Abstract
Aims: Alcohol abuse is associated with the development of the acute respiratory distress syndrome, a disorder characterized by abnormal alveolar-capillary permeability. We hypothesized that individuals with a history of alcohol abuse would have clinical evidence of abnormal alveolar-capillary permeability even in the absence of symptoms. This could contribute to their propensity for the development of this disorder. Methods: Thirty-three subjects with a history of alcohol abuse, but no other medical problems, and 13 age- and smoking-matched controls inhaled 99mTc–DTPA (technetium-labeled diethylenetriamine penta-acetate; an isotope used to measure lung permeability) for a 3-min period, and washout of this isotope was measured for a 90-min period. The rate at which it was cleared from the lungs was assessed and compared between subjects and controls. Results: The half-life of 99mTc–DTPA in the lungs of subjects with alcohol abuse was significantly shorter than that observed in matched controls, even when correcting for the effects of concomitant tobacco use. When the half-life of the isotope for smoking alcohol-abusing subjects and smoking controls were compared separately, there was a trend for the alcohol-abusing subjects to have a shorter half-life of the isotope present in the lungs. This was also true when non-smokers were compared. Conclusions: These observations provide further evidence that alcohol abuse affects the normal permeability of the alveolar-capillary barrier and thereby may contribute to the development of the acute respiratory distress syndrome in individuals with alcohol abuse.
Introduction
The acute respiratory distress syndrome (ARDS) is defined as refractory hypoxemia with bilateral infiltrates on chest radiograph in the absence of left atrial hypertension (Bernard et al., 1994). Pathophysiologically, ARDS is characterized by diffuse alveolar damage, alterations in pulmonary alveolar-capillary function and the subsequent accumulation of extravascular lung water (Ware and Matthay, 2000). ARDS affects ∼190,000 patients per year in the United States, and the mortality rate is 40–50% even in previously healthy individuals (Rubenfeld et al., 2005).
In two epidemiological studies of over 570 critically ill patients, a prior history of alcohol abuse was associated with an increased incidence and severity of ARDS (Moss et al., 1996, 2003). In addition, 50% of all patients with ARDS have a significant history of chronic alcohol abuse, making alcohol-related ARDS a common phenomenon and an enormous national health care problem (Ware and Matthay, 2000). Based on this observation, there are an estimated 30,000 deaths per year in the United States from alcohol-associated ARDS, a figure that exceeds the number of deaths due to many other alcohol-related diseases including cirrhosis of the liver and alcohol-related traffic accidents (CDC, 1993; Anderson, 2001). Therefore, improvements in the understanding of the pathogenesis of alcohol-associated ARDS and the development of therapies targeted for this population are important and necessary.
The alveolar-capillary membrane in the lung serves as a barrier to prevent the accumulation of fluid in the alveolar spaces. The thin alveolar-capillary barrier facilitates efficient gas exchange but severely restricts the diffusion of large solutes. In animal models, chronic ethanol feeding decreases integrity and increases permeability of the alveolar-capillary barrier (Guidot et al., 2000). Rats fed a liquid diet containing ethanol for 6 weeks had increased bidirectional protein permeability across the alveolar epithelium compared with rats fed a control diet (Guidot et al., 2000). Despite a reduced barrier function, ethanol-fed rats were still able to increase fluid transport in response to epinephrine stimulation, indicating that transcellular salt and water transport was still intact. In addition, when type II cells were isolated from ethanol-fed rats and placed in a culture for 8 days, they formed a more permeable cell layer (measured as permeability of 14C-inulin) than cells from control-fed animals.
The effects of alcohol abuse on pulmonary alveolar-capillary permeability in humans have never been fully examined. Based on the findings in the animal model, we hypothesize that alcohol abuse would be associated with alterations in alveolar-capillary barrier function in individuals with a history of alcohol abuse or dependence. In this study, we measured alveolarcapillary barrier function in individuals with a diagnosis of alcohol abuse or dependence using an inhaled radiolabeled isotope technique.
Materials and Methods
Within the Emory University hospital system, there are two sources for patients diagnosed with alcohol abuse or dependence: a four-bed chemical detoxification inpatient unit at the Veterans Administration (VA) Hospital and an outpatient Crisis Intervention Unit (CIU) at Grady Memorial Hospital. The unit at the VA admits ∼350 patients per year, and the CIU evaluates 8000 individuals per year with drug and alcohol abuse. Subjects were eligible to participate if they met all of the following criteria at study entry: (i) Short Michigan Screening Test (SMAST) score of >3, or an Alcohol Use Disorders Identification Test (AUDIT) score of ≥8, (ii) alcohol use within the 7 days prior to enrollment and (iii) age ≥21 and <55 years. The SMAST and AUDIT questionnaires are standardized surveys that can detect current and previous alcohol abuse and are validated in a variety of clinical settings (Selzer et al., 1975; Reinert and Allen, 2002). In order to determine the effect of current (not prior) alcohol abuse, we stipulated that our alcohol-consuming subjects should not have ceased drinking >7 days prior to their study being performed. Lastly, the choice of age range between 21 and 55 was made to ensure both that our subjects were legally consuming alcohol (drinking age in GA, USA, is 21) and to ensure that the presence of concomitant but asymptomatic co-morbidities would be minimized. The institutional review board at Emory University and the Atlanta VA Medical Center approved this study, and all subjects provided written informed consent prior to their participation in this protocol.
In order to isolate the effects of a history of alcohol abuse on the integrity of the alveolar-capillary barrier function and minimize the effects of co-morbidities on our outcome variable, subjects were ineligible to participate in the study if they met any of the following criteria: (i) prior medical history of liver disease (documented history of cirrhosis, total bilirubin ≥2.0 mg/dl, or albumin <3.0); (ii) prior medical history of gastrointestinal bleeding (due to the concern of varices); (iii) prior medical history of heart disease (documentation of ejection fraction <50%, myocardial infarction, or severe valvular dysfunction); (iv) prior medical history of renal disease (end-stage renal disease requiring dialysis, or a serum creatinine ≥2 mg/ dl); (v) prior medical history of lung disease defined as an abnormal chest radiograph or spirometry (FVC or FEV1 <75%); (vi) concurrent illicit drug use defined as a positive toxicology screen; (vii) prior history of diabetes mellitus; (viii) prior history of HIV infection; (ix) failure of the patient to provide informed consent; (x) refusal of the patient's attending physician to provide consent to participate; (xi) pregnancy; (xii) prior history of recent acetaminophen use due to the effects of this drug on hepatic glutathione concentrations (Schiodt et al., 1997) and (xiii) history of malnutrition defined as a Nutritional Risk Index of <90 (12). This index relies on the serum albumin concentration and the percentage of usual body weight in the following manner: NRI = 100 × [1.59 × albumin (g/l)] + [0.417 × (current weight/usual body weight in the past 6 months)]. In summary, the majority of these exclusion criteria were utilized in order to ensure a homogeneous population of alcoholic subjects and controls, to specifically focus on the effect of alcohol abuse on lung permeability.
In order to assess the presence of these exclusion criteria, all patients who met the inclusion criteria had a complete medical history obtained. In addition, a chest radiograph, routine blood chemistries, toxicology screen and spirometry were performed. We also performed the Subjective Global Assessment of Nutritional Status on all patients, in order to obtain a more complete index of nutritional status (Detsky et al., 1984, 1987). Normal controls subjects were recruited by placing fliers on bulletin boards throughout the Emory-affiliated hospitals. Control subjects had an AUDIT score of 0, had not ingested alcohol within the last 4 weeks and did not meet any of the exclusion criteria listed above.
Technetium-labeled diethylenetriamine penta-acetate (DTPA) clearance is the only commonly used measure of pulmonary epithelial permeability in clinical use today. The technique is based upon work published by Jones and co-workers, and involves the measurement of the rate of clearance of a small molecule from the airspaces of the lung (Jones et al., 1983). A faster clearance of 99mTc–DTPA implies a shorter half-life of this molecule in the lung, indicative of increased alveolar-capillary permeability. Numerous studies have shown that subjects who smoke have a much faster 99mTc–DTPA clearance than non-smokers; however, the effect of smoking on 99mTc–DTPA clearance time is rapidly reversible after smoking cessation for 24 h (Mason et al., 1983;Braude et al., 1986). Therefore, all smoking individuals (both subjects with a history of chronic alcohol abuse and controls) abstained from smoking for 24 h prior to the radionuclide scan.
Patients inhaled an aerosol of 99Tc–DTPA for a 3-min period with a 2-s breath hold at the end of each tidal breath in order to minimize tracheobronchial deposition. The aerosol was delivered with a disposable radio aerosol system for a ventilation scan. This apparatus produces a particle size with a mean mass diameter of 1.0 μ. A total of 30–40 mCi of 99mTc–DTPA were placed in the nebulizer in order for ∼1–3 mCi to be deposited on the alveolar membrane. The biodistribution and clearance of the 99mTc–DTPA in the lungs were imaged in the posterior projection using a large field of view gamma camera (>400 mm) with a low-energy all-purpose (LEAP) collimator. The matrix size was 256 × 256 word-mode, and the type of acquisition was planar dynamic mode. The frame rate that was used was the standard 1 min/frame. Immediately after the inhalation of the 99mTc–DTPA aerosol, the imaging began for a total duration of imaging of 90 min in a posterior projection with the patient in an upright position. Regions of interest (ROI) were placed over the right and the left lung and a rectangular background ROI was placed in the area below the right lung. Time activity curves (TAC) of the right and left lungs were generated after correcting the background counts and decay correction. Half-life values were calculated using a linear fit if the washout was linear or an exponential fit if the washout was exponential.
Using a Shapiro–Wilk W-test, the half-life values obtained from the subjects and controls were determined to be normally distributed. However, to be conservative and consistent in our analyses, all of the results are reported as a median and 25–75% quartiles. A Wilcoxon nonparametric test was used to determine whether the half-lives were different between any two groups, and the power of our result was determined for our primary outcome (http://www.dssresearch.com). An alpha value of 0.05 was used for all statistical tests.
Results
The demographic information for the 46 subjects (33 individuals with a diagnosis of alcohol abuse and 13 gender- and smoking-matched controls) is included in Table 1. The median SMAST score for those who used alcohol was 8 [7–11]. On average, these 33 individuals used alcohol 7 days per week for 28 years. In addition, 76% (25/33) of the individuals with alcohol dependence and 77% (10/13) of the controls had a history of current cigarette smoking. AUDIT scores were available for 21 of the individuals who used alcohol and their median AUDIT score was 21 [18–32].
Table 1.
Demographics of subjects with a history of alcohol abuse and control subjects
| Subjects with alcohol abuse (n = 33) | Control subjects (n = 13) | P value | |
|---|---|---|---|
| SMAST, median | 8 [7–11] | 0 | <0.0001 |
| AUDIT, mediana | 21 [18–32] | 0 | <0.0001 |
| Tobacco use | 76% | 77% | 0.93 |
| Gender, % men | 90% | 85% | 0.61 |
| Age, median | 48 | 48 | 0.36 |
| FEV1, % predicted | 94 [85–105]b | 93 [79–104] | 0.18 |
| FVC, % predicted | 108 [95–115]b | 86 [75–100] | 0.10 |
SMAST = Short Michigan Alcohol-Screening Test; AUDIT = Alcohol Use Disorders Identification Test.
aAvailable for 21 of 33 subjects with alcohol abuse.
bAvailable for smoking controls only.
All of the individuals with alcohol dependence underwent further testing with chest radiographs, pulmonary spirometry, liver function testing and urine toxicology screening. All of the chest radiographs were normal and all of the urine toxicology results were negative. Both the median FEV1 and FVC were normal in these individuals (FEV1 = 94% of predicted [85–105%] and FVC = 108% predicted [95–115%]). In subjects with alcohol abuse, the median serum glutamic-oxaloacetic tranaminase (SGOT) was 42 μ/l [24–59], and the median total bilirubin was 0.6 mg/dl [0.4–0.8]. The Nutritional Risk Index was >100 in all alcoholic subjects and controls, indicative of a normal nutritional status. Control subjects who smoked also had spirometry and chest radiographs performed. The median FEV1 and FVC were also normal in these smoking control subjects (FEV1 = 93% of predicted [79–104%] and FVC 86% predicted [75–100%]).
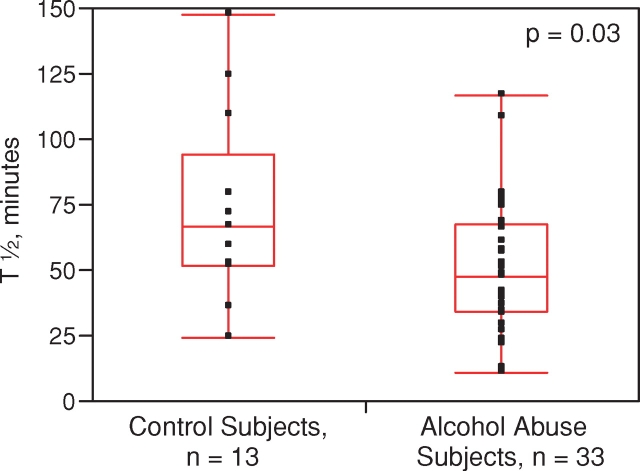
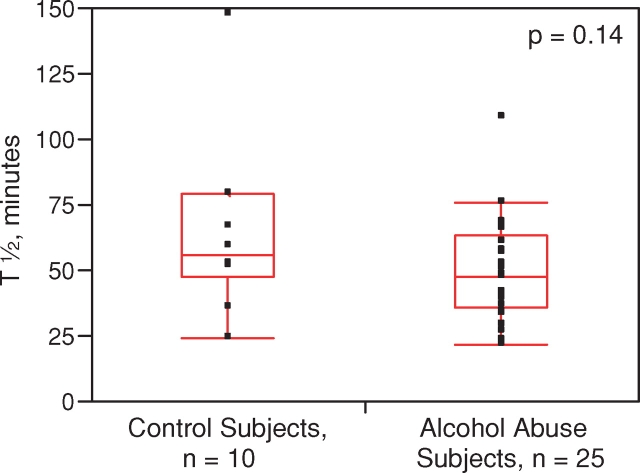
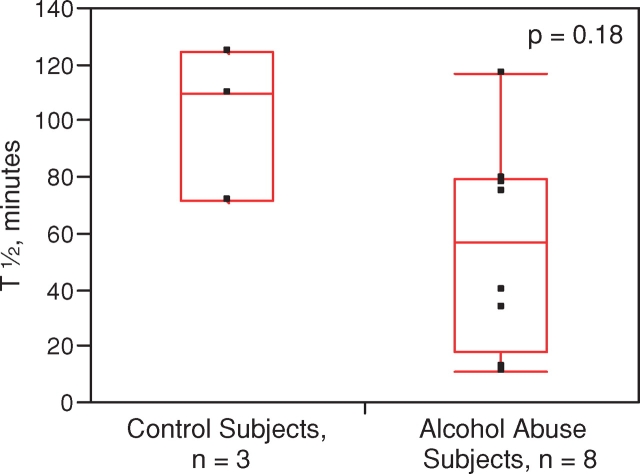
All of the 46 individuals tolerated the 99mTc–DTPA clearance test with no reported adverse events The median half-life was significantly less in the individuals with alcohol dependence (48 min [34–68]) compared to the smoking- and gender-matched controls (67 min [53–95]), indicative of increased alveolar-capillary permeability (P = 0.03) (Fig. 1). The statistical power of this finding was 70.2% utilizing an alpha of 0.05. We also performed a subgroup analysis of 99mTc–DTPA clearance stratified by smoking status. In the smoking subgroup (n = 35), the 99mTc–DTPA clearance was shorter in the alcoholic smoking cohort (n = 25) with a median half-life of 48 min [36–63] as compared to the smoking control group (n = 10) where the median half-life was 56 min [48–80], although this difference did not reach statistical significance (P = 0.14, Fig. 2). In the non-smoking subgroup (n = 11), the 99mTc–DTPA clearance was shorter in the alcoholic non-smoking cohort (n = 8) (58 min [18–80]) as compared to the non-smoking control group (n = 3) (110 min [72–125], though again, this difference did not reach statistical significance (P = 0.18, Fig. 3). In control subjects, abstinence from tobacco abuse for 24 h should lead to non-significant differences in the half-life of 99mTc–DTPA between smokers and non-smokers. Indeed, when the tracer's half-life in smoking controls (who should have been abstinent from tobacco prior to the test, n = 8) was compared to that of non-smoking controls (n = 3), no significant differences were observed (P = 0.11). Similarly, when the tracer's half-life between smoking (n = 25) and non-smoking (n = 8) alcoholic subjects was compared, there were no statistical differences (P = 0.72).
Fig. 1.
Measured half-life of 99mTc–DTPA tracer in lungs of subjects with alcohol abuse and age- and smoking-matched controls. Both smokers and non-smokers are included in this analysis. Middle of boxplot is the median, while bottom and top represent the 25th and 75th percentile of values, respectively. Whiskers represent the 5th and 95th percentile of values. This figure appears in colour in the online version of Alcohol and Alcoholism.
Fig. 2.
Measured half-life of 99mTc–DTPA tracer in lungs of subjects with alcohol abuse and controls who are smokers. Middle of boxplot is the median, while bottom and top represent the 25th and 75th percentile of values, respectively. Whiskers (where present) represent the 5th and 95th percentile of values. This figure appears in colour in the online version of Alcohol and Alcoholism.
Fig. 3.
Measured half-life of 99mTc–DTPA tracer in lungs of subjects with alcohol abuse and controls who are non-smokers. Middle of boxplot is the median, while bottom and top represent the 25th and 75th percentile of values, respectively. Whiskers (where present) represent the 5th and 95th percentile of values. This figure appears in colour in the online version of Alcohol and Alcoholism.
Discussion
In order to further explore the effects of alcohol abuse on alveolar-capillary barrier function, we use inhaled 99mTc–DTPA to provide an in vivo assessment of alveolar-capillary permeability. This study demonstrates that individuals with a history of alcohol abuse have a significantly shorter half-life of 99mTc–DTPA present within the lung, suggestive of increased alveolar-capillary permeability.
Radioaerosols delivered via nebulization have been used for many years to examine the blood–gas barrier of the lung and also in ventilation–perfusion testing to assess the presence of pulmonary embolism. The clearance of these aerosols depends on their size, lipophilicity and the permeability of the epithelium, with lipophilic compounds crossing the alveolar-capillary barrier much more quickly than hydrophilic ones (O’Doherty and Peters, 1997). 99mTc–DTPA is a small, hydrophilic solute that was initially studied in patients with interstitial lung disease. However, its clearance was noted to be significantly increased within the lungs of smokers who were otherwise healthy and even in those subjected to second-hand smoke (Jones et al., 1983; Beadsmoore et al., 2007). These smoking-related changes resolved after 24 h of smoking cessation. Therefore, we group matched our subjects with a history of alcohol abuse with controls on the basis of smoking history, and all subjects did not smoke during the 24 h prior to their study.
Alcohol abuse has been demonstrated to affect the permeability in a variety of organ systems. For example, both acute and chronic alcohol consumption has been linked with abnormal intestinal permeability and alterations in the mucosal barrier of the gut (Persson, 1991; Bjarnason et al., 1995). In addition, alcohol appears to alter normal permeability of the blood–brain barrier, perhaps through disruption of tight junctions normally found between brain endothelial cells (Haorah et al., 2005; Shiu et al., 2007). Alcohol in varying concentrations has also been reported to affect the permeability of the oral mucosa (Squier et al., 2003) that may contribute to the frequent oral and upper gastrointestinal tract pathology observed among those who both drink alcohol and smoke. Alcohol in this situation may act to inhibit the first-pass hepatic metabolism of carcinogens in cigarette smoke (Castonguay et al., 1984), thereby enhancing their ill effects. Alternatively, alcohol itself acts locally to increase the permeability of the mucosal barrier to carcinogens in cigarettes (Lieber et al., 1979).
Earlier work by our group has explored the effects of alcohol abuse on the alveolar-capillary barrier function in the lung using different techniques. Individuals with a history of alcohol abuse had increased protein concentrations in their bronchoalveolar lavage fluid when compared to age- and smoking-matched controls. After a 7-day period of abstinence, the protein concentrations remained abnormally elevated in the individuals with a history of alcohol abuse (Burnham et al., 2003). A history of alcohol abuse is also associated with an increased accumulation of extravascular lung water in patients at risk for the development of ARDS. In this study of 29 critically ill patients, the quantity of extravascular lung water was found to be almost twice as great among those who abused alcohol compared to those who did not (Martin et al., 2005). The data from this current study support these earlier results and provide in vivo evidence that the permeability of the lung in those who abuse alcohol is abnormal, even without overt, symptomatic lung disease.
Our work is not without limitations. When we stratified our subjects into smokers and non-smokers, although there was a trend for those with a history of alcohol abuse to have a shorter half-life of tracer present within the lung for each comparison, this did not achieve statistical significance. With a larger number of research subjects, more notable differences might have been observed, and our results would be strengthened. This is supported by our findings in the comparison of all alcohol-consuming subjects (smokers and non-smokers) to control subjects being statistically different and having a reasonable power (∼70%), despite the overall small sample size. A smaller percentage of non-smoking subjects with alcohol abuse is consistent with what is typically observed among this population; therefore, we focused our efforts in recruitment primarily on enrolling those who abused alcohol as well as smoked cigarettes. Our subjects and controls were admitted as outpatients to our clinical research area; therefore, compliance with not smoking cigarettes for 24 h prior to their nuclear medicine exam was possibly not 100%, and this may have affected our permeability results. However, the necessity to admit patients for a 24-h period or to obtain a urine cotinine test immediately prior to the study was not practical. Further, if the smoking subjects and controls were not compliant with abstaining from tobacco, they likely would have been so in equal proportions, and the effect would therefore cancel itself out between the two groups. Examining the control and alcoholic groups separately, comparing the 99 mTc–DTPA clearance between smokers and non-smokers in each group revealed no significant differences in clearance, a fact that strengthens our observation of differences between alcoholics and non-alcoholics. Nevertheless, matching our alcoholic subjects and controls on smoking was an important safeguard, since we were not observing them directly prior to performance of the study.
This study adds to the growing body of evidence examining the mechanism by which alcohol abuse increases susceptibility for the development of ARDS. Previous studies have suggested that abnormal oxidative stress is operative in the pathway of increased permeability in both the lung and gastrointestinal tract. Alcohol dehydrogenase (ADH) is a main determinant in alcohol elimination, where its presence results in the transformation of nicotinamide adenine dinucleotide (NAD) to its reduced form, NADH. NADH is in turn operative in the generation of reactive oxygen species (Lieber, 1997). The tripeptide glutathione is among the most important and substantive antioxidants within the lungs of normal humans. However, its pulmonary concentration in individuals with alcohol abuse is significantly decreased (Moss et al., 2000). The correction of an abnormal oxidative milieu within the lung using thiol-containing compounds, such as glutathione and procysteine, normalizes alveolar-capillary permeability (Guidot et al., 2000). Therefore, antioxidant replacement therapy may improve alveolar-capillary barrier function in individuals with a history of alcohol abuse.
In conclusion, subjects with alcohol abuse appear to have an increase in alveolar-capillary permeability as measured by inhaled radionuclide techniques when compared to age- and smoking-matched controls. That alcohol abuse would have effects on permeability of the lung is not surprising, given its reported effects on the permeability of other organ systems, but bears further investigation as the clinical effects of this enhanced lung permeability are not clear. It is not inconceivable that it might have an effect on the development of ARDS through its impact on fluid resorption within the lung. Further investigations are required to establish this mechanism in the causal pathway of ARDS.
Acknowledgments
This work was funded by R01-AA014435 and K23-AA13918.
References
- Anderson R. Deaths: leading causes for 1999. Natl Vital Stat Rep. 2001;49:6–13. [PubMed] [Google Scholar]
- Beadsmoore C, Cheow HK, Szczepura K, et al. Healthy passive cigarette smokers have increased pulmonary alveolar permeability. Nucl Med Commun. 2007;28:75–7. doi: 10.1097/MNM.0b013e328013eb1e. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, et al. The American–European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Macpherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1556–81. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Braude S, Nolop KB, Hughes JM, et al. Comparison of lung vascular and epithelial permeability indices in the adult respiratory distress syndrome. Am Rev Respir Dis. 1986;133:1002–5. doi: 10.1164/arrd.1986.133.6.1002. [DOI] [PubMed] [Google Scholar]
- Burnham EL, Brown LA, Halls L, et al. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res. 2003;27:1167–72. doi: 10.1097/01.ALC.0000075821.34270.98. [DOI] [PubMed] [Google Scholar]
- Castonguay A, Rivenson A, Trushin N, et al. Effects of chronic ethanol consumption on the metabolism and carcinogenicity of N-nitrosonornicotine in F344 rats. Cancer Res. 1984;44:2285–90. [PubMed] [Google Scholar]
- CDC. Reduction in alcohol-related traffic accidents—United States 1990–1992. Mortal Morb Wkly Rep. 1993;42:905–6. [PubMed] [Google Scholar]
- Detsky AS, Baker JP, Mendelson RA, et al. Evaluating the accuracy of nutritional assessment techniques applied to hospitalized patients: methodology and comparisons. JPEN J Parenter Enteral Nutr. 1984;8:153–9. doi: 10.1177/0148607184008002153. [DOI] [PubMed] [Google Scholar]
- Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- Guidot DM, Modelska K, Lois M, et al. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–35. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- Haorah J, Heilman D, Knipe B, et al. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res. 2005;29:999–1009. doi: 10.1097/01.alc.0000166944.79914.0a. [DOI] [PubMed] [Google Scholar]
- Jones JG, Royston D, Minty BD. Changes in alveolar-capillary barrier function in animals and humans. Am Rev Respir Dis. 1983;127(5 Pt 2):S51–9. [PubMed] [Google Scholar]
- Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Seitz HK, Garro AJ, et al. Alcohol-related diseases and carcinogenesis. Cancer Res. 1979;39:2863–86. [PubMed] [Google Scholar]
- Martin GS, Eaton S, Mealer M, et al. Extravascular lung water in patients with severe sepsis: a prospective cohort study. Crit Care. 2005;9:74–82. doi: 10.1186/cc3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GR, Uszler JM, Effros RM, et al. Rapidly reversible alterations of pulmonary epithelial permeability induced by smoking. Chest. 1983;83:6–11. doi: 10.1378/chest.83.1.6. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, et al. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. J Am Med Assoc. 1996;275:50–4. [PubMed] [Google Scholar]
- Moss M, Guidot DM, Wong-Lambertina M, et al. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–9. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–77. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- O’Doherty MJ, Peters AM. Pulmonary technetium-99 m diethylene triamine penta-acetic acid aerosol clearance as an index of lung injury. Eur J Nucl Med. 1997;24:81–7. doi: 10.1007/BF01728316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J. Alcohol and the small intestine. Scand J Gastroenterol. 1991;26:3–15. doi: 10.3109/00365529108996478. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res. 2002;26:272–9. [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Schiodt FV, Rochling FA, Casey DL, et al. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–7. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van RL. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36:117–26. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Shiu C, Barbier E, Di Cello F, et al. HIV-1 gp120 as well as alcohol affect blood-brain barrier permeability and stress fiber formation: involvement of reactive oxygen species. Alcohol Clin Exp Res. 2007;31:130–7. doi: 10.1111/j.1530-0277.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- Squier CA, Kremer MJ, Wertz MJ, et al. Effect of ethanol on lipid metabolism and epithelial permeability barrier of skin and oral mucosa in the rat. J Oral Pathol Med. 2003;32:525–9. doi: 10.1034/j.1600-0714.2003.00198.x. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]