Abstract
Background
Functional dependence and the risks of disability increase with age. The loss of independence is thought to be partially due to a decrease in physical activity. However, in populations, accurate measurement of physical activity is challenging and may not provide information on functional impairment.
Methods
This study therefore assessed physical functionality and physical activity level in a group of nonagenarians (11 men/11 women; 93 ± 1 years, 66.6 ± 2.4 kg, body mass index [BMI] = 24 ± 1 kg/m2) and a group of participants aged 60–74 years (17 men/15 women; 70 ± 1 years, 83.3 ± 3.0 kg, BMI = 29 ± 1 kg/m2) from the Louisiana Healthy Aging Study. Physical activity level was calculated from total energy expenditure (TEE) and resting metabolic rate (RMR). Physical functionality was assessed using the Reduced Continuous Scale Physical Functional Performance Test (CS-PFP10).
Results
Nonagenarians had lower absolute ( p < .001) and adjusted ( p < .007) TEE compared to participants aged 60–74 years which was attributed to a reduction in both RMR and physical activity level. Nonagenarians also had reduced functional performance ( p < .001) which was correlated with activity level (r = 0.68, p < .001).
Conclusions
When compared to individuals aged 60–74 years, 73% of the reduction in TEE in nonagenarians can be attributed to a reduction in physical activity level, the remaining being accounted for by a reduction in RMR. The reduced physical activity in nonagenarians is associated with less physical functionality. This study provides the first objective comparison of physical functionality and actual levels of physical activity in older individuals.
Functional dependence and the risks of disability increase with age. In 2003, more than one third of the older population aged > 65 years and 64% of individuals aged 85 years or older reported limitations in activities of daily life (1). The loss of independence is thought to be partially due to a decrease in physical activity; however, accurate measurements of physical activity are challenging in large populations (2,3). More importantly, the assessment of functionality in older, especially very old individuals (≥90 years) may provide more specific information on the severity of impairment as well as steps for the prevention and treatment of age-related disability.
One of the primary goals of gerontology research is the maintenance of health and independence in older adults, both of which are influenced by an individual’s physical activity level (PAL) (4,5). Doubly labeled water (DLW) is considered the reference method for the measurement of total energy expenditure (TEE) under free-living conditions (6). DLW studies not only allow the calculation of activity energy expenditure (AEE) by difference between TEE, the sum of resting metabolic rate (RMR), and the thermic effect of food (TEF), but also provide an objective measure of physical activity over periods of 1–2 weeks. While physical activity level is an important determinant of an individual’s health; it may be more important to know an elderly individual’s functional status and whether it is related to the actual current level of physical activity.
Tests of physical function in older individuals have gained popularity as alternatives to traditional laboratory measures of physical fitness (7). These tests have a number of advantages because they evaluate the ability to perform activities of daily living, provide more information on functional impairment, are less susceptible to floor and ceiling effects, and are applicable to a wider range of age and fitness levels than are more traditional fitness tests (7–9). The Reduced Continuous Scale-Physical Function Performance Test (CS-PFP10) is an adaptation of the full CS-PFP designed by Cress and colleagues (9) and is used in several physical domains and applied to a broad spectrum of abilities. The test has been validated against other measures of physical fitness including maximal oxygen consumption and measures of upper and lower body strength (9). However, whether the CS-PFP10 physical functionality score is associated with the level of physical activity in free-living conditions is currently unknown.
Therefore, the purpose of this study was to examine if there is a relationship in older individuals between physical functionality assessed by the CS-PFP10 and PAL estimated by DLW and RMR. A secondary aim was to examine whether nonagenarians were less functionally independent than aged (60- to 74-year-old) individuals and whether this dependence was related to a reduced level of physical activity.
METHODS
Participant Sampling and Recruitment
The participants included in this analysis represent a subset from a population-based study known as The Louisiana Healthy Aging Study (LHAS). The overall aim of the LHAS is to determine whether characteristics of an individual’s metabolism predispose to long (or not so long) life with the retention of physical and cognitive functionality associated with healthy aging. The total number of persons targeted in the overall project, which was related to the genetics of aging and longevity, was 877 with > 250 older than 90 years. After individuals are screened for the main project, eligibility is then determined for other projects including the present study and studies of cognitive functionality. Participant sampling was performed via random selection based on voter registration lists and The Medicare Beneficiary Enrollment Data File from the Center of Medicare and Medicaid Services. Method of recruitment included mail-outs, follow-up phone calls, and (in the case of the nonagenarians), a home visit made by members of the investigative team to explain the study in detail. The present study is part of a National Institutes of Health (NIH)-sponsored Program Project with two groups of older adults included—that is, those aged 60–74 years and ≥90 years. According to study design and the above eligibility criteria, 54 individuals were available for data analysis.
For this study, individuals were excluded if they had unstable cardiovascular disease, had a myocardial infarction, or cerebrovascular accident in the last 3 months; severe high blood pressure; blood vessel aneurysm; taking certain medications used for myasthenia gravis; have uncontrolled asthma, an asthma-like condition, or emphysema and/or chronic obstructive pulmonary disorder; thyroid disease; mental health problems requiring drug treatment; or a Mini-Mental State Examination Score < 23. Blindness was the only functional exclusion criterion. Participants provided written informed consent. The Institutional Review Boards at the Pennington Biomedical Research Center and at Louisiana State University approved the study.
DLW
Upon arriving at the Pennington Center, each participant provided a baseline urine sample and were then dosed with a mixture of 2.0 g of 10% enriched H2 18O and 0.12 g of 99% enriched 2H2O per kg of estimated total body water (55% of body weight) (Cambridge Isotopes, Cambridge, MA). The dose was followed by 100 mL of tap water used to rinse the dose container. Two more urine samples were collected at 4.5 and 6 hours after dosing. On the mornings of days 13 and 14, participants were instructed to discard their first morning urine and collect the second urine of the day. Samples were kept refrigerated in airtight containers and were picked up by the study staff. The 18O abundance was measured in duplicate on a Finnigan MAT 252 dual inlet Gas Isotope Ratio Mass Spectrometer (10). The 2H2 abundance was also measured in duplicate on the same Isotope Ratio Mass Spectrometer using a Finnigan H/D device (10). The enrichments of the postdose samples were compared to the enrichment of the baseline sample. The 2H and 18O isotope elimination rates (kD and kO) were calculated using linear regression following a log transformation. Total body water (N) was determined at enrichment time 0, obtained from the regression line of the H2 18O isotope. The rate of CO2 production was calculated using the equations of Schoeller and colleagues (11) and were later modified (12) as follows (11,12):
where rCO2 is the rate of carbon dioxide production; N is total body water calculated from NO/1.007; kO and kD represent the elimination rates of oxygen-18 and deuterium, respectively; and rGF is the rate of fractionated gaseous evaporative water loss, which is estimated to be 1.05N (1.007kO − 1.041kD). TEE was calculated as follows:
where RQ is the estimated respiratory quotient (estimated to be 0.88). The energy equivalent of CO2 (EeqCO2) was therefore 5.637 kcal/L CO2.
RMR
RMR was measured in the fasting condition using a Deltratrac II metabolic cart (Sensormedics, Yorba Linda, CA). The participants were required to rest for 30 minutes before the start of the test. Oxygen consumption and carbon dioxide production was measured for 30 minutes, and the last 20 minutes were used to calculate RMR. The cart was calibrated before each test using room air and a known calibration gas concentration (96% oxygen and 4% carbon dioxide).
PAL
AEE was determined by the following equation (13):
This approach assumes that the TEF is 10% of TEE (14).
The level of physical activity often referred to as PAL was calculated as the ratio of TEE/RMR. To avoid potential bias by using a ratio, PAL was also calculated using linear regression analysis to adjust TEE for RMR (15).
Body Composition
Body weight was measured in a hospital gown with an electronic scale (Detecto, Webb City, MO) that was checked daily with a standard 25-kg weight. Height was measured with a wall-mounted stadiometer (Holtain, Crymych, Dyfed, U.K.), and body mass index was calculated as mass/height2 (kg/m2). Body composition was measured by dual-energy x-ray absorptiometry (DEXA) (QDA 4500A; Hologics, Bedford, MA).
CS-PFP10
Participants performed the CS-PFP10 developed by Cress and colleagues (9). Briefly, the CS-PFP10 involves the performance of 10 tasks representing activities of daily living. Participants are instructed to perform the activities as quickly, but as comfortably as possible. Participants are allowed to use assistant devices such as a cane; however, no one in the current study requested the use of one. The test includes the following:
Weight carry test: Participant will carry two 5-lb sand-bags from one counter to another counter approximately 63 inches away.
Jacket test: Participant will put on a light windbreaker and zip the jacket completely.
Scarves test: Participant will pick up four scarves, one at a time, from the floor.
Reach test: Participant will reach as high as possible and place a sponge on a shelf, which is an 8-foot-high adjustable shelf mounted on the wall.
Floor sweep test: Participant will sweep up a ½ cup of kitty litter in a 4 × 3 block square.
Laundry test 1: Participant will empty clothes from a top-loading washer into a front-loading dryer.
Laundry test 2: Participant will unload a dryer and then place two sandbags into a laundry basket and move the basket to the cabinet, 36 inches high adjacent to the dryer.
Floor down/up test: Participants will start in the standing position and then sit down on the floor and then immediately stand up finishing with their arms at their side.
Stair climb test: Participants will climb one flight of stairs, 9–11 steps, 12 inches in depth, 6.5 inches high.
Grocery test: Participant will carry a comfortable amount of groceries 16 yards to a set of bus steps, ascend the bus steps, turn around, descend the steps, carry the bag to the door, and open and close the door. The total walking distance is 42 yards, excluding the steps. Participants may make more than one trip, and the maximum weight allowed is 30 lb over two trips. Scores are scaled from 0 to 100 utilizing a formula based on lower and upper extremes of performance from previously tested older adults.
The CS-PFP10 total score is the average score of all of the tests (8).
Statistical Analysis
Data are provided as means ± standard error of the mean (SEM). Statistical analysis was performed using SPSS (version 12; SPSS Inc., Chicago, IL). Multiple regression analysis was used to adjust TEE for fat-free mass (FFM), fat mass (FM), and sex in one model and for RMR in another model. The residual values represent the difference between predicted and measured values. Procedures for t tests were used to assess differences between the groups. Spearman correlation coefficients were run to assess relationships between measurements of physical activity.
RESULTS
Body Composition
A total of 54 participants were included in the analysis. Participant characteristics by age group and sex are displayed in Table 1. Nonagenarians weighed significantly less (p < .001) and had significantly less FFM (p < .005) and FM (p < .001) than did participants aged 60–74 years. As expected, in both groups, men were taller and weighed more than women (both, p = .05), and women had a higher percent body fat with less FFM than men (both, p < .05), but similar FM.
Table 1.
Participant Characteristics
| 60–74 Years Old | ≥90 Years Old | ||||
|---|---|---|---|---|---|
| Age | |||||
| Men | Women | Men | Women | Effect | |
| Characteristic | (N = 17) | (N = 15) | (N = 11) | (N = 11) | p Value |
| Age, y | 71 ± 1 | 70 ± 1 | 93 ± 1 | 93 ± 1 | — |
| Height, cm | 176 ± 1 | 159 ± 1* | 169 ± 1 | 158 ± 1* | .120 |
| Weight, kg | 90 ± 3 | 76 ± 5* | 71 ± 2 | 60 ± 4* | .001 |
| BMI, kg/m2 | 28 ± 1 | 30 ± 2 | 25 ± 1 | 24 ± 1 | .001 |
| Percent fat mass, % | 28 ± 1 | 41 ± 1* | 26 ± 2 | 35 ± 2* | .111 |
| Fat-free mass, kg | 64 ± 2 | 45 ± 2* | 53 ± 1 | 38 ± 2* | .005 |
| Fat mass, kg | 25 ± 2 | 32 ± 1* | 18 ± 1 | 21 ± 2 | .001 |
Notes: All values are mean ± standard error of the mean.
Sex difference p < .05.
BMI = body mass index.
Energy Expenditure and PAL
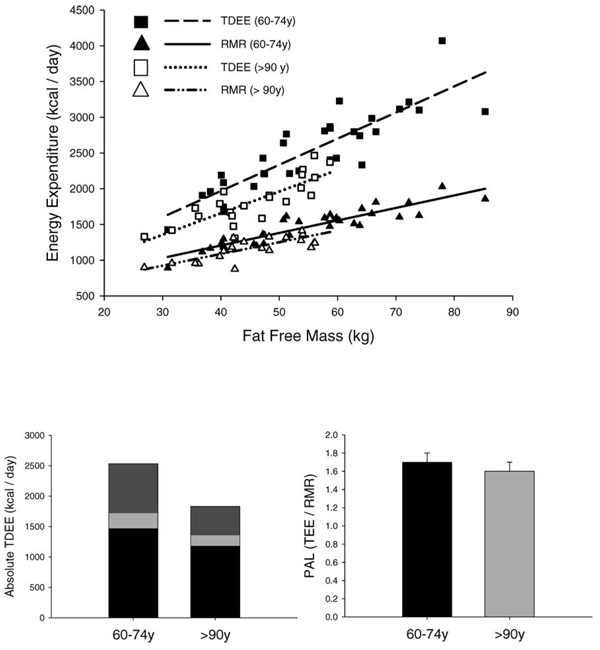
Nonagenarians had significantly lower RMR (p < .001) and TEE (p < .001) than individuals aged 60–74 y that were independent of differences in FFM, FM, and sex (Table 2 and Figure 1, top). After adjustment for FFM, FM, and sex, nonagenarians had a daily TEE that was 267 kcal/d lower than that of participants aged 60–74 years (p < .001). Similar results were noted when TEE was adjusted for differences in RMR (an indicator of metabolic body size) by regression analysis (data not shown). RMR in the nonagenarians was 72 kcal/d (p < .001) lower than that in individuals aged 60–74 years independent of differences in body weight and body composition; this finding accounted for 27% of the lower TEE in the nonagenarians (Figure 1, bottom left).
Table 2.
Energy Expenditure by Age Group
| 60–74 Years Old | ≥90 Years Old | ||||
|---|---|---|---|---|---|
| Age | |||||
| Energy | Men | Women | Men | Women | Effect |
| Expenditure | (N = 17) | (N = 15) | (N = 11) | (N = 11) | p Value |
| RMR (kcal/d) | 1627 ± 38 | 1297 ± 58 | 1296 ± 39 | 1066 ± 45 | .001 |
| TEE (kcal/d) | 2852 ± 112 | 2293 ± 176* | 2052 ± 80 | 1608 ± 62* | .001 |
| TEE (kcal/d)† | 88 ± 85 | 159 ± 149 | −136 ± 47 | −217 ± 78 | .007 |
| AEE (kcal/d) | 940 ± 80 | 767 ± 144 | 551 ± 59 | 381 ± 54 | .001 |
Notes: All values are mean ± standard error of the mean.
Sex difference p < .05.
Expressed as residual values (measured minus predicted value of the mean of the entire group). Adjusted for fat-free mass, fat mass, and sex.
RMR = resting metabolic rate; TEE = total energy expenditure; AEE = activity energy expenditure.
Figure 1.
Top: Relationship between total energy expenditure (TDEE) and resting metabolic rate (RMR) with fat-free mass. Closed squares: 60- to 74-year-old Total Daily Energy Expenditure; open squares: ≥ 90-year-old TDEE. Closed triangles: 60– to 74-year-old RMR; open triangles: ≥ 90-year-old RMR. Dashed line: Regression line of TEE in the 60- to 74-year-olds (r=.78, p < .001). Solid line: Regression line of resting metabolic rate in 60- to 74-year-olds (r=0.90, p < .001). Dotted line: Regression line of TEE in ≥90-year-olds. Dashed/dotted line: Regression line RMR ≥90-year-olds. Bottom left: TEE and its components. TEF, thermic effect of food. Black bar=RMR; light gray bar=TEF; dark gray bar=AEE. Both RMR (p < .001) and AEE (p < .001) were significantly lower in the nonagenarians. Bottom right: Physical activity level (PAL) in the two groups. PAL is a ratio; no units are assigned to it. PAL was significantly (p < .005) lower in the nonagenarians.
The energy cost of physical activity (AEE) was significantly lower in the nonagenarians (p < .001, Figure 1, bottom left). Adjusted AEE was 159 kcal/d lower in the nonagenarians; this finding accounted for 73% of the lower TEE. PAL was also significantly lower (60- to 74-year-olds, p < .005, Figure 1, bottom right) in the nonagenarians compared to the participants aged 60–74 years.
CS-PFP10
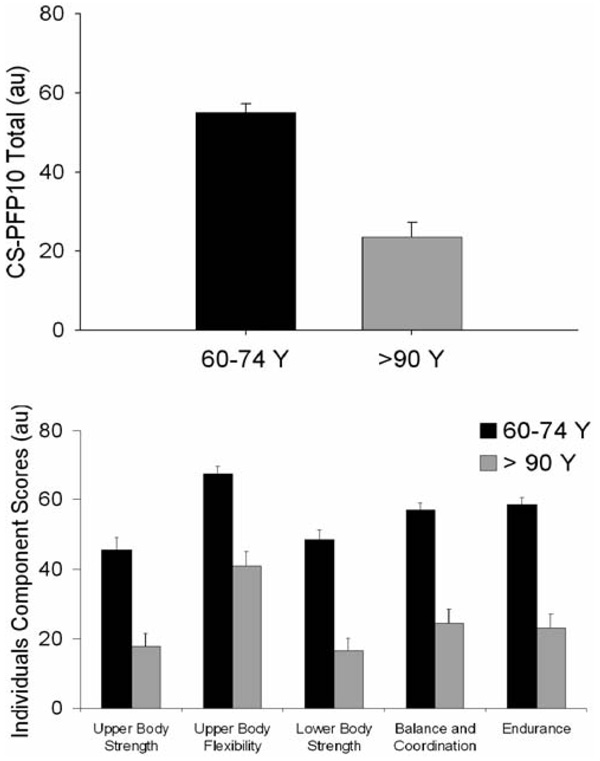
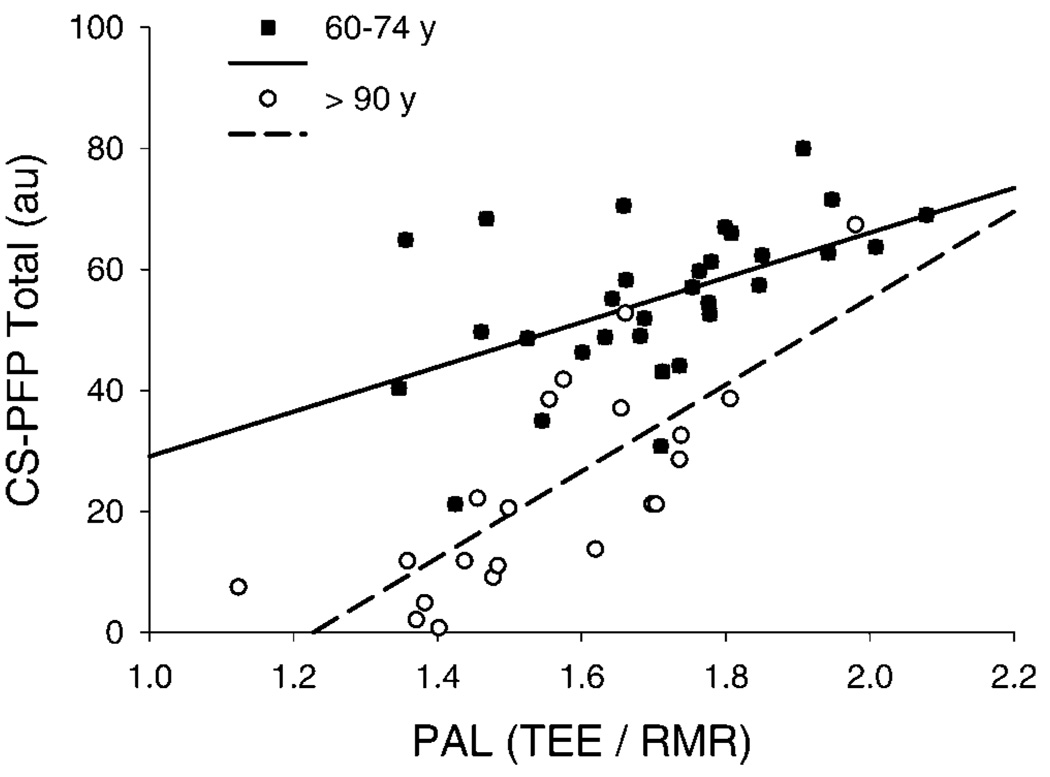
As expected, nonagenarians scored significantly lower on all aspects of the CS-PFP10 than the individuals aged 60–74 years (all, p < .001; Figure 2). In addition, the CS-PFP10 total score was highly correlated with PAL (r = 0.68, p < .001; Figure 3) and persisted when analyzing the nonagenarians (r = 0.78, p < .003) or the 60- to 74-year-old individuals (r = 0.52, p < .002) separately. There was also a significant relationship between CS-PFP10 score and TEE adjusted for FFM, FM, and sex (r=0.35, p < .009; data not shown), and this remained significant when analyzing the individuals aged 60–74 years (r = 0.52, p <.002) and the nonagenarians only (r = 0.61, p < .004).
Figure 2.
Physical functionality in the two groups. Top: Total score. Bottom: Individual components. The nonagenarians were significantly (all, p < .001) less functional than individuals aged 60 to 74 years. CS-PFP10 = Continuous Scale Physical Functional Performance Test.
Figure 3.
Top: Relationship between Continuous Scale Physical Functional Performance Test (CS-PFP10) score and physical activity level (PAL) (r=0.68, p < .001). The relationship between the CS-PFP10 and PAL was also significant when analyzing the nonagenarians (r=0.78, p < .003) and aged individuals (r= 0.52, p < .002) separately. TEE = total energy expenditure; RMR = resting metabolic rate.
DISCUSSION
To our knowledge, the Louisiana Healthy Aging Study is the first of its kind to examine the relationship between functional performance and levels of physical activity (PAL and AEE) in a group of elderly individuals. The results of this study support previous findings that both absolute TEE and TEE adjusted for body weight and body composition are lower in nonagenarians when compared to individuals aged 60–74 years and is attributed to a decrease in both RMR and PAL (16). The present study also demonstrates that nonagenarians have impaired functional performance compared to younger individuals and that this is associated with reduced physical activity. These findings in nonagenarians, a model of ‘‘successful aging,’’ imply that some degree of functional impairment is part of the natural aging process, but maintenance of physical activity may help to delay this age-related decline. Finally, this study provides for the first time evidence that the CS-PFP10 is associated with the level of free-living physical activity in elderly individuals.
Aging is associated with an increased risk of frailty, a dependent state of reduced physiological reserves characterized by generalized weakness, increased risk of fractures, impaired mobility, and poor endurance (17–20). Although most elderly individuals die from age-related illnesses such as cardiovascular disease and cancer, many of the oldest old, having escaped these age-related diseases will suffer from frailty, impaired physical function, and an inability to live independently (19). Physical activity helps to maintain strength and endurance and may be beneficial in the maintenance of functional independence (21–23). Physical function tests have gained popularity because they provide more specific information on the level of independence than do typical laboratory measures of aerobic fitness. In the current study, nonagenarians were less physically functional and less physically active than were participants 20 years younger. However, this relationship does not imply cause and effect but suggests the possibility of using the CS-PFP10 as an alternative test when DLW is not feasible (cost, length of measurement). Previous intervention studies have shown, however, that structured exercise results in significant improvement in functionality, and that detraining results in opposite changes (5,24–26). In the present study, PAL was lower in nonagenarians, accounting for approximately 60% of the decline in TEE. However, since PAL is the ratio between TEE and RMR, it can suffer from previously described artifacts (15). We therefore also confirmed the association between functionality and activity by adjusting TEE for RMR as an index of activity.
There are a number of factors that contribute to an individual’s PAL, including illness and disease as well as psychological and environmental factors. It was impossible in our study to determine the contribution of each of these to an individual’s activity status. However, study participants had to be well enough to come to the testing facility; this fact excluded individuals who were too ill to participate. It is also possible that some extremely old individuals choose not to engage in a more active lifestyle for reasons other than inability or infirmity, which may have been the case in the current study (27,28). Therefore, assessment of lifelong instead of present PAL may provide more information on the effect of physical activity on health and independence (29). Importantly, only longitudinal studies will provide more specific information on the changes in physical activity patterns and physical functionality over time (30).
Conclusion
This study is the first to demonstrate a relationship between physical functionality and PAL in older individuals. In addition, TEE is lower in nonagenarians independent of differences in body weight and body composition, and this is attributed to a lower RMR (≈70%) and reduced physical activity (≈73%). Furthermore, nonagenarians had reduced physical activity, which may lead to a deficit in physical functionality.
ACKNOWLEDGMENTS
This work was supported by the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund [HEF (2001-06) 02] and by the National Institute on Aging (PO1 AGO22064).
We acknowledge and thank everyone working on the Louisiana Healthy Aging Study from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge; Louisiana State University Health Sciences Center, New Orleans; and University of Alabama at Birmingham. We thank S. Michal Jazwinski, PhD, Mark Batzer, PhD, Pauline Callinan, Jerilyn Walker, Scott W. Herke, DeQuindra Rouzan, Jennifer Arceneaux, RN, Andrew Pellett, PhD, Henry Rothschild, MD, PhD, Crystal Traylor, APRN, MSN, WHNP, David Welsh, MD, Joseph Su, PhD, Yu-wen Chiu, Elizabeth Fontham, PhD, Cruz Velasco-Gonzalez, PhD, Jennifer Hayden, Matthew Leblanc, Li Li, MD, Sangkyu Kim, Hui-Yi Lin, PhD, Beth Schmidt, Jessi Thomspon, PhD, Valentina Greco, PhD, Beth Kimball, Meghan Allen, Donald Scott, PhD, John Mountz, PhD, MD, Hui-Chen Hsu, PhD, Pili Zhang, Kim Pederson, Juling Zhou, PhD, Tiffany Hall, Kim Landry, Mandy Shipp, Anita Smith, Laurie Byerley, PhD, James P DeLany, PhD, Robert Schwartz, PhD, Evest Broussard, Michael Businelle, Paula Geiselman, PhD, Darla Kendzor, Vijay Hegde, PhD, Robert Wood, PhD, Michael Welsch, PhD, Iina E. Antikainen, Fernanada Holton, Carl Lavie, MD, Artie Brown, Ryan Russell, Devon Dobrosielski, Arturo Ace, Katie Cherry, PhD, Karri Hawley, PhD, Emily Olinde, Jenny Denver, and Kay lopez, DSN.
REFERENCES
- 1.National Center for Health Statistics. Hyattsville, MD: U.S. Government Printing Office; Health, United States, 2005. 2005
- 2.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 3.Washburn RA, Janney CA, Fenster JR. The validity of objective physical activity monitoring in older individuals. Res Q Exerc Sport. 1990;61:114–117. doi: 10.1080/02701367.1990.10607489. [DOI] [PubMed] [Google Scholar]
- 4.Miszko TA, Cress ME, Slade JM, Covey CT, Agrawal SK, Doerr CT. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol Biol Sci Med Sci. 2003;58A:171–175. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- 5.Cress ME, Buchner DM, Questad KS, Esselman PC, DeLateur BJ, Schwartz RS. Exercise: effects on physical functional performance in independent older adults. J Gerontol Med Sci. 1999;54A:M242–M248. doi: 10.1093/gerona/54.5.m242. [DOI] [PubMed] [Google Scholar]
- 6.Schoeller DA, van Santen E. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol. 1982;53:955–959. doi: 10.1152/jappl.1982.53.4.955. [DOI] [PubMed] [Google Scholar]
- 7.Wood RH, Hondzinski JM, Lee CM. Evidence of an association among age-related changes in physical, psychomotor and autonomic function. Age Ageing. 2003;32:415–421. doi: 10.1093/ageing/32.4.415. [DOI] [PubMed] [Google Scholar]
- 8.Cress ME, Petrella JK, Moore TL, Schenkman ML. Continuous-scale physical functional performance test: validity, reliability, and sensitivity of data for the short version. Phys Ther. 2005;85:323–335. [PubMed] [Google Scholar]
- 9.Cress ME, Buchner DM, Questad KS, Esselman PC, DeLateur BJ, Schwartz RS. Continuous-scale physical functional performance in healthy older adults: a validation study. Arch Phys Med Rehabil. 1996;77:1243–1250. doi: 10.1016/s0003-9993(96)90187-2. [DOI] [PubMed] [Google Scholar]
- 10.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol. 1989;67:1922–1929. doi: 10.1152/jappl.1989.67.5.1922. [DOI] [PubMed] [Google Scholar]
- 11.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118:1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 12.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267(4 Pt 1):E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 13.Rising R, Harper IT, Fontvielle AM, Ferraro RT, Spraul M, Ravussin E. Determinants of total daily energy expenditure: variability in physical activity. Am J Clin Nutr. 1994;59:800–804. doi: 10.1093/ajcn/59.4.800. [DOI] [PubMed] [Google Scholar]
- 14.Ravussin E, Swinburn BA. Energy metabolism. In: Stunkard AJ, Wadden TA, editors. Obesity: Theory and Therapy. New York: Raven Press; 1993. pp. 97–123. [Google Scholar]
- 15.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644–652. [PubMed] [Google Scholar]
- 16.Roberts SB, Fuss P, Heyman MB, Young VR. Influence of age on energy requirements. Am J Clin Nutr. 1995;62 5 Suppl:1053S–1058S. doi: 10.1093/ajcn/62.5.1053S. [DOI] [PubMed] [Google Scholar]
- 17.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 20.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 21.Frontera WR, Hughes VA, Lutz KJ, Evan WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 22.Meredith CN, Frontera WD, Fisher EC, et al. Peripheral effects of endurance training in young and old subjects. J Appl Physiol. 1989;66:2844–2849. doi: 10.1152/jappl.1989.66.6.2844. [DOI] [PubMed] [Google Scholar]
- 23.Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol. 1984;57:1024–1029. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- 24.Ades PA, Savage PD, Cress ME, Brochu M, Lee NM, Poehlman ET. Resistance training on physical performance in disabled older female cardiac patients. Med Sci Sports Exerc. 2003;35:1265–1270. doi: 10.1249/01.MSS.0000079044.21828.0E. [DOI] [PubMed] [Google Scholar]
- 25.Brochu M, Savage P, Lee M, et al. Effects of resistance training on physical function in older disabled women with coronary heart disease. J Appl Physiol. 2002;92:672–678. doi: 10.1152/japplphysiol.00804.2001. [DOI] [PubMed] [Google Scholar]
- 26.Toraman NF, Ayceman N. Effects of six weeks of detraining on retention of functional fitness of old people after nine weeks of multicomponent training. Br J Sports Med. 2005;39:565–568. doi: 10.1136/bjsm.2004.015586. discussion 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg MR, Renne J. Where does walkability matter the most? An environmental justice interpretation of New Jersey data. J Urban Health. 2005;82:90–100. doi: 10.1093/jurban/jti011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks SE, Housemann RA, Brownson RC. Differential correlates of physical activity in urban and rural adults of various socioeconomic backgrounds in the United States. J Epidemiol Community Health. 2003;57:29–35. doi: 10.1136/jech.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillsdon MM, Brunner EJ, Guralnik JM, Marmot MG. Prospective study of physical activity and physical function in early old age. Am J Prev Med. 2005;28:245–250. doi: 10.1016/j.amepre.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Bijnen FC, Feskins EJ, Caspersen CJ, Mosterd WL, Kromhout D. Age, period, and cohort effects on physical activity among elderly men during 10 years of follow-up: the Zutphen Elderly Study. J Gerontol Med Sci. 1998;53A:M235–M241. doi: 10.1093/gerona/53a.3.m235. [DOI] [PubMed] [Google Scholar]