Abstract
Background
Both induction chemotherapy followed by irradiation and concurrent chemotherapy and radiotherapy have been reported as valuable alternatives to total laryngectomy in patients with advanced larynx or hypopharynx cancer. We report results of the randomized phase 3 trial 24954 from the European Organization for Research and Treatment of Cancer.
Methods
Patients with resectable advanced squamous cell carcinoma of the larynx (tumor stage T3–T4) or hypopharynx (T2–T4), with regional lymph nodes in the neck staged as N0–N2 and with no metastasis, were randomly assigned to treatment in the sequential (or control) or the alternating (or experimental) arm. In the sequential arm, patients with a 50% or more reduction in primary tumor size after two cycles of cisplatin and 5-fluorouracil received another two cycles, followed by radiotherapy (70 Gy total). In the alternating arm, a total of four cycles of cisplatin and 5-fluorouracil (in weeks 1, 4, 7, and 10) were alternated with radiotherapy with 20 Gy during the three 2-week intervals between chemotherapy cycles (60 Gy total). All nonresponders underwent salvage surgery and postoperative radiotherapy. The Kaplan–Meier method was used to obtain time-to-event data.
Results
The 450 patients were randomly assigned to treatment (224 to the sequential arm and 226 to the alternating arm). Median follow-up was 6.5 years. Survival with a functional larynx was similar in sequential and alternating arms (hazard ratio of death and/or event = 0.85, 95% confidence interval = 0.68 to 1.06), as were median overall survival (4.4 and 5.1 years, respectively) and median progression-free interval (3.0 and 3.1 years, respectively). Grade 3 or 4 mucositis occurred in 64 (32%) of the 200 patients in the sequential arm who received radiotherapy and in 47 (21%) of the 220 patients in the alternating arm. Late severe edema and/or fibrosis was observed in 32 (16%) patients in the sequential arm and in 25 (11%) in the alternating arm.
Conclusions
Larynx preservation, progression-free interval, and overall survival were similar in both arms, as were acute and late toxic effects.
Context and Caveats
Prior knowledge
For patients with advanced larynx or hypopharynx cancer, induction chemotherapy followed by irradiation and concurrent chemotherapy and radiotherapy appear to be clinically valuable alternatives to total laryngectomy, so that the larynx can be retained.
Study design
Phase 3 randomized trial of patients with resectable advanced larynx or hypopharynx cancer. Patients (n = 450) who responded to two cycles of cisplatin and 5-fluorouracil were randomly assigned to another two cycles, followed by radiotherapy (the sequential arm, n = 224), or to a total of four cycles of cisplatin and 5-fluorouracil with radiotherapy being given between cycles of chemotherapy (the alternating arm, n = 226).
Contribution
Larynx preservation, progression-free interval, and overall survival were similar in both arms, as were acute and late toxic effects.
Implications
The optimal approach for larynx preservation has not been identified, and so additional research is warranted.
Limitations
A restrictive definition for larynx preservation was used (ie, a larynx without tumor, tracheotomy, or use of a feeding tube). Data on smoking habits were not collected.
From the Editors
In the early 1980s, investigators at Wayne State University (1,2) published their experience with cisplatin and infusional 5-fluorouracil as induction chemotherapy for head and neck squamous cell carcinoma. They found that in previously untreated patients, induction chemotherapy with cisplatin and 5-fluorouracil was associated with response rates as high as 94% and that those responding to induction chemotherapy also proved to be good candidates for subsequent radiotherapy. These results were followed by the first-generation larynx preservation trials (3,4), which compared conventional treatment (total laryngectomy and postoperative radiotherapy) with an experimental approach consisting of induction chemotherapy with cisplatin and 5-fluorouracil, followed by radiotherapy among good responders (ie, those with a complete response or a partial response after induction chemotherapy) and by surgery and postoperative radiotherapy among poor responders (ie, those with either no change or progressive disease). Results of two large randomized trials comparing these two approaches have been reported: one for larynx cancer in the United States by the US Department of Veterans Affairs (3) and one for hypopharynx cancer in Europe by the European Organization for Research and Treatment of Cancer (EORTC) Head and Neck Cancer Cooperative Group, the EORTC 24891 trial (4). Results of these trials showed that the experimental approach consisting of induction chemotherapy with cisplatin and 5-fluorouracil followed by radiotherapy among good responders did not compromise either disease control or survival, compared with the control approach consisting of conventional treatment (total laryngectomy and postoperative radiotherapy), and that the larynx could be preserved in 40%–60% of the patients. In contrast, one smaller French randomized trial (5) for advanced larynx cancer (T3) found statistically significantly better survival for patients in the laryngectomy arm than for those in the no-surgery experimental arm. However, reliable data on the quality of laryngeal function were often missing (4,5), and radiotherapy alone for larynx preservation was not investigated in all three studies (3–5).
In the 1990s, when concurrent chemotherapy and radiotherapy appeared to be the most effective treatment for advanced head and neck cancer (6), two second-generation larynx preservation trials—the Radiation Therapy Oncology Group (RTOG) 91-11 trial (7) and the EORTC 24954 trial (the subject of this report)—were initiated to compare induction chemotherapy with concomitant chemotherapy and radiotherapy that was administered concurrently (7) or alternatively (this trial). The RTOG 91-11 trial is a three-arm randomized Intergroup trial (7), in which the first arm received induction chemotherapy with cisplatin and 5-fluorouracil, the second arm received concurrent chemotherapy with cisplatin and radiotherapy, and the third arm received radiotherapy alone. The EORTC 24954 trial, whose results we report in this article, is a collaboration between the EORTC Head and Neck Cancer Cooperative Group and the EORTC Radiation Oncology Group. In this randomized phase 3 trial, the control arm was the same as the experimental arm of EORTC protocol 24891, except that there were four cycles of chemotherapy instead of three cycles and patients in the experimental arm received an alternating schedule of four cycles of chemotherapy and three 2-week courses of radiotherapy, each of 20 Gy. Both the increased number of induction chemotherapy cycles in the control arm and the rapidly alternating split course of chemotherapy and radiotherapy in the experimental arm were selected on the basis of results from two earlier phase 3 randomized trials that were conducted in Italy (8,9). Both sequential or control and alternating or experimental arms in the EORTC 24954 trial have the same number of chemotherapy cycles (maximally four), but the alternating arm differed from the sequential arm in that a lower dose of 5-fluorouracil (200 mg/m2 per day instead of 1000 mg/m2 per day) was used in each chemotherapy cycle and radiotherapy was given at a lower total dose (60 Gy instead of 70 Gy) and for a longer time (8 weeks instead of 7 weeks). Overall results from the EORTC 24954 trial were reported at the 2007 annual meeting of the American Society of Clinical Oncology (10). We now present the detailed analysis of this trial.
Patients and Methods
Patient Eligibility
To be eligible for this study, patients had to have measurable or evaluable histologically proven advanced primary squamous cell carcinoma of the larynx (T2–T4 and N0–N2) or the hypopharynx (T2–T4 and N0–N2) that would have required a total laryngectomy under standard practice. In addition, patients had to be amenable to a conventional total laryngectomy (ie, those with squamous cell carcinoma of the larynx) or to a total laryngectomy with a partial pharyngectomy that would allow a primary closure without any kind of flap (ie, those with squamous cell carcinoma of the hypopharynx) to be eligible. Patients were not eligible for this study if they qualified for a partial laryngectomy or required a circumferential pharyngectomy and/or a bilateral internal jugular vein removal, and/or a tracheotomy before treatment.
Additional eligibility requirements included being aged 18–75 years, having no history of cancer or second primary tumor (except in situ carcinoma of the cervix or adequately treated basal or squamous cell carcinoma of the skin), being free of distant metastases, having a medical condition that allowed surgery under general anesthesia (ie, a World Health Organization [WHO] performance status of 0–2, the absence of poor nutritional status [ie, one that could not be restored to fair nutritional status within 3 weeks], or a serious nonmalignant systemic disease), and having adequate organ functions (ie, with a serum creatinine value of ≤1.5 mg/dL and/or a creatinine clearance value of ≥60 mL/min, a white blood cell count of ≥4 × 109 cells per liter, a platelet count of ≥100 × 109 platelets per liter, and transaminase and bilirubin values of less than twice the upper limit of normal range). Patients whose compliance to treatment and follow-up was compromised by a medical or psychological condition or by the geographical area in which they lived were also not eligible.
The ethics committees of all participating institutions approved the protocol and written patient information. All patients provided written informed consent.
Chemotherapy and Radiotherapy in the Sequential Arm
Induction chemotherapy consisted of cisplatin (100 mg/m2) given intravenously over a 1-hour period, followed by 5-fluorouracil (1000 mg/m2 per day) given as a 120-hour infusion over a 5-day period (for a total of 5000 mg/m2). Administration of supportive care with antiemetics and forced hydration followed standard practice at each institution.
Evaluation of response to treatment (either chemotherapy or irradiation) included computed tomography or magnetic resonance imaging measurements and endoscopy under general anesthesia. For the primary tumor, a complete response was defined as complete disappearance of all macroscopic disease, with a complete recovery of larynx mobility. For larynx cancers, a partial response was defined as a substantial regression of the tumor volume, with a complete disappearance of bulging valleculae, bulging hypothyroid membrane, deep invasion of the preepiglottic space, and at least a partial recovery of the larynx mobility. For hypopharynx cancers, a partial response was defined as a substantial regression of the tumor volume and at least a partial recovery of the larynx mobility.
The regression volume of palpable lymph node(s) was assessed separately by clinical examination and palpation and by serial imaging (computed tomography scan or magnetic resonance imaging). A complete lymph node response was defined as a complete disappearance of the enlarged lymph nodes. A partial response was defined as a 50% or more decrease of the sum of products of the largest and perpendicular diameters of the largest lymph node on imaging. Progressive disease was defined as a 25% or more increase in the size of the largest lymph node or the appearance of new enlarged lymph node(s). No change was defined as when neither a 50% decrease in lymph node size could be established nor a 25% increase in lymph node size could be demonstrated.
Response to chemotherapy in the sequential arm was evaluated 2 weeks after the second cycle (ie, on day 42 after the beginning of chemotherapy). Patients who did not achieve at least a partial response were scheduled to undergo immediate salvage surgery (ie, total laryngectomy) and postoperative radiotherapy. Patients with a partial or complete response were assigned to receive two additional cycles of chemotherapy with cisplatin and 5-fluorouracil, followed by radiotherapy. A subsequent endoscopic and radiological evaluation was performed 2 months after the end of radiotherapy to check for the absence of a residual tumor at the primary site or in the neck.
All patients in the sequential arm were to receive external radiotherapy beginning either postoperatively (4–6 weeks after surgery) or immediately after chemotherapy (ie, on day 80 after the initiation of treatment). The irradiated volumes for cancers of the larynx and hypopharynx included the primary site and both the left and the right sides of the neck. Patients received radiotherapy in a supine position, with megavoltage-level radiation by use of a conventional fractionation (one fraction of 2 Gy/d for 5 d/wk for 7 successive weeks). When definitive radiotherapy was administered after chemotherapy, a dose of 50 Gy was given to the whole irradiated volume, followed by a boost dose of 20 Gy that was restricted to the tumor site and to any palpable lymph node(s). When delivered postoperatively, a dose of 50 Gy was to be given on the entire remaining pharynx, both sides of the neck, and the tracheostoma, with a booster dose of 14 Gy if positive margins and/or extracapsular spread, and/or three or more positive lymph nodes were present. Thus, the total dose was 70 Gy for definitive radiotherapy and 50–64 Gy for postoperative radiotherapy. For all patients, the spinal cord had to be shielded after a dose of 45 Gy. Therefore, the superficial posterior neck lymph nodes were irradiated with direct low-energy electrons from a linear accelerator.
Chemotherapy and Radiotherapy in the Alternating Arm
Chemotherapy in the alternating arm was administered in weeks 1, 4, 7, and 10, and consisted of cisplatin administered intravenously at a dose of 20 mg/m2 per day on days 1–5 (for a total of 100 mg/m2) and 5-fluorouracil administered by bolus infusion at a dose of 200 mg/m2 per day on days 1–5 (for a total of 1000 mg/m2). Chemotherapy was alternated with three 2-week courses of radiotherapy (20 Gy per course) that were administered in weeks 2 and 3, weeks 5 and 6, and weeks 8 and 9.
In the alternating arm, cisplatin treatment was preceded by treatment with 20 mg of furosemide that was administered intravenously to guarantee adequate urinary flow and by antiemetic therapy that consisted of metoclopramide (1 mg/kg) or a 5-hydroxytryptamine-3 receptor antagonist (the choice of which antagonist to use was left to each institution and could include 8 mg of ondansetron, 1 mg of granisetron, or 5 mg of tropisetron) and/or 8 mg of dexamethasone. Immediately after the antiemetic therapy, 500 mL of normal saline (with potassium chloride at 6 milliequivalents [mEq]) was administered over a period of 30–40 minutes. Next, 500 mL of normal saline was administered over a period of 30–40 minutes, and at the same time, cisplatin (20 mg/m2) was administered in 100 mL of normal saline over 15 minutes. Posttreatment hydration was administered as 500 mL of normal saline containing 6 mEq of potassium chloride and 2 g of magnesium sulfate (over 30–40 minutes). This treatment was followed by another 500 mL of normal saline administered over 30–40 minutes and then by 5-fluorouracil (200 mg/m2) administered intravenously over 1–2 minutes. Thus, a total of 2 L of saline was administered with potassium chloride at 6 mEq/L and a total of 2 g of magnesium sulfate. This procedure was repeated every day for 5 days.
Because of a concern that the outcomes of surgical salvage in the alternating arm would be hampered if the dose of radiotherapy administered before surgery was too high, institutions were allowed to evaluate the intermediate response after a cumulative radiotherapy dose of approximately 50 Gy. Whether or not an institution used an intermediate evaluation had to be declared before random assignment of the first patient and had to be maintained by this institution throughout the trial. In the absence of a decrease in tumor size of more than 50%, chemotherapy and radiotherapy were to be halted, and patients were scheduled to undergo immediate salvage surgery (ie, total laryngectomy). It should be noted that all patients were to be evaluated for response to treatment 2 months after the end of treatment, whether or not their institutions had selected the intermediate evaluation.
Radiotherapy was delivered in the alternating arm by the same technique and schedule that were used in the sequential arm. The dose delivered to the whole irradiated volume, including the primary site and both left and right sides of the neck, was also 50 Gy, but the boost, which was delivered only to the tumor site and palpable lymph nodes, was limited to 10 Gy.
Surgery
In the sequential arm, patients who did not have a partial or complete response after the second cycle of induction chemotherapy with cisplatin and 5-fluorouracil had to undergo surgery as initially planned (ie, a total laryngectomy with or without a partial pharyngectomy). The interval between the last day of chemotherapy and surgery had to be at least 3 weeks. As initially planned, patients had to undergo surgery if they had residual disease 2 months after radiotherapy or if they had a complete response after the overall treatment but relapsed thereafter. For all patients, the use of flaps to cover the pharyngeal sutures was left to the surgeon’s discretion. In the alternating arm, as initially planned, patients had to undergo surgery if they had progressive disease or no change at the day 42 evaluation (for institutions that selected the intermediate evaluation), if they had persistent local disease 2 months after the last day of the fourth cycle of chemotherapy, or if they relapsed during the follow-up.
Quality Control
The study coordinator reviewed all patient files and performed regular written or telephone updates. The EORTC study team did the updates whenever eligibility concerns or compliance issues were suspected.
Statistical Analyses
The primary endpoint of this study was survival with a functional larynx. For this endpoint, events were defined as death from any cause, local progression or relapse, tracheotomy, feeding tube insertion, gastrostomy, or laryngectomy, whichever occurred first. Patients who did not experience an event were censored on the last date that they were known to be alive. From our previous experience with the EORTC 24891 trial (4), we estimated a 3-year survival with a functional larynx in place of 28% in the sequential arm, with a type I error of .05 (two-sided) and a power of 80%. The trial was originally designed to be powered to detect an absolute improvement in 3-year estimate of survival with a functional larynx in place of 10% (from a survival of 28% to a survival of 38%) and so required a total of 426 events. Two independent data monitoring committee analyses were planned, at the times that 100 and 200 events had occurred, by use of an O’Brien–Fleming correction (11). Because of lower than planned accrual of patients, the second interim analysis resulted in a recommendation to limit the accrual to a total of 450 patients and 350 events, which corresponded to a study powered to detect an 11% absolute improvement. A third, unplanned, submission to the independent data monitoring committee led to permission to perform the final analysis because it appeared (because of better than expected survival with a functional larynx in both arms) that 350 events for the primary endpoint would not occur for another 3 years.
Time-to-event curves were obtained by use of the Kaplan–Meier method. Treatment arms were compared by use of a two-sided log-rank test. Efficacy analyses were done under an intent-to-treat policy.
Secondary endpoints were disease-free survival, overall survival, larynx preservation (with the same definition as above, ie, larynx in place without local progression or relapse, tracheotomy, feeding tube insertion, gastrostomy, or laryngectomy), and quality of life. Time to locoregional progression, time to distant progression, larynx preservation, and time to second primary tumor were analyzed, with death as a competing event. For these competing risk analyses, cumulative incidences and their confidence intervals were calculated. Comparisons were made by use of the log-rank test while censoring for death. Toxic effects associated with radiotherapy were compared between arms by use of the Wilcoxon rank sum test on the patients’ worst grades.
The sequential arm was always the reference group for the comparison between both arms. All hazard ratios (HRs) are defined as the hazard in the alternating arm divided by the hazard in the sequential arm.
Because many covariates may affect clinical outcome in head and neck cancers, univariate (by the log-rank test) and multivariable (by Cox proportional hazards regression) analyses were carried out. A Cox proportional hazards model was built by both forward and backward selection, from patients’ characteristics, including sex (male or female), age (continuous), performance status (WHO 0, 1, or 2), and marital status (single, married, or separated), and tumor characteristics including primary site (supraglottis, glottis, transglottis, or hypopharynx), histological grading (well, moderate, or poor), and staging according to Union Internationale Contre le Cancer (International Union Against Cancer) classification guidelines (II, III, or IV). Qualitative graphical methods were used to check against deviations from the proportional hazards assumption. Eligible patients were centrally and randomly assigned to treatment at the EORTC headquarters by the minimization method (12), stratifying by institution, WHO performance status (0, 1, or 2), site (hypopharynx, epilarynx, or endolarynx), tumor category (T2, T3, or T4), and lymph node category (N0, N1, or N2). All statistical tests were two-sided.
Results
Patient Population
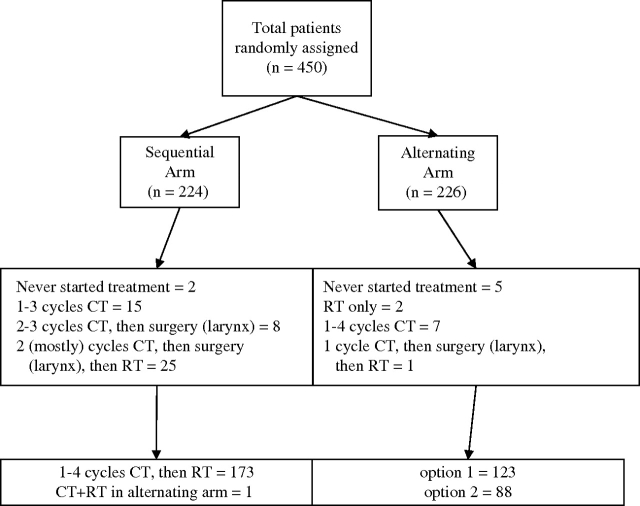
Between July 23, 1996, and April 26, 2004, 450 patients were randomly assigned to treatment in this trial (224 to the sequential arm and 226 to the alternating arm) by 19 different institutions (with 82% of patients being enrolled by eight institutions) (Figure 1). Among the 450 patients, 10 were found to be ineligible—four (2%) patients in the sequential arm and six (3%) patients in the alternating arm. Causes of ineligibility were presence of a second cancer (three patients), the site of their disease was not eligible (three patients), presence of distant metastases (one patient), previous tracheotomy (one patient), simultaneous cancer (one patient), and otologic contraindication to cisplatin (one patient). The median follow-up was 6.5 years (6.4 years in the sequential arm and 6.9 years in the alternating arm; P = .06).
Figure 1.
CONSORT diagram. The distribution of patients in the randomized phase 3 trial 24954 from the European Organization for Research and Treatment of Cancer, which compared induction CT (sequential or control arm) with a rapidly alternating CT (alternating or experimental arm) and then RT. All patients were analyzed under the intention-to-treat principle. Under option 1, patients were evaluated at the end of the protocol, and under option 2, patients had an intermediate evaluation at day 42. CT = chemotherapy; RT = radiotherapy.
The two arms were well balanced for sex, age, WHO performance status, primary tumor site and histology, tumor classification, lymph node classification, and stage grouping (Table 1). Four hundred two (89%) patients were male. Ages ranged from 35 to 76 years, with a median age of 55 years in both arms. Two hundred thirty-one (51%) of the 450 patients had hypopharyngeal squamous cell carcinoma and 218 (49%) had laryngeal squamous cell carcinoma (however, the primary site was not specified for one patient). Among the 218 patients with laryngeal squamous cell carcinoma, 155 (71%) had supraglottic diseases and 63 (29%) had glottic or transglottic diseases. Also among the 218 patients with laryngeal squamous cell carcinoma, 123 were considered to have epilaryngeal cancers and 95 were considered to have endolaryngeal cancers. Two hundred fifty-nine (58%) of the 450 patients had stage IV disease and only 16 (4%) had stage II disease.
Table 1.
Population characteristics
| Characteristic | Sequential arm, No. (%) | Alternating arm, No. (%) | Total, No. (%) |
| Total No. | 224 | 226 | 450 |
| Sex | |||
| Male | 201 (90) | 201 (89) | 402 (89) |
| Female | 23 (10) | 25 (11) | 48 (11) |
| Primary tumor site | |||
| Hypopharynx | 116 (52) | 115 (51) | 231 (51) |
| Supraglottis | 77 (34) | 78 (35) | 155 (34) |
| Glottis or transglottis | 31 (14) | 32 (14) | 63 (14) |
| Missing | 0 (0) | 1 (<1) | 1 (<1) |
| Tumor category | |||
| T2 | 29 (13) | 32 (14) | 61 (14) |
| T3 | 127 (57) | 125 (55) | 252 (56) |
| T4 | 68 (30) | 69 (31) | 137 (30) |
| Lymph node category | |||
| N0 | 80 (36) | 82 (36) | 162 (36) |
| N1 | 56 (25) | 60 (27) | 116 (26) |
| N2a | 26 (12) | 22 (10) | 48 (10.5) |
| N2b | 41 (18) | 44 (19) | 85 (19) |
| N2c | 21 (9) | 18 (8) | 39 (8.5) |
| Stage | |||
| II | 7 (3) | 9 (4) | 16 (4) |
| III | 91 (41) | 84 (37) | 175 (39) |
| IV | 126 (56) | 133 (59) | 259 (58) |
Protocol Compliance
Treatment was not started in two patients in the sequential arm (one ineligible patient and one because of medical decision) and in five patients in the alternating arm (three ineligible patients and two because of patient refusal). In the sequential arm, six patients received incorrect chemotherapy dosages (three overtreatment and three undertreatment). In the alternating arm, 14 patients received incorrect chemotherapy dosages (five undertreatment and nine overtreatment), and the timing of chemotherapy differed from that of the study protocol for one patient.
In the sequential arm, one patient had a total dose of irradiation higher than that in the protocol and three patients had a radiotherapy scheme different from that in the study protocol. In the alternating arm, all patients received radiotherapy according to protocol.
Salvage surgery was considered to be performed improperly for eight patients in the sequential arm and for two patients in the alternating arm. In the sequential arm, seven patients received salvage surgery after two cycles of chemotherapy, despite the absence of a partial response (six patients continued on chemotherapy and one patient was sent to radiotherapy, all by medical decision), and one patient refused to continue chemotherapy after the first cycle of chemotherapy and wanted to have surgery, despite a clear clinical response (remarkably, no residual disease was found in that patient's pathology specimen). In the alternating arm, one received partial salvage surgery instead of total salvage laryngectomy and one received a protocol continuation, despite the absence of a partial response at the intermediate evaluation.
Chemotherapy
In the sequential arm, 144 (64%) patients received all four cycles of induction chemotherapy, 12 patients received only one cycle of chemotherapy, 49 patients received two cycles, and 17 patients received three cycles. In the alternating arm, 156 (69%) patients received all four cycles of induction chemotherapy, 10 patients received only one cycle, 22 patients received two cycles, and 31 patients received three cycles (two patients received radiotherapy but no chemotherapy). It is reasonable that fewer patients received the planned total number of cycles in the sequential arm, which had a mandatory evaluation for response to chemotherapy after two cycles, than in the alternating arm, in which this evaluation was optional.
Among the 222 patients in the sequential arm who actually received chemotherapy, 177 (80%) received a relative dose intensity for 5-fluorouracil of more than 90% and 200 (91%) received a relative dose intensity for cisplatin of more than 90%. Reasons for dose modification were hematologic toxic effects (77 patients), nonhematologic toxic effects (51 patients), or both (14 patients). Further reasons were intercurrent diseases (eight patients), administrative and/or technical reasons (five patients), medical errors or protocol misinterpretation (four patients), and patient's decision (two patients). For two patients, we could not identify the reason.
Among the 219 patients in the alternating arm who actually received chemotherapy, 205 (94%) received a relative dose intensity for 5-fluorouracil of more than 90% and 200 (91%) received a relative dose intensity for cisplatin of more than 90%. Further reasons for dose modification were hematologic toxic effects (105 patients), nonhematologic toxic effects (25 patients), both hematologic and nonhematologic toxic effects (seven patients), intercurrent diseases (five patients), administrative and/or technical concerns (nine patients), mistake (one patient), and patient's decision (one patient).
Radiotherapy
In the sequential arm, 200 (89%) of the 224 patients received radiotherapy, with a median dose of 71.5 Gy (range = 14.0–79.3 Gy). Reasons for interrupting or delaying radiotherapy in 14 (7%) patients were toxic effects from radiotherapy (six patients), intercurrent diseases (four patients), and administrative and/or technical concerns (four patients). Reasons for discontinuing radiotherapy in four (2%) patients were intercurrent diseases (two patients) and administrative and/or technical concerns (two patients).
In the alternating arm, 220 (97%) of the 226 patients received radiotherapy, with a median dose of 62.8 Gy (range = 2.0–76.6 Gy). Reasons for interrupting or delaying radiotherapy in 53 (23%) of the 220 patients were toxic effects of radiotherapy (seven patients), toxic effects of chemotherapy (35 patients), intercurrent diseases (three patients), and administrative and/or technical concerns (eight patients). Reasons for discontinuing radiotherapy in eight (4%) patients were toxic effects of radiotherapy (one patient), toxic effects of chemotherapy (one patient), intercurrent diseases (two patients), and administrative and/or technical concerns (four patients).
Acute toxic effects (ie, functional mucosal reactions, objective mucosal reactions, and skin reactions) were statistically significantly higher in the sequential arm than in the alternating arm (all P < .001) (Table 2). This result is reasonable because patients in the sequential arm received a higher total dose as indicated in the protocol than those in the alternating arm. In contrast, late sequelae (ie, late mucosal sequelae, P = .5; late connective tissue sequelae, P = .029; and late nervous system sequelae, P = .71) were similar in both arms (Table 2). Late sequelae contraindicated salvage surgery for only two (1%) patients in each arm.
Table 2.
Radiotherapy-related toxic effects among 200 patients in the sequential arm and 220 patients in the alternating arm*
| Toxic effect | Sequential arm, No. of patients(%) | Alternating arm, No. of patients (%) | P values† |
| Acute functional mucosal reaction | <.001 | ||
| Liquid diet only | 66 (33) | 45 (20) | |
| No oral intake | 17 (9) | 17 (8) | |
| Acute objective mucosal reaction | <.001 | ||
| Grade 2 mucositis | 82 (41) | 76 (35) | |
| Grade 3–4 mucositis | 64 (32) | 47 (21) | |
| Acute skin reaction | <.001 | ||
| Grade 2 | 12 (6) | 2 (1) | |
| Grade 3–4 | 12 (6) | 0 (0) | |
| Late mucosal sequelae | .5 | ||
| Superficial necrosis or moderate edema | 50 (25) | 62 (28) | |
| Deep necrosis or severe edema | 19 (10) | 14 (6) | |
| Late connective tissue sequelae | .029 | ||
| Moderate fibrosis or sclerosis | 61 (31) | 62 (28) | |
| Severe fibrosis or sclerosis | 20 (10) | 16 (7) | |
| Late nervous system sequelae | .71 | ||
| Transient neuropathy | 11 (6) | 14 (6) | |
| Permanent neuropathy | 27 (14) | 24 (11) | |
| Radiotherapy-induced myelitis | 1 (1) | 1 (<1) | |
| Late sequelae contraindicating salvage surgery | 2 (1) | 2 (1) | .18 |
There were a total of 200 patients in the sequential arm and a total of 220 patients in the alternating arm who received radiotherapy.
The ordinal toxicity outcomes were compared by Wilcoxon test (two-sided). All statistical tests were two-sided.
Surgery
At the time of trial evaluation, 68 (30.1%) of the 224 patients in the sequential arm had had salvage surgery to the primary tumor: 33 during the study protocol, six at the end of the study protocol, and 29 during follow-up. In the alternating arm, 50 (22.1%) of the 226 patients had had surgery to the primary tumor: 11 during the study treatment protocol, 13 at the end, and 26 during follow-up. There was no statistically significant difference between both arms regarding surgical techniques, quality of surgical margins, and postoperative complications. No residual disease was found in the specimen from four patients in the sequential arm and from seven patients in the alternating arm. One postoperative death has been reported in each arm.
Seventy-seven (34.4%) of the 224 patients in the sequential arm and 56 (24.7%) of the 226 patients in the alternating arm had a neck dissection. There was no difference in surgical techniques and postoperative courses between both arms. Positive lymph nodes were found in 46 (59.7%) of the 77 surgical specimens of the sequential group and in 32 (57.1%) of the 56 surgical specimens of the alternating group, with capsular rupture in 31 of the 46 positive specimens in the sequential group and in 21 of the 32 positive specimens in the alternating group.
Larynx Preservation
At the time of evaluation in the sequential arm, a tracheotomy had been performed in 19 patients and was left in place for more than 3 months in all 19 patients, a gastrostomy had been performed or a feeding tube had been placed in 43 patients and was left in place for more than 3 months in 40 patients, and a laryngectomy had been performed in 67 patients. In the alternating arm, a tracheotomy had been performed in 19 patients and was left in place for more than 3 months in 18 patients, a gastrostomy had been performed or a feeding tube had been placed in 39 patients and was left in place for more than 3 months in 35 patients, and a laryngectomy had been performed in 52 patients.
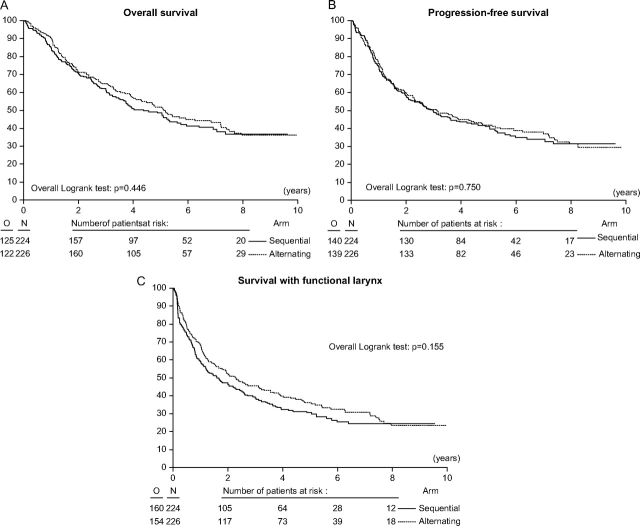
The primary endpoint was survival with a functional larynx in place. Although there was a trend for a better outcome for the alternating arm, the outcome was not statistically significantly different between the two treatment arms (Figure 2, C). The median survival with a functional larynx was 1.6 years (95% confidence interval [CI] = 1.1 to 2.4 years) in the sequential arm and 2.3 years (95% CI = 1.6 to 3.3 years) in the alternating arm (HR of event = 0.85, 95% CI = 0.68 to 1.06). Estimates of the 3-year survival with a functional larynx were 39.5% (95% CI = 33.0% to 45.8%) for the sequential arm and 45.4% (95% CI = 38.8% to 51.8%) for the alternating arm. Estimates of the 5-year survival with a functional larynx were 30.5% (95% CI = 24.5% to 36.8%) for the sequential arm and 36.2% (95% CI = 29.7% to 42.7%) for the alternating arm.
Figure 2.
A) Overall survival. Estimates of overall survival at 5 years were 48.5 (95% CI = 41.6 to 55.1) in the sequential arm and 51.9 (95% CI = 44.8 to 58.4) in the alternating arm. B) Progression-free interval. Estimates of progression-free interval at 3 years were 49.7 (95% CI = 43.0 to 56.1) in the sequential arm and 50.6 (95% CI = 43.8 to 56.9) in the alternating arm. C) Survival with a functional larynx. Estimates of survival with a functional larynx at 3 years were 39.5 (95% CI = 33.0 to 45.8) in the sequential arm and 45.4 (95% CI = 38.8 to 51.8) in the alternating arm. All statistical tests were two-sided. CI = confidence interval.
Overall Survival
With a median follow-up of 6.5 years, 125 (55.8%) of the 224 patients in the sequential arm and 122 (53.9%) of the 226 patients in the alternating arm had died. The median overall survival times were 4.4 years (95% CI = 3.5 to 5.6 years) in the sequential group and 5.1 years (95% CI = 4.0 to 7.2 years) in the alternating group. Estimates of the 3-year overall survival (Figure 2, A) were 62.2% (95% CI = 55.4% to 68.2%) in the sequential group and 64.8% (95% CI = 58.1% to 70.6%) in the alternating group. Estimates of the 5-year overall survival were 48.5% (95% CI = 41.6% to 55.1%) in the sequential group and 51.9% (95% CI = 44.8% to 58.4%) in the alternating group (HR of death = 0.91, 95% CI = 0.71 to 1.16).
Neither survival curves nor causes of death (Table 3) differed statistically significantly between treatment arms. Cancer progression was the cause of death in approximately half of all patients.
Table 3.
Causes of death*
| Cause of death | Sequential arm, No. (%) | Alternating arm, No. (%) |
| Cancer progression† | 61 (49) | 63 (52) |
| All complications | ||
| Toxic effects | 9 | 4 |
| Infection | 6 | 2 |
| Postoperative death | 1 | 1 |
| Second primary cancer | 19 (15) | 17 (14) |
| Not cancer related | 17 (14) | 22 (18) |
| Unknown | 12 (10) | 13 (11) |
There were a total of 125 patients in the sequential arm and a total of 122 patients in the alternating arm who were known to be dead by the time of analysis.
Cancer progression included local and/or regional, and/or distant progression.
In the multivariable analysis for overall survival, statistically significant risk factors were the sex (female patients survived longer than male patients, P = .013; HR of death = 1.88, 95% CI = 1.14 to 3.08, for men compared with women), age (younger patients survived longer than older patients, P = .023; HR = 1.20, 95% CI = 1.03 to 1.34, per 10 additional years of age), marital status (married patients survived longer than single patients, P = .022; HR = 0.60, 95% CI = 0.42 to 0.87, for married compared with single; HR = 0.70, 95% CI = 0.45 to 1.09, for separated compared with single), and stage (patients with stage IV disease had worse survival than those with stage II and III disease, P = .005; HR = 1.77, 95% CI = 0.72 to 4.38, for stage III compared with stage II; HR = 2.59, 95% CI = 1.06 to 6.31, for stage IV compared with stage II). For the primary endpoint of survival with a functional larynx, marital status was the only statistically significant risk factor (married patients fared better than single patients, P = .013; HR of event or death = 0.61, 95% CI = 0.43 to 0.87, for married compared with single; HR = 0.67, 95% CI = 0.43 to 1.04, for separated compared with single). When adding treatment arm to these multivariable models, the treatment comparison did not differ from that of the univariate analysis.
Pattern of Relapse
At the time of analysis, there was no statistically significant difference in relapse between the sequential arm, in which 93 (41.5%) of the 224 patients had progressed, and the alternating arm, in which 95 (42.0%) of the 226 patients had progressed. During follow-up, in the sequential arm, 60 (26.8%) of the 224 patients experienced local progression either isolated (28 patients) or associated with lymph node and/or distant progression (32 patients). Lymph node recurrence was observed in 37 (16.5%) patients, and it was isolated in nine of them. Distant metastases occurred in 34 (15.2%) patients, and it was the sole event in 19 of them. In addition, 32 (14.3%) patients developed a second primary tumor.
During follow-up, in the alternating arm, 52 (23.0%) of the 226 patients experienced local progression, either isolated (21 patients) or associated with lymph node and/or a distant progression (31 patients). Lymph node recurrence was observed in 47 (20.8%) patients, and it was isolated in 14 of them. Distant metastases occurred in 48 (21.2%) patients, and it was the sole event in 19 of them. In addition, 51 (22.6%) patients developed a second primary tumor.
At 5 years, in the sequential arm, the estimated cumulative incidence of locoregional recurrence was 31.8% (95% CI = 25.6% to 38.0%) compared with 32.3% (95% CI = 26.1% to 38.5%) in the alternating arm. Cumulative incidence of distant metastases was 15.5% (95% CI = 10.7% to 20.3%) in the sequential arm compared with 21.5% (95% CI = 16.0% to 27.0%) in the alternating arm. In the sequential arm, 13.3% (95% CI = 8.6% to 17.9%) had developed a second primary cancer compared with 20.2% (95% CI = 14.6% to 25.8%) in the alternating arm (Table 4).
Table 4.
Patterns of treatment failure among 224 patients in the sequential arm and 226 patients in the alternating arm*
| Type of treatment failure | Time of cumulative incidence estimate, y | Cumulative incidence estimate, % (95% CI) |
|
| Sequential arm | Alternating arm | ||
| Locoregional progression | 3 | 28.7 (22.8 to 34.7) | 30.3 (24.3 to 36.3) |
| 5 | 31.8 (25.6 to 38.0) | 32.3 (26.1 to 38.5) | |
| Distant progression | 3 | 13.1 (8.7 to 17.5) | 18.3 (13.2 to 23.3) |
| 5 | 15.5 (10.7 to 20.3) | 21.5 (16.0 to 27.0) | |
| Second primary cancer | 3 | 9.9 (6.0 to 13.9) | 11.6 (7.4 to 15.8) |
| 5 | 13.3 (8.6 to 17.9) | 20.2 (14.6 to 25.8) | |
CI = confidence interval.
The median progression-free interval did not differ statistically significantly between both arms (HR of death and/or an event = 0.96, 95% CI = 0.76 to 1.22) (Figure 2, B). The median progression-free interval was 3.0 years (95% CI = 2.1 to 4.2 years) in the sequential arm and 3.1 years (95% CI = 2.3 to 4.8 years) in the alternating arm. Estimates of the 3-year progression-free interval were 49.7% (95% CI = 43.0% to 56.1%) in the sequential arm and 50.7% (95% CI = 43.8% to 56.9%) in the alternating arm. The 5-year estimates were 41.04% (95% CI = 34.4% to 47.6%) in the sequential arm and 41.8% (95% CI = 35.1% to 48.4%) in the alternating arm.
Quality of the Laryngeal Function
There was a trend for a slightly higher rate of larynx preservation at 1 and 3 years in the alternating arm than in the sequential arm (Table 5). There was also a trend for a better function of the preserved larynx at 1 year in the alternating arm but with essentially no difference at 3 years between arms. Of note, 58 of the 61 patients who had had a laryngectomy achieved satisfactory voice rehabilitation (esophageal voice or tracheoesophageal prosthesis).
Table 5.
Quality of laryngeal function
| Function | No. of patients (%) |
|
| Sequential arm | Alternating arm | |
| 1 y after end of treatment* | ||
| Larynx in place | 142 (78) | 166 (84) |
| Intelligible or sociable voice | 127 (89) | 156 (94) |
| Normal intake† | 123 (87) | 151 (91) |
| Normal breathing | 127 (89) | 150 (90) |
| Laryngectomy | 39 (22) | 32 (16) |
| With voice rehabilitation | 38 (97) | 26 (81) |
| 3 y after end of treatment‡ | ||
| Larynx in place | 101 (75) | 116 (81) |
| Intelligible or sociable voice | 95 (95) | 110 (95) |
| Normal intake† | 87 (87) | 104 (90) |
| Normal breathing | 86 (86) | 104 (90) |
| Laryngectomy | 33 (25) | 28 (19) |
| With voice rehabilitation | 33 (100) | 25 (89) |
One year after the end of treatment, data on laryngeal function were available for 181 patients in the sequential arm and for 198 patients in the alternating arm.
Normal intake is the ability to eat orally any type of food without loss of weight.
Three years after the end of treatment, data on laryngeal function were available for 134 patients in the sequential arm and for 144 patients in the alternating arm.
Discussion
In this study, we found that survival with a functional larynx, overall survival, and progression-free interval were similar in both arms, as were acute and late toxic effects. At 5 years, approximately one-half of all patients were alive, and one-third of all patients were alive with a functional larynx in place. Grade 3 or 4 mucositis occurred in 64 (32%) of the 200 patients in the sequential arm who received radiotherapy and in 47 (21%) of the 220 patients in the alternating arm. Late severe edema and/or fibrosis was observed in 32 (16%) patients in the sequential arm and in 25 (11%) in the alternating arm.
For the past two decades, larynx preservation has been one of the most important achievements in head and neck oncology. The first generation of clinical trials (3–6), which evaluated induction chemotherapy followed by irradiation compared with the conventional surgical approach of total laryngectomy, showed that a total laryngectomy could be avoided in numerous patients without jeopardizing the chances of cure. In a large meta-analysis (6), concurrent administration of chemotherapy and radiotherapy resulted in the best survival outcome of all regimens of chemotherapy and radiotherapy examined. Consequently, the use of concurrent administration of chemotherapy and radiotherapy became a standard of care for advanced head and neck cancers. In addition, a large phase 3 US Intergroup trial (7,13) that examined larynx preservation also found better locoregional control with concurrent chemotherapy and radiotherapy than with sequential chemotherapy and radiotherapy or with radiotherapy alone. However, the concurrent regimen was associated with statistically significant severe toxic effects, and the overall survival, disease-free interval, and laryngectomy-free interval were similar between treatment groups (7,13). In addition, the randomized trials (3–7,13) that examined larynx preservation found that survival was similar in the larynx preservation group and in the group receiving surgery and postoperative radiotherapy. Survival was also similar when different larynx-preserving approaches (sequential, concurrent, or alternating) were compared (3–7,13,16), and the benefit of concurrent chemotherapy and radiotherapy has been restricted to better locoregional control, which results in better larynx preservation (7,13).
Further innovative approaches for larynx preservation include the following two developments. The first development is the addition of taxanes to induction chemotherapy regimens, which has renewed the interest in the sequential approach (14–16). Two randomized trials (14,15) of induction chemotherapy that compared the combination of cisplatin and 5-fluorouracil with the combination of cisplatin, 5-fluorouracil, and docetaxel, followed by standard radiotherapy alone or by concurrent chemotherapy with carboplatin and radiotherapy, showed that statistically significant improved overall survival and progression-free interval and fewer acute toxic effects were associated with the triplet induction regimen. A French randomized trial (16) compared induction chemotherapy with cisplatin, 5-fluorouracil, and docetaxel, or with cisplatin and 5-fluorouracil, followed by radiotherapy alone; larynx preservation was the primary endpoint. In this trial, the larynx preservation rate was higher with cisplatin, 5-fluorouracil, and docetaxel treatment than with cisplatin and 5-fluorouracil treatment, and treatment with cisplatin, 5-fluorouracil, and docetaxel had fewer side effects than treatment with cisplatin and 5-fluorouracil. However, no gain in survival was observed. The second development is the integration of molecular targeted therapy into multimodal approaches to locoregionally advanced head and neck cancer (17,18). A randomized phase 3 trial (17) compared radiotherapy alone or with cetuximab, a monoclonal antibody against the epidermal growth factor receptor 1, among patients with head and neck cancer. Both locoregional control and overall survival were statistically significantly improved by the use of cetuximab without increased acute mucosal reactions or late toxic effects. In the subset of patients with larynx or hypopharynx cancers, larynx preservation appeared to be higher in the arm with cetuximab (18). Results of our trial should be considered in the context of these results (14–18).
The aim of our trial was to identify a regimen that would mimic concomitant chemotherapy and radiotherapy without its increased toxic effects (19). Although we achieved the latter goal, acute toxic effects at the doses and schedule used were even less than those associated with induction chemotherapy, possibly because of the split course of radiotherapy, but efficacy was not improved. It should, however, be noted that despite lower total doses of chemotherapy (1000 mg/m2 of 5-fluorouracil per cycle vs 5000 mg/m2) and radiotherapy (60 vs 70 Gy) and a longer overall treatment time for radiotherapy in the alternating arm (8 vs 7 weeks), the outcome was not compromised. In fact, in the sequential arm, among the 200 patients who received radiotherapy after induction chemotherapy, 121 (60.5%) had the first day of radiotherapy within 1 month, 49 (24.5%) had the first day of radiotherapy between 1 and 2 months, and only 30 (15%) had the first day of radiotherapy more than 2 months after induction chemotherapy. In the alternating arm, 61 (28%) of 220 patients had a delay or interruption in radiotherapy. The cumulative incidence of locoregional progression at 3 years in the sequential arm was 31.4% (95% CI = 24.4% to 38.4%) in patients without a delay in radiotherapy vs 23.3% (95% CI = 8.2% to 38.5%) in patients with delayed radiotherapy. In the alternating arm, cumulative incidence of locoregional progression at 3 years was 29.2% (95% CI = 22.1% to 36.2%) for patients without an interruption or delay vs 31.2% (95% CI = 19.6% to 42.8%) for patients with an interruption or delay. Although these analyses are biased because they were stratified by treatment factors, there was no strong indication that these delays adversely affected outcome. The 6.7% higher rate of larynx preservation in the alternating arm than in the sequential arm was not statistically significant and did not translate into a statistically significant difference in survival with a functional larynx.
Even though there was the usual trend for higher survival rates in patients with squamous cell carcinoma of the larynx than in those with squamous cell carcinoma of the hypopharynx, there was no statistically significant difference in overall survival, progression-free interval, or survival with a functional larynx between these groups. In addition, even when we considered three cancer sites (hypopharynx, epilarynx, and endolarynx), there was no statistically significant difference in overall survival, progression-free interval, or survival with a functional larynx between the sequential arm and the alternating arm among the groups, and the usual trend was observed for a poorer overall survival in the patients with hypopharynx and epilarynx cancer than in those with cancer of the endolarynx (for epilarynx compared with hypopharynx, HR = 0.88, 95% CI = 0.66 to 1.18; and for endolarynx compared with hypopharynx, HR = 0.69, 95% CI = 0.49 to 0.98). However, again there was no statistically significant difference in the progression-free interval (for epilarynx compared with hypopharynx, HR = 1.01, 95% CI = 0.77 to 1.33, or for endolarynx compared with hypopharynx, HR = 0.80, 95% CI = 0.58 to 1.10) or overall survival with a functional larynx (for epilarynx compared with hypopharynx, HR = 1.00, 95% CI = 0.77 to 1.29; or for endolarynx compared with hypopharynx, HR = 0.95, 95% CI = 0.71 to 1.27) between the two arms. Although cross-trial comparisons are generally difficult because of differences in primary disease sites and in endpoint definitions, when overall survival of the patients with hypopharyngeal cancer in the sequential arm of the present trial was assessed, it was found to be identical to that observed in the sequential arm of the previous EORTC trial (4).
Our trial may have several limitations. First, we used the most restrictive definition for larynx preservation: survival with a normal larynx (ie, a larynx without tumor) and a functional larynx (ie, without tracheotomy and no feeding tube or gastrostomy for longer than 3 months). Use of this definition may have made a difference between both arms for larynx preservation assessment. The EORTC group firmly decided to use this restrictive definition because they considered that it reflected a real benefit for patients. Another limitation could be the absence of data on smoking habits. However, in Europe, at least 95% of patients with larynx or hypopharynx cancer are heavy smokers, and so the real cumulated dose of tobacco smoked before diagnosis is difficult to assess. In addition, none of the randomized trials on larynx preservation (3–7) were able to report smoking habits of their patients.
It is clear that the optimal approach for larynx preservation has still not been identified. Several treatment options are available, each with different levels of tolerability but little difference in outcome. Recent developments (14–18) have made it possible to explore new options that may lead to better tumor control, fewer toxic effects, and a better quality of life. The sequential use of more effective induction chemotherapy regimens followed by less toxic locoregional approaches is one option that urgently needs to be evaluated.
Funding
Cancer trial registry number for this trial is NCT00002839. This publication was supported by grants 2U10 CA11488-25 through 5U10 CA11488-37 from the National Cancer Institute (Bethesda, MD). This research project was supported by Fonds Cancer/FOCA (Belgium).
Footnotes
J. L. Lefebvre, L. Licitra, and J. B. Vermorken are members of Sanofi-Aventis and Merck Serono advisory boards. Other authors had no conflict of interests. J. L. Lefebvre, corresponding author, had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors gave their approval for publication. The authors had full responsibility for designing and analyzing the study, interpreting the data, writing the manuscript, and submitting it for publication.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The following investigators (by alphabetical order) have also contributed to this trial: Beauvillain de Montreuil, University Hospital, Nantes, France; R. J. Bensadoun, Centre Antoine Laccassagne, Nice, France; J. Buter, VU University Medical Center, Amsterdam, the Netherlands; B. Coche-Dequeant, Centre Oscar Lambret, France; M. Degardin, Centre Oscar Lambret, Lille, France; D. Dehesdin, University Hospital Charles Nicolle, Rouen, France; C. Duvillard, University Hospital, Dijon, France; A. Kutem, Rambam Medical Center, Israel; J. A. Langendiijk, VU University Medical Center, Amsterdam, the Netherlands; J. P. Rame, Centre Francois Baclesse, Caen, France; G. Truc, Centre Georges Francois Leclerc, Dijon, France; D. van den Weynaert, Middelheim, Belgium.
References
- 1.Decker DA, Drelichman A, Jacob J, et al. Adjuvant chemotherapy with cisdiamminodichloroplatinum II and 120-hour infusion 5-fluorouracil in stage III and IV squamous cell carcinoma of the head and neck. Cancer. 1983;51(8):1353–1355. doi: 10.1002/1097-0142(19830415)51:8<1353::aid-cncr2820510805>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Ensley J, Jacobs J, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54(5):811–814. doi: 10.1002/1097-0142(19840901)54:5<811::aid-cncr2820540508>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 5.Richard JM, Sancho-Garnier H, Pessey JJ, et al. Randomized trial of induction chemotherapy in larynx carcinoma. Oral Oncol. 1998;34(3):224–228. doi: 10.1016/s1368-8375(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 7.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 8.Paccagnella A, Orlando A, Marchiori C, et al. Phase III of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. 1994;86(4):265–272. doi: 10.1093/jnci/86.4.265. [DOI] [PubMed] [Google Scholar]
- 9.Merlano M, Vitale V, Rosso R, et al. Treatment of advanced squamous-cell carcinoma of the head and neck with alternating chemotherapy and radiotherapy. N Engl J Med. 1992;327(16):1115–1121. doi: 10.1056/NEJM199210153271602. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre JL, Rolland F, Tesselar M, et al. A phase III study on larynx preservation comparing induction chemotherapy and radiotherapy versus alternating chemo-radiotherapy in resectable hypopharynx and larynx cancers. Proc Am Soc Clin Oncol. 2007 J Clin Oncol. 2007;18s (25), abstract LBA 6016. [Google Scholar]
- 11.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 12.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 13.Forastiere AA, Maor M, Weber RS, et al. Long-term results of intergroup RTOG 91-11: a phase III trial to preserve the larynx-Induction cisplatin/5FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. Proc Am Soc Clin Oncol. 2006 J Clin Oncol. 2006;18s(24), abstract 5517; p 284s. [Google Scholar]
- 14.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 15.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 16.Calais G, Pointreau Y, Sire C, et al. Induction chemotherapy for larynx preservation. Updated results of the GORTEC 2000–01 randomized trial comparing docetaxel + cisplatinum + fluorouracil (TPF) versus cisplatinum + fluorouracil (PF) Proc European Cancer Conference ECCO 14. Abstract 5500. [Google Scholar]
- 17.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 18.Bonner JA. Improved preservation of larynx with the addition of cetuximab to radiation for cancer of the larynx and hypopharynx. Proc Am Soc Clin Oncol. 2005 J Clin Oncol. 2005;16s(23), abstract 5533; p508s. [Google Scholar]
- 19.Merlano M. Alternating chemotherapy and radiotherapy in locally advanced head and neck cancer: an alternative. Oncologist. 2006;11(2):146–151. doi: 10.1634/theoncologist.11-2-146. [DOI] [PubMed] [Google Scholar]