Abstract
Knockdown of the tumor suppressor phosphatase PTEN with shRNA in three estrogen receptor (ER)-positive breast cancer cell lines resulted in increased PI3K and AKT activities, resistance to tamoxifen and fulvestrant, and hormone-independent growth. PTEN knockdown induced the upregulation of ER transcriptional activity in MCF-7 cells, but decreased ER protein levels and transcriptional activity in T47D and MDA-361 cells. Tamoxifen and fulvestrant treatment inhibited estradiol-induced ER transcriptional activity in all shPTEN cell lines but did not abrogate the increased cell proliferation induced by PTEN knockdown. PTEN knockdown increased basal and ligand-induced activation of the IGF-I and ErbB3 receptor tyrosine kinases, and prolonged the association of the p85 PI3K subunit with the IGF-IR effector IRS-1 and with ErbB3, implicating PTEN in the modulation of signaling upstream of PI3K. Consistent with these data, PTEN levels inversely correlated with levels of tyrosine-phosphorylated IGF-IR in tissue lysate arrays of primary breast cancers. Inhibition of IGF-IR and/or ErbB2-mediated activation of ErbB3 with tyrosine kinase inhibitors restored hormone-dependence and the growth inhibitory effect of tamoxifen and fulvestrant on shPTEN cells, suggesting that co-targeting both ER and receptor tyrosine kinase pathways holds promise for the treatment of patients with ER+, PTEN-deficient breast cancers.
Keywords: PTEN, antiestrogen, breast cancer, IGF-IR, ErbB3
Introduction
Loss-of-function mutations of the Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) tumor suppressor gene occur in 5–45% of human cancers (1), with reduced PTEN protein found in 31–48% of breast cancers (2–4). The main tumor suppressive action of PTEN is its lipid phosphatase activity to antagonize phosphatidylinositol-3 kinase (PI3K) by dephosphorylating its product, phosphatidylinositol (3,4,5)-trisphosphate (PIP3), resulting in inhibition of the serine/threonine kinase AKT and other pleckstrin homology domain-containing proteins which modulate cell growth, survival, and angiogenesis. PTEN can also act as a protein phosphatase with targets including focal adhesion kinase (FAK) (5), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) (6), and itself (7), and as a binding partner to increase p53 activity (8).
Two-thirds of breast cancers express estrogen receptor-α (ER), which drives breast cancer cell growth. Although endocrine therapies designed to block estrogen action (e.g. tamoxifen, aromatase inhibitors) have changed the natural history of hormone-dependent breast cancer, many tumors exhibit de novo or acquired therapeutic resistance. Crosstalk between receptor tyrosine kinase (RTK) and ER signaling promotes resistance to endocrine therapy (9). Tumor overexpression of RTKs and RTK ligands, and increased RTK pathway activation, have been linked to antiestrogen resistance (10–12). For example, the RTK effectors AKT and MAPK can phosphorylate ER (13, 14), and MAPK can phosphorylate the ER coactivator AIB1, to promote ER transcriptional activity (15). In turn, ER drives transcription of insulin-like growth factor-I (IGF-I), IGF-II, IGF-I receptor (IGF-IR), and its effector insulin receptor substrate-1 (IRS-1). Activated IRS-1 induces PI3K activation (16) and is stabilized by ER (17). Furthermore, estrogen induces the expression of genes encoding the EGFR ligands TGFα and amphiregulin (18, 19) and can activate EGFR, HER2/ErbB2, and downstream signal transducers by transcription-independent mechanisms (20). While the traditional role of ER as a transcription factor is central to ER+ breast cancer, ER has also been implicated in non-genomic, plasma membrane-initiated signaling with IGF-IR, EGFR, and PI3K (21, 22).
Mutational activation of the PI3K pathway, by PTEN loss and/or gain-of-function mutations in PIK3CA (which encodes the PI3K p110α catalytic subunit), occurs in 56–62% of ER+ breast cancers (23, 24). Patients with cancers exhibiting a gene expression signature of PTEN loss show poor disease outcome (24). While PI3K mutations and PTEN loss are both thought to confer increased PI3K activity, the cellular effects of these mutations may be different, as suggested by the coexistence of these alterations in 5–14% of primary breast tumors (2, 23, 24). We therefore investigated the effects of PTEN loss in three ER+ human breast cancer cell lines on PI3K activation, hormone-independent growth, and response to antiestrogens.
Methods
Cell lines
MCF-7, T47D, and MDA-361 cells (ATCC) were stably transduced with retrovirus encoding shRNA targeting PTEN or mismatch control (shMM) (as in Supplementary Methods). Experiments were performed using phenol red-free IMEM + dextran-charcoal-treated-FBS (DCC-FBS, Hyclone) unless otherwise indicated.
Phospholipid analysis
MCF-7 lines were labeled × 16 hrs with 100 µCi/mL [32P]-orthophosphate (Perkin-Elmer) in phosphate-free DMEM + 10% dialyzed FBS (Hyclone). Radiolabeled lipids were extracted, concentrated, and separated by thin-layer chromatography as described (25). 32P incorporation into phosphatidylinositol species was detected by autoradiography.
Cell proliferations assays
Cells were seeded in triplicate in 12-well plates (2.5×104 per well). The next day, medium was changed to IMEM + DCC-FBS +/− 17-β-estradiol (E2), 4-hydroxytamoxifen (4-OH-T), fulvestrant (faslodex, ICI182780, gift from AstraZeneca), testosterone, letrozole (Lz, gift from Dean Evans, Novartis), the allosteric AKT1/2 inhibitor 0360263-1 [AKTi (26)], BEZ235 (27), AEW541 (28) (both provided by Carlos Garcia-Echeverria, Novartis), or lapatinib ditosylate (GW-572016, LC Laboratories). For siRNA of ErbB3/HER3, cells were transfected as in Supplementary Methods. Media were refreshed every 2–3 days, and after 5–8 days cells were trypsinized and counted using a Coulter counter.
ER transcriptional reporter assays
Cells were plated as above and transfected with pGLB-MERE (provided by Dorraya El-Ashry, Univ. of Miami) and pCMV-Renilla (Promega) luciferase plasmids. Cells were then treated as above, and luciferase activity was measured 16–20 hrs later as described (29).
Immunoprecipitation and immunoblotting
Cells were treated as indicated (IGF-I and heregulin-β1, R&D Systems). Cells were lysed in NP-40 buffer plus protease and phosphatase inhibitors, sonicated for 10 sec., centrifuged at 14k rpm for 10 min., and protein was quantitated using BCA assay (Pierce). Immunoprecipitations were performed using Dynal protein-G beads (Invitrogen) and p85 Ab (Upstate) as described (30). Immunoprecipitates and cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose. Primary antibodies for immunoblotting included PTEN, IGF-IRβ, HER3, ERα (Santa Cruz Biotechnology), AKT, P-AKTS473, P-HER2Y1248, P-HER3Y1289, P-IGF-IRβY1131 (Cell Signaling), PR (Dako), P-tyr (4G10, Vanderbilt Monoclonal Antibody Core), IRS-1 (Upstate), and actin (Sigma). Phospho-RTK arrays were performed as per manufacturer’s protocol (R&D Systems).
Reverse phase protein lysate microarray analysis (RPPA)
Three hundred and eighty-three hormone receptor-positive, primary breast tumor samples were obtained from the Breast Tissue Frozen Tumor Bank at M.D. Anderson Cancer Center. Specimens were collected under IRB-approved protocols. Tumor lysates were analyzed as in Supplementary Methods (31) using antibodies against PTEN, P-IGF-IRβY1135/1136 (may cross-react with P-InsRβY1150/1151), and IGF-IRβ. Relative protein levels were quantified, and PTEN scores were plotted against P-IGF-IRβ/IGF-IRβ ratio.
Statistical analysis
In cell proliferation assays and ER transcriptional reporter assays, significant differences were determined by two-tailed t-test. In RPPA, relative units for PTEN, P-IGF-IRβ, and IGF-IRβ levels were converted to logarithms, and the relationship between PTEN and ratio of P-IGF-IRβ/IGF-IRβ was analyzed using two-tailed t-test and Pearson correlation. p<0.05 was considered significant.
Results
PTEN loss results in hormone-independent growth and antiestrogen resistance
We stably knocked-down PTEN in MCF-7, T47D, and MDA-361 ER+ breast cancer cells using shRNA targeting PTEN or mismatch control (shMM). In shPTEN cell lines, PTEN protein was undetectable and AKT phosphorylation at Ser473 (P-AKT) was upregulated compared to shMM controls (Fig. 1A). All three of these cell lines harbor activating mutations in PIK3CA, which encodes the PI3K p110α catalytic subunit (2). In addition, PTEN loss increased levels of the PI3K product phosphatidylinositol (3,4,5)-trisphosphate (PIP3) (Fig. 1B). ER levels were unchanged by PTEN loss in MCF-7 cells, but were decreased in T47D and MDA-361 cells. PTEN loss reduced progesterone receptor (PR) levels in MCF-7 and T47D cells, which is consistent with the negative correlation between PTEN and PR levels observed in human breast cancers (2).
Fig. 1. PTEN loss increases PIP3 and P-AKT, and alters ER and PR levels.
A) Cells were treated with medium containing DCC-FBS (MCF-7: 2%; T47D, MDA-361: 0.5%) × 24 hrs, and lysates were analyzed by immunoblotting with the indicated antibodies. PR was not detected in MDA-361 cells. B) MCF-7 lines were metabolically labeled with 32P-orthophosphatase in 10% dialyzed FBS × 16 hrs. Lipids were extracted, resolved by thin-layer chromatography, and 32P-PIP species were detected by autoradiography (arrows). Origin of spotting is indicated. Cell lysates were also used for immunoblotting to confirm PTEN status. Fold-changes in PR isoforms (A) and PIP3 (B) normalized to actin were determined by densitometry analysis (bar graphs).
In cell proliferation assays, shPTEN cells significantly outgrew shMM controls under hormone-depleted and 17β-estradiol (E2)-induced conditions (Fig. 2A, Fig. S1, p<0.05). In 5/5 nude mice, MCF-7/shPTEN cells formed palpable tumors (≥ 3 mm in diameter) vs. only 1/5 animals injected with MCF-7/shMM cells (Fig. S1). Although all lines were sensitive to the inhibitory effects of the selective ER modulator (SERM) 4-hydroxy-tamoxifen (4-OH-T) and the ER downregulator fulvestrant, shPTEN cells exhibited significantly increased growth compared to shMM controls (Fig. 2A, p<0.05), indicative of relative antiestrogen resistance. The hormone-independent proliferation of MDA-361/shMM cells, but not MDA-361/shPTEN cells, was inhibited by 4-OH-T and fulvestrant. Further, testosterone induced the growth of MCF-7/shPTEN but not MCF-7/shMM cells (Fig. 2A-iv, p<0.05). This response was blocked by the aromatase inhibitor letrozole, suggesting that PTEN knockdown upregulated the cellular response to E2 produced by the aromatization of testosterone. Aromatase mRNA levels were unchanged by PTEN knockdown (determined by microarray analysis, not shown). Aromatase protein was undetectable by immunoblot analysis of MCF-7 lines, but low levels of aromatase are known to be expressed in MCF-7 cells (32).
Fig. 2. PTEN loss increases hormone-independent growth and antiestrogen resistance.
A) Cell proliferation assays. MCF-7 (i, iv), T47D (ii), and MDA-361 (iii) lines were treated with medium containing DCC-FBS (MCF-7: 2%; T47D, MDA-361: 0.5%) with the indicated compounds (E2-17-β-estradiol; 4-OH-T- 4-hydroxy-tamoxifen; Fulvest- Fulvestrant; Lz- letrozole). Media and drugs were refreshed every 2–3 days. Adherent cells were counted after 5–8 days. B) ER transcriptional reporter assays. MCF-7 (i, iv), T47D (ii), and MDA-361 (iii) lines were transfected with luciferase reporter plasmids. Cells were treated as in (A), and luciferase activities were measured after 16–20 hrs. RLU- relative light units (firefly/Renilla). All data are presented as % untreated shMM control, mean of triplicates +/− SD. * p<0.05 by t-test comparing shPTEN to shMM under each condition.
PI3K/AKT pathway activation has been associated with increased ER transcriptional activity and reduced ER expression in MCF-7 cells (14, 33). Here, MCF-7/shPTEN cells showed increased basal, E2-induced, and testosterone-induced ER transcriptional reporter activity compared to shMM cells (Fig. 2B-i,iv, and Fig. S2). However, PTEN loss decreased basal and E2-stimulated ER transcriptional activity in T47D and MDA-361 cells (Fig. 2B-ii,iii, and Fig. S2), reflective of the reduced ER levels in these cells (Fig. 1). We also observed variable effects of PTEN loss on the expression of the E2-inducible genes AREG and EGR3 (18). In MCF-7 and T47D cells, PTEN knockdown increased the basal and E2-induced mRNA levels of AREG and EGR3 compared to shMM controls (Fig. S3). Opposite effects were observed in MDA-361 cells. Treatment with 4-OH-T or fulvestrant did not consistently suppress the effects of PTEN knockdown on AREG or EGR3 expression, suggesting that PTEN loss alters their expression by both ER-dependent and ER-independent mechanisms. Even though treatment with 4-OH-T, fulvestrant, or letrozole did not abrogate the growth advantage of shPTEN cell lines (Fig. 2A), these inhibitors markedly suppressed E2- and testosterone-induced ER transcriptional activity in all lines (Fig. 2B, Fig. S4). Overall, these data suggest that the increased hormone-independent growth and relative resistance to 4-OH-T and fulvestrant conferred by PTEN loss cannot be solely explained by increased ER transcriptional activity.
PTEN loss increases IGF-IR- and ErbB3-mediated activation of PI3K
We next examined mechanisms which can activate PI3K in PTEN-deficient cells. Using phospho-RTK array analysis, we detected predominant tyrosine (tyr) phosphorylation of IGF-IRβ, ErbB3/HER3, insulin receptor-β (InsRβ), and EGFR in MCF-7 cells (Fig. 3A-i), and of EGFR, HER2, and HER3 in T47D and MDA-361 cells (Figs. 3A-ii,iii). PTEN loss upregulated tyr phosphorylation of EGFR, InsRβ, and IGF-IRβ in MCF-7 cells, and of EGFR, HER3, ErbB4/HER4, and ROR2 in T47D cells. We next used a loss-of-function approach to assess which RTKs activated PI3K, measuring P-AKTS473 as a surrogate of PI3K activity. The IGF-IR tyr kinase inhibitor (TKI) AEW541 reduced P-AKT in MCF-7 cells (Fig. 3B-i), whereas the EGFR/HER2 TKI lapatinib reduced P-AKT in T47D and MDA-361 cells (Figs. 3B-ii,iii). Treatment with lapatinib decreased P-HER3 in all three cell lines (Fig. S4), suggesting that HER3 activation is EGFR/HER2-dependent in these cells, and that HER3 is the predominant activator of PI3K in T47D and MDA-361 cells. In all shPTEN lines, the combination of AEW541 plus lapatinib inhibited P-AKT more effectively than either drug alone. The allosteric AKT1/2 inhibitor 0360263-1 (AKTi) and the PI3K/mTOR inhibitor BEZ235 decreased P-AKT in all lines.
Fig. 3. PTEN loss activates RTKs upstream of PI3K.
A) Lysates from MCF-7 (i), T47D (ii), and MDA-361 (iii) lines treated with medium containing DCC-FBS (MCF-7: 2%; T47D, MDA-361: 0.5%) overnight were used to probe phospho-RTK arrays. Antibodies against 42 RTKs are spotted in duplicate on a membrane. Membranes are incubated with cell lysates followed by probing with a P-tyr antibody. A positive signal is indicative of receptor phosphorylation. Blots from each pair of lines are exposure-matched. Tyr-phosphorylated RTKs are labeled as: 1-EGFR; 2-HER2; 3-HER3; 4-InsRβ; 5-IGF-IRβ; 6-ErbB4; 7-ROR2; 8-EphA1. Positive controls are spotted at corners. B) Lysates from MCF-7 (i), T47D (ii), and MDA-361 (iii) lines treated as in (A) overnight +/− the indicated kinase inhibitors were used for immunoblotting with the indicated antibodies. Short and long exposures (exp) are shown in (iii). C) Tumor lysates from 383 hormone receptor-positive breast cancers were analyzed by RPPA to quantify relative levels of PTEN, P-IGF-IRβY1135/1136, and IGF-IRβ. Shown is a scatterplot of PTEN vs. ratio of P-IGF-IRβ/IGF-IRβ (indicative of the fraction of activated IGF-IRβ). This relationship was analyzed using two-tailed t-test and Pearson correlation.
To ascertain whether PTEN loss is linked with IGF-IR activation in primary breast cancers, we analyzed 383 hormone receptor-positive tumors by reverse phase protein lysate microarray (RPPA) for levels of PTEN, tyr-phosphorylated IGF-IRβ (P-IGF-IRβ), and total IGF-IRβ. We observed a statistically significant inverse correlation between PTEN levels and P-IGF-IRβ/IGF-IRβ ratio (R2=0.182, p< 10−15, Fig. 3C), consistent with the results observed upon PTEN knockdown in MCF-7 cells (Fig. 3A). Since P-IGF-IRβ antibodies may cross-react with P-InsRβ, we cannot rule out that P-InsRβ may also contribute to this correlation.
Growth factor receptors activate PI3K by phosphorylating adaptor proteins such as GAB1, GAB2, IRS-1, IRS-2, and HER3. Tyr-phosphorylated adaptors engage the N-SH2 domain of the PI3K regulatory subunit p85, relieving the inhibition of the p110 catalytic subunit by p85, and recruiting the p85–p110 heterodimer to its substrate phosphatidylinositol-4,5-bisphosphate (PIP2) at the plasma membrane (34). p110 phosphorylates PIP2 to produce PIP3. The interaction between p85 and tyr-phosphorylated adaptors permits the identification of PI3K activators by their coprecipitation with p85 antibodies. Immunoprecipitation of p85 followed by immunoblot analysis showed increased p85-IRS-1 binding under basal and serum-stimulated conditions in MCF-7/shPTEN cells compared to shMM control (Fig. 4A). Similarly, T47D/shPTEN cells showed increased p85-HER3 association compared to shMM cells. PTEN loss did not significantly alter the high basal p85-HER3 association in MDA-361 cells. Additionally, MCF-7/shPTEN cells showed increased PI3K/AKT pathway sensitivity to IGF-I ligand compared to shMM control (Fig. 4B-i). In contrast, PTEN loss maximally activated PI3K under basal conditions in T47D and MDA-361 cells, while shMM cells showed increased P-AKT upon stimulation with serum or the HER3 ligand heregulin-β1 (Figs. 4B-ii,iii).
Fig. 4. PTEN loss increases PI3K activation and sensitivity to RTK ligands.
A) p85 was immunoprecipitated from cell lysates of MCF-7 (i), T47D (ii), and MDA-361 (iii) lines treated overnight +/− 10% DCC-FBS. Short and long exposures (exp) are shown in (ii). B) Immunoblotting with the indicated antibodies of i) lysates from MCF-7 cells serum-starved overnight, then treated with IGF-I (0–100 ng/mL × 15 min.); ii) lysates from T47D and MDA-361 lines treated overnight +/− 10% DCC-FBS; iii) lysates from T47D cells serum-starved overnight, then treated with heregulin-β1 (20 ng/mL × 0–12 min.).
A time course in IGF-I-stimulated MCF-7 cell lines showed that the association of p85 with IRS-1 upon ligand addition was enhanced by PTEN loss (Fig. 5A). P-IGF-IRβ was detectable after 5 min. of IGF-I stimulation and returned to baseline within 1 hr in MCF-7/shMM cells. In contrast, P-IGF-IRβ and the increased p85-IRS-1 association remained detectable for ≥3 hrs after IGF-I stimulation in MCF-7/shPTEN cells. Therefore, PTEN loss increased and prolonged the activation of IGF-IR and IRS-1. Prior work has shown that PI3K pathway activation suppresses IRS-1 expression (35). Indeed, extended IGF-I stimulation for 24 hrs modestly decreased IRS-1 levels in MCF-7/shPTEN but not MCF-7/shMM cells (Fig. S5). Therefore, PTEN loss may promote IRS-1 downregulation due to increased negative feedback from PI3K signaling.
Fig. 5. PTEN loss prolongs IGF-IR and HER3 signaling, and increases E2-induced non-genomic signaling via IGF-IR.
A) p85 was immunoprecipitated from lysates of MCF-7 cells that had been pretreated overnight with serum-free medium +/− AEW541 (1 µM), then stimulated +/− 100 ng/mL IGF-I +/− AEW541 × 5, 60, or 180 min. Arrowhead indicates P-IGF-IRβ. B) Lysates from MCF-7 cells pretreated as in (A) +/− AEW541 (1 µM) or lapatinib (1 µM), then stimulated +/− IGF-I (100 ng/mL) +/− inhibitors × 15 min. C) p85 was immunoprecipitated from lysates of T47D cells treated with 0.5% DCC-FBS +/− lapatinib [1 µM × 15, 30, 60, 120, or 180 min., or overnight (o/n)]. Short and long exposures (exp) for P-HER3 are shown. D) p85 was immunoprecipitated from lysates of MCF-7 cells pretreated overnight with 10% DCC-FBS +/− 1 µM 4-OH-T, 1 µM fulvestrant, 1 µM AEW541, or 1 µM lapatinib, and then stimulated +/− 1 nM E2 +/− inhibitors × 20 min. Arrowhead indicates tyr-phosphorylated IRS-1 (≈150 kDa). All immunoprecipitates and cell lysates were analyzed by immunoblotting with the indicated antibodies.
Additionally, we detected IGF-I-induced tyr-phosphorylation of HER3 (P-HER3, Fig. 5A), suggestive of crosstalk between IGF-IR and ErbB receptors. This phosphorylation was more robust and sustained in MCF-7/shPTEN compared to MCF-7/shMM cells and was inhibited by AEW541. IGF-I-induced activation of IGF-IR, HER3, and AKT was inhibited by either AEW541 or lapatinib, suggesting that EGFR/HER2 tyr kinase activity was required for HER3 activation in response to IGF-I (Fig. 5B, Fig. S7).
PTEN loss induced maximal activation of PI3K/AKT in T47D cells (Fig. 4B). Lapatinib inhibited PI3K in these cells, suggesting that PI3K activation was EGFR/HER2/HER3-dependent (Fig. 3A–B, Fig. 4A, Fig. S5). Therefore, we examined the temporal effect of lapatinib on HER3 inactivation and p85-HER3 interaction. T47D/shPTEN cells exhibited constitutive association between p85 and HER3, and higher levels of P-HER3 and P-AKT compared to shMM control (Fig. 5C, Fig. S7). In T47D/shPTEN cells, p85-HER3 association, P-HER3, and P-AKT remained detectable after overnight treatment with lapatinib, compared to near-complete inhibition after 2–3 hrs in T47D/shMM cells. These data suggest that, like for IGF-IR, PTEN knockdown increases and prolongs the activation of HER3 and PI3K. PTEN loss did not alter the ability of lapatinib to suppress P-HER3 and p85-HER3 association in MDA-361 cells (not shown).
PTEN loss increases non-genomic estrogen signaling via IGF-IR
Non-genomic estrogen signaling to activate PI3K and MAPK has been proposed as a mechanism of resistance to hormonal therapy (22). We found that PTEN loss enhances an E2-induced increase in P-AKT and p85-IRS-1 interaction in MCF-7 cells (Fig. 5D). This signaling was unaffected by pretreatment with 4-OH-T or fulvestrant, even though these compounds respectively increased and decreased ER protein levels. AEW541 but not lapatinib blocked p85-IRS-1 binding and P-AKT, suggesting that IGF-IR permits E2-induced PI3K activation in these cells. E2 did not increase P-AKT in T47D or MDA-361 cells (not shown).
Combined blockade of IGF-IR and ErbB signaling inhibits PTEN-deficient cell growth
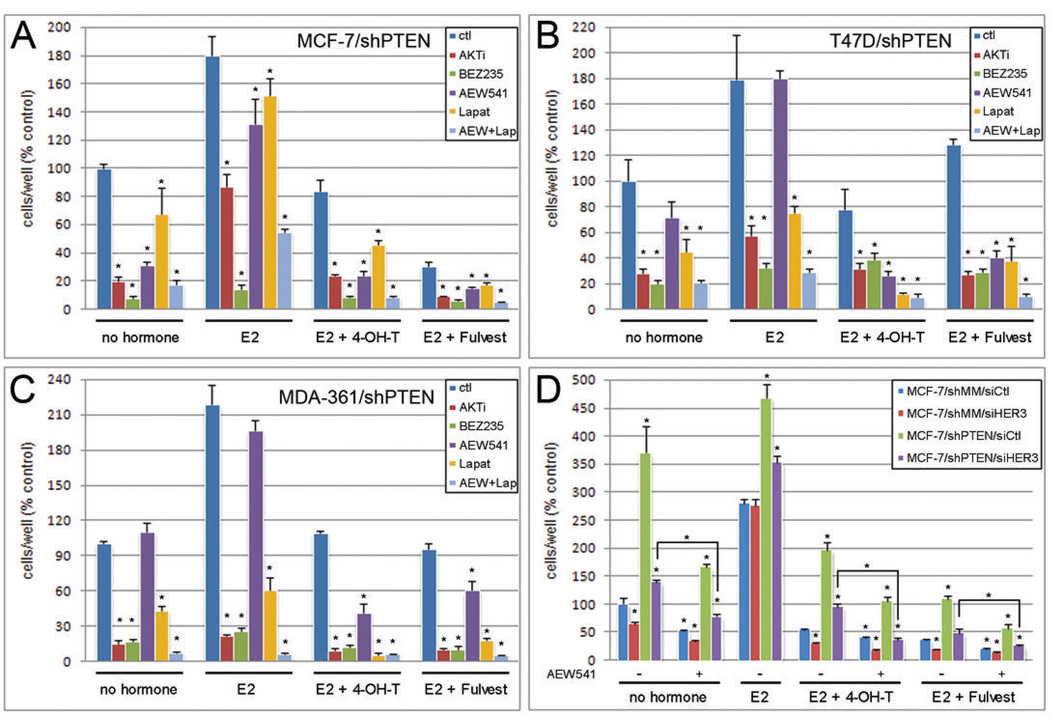
Treatment with AKTi, BEZ235, or AEW541 inhibited the growth of MCF-7/shPTEN cells in hormone-depleted medium and in the presence of tamoxifen or fulvestrant (Fig. 6A). In contrast, lapatinib was effective mainly when combined with 4-OH-T or fulvestrant. MCF-7/shPTEN cells showed increased sensitivity to AEW541 and the PI3K inhibitor LY294002 compared to shMM control (Fig. S7), suggesting that PTEN loss increases dependence upon IGF-IR and PI3K. Similarly, AKTi, BEZ235, and lapatinib significantly inhibited T47D/shPTEN (Fig. 6B) and MDA-361/shPTEN (Fig. 6C) cell growth. In these cells, AEW541 was only effective when combined with 4-OH-T or fulvestrant.
Fig. 6. Combined inhibition of IGF-IR and EGFR/HER2 synergizes with 4-OH-T, fulvestrant, and hormone-deprivation to block cell proliferation.
A) MCF-7/shPTEN, B) T47D/shPTEN, and C) MDA-361/shPTEN cells were treated with medium containing DCC-FBS (MCF-7: 2%; T47D, MDA-361: 0.5%) as indicated [0.1 nM E2 (MCF-7), 1 nM E2 (T47D, MDA-361), 1 µM 4-OH-T, 1 µM fulvestrant, 1 µM AKTi, 200 nM BEZ235, 1 µM AEW541, 1 µM lapatinib]. Media and drugs were refreshed every 2–3 days. Adherent cells were counted after 5–8 days. Data are presented as % untreated shMM control, mean of triplicates +/− SD. * p<0.05 by t-test comparing kinase inhibitor-treated cells to control (ctl) cells within each group. D) MCF-7/shPTEN and /shMM cells transfected with siRNA against HER3 or control (siCtl) were treated and analyzed as in (A). * p<0.05 by t-test compared to untreated shMM/siCtl within each group.
In all shPTEN lines, the combination of AEW541 plus lapatinib was significantly more inhibitory than either agent alone (Fig. 6). These results imply that in MCF-7 cells, IGF-IR mainly drives PI3K/AKT (Fig. 3–Fig. 5), but EGFR/HER2/HER3 may also contribute to hormone-independent growth and antiestrogen resistance (Fig. 6A). Indeed, siRNA-mediated knockdown of HER3 (verified in Fig. S8) inhibited the growth of MCF-7/shPTEN and /shMM cells in hormone-depleted medium, and sensitized them to 4-OH-T and fulvestrant (Fig. 6D). The inhibitory effect of HER3 knockdown was augmented by AEW541 treatment, implicating both HER3 and IGF-IR in the modulation of MCF-7/shPTEN cell growth. Similarly, HER3 drives PI3K in T47D and MDA-361 cells (Fig. 3–Fig. 5), but the synergistic effect of lapatinib plus AEW541 also implicates IGF-IR in the modulation of growth of T47D/shPTEN and MDA-361/shPTEN cells (Fig. 6B–C). Since these kinase inhibitors synergized with 4-OH-T, fulvestrant, and hormone-deprivation to block cell growth, PTEN-deficient, ER+ breast cancer patients may be effectively treated with drug combinations targeting ER and RTK pathways that activate PI3K.
Discussion
Herein, we show that shRNA-mediated knockdown of PTEN in three ER+ breast cancer cell lines resulted in antiestrogen resistance and hormone-independent growth by both genomic and non-genomic mechanisms. PTEN loss upregulated PI3K/AKT and enhanced IGF-IR/IRS-1 and HER3 signaling, implicating PTEN in the modulation of RTK signaling upstream of PI3K. Notably, all cell lines used herein harbor activating mutations in PIK3CA, the gene encoding p110α (2). These results suggest that 1) different signaling outputs result from PTEN loss versus PIK3CA mutations, and 2) PTEN loss is a more transforming event than PIK3CA mutations. This is consistent with reports that PIK3CA mutations and PTEN loss coexist in 5–14% of primary breast cancers (2, 23, 24). Crosstalk between IGF-IR and EGFR/HER2 was also enhanced by PTEN loss, as IGF-I-induced HER3 phosphorylation was inhibited by lapatinib (Fig. 5B, Fig. S7). Combined inhibition of these RTKs with TKIs and/or RNAi most effectively inhibited PI3K activation (Fig. 3B) and cell growth (Fig. 6).
The lipid phosphatase activity of PTEN mediates its tumor suppressive function via dephosphorylation of PIP3. However, evidence also suggests a tumor suppressor role for PTEN protein phosphatase activity. Firstly, a PTENG129E mutation was found in two kindreds of Cowden’s disease, a cancer predisposition disorder. PTENG129E lacks lipid phosphatase activity but retains protein phosphatase activity (36), inhibits cell migration (5) and epithelial-to-mesenchymal transition (37), and promotes G1 cell cycle arrest (38). Secondly, we found that PTEN loss dysregulates kinase signaling upstream of PI3K. PTEN directly modulates tyr phosphorylation of EGFR and PDGFR (6), and regulates IRS-1 activation and InsR-IRS-1 binding (39). We demonstrated that PTEN loss increases and prolongs IGF-IR and HER3 tyr-phosphorylation, p85-IRS-1 and p85-HER3 binding, and E2-induced, IGF-IR/IRS-1-dependent activation of PI3K/AKT (Fig. 3–Fig. 5, Fig. S7). Furthermore, PTEN levels were inversely correlated with P-IGF-IRβ in hormone receptor-positive breast cancers (Fig. 3C).
These findings collectively suggest that PTEN modulates RTKs and adaptors that activate PI3K, implicating PTEN in regulatory processes both upstream and downstream of PI3K. Since PTEN loss increases RTK activation and sensitivity to RTK ligands (Fig. 3–Fig. 5), we speculate that other RTK-initiated signaling pathways besides PI3K are likely to also be activated in PTEN-deficient cells. While the mechanism(s) by which PTEN regulates IGF-IR and HER3 remains unclear, possibilities include genomic effects, feedback signaling to RTKs or their adaptors (39), modulation of RTK ligand production, or PTEN binding and/or dephosphorylation of RTKs or adaptor proteins such as FAK (5). Notably, FAK is a PTEN substrate (5), and FAK phosphorylation has been implicated in IGF-IR (40), HER2/HER3 (41, 42), EGFR, and PDGFR signaling (43). To explore changes in RTK ligand production upon PTEN loss in MCF-7 cells, we utilized RT-PCR and ELISA assays for IGF-I and IGF-II, but we found no change in mRNA or protein levels (not shown).
We show that IGF-IR/ErbB crosstalk is enhanced by PTEN loss. IGF-I stimulation increased P-HER3 in direct correlation with P-IGF-IRβ, and these effects were prolonged in PTEN-deficient cells (Fig. 5A). These responses were blocked by lapatinib or AEW541 (Fig. 5B, Fig. S7), suggesting that IGF-IR and EGFR/HER2 kinases were required. IGF-IR has been shown to complex with P-HER2 upon stimulation with IGF-I or heregulin (44). IGF-IR has also been shown to activate EGFR (45). Interestingly, lapatinib suppressed IGF-I-induced IGF-IR phosphorylation (Fig. 5B, Fig. S7), suggesting that EGFR and/or HER2 kinases are also permissive of IGF-IR activation. This RTK crosstalk supports the synergistic inhibitory effect of AEW541 plus either lapatinib or HER3 knockdown on shPTEN cell growth (Fig. 6) and PI3K activation (Fig. 3B). We speculate that PTEN-deficient cancer cells utilize an alternative RTK pathway(s) to drive growth and survival when the primary PI3K-activating RTK is blocked. Dissecting the roles of IGF-IR and ErbB RTKs in heteromeric complex formation, stability, and signaling requires further evaluation, but these data suggest that inhibition of one RTK type (EGFR/HER2) may disrupt signaling of another RTK type (IGF-IR), and that combinations of TKIs should be considered for the treatment of PTEN-deficient cancers.
Breast cancer cells may maintain a balance between ER and RTK pathways. Upon treatment with 4-OH-T or hormone deprivation, breast cancer cells adapt by upregulating RTK network components (e.g. P-AKT, P-MAPK, RTK ligands) (46). In turn, RTK pathway activation modulates ER function. For example, activation of the Src tyr kinase can induce ER degradation (47). Prior findings with MCF-7 cells suggest that PI3K activation suppresses ER expression while increasing transcriptional activity (14, 33). However, we found that PTEN loss decreased both ER protein levels and transcriptional activity in T47D and MDA-361 cells (Fig. 1A, Fig. 2B, Fig. S3), indicating that the increase in ER transcriptional activity observed upon PTEN loss in MCF-7 cells (Fig. 2B, Fig. S3) is not widely applicable to all hormone-dependent breast cancers. The decreased levels of PR seen upon PTEN knockdown in MCF-7 and T47D cells (Fig. 1A) may be due to increased PR degradation or decreased gene expression (determined by microarray analysis, not shown). PI3K pathway activation has been shown to increase the expression of the E2-inducible gene Cyclin D1 while suppressing PgR expression (48). Further, heregulin-β1 stimulation of T47D cells increased PR phosphorylation at Ser294 (49) which, in turn, promotes PR degradation (50). Therefore, PI3K activation as a result of PTEN loss may upregulate or downregulate transcription of ER target genes, as well as modulate their gene products post-translationally.
In summary, we report that induced PTEN loss in hormone-dependent human breast cancer cells resulted in 1) hormone-independent growth and resistance to 4-OH-T and fulvestrant, 2) variable alterations in ER transcriptional activity, and 3) upregulation of kinase signaling upstream and downstream of PI3K. Furthermore, we showed that PTEN knockdown in three PIK3CA-mutant breast cancer cell lines confers gain-of-function effects, indicating different signaling outputs as a result of PTEN loss versus PIK3CA mutation. The use of three cell lines allowed us to discover pathways commonly dysregulated by PTEN loss in different systems, and we found that PTEN loss upregulates intact pathways upstream of PI3K. Inhibition of IGF-IR and ErbB signaling synergized with 4-OH-T, fulvestrant, and hormone-deprivation to overcome the growth advantage conferred by PTEN loss. These data hold promise for the treatment of PTEN-deficient, ER+ breast cancer patients with combinations of drugs targeting both ER and RTK/PI3K pathways.
Acknowledgments
Funding
This work was supported by the National Institutes of Health R01CA80195 (C.L.A.), F32CA121900 (T.W.M.), T32CA78136 (M.P-T.), K23CA121994, R21CA120248 (A.M.G.), Breast Cancer Specialized Program of Research Excellence (SPORE) P50CA98131, Vanderbilt-Ingram Comprehensive Cancer Center Support Grant P30CA68485; the Breast Cancer Research Foundation (C.L.A.); ACS Clinical Research Professorship Grant CRP-07-234 (C.L.A.); Kleberg Center for Molecular Markers, M. D. Anderson Cancer Center; American Society of Clinical Oncology [Career Development Award (A.M.G.); M.D. Anderson [Physician Scientist Award (B.T.H.)]; Susan G. Komen Breast Cancer Foundation [FAS0703849 (B.T.H., A.M.G., G.B.M.); American Cancer Society [IRG-58-009-49 (C.W.L.)].
References
- 1.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 2.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 3.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–676. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]
- 4.Perren A, Weng LP, Boag AH, et al. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol. 1999;155:1253–1260. doi: 10.1016/S0002-9440(10)65227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 6.Mahimainathan L, Choudhury GG. Inactivation of platelet-derived growth factor receptor by the tumor suppressor PTEN provides a novel mechanism of action of the phosphatase. J Biol Chem. 2004;279:15258–15268. doi: 10.1074/jbc.M314328200. [DOI] [PubMed] [Google Scholar]
- 7.Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A. Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science. 2004;303:1179–1181. doi: 10.1126/science.1092089. [DOI] [PubMed] [Google Scholar]
- 8.Freeman DJ, Li AG, Wei G, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 9.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the Estrogen Receptor and the HER Tyrosine Kinase Receptor Family: Molecular Mechanism and Clinical Implications for Endocrine Therapy Resistance. Endocr Rev. 2008 doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folgiero V, Avetrani P, Bon G, et al. Induction of ErbB-3 expression by alpha6beta4 integrin contributes to tamoxifen resistance in ERbeta1-negative breast carcinomas. PLoS ONE. 2008;3:e1592. doi: 10.1371/journal.pone.0001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurokawa H, Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin Cancer Res. 2003;9:511S–515S. [PubMed] [Google Scholar]
- 12.Massarweh S, Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer: new therapeutic opportunities. Clin Cancer Res. 2007;13:1950–1954. doi: 10.1158/1078-0432.CCR-06-2540. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 14.Martin MB, Franke TF, Stoica GE, et al. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 15.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadari YR, Tzahar E, Nadiv O, et al. Insulin and insulinomimetic agents induce activation of phosphatidylinositol 3'-kinase upon its association with pp185 (IRS-1) in intact rat livers. J Biol Chem. 1992;267:17483–17486. [PubMed] [Google Scholar]
- 17.Morelli C, Garofalo C, Bartucci M, Surmacz E. Estrogen receptor-alpha regulates the degradation of insulin receptor substrates 1 and 2 in breast cancer cells. Oncogene. 2003;22:4007–4016. doi: 10.1038/sj.onc.1206436. [DOI] [PubMed] [Google Scholar]
- 18.Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–1533. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- 19.Reddy KB, Yee D, Hilsenbeck SG, Coffey RJ, Osborne CK. Inhibition of estrogen-induced breast cancer cell proliferation by reduction in autocrine transforming growth factor alpha expression. Cell Growth Differ. 1994;5:1275–1282. [PubMed] [Google Scholar]
- 20.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 21.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song RX, Fan P, Yue W, Chen Y, Santen RJ. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocr Relat Cancer. 2006;13 Suppl 1:S3–S13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Tenorio G, Alkhori L, Olsson B, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 24.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–7569. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10870–10876. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- 26.Lindsley CW, Zhao Z, Leister WH, et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008 doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Echeverria C, Pearson MA, Marti A, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa H, Lenferink AE, Simpson JF, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 30.Engelman JA, Janne PA, Mermel C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibes R, Qiu Y, Lu Y, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 32.MacIndoe JH. Estradiol formation from testosterone by continuously cultured human breast cancer cells. J Clin Endocrinol Metab. 1979;49:272–277. doi: 10.1210/jcem-49-2-272. [DOI] [PubMed] [Google Scholar]
- 33.Stoica GE, Franke TF, Moroni M, et al. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. 2003;22:7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 34.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 35.Lee AV, Gooch JL, Oesterreich S, Guler RL, Yee D. Insulin-like growth factor I-induced degradation of insulin receptor substrate 1 is mediated by the 26S proteasome and blocked by phosphatidylinositol 3'-kinase inhibition. Mol Cell Biol. 2000;20:1489–1496. doi: 10.1128/mcb.20.5.1489-1496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers MP, Stolarov JP, Eng C, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie NR, Yang X, Downes CP, Weijer CJ. PtdIns(3,4,5)P(3)-dependent and - independent roles for PTEN in the control of cell migration. Curr Biol. 2007;17:115–125. doi: 10.1016/j.cub.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hlobilkova A, Guldberg P, Thullberg M, Zeuthen J, Lukas J, Bartek J. Cell cycle arrest by the PTEN tumor suppressor is target cell specific and may require protein phosphatase activity. Exp Cell Res. 2000;256:571–577. doi: 10.1006/excr.2000.4867. [DOI] [PubMed] [Google Scholar]
- 39.Vivanco I, Palaskas N, Tran C, et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. Fak and Igf-Ir Interact to Provide Survival Signals in Human Pancreatic Adenocarcinoma Cells. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vartanian T, Goodearl A, Lefebvre S, Park SK, Fischbach G. Neuregulin induces the rapid association of focal adhesion kinase with the erbB2-erbB3 receptor complex in schwann cells. Biochem Biophys Res Commun. 2000;271:414–417. doi: 10.1006/bbrc.2000.2624. [DOI] [PubMed] [Google Scholar]
- 42.Benlimame N, He Q, Jie S, et al. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J Cell Biol. 2005;171:505–516. doi: 10.1083/jcb.200504124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 44.Balana ME, Labriola L, Salatino M, et al. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene. 2001;20:34–47. doi: 10.1038/sj.onc.1204050. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad T, Farnie G, Bundred NJ, Anderson NG. The mitogenic action of insulin-like growth factor I in normal human mammary epithelial cells requires the epidermal growth factor receptor tyrosine kinase. J Biol Chem. 2004;279:1713–1719. doi: 10.1074/jbc.M306156200. [DOI] [PubMed] [Google Scholar]
- 46.Johnston SR, Martin LA, Leary A, Head J, Dowsett M. Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol. 2007;106:180–186. doi: 10.1016/j.jsbmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Chu I, Arnaout A, Loiseau S, et al. Src promotes estrogen-dependent estrogen receptor alpha proteolysis in human breast cancer. J Clin Invest. 2007;117:2205–2215. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui X, Zhang P, Deng W, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17:575–588. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 49.Labriola L, Salatino M, Proietti CJ, et al. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol Cell Biol. 2003;23:1095–1111. doi: 10.1128/MCB.23.3.1095-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]