biostatistician (J. M. Unger) had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the data and manuscript draft and approved the final version for submission.
The funding agency for the clinical trials analyzed herein (National Institutes of Health) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the article.
The authors thank the many Southwest Oncology Group Disease Committee chairs and investigators who conducted the trials that were used to form the database analyzed herein and Tina Rutschman for her secretarial assistance.
References
- 1.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 3.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19–30. [PubMed] [Google Scholar]
- 4.Underwood SM. Reducing the burden of cancer borne by African Americans: if not now, when? Cancer Epidemiol Biomarkers Prev. 2003;12(3):270s–276s. [PubMed] [Google Scholar]
- 5.Dignam JJ, Colangelo L, Tian W, et al. Outcomes among African-Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst. 1999;91(22):1933–1940. doi: 10.1093/jnci/91.22.1933. [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97(16):1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XL, Meyer TE, Franzini L. Meta-analysis of racial disparities in survival in association with socioeconomic status among men and women with colon cancer. Cancer. 2007;109(11):2161–2170. doi: 10.1002/cncr.22664. [DOI] [PubMed] [Google Scholar]
- 10.Du XL, Fang S, Coker AL, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106(6):1276–1285. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 11.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):451–481. [Google Scholar]
- 13.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grann V, Troxel AB, Zojwalla N, Hershman D, Glied SA, Jacobson JS. Regional and racial disparities in breast cancer-specific mortality. Soc Sci Med. 2006;62(2):337–347. doi: 10.1016/j.socscimed.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U.S. Department of Defense Healthcare system. Cancer. 2003;98(5):894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 16.Woodward WA, Huang EH, McNeese MD, et al. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107(11):2662–2668. doi: 10.1002/cncr.22281. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 18.Dignam JJ. Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin. 2000;50(1):50–64. doi: 10.3322/canjclin.50.1.50. [DOI] [PubMed] [Google Scholar]
- 19.Roach M, III, Cirrincione C, Budman D, et al. Race and survival from breast cancer: based on Cancer and Leukemia Group B trial 8541. Cancer J Sci Am. 1997;3(2):107–112. [PubMed] [Google Scholar]
- 20.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 21.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 22.Stewart SL, King JB, Thompson TD, Friedman C, Wingo PA. Cancer mortality surveillance—United States, 1990–2000. MMWR Surveill Summ. 2004;53(3):1–108. [PubMed] [Google Scholar]
- 23.Powell IJ, Dey J, Dudley A, et al. Disease-free survival difference between African Americans and whites after radical prostatectomy for local prostate cancer: a multivariable analysis. Urology. 2002;59(6):907–912. doi: 10.1016/s0090-4295(02)01609-6. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JH, Schoenbach VJ, Kaufman JS, et al. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States) Cancer Causes Control. 2006;17(6):803–811. doi: 10.1007/s10552-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 25.Thatai LC, Banerjee M, Lai Z, Vaishampayan U. Racial disparity in clinical course and outcome of metastatic androgen-independent prostate cancer. Urology. 2004;64(4):738–743. doi: 10.1016/j.urology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Barnholtz-Sloan JS, Tainsky MA, Abrams J, et al. Ethnic differences in survival among women with ovarian carcinoma. Cancer. 2002;94(6):1886–1893. doi: 10.1002/cncr.10415. [DOI] [PubMed] [Google Scholar]
- 27.McGuire V, Herrinton L, Whittemore AS. Race, epithelial ovarian cancer survival, and membership in a large health maintenance organization. Epidemiology. 2002;13(2):231–234. doi: 10.1097/00001648-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clin North Am. 2005;89(4):771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Sundararajan V, Grann VR, Jacobson JS, Ahsan H, Neugut AI. Variations in the use of adjuvant chemotherapy for node-positive colon cancer in the elderly: a population-based study. Cancer J. 2001;7(3):213–218. [PubMed] [Google Scholar]
- 30.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294(21):2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 31.Antman K, Amato D, Wood W, et al. Selection bias in clinical trials. J Clin Oncol. 1985;3(8):1142–1147. doi: 10.1200/JCO.1985.3.8.1142. [DOI] [PubMed] [Google Scholar]
- 32.Unger J, Green S, Albain K. Outcome of African American women with breast cancer in cooperative group clinical trials. In: Williams CKO, Olipade OI, Falkson CI, editors. Breast Cancer in Women of African American Descent. Springer; 2006. pp. 267–295. [Google Scholar]
- 33.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 34.Hershman D, Unger J, Barlow W, et al. Treatment quality and outcomes of African American vs White breast cancer patients: retrospective analysis of Southwest Oncology Group studies S8814/S8897. J Clin Oncol. 2009;27(13):2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillemette C, De Vivo I, Hankinson SE, et al. Association of genetic polymorphisms in UGT1A1 with breast cancer and plasma hormone levels. Cancer Epidemiol Biomarkers Prev. 2001;10(6):711–714. [PubMed] [Google Scholar]
- 36.Guillemette C, Millikan RC, Newman B, Housman DE. Genetic polymorphisms in uridine diphospho-glucuronosyltransferase 1A1 and association with breast cancer among African Americans. Cancer Res. 2000;60:950–956. [PubMed] [Google Scholar]
- 37.Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91(3):249–258. doi: 10.1007/s10549-004-7751-x. [DOI] [PubMed] [Google Scholar]
- 38.Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3(2):229–243. doi: 10.1517/14622416.3.2.229. [DOI] [PubMed] [Google Scholar]
- 39.Fu JB, Kau TY, Severson RK, Kalemkerian GP. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest. 2005;127(3):768–777. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 40.Albain KS, Unger J, Gotay CC, et al. Toxicity and survival by sex in patients with advanced non-small cell lung carcinoma on modern Southwest Oncology Group (SWOG) trials. J Clin Oncol. 2007;25(18S):7549. [Google Scholar]