Synopsis
We present here the identification and characterization of a small C-terminal domain (CTD) phosphatase-3 (SCP3) homologue in smooth muscle and show, for the first time, that it dephosphorylates CaMKII. SCP3 is a PP2C type phosphatase, which is primarily expressed in vascular smooth muscle tissues and specifically binds to association domain of the CaMKII gamma G-2 variant. The dephosphorylation is site specific, excluding the Thr287 associated with Ca2+/CaM-independent activation of the kinase. As a result, the autonomous activity of CaMKII gamma G-2 is not affected by the phosphatase activity of SCP3. SCP3 co-localizes with CaMKII gamma G-2 on cytoskeletal filaments, but is excluded from the nucleus in differentiated vascular smooth muscle cells. Upon depolarization induced Ca2+ influx, CaMKII gamma G-2 is activated and dissociates from SCP3. Subsequently, CaMKII gamma G-2 is targeted to cortical adhesion plaques. We show here that SCP3 regulates phosphorylation sites in the catalytic domain but not those involved in regulation of kinase activation. This selective dephosphorylation by SCP3 creates a constitutively active kinase that can then be differentially regulated by other phosphorylation-dependent regulatory mechanisms.
Keywords: Ca2+/ Calmodulin (CaM)-dependent protein kinase II, Small CTD Phosphatase, Vascular Smooth muscle, PP2C, Autophosphorylation
Introduction
Ca2+/ Calmodulin (CaM)-dependent protein kinase II (CaMKII) is a ubiquitous protein that has numerous functions in fundamental cellular processes including metabolism, cell cycle control [1], cell shape variation, gene transcription [2] and the regulation of ion channel and cytoskeletal function [3–5]. CaM kinase II is activated by Ca2+-bound CaM. There are 4 isoforms, alpha, beta, delta and gamma. In vitro studies have shown that CaM kinase II can autophosphorylate at several sites [6, 7]. Thr286 (numbering according to alpha isoform, Thr287 for beta, delta and gamma) is the best explored site and has been linked to the generation of a Ca2+/CaM-independent persistence of kinase activity. There are other known autophosphorylation sites (e.g. Thr253, Ser279, Thr305/306 and Ser314; numbering according to alpha isoform) but the in vivo functions of these sites are less well understood.
Previously, we reported the identification of six variants of CaM kinase II gamma in differentiated vascular smooth muscle (dVSM) [8]. CaMKII gamma G-2 has a novel association domain that is distinct from the common association domain found in all other CaMKII gamma variants. We have previously shown that CaMKII gamma G-2 translocates to cortical adhesion plaques upon activation and regulates contractility of smooth muscle through an ERK-mediated pathway [9].
Phosphorylated CaM kinase II is believed to be regenerated to its unphosphorylated state by the action of phosphatases in the cell. Accordingly, dephosphorylation of Thr286 by phosphatases in neurons has been reported [10]. A PP1 is reported to dephosphorylate CaM kinase II associated with postsynaptic densities, but the soluble form of CaM kinase II is reported to be dephosphorylated by a PP2A [11, 12]. PP1, PP2A [11, 12] and PP2C decrease autonomous activity of CaM kinase II but PP2B does not inhibit autonomous activity [10]. A PP2C has also been reported to dephosphorylate CaM kinase II in vitro [13], but its in vivo function has not been determined. The nature of the relevant CaMKII phosphatase in other cell types is not known.
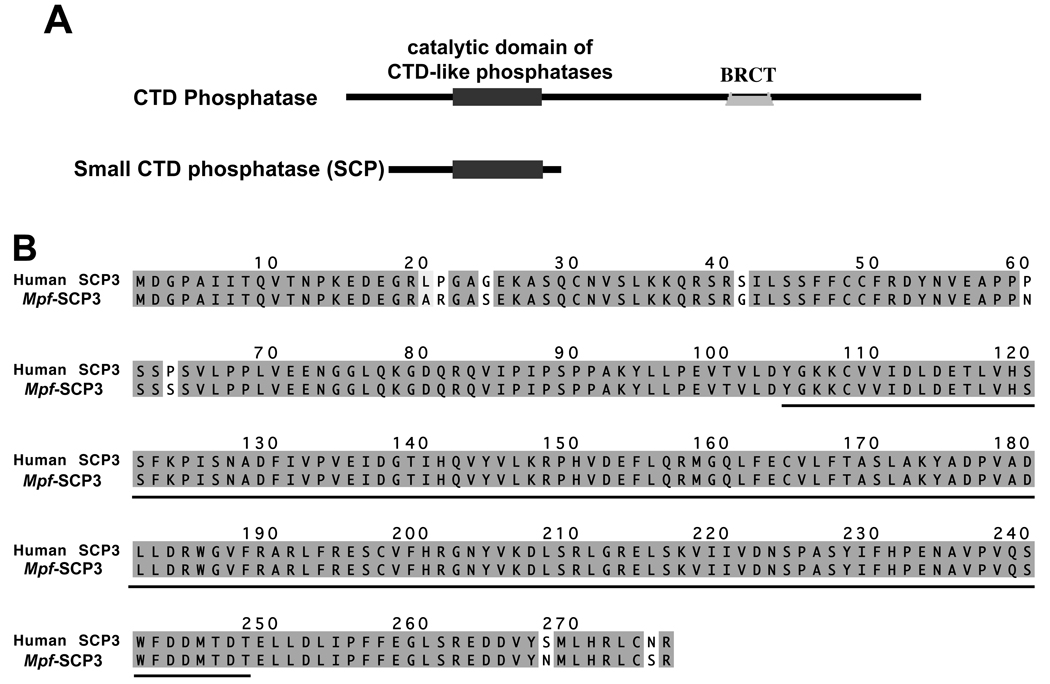
C-terminal domain (CTD) phosphatases are known to be involved in the dephosphorylation of the C-terminal domain of RNA polymerase II. CTD phosphatases consist of a phosphatase catalytic domain and a Breast Cancer 1 C-terminal (BRCT) domain. However, small CTD phosphatases (SCPs) lack the BRCT domain (figure1A). To date, three isoforms (SCP1-3) have been identified in the human. The only known functions of SCPs are that human SCP1 can dephosphorylate the C-terminal domain of the largest RNA polymerase II subunit in vitro [14] and that some human SCP isoforms are suggested to function as global silencers of neuronal genes [15].
Figure 1.
A: Diagrammatic presentation of the domains (as indicated) of CTD phosphatase and small CTD phosphatase. B: Alignment of Ferret (Mustela putorius furo, Mpf) SCP3 with Human (Hs) SCP3. The identical residues are shaded gray, similar residues are shaded light-gray and unmatched residues are not shaded. Catalytic domain of CTD phosphatase is underlined. GenBank accession number of Ferret SCP3 is DQ465034.
The present study was initiated to identify new CaMKII binding proteins. We have used the novel association domain of CaMKII gamma G-2 as a bait in a yeast 2-hybrid assay and here present the identification and characterization of a small CTD phosphatase (SCP) homologue in smooth muscle that binds to this novel association domain. SCP3 is a PP2C type phosphatase that is primarily expressed in vascular smooth muscle tissues. This phosphatase dephosphorylates CaMKII gamma G-2 but, as we show here, the dephosphorylation is site specific in that Thr287 is protected from dephosphorylation. We show that SCP3 regulates phosphorylation of CaMKII gamma G-2 at sites that are not involved in the autonomous activation of the kinase but that have been shown to regulate other functions such as targeting of the kinase.
Experimental
Yeast 2-hybrid assay
The cDNA corresponding to the novel 99 amino acid residue sequence was cloned into the bait vector pBD-GAL4 Cam (Stratagene,CA). A HybriZap 2.1 aortic cDNA 2-hybrid library was screened [8]. The method of screening was according to the instruction of the manufacturer (Stratagene,CA) [16].
Protein expression
The cDNA of SCP3, that of the novel G-2 association domain (corresponding to the 99 amino acid residues) and that of the association domain of CaMKII gamma C-1 (residues 331–495) were expressed and purified by IMPACT-CN system (Intein Mediated Purification with an Affinity Chitin-binding Tag; NE Biolabs Inc.). The procedures were according to the instruction of the supplier.
Antibodies
SCP3 antibody: E coli expressed pure protein was injected into rabbits and a polyclonal antibody was raised. The antibody was purified from the serum by an affinity column (AminoLink Kit, Pierce Biotechnology). The purified antibody was tested against recombinant SCP3 and aorta whole tissue homogenate. It recognizes the recombinant protein and also recognizes a single band of similar molecular weight on aorta tissue homogenate. This antibody was used for immunoblot and imaging studies.
The CaMKII gamma G-2 antibody is the same as used in our previous study [9] and pan CaMKII gamma antibody is the same as used in [8]. The phospho-Thr287 specific antibody is from Upstate (Upstate/Millipore). This is a mouse monoclonal antibody raised against a peptide corresponding to residues 281–294 of rat CaM kinase II alpha-subunit. This antibody has been extensively used and has been shown to not only be specific for activated CaMKII phosphorylated at Thr286 but also at the analogous Thr287 in the gamma isoforms [8]. Anti-Chitin binding domain (CBD) mouse monoclonal antibody is from New England Biolabs Inc (Ipswich, MA). Thr306 antibody is from PhosphoSolutions (Aurora, CO).
Affinity labeling of antibody
The fluorescent labeling of antibody was performed using kits from Molecular probes. The purified antibody was dialyzed against PBS to remove any ammonia or amines and the procedure of labeling was according to the instructions of the manufacturer.
Surface plasmon resonance analysis (Biacore)
The affinities and kinetics of the molecular interactions between SCP3 and the association domains of CaMKII gamma G-2 and C-1 were measured by surface plasmon resonance (SPR) analysis using a Biacore 300 instrument (Biacore, Piscataway, NJ). Purified SCP3 protein was immobilized to a CM-5 sensor chip (Biacore, Piscataway, NJ) using the standard amine coupling method. Approximately 500 resonance units of SCP3 were immobilized. A negative control sensor chip surface was prepared by activation and blocking with ethanolamine. All binding experiments were performed at 25 °C in phosphate-buffered saline, pH 7.4. To test for binding of CaMKII gamma G-2 and C-1 association domains to SCP3, 250 nM of each association domain was injected over both the SCP3-immobilized and negative control surface and the differential response was measured. For affinity and kinetic analysis of CaMKII gamma G-2 association domain binding to SCP3, serial dilutions from 250 to 0.3 nM of the former were injected over the SCP3-immobilized and control surfaces. Differential response curves were analyzed using the BIAevaluation 4.1 software (Biacore, Piscataway, NJ). The on- (ka) and off-rates (kd) were fitted simultaneously using a 1:1 Langmuir binding model, and KD values were determined by the ratio kd/ka.
Pull-down assay
Purified SCP3 was coupled to a resin by AminoLink kit from Pierce biotechnology Inc. Cleaned aorta tissue was attached to a force recording device to confirm viability and quick frozen with a dry ice/acetone slurry containing DTT. The tissue was homogenized in a homogenization buffer (20mM Tris-Hcl, pH 8.0, 50mM NaCl, 10% Glycerol, 0.1% Triton X-100, 5mM MgCl2, 1mM ATP and protease inhibitor cocktail). The coupled resin was removed from the column and it was incubated with the tissue homogenate under rotating conditions at 4°C overnight. The beads were washed with the washing buffer, which was identical to homogenization buffer except the NaCl concentration was 150mM. Finally, the beads were extracted with SDS-PAGE loading sample buffer containing SDS. The extracted proteins were electrophoresed and western blots were detected with a CaMKII gamma G-2 specific antibody. The same blot was stripped and labeled with anti-CBD antibody.
CaM kinase II gamma expression and purification
COS7/CHO cells were routinely maintained in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin, to avoid bacterial contaminations, at 37°C in the presence of 5% CO2. CaM kinase II gamma variant cDNAs as described previously [8] were cloned in to pcDNA4 His-Max TOPO vector. For transfection, cells were grown in 100mm petri-dishes to about 90% confluent culture. Each plate was transfected with 30–40µg of plasmid DNA and 50µl of lipofectamine 2000 transfection reagent (Invitrogen). After 48 hours of incubation, cells were harvested by scrubbing with rubber policeman and cell pellets were either stored at −80°C or proceed for purification.
Cell pellets from 10 plates were re-suspended in 2ml lysis buffer (50mM Phosphate buffer pH 7.0, 0.5M NaCl, 10% Glycerol, 0.1% Tween20 and a cocktail of protease inhibitors) and mixed by pipeting. After centrifugation at 15,000×g for 10 minutes, the supernatant was added to the talon spin column (BD Bioscience). After rocking for 30 minutes at room temperature, the column was washed with lysis buffer for 3 times followed by washing (4 times) with wash buffer (50mM Phosphate buffer pH 7.0, 1.0M NaCl, 10% Glycerol, 0.1% Tween20, 75mM Imidazole and a cocktail of protease inhibitors). Finally, the protein was eluted from the column with elution buffer (50mM Phosphate buffer pH 7.0, 50mM NaCl, 10% Glycerol, 200mM Imidazole and a cocktail of protease inhibitors) and concentrated by centrifugation with filter. The detailed method of purification was according to the instruction of the manufacturer of Talon column. The purified proteins were further purified by passing through a Superdex 75 size exclusion column (FPLC) with a buffer containing 50mM PIPES pH-7.0, 20mM NaCl, 0.5mM EDTA and 10% Glycerol. The CaMKII fractions were tested by activity measurements and pooled fractions were stored at −80°C in small aliquots.
Phosphatase assay
Phosphatase activity of SCP3 was measured with p-nitrophenyl phosphate (PNPP) as the substrate. The assay buffer contained 50mM Tris-acetate, 10mM MgCl2, 0.5mM DTT, 10% glycerol and 20mM PNPP. Purified SCP3 (0.5µg) was added to 200µl of the assay mixture and incubated at 30°C for 30 minutes. The reaction was stopped by the addition of 800µl 250mM NaOH and the absorbance of p-nitrophenol was measured at 410nm. For detection of dephosphorylation, 5µg of CaMKII gamma G-2 was incubated with 1µg of SCP3 (0.5 ml total volume) in the same assay buffer as indicated above in presence or absence of reagents described in the figure. The released phosphate was detected by incubation with 0.5 ml of Biomol Green Reagent (Biomol International) at 30°C for 30 minutes. Developed color was detected by OD measurement at 620nm.
Autophosphorylation of CaMKII, autonomous activity and dephosphorylation
Purified CaMKII gamma (1µg) proteins were autophosphorylated in the presence of Ca2+/CaM and ATP (or gamma 32P ATP) for 30 seconds in a 50µl reaction according to [8]. The reaction mixture was passed through a Micro Bio-Spin P-30 column (BioRad), pre-equilibrated with the phosphatase assay buffer to remove the unused ATP. The eluted protein was subjected to dephosphorylation with PP1 (NE Biolabs), PP2A (Upstate/Millipore) and purified SCP3 (equimolar to CaMKII protein) in the buffer as recommended by the suppliers (for PP1 and PP2A) and phosphatase assay buffer (for SCP3) at 30°C. At time intervals indicated in the figure, aliquots of 10 µl were withdrawn and the reaction stopped by mixing with sample loading buffer containing SDS. Thr287 dephosphorylation was measured by western blot with a phospho-Thr287 specific antibody and total dephosphorylation was tested by autoradiography of the electrophoresed samples. For the detection of Thr306 phosphorylation CaMKII gamma G-2 protein was autophosphorylated for 15 seconds at 30°C and EGTA was added to a final concentration of 3.3 mM. After incubation for 30 seconds at 30°C the reaction mixture was passed through the spin column as mentioned above and dephosphorylated by SCP3. Phosphorylation was detected by immuno blot developed with Anti-Phospho-Thr306 antibody.
For autonomous activity measurements in the presence of SCP3 the eluted protein from spin column was subjected to dephosphorylation with purified SCP3 (equimolar to CaMKII protein) in phosphatase assay buffer at 30°C for 10 minutes. Autonomous activity [(Ca2+ independent activity/total activity)×100] of SCP3 treated and untreated CaMKII gamma G-2 was measured according to [7].
Mass spectrometry
The phosphorylation site determination by mass spectrometry was performed by the Harvard Microchemistry and Proteomics Analysis Facility. The results were obtained by a single microcapillary reverse-phase HPLC run, directly coupled to the nano-electrospray ionization source of an ion trap mass spectrometer on a Finnigan LCQ DECA XP Plus quadrupole ion trap mass spectrometer. This instrument configuration is capable of acquiring individual sequence (MS/MS) spectra on-line at high sensitivity (<<<1 femtomole) for multiple peptides in the chromatographic run. These MS/MS spectra were then correlated with known sequences using the algorithm SEQUEST, developed at the Univ. of Washington and programs developed in the facility [17]. MS/MS peptide sequences were then reviewed for consensus with known proteins and the results were manually confirmed for fidelity.
Cell isolation from aorta tissue, staining and imaging
Ferrets (Marshall Farms, North Rose, NY) were anesthetized by an overdose of chloroform following procedures approved by the Institutional Animal Care and Use Committee and the abdominal aorta was excised quickly to a dish containing oxygenated Physiological Saline Solution (PSS). The tissue was cut to small strips and used for cell isolation. Single cells from the aorta tissue were isolated as previously described [18]. For each 100 mg of aorta (wet weight), the digestion medium A, consisted of 4.2 mg CLS 2 collagenase (type II, 390U/mg) dissolved in 4ml of Hanks Saline Solution, 4.8 mg elastase (3.63 U/mg) dissolved in 4 ml of Hanks Saline Solution, and 5000 U soybean trypsin inhibitor in final total volume of 7.5 ml Ca+2-Mg+2-free Hanks’ balanced saline solution. For all experiments, isolated cells on one coverslip were first tested to confirm that they shortened in response to Phenylephrine (PE). Confocal microscopy: Cells were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 10% goat serum and reacted with the appropriate concentration of fluorescent labeled primary and secondary antibodies. Images were obtained with a Kr/Ar laser (Radiance 2000) scanning confocal microscope equipped with Nikon X-60 (NA1.4)/ 40X (NA1.4) oil immersion objectives. Fluorescence microscopy: CHO cells were grown on coverslips and transfected with SCP3 cDNA. 48 hours after transfection cells were fixed with 4% paraformaldehyde, labeled with SCP3 antibody and Alexa-488 conjugated goat anti-rabbit secondary antibody. Images were taken with a Nikon Eclipse TE2000-E microscope.
Results
SCP3 interacts with the novel association domain of CaMKII gamma G-2
To identify the binding partners of the novel association domain of CaM kinase II gamma G-2, a yeast 2-hybrid assay was performed. The cDNA corresponding to the novel sequence of the CaMKII gamma G-2 association domain (residues 463–561) was used as a bait in screening a ferret aorta cDNA yeast 2-hybrid library [19]. A single, specific clone exhibited positive interactions, and was isolated. Analysis of the cDNA sequence by a Blast search revealed that the interacting protein is a homolog of a human SCP3, exhibiting a 97% sequence identity. The SCP3 homolog derived from the aorta cDNA library is a 31kDa protein with 276 amino acid residues. A catalytic domain of CTD-like phosphatases comprises residues 104–248. The amino acid sequence of the SCP3 homologue aligned with that of the human SCP3 isoform is shown in figure 1B.
CaMKII gamma G-2 association domain and SCP3 interact with high affinity
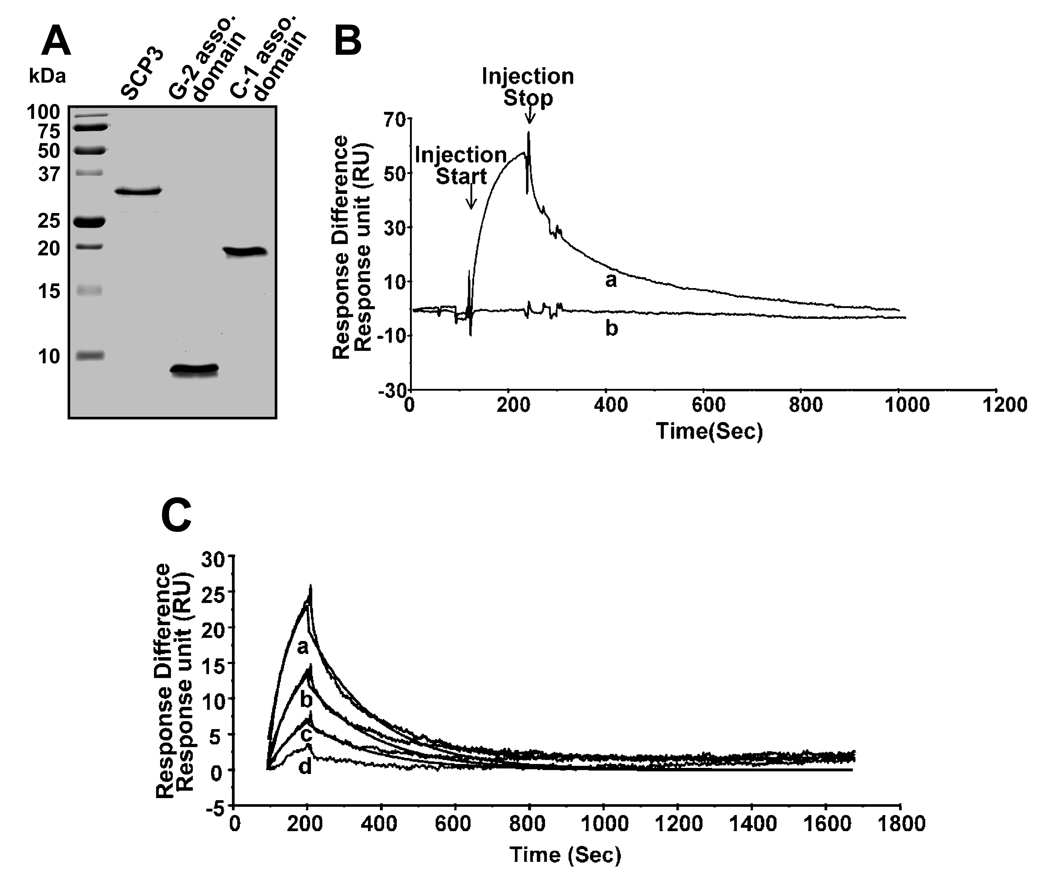
The in vitro interaction of SCP3 and CaMKII gamma G-2 was measured by surface plasmon resonance (SPR) analysis. Purified full-length recombinant SCP3, the novel 99 amino acid sequence of G-2 and the association domains of CaMKII gamma C-1 (residue 332–495) were used for these experiments (figure 2A). Injection of the G-2 association domain fragment through a flow cell to which SCP3 was immobilized produced a reproducible and reversible increase in the SPR response with the non-specific response to a blank control surface subtracted (figure 2B). Previously we have shown that G-2 forms a multimer in spite of having novel sequence in its association domain [8]. If the association between the CaMKII gamma G-2 association domain and SCP3 is due specifically to the novel sequence present in this variant, then the association domains of other CaMKII gamma variants will not bind. Indeed, this is what was observed. When a recombinant protein fragment corresponding to the association domain of CaMKII gamma C-1 (and identical to the association domains from all CaMKII gamma variants other than gamma G-2) was injected over the same flow cell, there was no detectable increase in the SPR response (figure 2B). In order to measure the affinity of the SCP3/CaMKII gamma G-2 association domain interaction, varying concentrations of CaMKII gamma G-2 association domain were injected and the kinetics of the specific association and dissociation with SCP3 were determined, with the resulting ratio of these rates corresponding to an affinity of 36 nM (figure 2C). Thus, the interaction between CaMKII association domains and SCP3 is specific to the G-2 variant of CaMKII and is of relatively high affinity.
Figure 2.
Protein expression and purification and surface plasmon resonance analysis of in vitro interactions. A: Coomassie stained protein gel of purified proteins (1µg each) as indicated. All three proteins were expressed in E coli (methods). B: An SPR sensorgram showing relative responses of injections of CaM kinase II gamma G-2 (a) and C-1(b) association domain, both at 250 nM concentration, over an SCP3-immobilized surface. C: Kinetic analysis of serial dilutions (250– 0.3 nM) of CaMKII gamma G-2 association domain binding to SCP3. The lines depict the relative responses of each injection (a-125nM; b-62nM; c-31nM and d-15nM of G-2 association domain) with the lines show the result of global curve-fitting of both ka and kd for three concentrations (a-125nM; b-62nM and c-31nM) using a 1:1 Langmuir binding model with the BIAevalution 4.1 software.
Full-length endogenous CaMKII gamma G-2 binds to SCP3
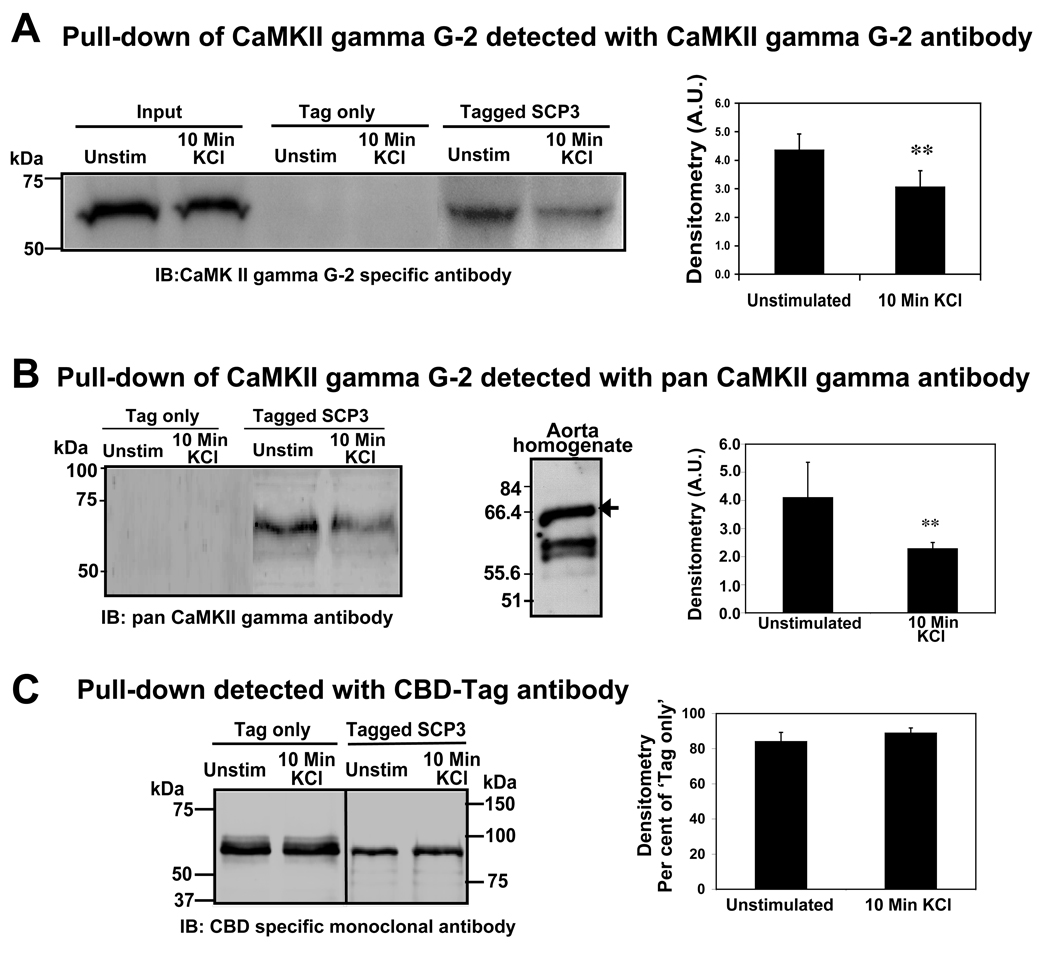
To test the interaction of full length, endogenous CaMKII gamma G-2 and SCP3, a pull-down assay was performed. SCP3 was expressed with an N-terminal chitin binding domain (CBD) tag and an affinity resin was made by immobilizing the tagged protein on chitin beads. The interactions were tested against ferret aorta tissue whole cell homogenates flash-frozen under these two conditions; (1) unstimulated tissue; and (2) tissue depolarized with physiological saline solution containing 51mM KCl. The pulled-down proteins were subjected to SDS-PAGE and immunoblotted with a CaMKII gamma G-2 specific antibody [9]. To eliminate the possibility of interaction between CaMKII gamma G-2 and the tag sequence, a negative control experiment was performed with the tag sequence alone. Immunoblot results (figure 3A) show that CaMKII gamma G-2 is pulled down with SCP3 labeled beads under both the conditions tested but that there is no signal detected for the tag only control. Quantitative densitometric measurements of bands (n=4) indicate that the interaction is significantly decreased (about 50%) in samples from depolarized muscle (right panel, figure 3A). The pulled down samples were further tested with a pan CaMKII gamma antibody (left panel, figure 3B). We have used this antibody against aorta whole cell homogenate and multiple bands are detected corresponding to the multiple CaMKII gamma variants in this tissue (middle panel, figure 3B). In contrast, in the pull-downs only a single band was detected with the pan CaMKII antibody. We found that the intensity of signal in 10 minute KCl treated tissue homogenate is significantly less (about 50%) than that from unstimulated tissue sample (right panel, figure 3B). The results from two antibodies describes a similarity in the decrease of signals in 10 minute KCl stimulated sample and suggest that hetero-oligomerization of G-2 with the other variants known to be present in this tissue does not occur. To test the linearity of loading and whether there was equally efficient CBD sedimentation in both the sets of tissue homogenates, the same blot was striped and labeled with anti-CBD monoclonal antibody. As seen in figure 3C the CBD signal levels remain unchanged in both negative controls (tag-only) and in pull-down samples (left panel, figure 3C). CaM kinases are autophosphorylated and activated upon depolarization of smooth muscle tissues (19) and thus, the interaction of CaMKII gamma G-2 and SCP3 is diminished during depolarization.
Figure 3.
Pull-down assay: detection of CaM kinase II gamma G-2 bound to SCP3 in tissue homogenate. A: Homogenates from unstimulated and KCl stimulated tissues were tested as indicated. IB: Immunoblot probed with CaMKII gamma G-2 specific antibody. Input: tissue homogenate, Tag only: pull-down with the chitin binding domain (CBD) protein tag as negative control, Tagged SCP3: pull-down with the chitin binding domain (CBD) tagged SCP3. Right panel represents the densitometry of immuno-blots probed with CaMKII gamma G-2 specific antibody. Densitometry data are normalized by dividing by the respective input signal (n=4). B: The signal as obtained from the pull-down blot by probing with a pan CaMKII gamma antibody (left panel). Middle panel represents western blot of aorta whole cell homogenate probed with pan CaMKII gamma antibody. The arrow corresponds to the G-2 variant. Right panel represents the densitometry of immuno-blots probed with a pan CaMKII gamma antibody and the data are the average absolute densitometric values of the digital signals (n=3). C: The signal as obtained from pull-downs probing with anti-CBD antibody. Right panel represents the densitometry of immunoblots probed with an anti-CBD antibody. Densitometry data are normalized by dividing by the respective tag-only signal (n=3). The two-tailed t-test P values <0.01 was denoted as **.
SCP3 is a cytosolic protein whose expression is vascular-specific
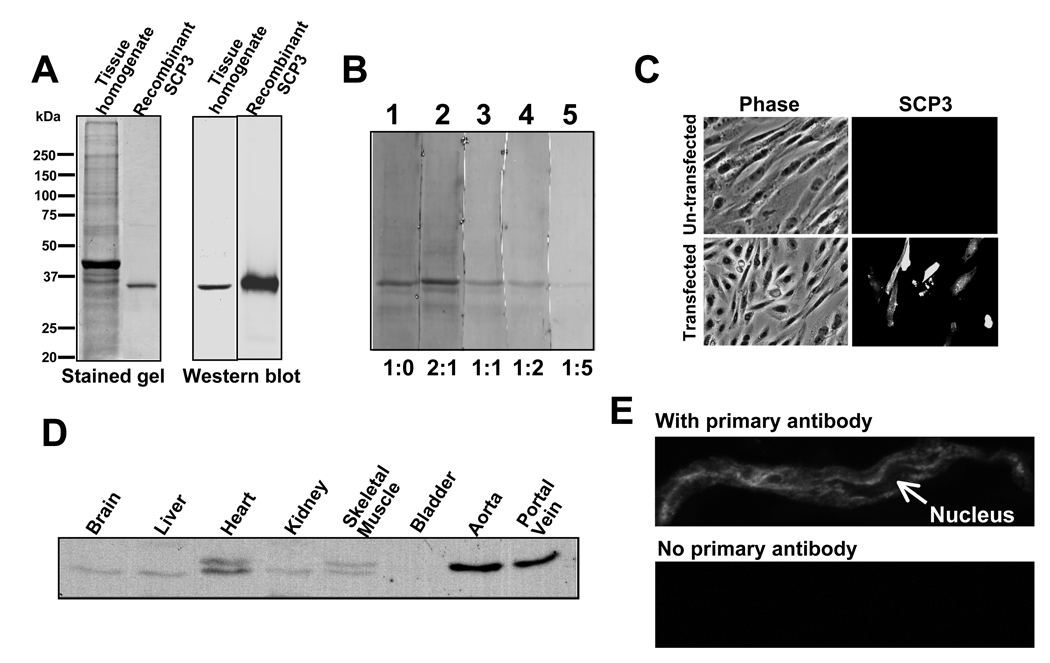
An anti-SCP3 antibody was raised to assess differential protein expression across tissues and at the sub-cellular level. This antibody recognizes a single band at a molecular weight appropriate for SCP3 (31 kDa) on a western blot of aorta whole cell homogenate (figure 4A). Binding of the antibody to the antigen on the blot is diminished by increasing amounts of the antigen in the antibody incubation buffer, demonstrating specificity (figure 4B). To test the specificity in a cellular environment we have stained CHO cells, both un-transfected and transfected with the expression construct of SCP3 cDNA, with the SCP3 antibody (figure 4C). The results show that the cells which are transfected with the cDNA of SCP3 and expressed the protein are stained with the antibody (lower right panel, figure 4C) and untransfected cells are not detectably stained (upper panel of figure 4C).
Figure 4.
Tissue/cellular distribution of SCP3. A: Left panel represents the Coomassie stained SDSPAGE gel picture of aorta tissue whole cell homogenate and recombinant E coli expressed SCP3. Right panel represents the western blot of corresponding proteins detected with SCP3 antibody as indicated. B: Immunoblot of aorta tissue whole cell homogenate probed with SCP3 antibody in the presence of antigen (SCP3 protein) with the antibody : antigen ratio indicated at the bottom (lane 1–5). C: Fluorescence microscopic images of CHO cells at identical microscope settings of both transfected and untransfected cells as indicated, stained with SCP3 antibody and Alexa 488 conjugated goat anti-rabbit secondary antibody. Fluorescence image (SCP3) and phase contrast image (Phase) were overlaid (Merge). D: Immunoblot of protein matched (20µg) whole cell homogenates of tissues as indicated detected by SCP specific antibody. E: Confocal image of freshly enzymatically isolated smooth muscle cell immunostained with SCP3 antibody (top); or (bottom) stained with secondary antibody and visualized at identical settings.
An immunoblot of a protein-matched tissue homogenate (figure 4D) shows that the expression of SCP3 is most pronounced in aorta and portal vein and that no signal is detected in bladder. Only faint signals are detected in brain, heart, liver, kidney and skeletal muscle and the faint signals could represent the vasculature of the tissues. Thus, the expression of SCP3 appears to be largely restricted to the vasculature. It is of note that doublets are visible in the samples from heart and skeletal muscle tissue homogenates. Our prediction is that this could be due to alternatively spliced isoforms, however, at the present time there is no report of alternative splicing of SCP3.
Previously described SCP family members have been associated with the functions of RNA polymerase dephosphorylation and gene silencing and, as a result, nuclear distribution of SCP3 was expected. Imaging of freshly dissociated differentiated vascular smooth muscle cells with the anti-SCP3 antibody shows that SCP3 is localized on filamentous structures but is not present in the nucleus (upper panel figure 4E,). We were unable to detect any signal from cells not treated with the primary (anti-SCP3) antibody (lower panel, figure 4E). These results imply a non-nuclear function of this small CTD phosphatase homologue.
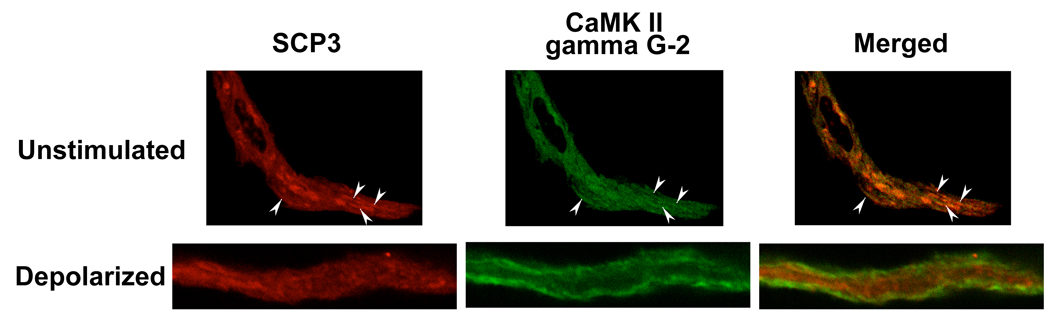
CaMKII gamma G-2 and SCP3 co-localize in unstimulated, but not depolarized dVSM cells
To evaluate if these two proteins interact in the cellular environment, we performed confocal imaging to assess their cellular localization. Since both the CaMKII gamma G-2 and SCP3 antibodies were raised in rabbit they had to be directly coupled to fluorophores resulting in somewhat diminished signals. Imaging of dissociated smooth muscle cells indicates that SCP3 partially co-localizes (arrowheads in red, green and merge images, figure 5) with CaMKII G-2 under unstimulated conditions. However, upon depolarization, CaMKII translocates to the cell cortex, as previously described [9], but, SCP3 does not and the two proteins no longer co-localize (figure 5). Thus, cellular SCP3 dissociates from CaMKII gamma G-2 upon depolarization, consistent with the pull-down experiments.
Figure 5.
Co-staining of freshly dissociated vascular smooth muscle cells. Cells fixed under unstimulated and depolarized condition co-stained with Alexa555 labeled SCP3 antibody (red) and Alexa465 labeled CaMKII gamma G-2 antibody (green). The arrowheads indicate co-localization of two proteins.
CaMKII gamma G-2 is dephosphorylated by SCP3
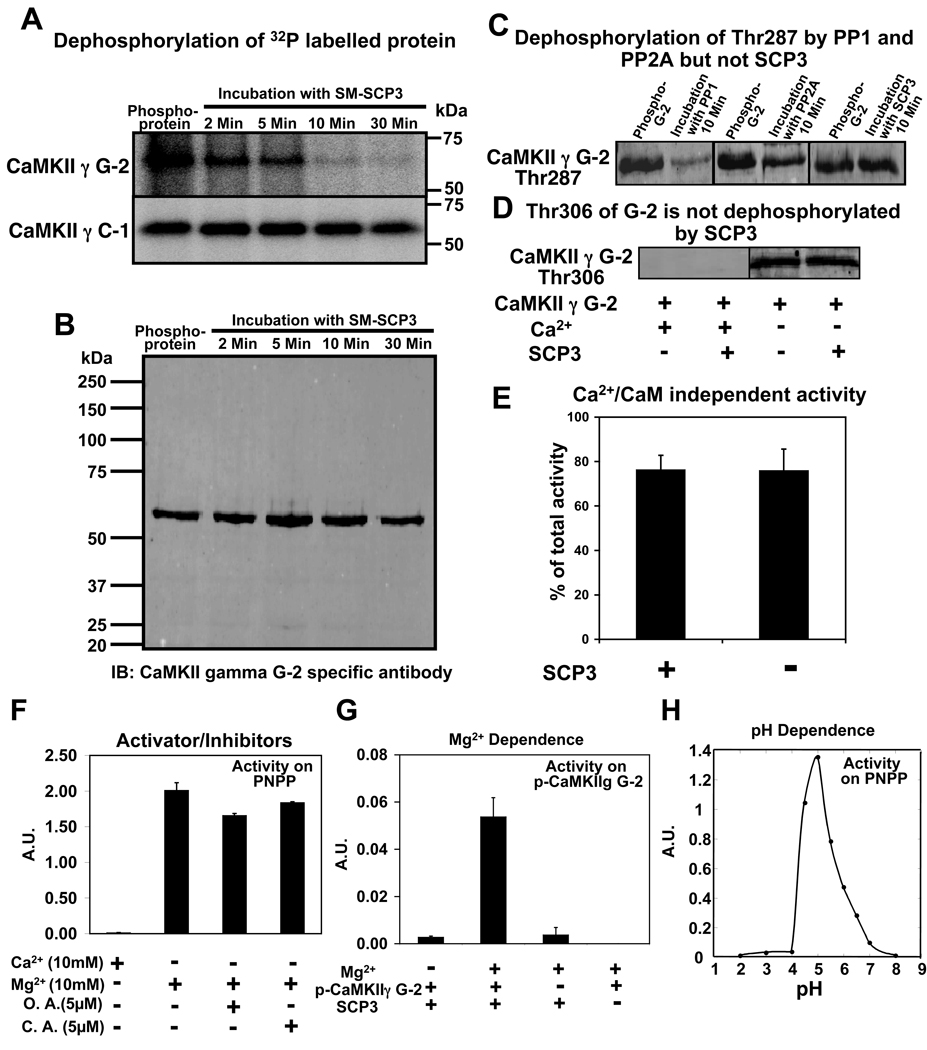
Multiple sites of CaM kinase II are known to be autophosphorylated in the presence of Ca2+/CaM and ATP [5, 10]. CaMKII gamma G-2 was autophosphorylated in the presence of Ca2+/CaM and gamma-32P ATP for 30 seconds so that the phospho-sites would be labeled with 32P. The labeled, phosphorylated CaMKII gamma G-2 was treated with recombinant SCP3. Under these conditions, CaMKII gamma G-2 was largely dephosphorylated within 10 minutes, leaving only a faint signal. This faint signal, although close to baseline, was maintained at up to 30 minutes of incubation (upper panel, figure 6A). A parallel control reaction of dephosphorylation in the absence of SCP3 (data not shown) did not detect any change in the 32P signal of CaMKII gamma G-2 for up to 30 minutes of observation. Some residual signal is expected due to the lack of dephosphorylation of Thr287 seen below with phospho-antibodies. An identical experiment using SCP3 and CaMKII gamma C-1 showed no change in phosphorylation up to 30 minutes of incubation (lower panel, figure 6A), consistent with the lack of binding between SCP3 and CaMKII gamma C-1 (fig 2B). A blot detected with the CaMKII gamma G-2 specific antibody from a parallel reaction of G-2 dephosphorylation shows the similarity of the lane loading and also ruled out the possibility of any degradation/proteolysis of the protein (figure 6B). Thus it appears that the anchoring association domain of G-2 is necessary for dephosphorylation by SCP3.
Figure 6.
Dephosphorylation of CaMKII gamma by SCP3 and function of SCP3 as a PP2C phosphatase. A: Autoradiogram of autophosphorylated CaMKII gamma G-2 and C-1 and dephosphorylation by SCP3 (0.1µg). Time of incubation in the presence of SCP3 is indicated above. B: A full length blot of autophosphorylated CaMKII gamma G-2 and subsequently dephosphorylated by SCP3 was probed with CaMKII gamma G-2 specific antibody. C: Immunoblots of phospho-CaMKII gamma G-2 detected by phospho-Thr287 specific antibody under conditions indicated above blots. D: Detection of dephosphorylation at Thr306 of G-2 in the presence and absence of SCP3. Autophosphorylation at Thr306 is seen only in the absence of Ca2+. E: Ca2+/CaM-Independent activity (per cent of total activity) of CaMKII gamma G-2 in the presence or absence of SCP3. F: Activity and Inhibition of SCP3 using PNPP as a substrate in the presence or absence of ions. Activity was measured (absorption unit, A.U.) as the absorption of para-Nitrophenol, the product of phosphatase reaction. O.A. is Okadaic acid and C.A. is Cyclosporin A. G: Activity of SCP3 on phospho-CaMKII gamma G-2 in the presence or absence of Mg2+ as indicated. The released phosphate was detected by Biomol Green Reagent at 620 nm. H: Plot of activity versus pH of SCP3. Activity was measured (absorption unit, A.U.) as the absorption of para- Nitrophenol, the product of phosphatase reaction.
Residue Thr287 of CaMKII is known to be involved in the generation of Ca2+/CaM-independent (e.g., autonomous) activity. Therefore, we tested whether Thr287 of CaMKII gamma G-2 was dephosphorylated by SCP3 using a phospho-Thr287 specific antibody. CaMKII gamma G-2 was autophosphorylated in the presence of Ca2+/CaM and ATP for 30 seconds, and was subsequently subjected to dephosphorylation for 10 minutes in the presence of PP1 (NE Biolabs) PP2A (Millipore) and SCP3. As seen in figure 6C (upper panel), there is dephosphorylation at Thr287 of G-2 by PP1 and also to a lesser extent by PP2A but there was no change in the signal of CaMKII gamma G-2 phosphorylated at Thr287, whether in the presence of SCP3 or not. Thus, SCP3 must decrease 32P labeled CaMKII gamma G-2 by dephosphorylation of residues other than Thr287.
We have also tested for possible dephosphorylation of the Thr306 site of G-2 by SCP3. The Thr306 site is known to be inhibitory with respect to kinase activity [3–5]. This site is phosphorylated only when Ca2+ is removed from the reaction mixture by the addition of EGTA after prior activation of CaMKII [3–5]. As shown in figure 6D, there is no detectable decrease in the phosphorylation of this site after incubation with SCP3. Thus, SCP3 does not globally dephosphorylate all sites other than Thr287; but, on the other hand, must dephosphorylate several sites, given the large decrease in the 32P signal.
Since the generation of Ca2+/CaM-independent activity of CaM kinase II is associated with its autophosphorylation at Thr287, we also measured Ca2+/CaM-independent activity of activated CaM kinase II gamma G-2 in the presence and absence of SCP3. Purified CaMKII gamma G-2 was autophosphorylated in the presence of Ca2+/CaM and ATP, subjected to dephosphorylation in the presence or absence of SCP3 for 10 minutes and autonomous activity was measured (figure 6E). The results show that the Ca2+/CaM-independent activity of CaMKII gamma G-2 is not affected by SCP3 treatment, consistent with the negative phospho-Thr287 immunoblot and confirming that CaMKII gamma G-2 dephosphorylated by SCP3 retains autonomous activity. These results also indicate that the sites dephosphorylated by SCP3 are not involved in the generation of autonomous activity and suggests that SCP3 instead may regulate other functions of CaMKII gamma G-2, which has a direct role in smooth muscle contractility [9].
The CaMKII-binding SCP3 is a PP2C type phosphatase
Phosphatases are classified according to their dependence on metal ions and their sensitivity to specific inhibitors. PP1 as well as PP2A phosphatases are Ca2+-independent, whereas PP2B phosphatases are Ca2+-dependent. PP2C phosphatases are known to be Mg2+-dependent. The activity of SCP3 on a generic phosphatase substrate para-Nitrophenyl Phosphate (PNPP) is dependent on Mg2+ ion and Ca2+ cannot substitute for Mg2+ (figure 6F). However, since anomalous results have been reported in some cases for the Mg2+ dependence of phosphatase activity on PNPP [20, 21], phosphatase activity was tested on phospho-CaMKII gamma G-2 as a substrate. SCP3 was found to be Mg2+ dependent with respect to G-2 dephosphorylation as well (figure 6G). Okadaic acid (inhibitor of PP1, PP2A type Ser/Thr phosphatases) [22] and cyclosporin-A (inhibitor of PP2B type Ser/Thr phosphatases) [23] did not inhibit the activity of SCP3 (figure 6F). The dependence on pH was tested in a range from pH 2–8 and optimum activity for SCP3 was obtained at pH 5.0 (figure 6H). This profile indicates that SCP3 functions as a PP2C type phosphatase.
CaMKII gamma is autophosphorylated at multiple sites
In an effort to investigate the sites of autophosphorylation on CaMKII gamma, we analyzed its phosphorylation state by mass spectrometry. We were unable to express large enough quantities of CaMKII gamma G-2 for phosphorylation site determination by mass spectrometry, but since the sequence of CaMKII gamma G-2 and that of the more easily expressed CaMKII gamma C-1 are identical in the catalytic/regulatory domain and the first one third of the association domain, we used the CaMKII gamma C-1 protein. Purified CaMKII gamma C-1 was autophosphorylated in the presence of Ca2+/CaM and ATP for 10 minutes, separated by SDS-PAGE, subjected to in-gel tryptic digestion and analyzed by microcapillary reverse-phase HPLC nano-electrospray tandem mass spectrometry (LC/MS-MS). The CaMKII gamma phosphorylation sites identified are illustrated in detail in supplementary figure 1 and figure 2. Some of these sites have been previously reported for the alpha and beta isoforms (Thr287, Thr254 and Ser280; numbering according to gamma isoform), however, we also found some unique phosphorylation sites (e.g. Ser26, Thr262, Ser319 and Ser350) not previously reported. These phosphorylation sites are found predominantly in the catalytic/regulatory domain of CaMKII gamma and at least one (Thr254) has been shown to regulate CaMKII targeting [24].
Discussion
We show here that SCP3 binds specifically to the CaMKII gamma G-2 association domain, a novel function for SCP phosphatases. SCP3 is primarily expressed in vascular tissues. Although only nuclear functions have previously been attributed to the SCP family of phosphatases, we show here that SCP3 is excluded from the nucleus in differentiated cells. Thus, despite containing a CTD phosphatase domain, it is unlikely that SCP3 dephosphorylates the C-terminal domain of RNA polymerase II, as has been described for SCP1. Based on the specific binding to the CaMKII gamma G2 association domain, selective dephosphorylation of sites on CaMKII gamma G2, as well as cellular co-localization and co-pull-down from homogenates, it appears that this SCP isoform regulates non-nuclear CaMKII.
The structurally and functionally diverse family of phosphatases have been grouped into three distinct gene families, namely PPP, PPM and PTP [25]. PP1, PP2A and PP2B belong to the PPP family and PP2C belongs to the PPM family. Besides the dependence on Mg2+ of PP2C phosphatases, they are also characterized by the insensitivity to the inhibitors okadeic acid and cyclosporine A [22, 23]. Thus, SCP3 has the functional properties of a PP2C-type phosphatase. PP2C family proteins contain 11 conserved domains [26], however, and SCP3 has little sequence homology to these domains.
Based on in vitro evidence, a presumed CaMKII phosphatase [27] has been reported that is Mn2+ dependent, but is a PP2C type phosphatase distinct from any class of phosphatases. A comparison of sequences between SCP3 and this phosphatase reveals negligible similarity. Thus, SCP3 is a unique CaMKII phosphatase.
In the present work we have tested the activity of SCP3 on phospho-CaMKII gamma. Multiple sites have been reported to be phosphorylated in the alpha and beta isoforms. Mass spectrometry data confirm that the gamma isoform is autophosphorylated at Thr287, Thr254 and Ser280, as previously reported [6, 28]. In addition, we report four novel sites, including Ser26, Thr262, Ser319 and Ser350. Autophosphorylation of Thr253 of the alpha isoform is known to be involved in the targeting of CaMKII to specific subcellular sites (postsynaptic densities) [24] and, taken together with our previous findings for CaMKII gamma G-2, our results may suggest that the dephosphorylation by SCP3 may perform a regulatory function such as targeting of the gamma G-2 variant of CaMKII.
When SCP3 dephosphorylates CaMKII gamma G-2, Thr287 is not dephosphorylated. As a result, the Ca2+/CaM-independent activity (commonly called “autonomous activity” or “memory”) [5] of the G-2 variant is maintained in the presence of SCP3. This makes possible a regulatory mechanism where the fully active kinase can be potentially regulated by the action of SCP3 on other phosphorylation sites of CaMKII G-2. We speculate that protection of Thr287 from dephosphorylation related to the conformational changes known to be caused by the autophosphorylation of the molecule in concert with its binding of potential substrates or docking proteins subsequent to the conformational shift. This could protect this site from binding to SCP3 or could remove it from the reach of SCP3 as it is bound to the association domain.
Binding between SCP3 and the novel association domain of CaMKII gamma G-2, as obtained by surface plasmon resonance analysis, reveals a relatively high affinity interaction. However, the amount of full length CaMKII gamma G-2 associated with the phosphatase in cells is regulated, since the amount of kinase pulled-down with SCP3 is decreased in homogenates from depolarized tissue compared to homogenates from unstimulated tissue. The association domain of CaMKII G-2 is unique amongst CaMKII gamma variants in containing polyproline, SH3 binding domains which we have shown are involved in the targeting of CaMKII gamma G-2 [9]. Our experimental result indicates that SCP3 does not bind to C-1 association domain and C-1 variant is not dephosphorylated by SCP3. These results imply that indeed binding is required for dephosphorylation.
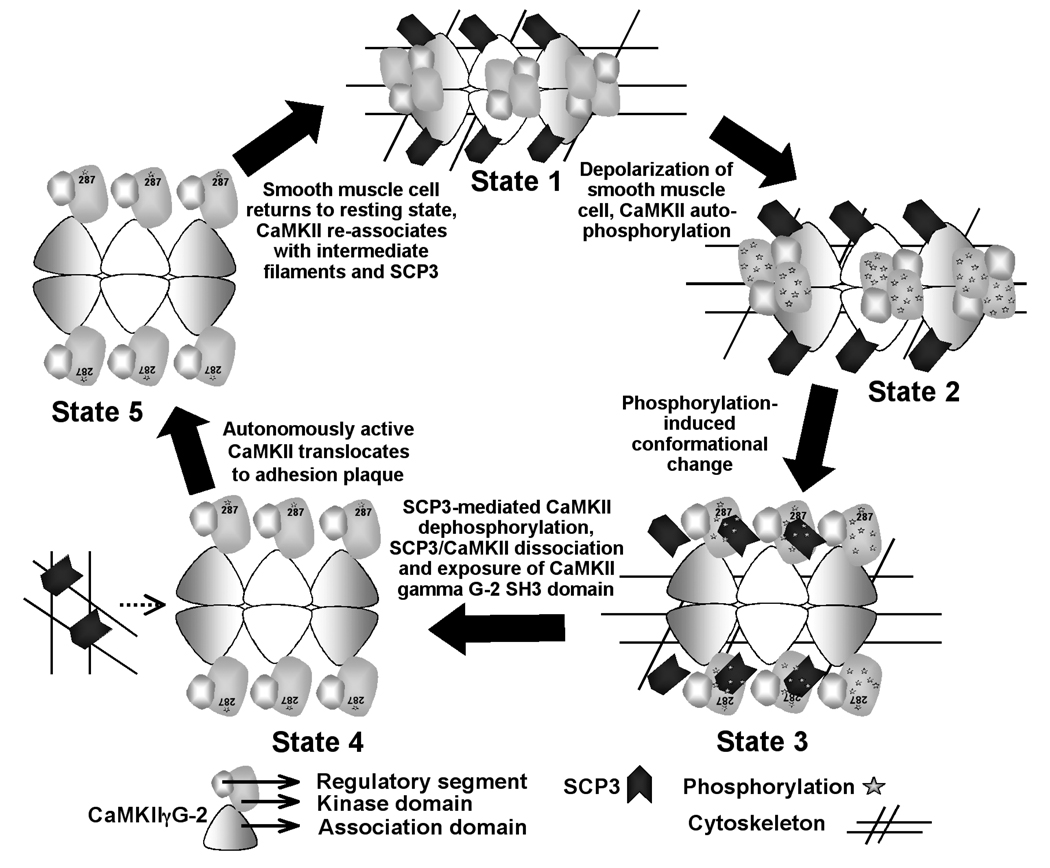
Considering the data from our previous work involving CaMKII gamma G-2 [9] and the results presented here, allows us to propose a possible mechanistic model by which SCP3 regulates CaMKII (figure 7). However, this is only one possible interpretation of the available data. We have previously shown that CaMKII gamma G-2 is associated with the cellular cytoskeleton in unstimulated cells [9]. Under these same unstimulated conditions, we now know that there is maximal association of CaMKII G-2 with SCP3 (state 1). However, when CaMKII gamma G-2 is activated in depolarized tissues [9] and consequently undergoes autophosphorylation (state 2), we have previously shown that CaMKII G-2 subsequently translocates from the cytoskeleton to cortical adhesion plaques. This translocation can be prevented by a polyproline decoy peptide. In this study, we show that after activation of the kinase (state 2), the association between SCP3 and CaMKII G-2 is diminished (state 3). Activation of CaMKII is known to cause conformational changes in the molecule. We speculate that either the conformational changes or the appearance of phosphorylation sites on the activated CaMKII catalytic domain attract the phosphatase, or both, and consequently weaken the association of SCP3 with the G-2 association domain (state 3, figure 7). Dephosphorylation of CaMKII at sites other than Thr287 and Thr 306 by SCP3 are then expected to lead to the completion of dissociation of the phosphatase (state 4), exposing the polyproline regions in the G-2 association domain required for targeting of CaMKII gamma G-2 to cortical adhesion plaques [9] (state 5, figure 7). Adhesion plaque proteins such as p130 Cas are known to contain SH3 domains that may attract CaMKII gamma G-2 to those sites [29].
Figure 7.
Proposed model for SCP3 interactions with CaMKII gamma G-2 in the smooth muscle cell. The G-2 oligomer is drawn following Rosenberg et al., 2005 [30]. The holoenzyme is composed of two stacked hexameric rings. A side view of the molecule is shown. The dodecameric-holoenzyme is modeled as a 6-mer for simplicity. State 1: Under unstimulated conditions there is maximal association of CaMKII gamma G-2 with SCP3. State 2: In depolarized tissues CaMKII gamma G-2 undergoes autophosphorylation. State 3: After the activation-induced conformational changes and phosphorylation of the kinase, SCP3 is associated with phosphorylation sites on CaMKII gamma G-2. State 4: Dephosphorylation of CaMKII by SCP3 leads to the completion of dissociation of SCP3 from CaMKII. State 5: The dissociated CaMKII gamma G-2 is targeted to the adhesion plaques. See text for details.
We have previously reported that CaMKII gamma G-2 directly binds to the cytoskeletal proteins vimentin and alpha actinin and colocalizes with them in unstimulated cells [9]. When G-2 translocates to the cortical adhesion plaques one of two mechanisms could explain its dissociation from the cytoskeleton: 1) CaMKII gamma G-2 is not directly bound to the cytoskeleton but it is bound via SCP3. Thus, dissociation of G-2 from SCP3 releases G-2 from the cytoskeleton; or, 2) CaMKII gamma G-2 upon activation phosphorylates the cytoskeleton, causing a disruption of the association of CaMKII and the cytoskeleton.
In conclusion, this study provides the identification of a SCP3 homologue (SCP3) predominantly expressed in smooth muscle, which is non-nuclear in differentiated cells and interacts with a novel variant of CaMKII gamma (variant G-2) through its association domain. After activation of CaMKII, SCP3 dephosphorylates CaMKII at sites implicated regulation of the kinase, but in a manner that we have shown maintains the autonomous activity of the kinase.
Supplementary Material
Acknowledgements
This work was supported by NIH grants HL31704, HL80003, HL86655 and HD43054 (to K.G.M.); HL074470 (to S.S.G.).
Abbreviations
- CaM
Calmodulin
- CaMKII
Ca2+/ Calmodulin (CaM)-dependent protein kinase II
- CTD
c-Terminal Domain
- SCP
Small CTD Phosphatase
Footnotes
GenBank accession number: DQ465034
References
- 1.Lorca T, Abrieu A, Means A, Doree M. Ca2+ is involved through type II calmodulin-dependent protein kinase in cyclin degradation and exit from metaphase. Biochim. Biophys. Acta. 1994;1223:325–332. doi: 10.1016/0167-4889(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 3.Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu. Rev. Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 4.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulindependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: The role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 6.Miller SG, Patton BL, Kennedy MB. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 7.Hanson PI, Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin- dependent protein kinase analyzed by site-directed mutagenesis. J. Biol. Chem. 1992;267:17216–17224. [PubMed] [Google Scholar]
- 8.Gangopadhyay SS, Barber AL, Gallant C, Grabarek Z, Smith JL, Morgan KG. Differential functional properties of calmodulin-dependent protein kinase IIgamma variants isolated from smooth muscle. Biochem. J. 2003;372:347–357. doi: 10.1042/BJ20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a novel Ca+2/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circ. Res. 2005;97:541–549. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- 10.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem. J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields SM, Ingebritsen TS, Kelly PT. Identification of protein phosphatase 1 in synaptic junctions: dephosphorylation of endogenous calmodulin-dependent kinase II and synapse-enriched phosphoproteins. J. Neurosci. 1985;5:3414–3422. doi: 10.1523/JNEUROSCI.05-12-03414.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga K, Kobayashi T, Tamura S, Miyamoto E. Dephosphorylation of autophosphorylated Ca2+/calmodulin-dependent protein kinase II by protein phosphatase 2C. J. Biol. Chem. 1993;268:133–137. [PubMed] [Google Scholar]
- 14.Yeo M, Lin PS, Dahmus ME, Gill GN. A novel RNA polymerase II C-terminal domain phosphatase that preferentially dephosphorylates serine 5. J. Biol. Chem. 2003;278:26078–26085. doi: 10.1074/jbc.M301791200. [DOI] [PubMed] [Google Scholar]
- 15.Yeo M, Lee SK, Lee B, Ruiz EC, Pfaff SL, Gill GN. Small CTD phosphatases function in silencing neuronal gene expression. Science. 2005;307:596–600. doi: 10.1126/science.1100801. [DOI] [PubMed] [Google Scholar]
- 16.Je HD, Gangopadhyay SS, Ashworth TD, Morgan KG. Calponin is required for agonist-induced signal transduction - evidence from an antisense approach in ferret smooth muscle. J. Physiol. 2001;537:567–577. doi: 10.1111/j.1469-7793.2001.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chittum HS, Lane WS, Carlson BA, Roller PP, Lung FD, Lee BJ, Hatfield DL. Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry. 1998;37:10866–10870. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- 18.Shin HM, Je HD, Gallant C, Tao TC, Hartshorne DJ, Ito M, Morgan KG. Differential association and localization of myosin phosphatase subunits during agonist-induced signal transduction in smooth muscle. Circ. Res. 2002;90:546–553. doi: 10.1161/01.res.0000012822.23273.ec. [DOI] [PubMed] [Google Scholar]
- 19.Gangopadhyay SS, Takizawa N, Gallant C, Barber AL, Je HD, Smith TC, Luna EJ, Morgan KG. Smooth muscle archvillin: a novel regulator of signaling and contractility in vascular smooth muscle. J. Cell. Sci. 2004;117:5043–5057. doi: 10.1242/jcs.01378. [DOI] [PubMed] [Google Scholar]
- 20.Fjeld CC, Denu JM. Kinetic analysis of human serine/threonine protein phosphatase 2Calpha. J. Biol. Chem. 1999;274:20336–20343. doi: 10.1074/jbc.274.29.20336. [DOI] [PubMed] [Google Scholar]
- 21.Marley AE, Sullivan JE, Carling D, Abbott WM, Smith GJ, Taylor IW, Carey F, Beri RK. Biochemical characterization and deletion analysis of recombinant human protein phosphatase 2C alpha. Biochem. J. 1996;320(Pt 3):801–806. doi: 10.1042/bj3200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 24.Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim AT, Dickson PW, Rostas JA. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J. Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- 25.Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem. Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- 26.Bork P, Brown NP, Hegyi H, Schultz J. The protein phosphatase 2C (PP2C) superfamily: detection of bacterial homologues. Protein. Sci. 1996;5:1421–1425. doi: 10.1002/pro.5560050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida A, Kameshita I, Fujisawa H. A novel protein phosphatase that dephosphorylates and regulates Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1998;273:1904–1910. doi: 10.1074/jbc.273.4.1904. [DOI] [PubMed] [Google Scholar]
- 28.Dosemeci A, Gollop N, Jaffe H. Identification of a major autophosphorylation site on postsynaptic density-associated Ca2+/calmodulin-dependent protein kinase. J. Biol. Chem. 1994;269:31330–31333. [PubMed] [Google Scholar]
- 29.Lo SH. Focal adhesions: what's new inside. Dev. Biol. 2006;294:280–291. doi: 10.1016/j.ydbio.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.