Abstract
In the human brain, the morphology of cortical gyri and sulci is complex and variable among individuals, and it may reflect pathological functioning with specific abnormalities observed in certain developmental and neuropsychiatric disorders. Since cortical folding occurs early during brain development, these structural abnormalities might be present long before the appearance of functional symptoms. So far, the precise mechanisms responsible for such alteration in the convolution pattern during intra-uterine or post-natal development are still poorly understood. Here we compared anatomical and functional brain development in vivo among 45 premature newborns who experienced different intra-uterine environments: 22 normal singletons, 12 twins and 11 newborns with intrauterine growth restriction (IUGR). Using magnetic resonance imaging (MRI) and dedicated post-processing tools, we investigated early disturbances in cortical formation at birth, over the developmental period critical for the emergence of convolutions (26–36 weeks of gestational age), and defined early ‘endophenotypes’ of sulcal development. We demonstrated that twins have a delayed but harmonious maturation, with reduced surface and sulcation index compared to singletons, whereas the gyrification of IUGR newborns is discordant to the normal developmental trajectory, with a more pronounced reduction of surface in relation to the sulcation index compared to normal newborns. Furthermore, we showed that these structural measurements of the brain at birth are predictors of infants’ outcome at term equivalent age, for MRI-based cerebral volumes and neurobehavioural development evaluated with the assessment of preterm infant's behaviour (APIB).
Keywords: cortex, development, gyrification, newborn, premature, twin, IUGR, MRI, APIB
Introduction
In the human brain, the cerebral cortex is divided into gyri and sulci whose morphology is variable among individuals (Ono et al., 1990; Welker, 1990). This anatomical organization is partly related to the cortical functional organization, and it may reflect pathological functioning as specific abnormalities in the gyral and sulcal pattern are observed in certain developmental and neuropsychiatric disorders such as autism, Williams syndrome and schizophrenia (Galaburda and Bellugi, 2000; Van Essen et al., 2006; Cachia et al., 2007; Nordahl et al., 2007). While these observations have been performed on adult brains, such structural abnormalities might be present long before the appearance of functional symptoms since cortical folding occurs early during brain development (Chi et al., 1977; Hansen et al., 1993; Garel et al., 2001). However, the precise mechanisms which may be responsible for alterations in the convolution pattern during intra-uterine or post-natal development are still poorly understood.
So far, several hypotheses have been put forward on the processes that underlie the normal gyrification in the foetal brain, such as genetic control (Rakic, 2004; Piao et al., 2004) supplying a protomap of sulcal roots (Régis et al., 2005), active growth of convolutions during gyrogenesis (Welker, 1990), differential growth of inner and outer cortical layers (Richman et al., 1975), cytoarchitectonic differentiation (Connolly, 1950), cortical growth (Toro and Burnod; 2005) and tension from white matter axonal fibres (Goldman-Rakic and Rakic, 1984; Van Essen, 1997; Hilgetag and Barbas, 2005). Furthermore, as most observations relied on post-mortem foetal brains (Feess-Higgins and Laroche, 1987), the potential influence of epigenetic or prenatal and postnatal environmental factors is unknown. To deal with such issue, we recently implemented an approach to reliably measure the normal cortical folding process in vivo in human premature newborns (Dubois et al., 2008), based on non-invasive magnetic resonance images (MRI) (Hüppi and Inder, 2001) and dedicated post-processing tools (Hüppi et al., 1998; Cachia et al., 2003).
Using this approach, we investigated in the current study if early alteration of cortex formation can be highlighted at birth according to intra-uterine environment, and if early brain morphology can be related to infants’ outcome at term equivalent age. To do so, development was compared among premature newborns who experienced different prenatal conditions: normal singletons, twins and newborns with intra-uterine growth restriction (IUGR). In comparison with singletons, early autopsy studies have described a global delay in body and brain maturation of twins (Chi et al., 1977). Besides, IUGR often results from a chronic fetal undernutrition due to placental insufficiency several weeks before birth. It is known to increase cortisol exposure and to alter the fetal neuroendocrine environment (Seckl, 1997). At birth and at term equivalent age, the cortical volume of IUGR newborns is decreased (Tolsa et al., 2004) and, as a functional consequence, IUGR is a known independent risk factor for the development of attention deficit hyperactivity disorder (ADHD) and schizophrenia (Geva et al., 2006).
In this study, early ‘endophenotypes’ (Leonard et al., 2006) of sulcal development were defined in the immature brain at birth, over the developmental period critical for the emergence of cortical convolutions (26–36 weeks of gestational age). Such measurements were further correlated to infants’ outcome at term equivalent age, for MRI-based cerebral volumes and neurobehavioural development evaluated with the assessment of preterm infant's behaviour (APIB).
Materials and Methods
Subjects
Forty-five preterm newborns were included in this study (gestational age—GA—at birth GAb: 25.6–35.6 weeks, mean 30.5 ± 2.7 weeks) (Table 1). Two MRI examinations were performed, the first one shortly after birth (mean GA1: 32.2 ± 2.6 weeks) and the second one around term equivalent age (mean GA2: 40.7 ± 1.1 weeks). The newborns were classified into three groups including 22 normal singletons, 12 normal twins and 11 IUGR newborns. No brain lesion was detected for any newborn on the MRI examinations performed at birth and at term equivalent age. All twin newborns were from a bi-amniotic pregnancy (nine bi-chorial, three mono-chorial), and three newborns among 12 were mono-zygotic twins. IUGR was defined as birth weight below the 10th percentile for gestational age and gender, and on criterion of placental insufficiency according to intra-uterine growth assessment, prenatal ultrasound and Doppler measurements within the umbilical artery (Tolsa et al., 2004). All these newborns were from a single pregnancy.
Table 1.
Preterm groups
| Group | All | Normal singleton | Normal twin | Singleton IUGR |
|---|---|---|---|---|
| Newborn count MRI | 45 | 22 | 12 | 11 |
| Gender repartition | 26 F, 19 M | 10 F, 12 M | 8 F, 4 M | 8 F, 3 M |
| GAb at birth: mean ± SD [min–max] (weeks) | 30.5 ± 2.7 [25.6–35.6] | 30.2 ± 2.6 [25.6–35.6] | 29.8 ± 2.5 [27.7–34.0] | 31.8 ± 2.9 [25.7–35.0] |
| GA1 at MRI around birth: mean ± SD [min–max] (weeks) | 32.2 ± 2.6 [26.7–36.1] | 31.5 ± 2.5 [26.7–35.7] | 31.9 ± 2.6 [29.1–36.0] | 34.1 ± 2.2 [28.9–36.1] |
| GA2 at MRI at term: mean ± SD [min–max] (weeks) | 40.7 ± 1.1 [38.7–44.4] | 40.6 ± 1.1 [38.9–43.9] | 40.9 ± 1.3 [39.0–44.4] | 40.4 ± 0.8 [38.7–41.3] |
| Newborn count APIB | 35 | 16 | 9 | 10 |
| 23 F, 12 M | 9 F, 7 M | 6 F, 3 M | 8 F, 2 M |
Gender repartition (F: female, M: male), gestational age (GA in weeks) at birth and at MRI examinations (around birth and at term equivalent age), and newborn counts at MRI and at APIB assessment (at term).
Data acquisition
Newborns underwent MRI examinations on a 1.5T system, during post-prandial sleep without sedation. They were fixed within a vacuum pillow, and special ‘mini-muffs’ were applied on their ears to minimize noise exposure. Coronal brain images were acquired using anatomical sequences (T2-weighted fast spin echo sequence and 3D T1-weighted fast gradient recovery sequence), with high spatial resolution (0.7 × 0.7 × 1.5 mm3 or 0.8 × 0.8 × 1.2 mm3). Protocol was approved by the local ethical committee and all parents gave written informed consent.
Data post-processing
Two complementary approaches were used to evaluate anatomical maturation by quantifying cerebral tissue volumes and cortical surface.
Volumetric quantification
First, for both the first and second MRI examinations, quantitative measurements of cerebral tissue volumes (cortical grey matter, basal ganglia/thalami, unmyelinated and myelinated white matter and cerebrospinal fluid) were performed using an optimal non-parametric density estimator with k-nearest neighbour (KNN) classification, based both on the MRI signal intensity of the registered T2- and T1-weighted images, and on anatomic location to differentiate cortex and central grey matter according to basal ganglia atlases for the preterm and at-term groups (Hüppi et al., 1998; Warfield et al., 2000; Inder et al., 2005). Initial estimates for the tissue classifiers were manually defined. For correlations with cortical surface, we focused on the volumes of cortex (C) and unmyelinated white matter (W), as myelinated white matter is only present in the midbrain and internal capsule at these gestational ages.
Quantification of brain surface
Second, we applied an original approach recently implemented to assess the cortex gyrification on the inner cortical surface from T2-weighted images of the first MRI examination (Dubois et al., 2008). Using a semi-automatic sequence of image post-processing tools based on thresholding and mathematical morphology (Cachia, et al., 2003; Mangin et al., 2004), the interface between cortex and white matter was segmented and reconstructed in 3D. This inner cortical surface highlighted the sulcation pattern better than the outer surface, which was not segmented because the thinness of the cortical ribbon in the neonatal brain and partial volume effects make the sulci sides to touch. For quantification, the surface area (S) was computed, as well as the sulcation index (SI) which highlights the proportion of ventral, lateral and vertex cortical sulci areas according to brain size, computed after inter-hemispheric splitting (see details in Dubois et al., 2008). Partial volume effects resulting from important gyrification in the medial surface precluded its correct segmentation. A rough measurement of apparent cortical thickness was estimated according to the ratio between cortical volume and surface (C/S). For the second MRI examination, the increased complexity of the surface, the heterogeneous myelination of white matter and partial volume effects with the current image acquisition protocol precluded the application of such approach to segment the inner cortical surface.
Neurobehavioural assessment at term equivalent age
For 35 newborns (Table 1), functional development was measured at term equivalent age, the same day as the second MRI examination, by a specialized neurobehavioural assessment specifically designed for the evaluation of brain functioning in preterm infants in the newborn period (APIB, Als et al., 1982, 2005). Different ‘system scores’ were measured (from 1 to 9, characterizing best to compromised performances, respectively): the autonomic or physiologic system (respiration, digestion, colour), the motor organizational system (tone, movement, postures), the state organizational system (range, robustness, transition patterns), the attention-interaction system (attention capacity, interaction robustness, transitions) and the self-regulation system (effort, success).
Statistical analysis of the preterm brain development
Cerebral maturation at birth: comparison of normal singletons, twins and IUGR newborns
We assessed differences at birth among the three groups by implementing general linear models for the volumes of cortex (C1) and white matter (W1), the inner cortical surface (S1), the sulcation index (SI1) and the apparent cortical thickness (C1/S1), with gestational age at first MRI examination (GA1) as co-variable, group and gender as co-factors (the small numbers of males and females per group precluded separating analyses for each gender). We further compared these four structural parameters among groups, independently of the effect of age: the cortical surface was correlated to the volumes on a logarithm scale (Kapellou et al., 2006), and the sulcation index to the surface, through linear models with group and gender as co-factors (Dubois et al., 2008).
Cerebral maturation at term equivalent age
In the same way, possible group differences were evaluated at term equivalent age. General linear models for cerebral volumes (C2, W2) and APIB scores were implemented with gestational ages at birth (GAb) and at second MRI examination (GA2) as co-variables, group and gender as co-factors.
Relationships between structural measurements at birth and development at term
Finally, we evaluated how brain structural measurements at birth can predict outcome at term equivalent age, in terms of MRI volumetric measurements and functional assessment. To do so, volumes of cortex (C2) and white matter (W2) at term were modelled successively by either one of the birth parameters (C1 or W1, S1, SI1, C1/S1), using general linear models with the time interval between both MRI examinations (GA2–GA1) as second co-variable, and group and gender as co-factors. A logarithm scale was considered for correlations between volumes, surface and apparent thickness. APIB scores at term were also modelled with birth cortical measurements (C1, S1, SI1, C1/S1) and time interval (GA2–GA1) as co-variables, group and gender as co-factors.
In supplemental information are presented further analyses on early pre- and post-natal environment (antenatal exposure to steroids, number of days with supplemental oxygen use, mechanical ventilation, continuous positive airway pressure and days in the neonatal intensive care unit), and on perinatal adaptation (Apgar scorings).
Results
Cerebral maturation at birth: comparison of normal singleton, twin and IUGR newborns
Based on high-resolution MR images of the newborn brain at birth (Fig. 1A and B), cerebral tissues were classified (Fig. 1C), and, using our original approach (Dubois et al., 2008), the inner cortical surface was segmented and reconstructed in 3D (Fig. 1D and E), and the cortical sulci were identified (Fig. 1F). For each newborn, the segmentations were visually checked and no gross error was detected. Volume and surface measurements were then compared.
Fig. 1.
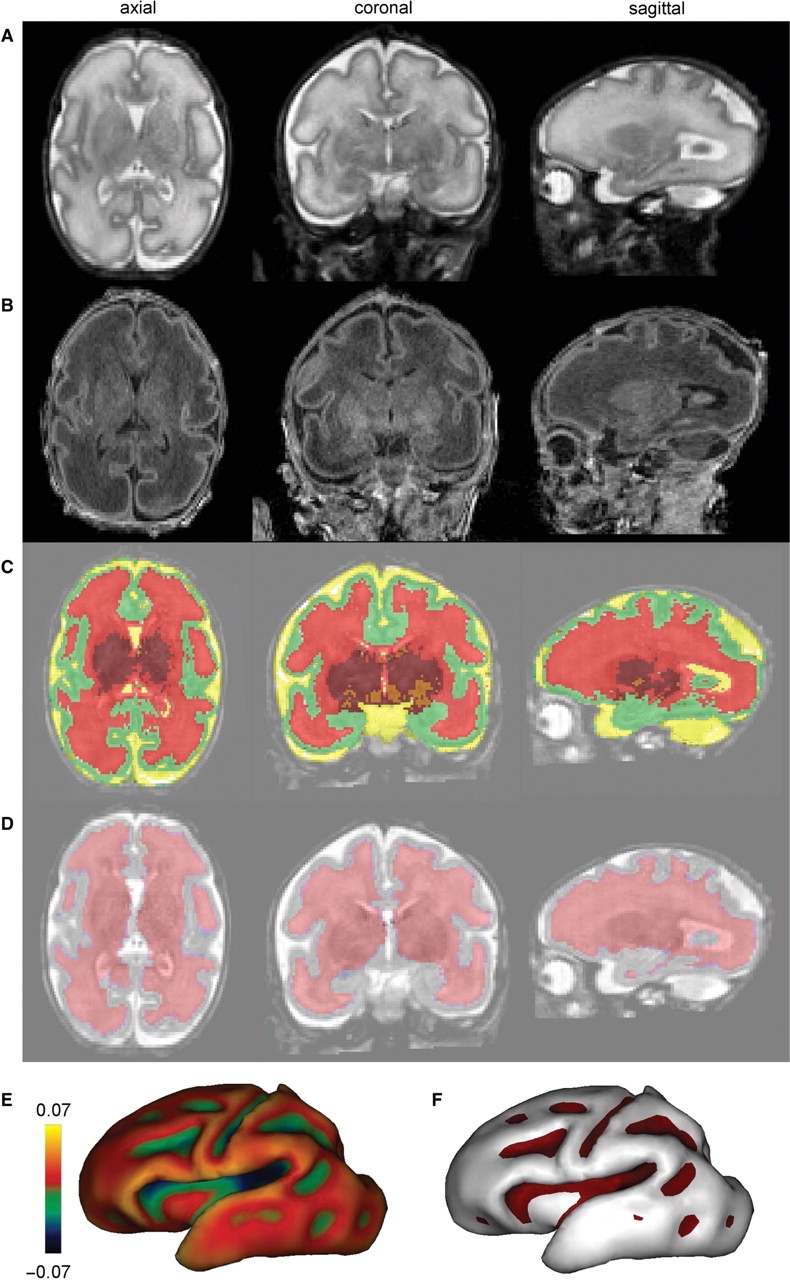
Volumetric and surfacic identification at birth: Using T2- and T1-weighted MR images (A and B), post-processing enabled the classification of cerebral tissues for volumetric measurements [(C) green: cortex, red: unmyelinated white matter, orange: myelinated white matter, maroon: basal ganglia/thalami, yellow: cerebrospinal fluid], and the segmentation of the interface between cortex and white matter for surfacic measurements (D). Based on this segmentation, the inner cortical surface was reconstructed in 3D [(E) the surface curvature is colour-coded] and the cortical sulci were identified according to negative curvatures [(F) sulci are outlined in purple], which enabled the computation of the sulcation index. Examples are presented for a newborn of 31.1-week-old GA.
Delayed maturation in twin and IUGR newborns
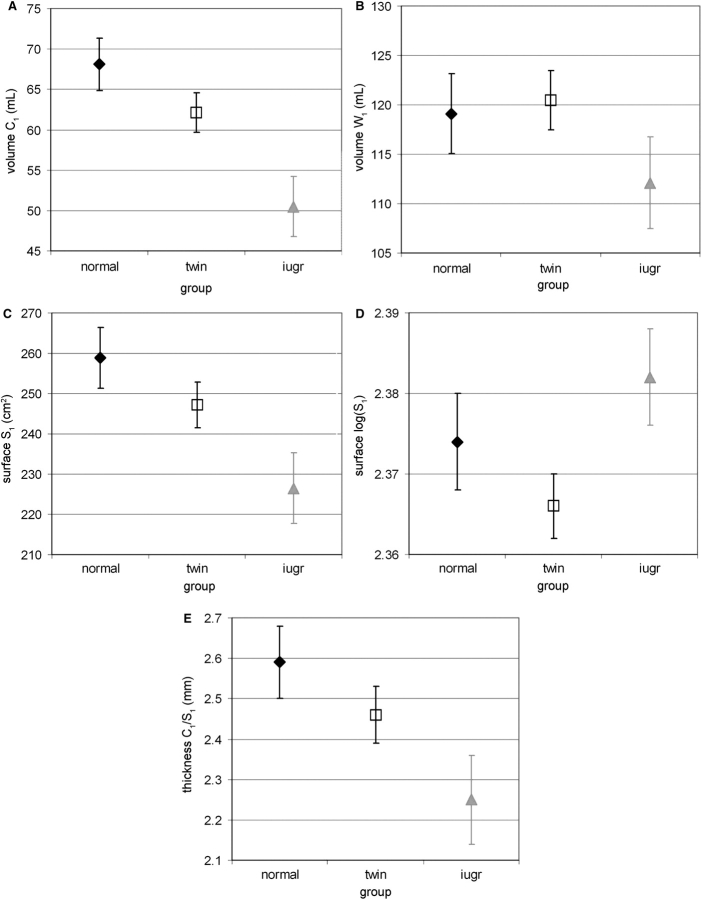
Besides the age-related effects, the general linear models with age at first MRI examination as co-variable, group and gender as co-factors, showed that cortical parameters at birth were significantly different in both twin and IUGR newborns in comparison with normal singletons (Table 2). For equivalent age, the cortical volume C1 was reduced in both groups (group effect: F = 7.1, P = 0.002, Fig. 2A), as well as the cortical surface S1 (group effect: F = 4.4, P = 0.019, Fig. 2C), the apparent cortical thickness C1/S1 (group effect: F = 3.3, P = 0.048, Fig. 2E) and the sulcation index SI1 (group effect: F = 5.2, P = 0.010, Fig. 3A). A trend towards lower white matter volume W1 was observed in IUGR newborns (Fig. 2B).
Table 2.
Group comparison at birth
| Parameters of interest | Model fit (R2) | Co-variables |
Co-factors |
||||
|---|---|---|---|---|---|---|---|
| GA1 | log(C1) | log(W1) | S1 | Group | Gender | ||
| C1 | 0.80 | 136.7/<0.001 | 7.1/0.002 | 1.1/NS | |||
| W1 | 0.80 | 122.7/<0.001 | 1.0/NS | 1.5/NS | |||
| S1 | 0.88 | 248.9/<0.001 | 4.4/0.019 | 0.5/NS | |||
| log(S1) | 0.98 | 130.0/<0.001 | 174.2/<0.001 | 1.9/NS | 4.3/0.045 | ||
| SI1 | 0.94 | 510.5/<0.001 | 5.2/0.010 | 1.1/NS | |||
| SI1 | 0.95 | 630.7/<0.001 | 10.9/<0.001 | 2.9/NS | |||
| C1/S1 | 0.34 | 11.4/0.002 | 3.3/0.048 | 1.9/NS | |||
Statistical results of the general linear models for the parameters of interest at birth (cortical volume C1, white matter volume W1, inner cortical surface S1, on a logarithm scale log(S1), sulcation index SI1, apparent cortical thickness C1/S1) according to co-variables [age GA1, log(C1), log(W1), S1] and co-factors (group, gender). Each line corresponds to a specific model. The fit quality is expressed for each analysis in term of percentage of variance explained by the linear model (R2). Bold value denotes the highest significant R2 for each parameter of interest, and italic value denotes non-significant R2 (P > 0.05). The influence of the specified co-variable and co-factor is highlighted by the F- and P-values (F/P), and significant effects are considered at the level of P < 0.05 (NS: not significant).
Fig. 2.
Volumetric and surfacic measurements among groups at birth: (A) Average of the cortical (C1) and (B) white matter (W1) volumes, of the (C) inner cortical surface (S1), and of the (E) apparent cortical thickness (C1/S1) according to group (with standard error in plot bars), estimated by the models with age GA1 as co-variable (Table 2, mean GA1 = 32.2 weeks), showing lower cortical volume (A), surface (C) and apparent thickness (E) in twins and IUGR newborns compared with singletons of equivalent age. (D) Average of the logarithmic inner cortical surface, log (S1), according to group (with standard error in plot bars), estimated by the models with logarithmic cortical, log (C1), and white matter log (W1) volumes as co-variables [Table 2, mean log(C1) = 1.76, mean log(W1) = 2.06], suggesting that higher surface is related to equivalent volumes in IUGR newborns.
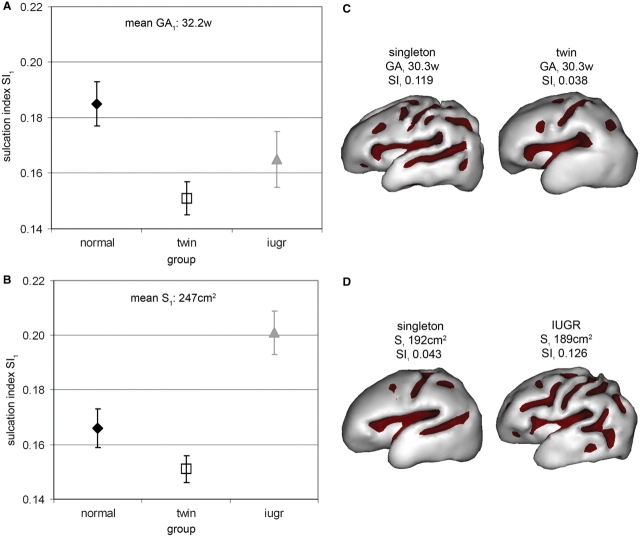
Fig. 3.
Sulcation index quantification among groups at birth. (A and B) Average of the sulcation index (SI1) according to group (with standard error in plot bars), estimated by a model with age GA1 [(A) mean GA1 = 32.2 weeks] or cortical surface S1 [(B) mean S1 = 247 cm2] as co-variable (Table 2), showing lower index in twins and IUGR newborns compared with singletons of equivalent age (A) but higher index in IUGR newborns compared with singletons and twins with similar surface (B). (C and D) Examples of inner cortical surface, with sulci outlined in colour, for a singleton and a twin of equivalent age [(C) GA1 30.3/30.3 weeks; S1 224/164 cm2; SI1 0.119/0.038], and for a singleton and an IUGR newborn with similar surface [(D) GA1 28.6/32.1 weeks; S1 192/189 cm2; SI1 0.043/0.126].
Impaired maturation in IUGR newborns
We then investigated whether these alterations rely on a delay or alteration in maturation processes by comparing the four structural parameters (C1, W1, S1, SI1), independently of the effect of age (Table 2). On a logarithm scale, the inner cortical surface log(S1) was highly correlated with cortical and white matter volumes log(C1) and log(W1), with a non-significant trend towards higher surface for equivalent volumes in IUGR newborns (Fig. 2D), related to decreased apparent cortical thickness. The sulcation index was linearly correlated to cortical surface, and IUGR newborns presented a higher index in proportion to surface (group effect: F = 10.9, P < 0.001, Fig. 3B). On the contrary, normal singletons and twins showed a similar pattern in cortical folding for equivalent surface.
These surfacic and volumetric results at birth suggested that normal twins had a delayed maturation of cortical volume and gyrification according to age (Fig. 3C), but this delay was harmonious in regard to the developmental profile. IUGR newborns also demonstrated a delay, but the cortical growth and folding was discordant to the normal developmental trajectory, as the alterations in sulcation index were not proportional to the low surfacic and volumetric growth (Fig. 3D).
Cerebral maturation at term equivalent age
At term, models implemented for cerebral volumes showed reduced influence of the ages at examination (GA2) and at birth (GAb), and of the group and gender (Table 3). Nevertheless, we observed a trend towards lower cortical and white matter volumes in IUGR newborns (Fig. 4A and B). Concerning neurobehavioural development at term, the age at examination influenced all APIB scores except for the state organizational system, and the age at birth influenced the scores for the motor organizational and self-regulation systems (Table 3). A statistically significant difference was detected between the three groups for the score of the attention–interaction system, with higher score in IUGR newborns (group effect: F = 3.5, P = 0.045, Fig. 4F), and a similar trend was observed for the other APIB scores (Fig. 4C–G).
Table 3.
Group comparison at term equivalent age
| Parameters of interest | Model fit (R2) | Co-variables |
Co-factors |
||
|---|---|---|---|---|---|
| GAb | GA2 | Group | Gender | ||
| C2 | 0.21 | 0.7/NS | 7.3/0.010 | 1.1/NS | 0.0/NS |
| W2 | 0.27 | 0.2/NS | 3.2/NS | 1.6/NS | 3.8/NS |
| Physiologic | 0.37 | 3.2/NS | 7.3/0.011 | 1.3/NS | 2.0/NS |
| Motor | 0.34 | 4.2/0.05 | 4.6/0.041 | 1.9/NS | 1.3/NS |
| State | 0.25 | 3.3/NS | 1.0/NS | 2.0/NS | 1.0/NS |
| Attention | 0.37 | 2.4/NS | 8.0/0.008 | 3.5/0.045 | 0.0/NS |
| Regulation | 0.36 | 4.9/0.035 | 6.1/0.020 | 1.7/NS | 1.1/NS |
Statistical results of the general linear models for the parameters of interest at term (cortical volume C2, white matter volume W2, APIB scores: autonomic or physiologic, motor organizational, state organizational, attention–interaction and self-regulation systems) according to co-variables (ages GAb and GA2) and co-factors (group, gender). See Table 2 legend for details.
Fig. 4.
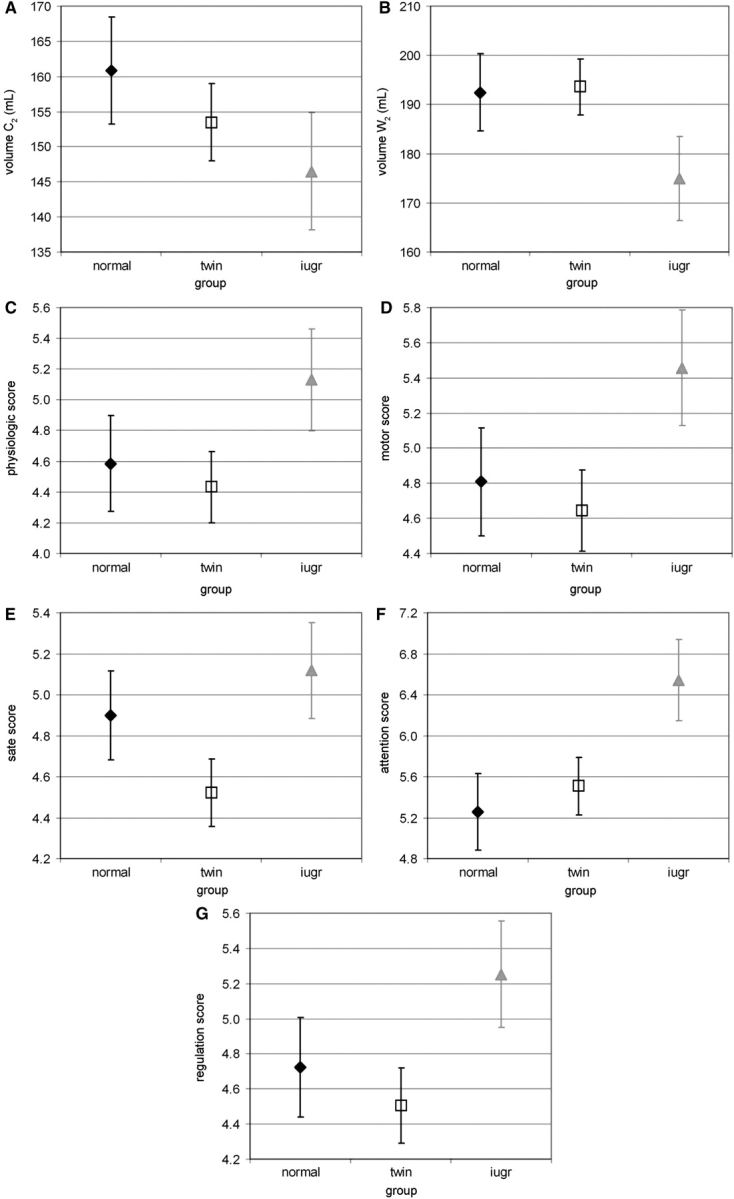
Volumetric and neurobehavioural measurements among groups at term equivalent age: (A and B) Average of the cortical (A: C2) and white matter (B: W2) volumes according to group (with standard error in plot bars), estimated by the models with ages GAb and GA2 as co-variables (Table 3, mean GAb = 30.5 weeks, mean GA2 = 40.7 weeks), showing a trend towards lower volumes in IUGR newborns compared with singletons of equivalent age. (C–G) Average of the APIB scores [(C) autonomic or physiologic, (D) motor organizational, (E) state organizational, (F) attention–interaction, (G) self-regulation systems] according to group (with standard error in plot bars), estimated by the models with ages GAb and GA2 as co-variables (Table 3, mean GAb = 30.8 weeks, mean GA2 = 40.8 weeks), showing a trend towards higher scores in IUGR newborns compared with singletons of equivalent age.
Structural measurements at birth predict development at term
Furthermore, anatomical and functional measurements at term equivalent age were strongly correlated to structural measurements at birth.
Volumetric measurements at term equivalent age
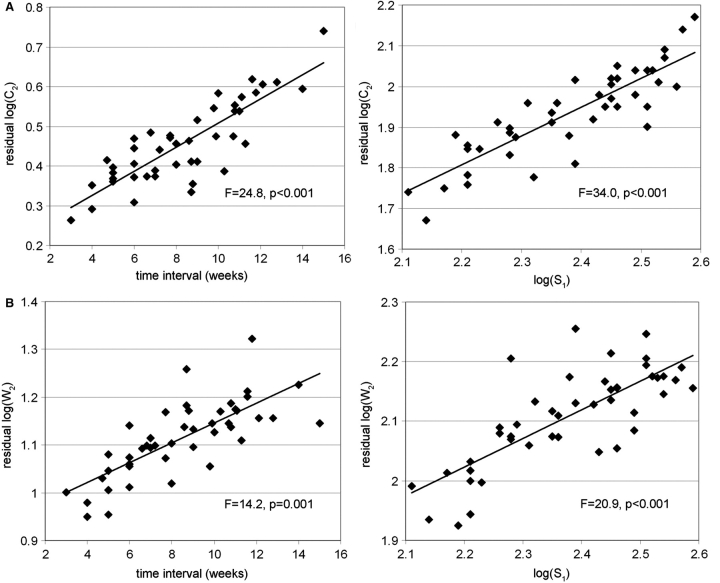
First, we showed that cortical and white matter volumes C2 and W2, measured at term equivalent age by the second MRI examination, were explained by the time interval between examinations (GA2–GA1) and the first cerebral measurements, except the apparent cortical thickness C1/S1 (Table 4). No group or gender effect was observed. For both tissues, the model correlating volumes and surface on a logarithm scale had the highest R2, suggesting that inner cortical surface S1 was the co-variable at birth that best correlated with the anatomical development at term in comparison with volumes C1, W1, sulcation index SI1 or apparent cortical thickness C1/S1. For equivalent time interval between MRI examinations, higher volumes at term relied on higher surfaces at birth on a logarithm scale (Fig. 5).
Table 4.
Relationships between structural measurements at birth and at term equivalent age
| Parameters of interest | Model fit (R2) | Co-variables |
Co-factors |
||||||
|---|---|---|---|---|---|---|---|---|---|
| GA2–GA1 | C1 | W1 | log(S1) | SI1 | log(C1/S1) | Group | Gender | ||
| C2 | 0.46 | 20.8/<0.001 | 29.3/<0.001 | 0.3/NS | 1.0/NS | ||||
| 0.40 | 18.7/<0.001 | 22.6/<0.001 | 0.8/NS | 0.0/NS | |||||
| log(C2) | 0.49 | 24.8/<0.001 | 34.0/<0.001 | 0.1/NS | 0.9/NS | ||||
| 0.09 | 1.0/NS | 2.1/NS | 0.1/NS | 0.1/NS | |||||
| W2 | 0.47 | 11.8/0.002 | 20.0/<0.001 | 1.9/NS | 1.5/NS | ||||
| 0.28 | 3.3/NS | 4.0/NS | 2.2/NS | 3.5/NS | |||||
| log(W2) | 0.48 | 14.2/0.001 | 20.9/<0.001 | 1.4/NS | 1.9/NS | ||||
| 0.23 | 0.2/NS | 1.4/NS | 2.7/NS | 4.0/NS | |||||
Statistical results of the general linear models for the cerebral volumes at term [cortical volume C2, white matter volume W2, on a logarithm scale log(C2), log(W2)] according to co-variables [time interval between examinations: GA2–GA1; cerebral measurements at birth: C1, W1, log(S1), SI1, log(C1/S1)] and co-factors (group, gender). See Table 2 legend for details.
Fig. 5.
Relationships between cortical surface at birth and cerebral volumes at term equivalent age: Models of cortical and white matter volumes at term [(A) log(C2), (B) log(W2)] are considered on a logarithm scale, with time interval between examinations (GA2–GA1) and inner cortical surface at birth, log(S1), as co-variables (Table 4). The plots represent the variations of ‘residual volumes’ as function of time interval, after correction for the surface effect (left column), and the variations of ‘residual volumes’ as function of surface, after correction for the time interval effect (right column). For equivalent time interval, higher volumes at term relied on higher surfaces at birth.
APIB assessment at term equivalent age
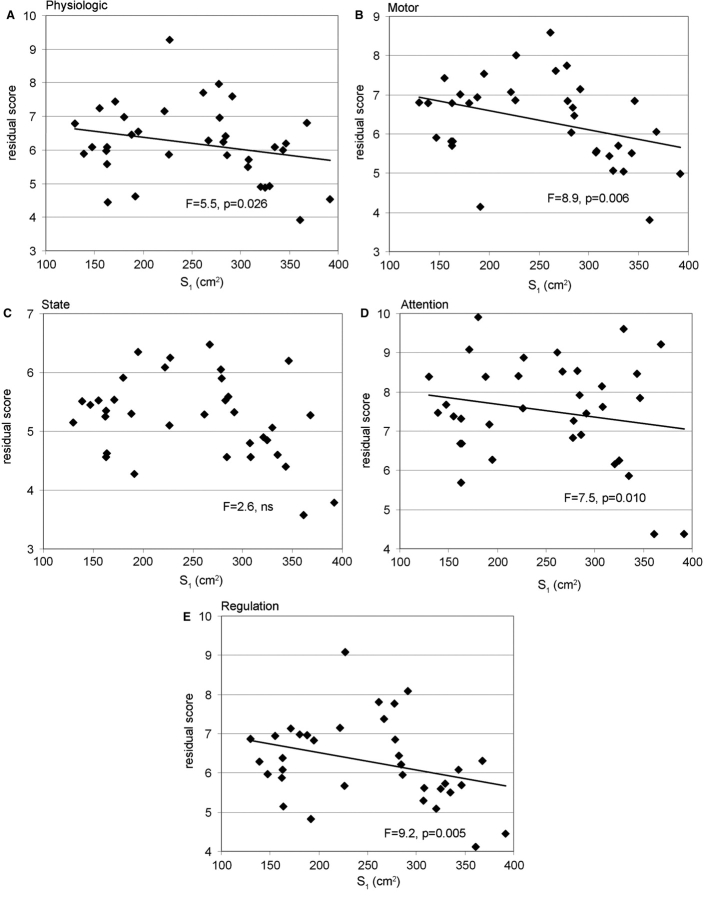
In the same way, linear models for the APIB scores demonstrated significant correlations between functional assessments at term and cortical measurements at birth, except for the state organizational system (Table 5). Time interval between examinations (GA2–GA1) had a reduced influence, and no independent group or gender effect was observed for any APIB scores. Given the R2, the inner cortical surface S1 was the birth parameter that described the most precisely the scores variability for the motor organizational, attention–interaction and self-regulation systems, whereas the sulcation index SI1 did for the autonomic–physiologic system. For equivalent time interval between examinations, higher surfaces at birth implied a better functional development at term, as underlined by lower APIB scores (Fig. 6).
Table 5.
Relationships between structural measurements at birth and neurobehavioural assessment at term equivalent age
| Parameters of interest | Model fit (R2) | Co-variables |
Co-factors |
|||||
|---|---|---|---|---|---|---|---|---|
| GA2–GA1 | C1 | S1 | SI1 | C1/S1 | Group | Gender | ||
| Physiologic | 0.31 | 2.9/NS | 5.7/0.023 | 0.4/NS | 1.8/NS | |||
| 0.30 | 3.1/NS | 5.5/0.026 | 0.8/NS | 1.4/NS | ||||
| 0.33 | 4.3/0.047 | 6.6/0.016 | 1.6/NS | 2.6/NS | ||||
| 0.20 | 0.1/NS | 1.1/NS | 0.4/NS | 3.0/NS | ||||
| Motor | 0.32 | 1.5/NS | 5.7/0.024 | 0.7/NS | 1.0/NS | |||
| 0.38 | 3.3/NS | 8.9/0.006 | 1.4/NS | 0.5/NS | ||||
| 0.33 | 2.6/NS | 6.2/0.019 | 2.3/NS | 1.6/NS | ||||
| 0.19 | 0.4/NS | 0.1/NS | 1.0/NS | 2.3/NS | ||||
| State | 0.28 | 0.5/NS | 3.6/NS | 1.5/NS | 0.6/NS | |||
| 0.26 | 0.4/NS | 2.6/NS | 1.9/NS | 0.5/NS | ||||
| 0.23 | 0.2/NS | 1.5/NS | 2.3/NS | 1.2/NS | ||||
| 0.22 | 0.2/NS | 1.0/NS | 1.4/NS | 1.3/NS | ||||
| Attention | 0.29 | 2.1/NS | 4.9/0.034 | 1.1/NS | 0.0/NS | |||
| 0.34 | 3.8/NS | 7.5/0.010 | 0.1/NS | 0.9/NS | ||||
| 0.30 | 3.2/NS | 5.5/0.026 | 3.0/NS | 0.1/NS | ||||
| 0.19 | 0.0/NS | 0.8/NS | 1.3/NS | 0.3/NS | ||||
| Regulation | 0.36 | 3.5/NS | 9.0/0.006 | 0.6/NS | 0.8/NS | |||
| 0.36 | 4.0/NS | 9.2/0.005 | 1.2/NS | 0.5/NS | ||||
| 0.36 | 4.9/0.036 | 9.1/0.005 | 2.3/NS | 1.5/NS | ||||
| 0.20 | 0.0/NS | 1.4/NS | 0.6/NS | 1.9/NS | ||||
Statistical results of the general linear models for the APIB scores at term (autonomic or physiologic, motor organizational, state organizational, attention–interaction and self-regulation systems) according to co-variables (time interval between examinations: GA2–GA1; cortical measurements at birth: C1, S1, SI1, C1/S1) and co-factors (group, gender). See Table 2 legend for details.
Fig. 6.
Relationships between cortical surface at birth and neurobehavioural assessment at term equivalent age: Models of APIB scores at term [(A) autonomic or physiologic, (B) motor organizational, (C) state organizational, (D) attention–interaction, (E) self-regulation systems] are considered with time interval between examinations (GA2–GA1) and inner cortical surface at birth (S1) as co-variables (Table 5). The plots represent the variations of ‘residual scores’ as function of surface, after correction for the time interval effect. The variations of ‘residual scores’ as function of time interval, after correction for the surface effect, are not presented because of their non-significance. For equivalent time interval, higher surfaces at birth implied lower APIB scores at term.
All these results suggested that structural parameters measured at birth in the premature brain, particularly the inner cortical surface, predicted inter-individual variability in anatomical and functional outcome, at term equivalent age, more reliably and precisely than group or gender belonging alone.
Discussion
This study reports for the first time the influence of brain cortical development in relation to functional development in human premature newborns studied in vivo at birth and at term equivalent age. The cortical maturation and gyrification were successfully mapped at birth using original complementary approaches of MR images post-processing. Early differences were highlighted among newborns who experienced different intra-uterine environment, with harmonious delay in twins and early impairment in IUGR newborns. Furthermore, we demonstrated that the early characterization of inner cortical surface at birth was a good marker of anatomical and functional outcome at term equivalent age, indicating that cortical morphology at birth may represent an early endophenotype of later development.
Cerebral development was compared among three groups of premature newborns. We considered that infants born prematurily, from a single pregnancy and without apparent lesions on MRI examinations, were normal controls in reference to premature twin and IUGR newborns. These newborns may not be considered as ‘normal’ compared with newborns born at term, since prematurity and subsequent neonatal intensive care have been shown to influence brain development at term equivalent age (Inder et al., 2005; Kapellou et al., 2006). However, the influence of extra-uterine development was here negligible for the first MRI examination which was performed in the first post-natal days.
Early dissimilarities were outlined among newborn groups at birth. In comparison with normal singletons, twins had lower cortical volume, surface and sulcation index at birth, which underlined a harmonious delay in cortical development during the last gestational weeks, coherent with previous post-mortem observations on brain and body maturation (Chi et al., 1977). The factors involved in such a delay, like sharing the intra-uterine space, the placenta or the amniotic cavity, are still unclear and should be investigated over a larger cohort of subjects from various pregnancies (mono/di-zygotic, mono/di-chorionic, mono/di-amniotic, spontaneous or fertility treatment induced). This study was not set out to evaluate differences between mono-zygotic and di-zygotic twins.
Besides, IUGR newborns also demontrated an important delay in cortical development at birth, in agreement with a previous study which indicated a reduction in cortical volume (Tolsa et al., 2004), but here we observed a disruption of the developmental profile. The gyrification was not as delayed according to age as it should be in relationship with the delayed volumetric and surfacic growth, and the sulcation index was too high for equivalent surface in IUGR newborns compared with normal newborns. This is thought-provoking on the mechanisms that underlie the folding process. This progression in sulci and gyri formation despite the reduced cortical expansion suggests the importance of external factors like the tension from white matter fibres (Goldman-Rakic and Rakic, 1984; Van Essen, 1997; Hilgetag and Barbas, 2006). As described in some cortical developmental dysplasias such as polymicrogyria, the increased cortical sulcation in proportion to surface in IUGR newborns may also be related to a thinner thickness of the cortex, which was the case in our study. Such structural abnormalities in the IUGR newborn brain at birth might represent an early marker for the later appearance of functional disturbance (ADHD, schizophrenia), and may be in part responsible for the lower IQ measured in children born with IUGR (Geva et al., 2006). In this perspective, ADHD disorder has been associated with thinner cortex in the cortical networks that modulate attention and executive functions in adults (Makris et al., 2007), and with a delay in brain maturation in children and adolescents (Shaw et al., 2007). In the future, further longitudinal follow-up studies will help to investigate this issue (Kraemer et al., 2000).
At term equivalent age, we only observed in IUGR newborns trends towards lower cerebral volumes and less mature APIB scores, with a statistically significant difference in the score for the attention–interaction system, as demonstrated in a previous study (Tolsa et al., 2004). In comparison with this study, the discrepancy in significance for the cortical volume decrease may be attributed to the small number of subjects in our study, or to the diverging analyses: instead of considering age as a continuous co-variable, matching newborns for equivalent gestational ages at birth and at second MRI examination may be required to underline subtle differences between groups. Anatomical and functional outcomes at term equivalent age were here better correlated with early structural parameters at birth. This suggests that the inter-individual variability within our global population of preterm newborns studied at term was higher than variability across groups, and that MRI-based measurements were more reliable and precise than classification by group or gender belonging for infant follow-up, which showed the importance of structural brain maturation for functional outcome. To that purpose, the early inner cortical surface appeared as the most exquisite parameter to describe cerebral volumes at term, highlighting the high reliability of the original approach we recently proposed (Dubois et al., 2008). For equivalent time interval between MRI examinations, higher volumes at term coherently relied on higher surfaces at birth. The apparent cortical thickness estimated at birth as the ratio between cortical volume and surface was not a reliable parameter to predict measurements at term, probably in part because it was a rough computation from parameters obtained by two different approaches. Using other segmentation approaches suggested for the developing brain (Xue et al., 2007) would be necessary to precisely measure the birth cortical thickness and investigate its impact on term cerebral volumes.
Furthermore, we showed that the potential of early MRI measurements goes beyond anatomical quantification since we observed that neurobehavioural scores obtained with APIB assessment at term equivalent age were correlated with cortical development at birth, except for the state organizational system, which would make sense as sleep–wake cycles are mainly controlled by distinct cellular systems in the hypothalamus, not measured with our approach. Cortical surface was again the most explanatory variable for three scores over five (motor organizational, attention–interaction and self-regulation systems). For equivalent time interval between first MRI and APIB examination, a better functional outcome at term (lower scores) relied on a higher surface at birth. Whereas precisely localizing the neurobehavioural functions is difficult in the developing newborn brain, we initially hypothesized that cortical parameters at birth would particularly influence the attention–interaction system score, which characterizes the newborn's degree of arousal and attention, and its ability to react to external stimuli (light, face, voice, sound) which rely on frontal brain maturation. Besides, the motor organizational system score probably depends on the development of the cortical central region, around the central sulcus, whereas the autonomic or physiological system score may rather rely on basal ganglia and midbrain.
The correlation results between cortical surface at birth and neurobehavioural scores at term highlighted close links between structure and function in the developing human brain, which is particularly interesting as imaging cerebral anatomy is generally easier than testing the psycho-motor development of non-cooperating newborns or infants. Our study indicates that early brain development alteration could constitute the first step in the cascade of functional impairment underlying neurobehavioural pathologies. Unfortunately, we were not able to compare structural measurements with later functional outcome during infancy and childhood because the reduced number of subjects precluded a classification regarding criteria of family and socio-economic environment, which is to play a major role on cognitive development at these ages but not yet at term age. Brain characteristics in preterm infants at birth may influence later structural and functional development, which is highly relevant for the understanding of the contribution of antenatal conditions and neonatal intensive care on the functional brain deficits observed after premature birth.
Accurate segmentation of the neonatal brain is a significant challenge, as neonatal MRI scans exhibit partial volume effects and limited contrast between different tissues that are more difficult to overcome than in adult brains, due to the difference in size of the structures of interest, and the age-dependent maturation of the different brain tissues. To address these issues, we used MR images of high quality and only subjects with T2w and T1w images with no or slight motion artefacts were included in the analyses. Both the volume (Warfield et al., 2000) and surface analysis approaches described here were designed specifically for the immature brain. To address tissue misclassification resulting from partial volume effects and variation in signal intensity in the immature white matter, all segmentations were visually checked and mislabelled voxels were manually corrected if necessary.
Conclusion
Finally, the early characterization of cerebral volumes, cortical surface and gyrification, with in vivo MRI-based complementary post-processings of brain images at birth, opens up the possibility to study the environmental effects on the cortical folding process in human premature newborns. We here presented data indicating that developmental adaptations in multi-fetal growth are not identical to growth restriction in singleton pregnancy. These distinct differences may well represent early surrogate markers of the later appearance of developmental disorders, as the structural measurements at birth correlated with anatomical and functional outcome at term equivalent age.
Supplementary Material
Acknowledgements
This work was supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne, by the Swiss National Foundation (grants nos 32-56927, 3200B0-102127; P.S.H.), by the EU grant NEOBRAIN (www.neobrain.eu, grant no 036534; PSH), by the French National Agency for Research (grant PSYMARKER/APV05137LSA; A.C.) and by the NIH (grants nos R01 RR021885, R01 GM074068 and P30 HD018655; S.K.W.).
Glossary
Abbreviations:
- IUGR
intrauterine growth restriction
- APIB
assessment of preterm infant's behaviour
- MRI
magnetic resonance imaging
- ADHD
attention deficit hyperactivity disorder
References
- Als H, Butler S, Kosta S, McAnulty G. The Assessment of Preterm Infants’ Behavior (APIB): furthering the understanding and measurement of neurodevelopmental competence in preterm and full-term infants. Ment Retard Dev Disabil Res Rev. 2005;11:94–102. doi: 10.1002/mrdd.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als H, Lester B, Tronick E, Brazelton T. Towards a research instrument for the assessment of preterm infant's behavior (APIB) and manual for the assessment of preterm infant's behavior (APIB) In: Fitzgerald HE, Lester BM, Yogman MW, editors. Theory and research in behavioral pediatrics. New York: Plenum Publishing; 1982. pp. 35–63. [Google Scholar]
- Cachia A, Mangin JF, Riviere D, Kherif F, Boddaert N, Andrade A, et al. A primal sketch of the cortex mean curvature: a morphogenesis based approach to study the variability of the folding patterns. IEEE Trans Med Imaging. 2003;22:754–65. doi: 10.1109/TMI.2003.814781. [DOI] [PubMed] [Google Scholar]
- Cachia A, Paillère-Martinot ML, Galinowski A, Januel D, de Beaurepaire R, Bellivier F, et al. Neuroimage. 2007. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. DOI 10.1016/j.neuroimage.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Connolly C. External morphology of the primate brain. Springfield, Illinois: Charles C Thomas; 1950. [Google Scholar]
- Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18:1444–54. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- Feess-Higgins A, Laroche JC. Development of the human foetal brain: an anatomical atlas. Paris, France: Inserm-CNRS, Masson; 1987. [Google Scholar]
- Galaburda AM, Bellugi UV. Multi-level analysis of cortical neuroanatomy in Williams syndrome. J Cogn Neurosci. 2000;12(Suppl 1):74–88. doi: 10.1162/089892900561995. [DOI] [PubMed] [Google Scholar]
- Garel C, Chantrel E, Brisse H, Elmaleh M, Luton D, Oury JF, et al. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR. 2001;22:184–9. [PMC free article] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118:91–100. doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Rakic P. Experimental modification of gyral patterns. In: Geschwind N, Galaburda A, editors. Cerebral dominance. Cambridge (Massachusetts): Harvard University Press; 1984. pp. 179–92. [Google Scholar]
- Hansen PE, Ballesteros MC, Soila K, Garcia L, Howard JM. MR imaging of the developing human brain. Part 1. Prenatal development. Radiographics. 1993;13:21–36. doi: 10.1148/radiographics.13.1.8426929. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol. 2005;210:411–7. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppi PS, Inder TE. Magnetic resonance techniques in the evaluation of the perinatal brain: recent advances and future directions. Semin Neonatol. 2001;6:195–210. doi: 10.1053/siny.2001.0039. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–35. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–94. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, et al. Anormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–71. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Kuldau JM. Exploiting human anatomical variability as a link between genome and cognome. Genes Brain Behav. 2006;5(Suppl 1):64–77. doi: 10.1111/j.1601-183X.2006.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–75. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, et al. A framework to study the cortical folding patterns. Neuroimage. 2004;23(Suppl 1):S129–38. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 2007;27:11725–35. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. New York: Thieme Medical Publishers; 1990. [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–6. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuroscience. Genetic control of cortical convolutions. Science. 2004;303:1983–4. doi: 10.1126/science.1096414. [DOI] [PubMed] [Google Scholar]
- Régis J, Mangin JF, Ochiai T, Frouin V, Riviére D, Cachia A, et al. ‘Sulcal root’ generic model: a hypothesis to overcome the variability of the human cortex folding patterns. Neurol Med Chir. 2005;45:1–17. doi: 10.2176/nmc.45.1. [DOI] [PubMed] [Google Scholar]
- Richman DP, Stewart RM, Hutchinson JW, Caviness VS Mechanical model of brain convolutional development. Science. 1975;189:18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, feto-placental 11 beta-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids. 1997;62:89–94. doi: 10.1016/s0039-128x(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Proc Natl Acad Sci USA. 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. DOI:10.1073/pnas0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004;56:132–8. doi: 10.1203/01.PDR.0000128983.54614.7E. [DOI] [PubMed] [Google Scholar]
- Toro R, Burnod Y. A morphogenetic model for the development of cortical convolutions. Cereb Cortex. 2005;15:1900–13. doi: 10.1093/cercor/bhi068. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–8. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26:5470–83. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield SK, Kaus M, Jolesz FA, Kikinis R. Adaptive, template moderated, spatially varying statistical classification. Med Image Anal. 2000;4:43–55. doi: 10.1016/s1361-8415(00)00003-7. [DOI] [PubMed] [Google Scholar]
- Welker W. Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In: Jones E, Peters A, editors. Comparative structure and evolution of cerebral cortex. Part II. 8B. New York: Plenum; 1990. pp. 3–136. [Google Scholar]
- Xue H, Srinivasan L, Jiang S, Rutherford M, Edwards AD, Rueckert D, et al. Automatic segmentation and reconstruction of the cortex from neonatal MRI. Neuroimage. 2007;38:461–77. doi: 10.1016/j.neuroimage.2007.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.