Abstract
After-effects are perceptual illusions caused by visual adaptation to one or more stimulus attribute, such as orientation, motion, or shape, and are generally characterized by a repulsive shift in the perception of the adapted features in a corresponding test stimulus. Neurophysiological studies seeking to understand the basis of adaptation have observed firing rate reduction and changes in tuning of sensory neurons during periods of prolonged stimulation. In the domain of shape, recent psychophysical work has shown that adaptation to a convex pattern induces a subsequently seen rectangle to appear slightly concave. In the present study, we investigate the possible contribution of V4 neurons, which are thought to be involved in the coding of convexity, to such shape-specific adaptation. Visually responsive neurons were monitored during the brief presentation of simple shapes varying in their convexity level. Each test presentation was preceded by either a blank period or several seconds of adaptation to a convex or concave stimulus, presented in two different sizes. Adaptation consistently changed the tuning of neurons away from the convex or concave adaptor, shifting the response to the neutral rectangle in the direction of the opposite convexity. This repulsive shift was consistent with the known perceptual distortion associated with adaptation to such stimuli. Adaptation also caused a nonspecific decrease in firing, as well as the shape-selective suppression for the repeated presentation of the adaptor stimulus. The latter effects were observed whether or not the adapting and test stimuli matched closely in their size. Taken together, these results provide evidence for shape-specific adaptation of neurons in area V4, which may contribute to the perception of the convexity aftereffect.
A visual stimulus presented on two separate occasions will inevitably elicit different responses from the brain (Faisal et al., 2008). Response variability of single neurons is contributed in part by endogenous factors such as ongoing activity (Arieli et al., 1995) and cognitive variables such as attention (Desimone and Duncan, 1995). At the same time, exogenous factors such as stimulation history can significantly influence the response elicited by a given presentation. For example, the prolonged or repeated exposure to a visual stimulus, often termed adaptation, can significantly shape the response of neurons at all stages of visual processing. Repeated stimulation of the same portion of the retina leads to declining responses from ganglion cells (Enroth-Cugell and Shapley, 1973; Brown and Masland, 2001) as well as neurons throughout the visual brain (Maffei et al., 1973; Miller et al., 1991; Lisberger and Movshon, 1999). At the behavioral level, visual adaptation can alter the subsequent perception of visual stimuli in many visual domains such as orientation (Gibson, 1937; Gibson and Radner, 1937), motion (Anstis et al., 1998; Addams, 1834), spatial configuration (Köhler and Wallach, 1944), color (Webster and Mollon, 1991), or complex shape (Webster and MacLin, 1999), with perceptual aftereffects serving as an important avenue for visual scientists to understand neural principles of stimulus encoding (Frisby, 1979). Recent work has highlighted the usefulness of adaptation for studying shape perception (Gheorghiu and Kingdom, 2008; Webster and MacLin, 1999; Leopold et al., 2001). In a series of studies, Suzuki (Suzuki, 2001; Suzuki, 2003), demonstrated that aftereffects for shape could be observed following adaptation to intermediate-level stimulus features such as convexity and concavity. Specifically, brief exposure to convex shapes was shown to cause subsequently presented rectilinear shapes to appear concave and vice versa; an attention-dependent effect the author took as evidence for opponency in the domain of shape. In considering its neural basis, Suzuki initially postulated that neurons in the inferotemporal cortex could underlie the shape specific aftereffect, based largely on the fact that aftereffect could be observed despite a mismatch in the relative scales of the adapting and test stimuli (Suzuki, 2001; Suzuki and Cavanagh, 1998). Based on further experiments (Suzuki, 2003), along evidence from the neurophysiology of attention (Reynolds et al., 1999), area V4 was identified as a more likely candidate. This speculation resonates with other studies that have identified area V4 as being instrumental in the intermediate level processing of shape, including both lesion studies (Merigan and Pham, 1998; Merigan, 2000) and microelectrode recordings (Kobatake and Tanaka, 1994; Pasupathy and Connor, 2001; Pasupathy and Connor, 2002; Hegde and Van Essen, 2007). In a pivotal study Pasupathy and Connor (Pasupathy and Connor, 1999) concluded that V4 neurons express a systematic tuning to contour features and, based on tuning to a large number of parametrically manipulated black and white shapes, singled out convexity as a key determinant of V4 cell responses.
The present study directly examines the effects of adaptation upon the responses to convex and concave stimuli in area V4. We focus on the relationship of neural adaptation effects to the previously reported perceptual aftereffect as well as the robustness of adaptation effects to the size of the adapting stimuli.
Experimental Procedures
Surgery and Animal Handling
All described procedures were carried out under a protocol approved by the Animal Care and Use Committee of the National Institute of Mental Health. They are in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Two male adult rhesus macaques (Macaca mulatto, “monkey B” (7.8kg) and “monkey E” (8.0kg)) underwent surgery under sterile conditions and deep isoflurane anesthesia. Custom head restraints constructed from G10 fiberglass were implanted using transcranial ceramic screws (Thomas Recording, Giessen, Germany) and self curing dental acrylic (Lang Inc., Wheeling IL). Scleral search coils were implanted (Judge et al., 1980). Then, the animals were trained to maintain their gaze within a window of .50º radius using apple juice as positive reinforcement. In a later surgery, each monkey was implanted with a custom-made recording chamber targeting ventral V4, which was installed using MRI guided surgery software (BrainSight Primate, Rogue Research, Montreal QC), and craniotomy was performed. Recording sites were determined by MRI anatomical criteria (Saleem and Logothetis, 2007; Paxinos et al., 2000). In order to acquire anatomical images, a 4.7T, 60 cm vertical monkey scanner (Bruker Biospec) equipped with a Siemens AC44 gradient coil was used. Using positive reinforcement, monkeys were trained to accept the scanner environment and sat in a custom-made MR-compatible chair without the necessity for anesthesia. The animals were scanned using custom-made radiofrequency coils. Coronal anatomical images were acquired
Equipment and Data Acquisition
Stimuli were created on a PC workstation and in the case of monkey B displayed on a 33.5 × 59.5cm / 1024 × 768 pixels screen with a viewing distance of 100cm. For monkey E a 38 × 65cm / 1024 × 768 pixels screen was used with a viewing distance of 88cm. Custom written software was used for stimulus presentation (Stim, QPCS, courtesy David Sheinberg).
Single units were monitored in ventral cortical area V4 with standard extracellular recording techniques, using commercially available 20µm diameter tungsten electrodes coated with glass (outer diameter: 80 µm; impedance: .7 − 2.0 MΩ at 1kHz; Thomas Recording, Giessen Germany). At the beginning of each recording session, an Eckhorn multielectrode array (Thomas Recording, Giessen Germany) was used to lower 2–7 microelectrodes simultaneously into the brain through a 25 gauge guide tube. The microelectrodes were advanced individually and positioned within area V4. A metallic plate located in the saline filled chamber was used as both reference and ground. The raw signal was preamplified within the Eckhorn array and subsequently amplified, high-pass filtered, and digitized (Plexon Inc, Dallas TX). Spike candidates were identified online through RASPUTIN software (Plexon Inc., Dallas TX) on a PC receiving the digitized signals. We adjusted the waveform thresholds manually for each channel immediately before the recording file was started. After the experiment, units were isolated in PCA space in the commercially available “Offline Sorter” (Plexon Inc, Dallas TX), and timestamps were saved for further processing.
Receptive Field Mapping
Once a neuron was identified on an oscilloscope, the extent of its receptive field (minimal response field) was determined using custom written software. The monkey fixated a square subtending as little as 3 minutes of visual angle, while white bars on a black background were manually swept across the screen to identify the respective area evoking neural responses. During the sessions with more than one intact electrode, the mapping procedure was applied to all channels. Since all electrodes in a given session were separated by less than 1mm, the receptive fields were largely overlapping.
Task and Stimuli
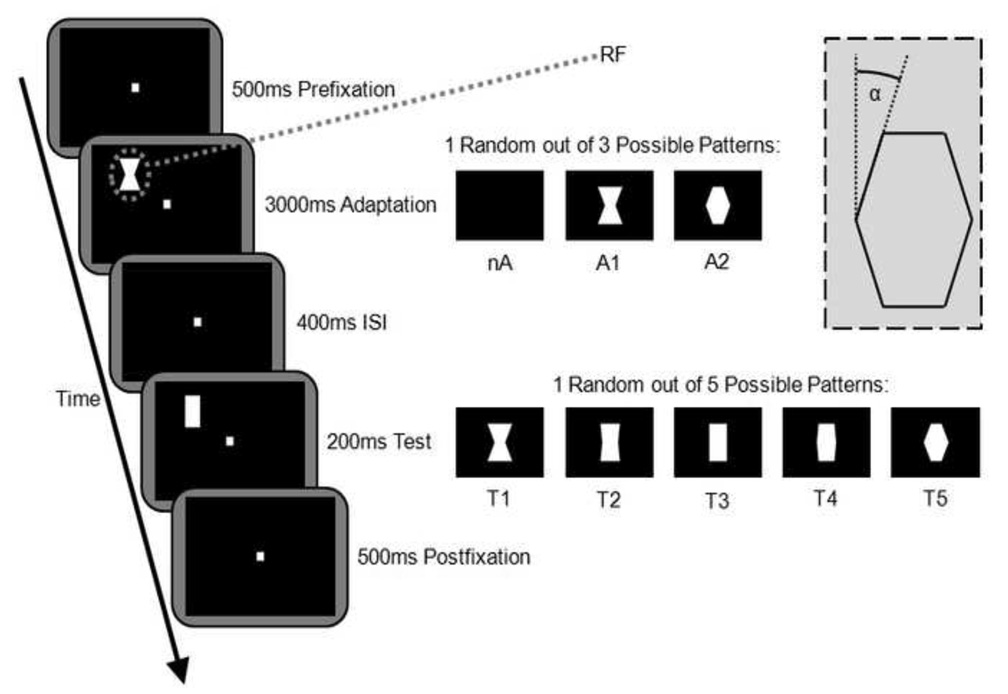
Based on the receptive field plotting, the stimuli were adjusted to be smaller than the receptive fields, and fit completely into the measured boundaries. The visual paradigm is depicted in Fig. 1. Once the monkey acquired fixation, the sequence of visual stimuli started: Following a 500ms baseline period, an adaptation pattern was shown for 3000ms. Then, after a 400ms blank interval, the test stimulus was shown for 200ms. A 500ms post stimulation fixation period followed. The adaptation phase was characterized by either no visual stimulation (nA) or else concave or convex adapters of the same size as the test stimuli (A1 or A2). In all sessions, larger adaptors of the same shape with an area 2.25 times the standard stimulus size were presented as well (not shown in Fig. 1). Top and bottom edges of the larger adaptors were outside the receptive field. All stimuli were solid white (luminance 41 cd/m2) on a black background (luminance 0.1 cd/m2) and were always presented in the upright orientations as shown in Figure 2. We define the level of convexity through angle α as illustrated in Fig. 1. For adaptors, two levels of convexity were used: α=−21.8º (concave, A1) and α=21.8º (convex, A2). The test stimuli consisted of 5 different convexity levels: α=−21.8º (T1), α=−5.7º (minimally concave, T2), α=0º (rectangular, aspect ratio ••• (width/height), T3), α=5.7º (minimally convex, T4), and α=21.8º (T5). All test stimuli and standard size adaptors (A1 and A2) were of the same area. Overall this stimulus arrangement resulted in 25 different combinations of adaptation and test stimuli. These 25 combinations were presented in random order. A session lasted 500 trials, providing 20 trials per condition on average.
Figure 1.
Visual stimulation paradigm. Monkeys held their gaze on a fixation dot while stimuli were presented completely within a previously mapped receptive field. On adaptation trials, 3sec exposure to A1 or A2 was followed by the presentation of the test pattern. Baseline tuning was determined in the absence of any adaptation pattern (nA). Completion of successful trials was rewarded with apple juice. The inset illustrates angle α; see “Materials and Methods” section for details (nA, A1, A2 = adaptation stimuli; T1T5 = test stimuli; RF=schematic of manually mapped receptive field; ISI = inter stimulus interval).
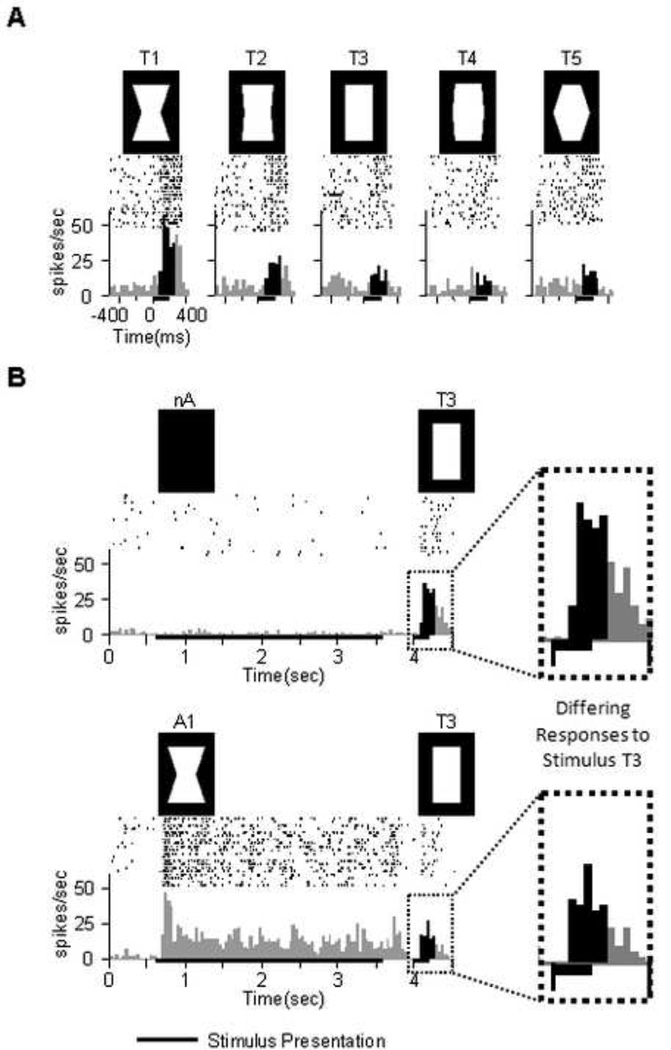
Figure 2.
Typical V4 responses to stimulus set. Spike rasters are shown above with peristimulus time histograms below. Black vertical histogram bars designate the analysis window (80ms–280ms post test onset). (A) Selective response of a unit in the absence of adaptation (cf. Fig1: nA). (B) A different unit. In this example as well as on the average population level adaptation reduced the response.
The animal was required to maintain fixation over the length of a trial (4600ms) in a .7º radius window (occasionally this was changed to other values between .5º and .8º, e.g. due to training or motivational purposes). Successful fixation was rewarded with a drop of apple juice. Breaking fixation would abort the trial immediately and prevent the animal from being rewarded. Aborted trials were excluded from further analysis. Between trials there was a 2000ms break.
Analysis
Analysis was performed using custom written code in MatLab 7.4 (The Mathworks Inc., Natick MA). Neural responses were analyzed in a window from 80ms after test stimulus onset until 80ms after test stimulus offset (i.e. 80ms–280ms post test stimulus onset). This time range ensured the inclusion of the full visual response to the test stimulus while excluding potential “offset responses” as observed in several neurons. We also tested other plausible analysis windows, but the results did not change.
Results
We recorded from 104 visually responsive single units in ventral area V4 of two monkeys in the context of an adaptation paradigm similar to that used to demonstrate the attention-dependent psychophysical aftereffect for convexity (Suzuki, 2001; Suzuki, 2003). The monkeys did not report their percept, but were required to maintain fixation steadily through the adaptation and test periods. We began by evaluating the convexity tuning of each neuron in the absence of any adaptation. In this baseline condition thirty-eight (37%) of the neurons responded selectively as a function of convexity level (univariate ANOVA, p<.05). An example of such a neuron, tuned for the most concave stimulus (T1), is shown in Fig. 2A. Of the selective neurons, 68% (26 out of 38) responded strongest to the most concave stimulus, 8% (3 out of 38) to the most convex stimulus, and 24% (9 out of 38) to the neutral stimulus or one of the minimal deviations thereof.
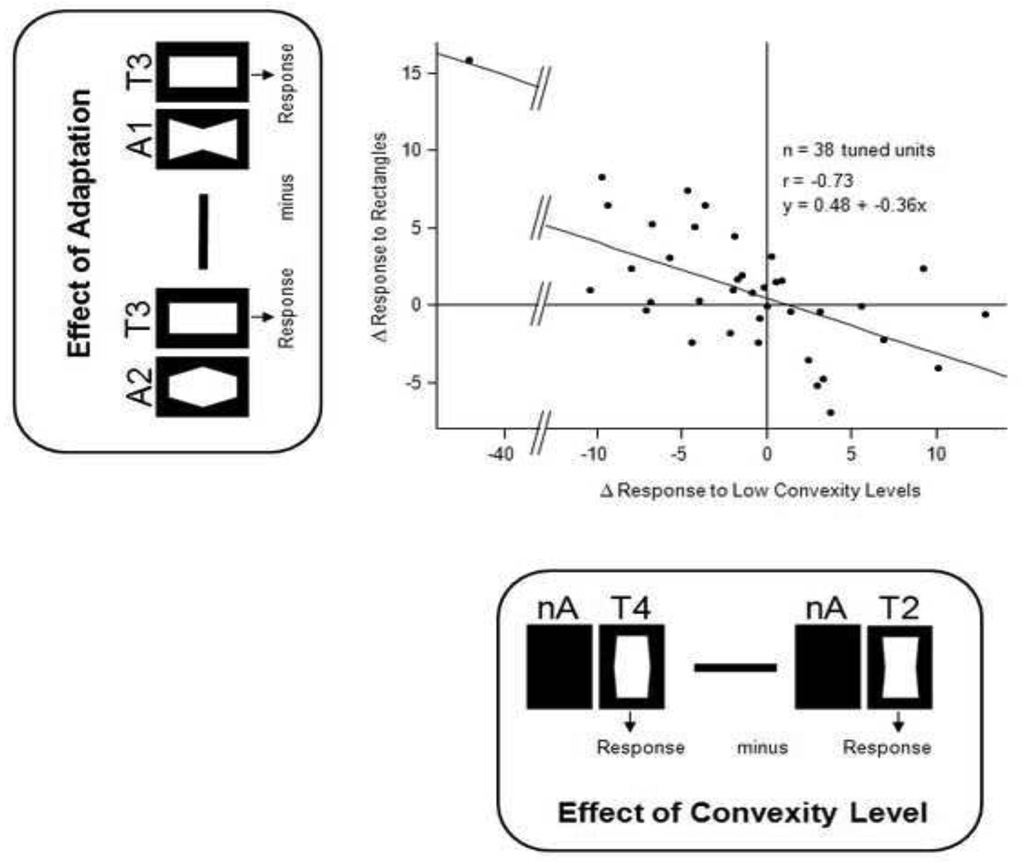
We next asked how the pattern of response to the test stimuli might be influenced by a period of prior shape adaptation, focusing first on responses to the neutral (i.e. rectangular) test stimulus. Adaptation significantly affected the responses of 44% of the neurons tested (univariate ANOVA over three adaptation condition mean values, p<.05), leading to a average absolute shift in firing rate of 5.9 spikes/sec (mean over all unadapted compared to mean over all adapted). Across the population, this adaptation was shape-specific, inducing a change in firing rate consistent with the expectations of a negative perceptual aftereffect. We assessed this by comparing the responses to the neutral test stimulus following adaptation to those elicited by low convexity levels in the absence of adaptation. The repulsive shift in convexity tuning following adaptation can be seen in the negative slope of the correlation in Figure 3, which plots the differential spike rate measured between two conditions.
Figure 3.
Negative correlation between convexity tuning and the effects of adaptation on the response to the zero-convexity test stimulus. All single units with significant tuning to one of the 5 test stimuli were included in this analysis, with each dot corresponding to one unit. Values on the x-axis show responses to low convexity levels without previous adaptation (nA): Mean firing rate to T2 was subtracted from mean firing rate to T4. The y-axis shows differential effects of convex (A2) and concave (A1) adaptation on response to test rectangle (T3). Note the high correlation with negative slope between the two differential measures, indicative of a negative aftereffect.
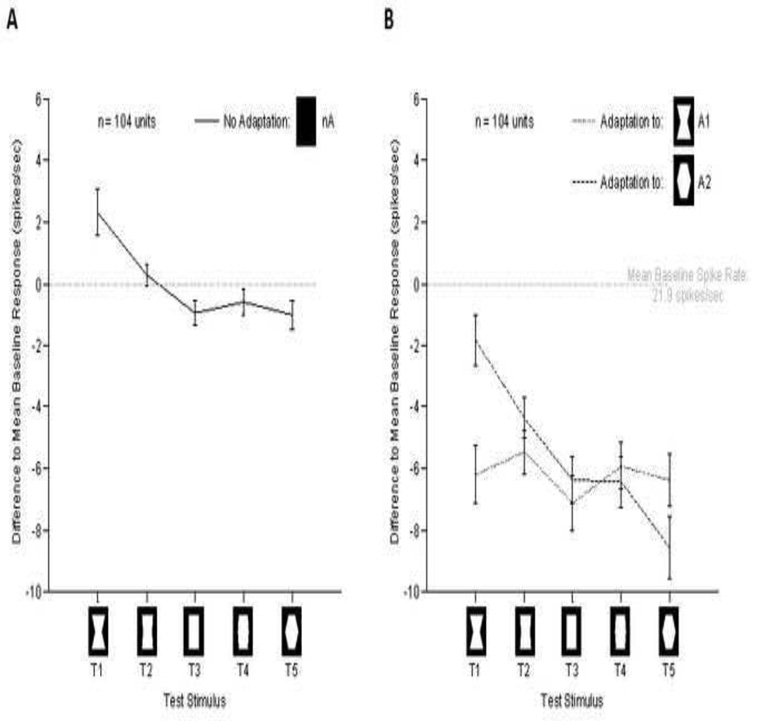
Next, we analyzed neural responses to five different convexity levels following three seconds of exposure to convex or concave adaptors (see Figure 2B). The relative responses were computed for each unit prior to averaging by subtracting the mean baseline response to the 5 test stimuli. The mean results across the population (n=104) are shown in Figure 4A. In the absence of adaptation, the most concave stimulus gave a slightly but significantly higher response compared to the other stimuli. Note that this finding differs from previous work in dorsal V4 (Pasupathy and Connor, 1999), and TE (Kayaert et al., 2005), where convex patterns were shown to be preferred over concave ones across the population.
Figure 4.
Nonspecific and specific effects of adaptation. The y-axis marks the difference to the mean baseline activity. X-axis shows the different test stimuli. Error bars represent the standard error of the mean. (A) Average response to the test stimuli across the population. Note the overall preference to the globally concave stimulus. (B) Population tuning following adaptation. Note that the most prominent effect was a significant overall decrease in firing following adaptation. In addition there was a smaller stimulus specific effect of adaptation, which can be inferred by comparing the responses to T1 and T5 following different adaptors.
Figure 4B shows that, in addition to the shape-specific effects on the neutral stimulus reported above, adaptation caused a large, nonspecific decrease in responses for all test stimuli across the population (concave adaptor: F(1,9)=262.0, p<.001; convex adaptor: F(1,9)=186.1,p<.001). In addition, this analysis revealed a second shape-specific component that affected the responses to the most extreme values, which had the same shape as the adaptor stimuli. The latter effect is similar to the suppression to repeated visual stimuli commonly observed in more rostral temporal areas (Miller et al., 1991; Sobotka and Ringo, 1994; Brown and Xiang, 1998) but also well documented for the early visual cortex (Carandini et al.. 1997). The mean effects of adaptation for individual neurons across the population can comparing the effects of adaptation on rectangular stimuli (i.e. analysis similar to Figure 3).
Discussion
Neural responses in area V4 were strongly influenced by prior adaptation, and this influence consisted of three components. The first, nonspecific, component resulted in a suppression of neural firing rate across the population regardless of the identity of the test and adapting stimuli (see Fig. 4). The second, shape-specific, component resembled the well-studied phenomenon of repetition suppression. This effect is characterized by prior exposure to the same stimulus inducing a stronger suppression than prior exposure to a different stimulus (see Fig 5). Repetition suppression contingent upon specific stimuli has been reported in different visual areas of the ventral stream preceding and following V4 (Miller et al., 1991; Carandini et al., 1997; Sawamura et al., 2006) The third and most complex component of adaptation was both shape-and tuning-specific (Fig 3). In this component, which can be compared directly to the corresponding shape aftereffect, the neural response to a neutral stimulus was shifted away from the adapting stimulus according to each neuron’s tuning function. These findings are consistent with simple cellular models proposing stronger suppression as a result of higher previous activity.
Figure 5.
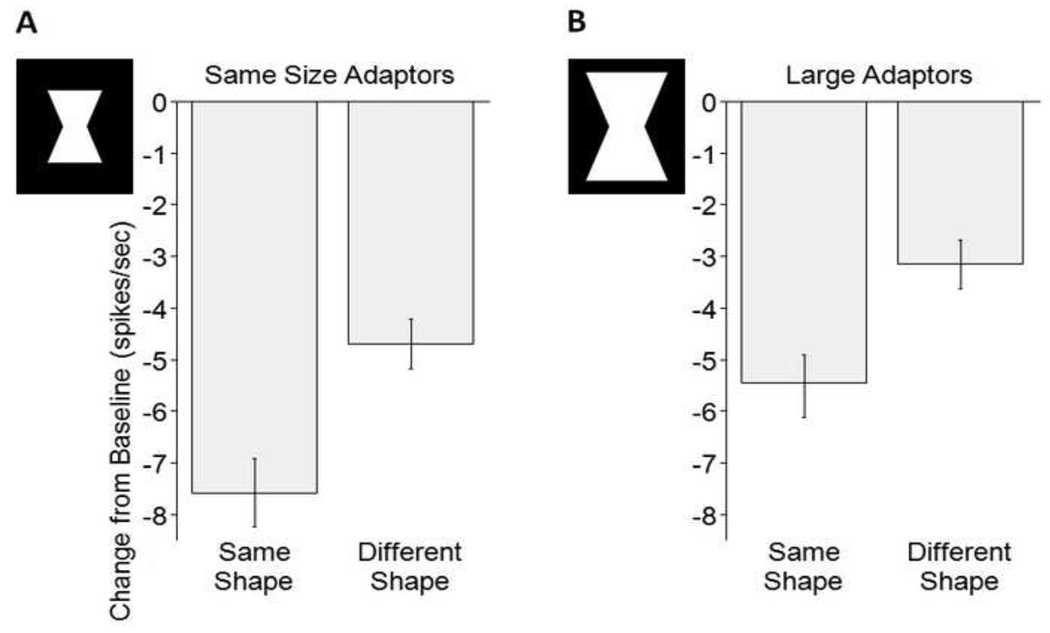
Size-independent pattern specificity of adaptation (N=104 single units, 2 conditions per unit combined in each data point). Here, the pooled effects of the convex and the concave adaptors onto T1 or T5 respectively are depicted. The stimuli illustrate the size difference. Convex adaptors were of the same area as the concave adaptors shown here. (A) Average difference between adapted and baseline firing is 7.6 spikes/sec for the same shapes vs. 4.7 spikes/sec for different shapes. Error bars indicate standard error of the mean. (B) Larger size refers to adaptors of the same shapes but 2.25 times the area. Note that the pattern selective adaptation effect remains despite a change in the adaptor size.
Adaptation in the early visual system is well documented, and may account for the nonspecific component in the present study. In the precortical cascade of events from photoreceptors through ganglion cells and thalamic relay neurons, adaptation effects have been demonstrated (Perlman and Normann, 1998; Smirnakis et al., 1997; Sanchez-Vives et al., 2000) and have been characterized by decline of the spike rate and post-adaptation firing reduction for several seconds. One common difficulty in drawing conclusions based on the neural effects of visual adaptation is that adaptation simultaneously influences neurons at many stages of visual processing, and potentially in different ways. While at any point in the visual pathway, some aspects of adaptation will be inherited from previous processing stages, others may be generated locally. For contrast adaptation a disproportionately high fraction of adaptation mechanisms is well documented to be of early cortical origin (Maffei et al., 1973; Ohzawa et al., 1985; Sanchez-Vives et al., 2000). This component of V1 adaptation correlates with a tonic hyperpolarization of the cell membranes, which is likely to be the cause for the decline in spike rate and postadaptation firing reduction (Carandini and Ferster, 1997; Sanchez-Vives et al., 2000). Similarly, evidence suggests that the adaptation measured in neurons of area MT also has its origins in the striate cortex (Kohn and Movshon, 2003). It would therefore be expected that a large fraction of the nonspecific adaptation effects, which were on average suppressive, have their origins in primary visual cortex.
The shape-and tuning-specific component provides the first link between neural activity in V4 and a perceptual aftereffect. The parameters of the minimally convex and minimally concave stimuli were chosen to resemble the visual aftereffect (Suzuki, 2001). Previous studies examining parametric tuning characteristics of V4 neurons have concluded that shape (Pasupathy and Connor, 2002; Pasupathy and Connor, 2001; Gallant et al., 1996; Hegde and Van Essen, 2007) and particularly convexity (Pasupathy and Connor, 1999), are important coding features of V4. The present findings suggest that this shape feature can be specifically adapted in V4 neurons, and that this adaptation results in a predictable change in the tuning of the neuron. The three components of adaptation in our results have been reported in other adaptation studies in cat V1 (Dragoi et al., 2001; Dragoi et al., 2000; Ghisovan et al., 2008), macaque V1 (Carandini et al., 1997; Dragoi et al., 2002) as well as macaque MT (Kohn and Movshon, 2004;
Krekelberg et al., 2006). In V1 the tuning shifts away from the adapting stimulus, while in MT, there is an attractive shift towards the adaptor. The negative correlation we observed in V4 between the tuning to low convexity levels and the rectangle following adaptation to high convexity levels is more similar to the repulsive shift in V1 than the attractive shift in MT. However, note that tuning in V4 is not as well understood as in V1 or MT, thus analyzing data in the traditional way turned out impossible for the current data set.
Of particular importance is the observation that the shape specific adaptation effects were tolerant to a substantial change in the size of the adapting pattern. This observation eliminates adaptation within strict retinotopic areas as being responsible for the observed change in responsiveness, and thus argues that it is convexity itself that is being adapted. Also, the negative shift observed in the third component (shape-and tuning-specific) closely mirrors the expectations of the convexity aftereffect (Suzuki, 2001). In that case, adaptation to a convex pattern causes the subsequent presentation of a rectangle to appear concave, and vice versa. Our observation, that differential neural responses to rectangles are shifted away from the adaptation stimulus toward the opposite convexity level, suggest that not only are V4 neurons involved in analyzing this stimulus dimension, but that shifts in their responses may contribute to the convexity aftereffect.
Supplementary Material
Acknowledgements
We thank our collaborators: N. Phipps and K. Smith for assistance with animal training and experimentation; C. Zhu and Dr. F. Ye for help with MRI scans; Dr. D. Sheinberg for providing some of the stimulation software; Dr. A. Maier and Dr. D. McMahon for insightful suggestions concerning data acquisition, analysis, and previous versions of this manuscript. This work was supported by the NIMH, NINDS, and NEI Intramural Research Programs.
Reference List
- Addams R. An account of a peculiar optical phenomenon seen after having looked at a moving body. London & Edinburgh Philosophical Magazine and Journal of Science. 1834;5:373–374. [Google Scholar]
- Anstis S, Verstraten FAJ, Mather G. The Motion Aftereffect. Trends Cogn Sci. 1998;2:111–117. doi: 10.1016/s1364-6613(98)01142-5. [DOI] [PubMed] [Google Scholar]
- Arieli A, Shoham D, Hildesheim R, Grinvald A. Coherent spatiotemporal patterns of ongoing activity revealed by real-time optical imaging coupled with single-unit recording in the cat visual cortex. J. Neurophysiol. 1995;73:2072–2093. doi: 10.1152/jn.1995.73.5.2072. [DOI] [PubMed] [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog. Neurobiol. 1998;55:149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Brown SP, Masland RH. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat. Neurosci. 2001;4:44–51. doi: 10.1038/82888. [DOI] [PubMed] [Google Scholar]
- Carandini M, Barlow HB, O'Keefe LP, Poirson AB, Movshon JA. Adaptation to contingencies in macaque primary visual cortex. Philos. Trans. R. Soc. Lond B Biol. Sci. 1997;352:1149–1154. doi: 10.1098/rstb.1997.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dragoi V, Rivadulla C, Sur M. Foci of orientation plasticity in visual cortex. Nature. 2001;411:80–86. doi: 10.1038/35075070. [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat. Neurosci. 2002;5:883–891. doi: 10.1038/nn900. [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Sur M. Adaptation-induced plasticity of orientation tuning in adult visual cortex. Neuron. 2000;28:287–298. doi: 10.1016/s0896-6273(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Shapley RM. Adaptation and dynamics of cat retinal ganglion cells. J. Physiol. 1973;233:271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat. Rev. Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby JP. Seeing: Illusion, brain and mind. Oxford, England: Oxford University Press; 1979. [Google Scholar]
- Gallant JL, Connor CE, Rakshit S, Lewis JW, Van E. Neural responses to polar, hyperbolic, and Cartesian gratings in area V4 of the macaque monkey. J. Neurophysiol. 1996;76:2718–2739. doi: 10.1152/jn.1996.76.4.2718. [DOI] [PubMed] [Google Scholar]
- Gheorghiu E, Kingdom FA. Spatial properties of curvature-encoding mechanisms revealed through the shape-frequency and shape-amplitude after-effects. Vision Res. 2008;48:1107–1124. doi: 10.1016/j.visres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Ghisovan N, Nemri A, Shumikhina S, Molotchnikoff S. Synchrony between orientation-selective neurons is modulated during adaptation-induced plasticity in cat visual cortex. BMC. Neurosci. 2008;9:60. doi: 10.1186/1471-2202-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. Adaptation, after-effect, and contrast in the perception of tilted lines. II. Simultaneous contrast and the areal restriction of the after-effect. J. Exp. Psychol. 1937;20:553–569. [Google Scholar]
- Gibson JJ, Radner M. Adaptation, after-effect, and contrast in the perception of tilted lines. J. Exp. Psychol. 1937;20:453–467. [Google Scholar]
- Hegde J, Van Essen DC. A comparative study of shape representation in macaque visual areas v2 and v4. Cereb. Cortex. 2007;17:1100–1116. doi: 10.1093/cercor/bhl020. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kayaert G, Biederman I, Op de Beeck HP, Vogels R. Tuning for shape dimensions in macaque inferior temporal cortex. Eur. J. Neurosci. 2005;22:212–224. doi: 10.1111/j.1460-9568.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J. Neurophysiol. 1994;71:856–867. doi: 10.1152/jn.1994.71.3.856. [DOI] [PubMed] [Google Scholar]
- Köhler W, Wallach H. Figural After-Effects: An Investigation of Visual Processes. Proceedings of the American Philosophical Society. 1944;88:269–357. [Google Scholar]
- Kohn A, Movshon JA. Neuronal adaptation to visual motion in area MT of the macaque. Neuron. 2003;39:681–691. doi: 10.1016/s0896-6273(03)00438-0. [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat. Neurosci. 2004;7:764–772. doi: 10.1038/nn1267. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, van Wezel RJ, Albright TD. Adaptation in macaque MT reduces perceived speed and improves speed discrimination. J. Neurophysiol. 2006;95:255–270. doi: 10.1152/jn.00750.2005. [DOI] [PubMed] [Google Scholar]
- Leopold DA, O'Toole AJ, Vetter T, Blanz V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nat. Neurosci. 2001;4:89–94. doi: 10.1038/82947. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Movshon JA. Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. J. Neurosci. 1999;19:2224–2246. doi: 10.1523/JNEUROSCI.19-06-02224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A, Bisti S. Neural correlate of perceptual adaptation to gratings. Science. 1973;182:1036–1038. doi: 10.1126/science.182.4116.1036. [DOI] [PubMed] [Google Scholar]
- Matsui T, Koyano KW, Koyama M, Nakahara K, Takeda M, Ohashi Y, Naya Y, Miyashita Y. MRI-based localization of electrophysiological recording sites within the cerebral cortex at single-voxel accuracy. Nat. Methods. 2007;4:161–168. doi: 10.1038/nmeth987. [DOI] [PubMed] [Google Scholar]
- Merigan WH. Cortical area V4 is critical for certain texture discriminations, but this effect is not dependent on attention. Vis. Neurosci. 2000;17:949–958. doi: 10.1017/s095252380017614x. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Pham HA. V4 lesions in macaques affect both single-and multiple-viewpoint shape discriminations. Vis. Neurosci. 1998;15:359–367. doi: 10.1017/s0952523898152112. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Vis. Neurosci. 1991;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat' visual system. J. Neurophysiol. 1985;54:651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Responses to contour features in macaque area V4. J. Neurophysiol. 1999;82:2490–2502. doi: 10.1152/jn.1999.82.5.2490. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Shape representation in area V4: position-specific tuning for boundary conformation. J. Neurophysiol. 2001;86:2505–2519. doi: 10.1152/jn.2001.86.5.2505. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Population coding of shape in area V4. Nat. Neurosci. 2002;5:1332–1338. doi: 10.1038/nn972. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain. London: Academic Press; 2000. [Google Scholar]
- Perlman I, Normann RA. Light adaptation and sensitivity controlling mechanisms in vertebrate photoreceptors. Prog. Retin. Eye Res. 1998;17:523–563. doi: 10.1016/s1350-9462(98)00005-6. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. London: Academic Press; 2007. A combined MRI and histology atlas of the teh rhesus monkey brain in stereotaxic coordinates. (Elsevier Limited) [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J. Neurosci. 2000;20:4267–4285. doi: 10.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Smirnakis SM, Berry MJ, Warland DK, Bialek W, Meister M. Adaptation of retinal processing to image contrast and spatial scale. Nature. 1997;386:69–73. doi: 10.1038/386069a0. [DOI] [PubMed] [Google Scholar]
- Sobotka S, Ringo JL. Stimulus specific adaptation in excited but not in inhibited cells in inferotemporal cortex of macaque. Brain Res. 1994;646:95–99. doi: 10.1016/0006-8993(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Attention-dependent brief adaptation to contour orientation: a high-level aftereffect for convexity? Vision Res. 2001;41:3883–3902. doi: 10.1016/s0042-6989(01)00249-8. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Attentional selection of overlapped shapes: a study using brief shape aftereffects. Vision Res. 2003;43:549–561. doi: 10.1016/s0042-6989(02)00683-1. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Cavanagh P. A shape-contrast effect for briefly presented stimuli. J. Exp. Psychol. Hum. Percept. Perform. 1998;24:1315–1341. doi: 10.1037//0096-1523.24.5.1315. [DOI] [PubMed] [Google Scholar]
- Webster MA, MacLin OH. Figural aftereffects in the perception of faces. Psychon. Bull. Rev. 1999;6:647–653. doi: 10.3758/bf03212974. [DOI] [PubMed] [Google Scholar]
- Webster MA, Mollon JD. Changes in colour appearance following post-receptoral adaptation. Nature. 1991;349:235–238. doi: 10.1038/349235a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.