Abstract
We hypothesized that abnormal fetal heart rate monitoring patterns (FHR-MP) occur more often in pregnancies complicated by intra-amniotic inflammation. Therefore, our objective was to examine the relationships between FHR-MP abnormalities, intra-amniotic inflammation and/or infection, acute histological chorioamnionitis and early-onset neonatal sepsis (EONS) in pregnancies complicated by preterm birth. Additionally, the ability of various FHR-MPs to predict EONS was investigated. FHR-MP from 87 singleton premature neonates delivered within 48 hours from amniocentesis [gestational age: 28.9 ± 3.3 weeks] were analyzed blindly using strict NICHD criteria. Strips were evaluated at three time points: at admission, at amniocentesis and prior to delivery. Intra-amniotic inflammation was established based on a previously validated proteomic fingerprint (MR score). Diagnoses of histological chorioamnionitis and EONS were based on well-recognized pathological, clinical and laboratory criteria. We determined that fetuses of women with severe intra-amniotic inflammation had a higher FHR baseline throughout the entire monitoring period and an increased frequency of a non-reactive FHR-MP at admission. Of all FHR-MP, a non-reassuring test at admission had 32% sensitivity, 95% specificity, 73% positive predictive value, 77% negative predictive value, and 76% accuracy in predicting EONS. Although a non-reassuring FHR-MP at admission was significantly associated with EONS after correcting for gestational age (OR: 5.6 [95%CI: 1.2–26.2], p=0.030), the majority of the neonates that developed EONS had an overall reassuring FHR-MP. Non-reassuring FHR-MPs at either amniocentesis or delivery had no association with EONS. We conclude that in cases complicated by preterm birth, a non-reassuring FHR-MP at the initial evaluation is a specific but not a sensitive predictor of EONS. An abnormal FHR-MP can thus raise the level of awareness that a fetus with EONS may be born, but is not a useful clinical indicator of the need for antibiotic treatment of the neonate.
INTRODUCTION
The autonomic nervous system is a key component of the fetal neuroendocrine response to stress. 1 The primary function of the parasympathetic element is to coordinate the behavior related to preservation and conservation of fetal body energy via the vagus nerve, whose stimulation by the cardio-inhibitory centers of the medulla oblongata force fetal bradycardia. In contrast, sympathetic stimulation is followed by vasoconstriction and fetal tachycardia preparing the fetus for the challenges of a stressful situation. It is generally assumed that fetal heart rate (FHR) variability increases with gestation, reflecting maturation of the fetal autonomic nervous system.2 Therefore, maturation and maintenance of the structural and functional integrity of the fetal autonomic nervous system is responsible for the changes in FHR activity observed in utero, in both normal and abnormal conditions such as intra-amniotic inflammation.3
The fetus relies on the placenta to assure adequate oxygen and nutritional transfer from the mother. However, in the context of an acute or chronic placental inflammatory dysfunction, abnormal biophysical profile scores and FHR monitoring patterns (FHR-MP) may become the first clinical manifestation of such an intra-uterine process.3,4 This paradigm is supported by the clinical and histopathological evidence that abnormal FHR-MP are more frequently present in association with acute umbilical cord vasculitis (funisitis) and clinical chorioamnionitis. 5
We have recently demonstrated that proteomic analysis of amniotic fluid (AF) shows presence of biomarkers characteristic of intrauterine inflammation. 6 We prospectively validated the clinical utility of one such proteomic profile, the Mass Restricted (MR) score as a diagnostic test of intra-amniotic inflammation. We have demonstrated that women with MR scores 3–4 are more likely to have preterm birth, histological chorioamnionitis, funisitis, and deliver babies with EONS. 6,7,8 With regard to preterm birth, our prospective study further determined that even women with “minimal” inflammation (MR scores 1–2) have a shorter interval to delivery compared to the women with “no” inflammation (MR score 0). 6 The amniocentesis however, can be technically difficult and its role in the clinical management of patients presenting with preterm labor (PTL) or signs or symptoms of chorioamnionitis is subject to debate. Therefore, identification of non-invasive surrogate markers that can diagnose histological chorioamniontis and predict EONS is critical.
Previous studies found that abnormal FHR-MPs are associated with histological evidence of inflammation and neonatal sepsis 3,5,9,10,11 However, in the absence of an antenatal assessment of the intra-amniotic environment for inflammation and infection such relationships remain with limited significance since histological markers of acute chorioamnionitis are also found in relationship to labor at term and vary significantly with the site of membrane rupture.12 We hypothesize that abnormal FHR-MPs occur more often in pregnancies complicated by intra-amniotic inflammation. Therefore, the purpose of this study was to examine the relationships between FHR-MP abnormalities, intra-amniotic inflammation, histological chorioamnionitis and early-onset neonatal sepsis (EONS) in pregnancies complicated by preterm birth. Additionally, the ability of various FHR-MPs to predict EONS at birth was investigated.
METHODS
Patient population and study design
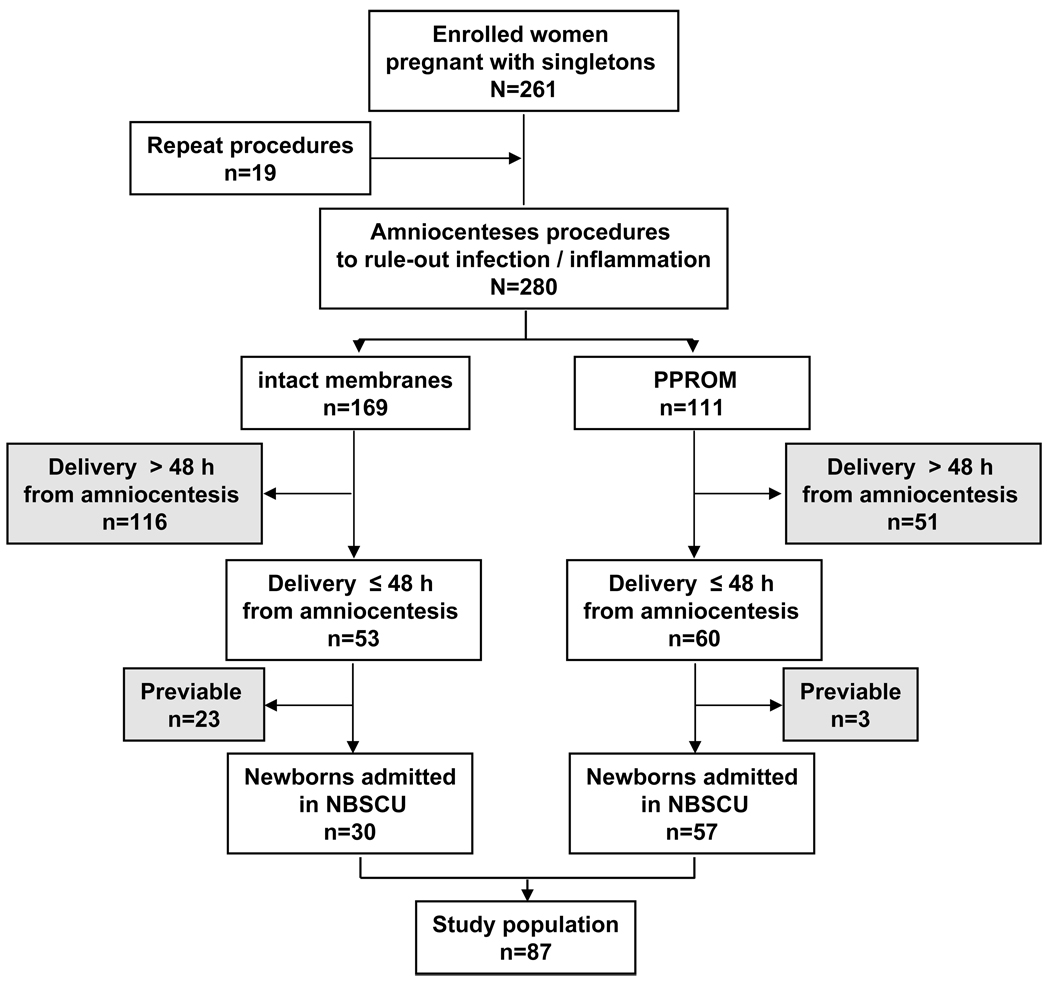
We analyzed a prospective cohort of 261 women pregnant with singletons recruited following admission to the Labor and Birth unit or the High Risk Service at Yale New Haven Hospital (YNHH) from February 2004 to April 2007. The Human Investigation Committee of Yale University approved the study and written informed consent was obtained from all participants. In all participants, following admission with preterm labor and intact membranes or preterm premature rupture of membranes, an ultrasound-guided amniocentesis procedure was performed. Eligibility criteria for enrolment were a gestational age (GA) at amniocentesis ≤34 weeks and a clinically indicated amniocentesis to rule-out intra-amniotic inflammation and/or infection. Exclusion criteria for amniocentesis included: presence of FHR-MP requiring immediate delivery at admission (bradycardia, repeat or prolonged variable decelerations), anhydramnios, human immunodeficiency virus or hepatitis infections. Nineteen women had more than one amniocentesis procedure performed thus a total of 280 procedures were performed in the study period.
Gestational age was established based on an ultrasonographic examination prior to 20 weeks. We defined preterm labor as the presence of regular uterine contractions and documented cervical effacement and/or dilatation (≥3 cm).13. PPROM was confirmed by vaginal AF “pooling”, “nitrazine”, “ferning” or amniocentesis-dye positive tests. In all cases, clinical management was made independent of our research protocol. In the absence of clinical laboratory results suggestive of infection, or signs/symptoms of clinical chorioamnionitis (fever ≥100.4° F, abdominal tenderness, fetal tachycardia), and/or abnormalities of FHR (variable or late decelerations), and/or abruption, PPROM was managed expectantly. Following amniocentesis, we prospectively followed each woman up to the time of delivery. Induction of labor or a surgical delivery (indicated delivery) was performed in 66% (57/87) of the women for clinical indications such as AF laboratory results traditionally considered to indicate intra-amniotic inflammation/infection, prolapsed umbilical cord and/or GA ≥34 weeks in the context of PPROM. 14,15 For the purpose of the current investigation, and similar to other authors, 9 we restricted our analysis of FHR-MP to the cases where delivery (either spontaneous or indicated) has occurred within 48 hours from the time of the amniocentesis (n=113) and resulted in a viable newborn admitted in the Newborn Special Care Unit at YNHH (n=87). The standardization of the amniocentesis-to-delivery interval allowed us to minimize the bias to the null impacted by events occurring post-amniocentesis. A flow-chart of the cases analyzed as part of the present study relative to the prospective cohort of enrolled patients is provided in Figure 1.
Figure 1. Flow-chart of women enrolled in the study and included in the final analysis.
Abbreviations: PPROM: Preterm premature rupture of membranes; NBSCU: Newborn Special Care Unit at Yale New Haven Hospital
Forty-five percent (39/87) of women were exposed to corticosteroids within 24 hours prior to amniocentesis and 63% (55/87) received at least one dose of steroids 72 hours prior to delivery. Eighty-six percent of women (75/87) received at least one dose of antibiotic prior to birth based on the American Collage of Obstetrics and Gynecology protocols recommended for management of PPROM or prophylaxis for group B Streptococcus infection. 16,17
FHR monitoring and pattern interpretation
In all 87 fetuses included in the analysis continuous FHR monitoring by cardiotocography was initiated at the time of admission and continued up to the point of delivery. For each case we prospectively recorded the time of amniocentesis procedure, steroid administration and the time of delivery. We applied our analysis of the FHR-MP to three segments of the monitoring period: time of hospital admission (annotated as initial evaluation: IEV), time of amniocentesis (annotated as amniocentesis evaluation: AEV - as close as possible to the time of amniocentesis) and prior to delivery (annotated as delivery evaluation: DEV - as close as possible from the time of delivery, preferably within 1 hour). Tracings were evaluated and scored independently by three Maternal-Fetal Medicine specialists (SAR, MC, CMP), but the final data entries were only following full agreement among the three investigators. All three researchers were certified by the National Certification Corporation (www.nccnet.org) for reading and interpretation of FHR-MP based on the National Institute of Child Health and Human Development (NICHD) research guidelines recommendations.18 In all cases the FHR-MP interpretation team was blinded to the results of the AF analysis, pathological examination of the placenta, neonatal hematological indices and sepsis categorization. Briefly, we interpreted the characteristics of the FHR-MP based on GA.18 We followed the NICHD guidelines to define and interpret the FHR baseline rate and variability, presence or absence of accelerations or of episodic variable decelerations, as well as any changes or trends in the FHR-MP over time. Bradycardia was defined as a baseline FHR < 110 beats/minute, while tachycardia was established when the baseline FHR was > 160 beats/minute. The NICHD guidelines define an acceleration as 10 beats/minute × 10 seconds at < 32 weeks GA and as 15 beats/minute × 15 seconds after 32 weeks GA. We used presence of 2 or more accelerations in 20 minutes of recording to define a reactive non-stress test. We defined a non-reassuring FHR-MP in the presence of recurrent late deceleration, severe variable deceleration, prolonged deceleration and fetal bradycardia with absent FHR variability. 18,19
Diagnosis of intra-amniotic inflammation and infection
Following amniocentesis the AF was cultured for aerobic and anaerobic bacteria, and Ureaplasma and Mycoplasma species. Rapid clinical laboratory results for glucose (cut-off ≤15 mg/dL), 20 lactate dehydrogenase (LDH) activity (cut-off ≥419 U/L), 21 Gram stain and white blood cell count (WBC) were available to the primary care providers for clinical management.
We generated a proteomic MR score profile immediately after the procedure by using fresh biological samples of AF in a research setting. The MR score was generated using SELDI (surface-enhanced-laser-desorption-ionization) mass spectrometry. The methodology for generation of the MR score has been previously described. 22 Briefly, 5-µl of AF was placed on spots of duplicate H4 arrays (8-spot H4 array, Ciphergen Biosystems, Fremont, California). After 1-h of incubation the arrays were read in the ProteinChip Reader (Model PBS IIC) (Ciphergen Biosystems) using the ProteinChip Software 3.1.1. Peaks comprising the MR score were identified by their conspicuous aspect at or in proximity of their known respective masses: 3377.0 and 3448.1 Da (corresponding to defensin-2 and defensin-1, respectively) and at 10,443.8 and 10,834.5 Da (corresponding to calgranulins C and A, respectively). 22 The MR score ranges from 0 to 4, depending upon the presence or absence of each of the four protein biomarkers. 22 A categorical value of 1 is assigned if a biomarker peak is present and 0 if absent. We also stratified the study population based on the “severity” of inflammation (MR=0 “no” inflammation; MR=1–2 “minimal” inflammation; MR=3–4 “severe” inflammation).6 The protein chip assays were scored “blindly” by an investigator (IAB) unaware of the FHR-MP, or either clinical presentation or outcome.
Immunoassay procedures
We performed ELISAs for human IL-6 (Pierce-Endogen, Rockford, IL) and MMP-8 (R&D Systems, Minneapolis, MN) in duplicate according to the manufacturers’ instructions by investigators unaware of sample origin. The minimal detectable concentration for interleukin −6 (IL-6) was 1 pg/mL and less than 0.02 ng/mL for matrix metalloprotease-8 (MMP-8). The inter- and intra-assay coefficients of variation were <10% for IL-6 and <6% for MMP-8, respectively. An AF concentration above 11.4-ng/mL for IL-6 and 23-ng/mL for MMP-8 were considered indicative of intra-amniotic inflammation/infection. 23,24
Histological evaluation of the placenta for acute inflammation
We had tissue sections available for histological analysis in 81/87 of the women who also provided AF samples. Sections were read by a perinatal pathologist (EZ), unaware of the results of the FHR-MP, proteomic profiling of the AF or the neonatal hematological indices and sepsis categorization. From each placenta, sections of chorionic plate, extraplacental membranes and umbilical cord were examined systematically for inflammation. Three histological stages of acute chorioamnionitis were evaluated in the chorionic plate 25 (stage I: subchorionic intervillositis, stage II: chorionic inflammation, and stage III: full thickness inflammation of both chorion and amnion), complemented by the histological grading system devised by Salafia et. al. which includes four grades of inflammation of the amnion, chorion-decidua, and umbilical cord. Funisitis was diagnosed when neutrophils infiltrated the umbilical vessels walls or Wharton’s jelly. Funisitis results were expressed using the histological grading system devised by Salafia et al. 12 as: Grade 0: no neutrophils observed; Grade 1: neutrophils within the inner third of the umbilical vein wall (umbilical phlebitis); Grade 2: neutrophils within the inner third of at least two umbilical vessels walls; Grade 3: neutrophils in the perivascular Wharton jelly or Grade 4: panvasculitis and funisitis extending deep into the Wharton jelly.
Evaluation of early-onset neonatal sepsis
With the exception of one early premature neonate who expired shortly after birth all neonates of mothers enrolled in this study (86/87) were admitted to the Newborn Special Care Unit (NBSCU) and evaluated for EONS based on hematological indices from blood specimens and cultures obtained in the first hour after birth.26,27 EONS evaluation was performed by one of the investigators (VB) who was unaware of the results of the FHR-MP interpretation, proteomic profiling of the AF or histological evaluation of the placenta. EONS was diagnosed in the presence of clinical suspicion of sepsis (signs/symptoms of which included lethargy, apnea, respiratory distress, hypoperfusion and shock) with support from laboratory results. Laboratory criteria were based on modification of the criteria of Rodwell et al. 26,27 when ≥2 of the following were observed: absolute neutrophil count (ANC) <7,500/mL or >14,500/mL, absolute band count (ABC) >1,500/mL, immature/total neutrophil ratio (I:T) ratio >16%, platelet count <150,000 cells/mm3 or abnormal spinal tap. We confirmed sepsis when either the blood and/or cerebrospinal fluid cultures were positive. For the purpose of data analysis EONS was dichotomized into present (when sepsis was either confirmed or suspected) or absent. All neonates received antibiotic therapy for at least 48 hours. The antibiotics were continued for at least 7 days if results of the blood or CSF cultures were positive.
Statistical analysis
We tested our data for normality of distribution using the Kolmogorov-Smirnov method. We present our results as mean and standard deviations (for normally distributed data sets) or medians and interquartile ranges (for non-normally distributed data). We used Student t-tests (two groups, parametric), Mann-Whitney tests (two groups, non-parametric), one-way ANOVA followed by Student-Newman-Keuls tests (three groups, parametric) or Kruskal-Wallis on ranks followed by Dunn’s tests (three groups, non-parametric) for comparisons between groups. Our correlation analyses were performed with Pearson or Spearman tests and differences between proportions were identified with the aid of Chi-square or Fisher’s exact tests. We measured test accuracy (cases correctly classified / total number of cases), sensitivity, specificity, positive (PPV) and negative predictive values (NPV) for FHR to identify fetuses with EONS by examining the distribution of individual components of the FHR on receiver operator characteristic (ROC) plots. We adjusted the odds ratios (OR) and p values with the use of multiple stepwise logistic or linear regression analysis, as appropriate. We entered the variables into the model if p< 0.05 and removed them if p> 0.1. We employed multiple stepwise linear and logistic regression analyses to adjust p values and odds ratios, respectively, for potential influences of GA or other parameters. We used Sigma Stat (v.2.03, SPSS Inc., Chicago, IL) and MedCalc (Broekstraat, Belgium) statistical softwares as aids for our analysis and considered a p <0.05 to indicate statistical significance.
RESULTS
Characteristics of women, neonates and clinical outcomes
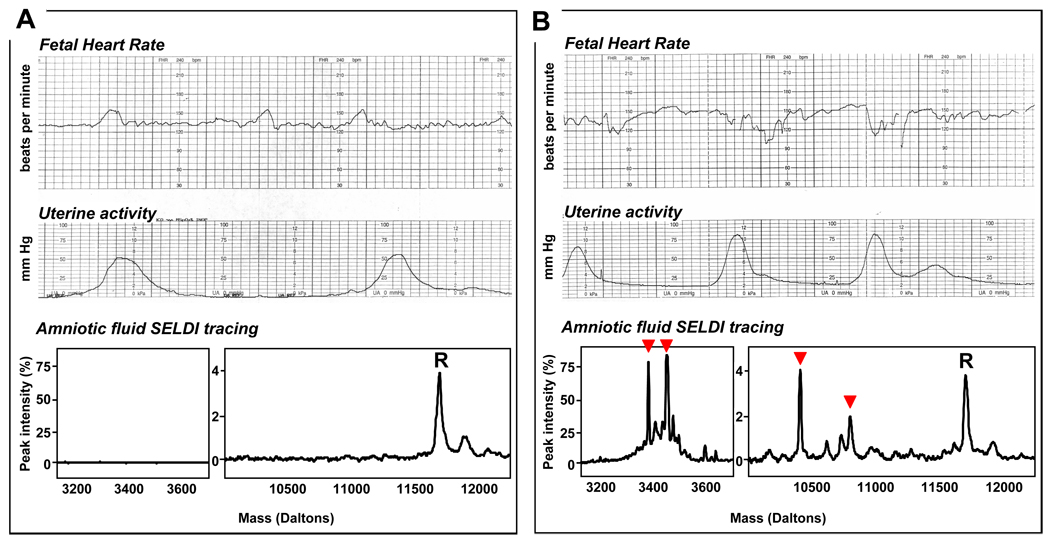
In Figure 2 we illustrate representative FHR-MPs and AF SELDI tracings of women without (Figure 2A) and with (Figure 2B) intra-amniotic inflammation. In Table 1 we present the clinical characteristics of the women at amniocentesis and the pregnancy outcome based on grades of intra-amniotic inflammation. We determined that women with “severe” intra-amniotic inflammation (MR 3–4) were more often of non-caucasian race, and of lower GA at amniocentesis and delivery compared to the other two groups. More women in the “severe inflammation” group were exposed to corticosteroids at any time during pregnancy. Lastly, women with MR scores 3–4 delivered fetuses with significantly lower birthweight (p<0.001) and lower 5-min Apgar scores compared to the women with “no” (MR 0) or “mild” (MR 1–2) AF inflammation. There was no significant difference in acid-base status at birth of the fetuses among the three groups. As expected, women with MR scores 3–4 had lower AF glucose levels, higher LDH activity and higher WBC counts compared to the women with MR score 0 or 1–2 (Table 2). A higher percentage of women with “severe” inflammation (MR 3–4) had a positive Gram stain and/or positive microbial culture result. Similarly, women with MR scores 3–4 had higher IL-6 and MMP-8 levels. These results confirm the biological and clinical relevance of the MR score for case classification.
Figure 2. Representative cardiotocographic recordings and amniotic fluid proteomic tracings Panel A.
Reactive and reassuring tracing in the context of absence of intra-amniotic inflammation (MR score of 0: none of the four biomarkers present). R represents a “reference” SELDI (surface-enhanced-laser-desorption-ionization) peak, present in all samples of amniotic fluid and corresponds to beta2-microglobulin. Panel B: Non-reactive, non-reassuring tracing in the context of intra-amniotic infection and inflammation (MR score of 4: all four biomarkers present and marked in order by arrowheads from the left to the right: neutrophil defensin-2, neutrophil defensin-1, calgranulin C and calgranulin A).
Table 1.
Demographic and clinical characteristics of women
| Variable | All | Groups | P value | ||
|---|---|---|---|---|---|
| MR 0 | MR 1–2 | MR 3–4 | |||
| n=87 | n=13 | n=23 | n=51 | ||
| Characteristics at enrollment and hospital course | |||||
| Age, years† | 28.9 ± 6.6 | 28.5 ± 6.2 | 27.4 ± 5.5 | 29.6 ± 7.0 | 0.391 |
| Race ‡ | 0.011 | ||||
| Caucasian | 35 (40) | 6 (7) | 16 (18) | 13(15) | |
| African-American | 36 (41) | 5 (6) | 5 (6) | 26 (30) | |
| Hispanic | 13 (15) | 1 (1) | 2 (2) | 10 (11) | |
| Other | 3 (3) | 1 (1) | 0 (0) | 2 (2) | |
| Non-caucasian race § | 52 (60) | 7 (54) | 7 (30) | 38 (75) | 0.001 |
| Gravidity ‡ | 2 [1–4] | 2 [1–3] | 2 [1–4] | 3 [2–5] | 0.365 |
| Parity ‡ | 1 [0–2] | 1 [0–1] | 0 [0–1] | 1 [0–2] | 0.627 |
| Gestational age, weeks† | 28.9 ± 3.3 | 31.1 ± 2.5 | 30.5 ± 2.4 | 27.6 ± 3.2 | < 0.001 |
| Ruptured membranes § | 57 (66) | 12 (92) | 14 (61) | 31 (61) | 0.088 |
| Uterine contractions § | 47 (54) | 5 (38) | 14 (61) | 28 (55) | 0.424 |
| Clinical chorioamnionitis § | 11 (13) | 0 (0) | 2 (9) | 9 (18) | 0.177 |
| History of preterm birth § | 24 (28) | 4 (31) | 7 (32) | 13 (26) | 0.859 |
| Steroid exposure during pregnancy § | 77 (89) | 9 (69) | 19 (83) | 49 (96) | 0.015 |
| Antibiotic treatment § | 75 (86) | 11 (85) | 20 (87) | 44 (86) | 0.981 |
| Tocolytic treatment § | 51 (59) | 10 (77) | 11 (48) | 30 (59) | 0.234 |
| Outcome characteristics | |||||
| Amniocentesis-to-delivery, hours‡ | 10.7 [3.6–20.6] | 16.2 [8.1–30.0] | 16.6 [4.9–29.4] | 8.3 [3.2–15.5] | 0.032 |
| Gestational age at delivery, weeks† | 29.1 ± 3.3 | 31.7 ± 2.5 | 30.6 ± 2.4 | 27.8 ± 3.1 | < 0.001 |
| Birthweight, grams† | 1,414 ± 589 | 1,863 ± 589 | 1,645 ± 395 | 1195 ± 564 | < 0.001 |
| Male sex § | 45 (52) | 8 (62) | 15 (65) | 22 (43) | 0.158 |
| Cesarean delivery § | 34 (39) | 3 (23) | 8 (35) | 23 (45) | 0.308 |
| Apgar score at 1 minute ‡ | 6 [4–8] | 7 [5–8] | 7 [5–8] | 6 [3–8] | 0.105 |
| Apgar score at 5 minutes‡ | 8 [7–9] | 9 [8–9] | 9 [8–9] | 8 [6–9] | 0.041 |
| Umbilical artery pH † | 7.32 ± 0.07 | 7.33 ± 0.04 | 7.28 ± 0.03 | 7.33 ± 0.06 | 0.161 |
| Umbilical vein pH † | 7.36 ± 0.07 | 7.36 ± 0.03 | 7.34 ± 0.09 | 7.36 ± 0.06 | 0.636 |
| Umbilical artery base deficit mmols/L† | 4.7 ± 3.0 | 3.9 ± 0.5 | 5.4 ± 3.6 | 4.5 ± 2.9 | 0.683 |
| Umbilical vein base deficit, mmols/L † | 3.7 ±2.4 | 4.0 ± 0.7 | 4.2 ± 3.1 | 3.4 ± 2.1 | 0.619 |
Data presented as mean ± standard deviation and analyzed by One-Way ANOVA among the 3 groups.
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA among the 3 groups.
Data presented as n (%) and analyzed by Chi square among the 3 groups. MR: mass restricted score.
Table 2.
Amniotic fluid analysis
| Variable | All | Groups | P value | ||
|---|---|---|---|---|---|
| MR 0 | MR 1–2 | MR 3–4 | |||
| n=87 | n=13 | n=23 | n=51 | ||
| Clinical laboratory analysis | |||||
| Glucose, mg/dL‡ | 12 [2–24] | 28 [16–40] | 23 [14–44] | 3 [2–10] | < 0.001 |
| Glucose < 10 mg/dL§ | 48 (55) | 2 (15) | 5 (23) | 41 (82) | < 0.001 |
| Glucose < 15 mg/dL§ | 39 (45) | 1 (8) | 1 (5) | 37 (74) | < 0.001 |
| LDH, U/L‡ | 266 [123–893] | 104 [26–162] | 185 [2–6470] | 811 [280–1,343] | < 0.001 |
| LDH > 419 U/L§ | 28 (37) | 1 (9) | 2 (9) | 25 (58) | < 0.001 |
| WBC, cells/mm3 | 80 [5–1,135] | 4 [2–14] | 5 [2–14] | 783 [127–2,132] | < 0.001 |
| WBC > 50 cells/mm3§ | 46 (52) | 1 (8) | 4 (17) | 41 (80) | < 0.001 |
| WBC > 100 cells/mm3§ | 41 (47) | 1 (8) | 2 (9) | 38 (75) | < 0.001 |
| RBC, cells/mm3 | 246 [19–1,045] | 250 [21–442] | 133 [7–433] | 350 [27–1,272] | 0.088 |
| Positive Gram stain § | 37 (43) | 2 (15) | 6 (26) | 29 (57) | 0.005 |
| Positive cultures § | 46 (53) | 2 (15) | 5 (22) | 39(76) | < 0.001 |
| Research laboratory analysis | |||||
| IL-6, ng/mL‡ | 7.5 [1.2–24.2] | 0.5 [0.2–0.9] | 1.5 [0.5–3.9] | 14.9 [9.0–56.6] | < 0.001 |
| IL-6 > 11.4 ng/mL§ | 31 (39) | 0 (0) | 1 (5) | 30 (65) | < 0.001 |
| MMP-8, ng/Ml‡ | 171.5 [26.1–502.8] | 7.8 [4.4–17.1] | 33.1 [8.3–49.7] | 449.2 [236.1–1,178.6] | < 0.001 |
| MMP-8 >23 ng/mL§ | 61 (76) | 3 (23) | 12 (57) | 46 (100) | < 0.001 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA among the 3 groups.
Data presented as n (%) and analyzed by Chi square among the 3 groups. (mg): milligrams; (ng): nanograms; (dL): decilitre; (U): units; (L): litre; (LDH); lactate dehydrogenase; (WBC): white blood cells; (RBC): red blood cells; (IL-6): interleukin 6; (MMP-8): matrix metalloprotease 8; MR: mass restricted score.
We present the distribution of histological acute vasculitis (funisitis) and histological chorioamnionitis in Table 3. Cases of “severe” inflammation (MR 3–4) were more likely to have advanced grades of funisitis. We further determined that the severity of chorionic plate inflammation, amnionitis and chorio-decidual inflammation was significantly higher in women with MR scores 3–4. In a prior study we have showed that only funistis grades 2–4 was significantly associated with EONS and thus deemed by us to be clinically relevant from a prognostic perspective.7 The prevalence of funistis grades 2–4 in the present study cohort reached 40% (33/81) thus confirming that a significant proportion of the cases analyzed as part of this study had advanced grades of funisitis.
Table 3.
Results of histological analysis of the umbilical cord, chorionic plate and placental membranes for acute inflammation
| Variable | All | Groups | P value | ||
|---|---|---|---|---|---|
| MR 0 | MR 1–2 | MR 3–4 | |||
| n=81 | n=11 | n=21 | n=49 | ||
| Funisitis, grades‡ | 0 [0–3] | 0 [0–0] | 0 [0–0] | 3 [0–4] | < 0.001 |
| Funisitis grade distribution§ | < 0.001 | ||||
| grade 0 (absent) | 43 (53) | 9 (11) | 18 (22) | 16 (20) | |
| grade 1 | 5 (6) | 1 (1) | 1 (1) | 3 (4) | |
| grade 2 | 5 (6) | 0 (0) | 1 (1) | 4 (5) | |
| grade 3 | 10 (12) | 1 (1) | 0 (0) | 9 (11) | |
| grade 4 | 18 (22) | 0 (0) | 1 (1) | 17 (21) | |
| Chorioamnionitis, stages‡ | 2 [0–3] | 0 [0–1] | 0 [0–1] | 3 [2–3] | < 0.001 |
| Chorioamnionitis stage distribution § | < 0.001 | ||||
| Stage 0 (absent) | 22 (27) | 7 (9) | 11 (14) | 5 (5) | |
| Stage I | 10 (12) | 2 (2) | 5 (6) | 3 (4) | |
| Stage II | 12 (15) | 1 (1) | 1 (1) | 10 (12) | |
| Stage III | 37 (46) | 1 (1) | 4 (5) | 32 (40) | |
| Amnionitis, grades‡ | 2 [0–3] | 0 [0–0] | 0 [0–0] | 3 [2–3] | < 0.001 |
| Amnionitis gr. 2–4 § | 43 (53) | 10 (9) | 4 (19) | 38 (78) | < 0.001 |
| Choriodeciduitis, grades‡ | 3 [2–3] | 0 [0–2] | 1 [0–3] | 3 [2–3] | < 0.001 |
| Choriodeciduitis gr. 2–4 § | 62 (57) | 5 (45) | 10 (48) | 47 (98) | < 0.001 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA among the 3 groups.
Data presented as n (%) and analyzed by Chi square among all groups. MR: mass restricted score
In Table 4 we show the relationship between the severity of intra-amniotic inflammation by MR score and neonatal hematological indices used to define EONS. We determined that fetuses delivered by mothers with MR scores 3–4 were more frequently anemic, lymphopenic, had a higher neutrophil count with significant bandemia. These changes resulted in significantly altered ABC and I:T ratios in the neonates born from mothers with AF MR scores 3–4 compared to those from pregnancies with MR scores of either 0 or 1–2. Further, fetuses delivered by women with “severe” intra-amniotic inflammation were more often diagnosed with EONS. The prevalence of newborns diagnosed with EONS in this study cohort was 30% (26/86). Four neonates had positive blood cultures. All were delivered by mothers who had AF proteomic MR scores of 4 and all had funisitis grades 3 or 4. There was a significant correlation between an MR score 3–4, presence of grade 2–4 funisitis (Spearman, r=0.516, p<0.001) and a diagnosis of EONS at birth (r=0.302, p=0.005). Similarly, newborns with a diagnosis of EONS were more likely to have a grade 2–4 of histological funisitis (r=0.360, p=0.001).
Table 4.
Results of haematological indices of the neonates admitted to Newborn Special Care Unit
| Variable | All | Groups | P value | ||
|---|---|---|---|---|---|
| MR 0 | MR 1–2 | MR 3–4 | |||
| n=86 | n=13 | n=23 | n=50 | ||
| Hematological indices | |||||
| Hematocrit, %† | 46.5 ± 5.9 | 50.0 ± 4.8 | 48.2 ± 4.6 | 45.0 ± 6.3 | 0.024 |
| Hemoglobin, g/dL† | 15.0 ± 1.9 | 15.8 ± 1.5 | 15.5 ± 1.5 | 14.5 ± 2.1 | 0.030 |
| WBC, cells × 1000/mm3† | 12.2 ± 6.2 | 10.9 ± 3.6 | 9.9 ± 3.3 | 13.6 ± 7.3 | 0.045 |
| Platelets, cells × 1000/mm3† | 256.7 ± 71.4 | 235.5 ± 84.6 | 258.7 ± 55.8 | 261.2 ± 74.5 | 0.509 |
| Segmented, %† | 32.6 ± 13.5 | 35.8 ± 14.9 | 32.3 ± 10.4 | 31.9 ± 14.4 | 0.637 |
| Bands, % ‡ | 5 [1–14] | 2 [1–6] | 1 [0–3] | 10 [4–17] | < 0.001 |
| Lymphocytes, %‡ | 44 [29–56] | 47 [31–61] | 49 [44–62] | 36 [24–51] | 0.002 |
| ANC, cells/mm3‡ | 3696 [1872–5481] | 3780 [2687–6120] | 3388 [1813–4058] | 3958 [1848–6536] | 0.532 |
| ABC, cells/mm3‡ | 443 [132–1755] | 310 [62–769] | 83 [0–386] | 785 [295–2500] | < 0.001 |
| I:T ratio, % ‡ | 45 [10–140] | 20 [8–63] | 10 [0–30] | 95 [40–170] | < 0.001 |
| Assessment for early onset neonatal sepsis (EONS) | |||||
| Diagnosis of EONS § | 26 (30) | 2 (15) | 3 (13) | 21 (42) | 0.020 |
| Positive blood cultures § | 4 (5) | 0 (0) | 0 (0) | 4 (8) | 0.221 |
Data presented as mean ± standard deviation and analyzed by One-Way ANOVA among the 3 groups;
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA among the 3 groups.
Data presented as n (%) and analyzed by Chi square among the 3 groups. MR: mass restricted score; (WBC): white blood cells; ANC: absolute neutrophil count; ABC: absolute band count; (I:T): immature/total neutrophil ratio.
Characteristics of the FHR monitoring patterns. Relationships with severe intra-amniotic inflammation and funisitis
Given that we did not find statistically significant differences for any of the variables presented in Table 1–Table 4 between the fetuses with MR scores of 0 and MR scores of 1–2, we further grouped fetuses with MR scores of 0, 1 and 2 as we did in our initial study 22 and analyzed them comparatively for changes in FHR-MP against fetuses with “severe inflammation” (MR scores 3 or 4). In Table 5 we outline the time frames of our FHR-MP analysis relative to the time of initial evaluation (IEV), amniocentesis (AEV) and delivery (DEV). In 80% (69/87) of the women we conducted our analysis of the FHR-MP at the time of IEV on a segment of the FHR record which was different from that of the AEV. In the remaining 20% (18/87) of the women, the AEV coincided with IEV, suggesting that in such clinical scenarios the amniocentesis was performed immediately following admission. Altogether, we performed our analysis for the IEV 3.4 [1.0–8.7] hours prior to the time of amniocentesis and 17.2 [8.1–30.1] hours prior to delivery. When we excluded the 18 women where IEV coincided with AEV time, the IEV preceded AEV by 4.9 [2.0–10.0] hours. Evaluation of the FHR-MP adjacent to the amniocentesis concentrated on an interval of ± 19 [0–53] minutes from the recorded time of the procedure and preceded the time of birth by 10.0 [4.0–19.7] hours. The final analysis of the FHR-MP focused on a time interval which occurred approximately 39.0 [8–65] minutes prior to delivery. There were no significant differences in these time intervals among MR score categories.
Table 5.
Time frames of the fetal heart rate tracing analysis
| Variable | All | Groups | P value | ||
|---|---|---|---|---|---|
| MR 0 | MR 1–2 | MR 3–4 | |||
| n=87 | n=13 | n=23 | n=51 | ||
| Time IEV-to-amniocentesis, hours‡ | 3.4 [1.0–8.7] | 2.5 [1.2–5.4] | 2.6 [0–8.6] | 3.7 [1.5–10.5] | 0.365 |
| Time IEV-to-delivery, hours‡ | 17.2 [8.1–30.1] | 19.4 [7.5–33.2] | 21.9 [11.3–34.8] | 15.9 [7.5–27.0] | 0.430 |
| Time IEV-to-AEV, hours‡ | 3.1 [1.0–8.9] | 3.4 [1.3–5.4] | 2.4 [0.0–8.0] | 3.0 [1.5–10.5] | 0.479 |
| Time AEV-to-amniocentesis *, min‡ | 19 [0–53] | 46 [4–65] | 11 [0–68] | 15 [0–40] | 0.332 |
| Time AEV-to-delivery, hours‡ | 10.0 [4.0–19.7] | 16.2 [7.0–28.7] | 16.2 [5.1–28.9] | 8.3 [3.3–14.8] | 0.057 |
| Time AEV-to-DEV, hours‡ | 9.9 [2.8–19.6] | 16.2 [6.8–24.0] | 15.8 [5.0–28.0] | 6.4 [2.2–14.3] | 0.042 |
| Time IEV-to-DEV, hours‡ | 16.8 [6.8–29.3] | 18.0 [7.2–28.6] | 20.7 [10.3–34.2] | 14.2 [5.6–25.7] | 0.305 |
| Time DEV-to-delivery, min‡ | 39 [8–65] | 19 [0–59] | 38 [4–59] | 51 [9–85] | 0.345 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA among the 3 groups.
Time relative to amniocentesis in absolute value (i.e. irrespective if earlier or later); MR: mass restricted score; (IEV): initial evaluation; (AEV): time of amniocentesis; (DEV): delivery evaluation.
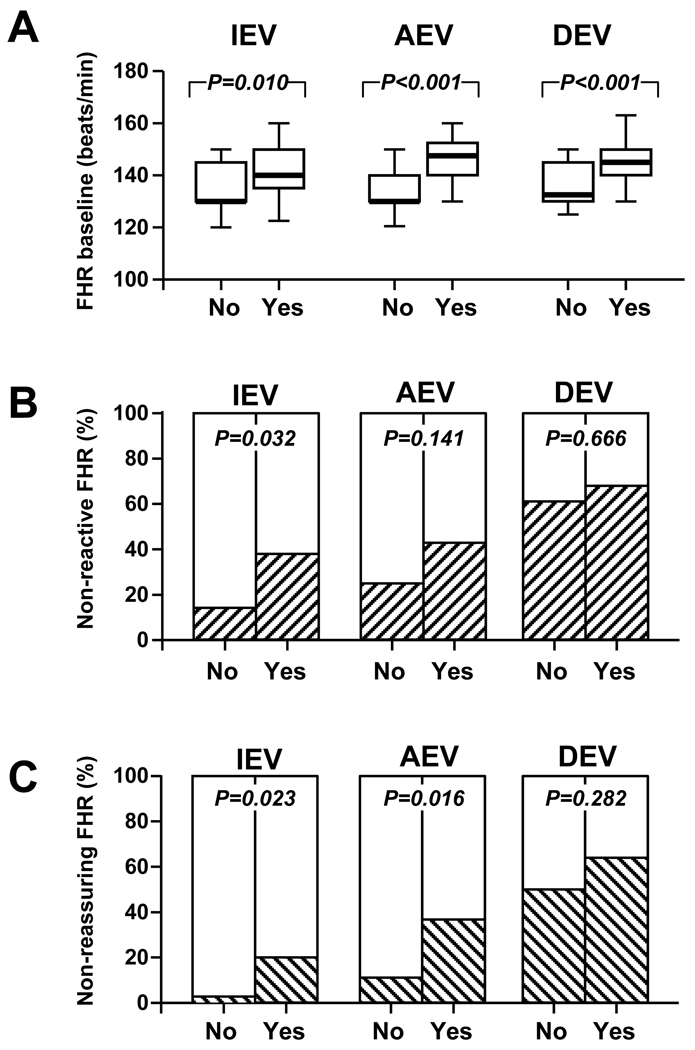
We analyzed the FHR baseline and provided a qualitative interpretation of the pattern as reactive/non-reactive and reassuring/non-reassuring relative to the presence of an MR score indicative of “severe” inflammation (MR score 3–4) at the time of IEV, AEV and DEV (Figure 3). In essence, we determined that fetuses of the women with “severe inflammation” had a higher FHR baseline throughout the entire monitoring period (Figure 3A) and an increased frequency of a non-reactive FHR-MP at IEV (Figure 3B). We observed that out of the 87 fetuses included in our study, only eight had at least one episode of fetal tachycardia (7/87) or bradycardia (1/87) in any of the intervals we assessed. Interestingly, all eight fetuses had MR scores 3–4 and positive AF culture results. All fetuses had histological evidence of funisitis at delivery and 7/8 had grades 3–4 vasculitis. Three neonates had EONS. However, although the occurrence of fetal tachycardia was statistically higher in fetuses with “severe” intra-amniotic inflammation (Fisher’s exact: p=0.038), in only 2/8 cases maternal fever was a confounder. In these select cases, all changes in FHR occurred in the context of a normal acid base status (median ± SD, arterial pH at birth: 7.31±0.03). “Severe” intra-amniotic inflammation manifested with an increased frequency of a non-reassuring FHR at IEV and AEV (Figure 3C).
Figure 3. Fetal heart monitoring patterns and severe intra-amniotic inflammation by MR score Panel A.
FHR baseline levels of fetuses with amniotic fluid MR scores 3 or 4 (Yes, n=51) versus those with MR scores 0, 1 or 2 (No, n=36). The ends of the boxes define the 25th and 75th percentiles, the line inside the box defines the median and the whiskers show the largest and smallest values. Statistical comparisons: Mann-Whitney tests. Panel B: Percent of fetuses with non-reactive FHR monitoring patterns (hashed bars) in the group with MR scores 3 or 4 (Yes) versus those with MR scores 0, 1 or 2 (No). Statistical comparisons: Chi square tests. Panel C: Percent of fetuses with non-reassuring FHR monitoring patterns (hashed bars) in the group with MR scores 3 or 4 (Yes) versus those with MR scores 0, 1 or 2 (No). Statistical comparisons: Chi square tests. IEV (initial evaluation), AEV (amniocentesis evaluation) and DEV (delivery evaluation) represent the times when the strips were scored.
When we evaluated the FHR-MP by the results of the AF cultures we found that fetuses with positive cultures more often had higher FHR median baselines at AEV (p=0.006) and DEV (p=0.008), and a non-reassuring pattern at IEV (p=0.017) and AEV (p=0.002) compared to women with negative AF cultures.
We further performed analysis of the relationship between the FHR-MP and presence of clinically relevant histological funisitis (grade 2–4) (Figure 4). We determined that fetuses with histological funisitis are more likely to have a higher FHR baseline at IEV, AEV and DEV in comparison to fetuses without histological funistis (Figure 4A). We noted a higher frequency of a non-reactive, non-reassuring FHR-MP of the fetuses with funisitis at the time of IEV (Figure 4B & 4C).
Figure 4. Fetal heart monitoring patterns and funisitis (grades 2–4) Panel A.
FHR baseline levels of fetuses with grades 2–4 funisitis (Yes, n=33) versus those without funisitis or with grade 1 funisitis (No, n=48). The ends of the boxes define the 25th and 75th percentiles, the line inside the box defines the median and the whiskers show the largest and smallest values. Statistical comparisons: Mann-Whitney tests. Panel B: Percent of fetuses with non-reactive FHR monitoring patterns (hashed bars) in the group of fetuses with grades 2–4 funisitis (Yes, n=33) versus those without or with grade 1 funisitis (No, n=48). Statistical comparisons: Chi square tests. Panel C: Percent of fetuses with non-reassuring FHR monitoring patterns (hashed bars) in the group with MR scores 3 or 4 (Yes) versus those with MR scores 0, 1 or 2 (No). Statistical comparisons: Fisher’s exact or Chi square tests. IEV (initial evaluation), AEV (amniocentesis evaluation) and DEV (delivery evaluation) represent the times when the strips were scored.
A non-reactive or a non-reassuring FHR-MP at delivery had no relationship with either intra-amniotic inflammation (non-reactive: r=0.071, p=0.513; non-reassuring: r=0.140, p=0.198) or funisitis (non-reactive: r=0.097, p=0.390; non-reassuring: r=0.166, p=0.141). Sixty percent (56/87) of all fetuses had a non-reactive FHR-MP, and at least 50% (50/87) a non-reassuring FHR-MP independent of intra-amniotic infection, inflammation, funisitis and acid-base abnormalities (p>0.05). Events related to labor management (i.e. medication, steroids) may significantly impact on FHR and transiently suppress several parameters proposed as reflective of fetal well-being, leading to an erroneous diagnosis of fetal distress. 28,29 Therefore, we aimed to determine the possible effect of steroids on FHR-MPs in women with intra-amniotic inflammation. We first determined that out of all the fetuses exposed to steroids within 72 hours prior to delivery, 56% (31/55) have been already exposed prior to our IEV and 84% (46/55) prior to AEV. This was indicative that the cumulative proportion of fetuses that were exposed to at least one dose of steroids prior to the windows of our analysis increased (Chi square p<0.001). For the exposed fetuses there was no difference in the median time exposure prior to IEV and AEV (IEV: 4.3 [1.3–9.0] vs. AEV: 5.5 [2.2–13.8] hours, p=0.483). The time of exposure prior to DEV was significantly longer (18.3 [7.8–38.3] hours, p<0.001) compared to that prior to either IEV or AEV. In logistic regression we determined that a non-reactive or a non-reassuring FHR-MP at IEV were significantly dependent on GA at assessment (non-reactive p=0.009, non-reassuring p=0.013) but not on steroid exposure. A non-reactive or a non-reassuring FHR-MP at AEV was significantly related to a steroid exposure within 24 hours from the assessment (p=0.027) and an MR score 3–4 (p=0.006). A non-reassuring but not a non-reactive pattern at DEV was determined by the steroid exposure within 24 hours from the assessment (p=0.002) and GA (p=0.022).
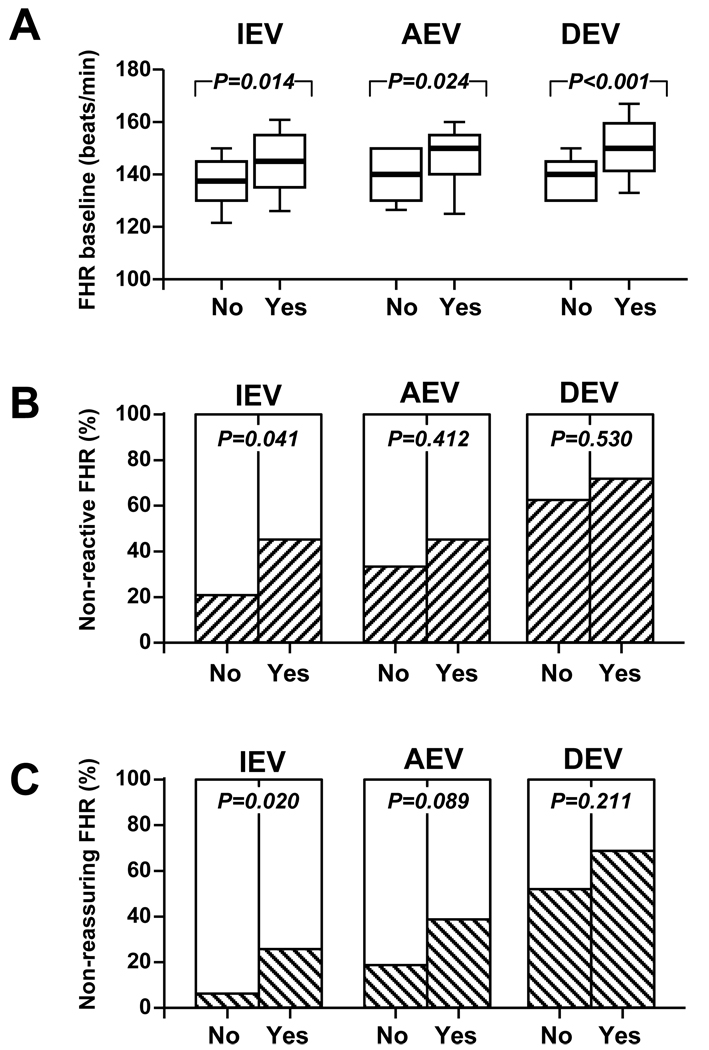
FHR (FHR) monitoring patterns (FHR-MP) and prediction of EONS
In Figure 5 we illustrate the FHR baseline (Figure 5A) and qualitative interpretations of the FHR-MP as reactive/non-reactive (Figure 5B) and reassuring/non-reassuring (Figure 5C) in relationship to a diagnosis of EONS. We determined that only 5% of the neonates without EONS (3/60) had a non-reassuring FHR-MP at IEV. This was in significant contrast with the 35% (9/26) of the fetuses with EONS who presented with a non-reassuring FHR (no EONS vs. yes EONS, Fisher’s exact p=0.002, Spearman r=0.365, p<0.001) (Figure 5C). This suggests that at the time of IEV, the majority of the neonates diagnosed with EONS had an overall reassuring FHR-MP. There were no other significant relationship between FHR-MP and EONS. Regarding the four fetuses with positive blood cultures, two had reassuring FHR patterns at all times of assessment. Given our observations, we calculated the sensitivity, spcificity, PPV, NPV and accuracies of each of the analyzed FHR-MP in predicting EONS in the newborn (Table 6). Accuracies ranged from 41% (lowest, of a non-reactive pattern at delivery) to 76% (highest, of a non-reassuring pattern at admission). The ability of a non-reassuring FHR-MP at admission (IEV) to predict EONS was maintained after correcting for GA in multivariate logistic regression analysis (OR: 5.6 [95%CI: 1.2–26.2], p=0.030). Other variables excluded from the model were status of the membranes, antibiotics, tocolytics, time interval IEV-to-delivery, steroid exposure and number of steroid doses.
Figure 5. Fetal heart monitoring patterns and early-onset neonatal sepsis (EONS) Panel A.
FHR baseline levels of fetuses diagnosed with EONS in the immediate postnatal period (<3 days after birth) (Yes, n=26) versus those without or EONS (No, n=60). The ends of the boxes define the 25th and 75th percentiles, the line inside the box defines the median and the whiskers show the largest and smallest values. Statistical comparisons: Mann-Whitney tests Panel B: Percent of fetuses with non-reactive FHR monitoring patterns (hashed bars) in the group of fetuses diagnosed postnatally with EONS (Yes) versus those without EONS (No). Panel C: Percent of fetuses with non-reassuring FHR monitoring patterns (hashed bars) in the group of fetuses diagnosed postnatally with EONS (Yes) versus those without EONS (No). Statistical comparisons: Fisher’s exact or Chi square tests. IEV (initial evaluation), AEV (amniocentesis evaluation) and DEV (delivery evaluation) represent the times when the strips were scored.
Table 6.
Accuracy of fetal heart rate (FHR) monitoring patterns (FHR-MP) in identifying newborns diagnosed with early onset neonatal sepsis (EONS).
| Variable | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| FHR baseline* | |||||
| >150 beats/min. at IEV | 28 [10–46] | 90 [82–98] | 54 [27–81] | 75 [65–85] | 71 [62–81] |
| >130 beats/min. at AEV | 83 [68–98] | 37 [25–50]] | 35 [23–47] | 85 [71–98] | 51 [40–61] |
| >130 beats/min. at DEV | 88 [76–101] | 36 [24–49] | 38 [26–51] | 88 [74–101] | 52 [42–63] |
| Non-reactive FHR-MP | |||||
| at IEV | 40 [21–59] | 78 [67–89] | 43 [23–64] | 75 [65–86] | 67 [57–77] |
| At AEV | 36 [17–55] | 66 [54–78] | 31 [14–48] | 28 [10–46] | 57 [47–68] |
| At DEV | 62 [43–80] | 32 [20–44] | 29 [17–40] | 28 [10–46] | 41 [31–52] |
| Non-reassuring FHR- MP | |||||
| At IEV | 32 [14–50] | 95 [89–101] | 73 [46–99] | 77 [67–86] | 76 [67–85] |
| At AEV | 40 [21–59] | 80 [69–90] | 45 [25–66] | 76 [65–86] | 68 [58–78] |
| At DEV | 54 [35–73] | 41 [28–53] | 29 [26–41] | 67 [51–82] | 45 [30–55] |
cut-off values correspond to the highest accuracy (minimal false negative and false positive results) based on ROC analysis. 95% confidence intervals are given in brackets. PPV: positive predictive value; NPV: negative predictive value; (IEV): initial evaluation; (AEV): time of amniocentesis; (DEV): delivery evaluation.
DISCUSSION
Hypoxic-ischemic brain injury that occurs during the perinatal period is one of the most recognized causes of severe, long-term neurological deficits in children. 30 However, the concept that cerebral palsy is due only to acquired insults such as perinatal asphyxia has been fundamentally challenged in the past decade. The current view is that the vast majority of injuries are the result of interplay between several risk factors such as genetic background, acute intrapartum hypoxia, excitotoxicity, oxidative stress, sepsis and an exacerbated inflammatory fetal and placental host response. 30,31 Discerning among these factors is important not only when formulating antenatal preventative or therapeutic strategies to avert cerebral palsy but also within medico-legal contexts. In regard to the latter, clinical practice guidelines indicate the importance of evaluation of umbilical cord blood gases and acid-base status of the premature neonate at the time of birth in order to document or exclude possible intrapartum hypoxic events.32 Furthermore, exclusion of other identifiable causes (i.e. trauma, coagulation disorder, or infectious or genetic conditions) is vital before declaring an anoxic intra-partum event as the definitive cause of poor neuro-developmental outcome.
A series of studies formally addressed the question of whether by using non-invasive clinical tests, septic fetuses can be accurately recognized in utero. 3,9,33,34 While some felt that fetal breathing, biophysical profile, AF index, and non-stress test were useful tools for evaluating fetuses at risk for infection, 3,9,10 others disagreed. 11,34,35 The differences in opinion among authors may be due to the retrospective nature of most of the above studies, a wide variability in the time interval between the assessment of the FHR-MP and delivery of the fetus, along with the inability to simultaneously integrate in one project the information pertaining to FHR-MP, inflammatory status of the AF and placenta, and EONS. Given the above limitations, the lack of consensus in the literature, and the significant changes in the NICHD recommended guidelines for interpretation of the FHR, we felt compelled to readdress the question by analyzing a prospective cohort of fetuses while standardizing variables (such as the times of FHR assessment) and interval to delivery in order to minimize bias. In this context, we found that fetuses with severe intra-amniotic inflammation are more likely to have some, but not all FHR-MPs altered. Interestingly the most specific changes (increased FHR baseline compared to those without inflammation, and a non-reactive, non-reassuring FHR-MP) were those at the time of IEV due to an increased proportion of fetuses exhibiting non-reactive and/or non-reassuring patterns closer to delivery. However, the increased inflammatory status of the AF cannot independently explain the possible association between an abnormal FHR-MP and EONS.
It was hypothesized that “sensitization” of the umbilical cord blood vessels by the inflammatory cytokines alters the vasomotor response to mechanical compression which may occur secondarily to uterine contractions or fetal movement.3 We analyzed the FHR-MP in regard to the presence or absence of histological funisitis (grade 2–4). We determined that an advanced grade of arteritis and phlebitis is associated with a higher FHR baseline and a non-reactive, non-reassuring FHR-MP at the time of IEV. The finding of a similar distribution of uterine contractions among groups at the time of IEV but also at AEV and DEV suggests mechanical compression of the cord is not solely responsible for an abnormal FHR-MP and provides support for the above hypothesis. Conversely, all episodes with fetal tachycardia occurred in the context of severe intra-amniotic inflammation, infection and funisitis and in most of the cases in the absence of maternal fever. This suggests that in certain clinical scenarios evaluation of the intra-uterine environment may be warranted even in the absence of maternal signs or symptoms of chrioamnionitis.
Recognition of EONS is difficult 36 and clinicians routinely over treat neonates with antibiotics, under the assumption that an infant is more likely to suffer if infection is not diagnosed rather than if the infant is treated unnecessarily. Furthermore, 75% of neonates in our study have also been exposed to antibiotics antenatally. Therefore, the small number of neonates with proven sepsis should not come as a surprise. To overcome this specific difficulty, neonatologists often have to rely on standardized hematological criteria to diagnose EONS.26,27 Because none of the prior studies that analyzed FHR-MPs in relationship with perinatal sepsis have assessed intra-amniotic inflammation directly, it was important for us to analyze the relationships of the various FHR-MP, placental inflammation and EONS separately. We show that the only FHR-MP at the time of IEV significantly associated with EONS is a non-reassuring FHR. Although the odds ratio of a non-reassuring FHR-MP at the time of IEV to predict EONS was higher than that of any other FHR element taken into consideration, this pattern was neither a sensitive nor a highly accurate test to predict EONS antenatally. Our analysis determined that the two fetuses with proven sepsis by positive blood cultures and most of the fetuses diagnosed with EONS using hematological findings had in fact a reassuring non-stress test at the time of IEV. Because a non-reassuring FHR is a clinical indicator for rapid delivery of the fetus, the clinical utility of a non reassuring non-stress test can, at best, increase the level of clinical awareness that a fetus with EONS may be born but it cannot be used at this time to dictate whether or not neonatal antibiotic treatment should be instituted at the time of delivery. Since our data indicate that more than half of fetuses studied showed at some point an altered FHR-MP and none in the context of abnormal umbilical artery pH values, it can only underscore the importance of documenting the acid base status at delivery in instances with abnormal FHR-MP especially if amniocentesis results are not available for reference.
Finally, we have to acknowledge the persistence of several biases inherent to the population analyzed. The exclusion criteria for amniocentesis biases to the null (i.e. we may have missed patients presenting with abnormal FHR-MP requiring immediate delivery). Conversely, our restriction of the analysis to women delivering within 48 hours from amniocentesis biases away from the null given the higher prevalence of intra-amniotic infection managed with indicated delivery in this subgroup. Previous studies have shown that steroids can cause transient but profound suppression of FHR parameters, which can mimic distress.37 Our analysis demonstrated that steroid exposure was indeed associated with non-reassuring FHR-MPs at both AEV and DEV. This may explain the increased frequency of abnormal FHR-MP at these later times irrespective of intra-amniotic inflammation.
ACKNOWLEDGEMENT
This work was supported from National Institutes of Health Grant RO1 HD 047321 (IAB)
We are indebted to the nurses, residents, clinical fellows and patients at Yale New Haven Hospital, Department of Ob./Gyn. and Reprod. Sci, who participated in the recruitment process and collection of biological samples.
REFERENCES
- 1.Renou P, Newman W, Wood C. Autonomic control of FHR. Am J Obstet Gynecol. 1969;105:949–1053. doi: 10.1016/0002-9378(69)90103-3. [DOI] [PubMed] [Google Scholar]
- 2.Van Leeuwen P, Cysarz D, Lange S, Gronemeyer D. Increase in regularity of FHR variability with age. Biomed Tech (Berl) 2006;51:244–247. doi: 10.1515/BMT.2006.047. [DOI] [PubMed] [Google Scholar]
- 3.Salafia CM, Ghidini A, Sherer DM, Pezzullo JC. Abnormalities of the FHR in preterm deliveries are associated with acute intra-amniotic infection. J Soc Gynecol Investig. 1998;5:188–191. doi: 10.1016/s1071-5576(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 4.Fleming AD, Salafia CM, Vintzileos AM, Rodis JF, Campbell WA, Bantham KF. The relationships among umbilical artery velocimetry, fetal biophysical profile, and placental inflammation in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1991;164:38–41. doi: 10.1016/0002-9378(91)90619-3. [DOI] [PubMed] [Google Scholar]
- 5.Salafia CM, Mangam HE, Weigl CA, Foye GJ, Silberman L. Abnormal FHR patterns and placental inflammation. Am J Obstet Gynecol. 1989;160:140–147. doi: 10.1016/0002-9378(89)90107-5. [DOI] [PubMed] [Google Scholar]
- 6.Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, et al. Proteomic Profiling of the Amniotic Fluid to Detect Inflammation, Infection, and Neonatal Sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, et al. Proteomic Biomarkers of Intra-amniotic Inflammation: Relationship with Funisitis and Early-onset Sepsis in the Premature Neonate. Pediatr Res. 2007;61:318–324. doi: 10.1203/01.pdr.0000252439.48564.37. [DOI] [PubMed] [Google Scholar]
- 8.Buhimschi IA, Zambrano E, Pettker CM, Bahtiyar MO, Paidas M, Rosenberg VA, Thung S, Salafia CM, Buhimschi CS. Using proteomic analysis of the human amniotic fluid to identify histological chorioamnionitis. Obstet Gynecol. doi: 10.1097/AOG.0b013e31816102aa. in press. [DOI] [PubMed] [Google Scholar]
- 9.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149–153. doi: 10.1016/0002-9378(86)90100-6. [DOI] [PubMed] [Google Scholar]
- 10.Schiano MA, Hauth JC, Gilstrap LC., 3rd Second-stage fetal tachycardia and neonatal infection. Am J Obstet Gynecol. 1984;148:779–781. doi: 10.1016/0002-9378(84)90566-0. [DOI] [PubMed] [Google Scholar]
- 11.Day D, Ugol JH, French JI, Haverkamp A, Wall RE, McGregor JA. Fetal monitoring in perinatal sepsis. Am J Perinatol. 1992;9:28–33. doi: 10.1055/s-2007-994665. [DOI] [PubMed] [Google Scholar]
- 12.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 13.Lewis DF, Bergstedt S, Edwards MS, Burlison S, Gallaspy JW, Brooks GG, Adair CD. Successful magnesium sulfate tocolysis: is "weaning" the drug necessary? Am J Obstet Gynecol. 1997;177:742–745. doi: 10.1016/s0002-9378(97)70261-8. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnosis performance of amniotic fluid glucose, white blood cell count, interleukin-6, and Gram stain in the detection of microbial invasion in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 15.Naef RW, 3rd, Allbert JR, Ross EL, Weber BM, Martin RW, Morrison JC. Premature rupture of membranes at 34 to 37 weeks’ gestation: aggressive versus conservative management. Am J Obstet Gynecol. 1998;178:126–130. doi: 10.1016/s0002-9378(98)70638-6. [DOI] [PubMed] [Google Scholar]
- 16.Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2002;100:1405–1412. doi: 10.1016/s0029-7844(02)02629-7. ACOG Committee Opinion: number 279, December 2002. [DOI] [PubMed] [Google Scholar]
- 17.Premature rupture of membranes. Obstet Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. ACOG Practice Bulletin: number 80, April 2007. [DOI] [PubMed] [Google Scholar]
- 18.Electronic FHR monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385–1390. [PubMed] [Google Scholar]
- 19.Sameshima H, Ikenoue T, Ikeda T, Kamitomo M, Ibara S. Association of nonreassuring FHR patterns and subsequent cerebral palsy in pregnancies with intrauterine bacterial infection. Am J Perinatol. 2005;22:181–187. doi: 10.1055/s-2005-867090. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RK, Clark P, Locksmith Gregory J, Duff P. Performance characteristics of putative tests for subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 2001;9:209–214. doi: 10.1155/S1064744901000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–1341. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 22.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Romero R, Yoon B, Moon J, Oh S, Han S, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 24.Harirah H, Donia S, Hsu C. Amniotic fluid matrix metalloproteinase-9 and interleukin-6 in predicting intra-amniotic infection. Obstet Gynecol. 2002;99:80–84. doi: 10.1016/s0029-7844(01)01632-5. [DOI] [PubMed] [Google Scholar]
- 25.Naeye RL. Disorder of the Placenta, Fetus and Neonate: Diagnosis and Clinical Significance. St. Louis: Mosby; 1992. Disorders of the placenta and decidua; pp. 118–247. [Google Scholar]
- 26.Smulian JC, Bhandari V, Campbell WA, Rodis JF, Vintzileos AM. Value of umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med. 1997;6:254–259. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<254::AID-MFM2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 27.Rodwell RL, Leslie AL, Tudehope DI. Early diagnosis of neonatal sepsis using a hematologic scoring system. J Pediatr. 1988;112:761–767. doi: 10.1016/s0022-3476(88)80699-1. [DOI] [PubMed] [Google Scholar]
- 28.Hill JB, Alexander JM, Sharma SK, McIntire DD, Leveno KJ. A comparison of the effects of epidural and meperidine analgesia during labor on FHR. Obstet Gynecol. 2003;102:333–337. doi: 10.1016/s0029-7844(03)00567-2. [DOI] [PubMed] [Google Scholar]
- 29.Rotmensch S, Lev S, Kovo M, Efrat Z, Zahavi Z, Lev N, Celentano C, Ben-Rafael Z. Effect of betamethasone administration on FHR tracing: a blinded longitudinal study. Fetal Diagn Ther. 2005;20:371–376. doi: 10.1159/000086815. [DOI] [PubMed] [Google Scholar]
- 30.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 31.Redline RW. Placental pathology and cerebral palsy. Clin Perinatol. 2006;33:503–516. doi: 10.1016/j.clp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108:1319–1322. doi: 10.1097/00006250-200611000-00058. ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 348, November 2006. [DOI] [PubMed] [Google Scholar]
- 33.Roberts AB, Goldstein I, Romero R, Hobbins JC. Comparison of total fetal activity measurement with the biophysical profile in predicting intra-amniotic infection in preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 1991;1:36–39. doi: 10.1046/j.1469-0705.1991.01010036.x. [DOI] [PubMed] [Google Scholar]
- 34.Carroll SG, Papaioannou S, Nicolaides KH. Assessment of fetal activity and amniotic fluid volume in the prediction of intrauterine infection in preterm prelabor amniorrhexis. Am J Obstet Gynecol. 1995;172:1427–1435. doi: 10.1016/0002-9378(95)90473-5. [DOI] [PubMed] [Google Scholar]
- 35.Day D, Ugol JH, French JI, Haverkamp A, Wall RE, McGregor JA. Fetal monitoring in perinatal sepsis. Am J Perinatol. 1992;9:28–33. doi: 10.1055/s-2007-994665. [DOI] [PubMed] [Google Scholar]
- 36.Mishra UK, Jacobs SE, Doyle LW, Garland SM. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2006;91:F208–F212. doi: 10.1136/adc.2004.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotmensch S, Lev S, Kovo M, Efrat Z, Zahavi Z, Lev N, Celentano C, Ben-Rafael Z. Effect of betamethasone administration on fetal heart rate tracing: a blinded longitudinal study. Fetal Diagn Ther. 2005;20:371–376. doi: 10.1159/000086815. [DOI] [PubMed] [Google Scholar]