Abstract
Deep brain stimulation of the subthalamic nucleus (STN DBS) improves motor symptoms in idiopathic Parkinson's disease, yet the mechanism of action remains unclear. Previous studies indicate that STN DBS increases regional cerebral blood flow (rCBF) in immediate downstream targets but does not reveal which brain regions may have functional changes associated with improved motor manifestations. We studied 48 patients with STN DBS who withheld medication overnight and underwent PET scans to measure rCBF responses to bilateral STN DBS. PET scans were performed with bilateral DBS OFF and ON in a counterbalanced order followed by clinical ratings of motor manifestations using Unified Parkinson Disease Rating Scale 3 (UPDRS 3). We investigated whether improvement in UPDRS 3 scores in rigidity, bradykinesia, postural stability and gait correlate with rCBF responses in a priori determined regions. These regions were selected based on a previous study showing significant STN DBS-induced rCBF change in the thalamus, midbrain and supplementary motor area (SMA). We also chose the pedunculopontine nucleus region (PPN) due to mounting evidence of its involvement in locomotion. In the current study, bilateral STN DBS improved rigidity (62%), bradykinesia (44%), gait (49%) and postural stability (56%) (paired t-tests: P < 0.001). As expected, bilateral STN DBS also increased rCBF in the bilateral thalami, right midbrain, and decreased rCBF in the right premotor cortex (P < 0.05, corrected). There were significant correlations between improvement of rigidity and decreased rCBF in the SMA (rs = –0.4, P < 0.02) and between improvement in bradykinesia and increased rCBF in the thalamus (rs = 0.31, P < 0.05). In addition, improved postural reflexes correlated with decreased rCBF in the PPN (rs = –0.38, P < 0.03). These modest correlations between selective motor manifestations and rCBF in specific regions suggest possible regional selectivity for improvement of different motor signs of Parkinson's disease.
Keywords: Parkinson's disease, deep brain stimulation, subthalamic nucleus, pedunculopontine nucleus, positron emission tomography
Introduction
Parkinson's disease is a neurodegenerative disorder with striatal dopamine deficiency characterized by bradykinesia, rigidity, tremor and impaired balance. Levodopa and other medications provide symptomatic relief for a limited period of time, but as the disease progresses, patients frequently develop drug-induced dyskinesias, motor fluctuations and dose failures. Recently, deep brain stimulation (DBS) of the subthalamic nucleus (STN) has provided marked benefit for motor symptoms (Krack et al., 2003), yet the mechanism of DBS action remains unclear (McIntyre and Thakor, 2002; Perlmutter and Mink, 2006).
Much research has focused on how DBS affects neuronal activity. Electrophysiologic methods using extracellular electrodes can measure single unit electrical activity or field potentials in selected downstream targets in response to DBS (Hashimoto et al., 2003; Brown et al., 2004), whereas PET or SPECT imaging can measure DBS-induced changes in regional cerebral blood flow (rCBF) throughout the entire brain. In a previous study, we demonstrated that STN DBS increases blood flow in midbrain, globus pallidus and thalamus but reduces blood flow in superior frontal gyrus supplementary motor area (SMA) and middle frontal gyrus (premotor cortex), suggesting that STN DBS increases the net output from STN (Hershey et al., 2003). Similarly, electrophysiologic recordings demonstrated increased firing rate of globus pallidus pars interna (Gpi) neurons during STN DBS (Hashimoto et al., 2003). Although these studies address the effects of DBS on neuronal activity, they have not yet determined how this translates into clinical benefit. The purpose of this study was to investigate whether STN DBS-induced rCBF changes in selected brain regions correlate with improvements in several key aspects of motor performance in Parkinson's disease: locomotion, rigidity and bradykinesia. Regions of interest were identified a priori based on the current knowledge of basal ganglia circuitry (Mink, 2003), our previous findings of cortical regions that respond to STN DBS (Hershey et al., 2003) and regions thought to be critical for optimal motor function. We determined the significance of correlations between STN DBS-induced changes in rigidity and bradykinesia scores and rCBF responses in bilateral thalamus, sensorimotor cortex (SMC) and SMA. Given the increasing focus on the role of the pedunculopontine nucleus (PPN) in locomotion, we also determined the significance of correlations between gait and postural stability score changes and rCBF responses in the PPN (Pahapill and Lozano, 2000).
Methods
Subjects
All 48 patients met the diagnostic criteria for clinically definite Parkinson's disease (Hughes et al., 1992) and had bilateral STN stimulators implanted at least 3 months prior to the study to permit optimal programming of DBS (Tabbal et al., 2007b). Exclusionary criteria included history of encephalitis, dementia, stroke, serious head injury or inability to hold the head still while lying supine with DBS turned OFF (e.g. due to severe resting jaw tremor or neck dystonia). All participants provided informed written consent. The study protocol was approved by the Human Research Protection Office and the Radioactive Drug Research Committee of Washington University in St Louis.
Procedure
Subjects refrained from taking any antiparkinsonian medications overnight while stimulators were left ON. Subjects were scanned on the Siemens/CTI ECAT EXACT HR 47 tomograph (Wienhard et al., 1994) in 2D mode. We placed a 20-gauge plastic catheter in an antecubital vein for [15O]-water injection. Each subject was positioned in the PET scanner using cross laser lines and a polyform mask (Roylan Industries, Menomonee Falls, WI). The mask and face were marked to detect any change in head position relative to the mask. Attenuation was measured using three rotating rod sources of 68Ge/68Ga. During each scan the lights were dimmed and the room was quiet while the subject kept the eyes closed. About 50 mCi of [15O]-water was injected intravenously as a bolus followed by data acquisition for 2 min. PETs were collected 14 min apart to permit adequate radioactive decay.
DBS conditions
PET scans were done with either both STN DBS OFF or both ON. The order of conditions was chosen randomly on the morning of the study (ON then OFF versus OFF then ON) to reduce any bias introduced by progressive worsening of parkinsonian signs due to potential stress of prolonged testing or longer withholding of medication. Up to six PET scans were done as a block in each condition. The first PET scan in each block (ON or OFF) was performed at least 42 min after the change in the stimulator settings. The Unified Parkinson Disease Rating Scale (UPDRS) measurement was performed at the end of the block. The time between change in settings and the UPDRS measurement was therefore about 3 h. Individual components of the UPDRS 3 were assessed after each scan (tremor, rigidity and bradykinesia of each limb) to confirm no change in ratings across each block of PET scans. About 80% of the effect of DBS on rigidity and bradykinesia abates by 30 min after stopping DBS, whereas benefit to gait drops to a similar level after an hour (Temperli et al., 2003). It should be noted that there are no data available on the washout period for the DBS-induced changes in rCBF. In addition, we were able to control in part for individual differences in residual DBS effects using percent change in rCBF between ON and OFF scans.
Monitoring
During each PET scan, the subject was watched by at least one movement disorders specialist for visible tremor or other movements. Eight-channel surface EMG recordings were collected to detect evidence of substantial muscle activity not otherwise visible. In addition, all subjects were videotaped with two cameras, one aimed at the head and the other at the full length of the body, during scans to permit post-hoc review for questionable movement.
Surface EMG
Surface EMG electrodes were applied to bilateral biceps, wrist flexors, quadriceps femoris and gastrocnemius muscles. EMG signals were amplified at a gain of 2000, filtered on line with a band pass of 10 Hz to 1000 kHz and stored on computer using a CED Micro 1401 interface (Cambridge Electronic Design). Codes were inserted into the data stream to coincide with the onset and offset of each scan as well as to denote any movement, loud noise or other possibly confounding event.
PET data analysis
Data from the initial 40 s after the arrival time of the radioactive water in the brain were analysed. PET emission scans were reconstructed using filtered back projection, measured attenuation and scatter correction. To reduce noise, the two transmission images from each subject were co-registered to each other and averaged. This average attenuation image was re-sliced to match each emission image, and new attenuation corrections were forward projected. All emission scans were reconstructed a final time using these co-registered, averaged attenuation corrections. Emission images were smoothed with a 3D Gaussian filter to a final resolution of 16 mm full width at half maximum (FWHM). We used unfiltered images with a FWHM of 4.4 mm for analysis of the PPN region, since any filtering would reduce the resolution and thusly the probability of detecting rCBF changes in a small volume of interest (VOI). All emission images were co-registered to the initial emission image. These images then were co-registered and re-sliced to a standard mean blood flow image in Talairach atlas space (Talairach and Tournoux, 1988; Woods et al., 1992). Individual images were normalized using mean whole brain counts and masked to include only the voxels in common among all scans. All blood flow measurements were qualitative and globally normalized.
To eliminate behavioral confounds, all PET scans collected while the subject had tremor, other movements, or sustained substantial EMG activity above background noise before or within the 40 s of data acquisition were excluded from further analysis (Hershey and Mink, 2006b). Subjects were retained for analysis if they had at least one usable scan per condition.
Whole brain analysis
SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) was used for a global voxel-based comparison between average OFF and ON scans with paired t-tests to reveal significant STN DBS-induced increases and decreases in rCBF. A t-value was assigned to each voxel in the brain, and the t-map was examined for voxels that exceeded a height threshold (t = 3.38; P < 0.001). The program identified clusters of greater than 20 height-thresholded voxels and applied a multiple comparisons correction for the number of possible clusters of this size and magnitude of the brain volume. Clusters that reached a corrected P value of less than 0.05 were considered significant.
VOI definition
All VOIs were selected a priori. Bilateral thalami were defined by tracing the outlines of the structures on a Talairach atlas overlay registered to the images (Talairach and Tournoux, 1988). We chose separate VOIs for SMC and SMA and avoided motor task-activated cortical regions, since there are various reports in the literature regarding their involvement (Thobois et al., 2002; Strafella et al., 2003; Payoux et al., 2004; Asanuma et al., 2006). The SMC region was identified by an area of increased rCBF in response to hand vibration in normal subjects (Perlmutter and Mink, 2006). The SMA region was defined based on an area with significant blood flow decrease with bilateral DBS as published previously (Hershey et al., 2003).
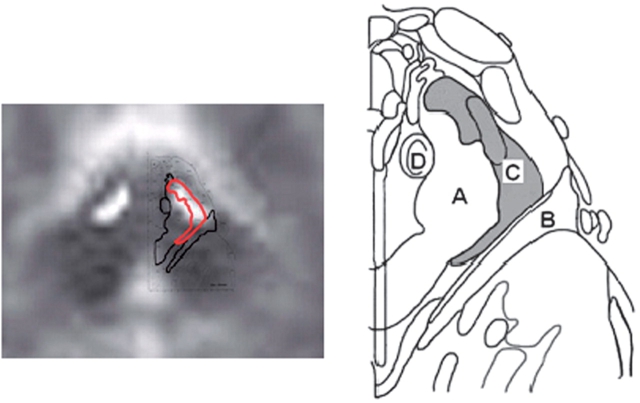
In addition, we selected the PPN, a region thought to be involved in locomotion (Pahapill and Lozano, 2000). The PPN is bordered medially by superior cerebellar peduncle, laterally by medial lemniscus, rostrally by substantia nigra and the retrorubral field and caudally by locus coeruleus (Pahapill and Lozano, 2000). Just posterior to the cerebral peduncles we defined a region containing 66 voxels extending 4–8 mm from midline and extending from z = –10 mm through –22 mm craniocaudally. The PPN VOI covered parts of superior cerebellar peduncle and medial lemniscus as illustrated in Fig. 1 (Paxinos and Huang, 1995).
Fig. 1.
Overlay of the average MRI of participants with available MRIs (n = 11) and PPN region defined using Paxinos and Huang Atlas of the Human Brainstem. (A) Superior cerebellar peduncle, (B) Medial lemniscus, (C) PPN AND (D) central tegmental tract.
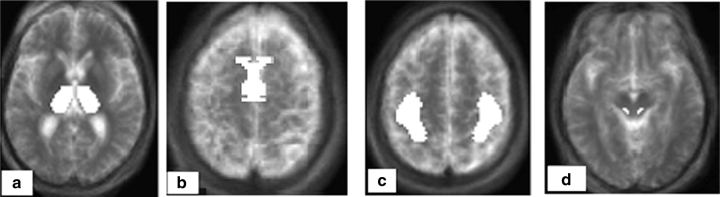
Since PPN is relatively small, we chose to extract blood flow data from unfiltered images to maximize resolution (FWHM = 4.4 mm), despite the increased noise. We also defined a small VOI for PPN to minimize volume averaging from nearby regions. Other grey matter structures that are in close proximity include SN and, to a lesser extent, red nucleus. Table 1 provides the volume and Fig. 2 displays a transverse section of each VOI.
Table 1.
List of a priori defined VOIs with their volume and voxel number
| Brain region | Number of voxels | Total volume (cc) |
|---|---|---|
| PPN | 66 | 0.528 |
| SMA | 945 | 7.560 |
| Thalamus | 1404 | 12.032 |
| SMC | 1366 | 10.204 |
Fig. 2.
A priori defined VOIs: (A) bilateral thalami, (B) bilateral SMA, (C) bilateral SMC, (D) bilateral PPN region. The VOIs are overlaid on Talairach atlas co-registered average MRI of 11 participants.
Motor measurements
For each DBS condition subjects were removed from the scanner at the end of six PET scans and underwent a complete UPDRS 3 motor rating. Each examiner, either a movement disorder specialist or a research nurse, was trained and validated in UPDRS 3 ratings (interclass correlations >0.88 comparing blinded UPDRS motor ratings on videos of 10 people with Parkinson's disease). Attempted blinding of raters and examiners failed since there was a dramatic difference between the DBS OFF and ON conditions. We used the sum of the bradykinesia subscores averaged for arms (finger tapping, hand opening/closing, hand rotation) and foot tapping bilaterally yielding a total bradykinesia score from 0 to 16. Similarly, we used the sum of the rigidity subscores for neck, arms and legs with a total rigidity score range from 0 to 20. We used the pull test subscore (0–4) for postural stability and the gait subscore (0–4) for gait performance. We also calculated the sum of scores for UPDRS 3 items 27–30 as an overall measure of axial signs. We did not include the tremor subscores, as the high variability of the tremor within the same condition would make tremor scoring less reproducible.
Correlation analysis
The mean regional activity corresponding to rCBF was computed by applying the VOIs (thalami, bilateral SMA, bilateral SMC and bilateral PPN region) to the average ON and average OFF blood flow images for each subject. Each VOI was assigned a value calculated as the mean of the right and left activity. The percent change [100 × (ON − OFF)/OFF] between ON and OFF activity was correlated (Spearman) with the change in motor performance between the ON and OFF conditions. We chose to use a difference score and not to use percent change of motor performance, since the motor performance was measured on relatively restricted scales; using percent change would thusly risk exaggerating a small absolute change in the score. Significance level was set at P < 0.05. We did not correct for multiple comparisons since the VOI regions were chosen a priori as part of separately formulated hypotheses for each motor aspect of Parkinson's disease. Our hypotheses were based upon current knowledge of basal ganglia-thalamo-cortical circuits and not post-hoc analysis of the current data. Thus we believe that correction for multiple comparisons across all motor correlations would be too conservative.
Results
Forty-eight subjects fulfilled the original inclusion criteria. Seventeen were excluded since all their OFF scans were affected by tremor, substantial EMG activity or movement. A total of 111 OFF PET scans and 34 ON PET scans were excluded.
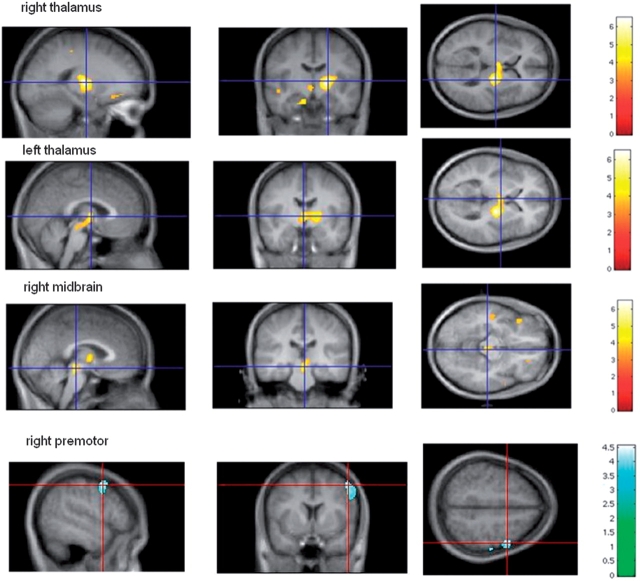
This left 31 subjects for final data analysis. The demographics of these subjects and their DBS characteristics are summarized in Table 2. A total of 87 PET scans were excluded due to tremor (visible or recorded on EMG) and 58 PET scans due to other reasons such as head movement, difficulty during injection of radioactive water, loud noise, or volitional movements during the scan. Eleven individuals were excluded because all of their OFF scans were affected by tremor. The average age of subjects was 61 ± 10 years and average duration of disease was 14 ± 8 years. Eleven of the 31 subjects included in analyses were female. Three subjects had missing bradykinesia ratings, and one subject had missing rigidity ratings. There was no statistical difference between the mean amount of radioactivity injected for ON and OFF conditions. All subjects experienced improvement in motor symptoms with bilateral STN DBS ON. Bilateral STN DBS improved rigidity (62%), bradykinesia (44%), gait (49%) and postural stability (56%) (paired t-tests: P < 0.001). SPM5 with paired t-tests between average ON and average OFF scans from the remaining 31 subjects revealed significant rCBF increases in bilateral thalami and right midbrain and significant rCBF decrease in right premotor cortex (P < 0.05, corrected at cluster level; see Fig. 2).
Table 2.
Demographics and stimulator settings
| Category | Satistics | |
|---|---|---|
| Demographics | ||
| Gender | ||
| Males | 19 (61.3%) | |
| Females | 12 (39.7%) | |
| Age at time of surgery (years) | 61 ± 10 | |
| Disease duration at time of surgery (years) | 14 ± 8 | |
| Statistics at time of study | ||
| Interval between surgery and study (days) | 376 ± 355 | |
| Laterality of symptoms based on mean rigidity and bradykinesia scores | ||
| Left | 2.10 ± 0.03 | |
| Right | 2.16 ± 0.04 | |
| Mean UPDRS | ||
| Med OFF-stim OFF | 42.1 ± 13.7 | |
| Med OFF-stim ON | 19.6 ± 9.1 | |
| Improvement | 53.3776% | |
| H&Y Med OFF-DBS OFF | ||
| 2 | 3 (9.7%) | |
| 2.5 | 4 (12.9%) | |
| 3 | 5 (16.13%) | |
| 4 | 11 (35.48%) | |
| 5 | 8 (25.81%) | |
| Mean levodopa equivalent doses | ||
| Before surgery | 1280 ± 757 | |
| After surgery | 795 ± 574 | |
| Reduction | 38% |
| Stimulation | Left | Right |
|---|---|---|
| Voltage | 3.06 ± 0.50 | 3.14 ± 0.60 |
| Pulse width | 65.7 ± 11.7 | 67.0 ± 12.9 |
| Impedance | 1037.5 ± 176.4 | 1076.3 ± 176.4 |
| Rate | 185 | 185 |
| Active contact | 27 monopolar 4 bipolar | 27 monopolar 4 bipolar |
The levodopa equivalent dose is calculated as 100 mg of levodopa equivalent to 125 mg of controlled-release levodopa, 1 mg of pergolide, 1.5 mg of pramipexole, 5 mg of ropinirole or 10 mg of bromocriptine (Kleiner-Fisman et al. 2003).
Table 3 summarizes the coordinates and Z-score of these clusters and %rCBF response.
Table 3.
Talairach coordinates and Z-scores of peak intensities of clusters with significant rCBF response to bilateral STN DBS with corrected P < 0.05 and their %rCBF response
| Brain region | Coordinates |
||||
|---|---|---|---|---|---|
| X | Y | Z | Z-score | % rCBF change | |
| R thalamus | 16 | −16 | 2 | 5.10 | 2.88 |
| L thalamus | −3 | −10 | 1 | 4.09 | 2.73 |
| R midbrain | 1 | −27 | −13 | 4.37 | 1.95 |
| R premotor | 48 | 4 | 44 | 3.60 | −1.97 |
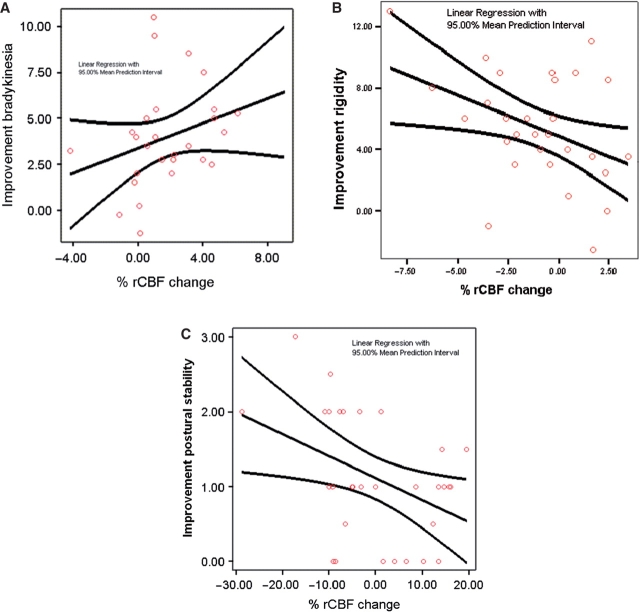
Greater improvement of bradykinesia correlated with greater increases in thalamic rCBF; rs = 0.31, P = 0.05, n = 28 (Fig. 3A). In contrast, greater improvement in rigidity correlated with greater decreases in SMA rCBF: rs = –0.381, P = 0.038, n = 30 (Fig. 3B). Finally, greater improvement in postural stability, as measured by the UPRDS 3 pull test, correlated with greater decreases in rCBF in the PPN region: rs = –0.38, P < 0.03, n = 31 (Fig. 3C). Table 4 summarizes the correlation analyses including the correlations not considered a priori but included to reveal other potential relationships. Age and disease severity also did not correlate significantly with change in rigidity, bradykinesia, postural stability or rCBF changes. The correlations between rCBF responses and changes in bradykinesia and postural stability remained significant even after covarying for age and disease severity (rs = 0.4, P < 0.03; two subjects excluded as outliers for disease duration). The correlation between rigidity changes and SMA blood flow changes persisted (rs = –0.3) but did not maintain significance (P = 0.1).
Fig 4.
(A) Improvement of bradykinesia correlates with thalamic rCBF increase; rs = 0.31, corrected P < 0.05, n = 28. (B) Improvement of rigidity correlates with SMA rCBF decrease in: rs = −0.381, corrected P < 0.038, n = 30. (C) Improvement in postural stability correlates with rCBF decrease in the PPN region: rs = −0.38, corrected P < 0.03, n = 31.
Fig. 3.
(A) Bilateral DBS STN leads to significant rCBF increase in bilateral thalami and right midbrain, corrected P < 0.05 at cluster level, n = 31. (B) Bilateral DBS STN leads to significant rCBF decrease in the right premotor region, corrected P < 0.05 at cluster level, n = 30.
Table 4.
Summary of all investigated correlations between rCBF responses in a priori IOVs and selected UPDRS subscores. The statistically significant correlations (P < 0.05) are in bold
| Score | Correlation with bilateral rCBF response |
|||
|---|---|---|---|---|
| Thalamus | SMC | SMA | PPN | |
| UPDRS subcategory | ||||
| Bradykinesia | rs = 0.31 (P < 0.05) | rs = −0.25 (P < 0.21) | rs = −0.35 (P < 0.07) | rs = 0.17 (P < 0.38) |
| Rigidity | rs = 0.21 (P < 0.25) | rs = −0.17 (P < 0.38) | rs = −0.38 (P < 0.04) | rs = −0.09 (P < 0.65) |
| Postural stability | rs = −0.06 (P < 0.76) | rs = −0.29 (P < 0.12) | rs = −0.28 (P < 0.13) | rs = −0.38 (P < 0.03) |
| Gait | rs = 0.39 (P < 0.03) | rs = −0.01 (P < 0.47) | rs = −0.17 (P < 0.37) | rs = −0.05 (P < 0.80) |
| Axial scores | rs = 0.15 (P < 0.44) | rs = −0.24 (P < 0.22) | rs = −0.38 (P < 0.05) | rs = −0.09 (P < 0.69) |
In addition, we examined the rCBF responses to DBS in the a priori VOIs using paired t-tests. We found significant rCBF change in bilateral thalami (P < 0.001) in concordance with the whole brain analysis. Lack of significant rCBF change in other VOIs was consistent with the notion that significant correlation between rCBF response and a certain motor aspect does not depend on the magnitude of the rCBF response.
Discussion
This study demonstrates that bilateral STN DBS-induced rCBF responses in specific regions of the brain correlate with improvement of selected motor signs in Parkinson's disease. These findings suggest possible regional selectivity for improvement of different motor characteristics of Parkinson's disease such as PPN for postural reflexes, thalamus for bradykinesia, and SMA region for rigidity.
According to traditional basal ganglia circuit models, parkinsonian symptoms are explained by excessive inhibition of thalamus and subsequently frontal cortex. This also would explain how an STN lesion can improve parkinsonian symptoms by decreasing the inhibitory effect on thalamus thereby increasing frontal cortical activity (Alexander et al., 1990). Our findings, however, are not consistent with this model. The net effect of STN DBS may be to increase the activity of output axons with increased excitation of pallidal neurons. This, in turn, would increase inhibitory output from pallidum to thalamus, consequently decreasing thalamic excitatory output to cortex, leading to decreased cortical activity (Hershey et al., 2003). Numerous neuroimaging studies have concordant findings (Payoux et al., 2004; Asanuma et al., 2006; Grafton et al., 2006) and electrophysiologic recordings support this effect of STN DBS (Hashimoto et al., 2003). Furthermore, measurement of pallidal local field potentials have been supportive of STN DBS-induced synchronization of high frequency oscillation in GPi (Brown et al., 2004). Together these findings and previous work suggest that patterns of firing rather than absolute changes in firing rates may be crucial for generation of pathologic symptoms (Marsden and Obeso 1994; Vitek et al., 1999).
The current results (n = 31) confirm our previous findings (n = 9) (Hershey et al., 2003) that STN DBS significantly increases rCBF in thalamus and midbrain and decreases rCBF in frontal cortical areas. This new study extends the previous investigation in several ways. First and most importantly, there is sufficient power to test for and identify significant correlations between rCBF responses and motor manifestations. We also were able to detect significant thalamic responses in the whole brain analysis. Moreover, the blood flow response in the frontal cortical region is more clearly localized, with the statistical peak in premotor cortex. Although there was a difference in lateralization of blood flow responses in the two studies, it is possible that there are bilateral effects not reaching statistical significance. Hershey et al. speculated that the left-sided lateralization was possibly related to the higher impedance in the right stimulator. However, this argument would not explain the right-sided lateralization in the current study, since the right impedance was slightly higher, and right-sided clinical symptoms in DBS OFF condition were slightly worse (Table 2). Overall, one could argue that the present study has a much larger number of subjects with the same stringent methods; hence its findings are more likely to be a reliable reflection of the true effect than the previous study by Hershey et al. Nevertheless, the laterality of the rCBF response requires more direct investigation which should be addressed in future studies.
There are numerous reports of rCBF or metabolic responses to STN DBS, most of them contrasting brain activity patterns during movement as compared to resting state (Thobois et al., 2002; Strafella et al., 2003; Grafton et al., 2006a). Interpretation of these studies may be confounded by differences in activity versus resting state, as performance-related feedback to brain could influence the brain responses identified (Hershey and Mink, 2006b; Perlmutter and Mink, 2006). We chose to focus on changes in resting state, eliminating any scans collected during movement or tremor that would reflect a behavioral change. This permitted us to isolate the effects of STN DBS on brain responses as measured by PET. Overall, most other papers report a larger number of regions with significant rCBF or metabolic changes. This discrepancy could be due in part to the fact that some studies report findings without correcting for multiple comparisons, risking potential false positive results.
Increases in thalamic blood flow and decreases in SMA blood flow are related to improvements in bradykinesia and rigidity respectively. This general pattern fits well with the proposed function of the cortical-basal ganglia-thalamic motor circuit. Changes in rCBF reflect altered local neuronal activity or changed input into the region of measurement (Hershey et al., 2003; Sestini et al., 2005; Herzog et al., 2006). Therefore, increased blood flow in thalamus could indicate increased inhibitory firing of neurons that project from GPi to thalamus, assuming that the net effect of STN DBS is to increase firing of output axons (Perlmutter and Mink, 2006). This increased thalamic inhibition would reduce thalamo-cortical output, including to the SMA. With decreased output from thalamus, we would anticipate reduced input to cortical targets such as SMA. Therefore, blood flow increase in thalamus and decrease in SMA in response to DBS STN likely reflect changes in signaling through these motor circuit pathways that interfere with abnormal signal transmission.
However, the observed relationships were more specific: improvement in rigidity correlated with blood flow responses in SMA but not thalamus, whereas improvement in bradykinesia correlated with blood flow response in thalamus but not SMA. Given an unchanged EMG background and absence of visible movement, the SMA rCBF changes are more likely related to changes in control of muscle tone. Such a regional selectivity for rigidity and bradykinesia could be driven by the different pathophysiologies underlying these motor abnormalities.
Rigidity may be related to the long-latency reflex magnitude and duration (Berardelli et al., 1983) and has been associated selectively with SMA in some studies. For example, increases in fMRI measured BOLD signal in motor cortex in response to paced thumb pressing movements at short and long time intervals positively correlated with arm rigidity but not with bradykinesia (Yu et al., 2007). In addition, others have reported a significant decrease in the threshold for effective motor cortex stimulation contralateral to the rigid hand compared to the non-rigid hand in Parkinson's disease patients (Cantello et al., 1991). Finally, improvement in the UPDRS rigidity score correlated with increased inhibition of motor evoked potentials (MEP) (Pierantozzi et al., 2001).
Underscaling muscle force may cause bradykinesia. This may be due to impaired reinforcement of appropriate cortical activity patterns and possibly poor sensorimotor integration (Berardelli et al., 2001). More recently, bradykinesia has been viewed in a new light assuming that dopaminergic input from substantia nigra to striatum carries a signal for motor motivation similar to dopaminergic projections from ventral tegmentum to frontal cortex involved in implicit decision making (Mazzoni et al., 2007). Others have found overactivity in SMA region possibly associated with akinesia (Sabatini et al., 2000). Strafella et al. (2003) report significant correlation between increased rCBF in SMA and anterior cingulate cortex and speed of hand movement. This finding is suggestive of a correlation between changes in bradykinesia score and rCBF responses. However, it should be noted that they used movement-related rCBF responses for the correlation which might be markedly different from DBS effects on resting blood flow as used in our correlation analyses. Regardless, one must not conclude that our data exclude correlation between SMA rCBF responses and bradykinesia, since our study has relatively low power, and the UPDRS ratings of these manifestations are not interval scales thereby potentially reducing the sensitivity of this analyses (Bohnen et al., 2006).
Our results also provide evidence for a relationship between activity in the PPN and postural stability. PPN receives inhibitory GABAergic afferents from GPi/SNr neurons with efferents (probably cholinergic) projecting to thalamus, basal ganglia, cerebral cortex and spinal cord (Shink et al., 1997). Pavese et al. (2006) demonstrated correlation between rigidity, bradykinesia and striatal dopamine level. However, they could not show such a correlation between axial symptoms and striatal dopamine levels, suggesting involvement of a more indirect mechanism. Such a mechanism could involve the PPN region. Indeed, MPTP treated monkeys were found to have increase in 2-deoxyglucose uptake in PPN suggestive of increased activity in GPi neurons projecting to PPN (Gnanalingham et al., 1995).
Pahapill and Lozano (2000) postulate that any intervention that reduces overactive inhibitory outflow from BG to PPN could potentially restore regular function with improved gait and postural stability. Based upon this rationale, they found that DBS of PPN and STN produced greater postural improvement than stimulation of only STN (Stefani et al., 2007). Furthermore, Pierantozzi et al. (2008) showed that PPN DBS can normalize the increased soleus Hoffman reflex in Parkinson's disease patients, suggesting that PPN may play a role in control of spinal excitability.
The negative correlation between rCBF in PPN region and improved postural stability supports Pahapill and Lozano's rationale, since decreased blood flow may reflect decreased input to PPN. However, this observation cannot be explained by a simple BG circuit with both thalamus and PPN as direct downstream targets of GPi with one (thalamus) receiving increased input while the other (PPN) is receiving decreased input from GPi. A possible explanation is that the critical effect of DBS may be to alter neuronal firing patterns (Hashimoto et al., 2003) with the speculation that some firing pattern changes may be associated with increased and others with decreased blood flow. Thus, opposite blood flow responses in two different downstream targets of DBS may reflect different blood flow ‘costs’ of the firing pattern change in these two different pathways.
The extension of the correlation analysis beyond the original hypothesis demonstrated significant correlation between the rCBF response in thalamus and gait as well as rCBF response in SMA and the sum of the axial scores. Thalamus receives afferents from PPN, and it is not surprising to detect a correlation between increased thalamic input and gait improvement. However, one would expect to find a similar correlation between gait and PPN rCBF response as well. Such a discrepancy can be addressed more adequately with detailed gait analysis and a more robust, validated interval scale of stability and gait. The sum of axial scores is a conglomerate, and the correlation with SMA rCBF responses could be explained by its wide role in planning movements (Hoshi et al., 2004; Iseki et al., 2008).
It is important to be cautious when interpreting the correlations between selected brain region responses and changes in specific motor manifestations. First, unlike the total UPDRS 3 motor score, the reliability of individual motor subscores is poor. Even PET measures of nigrostriatal neurons have variable degrees of correlation with UPDRS ratings (Ishikawa et al., 1996; Fuente-Fernandez et al., 2003; Bohnen et al., 2006). Second, the relatively low correlation coefficients in our study reflect the limitations of PET imaging of blood flow responses that have relatively low magnitude, and both PET measures and individual motor ratings have high variability thereby limiting our power to detect correlations.
These modest correlations between selective motor manifestations and rCBF in specific regions suggest possible regional selectivity for improvement of different motor signs of Parkinson's disease. Such insights support more target-oriented interventions such as PPN stimulation for those with predominant postural instability.
Acknowledgements
This study was supported by NIH/NINDS grants NS050425, NS41509, P30NS057105 (the Neuroscience Blueprint Core Grant to Washington University), CO6 RR020092 and UL1 RR024992 (CTSA at Washington University); the Greater St. Louis Chapter of American Parkinson's Disease Association (APDA); the APDA Advanced Center for Parkinson's disease Research at Washington University; and the Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and the Jack Buck Fund for Parkinson's Research). Dr Karimi and Dr Revilla have received partial fellowship funding from Medtronic, Inc., the manufacturer of the implanted stimulators; no other authors have any conflicts of interest to disclose.
Glossary
Abbreviations:
- DBS
deep brain stimulation
- Gpi
globus pallidus pars interna
- H&Y
Hoehn and Yahr
- PPN
pedunculopontine nucleus
- rCBF
regional cerebral blood flow
- SMA
supplementary motor area
- SMC
sensorimotor cortex
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson Disease Rating Scale
- VOI
volume of interest
References
- Alexander GE, Crutcher M, DeLong M. Basal ganglia-thalamo-cortical circuits: Parallel substrates for motor, oculomotor, “prefrontal,” and “limbic” functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, et al. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667–78. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–46. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Sabra AF, Hallett M. Physiological mechanisms of rigidity in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1983;46:45–53. doi: 10.1136/jnnp.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson's disease. Exp Neurol. 2004;188:480–90. doi: 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Bettucci D, Civardi C, De Angelis MS, Mutani R. Parkinson's disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology. 1991;41:1449–56. doi: 10.1212/wnl.41.9.1449. [DOI] [PubMed] [Google Scholar]
- Fuente-Fernandez R, Lim AS, Sossi V, Adam MJ, Ruth TJ, Calne DB, et al. Age and severity of nigrostriatal damage at onset of Parkinson's disease. Synapse. 2003;47:152–8. doi: 10.1002/syn.10160. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Milkowski NA, Smith LA, Hunter AJ, Jenner P, Marsden CD. Short and long-term changes in cerebral [14C]-2-deoxyglucose uptake in the MPTP-treated marmoset: relationship to locomotor activity. J Neural Transm Gen Sect. 1995;101:65–82. doi: 10.1007/BF01271546. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Turner RS, Desmurget M, Bakay R, DeLong M, Vitek J, et al. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–99. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Mink JW. Using functional neuroimaging to study the brain's response to deep brain stimulation. Neurology. 2006b;66:1142–3. doi: 10.1212/01.wnl.0000216425.34178.dd. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, McGee-Minnich L, Antenor JV, Videen TO, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–21. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, et al. Subthalamic stimulation modulates cortical control of urinary bladder in Parkinson's disease. Brain. 2006;129:3366–75. doi: 10.1093/brain/awl302. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: from information retrieval to motor planning and execution. J Neurophysiol. 2004;92:3482–99. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 2008;41:1021–31. doi: 10.1016/j.neuroimage.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Dhawan V, Kazumata K, Chaly T, Mandel F, Neumeyer J, et al. Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760–65. [PubMed] [Google Scholar]
- Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–95. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994;117(Pt 4):877–97. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–16. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Thakor NV. Uncovering the mechanisms of deep brain stimulation for Parkinson's disease through functional imaging, neural recording, and neural modeling. Crit Rev Biomed Eng. 2002;30:249–81. doi: 10.1615/critrevbiomedeng.v30.i456.20. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123(Pt 9):1767–83. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67:1612–7. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X. Atlas of the human brainstem. Elsevier; 1995. [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61:1307–13. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–57. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Galati S, Stanzione P, Peppe A, Tropepi D, et al. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson's disease patients. J Neural Transm. 2008;115:731–5. doi: 10.1007/s00702-007-0001-8. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Palmieri MG, Marciani MG, Bernardi G, Giacomini P, Stanzione P. Effect of apomorphine on cortical inhibition in Parkinson's disease patients: a transcranial magnetic stimulation study. Exp Brain Res. 2001;141:52–62. doi: 10.1007/s002210100839. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson's disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med. 2005;46:1444–54. [PubMed] [Google Scholar]
- Shink E, Sidibe M, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol. 1997;382:348–63. [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Dagher A, Sadikot AF. Cerebral blood flow changes induced by subthalamic stimulation in Parkinson's disease. Neurology. 2003;60:1039–42. doi: 10.1212/01.wnl.0000052691.48076.92. [DOI] [PubMed] [Google Scholar]
- Tabbal SD, Revilla FJ, Mink JW, Schneider-Gibson P, Wernle A, De Erausquin GA, et al. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson's disease. Neurosurgery. 2007;61:119–27. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- Talairach J. Co-planar stereotaxic atlas of the human brain. New York: Theime Verlag; 1988. [Google Scholar]
- Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Thobois S, Dominey P, Fraix V, Mertens P, Guenot M, Zimmer L, et al. Effects of subthalamic nucleus stimulation on actual and imagined movement in Parkinson's disease: a PET study. J Neurol. 2002;249:1689–98. doi: 10.1007/s00415-002-0906-y. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, Triche S, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wienhard K, Dalhbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner. J Comput Assist Tomogr. 1994;18:110–18. [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziota JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]