Abstract
Aims
Ventricular interactions may be mediated by loading conditions and biventricular timing and coordination. We sought to understand the relationships between right (RV) and left ventricular (LV) function and dyssynchrony, examine the RV correlates of LV dyssynchrony, and determine whether improved loading conditions affect inter-ventricular interaction.
Methods and results
In 25 heart failure patients [15 with left ventricular ejection fraction (LVEF) < 40%; 10 with LVEF ≥ 50%], Doppler echocardiography and invasive bi-ventricular pressure–volume haemodynamics were obtained at baseline and 30 min after infusion of the recombinant B-type natriuretic peptide vasodilator nesiritide. RV and LV intra-ventricular dyssynchrony was measured invasively using a pressure–conductance catheter. Patients with reduced LVEF had greater LV dyssynchrony (31 ± 3 vs. 24 ± 7%; P = 0.003) compared to those with preserved LVEF. Tricuspid annular plane systolic excursion (TAPSE) had the highest correlation with LV dyssynchrony (r = −0.52; P = 0.0002) compared to other RV echocardiographic parameters. The association between TAPSE and LV dyssynchrony was independent of RVEF and LVEF (P = 0.008). There were no acute changes in the correlations between LV dyssynchrony and TAPSE after nesiritide.
Conclusion
TAPSE and LV dyssynchrony are strongly associated, independent of RV and LV ejection fraction. Of the RV echocardiographic parameters, TAPSE has the highest predictive value of LV dyssynchrony, and remained significant after vasodilator unloading.
KEYWORDS: Heart failure, Dyssynchrony, Brain natriuretic peptide, hemodynamics
Introduction
With the development of cardiac resynchronization therapy (CRT) for left ventricular (LV) failure, there has been greater interest in the assessment of ventricular dyssynchrony. A substantial proportion of heart failure patients with a prolonged QRS duration, and even those with normal conduction, may exhibit ventricular dyssynchrony.1 As the QRS complex duration is not the sole determinant of systolic dyssynchrony, accurate assessment of ventricular dyssynchrony may be more important than QRS duration alone in selecting patients for cardiac resynchronization treatment.2 The mechanisms of dyssynchrony remain poorly understood in patients with heart failure with normal ejection fraction (HFNEF), and ventricular interaction may be important.
The available methods for measuring ventricular dyssynchrony include specialized echocardiographic software,3 nuclear scintigraphic imaging, magnetic resonance imaging,4 and invasive pressure–conductance catheter measurements.5 Current echocardiographic techniques are labour-intensive and are not in widespread use due to the need to acquire specialized software and training.3,6 Magnetic resonance imaging is limited by time and cost constraints as well as inability to use this methodology in patients with implanted devices. Although invasive pressure–conductance analysis is not practical for serial or routine clinical use, it is a good experimental tool that provides additional information about bi-ventricular loading conditions.
In patients with LV failure, right ventricular (RV) dysfunction is an independent predictor of an adverse prognosis.7 Physiologic RV dysfunction may be a direct consequence of LV dysfunction. This association is mediated largely by the septum, but also by LV free wall.8 Therefore, one may expect that tricuspid annular displacement is influenced by LV function, particularly as the septum shortens in the long-axis vector.
The evaluation of tricuspid annular plane systolic excursion (TAPSE) using Doppler tissue echocardiographic imaging provides a simple, rapid, quantitative, and non-invasive tool for assessing the RV systolic function in patients with heart failure.9,10 There is a direct correlation between TAPSE and RV ejection fraction as assessed by radionuclide angiography.11,12 The approach is reproducible, and has been shown to be a strong predictor of prognosis in heart failure.13
In large CRT trials, ∼20–30% of patients did not respond clinically. In addition, there may be patients with a normal QRS duration and significant dyssynchrony who may be candidates for CRT. Moreover, a readily available echocardiographic measure of ventricular dyssynchrony would be clinically useful. We hypothesized that RV and LV interaction contributes to dyssynchrony. To test this hypothesis, we invasively examined bi-ventricular loading conditions by measuring RV and LV pressure–volume haemodynamics, RV and LV intra-ventricular dyssynchrony, and echocardiographic parameters at baseline and acutely after vasodilator therapy with nesiritide, a recombinant B-type natriuretic peptide (BNP). We evaluated the echocardiographic parameters of RV function to determine whether they were predictive of LV dyssynchrony.
Methods
Study participants
This was a prospective cohort study that enrolled adults with heart failure referred for right and left heart catheterization and coronary angiography. All patients had a clinical history of symptomatic heart failure diagnosed by an experienced clinical cardiologist. Patients were required to have either HFREF [left ventricular ejection fraction (LVEF) < 40%] or HFNEF (LVEF ≥ 50%). Specifically, patients with LVEF 40–49% were excluded from the study. Additional exclusion criteria included age <18 years old, systolic blood pressure <95 mmHg, heart rate <50 bpm, intravenous vasopressor, inotropic, or vasodilator pharmacotherapy, cardiac rhythm other than a sinus or paced atrial rhythm, severe stenotic valvular disease, complex congenital heart disease, hypertrophic or obstructive cardiomyopathy, constrictive pericarditis, severe pre-capillary pulmonary hypertension, and mechanical ventilation. All patients gave written informed consent prior to the procedure, and the protocol was approved by the Committee on Human Research.
Study procedures
Right and left heart catheterization and coronary angiography were performed. Transthoracic echocardiography was performed for the assessment of RV and LV function. A four-French high-fidelity micromanometer pressure–volume conductance pigtail catheter (CD Leycom/CardioDynamics BV, Zoetermeer, Netherlands) was advanced to the right and left ventricles through a 6F guiding catheter for invasive pressure–volume measurements. All patients received 2 L/min of nasal cannula oxygen throughout the study period, and the level of conscious sedation was continued at a steady level throughout the study period.
Nesiritide (Natrecor®, Scios Inc., Mountain View, CA, USA; 2 mg/kg IV bolus followed by 0.01 mg/kg/min) was infused via a peripheral IV for 30 min.
At 30 min, echocardiography, right and left heart catheterization, and invasive bi-ventricular haemodynamics using the pressure–volume catheter were repeated.
Invasive pressure–volume ventricular haemodynamics
The four-French pressure–volume catheter has one solid-state micromanometer pressure sensor, 12 electrodes with 6 mm spacing, and measures volume from seven segments. Under fluoroscopic guidance, the catheter was advanced to the ventricular apex. Volume segments that were not in the ventricle were not included in the analyses. Measurements from this catheter were recorded using the Leycom CFL 512 for off-line analysis of cardiac function using the Conduct NT software (version 2.8, CD Leycom/CardioDynamics BV, Zoetermeer, Netherlands).
At each time-point, a segmental signal was defined as dyssynchronous if its change (i.e. dVseg/dt) was opposite to the simultaneous change in the total ventricular volume (dV/dt). Segmental dyssynchrony is quantified by calculating the percentage of time within the cardiac cycle, i.e. dyssynchronous. Overall ventricular dyssynchrony was calculated as the mean of the segmental dyssynchronies.14 Dyssynchrony was recorded both during the systolic and diastolic periods. In the right and left ventricles, end-diastolic pressure and maximal +dP/dt were recorded.
Echocardiography
Transthoracic echocardiography was performed at baseline and 30 min after beginning the nesiritide infusion (Acuson Sequoia, Siemens, Malvern, PA, USA). Echocardiographic contrast (Optison®, Amersham, Little Chalfont, UK; 0.3–0.5 mL injected into a peripheral vein) was administered, when required, to improve endocardial border detection and enhance Doppler signals. Echocardiographic data were stored on magneto-optical disks and analysed off-line by a single experienced reader.
RV end-diastolic area and RV end-systolic area were calculated from the apical four-chamber view, and the calculation of RV fractional area change (FACRV) used to determine global RV systolic function was performed using the following formula: FACRV = [(RV end-diastolic area − RV end-systolic area)/RV end-diastolic area] × 100%. RV fractional shortening was calculated as (RVIDed−RVIDes)/RVIDed, where ed and es are the RV internal diameters at end-diastole and end-systole from the apical four-chamber view, respectively. LV ejection fraction calculations were done using the biplane Simpson’s rule algorithm, and the following formula was used: LV ejection fraction = [(LV end-diastolic volume−LV end-systolic volume)/LV end-diastolic volume] × 100%. LV end-diastolic volume and LV end-systolic volume were traced from the apical four-chamber and apical two-chamber views in accordance with published data. The Tei myocardial performance index was calculated as the sum of isovolumetric contraction and relaxation times divided by the ventricular ejection time. The sum of RV isovolumetric contraction time and isovolumetric relaxation time was obtained by subtracting ventricular ejection time from the interval between cessation and onset of the tricuspid inflow velocities with pulsed-wave Doppler echocardiography.
To determine TAPSE, the M-mode cursor was oriented to the junction of the tricuspid valve plane with the RV free wall using the apical four-chamber view.15 The echoes generated were received and registered as motion of the RV base. Maximal TAPSE was determined by the total excursion of the tricuspid annulus from its highest position after atrial ascent to the peak descent during ventricular systole, as measured from the apical four-chamber view. All annular M-mode images were obtained at the end of expiration to minimize changes in the longitudinal movement of the tricuspid plane; to improve reproducibility, we used data from ≥3 beats in each patient.
Statistical analysis
Data are presented as mean values and standard deviations for continuous variables. Differences between baseline and nesiritide treatment values were assessed using a paired two-tailed Student’s t-test for paired observations or repeated-measures ANOVA where appropriate. Differences between those with reduced EF vs. normal EF were performed with χ2 and unpaired t-tests for dichotomous and continuous variables, respectively. Linear regression modelling was performed to identify independent predictors of LV dyssynchrony. Ventricular dyssynchrony was dichotomized at the median value. Two-tailed probability (P) values <0.05 were considered significant. All analyses were performed using STATA version 9.2 (StataCorp., College Station, TX, USA).
Results
Demographic and clinical characteristics
Of the 25 patients enrolled, 15 had reduced LVEF while 10 had normal LVEF (Table 1). Those with reduced LVEF had a higher prevalence of prior myocardial infarction and a lower prevalence of hypertension. Renal dysfunction tended to be worse in those with HFNEF. BNP levels were comparable in both groups.
Table 1.
Baseline demographic and clinical characteristics by type of heart failure
| Characteristics | Total cohort | LVEF < 40% | LVEF ≥ 50% | P-value |
|---|---|---|---|---|
| Number | 25 | 15 | 10 | |
| Age, years (mean) | 59.9 ± 11.2 | 59.5 ± 12.4 | 60.5 ± 9.7 | 0.83 |
| Male, n (%) | 12 (48%) | 9 (60%) | 3 (30%) | 0.14 |
| Body mass index (kg/m2) | 29.0 ± 8.1 | 29.2 ± 6.3 | 28.8 ± 10.7 | 0.91 |
| Diabetes mellitus, n (%) | 8 (32%) | 3 (20%) | 5 (50%) | 0.12 |
| Coronary artery disease, n (%) | 14 (56%) | 8 (53%) | 6 (60%) | 0.74 |
| Hypertension | 16 (64%) | 6 (40%) | 10 (100%) | 0.002 |
| Prior myocardial infarction | 9 (36%) | 8 (53%) | 1 (10%) | 0.027 |
| History of CABG | 4 (16%) | 2 (13%) | 2 (20%) | 0.66 |
| History of PCI | 5 (20%) | 3 (20%) | 2 (20%) | 1.00 |
| NYHA functional class | ||||
| I | 3 (12%) | 1 (7%) | 2 (20%) | 0.50 |
| II | 9 (36%) | 5 (33%) | 4 (40%) | |
| III | 11 (44%) | 7 (47%) | 4 (40%) | |
| IV | 2 (8%) | 2 (13%) | 0 (0%) | |
| Creatinine (mg/dL) | 1.5 ± 1.1 | 1.2 ± 0.4 | 1.9 ± 1.6 | 0.15 |
| BNP, pg/mL (median, IQR) | 231 (94–687) | 280 (73–1050) | 219 (190–301) | 0.48 |
Echocardiographic characteristics
Patients with reduced LVEF had significantly larger LV volumes compared to those with normal LVEF (Table 2). RV ejection fraction, volume, fractional shortening, and fractional area change were similar between these two groups. TAPSE was significantly lower for those with reduced LVEF.
Table 2.
Echocardiographic and invasive characteristics by type of heart failure
| Characteristics | Systolic HF | HFNEF | P-value |
|---|---|---|---|
| Echocardiographic | |||
| LV ejection fraction (%) | 32.1 ± 11.2 | 64.0 ± 7.4 | <0.001 |
| LV end-diastolic volume index (mL/m2) | 91.1 ± 37.6 | 47.5 ± 18.0 | 0.002 |
| LV end-systolic volume index (mL/m2) | 64.6 ± 34.1 | 16.9 ± 6.1 | <0.001 |
| RV ejection fraction (%) | 47.0 ± 19.3 | 51.6 ± 22.0 | 0.62 |
| RV fractional shortening | 0.26 ± 0.13 | 0.22 ± 0.10 | 0.50 |
| RV fractional area change | 32.0 ± 16.3 | 37.0 ± 12.6 | 0.49 |
| TAPSE (cm) | 0.95 ± 0.69 | 1.78 ± 0.84 | 0.02 |
| Invasive | |||
| LVEDP (mmHg) | 20.3 ± 10.8 | 21.1 ± 6.9 | 0.83 |
| LV Maximal +dP/dt (mmHg/s) | 861 ± 221 | 1142 ± 213 | 0.07 |
| LV dyssynchrony (%) | 30.6 ± 3.4 | 23.8 ± 6.6 | 0.003 |
| RV maximal +dP/dt (mmHg/s) | 308 ± 102 | 347 ± 148 | 0.48 |
| RV dyssynchrony (%) | 26.6 ± 6.9 | 22.3 ± 6.0 | 0.17 |
HFNEF, heart failure with normal ejection fraction; LV, left ventricular; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; LVEDP, left ventricular end-diastolic pressure.
Invasive measures of ventricular function
Those with reduced LVEF had significantly greater LV dyssynchrony compared to those with normal LVEF (Table 2). LV end-diastolic pressure was elevated in both groups. While LV maximal positive dP/dt tended to be lower in those with reduced LVEF, there was no difference in RV dP/dt or dyssynchrony between the two groups.
TAPSE and dyssynchrony
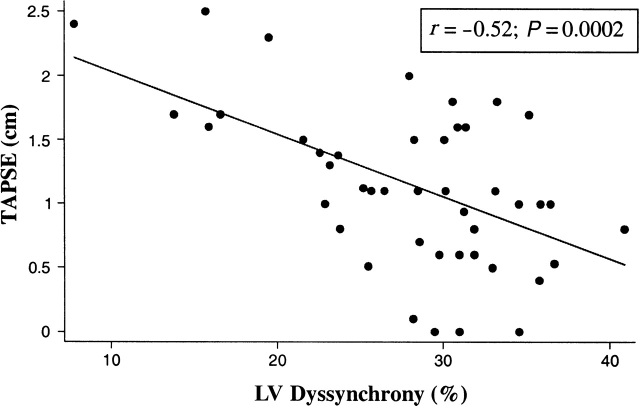
Linear regression analysis showed that for each 1 cm decrease in TAPSE, there was an absolute 5.6% increase in LV dyssynchrony (P < 0.001). Multivariable linear regression controlling for left and RV ejection fraction showed that TAPSE and LV dyssynchrony were independently associated (P = 0.008). The correlation coefficients for TAPSE and LVEF, +dP/dT, and LV dyssynchrony were r = 0.35 (P = 0.02), r = 0.24 (P = 0.11), and r = −0.52 (P = 0.0002), respectively (Table 3; Figure 1). TAPSE had the highest correlation with LV dyssynchrony compared to other RV echocardiographic parameters including RVEF, fractional shortening, fractional area change, and the myocardial performance index (Table 3). The area under the receiver operator curve for TAPSE was 0.75 to predict LVEF < 40%, and was 0.64 to predict an elevated LV dyssynchrony level >29%. A TAPSE cut-off <1.3 cm had 76% sensitivity and specificity to predict low LVEF. LV dyssynchrony was 24.1 ± 7.6% for patients with a TAPSE >1.3 cm, compared to 29.2 ± 6.5% for those with a TAPSE < 1.3 cm (P = 0.02). TAPSE was highest for those without dyssynchrony, and lowest for those with bi-ventricular dyssynchrony (Figure 2). After nesiritide infusion, there was no significant acute change in TAPSE. Post-nesiritide measurements did show reduced bi-ventricular filling pressure, but no change in RV or LV ejection fraction or dyssynchrony.
Table 3.
Correlation coefficients for right ventricular echocardiographic variables and left ventricular dyssynchrony
| Variable | Correlation coefficients for left ventricular dyssynchrony | P-value |
|---|---|---|
| TAPSE (cm) | −0.52 | 0.0002 |
| RV ejection fraction (%) | −0.29 | 0.059 |
| RV fractional shortening | −0.18 | 0.24 |
| RV fractional area change | −0.37 | 0.015 |
| RV myocardial performance index | 0.21 | 0.29 |
Figure 1.
Scatterplot of echocardiographic tricuspid annular plane systolic excursion (TAPSE) measurements and invasively determined left ventricular dyssynchrony. Using measurements both prior to and 30 min after nesiritide infusion, the correlation coefficient was −0.52 (P = 0.0002).
Figure 2.
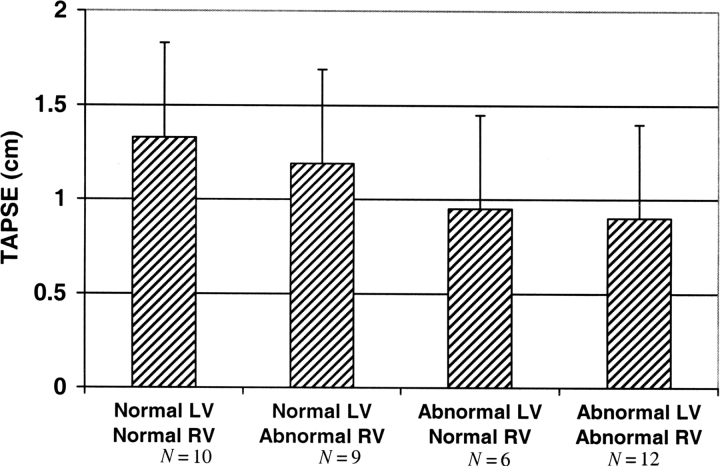
Bar graph showing the mean tricuspid annular plane systolic excursion (TAPSE) measurements based on bi-ventricular dyssynchrony.
Discussion
This study compared the utility of TAPSE with other traditional RV echocardiographic parameters to predict LV dyssynchrony in a cohort of heart failure patients with a wide spectrum of ventricular dyssynchrony severity. TAPSE correlated with invasive and echocardiographic measures of dyssynchrony and LV systolic function, respectively. TAPSE was the best performing RV echocardiographic predictor of LV dyssynchrony. RVEF and FACRV correlated fairly well with LV dyssynchrony, while there was no significant association for RV fractional shortening or the RV myocardial performance index. The association between TAPSE and LV dyssynchrony was significantly independent of RV and LV ejection fraction. Although nesiritide has acute effects in reducing preload and afterload, this vasodilator did not change RV or LV dyssynchrony. It is noteworthy that TAPSE was lowest for patients with bi-ventricular dyssynchrony, and highest for those without dyssynchrony. TAPSE may, therefore, give further quantitative insight into bi-ventricular dyssynchrony. Among the various other echocardiographic measures for the assessment of RV function, TAPSE is relatively easy to measure, and has less inter-observer variability.
RV function may be impaired either by primary right-sided heart disease, or secondary to left-sided cardiomyopathy or valvular heart disease. Historically, it was believed that the RV played only a minor role in maintenance of normal cardiac pump function.16 Over the last several years, there has been an increasing recognition of the vital role played by the RV. Moreover, RV function has been shown to be a major determinant of clinical outcome.10,17 For example, patients with inferior MI who also have RV myocardial involvement have been demonstrated to be at increased risk of death, shock, and arrhythmias, the elevated risk being related to the presence of RV myocardial involvement itself rather than the extent of LV myocardial damage.18 RV dysfunction may impair LV function by attenuating LV preload and adversely affecting the systolic and diastolic interaction via the intra-ventricular septum and the pericardium (ventricular interdependence). In patients with significant LV dysfunction, changes in RV function may be a sensitive indicator of changes in LV performance. Popescu et al.19 confirmed the role of IVS motion in RV performance demonstrating that RV performance is dependent on LV septal contractile contributions transmitted through systolic ventricular interaction.
Several invasive and non-invasive techniques have been used for estimating RV function. The assessment of RV function is difficult because of its complex geometry, pronounced translational motions, and the relationship between intrinsic myocardial performance and RV loading conditions. Commonly used parameters for echocardiographic assessment of RV function include RV ejection fraction,20,21 RV fractional shortening,22 FACRV, TAPSE, and the RV Tei (myocardial performance) index.23,24 In patients with moderate congestive heart failure, RV function as assessed by RV ejection fraction is an independent predictor of survival in patients with moderate heart failure.25
There is evidence that TAPSE may reflect abnormal LV function.26 The association between TAPSE and bi-ventricular dyssynchrony has not been previously examined. We sought to assess the clinical diagnostic accuracy of TAPSE for ventricular dyssynchrony. In addition to investigating a possible clinical tool for detecting ventricular dyssynchrony, we sought to assess the physiologic mechanism of ventricular dyssynchrony. We compared the utility of TAPSE with other traditional RV echocardiographic parameters to predict LV dyssynchrony in a cohort of heart failure patients with a wide spectrum of ventricular dyssynchrony.
This study has several limitations. First, the sample size was small. The complex geometry of the RV does not lend itself to accurate assessment by two-dimensional echocardiography. One needs to be cautious about labelling TAPSE as normal if there is significant RV dyssynchrony, particularly in those settings where there might be apical dyskinesis. None of the patients in this study had moderate or severe RV apical dyskinesis.
In conclusion, we have shown that TAPSE is significantly reduced in patients with LV systolic dysfunction. TAPSE was lowest for those with bi-ventricular dyssynchrony, and highest for those without dyssynchrony. The association between TAPSE and LV dyssynchrony is independent of the RV and LV ejection fraction. Because of its ease of measurement and reproducibility, TAPSE may serve as a useful indicator of LV dyssynchrony. Further study of this relationship is warranted, particularly with comparison of newer imaging modalities for assessment of ventricular dyssynchrony as well as after CRT.
Funding
This study was partially funded from Scios Inc (Mountain View, CA, USA). A.D.M. is supported by the NIH Mentored Patient-Oriented Research Career Development Award (K23 RR018319-01 A3).
Acknowledgements
We would like to thank Lillian Khor, MD, for her helpful review of this manuscript.
Conflict of interest: none declared.
References
- 1.Ghio S, Constantin C, Klersy C, Serio A, Fontana A, Campana C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of qrs duration. Eur Heart J. 2004;25:571–8. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal qrs duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorcsan J, III, Tanabe M, Bleeker GB, Suffoletto MS, Thomas NC, Saba S, et al. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol. 2007;50:1476–83. doi: 10.1016/j.jacc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Westenberg JJM, Lamb HJ, van der Geest RJ, Bleeker GB, Holman ER, Schalij MJ, et al. Assessment of left ventricular dyssynchrony in patients with conduction delay and idiopathic dilated cardiomyopathy: head-to-head comparison between tissue Doppler imaging and velocity-encoded magnetic resonance imaging. J Am Coll Cardiol. 2006;47:2042–8. doi: 10.1016/j.jacc.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 5.Byrne MJ, Helm RH, Daya S, Osman NF, Halperin HR, Berger RD, et al. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol. 2007;50:1484–90. doi: 10.1016/j.jacc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Arita T, Sorescu GP, Schuler BT, Schmarkey LS, Merlino JD, Vinten-Johansen J, et al. Speckle-tracking strain echocardiography for detecting cardiac dyssynchrony in a canine model of dyssynchrony and heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H735–42. doi: 10.1152/ajpheart.00168.2007. [DOI] [PubMed] [Google Scholar]
- 7.Zornoff LAM, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–5. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 8.Dong SJ, Smith ER, Tyberg JV. Changes in the radius of curvature of the ventricular septum at end diastole during pulmonary arterial and aortic constrictions in the dog. Circulation. 1992;86:1280–90. doi: 10.1161/01.cir.86.4.1280. [DOI] [PubMed] [Google Scholar]
- 9.Meluzin J, Spinarova L, Bakala J, Toman J, Krejci J, Hude P, et al. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion. A new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22:340–8. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 10.Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TAM, et al. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92:i19–26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–31. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 12.Ueti OM, Camargo EE, de A Ueti A, de Lima-Filho EC, Nogueira EA. Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuclide angiography. Heart. 2002;88:244–8. doi: 10.1136/heart.88.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–42. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 14.Steendijk P, Tulner SA, Schreuder JJ, Bax JJ, van Erven L, van der Wall EE, et al. Quantification of left ventricular mechanical dyssynchrony by conductance catheter in heart failure patients. Am J Physiol Heart Circ Physiol. 2004;286:H723–30. doi: 10.1152/ajpheart.00555.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hammarstrom E, Wranne B, Pinto FJ, Puryear J, Popp RL. Tricuspid annular motion. J Am Soc Echocardiogr. 1991;4:131–9. doi: 10.1016/s0894-7317(14)80524-5. [DOI] [PubMed] [Google Scholar]
- 16.Starr I, Jeffers WA, Meade JRH. The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am Heart J. 1943;26:291–301. [Google Scholar]
- 17.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 18.Mehta SR, Eikelboom JW, Natarajan MK, Diaz R, Yi C, Gibbons RJ, et al. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37:37–43. doi: 10.1016/s0735-1097(00)01089-5. [DOI] [PubMed] [Google Scholar]
- 19.Popescu BA, Antonini-Canterin F, Temporelli PL, Giannuzzi P, Bosimini E, Gentile F, et al. Right ventricular functional recovery after acute myocardial infarction: relation with left ventricular function and interventricular septum motion. Gissi-3 echo substudy. Heart. 2005;91:484–8. doi: 10.1136/hrt.2003.028050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–24. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 21.Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–80. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 22.Karatasakis GT, Karagounis LA, Kalyvas PA, Manginas A, Athanassopoulos GD, Aggelakas SA, et al. Prognostic significance of echocardiographically estimated right ventricular shortening in advanced heart failure. Am J Cardiol. 1998;82:329–34. doi: 10.1016/s0002-9149(98)00344-0. [DOI] [PubMed] [Google Scholar]
- 23.Eidem BW, O’Leary PW, Tei C, Seward JB. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol. 2000;86:654–8. doi: 10.1016/s0002-9149(00)01047-x. [DOI] [PubMed] [Google Scholar]
- 24.Meluzin J, Spinarova L, Hude P, Krejci J, Kincl V, Panovsky R, et al. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J Am Soc Echocardiogr. 2005;18:435–44. doi: 10.1016/j.echo.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 25.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–54. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Candales A, Rajagopalan N, Saxena N, Gulyasy B, Edelman K, Bazaz R. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol. 2006;98:973–7. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]