Abstract
The identity and functional potential of dopamine neurons derived in vitro from embryonic stem cells are critical for the development of a stem cell-based replacement therapy for Parkinson's disease. Using a parthenogenetic primate embryonic stem cell line, we have generated dopamine neurons that display persistent expression of midbrain regional and cell-specific transcription factors, which establish their proper identity and allow for their survival. We show here that transplantation of parthenogenetic dopamine neurons restores motor function in hemi-parkinsonian, 6-hydroxy-dopamine-lesioned rats. Exposure to Wnt5a and fibroblast growth factors (FGF) 20 and 2 at the final stage of in vitro differentiation enhanced the survival of dopamine neurons and, correspondingly, the extent of motor recovery of transplanted animals. Importantly for future development of clinical applications, dopamine neurons were post-mitotic at the time of transplantation and there was no tumour formation. These data provide proof for the concept that parthenogenetic stem cells are a suitable source of functional neurons for therapeutic applications.
Keywords: stem cells, transplantation, midbrain, Parkinson's disease, parthenogenesis
Introduction
Embryonic stem (ES) cells are largely seen as a potential source of specific cells for replacement therapies (McKay, 2000) and disease modelling. Parkinson's disease is a likely candidate for stem cell therapy because of the selective nature of the degenerative process and the feasibility of functional cell-replacement, as shown by foetal transplants in some Parkinson's disease patients (Mendez et al., 2005). Moreover, in vitro and in vivo, mouse ES cells can differentiate into functional DA neurons and upon transplantation improve motor deficits in animal models of Parkinson's disease (Bjorklund et al., 2002; Kim et al., 2002; Rodriguez-Gomez et al., 2007). However, in vivo studies using human and non-human primate ES cells have been disappointing. In several studies, the transplantation of early or poorly specified neurons or progenitors (to overcome the limited survival of mature neurons) has resulted in graft overgrowth and teratoma formation (Roy et al., 2006; Sonntag et al., 2007) and incomplete or questionable motor benefit in parkinsonian animals (Ben-Hur et al., 2004; Takagi et al., 2005; Ferrari et al., 2006; Roy et al., 2006; Sonntag et al., 2007). We have undertaken a stepwise approach to overcome these problems, focusing first on the derivation of DA neurons with a correct regional and cellular identity, using a sequential inductive protocol based on the developmental signals that guide the differentiation of floor plate neuroepithelial cells during embryogenesis (Perrier et al., 2004; Sanchez-Pernaute et al., 2005; Ferrari et al., 2006). In the brain, several neuronal populations express tyrosine hydroxylase (TH) the rate-limiting enzyme in DA synthesis and different classes of TH+ neurons can be derived from ES cells, including forebrain neurons (Yan et al., 2005) and neural crest derivatives (Lee et al., 2007; Elkabetz et al., 2008). Some of these TH+ neurons produce DA but have different axonal connectivity and physiology from those in the midbrain substantia nigra (SN) that are lost in Parkinson's disease.
Parthenogenesis has attracted attention as an alternative way to derive pluripotent stem cell lines that does not involve the destruction of viable embryos. However, parthenogenetic cells lack paternal imprinting and disorders related to aberrant monoparental imprinting affect the brain prominently (Wilkinson et al., 2007). A recent study suggested that parthenogenetic mouse ES cells were less efficient in producing terminally differentiated neuronal phenotypes (Hikichi et al., 2007), but, remarkably, this defect could be overcome without inducing significant changes in the methylation pattern at the H19/IGF2 region [which is imprinted during spermatogenesis and is critical for parthenote embryo viability (Leighton et al., 1995; Lucifero et al., 2002; Kono et al., 2004)]. Furthermore, fertilized ES cell lines often show abnormal imprinting (Humpherys et al., 2001) and a recent study of 17 primate fertilized ES cell lines, (Mitalipov et al., 2007) found abnormal hypermethylation of the IGF2/H19 imprinting centre in all the tested lines. Although imprinting defects affect the viability of the embryo and the capacity to form chimeras, the impact on the phenotype of the cell lines is unclear (Kim et al., 2007a), in a similar way to ES cell lines derived by somatic nuclear transfer (Dean et al., 1998; Humpherys et al., 2001; Mitalipov et al., 2007). Parthenogenetic ES cell lines from mouse (Kim et al., 2007a) and primate origins are pluripotent and can generate neurons that show physiological properties in vitro (Cibelli et al., 2002). Recently, efficient derivation of human parthenogenetic cell lines has been reported (Revazova et al., 2007) and analysis of the recombination pattern of the disputed human somatic nuclear transfer line has shown it to be parthenogenetic (Kim et al., 2007b). New advances in the generation of parthenogenetic and ES cell lines under good manufacturing practice quality conditions (Skottman et al., 2006) should make it possible to derive neurons suitable for clinical applications in the future.
Here, we report successful restoration of motor function in hemi-parkinsonian rats mediated by midbrain-like DA neurons generated from parthenogenetic primate ES cells. We demonstrate that post-mitotic DA neurons born in vitro are viable and have the capacity to re-establish synaptic contacts in the host striatum. Furthermore, we found that late in vitro exposure to specific signalling factors, normally expressed by midbrain glia, like fibroblast growth factor (FGF) 2 (Timmer et al., 2007), FGF20 (Ohmachi et al., 2000) and Wnt5a (Castelo-Branco et al., 2006), have a positive effect on the maturation and in vivo survival of DA neurons.
Material and Methods
In vitro differentiation
All experiments were performed using a non-human primate parthenogenetic stem cell line, Cyno1 (Cibelli et al., 2002). Neural differentiation was induced by co-culture on a stromal feeder cell line (MS5) as described (Barberi et al., 2003) with the addition of noggin during the first week (see Supplementary Fig. 1). At day 20, rosette structures were manually picked, plated on coated dishes as monolayer cultures and expanded in the presence of brain-derived neurotrophic factor (BDNF) and ascorbic acid (0.2 mM, Sigma–Aldrich) for 2 weeks. At the end of this period, cultures were differentiated by removing sonic hedgehog (SHH) and FGF8 and by adding dibutyryl cAMP (1 mM, Sigma–Aldrich), TGFβ3 and glial cell-derived neurotrophic factor (GDNF) (BCTG, control differentiation) and Wnt5a, FGF2, FGF20 alone or in combination. SHH (200 ng/ml), FGF8 (100 ng/ml), BDNF (20 ng/ml), GDNF (20 ng/ml), TGFβ3 (1 ng/ml), FGF2 (20 ng/ml), FGF20 (100 ng/ml), Wnt5a (100 ng/ml) were all purchased from R&D Systems.
Cell preparation and transplantation procedures
Five days after changing into the final differentiation media, cells were lightly tripsynized (0.025% trypsin/EDTA in HBBS for 4 min) into a cell suspension. Acridine orange/ethidium bromide staining was used to assess cell viability. Cells were counted and re-suspended at ∼25 000 viable cells/µl in media. The same cell suspension was re-plated for further immunocytochemistry characterization and sister cultures were used for RNA collection.
All animal procedures were performed following NIH guidelines and were approved by the IAUCC at McLean Hospital and Harvard Medical School. Female Sprague-Dawley rats (200–250 g) with 6-OHDA lesions were purchased from Taconic, and housed under standard conditions, with three to four per cage, in the animal facility at McLean Hospital. Transplantation was performed as described (Sanchez-Pernaute et al., 2001; Bjorklund et al., 2002). A total of 4 µl (∼100 000 cells in the same media they were differentiated in) were deposited in two sites in the right striatum (AP + 0.6, lat −2.8, VD 5.5–4 and AP −0.6, lat −3.2, VD 5.5–4 (0.5 µl/0.5 mm/min). To prevent rejection of grafted primate cells, rats were immunosupressed with cyclosporin A (15 mg/kg/day, Sandimmune, Sandoz, East Hannover, NJ) starting prior to surgery. After 10 weeks, the dose of cyclosporin was reduced to 10 mg/kg/day. Four months, or 4 weeks for the short-term experiments, after implantation, animals were terminally anesthetized by an i.p. overdose of pentobarbital (150 mg/kg), and perfused intracardially with heparinized saline (0.1% heparin in 0.9% saline) followed by paraformaldehyde (4% in PBS). Brains were removed, post-fixed for 4 h in 4% paraformaldehyde, equilibrated in 20% sucrose and sectioned on a freezing microtome in 40 µm slices that were serially collected.
Behavioural tests
The rotational response to apomorphine (0.05 mg/kg, 30 min) was examined 3 weeks after the 6-OHDA lesion and 3 weeks later to amphetamine (4 mg/kg, 90 min) as described previously (Ferrari et al., 2006). Post-transplantation the response to apomorphine was evaluated at 15 weeks and the response to amphetamine was tested at 6, 9, 12 and 16 weeks (Fig. 1C). The spontaneous use of the forelimbs was analysed using the cylinder test as described (Sanchez-Pernaute et al., 2004). Rats were placed individually in a plastic cylinder (21 × 34 cm) and videotaped for 5 min. The number of contacts on the cylinder wall during rearing was counted for each side. Data are shown as percentages of left forelimb (contralateral to 6-OHDA and graft) contacts over total contacts. A group of lesioned-only rats matched for the severity of baseline amphetamine rotation served as control (n = 6). These animals did not receive cyclosporin A. Cyclosporin A does not appear to modify the response to DA agonists (Schwarz et al., 2006).
Fig. 1.
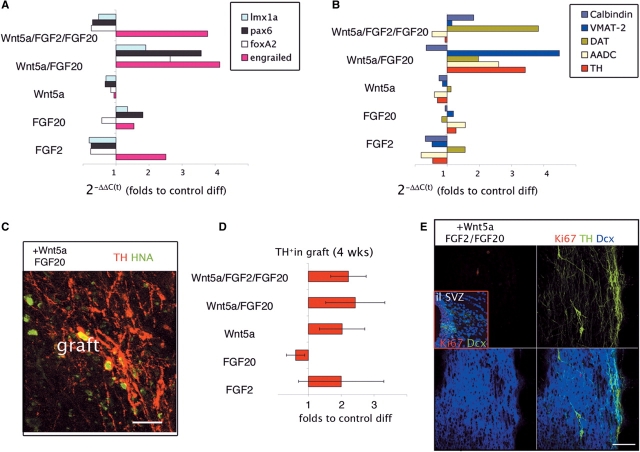
(A) Engrailed (red) and TH (green) expression in differentiated Cyno1 cells 2 days before transplantation, after 3 days in control (BCTG) or BCTG +Wnt5a/FGF2/FGF20 conditions (in vitro day 39). (B) Engrailed expression was higher in the presence of Wnt5a/FGF2/FGF20 (see also Supplementary Fig. 1). Sister cultures were harvested 2 days later for transplantation into 6-OHDA lesioned rats (n = 25). (C) Time line of in vivo studies. (D) Amphetamine response was examined before and at 6, 9, 12 and 16 weeks post-transplantation. Animals in both groups showed a progressive decline in ipsilateral rotation (CW) and an increase in contralateral (CCW) rotation (ANOVA repeated-measures over time P < 0.0001). Lesion-only animals (n = 6, not shown) did not show significant change in rotation over time (1069 +/− 71). (E) The net (CW–CCW) rotation was significantly correlated with the number of TH+ neurons in the grafts (n = 22, P < 0.05). (F) Apomorphine response was tested at 15 weeks and both groups showed a significant reduction in the response compared to pre-transplantation scores (t = −7, P < 0.001; t = −25, P < 0.0001). (G) There was a significant improvement in the use of the contralateral paw in the cylinder test in the group of animals receiving cells treated with Wnt5a/FGF2/FGF20 (n = 14, 33 ± 4%) compared to lesion-only animals (n = 6, 14 ± 5%, t = 2.44, P < 0.05). Amp = d-amphetamine; Apo = apomorphine; Ctrl = control (BCTG); Cyl = cylinder paw reaching test; CW = clockwise (ipsilateral to lesion); CCW = counter-clockwise (contralateral to lesion).
BrdU administration
To label TH neurons born in vivo from immature precursors, we administered BrdU in the drinking water (2.5 mg/ml for a daily dose of 250 mg/kg). To avoid cumulative toxicity rats were randomly allocated into three groups to receive BrdU for 2 weeks post-transplantation: 0–2 weeks (B1, N = 9 3/6), 2–4 weeks (B2, N = 9 3/6) and 4–6 weeks (B3, N = 7 3/4).
Immunohistochemistry and stereological procedures
Immunohistochemistry was performed on free-floating coronal sections as previously described (Sanchez-Pernaute et al., 2001; Bjorklund et al., 2002). Donor cells were identified in the rodent by using primate-specific monoclonal antibodies against human nuclear antigen (HNA), human-specific synaptobrevin (hVAMP), and primate-specific neural cell adhesion molecule (NCAM). Characterization of the grafted cell phenotypes was performed using multiple immunofluorescence labelling (primary antibody sources and concentrations are listed in Supplementary Table 1). For light microscopy, a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA; 1 : 300) was used to detect anti-TH (Pel-Freeze, AK, 1 : 1000), followed by incubation in streptavidin–biotin complex (Vectastain ABC Kit Elite, Vector Laboratories) for 60 min at room temperature and visualized by incubation in 3,3'-diaminobenzidine (DAB) solution with nickel enhancement (Vector Laboratories). For detection of BrdU, sections were first digested with 0.2–0.5% Triton-X, denaturated 20–40 min in 2 N HCl at 37°C and incubated overnight in anti-BrdU.
Confocal analysis was performed using a Zeiss LSM510/Meta station (Thornwood, NY). Qualitative analysis was performed by systematic evaluation of series of sections spanning the grafts. For identification of signal co-localization within a cell, optical sections were kept to a minimal thickness and orthogonal reconstructions were obtained with automated 2D-deconvolution (LSM software, Carl Zeiss MicroImaging Inc., Thornwood, NY) for high power images.
Quantification of TH+ neurons was performed on DAB-stained sections in every section where a graft was identifiable (1/6th section) in 22 animals, because one brain from the control group and two from the +Wnt5a/FGF2/FGF20 group were poorly fixed or processed and were not included in the analyses. Cell counts from serial sections were corrected and extrapolated for whole graft volumes and cell diameters using the Abercrombie method (Abercrombie, 1946). All other quantitative analyses were performed using StereoInvestigator (MicroBrightField, Williston, VT) and a Zeiss Axioplan microscope. Graft volumes were calculated using the Cavalieri estimator probe on HNA-stained sections (N = 14). Double-labelled cell counts were performed using the optical fractionator probe with either a 40× or a 63× (for nuclear staining) lens. For the estimation of the expression of BrdU and Ki67 over HNA, counts were done using the optical fractionator probe in randomly chosen fields containing the graft core within one series (500–1500 Hoechst+ nuclei) in two to four representative animals for each condition and results were expressed as percentages.
Quantitative-polymerase chain reaction (Q-PCR)
RNA extraction and cDNA syntheses were performed as described (Sonntag et al., 2005). For Q-PCR, cDNA samples (∼25–50 ng/µl mRNA equivalent) were analyzed using the SYBR Green PCR MasterMix (ABI, Foster City, CA) and an Opticon MJ thermocycler (BioRad, Hercules, CA) in total volumes of 25 µl with 40 nM primers for each reaction. Linearity and detection limit of the assay were performed in 10-fold serial dilutions (linear curves r = 0.9 or better) to determine the optimal template amounts. Quantification was performed at a threshold detection line (‘threshold cycles’, Ct value). The Ct of each gene product was normalized against that of the housekeeping gene β-actin, which was run simultaneously for each marker. Data were expressed as mean ± SEM. The ΔCt and ΔΔCt for each candidate were calculated according to the methods of Livak and Schmittgen (2001) and plotted as relative levels of gene expression using the control differentiation as reference. Data were analyzed by a two-tailed Student's t-test and statistical significance was set at P < 0.05. (Primers used are listed in Supplementary Table 2).
Statistical analysis
Results are shown as mean ± standard error. Repeated-measure ANOVA was used to evaluate treatment effects on rotational behaviour over time; unpaired two-tailed Student's t-tests or one-way ANOVA were performed to make comparisons between the groups (by graft type or BrdU administration). Simple regression analyses were performed to evaluate correlation between volumes and cell types. Significance was considered for P < 0.05. Statistical analyses were made using Statview software (SAS Institute Inc, Carny, North Carolina).
Results
For this study we used an in vitro differentiation protocol (Perrier et al., 2004; Sanchez-Pernaute et al., 2005; Ferrari et al., 2006), implemented by the addition of noggin [a bone morphogenetic protein antagonist that favours neural induction (Sonntag et al., 2007)] and early exposure to SHH protein (Supplementary Fig. 1A). Following the withdrawal of SHH and FGF8, at in vitro day 37, we applied two differentiation conditions. Cells in the control condition were exposed to BDNF, GDNF, TGF-β3, ascorbic acid and cAMP, as in our previous studies (Perrier et al., 2004; Sanchez-Pernaute et al., 2005; Ferrari et al., 2006). In the second condition, cells were exposed, in addition to the above, to a combination of Wnt5a, FGF2 and FGF20.
Transplantation of parthenogenetic DA neurons into 6-OHDA-lesioned rats restores motor function
Cells differentiated in control and (+)Wnt5a/FGF2/FGF20 conditions were harvested after 5 days (in vitro day 42) and transplanted into the striatum of 6-OHDA-lesioned rats (n = 25). Sister cultures were stained 2 days before harvesting to verify expression of engrailed (En1/2) and TH (Fig. 1A). The expression of En1 was higher in the (+)Wnt5a/FGF2/FGF20 condition by immunocytochemistry and RT-PCR analysis (Fig. 1B). Further in vitro characterization of the cells used in this grafting experiment is available in Supplementary information (Fig. S1).
A total of 100 000 cells in 4 µl were transplanted into the right striatum of 6-OHDA-lesioned hemi-parkinsonian rats (n = 9 for control cells, n = 16 for (+)Wnt5a/FGF2/FGF20). All the rats displayed severe motor asymmetry in response to DA agonists (apomorphine and d-amphetamine) in pharmacological rotational tests before transplantation. These pharmacological tests were repeated at different time points (Fig. 1C) to evaluate the effect of the grafted neurons that typically require several weeks to complete maturation in vivo. The response to d-amphetamine (an indirect DA agonist) was evaluated at 6, 9, 12 and 16 weeks post-transplantation (Fig. 1D). Animals in both groups showed a progressive decline in amphetamine-induced ipsilateral rotation (clockwise, CW) [ANOVA repeated-measures over time, F(4,18) = 34; P < 0.0001] and many of them displayed contralateral (counter-clockwise, CCW) rotation, that increased over time [ANOVA repeated-measures, F(3,16) = 5, P < 0.01]; and was more pronounced in the group of animals receiving cells treated with Wnt5a/FGF2/FGF20, although the differences between the groups did not reach significance (P = 0.07 at 16 weeks). Non-grafted animals (lesion-only, n = 6) did not show any improvement over time (average rotation at 16 weeks was 1069 ± 71). Taking both transplanted groups together, the net (CW–CCW) number of rotations in the last test before sacrifice was significantly correlated with the number of TH+ neurons present in the grafts in the post-mortem analysis (N = 22, r = 0.55, P < 0.05, Fig. 1E).
The post-synaptic supersensitivity induced by the 6-OHDA denervation, as measured by the rotational response to apomorphine (0.05 mg/kg, a direct DA agonist) was significantly attenuated at 15 weeks post-transplantation in both groups (t = −7, P < 0.01 and t = −15, P < 0.0001 for control and (+)Wnt5a/FGF2/FGF20-treated cells, respectively) (Fig. 1F). This effect on apomorphine response indicates that grafted DA neurons function towards normalization of basal DA striatal levels and post-synaptic receptor state.
The positive effect of the grafted neurons on motor behaviour was manifested in an increased use (reduced akinesia) of the forelimb contralateral to the 6-OHDA lesion, during spontaneous exploration in the cylinder test (Fig. 1G). The use of the contralateral paw in the group of rats receiving (+)Wnt5a/FGF2/FGF20 treated cells (0.33 ± 0.04) was significantly better than that of lesioned-only animals (0.14 ± 0.06, PLSD P < 0.05). Overall, these behavioural effects demonstrate that grafted DA neurons derived from parthenogenetic Cyno1 stem cells are functional in vivo.
Analysis of dopamine neurons in the grafts
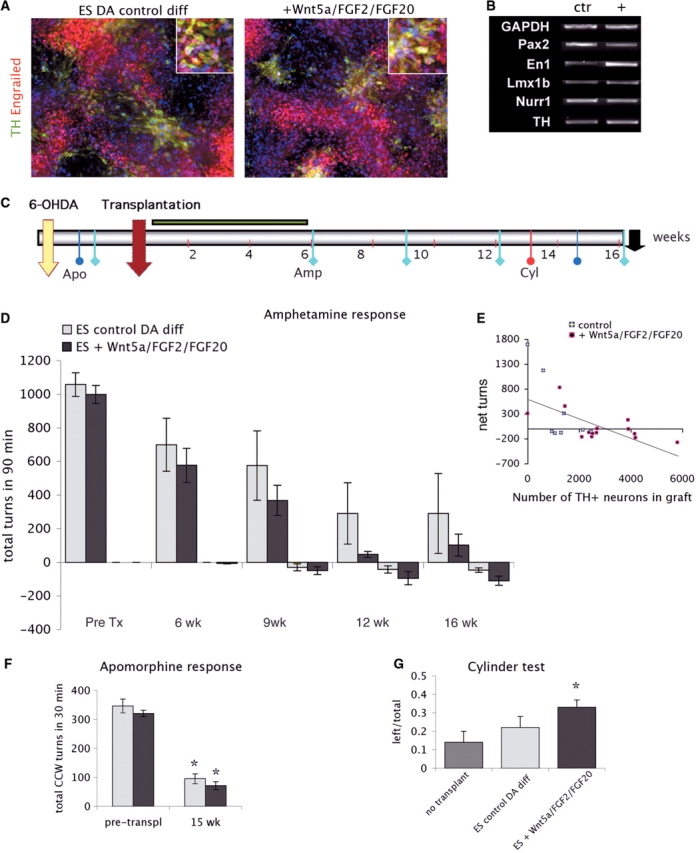
TH+ cells in the grafts had a mature morphology and were typically located at the host–graft interface in both groups (Fig. 2A). The number of TH+ neurons was significantly higher in the grafts from cells differentiated in (+) Wnt5a/FGF2/FGF20 conditions (Fig. 2B). Because in this group the grafts appeared to be larger, we performed stereological estimation of the graft volumes using the expression of HNA+ to identify the core of the graft. The difference in graft volumes between the groups was significant (P < 0.001) and there was a direct correlation between the number of TH+ neurons and the graft volumes (R2 = 0.87, P < 0.01) (Fig. 2C). The density of TH+ neurons (TH+/mm3) was similar in the two groups (781 and 798 TH+/mm3, respectively, P = 0.3) which represents ∼40% of the TH+ cell density reported in functional human foetal ventral midbrain grafts (Mendez et al., 2005). Therefore, the addition of Wnt5a/FGF2/FGF20 at the end stage of the differentiation (and importantly in the cell suspension) improved the survival of the grafted cell population, but did not result in a selective enrichment in TH+ neurons in vivo. We next examined the phenotype of TH+ neurons and found no differences between groups in the expression of characteristic midbrain transcription factors such as Pitx3, Foxa2 (HNF3β) (Fig. 2D–F) and En1/2 (not shown and Ferrari et al., 2006). Both the morphology and the expression of regional-specific transcription factors were similar to that of host DA neurons in the contralateral SN (Fig. 2D and E). Importantly, these transcription factors may be involved not only in specification but also in survival of midbrain DA neurons (Alberi et al., 2004; Sgado et al., 2006; Kittappa et al., 2007). We examined the co-expression of calbindin (CB) and Girk2 in TH+ neurons (Fig. 2G). CB is expressed preferentially by the medial DA neurons subpopulations projecting to limbic territories and not in the ventral subpopulation mostly affected in Parkinson's disease, the ventral tier of the SN pars compacta (Mendez et al., 2005) and, in vitro, FGF20 enhances TH+ CB– neuron survival (Murase and McKay, 2006). In the grafts, the proportion of TH+ CB+ neurons was similar in both groups (49.5 ± 5, N = 5 and 47.8 ± 5%, N = 8, P = 0.8). Girk2 is expressed by the TH+ neurons in the ventral tier of the SN. Girk2 was also expressed by TH+ neurons in the grafts (Fig. 2G). In summary, the addition of Wnt5a/FGF2/FGF20 increased overall grafted cell survival including that of TH+ neurons without noticeable modification of DA phenotypes in vivo.
Fig. 2.
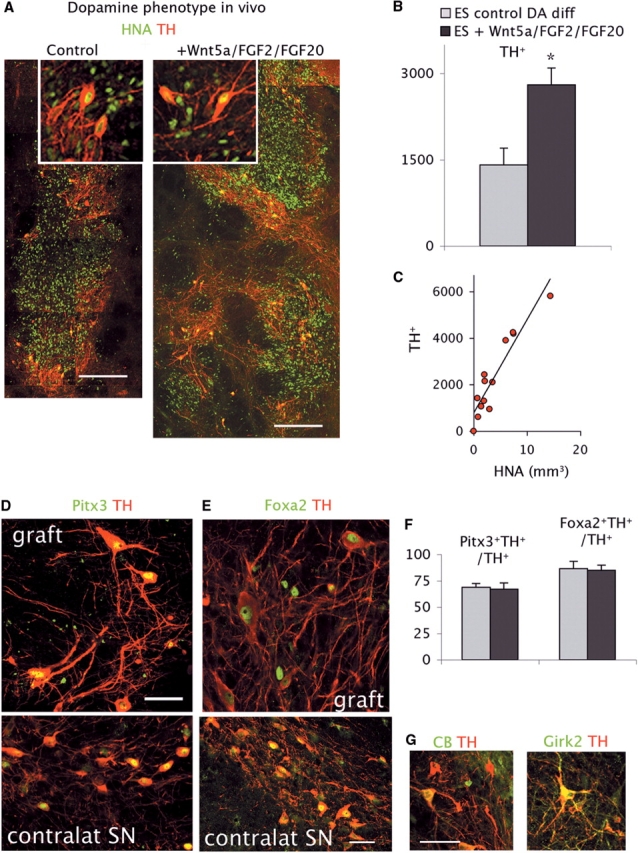
(A) Confocal reconstruction of representative grafts from control and cells treated with Wnt5a/FGF2/FGF20. Grafted cells are identified by expression of the human-specific nuclear antigen (HNA, green). Scale bars: 200 µm. Insets show higher magnification of TH+ neurons. (B) Total number of TH+ neurons was significantly higher in grafts from cells treated with Wnt5a/FGF2/FGF20 (P < 0.01) than in control grafts. (C) The number of TH+ neurons in the grafts was directly correlated with the graft volume (n = 14; r = 0.93, P < 0.01). (D–F) TH+ neurons expressed the midbrain transcription factors Pitx3 (green in D), Foxa2 (green in E) and En1 (not shown) as do host midbrain DA neurons in the contralateral (unlesioned) SN (E and F lower panels). Scale bars: 50 µm. There was no difference in the proportion of TH neurons expressing these transcription factors between the two cell conditions (n = 3/4 per group). (G and H) TH+ neurons in the grafts expressed the calcium-binding protein, CB D-28k, and Girk-2. Scale Bar: 50 µm.
Neurite outgrowth and synaptic formation
TH+ fibres from the grafted neurons grew several hundred microns into the host striatum (Fig. 3A–C), and established synaptic contacts on striatal medium spiny neurons, identified by PSD-95 immunostaining at the post-synaptic level (Fig. 3D and E). PSD-95 immunoreactivity was reduced in the grafted (lesioned) striatum (Fig. 3D), in concordance with the 75% decrease of PSD-95 in striatal membrane homogenates reported in 6-OHDA-lesioned rats (Nash et al., 2005). PSD-95 is an abundant post-synaptic density protein in glutamatergic synapses that regulates maturation and synaptic strength. In the striatum the DA synapses on D1 DA receptors, located in the neck of the dendritic spines of medium spiny neurons, are in close spatial association with cortical glutamate synapses, forming a functional synaptic triad that appears to be required for the converging actions of these pathways on striatal transmission (Yao et al., 2004). Confocal microscopy allows for the identification of synaptic contacts defined by the apposition of pre- and post-synaptic processes and the presence of specific synaptic proteins using multiple fluorescence labelling (Wouterlood et al., 2003). We found that TH+ fibres grew out from the graft and established contacts on DARPP-32+ neurons, closely apposed to PSD-95 immunoreactive synapses (Fig. 3D), thus appearing able to re-establish normal synaptic architecture. TH+ fibres expressed pre-synaptic proteins such as synapsin (not shown) and synaptobrevin (VAMP) (Fig. 3F–H). We confirmed that the TH+ axons were derived from grafted primate neurons (not remaining host axons) by co-labelling with a monoclonal antibody against human (h)VAMP that does not recognize the rat protein (Fig. 3F–H).
Fig. 3.
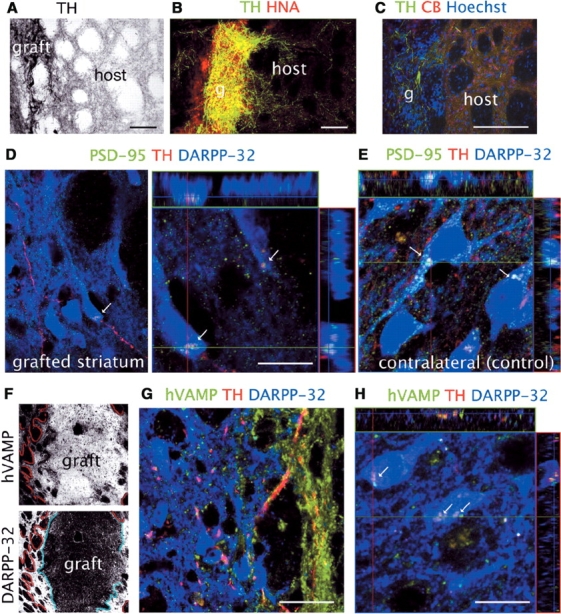
Neurite outgrowth. (A–C) Low power microphotographs demonstrated TH+ fibres extending from the graft into the host striatum. Scale bars: 200 µm. (D) Using confocal microscopy TH axons (red) were observed in contact to host DARPP-32 neurons (blue), closely apposed to PSD-95 (green) post-synaptic buttons, in a similar pattern to that observed in the control, contralateral striatum (E) Scale bars: 10 µm. (F–H) Grafted TH+ axons were identified by the co-expression of hVAMP (green). The graft profile outlined by the hVAMP immunoreactivity (red line) in the axons spread out of the graft bed, marked by the border of host DARPP-32 immunoreactivity (blue line), revealing a halo of dense primate axonal arborisation extending ∼200 µm from the core. (G and H) High magnification confocal images taken at the graft–host interface showing the localization of hVAMP on TH+ fibres (red in G and H) and in the host striatum synapsing on DARPP-32 neurons. Contacts are shown on the cell soma for clarity. Scale bars: 10 µm.
Dopamine neurons were born in vitro
To determine whether DA neurons present in the grafts were generated in vitro and survived transplantation or were born in vivo from transplanted progenitors, we administered BrdU to the rats for the first 6 weeks after transplantation. In order to avoid BrdU toxicity, animals were allocated into three groups to receive BrdU in the drinking water for 2 weeks (0–2 weeks post-transplantation, B1, N = 9; 2–4 weeks, B2, N = 9 and 4–6 weeks, B3, N = 7) (Fig. 4A). BrdU labelled cells were present in all animals in the graft and in other brain regions, including the sub-ventricular zone and endothelial cells in blood vessels. Within the core of the grafts, the majority of BrdU+ labelled cells were derived from the graft and co-expressed the primate marker HNA (HNA+, 88 ± 5%) and 22 ± 8% of the graft HNA+ were BrdU+ (Fig. 4B). There were no significant differences in the percentage of labelled cells between cell differentiation conditions (Fig. 4B). Taken together there were no significant differences in the percentage of BrdU labelled cells between the three periods of administration (Fig. 4C). Regardless of the period of BrdU administration, we never observed TH+ neurons labelled by BrdU in any group (Fig. 4D). During development midbrain DA neurons are born between days 35 and 42 in macaques (Levitt and Rakic, 1982) and in short-term experiments (see the Effects of Wnt5a, FGF20 and FGF2 section that follows) TH+ mature neurons were present at 4 weeks and were Ki67 negative (Fig. 5E). Thus the absence of labelling within the 6-week period of BrdU administration indicates that DA neurons present in the grafts were not born in vivo. BrdU + labelled doublecortin (Dcx+) neurons were observed in the group receiving BrdU from 4 to 6 weeks post-transplantation (B3, Fig. 4E and F), suggesting that, in contrast to DA neurons, Dcx+ neuroblasts were born in vivo during the second month post-transplantation. These results also suggest that it was unlikely that the regime of BrdU administration caused significant neurotoxicity, as during neurogenesis cortical neurons are more vulnerable than mesencephalic neurons (Kuwagata et al., 2007) to BrdU genotoxicity. There was no difference in the intensity of DCx labelling and chain formation at the edge of the grafts, between cell conditions, and the core of the grafts was intensely positive for this neuronal protein while nestin was only weakly expressed (see Supplementary Figs S2 and S3). To evaluate whether there was ongoing proliferation in the grafts at the time of sacrifice (4 months post-transplantation) we examined Ki67 immunoreactivity in the brain of representative animals from both groups (n = 4). Most cells in the ventricular lining were positive (Fig. 4G) and mitotic figures were easily identified in host cells (inset). A few HNA+ cells were observed close to the SVZ (Fig. 4G) as described previously (Sanchez-Pernaute et al., 2005; Tabar et al., 2005). Within the grafts, between 1–4% of HNA+ cells were Ki67+ (and ∼40% of Ki67+ were HNA+) and there were no differences between the two conditions (Fig. 4H and I). These results demonstrate that the majority of transplanted cells were terminally differentiated in vivo by 4 months.
Fig. 4.
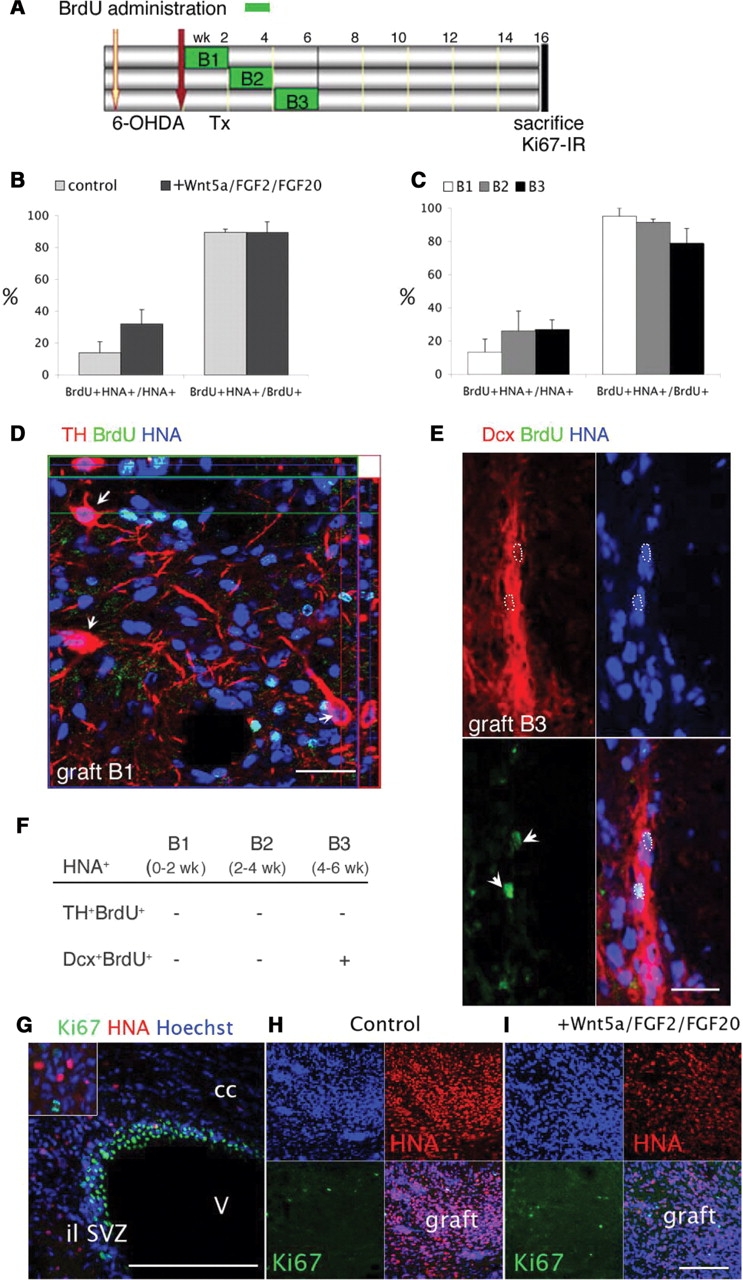
(A) Schematic representation of the time of BrdU administration in the drinking water. (B) There was no significant difference in BrdU labelling between control and +Wnt5a/FGF2/FGF20 conditions (quantification was performed for n = 4 per group, P = 0.15) in the relative percentage of cells in the graft core that incorporated BrdU (BrdU+ HNA+ over HNA+, 22 ± 8%) or in the percentage of proliferating cells that were graft derived (BrdU+ HNA+ over total BrdU+, 88 ± 5%). (C) There were no significant differences in labelling between times of administration. (D) TH neurons did not incorporate BrdU in vivo. Confocal orthogonal reconstruction showing BrdU+ (green) and HNA+ (blue, nuclear co-expression appears cyan) cells in a representative graft from an animal that received BrdU during the first 2 weeks post-transplantation. TH+ (red) HNA+ neurons were not labelled (white arrows). Scale bar: 40 µm. (E and F) In the group receiving BrdU 4–6 weeks post-transplantation (B3) a few grafted neurons expressing doublecortin (Dcx, red) were labelled by BrdU (green, white arrows) Scale bar: 20 µm. (G and H) Ki67 immunoreactivity was used to evaluate active proliferation at the time of sacrifice. (G) Expression was ubiquitous in the ventricular lining/sub-ventricular zone (shown is the lateral ventricle ipsilateral to the grafted striatum) in mitotic host cells (inset) Scale bar: 100 µm. (H) Representative view of the grafts revealed low proliferation and no differences between the two cell conditions in the percentage of HNA+ cells expressing Ki67 (1–4%) at this time. Scale bar: 200 µm.
Fig. 5.
(A) Five differentiation conditions were compared for the effects on DA specification in vitro (A and B) and DA neuronal survival in vivo (C–E). (A) Q-PCR analyses of midbrain transcription factors, Foxa2, Lmx1a and En1/2 and the dorsal marker Pax6, calculated over the levels in the control differentiation condition (BCTG only) and (B) DA phenotypic markers necessary for DA neurotransmission (TH, AADC, VMAT-2, DAT) and CB expression in the differentiation conditions examined, with reference to control differentiation (BCGT). (C) Representative confocal image of TH+ neurons in a graft from the Wnt5a/FGF20 differentiation condition at 4 weeks post-transplantation. Scale bar: 50 µm. (D) Number of TH+ cells in vivo 4 weeks after transplantation into rat striatum relative to control differentiation suggested that all tested conditions, except FGF20 alone, enhanced survival of TH+ neurons in vivo. (E) TH+ neurons had a mature morphology and did not express Ki-67 at 4 weeks. DCx was highly expressed in the grafts. Scale bar: 200 µm
Effects of Wnt5a, FGF20 and FGF2
To examine the contribution of these factors to the increase in number of DA neurons in the grafts, we performed a short-term (4 week) in vivo transplantation experiment in which the cells were exposed at the last stage (37–42 in vitro days) to either FGF2, FGF20, Wnt5a or FGF20/Wnt5a (in addition to BCTG) and compared the effects on TH+ cells in the grafts with control and Wnt5a/FGF2/FGF20 differentiation conditions (Fig. 5). We examined the expression of DA transcription factors and phenotypic markers by Q-PCR at the time of harvesting (day 42, Fig. 5A and B). The profile of gene expression (over control differentiation, Fig. 5A) revealed a 3-fold increase in engrailed for Wnt5a/FGF20 and FGF2/FGF20/Wnt5a, which is consistent with the results of the long-term experiment (Fig. 1B) and our preliminary data (Supplementary Fig. 1). The conditions using a combination of Wnt5a and FGF20 appeared to be the most favourable for DA differentiation and maturation with increases in specific proteins necessary for synthesis, storage and regulated release of DA (TH, DAT, VMAT2 and AADC) with respect to the control condition. In the post-mortem analysis of the grafts (n = 4–7 per condition), the number of TH+ neurons was significantly higher in conditions including Wnt5a (alone or in combination) [F(1,26)= 6.89, P ≤ 0.01]; relative to control differentiation all conditions except FGF20 tended to increase the number of TH+ neurons in the grafts (Fig. 5). TH density (+cells/mm3 of HNA+ volume) was highest in the Wnt5a/FGF20 condition (815 ± 200) and not different from the long-term study. TH+ neurons were negative for the proliferation marker Ki-67 at 4 weeks post-grafting (Fig. 5E), corroborating the data collected using BrdU labelling in the long-term study, while some Dcx+ neuroblasts were positive at this time (see Supplementary Fig. S2). Taken together our data strongly suggests that the absence of BrdU labelling in the long-term study was not due to neurotoxicity, but to a lack of mitotic activity in the TH population post-transplantation. The proportion of TH+ CB+ neurons in the grafts did not differ between conditions. Based on these results we conclude that the addition of Wnt5a and FGF20 at the end of the differentiation enhances the yield of grafted TH+ neurons generated in vitro that can survive in vivo.
Discussion
The functional effects of neurons derived from human and primate ES cells in vivo have been inconsistent. Furthermore, no previous studies have shown that parthenogenetic cells function in vivo. In this study we show for the first time a robust motor recovery in hemi-parkinsonian rats that was directly correlated with the number of parthenogenetic ES-derived DA neurons present in the grafts. Modifications of our in vitro differentiation protocol led to an increase in the number of DA neurons and to an improved survival, which in turn resulted in a sufficient number of midbrain-like DA neurons in the grafts (Ferrari et al., 2006) to mediate a reversal of typical parkinsonian motor deficits. In addition to the remarkable effect on amphetamine-induced rotation, which is a useful indicator of the presence of mature DA neurons in the graft, because it requires localization of the DA transporter to the cell membrane and the synthesis and release of DA, we also observed a significant attenuation in apomorphine-induced rotation and, importantly, an improvement in spontaneous exploratory behaviour to the same extent reported for l-DOPA in the cylinder test (Mela et al., 2007). These behaviours are indicative of a substantial degree of graft integration, because basal (spontaneous) release of DA by grafted neurons is necessary to attenuate the post-synaptic adaptations that mediate apomorphine-induced rotational response and to restore symmetric exploratory behaviour (Dunnett et al., 1988; Nikkhah et al., 1993). Axonal outgrowth and synaptic contacts between grafted DA neurons and host DARPP-32 striatal neurons was confirmed by the post-mortem analysis at the cellular level, using confocal imaging. Other factors, in particular compression, or lesion of striatofugal fibres, may indeed diminish the rotational response to apomorphine (Marshall and Ungerstedt, 1977; Christophersen and Brundin, 2007), but such complications were never observed in these animals. Analysis of graft cell composition revealed uniform expression of neuronal proteins such as neural cell adhesion molecule and Dcx, with little expression of nestin within the grafts at 16 weeks post-transplantation and no evidence of teratoma formation in any of the experimental groups. While nestin expression was low, the presence of uncommitted cells within the grafts is still a matter of great concern and emphasizes the need for selection procedures.
Another fundamental contribution of this study is the demonstration of the absence of BrdU incorporation by DA neurons after transplantation. Knowing whether DA neurons present in the grafts are the ones differentiated in vitro, or are born in the transplants is relevant for implementing selection and transplantation methods, in particular for negative selection of proliferating cells and cell dose optimization. Indeed, the developmental stage is crucial in terms of predictability of graft composition and phenotypic stability based on in vitro characterization of the cells (on which transplantation parameters are necessarily based). Because post-mitotic neurons are fragile, many in vivo studies have been performed using minimally differentiated neural progenitors or spheres from human (Ben-Hur et al., 2004; Roy et al., 2006; Ko et al., 2007) or primate (Takagi et al., 2005) ES cells for transplantation into parkinsonian animals. In those studies, the TH+ neurons identified in the grafts were most likely born in vivo (see Discussion in Brederlau et al., 2006) and the regional phenotype was not established. Moreover, while such an approach sometimes enhances grafted cell survival, the lack of control over proliferation and differentiation makes it unsuitable for therapeutic applications, because of the development of teratomas or graft overgrowth (Roy et al., 2006). In our study, administration of BrdU was designed specifically to detect DA neurons born in vivo and our results demonstrate that there were none. The absence of BrdU labelling in TH+ neurons in the grafts indicates that these neurons were born in vitro. It is very unlikely that there were proliferating DA precursors that died because of BrdU neurotoxicity, as we found doublecortin+ neurons that were BrdU+. The fact that very few cells were Ki67+ at the end of the study supports the idea that the cells that are proliferating in vivo are not stem cells but committed precursor cells, close to their developmental cell-cycle exit. Consistently, many double-labelled HNA+/BrdU+ cells expressed Dcx. Importantly, our results demonstrate that it is feasible to harvest and transplant in vitro born post-mitotic DA neurons that function in vivo; this post-mitotic stage would be preferable in terms of safety and reproducibility for clinical applications. Notably, the low numbers of Ki67+ cells in the graft, together with the fact that some BrdU labelled cells were detectable 10 to 14 weeks post-oral administration emphasizes the low proliferation rate when ES cells are differentiated for long time periods in vitro, like in the present study (6 weeks).
We found here that the addition of factors (Wnt5a/FGF20/FGF2), which are normally secreted by mesencephalic glia, resulted in a significant increase of TH+ neurons and enhanced survival. In vitro (Murase and McKay, 2006), FGF20 has been shown to promote survival of the CB– subpopulation of DA neurons when the cells were subjected to oxidative stress. Although we did not observe a effect on the TH+CB– population, the presence of FGF20 in the last stage of differentiation may have contributed to the enhanced survival to transplantation. In a recent publication (Correia et al., 2007) FGF20 appeared to promote DA differentiation from hES cells in vitro, using PA6 co-culture inductive protocols. FGF2 trophic support appears to be critical for post-mitotic DA neurons while it maybe redundant (i.e. compensated by other trophic factors) during development (Timmer et al., 2007). Our data suggest that Wnt5a had a strong effect in vitro and in vivo. Wnt5a promotes the differentiation of Nurr-1+ precursors to DA neurons and has been shown to induce a significant increase in TH+ neurons in vitro, in primary ventral midbrain DA cultures (Castelo-Branco et al., 2006; Castelo-Branco et al., 2003) and in locus coeruleus noradrenergic cultures (Holm et al., 2006). Interestingly, in those in vitro studies, the effect of Wnt5a (at late developmental stages that roughly correspond with the time of in vitro exposure in our study) was hypothesized to be due to direct TH induction or phenotypic stabilization in post-mitotic progenitors (Holm et al., 2006). In addition, this group has recently shown that Wnt5a over-expression in foetal ventral midbrain neurons is related to better survival of transplanted DA neurons and to faster functional recovery in vivo (Parish et al., 2008), like in our study, without having a direct impact on either proliferation or cell death. While Wnt5a promotes maturation, transcriptional stabilization and cell-cycle exit of DA precursors (Castelo-Branco et al., 2003) and probably enhanced the generation and/or maturation of DA neurons in our paradigm in vitro, it is likely that FGFs acted synergistically to improve overall survival to transplantation. It is possible that this particular trophic factor combination enhanced not only survival, but also proliferation of non-DA cells in vivo, early after transplantation, yet additional labelling studies would be required to identify such effects.
Our results here demonstrate that parthenogenetic stem cell lines could be utilized to derive functional DA neurons for cell therapy in Parkinson's disease patients. Recent studies have pointed to substantial differences between hES cell lines upon differentiation (Wu et al., 2007). Whether a propensity to generate neurons, or, more specifically, ventral neuronal phenotypes may be related to the parthenogenetic origin is currently under investigation (for example, there may be maternally imprinted genes involved in the patterning, differentiation or maturation of these neuronal populations or lack of paternally imprinted genes facilitating it). Nevertheless, while we acknowledge that further enrichment and purification of neurons derived from ES cells is required (Hedlund et al., 2008), this study conclusively demonstrates that is feasible to restore motor function in parkinsonian animals using DA neurons derived in vitro from primate parthenogenetic stem cells.
Supplementary material
Supplementary material is available at Brain online
Acknowledgements
This study was supported by the Harvard Stem Cell Institute, the Starr Fundation and the National Institutes of Health NINDS P50 NS-39793 and R01 NS-052671. We are grateful to Yalda Sadeghi and Shreeya Karki for technical help.
Glossary
Abbreviations:
- BDNF
brain-derived neurotrophic factor
- ES
Embryonic stem
- FGF
fibroblast growth factors
- GDNF
glial cell-derived neurotrophic factor
- HNA
human nuclear antigen
- hVAMP
human-specific synaptobrevin
- SHH
sonic hedgehog
- SN
substantia nigra
- TH
tyrosine hydroxylase
References
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–47. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Alberi L, Sgado P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131:3229–36. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–7. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–55. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99:2344–9. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–40. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol Cell Neurosci. 2006;31:251–62. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747–52. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen NS, Brundin P. Large stem cell grafts could lead to erroneous interpretations of behavioral results? Nat Med. 2007:118–9. doi: 10.1038/nm0207-118a. 13: 118; author reply. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Grant KA, Chapman KB, Cunniff K, Worst T, Green HL, et al. Parthenogenetic stem cells in nonhuman primates. Science. 2002;295:819. doi: 10.1126/science.1065637. [DOI] [PubMed] [Google Scholar]
- Correia AS, Anisimov SV, Roybon L, Li JY, Brundin P. Fibroblast growth factor-20 increases the yield of midbrain dopaminergic neurons derived from human embryonic stem cells. Front Neuroanat. 2007;1:1–6. doi: 10.3389/neuro.05.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean W, Bowden L, Aitchison A, Klose J, Moore T, Meneses JJ, et al. Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development. 1998;125:2273–82. doi: 10.1242/dev.125.12.2273. [DOI] [PubMed] [Google Scholar]
- Dunnett S, Hernandez T, Summerfield, Jones G, Arbuttnott G. Graft-derived recovery from 6-OHDA lesions: specificity of ventral mesencephalic graft tissue. Exp Brain Res. 1988;71:411–24. doi: 10.1007/BF00247501. [DOI] [PubMed] [Google Scholar]
- Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Sanchez-Pernaute R, Lee H, Studer L, Isacson O. Transplanted dopamine neurons derived from primate ES cells preferentially innervate DARPP-32 striatal progenitors within the graft. Eur J Neurosci. 2006;24:1885–96. doi: 10.1111/j.1460-9568.2006.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund EM, Pruszak J, Lardaro T, Ludwig W, Vinuela A, Kim KS, et al. Embryonic stem (ES) cell-derived Pitx3-eGFP midbrain dopamine neurons survive enrichment by FACS and function in an animal model of Parkinson's disease. Stem Cells. 2008;26:1526–36. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikichi T, Wakayama S, Mizutani E, Takashima Y, Kishigami S, Van Thuan N, et al. Differentiation potential of parthenogenetic embryonic stem cells is improved by nuclear transfer. Stem Cells. 2007;25:46–53. doi: 10.1634/stemcells.2006-0439. [DOI] [PubMed] [Google Scholar]
- Holm PC, Rodriguez FJ, Kele J, Castelo-Branco G, Kitajewski J, Arenas E. BMPs, FGF8 and Wnts regulate the differentiation of locus coeruleus noradrenergic neuronal precursors. J Neurochem. 2006;99:343–52. doi: 10.1111/j.1471-4159.2006.04039.x. [DOI] [PubMed] [Google Scholar]
- Humpherys D, Eggan K, Akutsu H, Hochedlinger K, Rideout WM, Biniszkiewicz D, et al. Epigenetic instability in ES cells and cloned mice. Science. 2001;293:95–7. doi: 10.1126/science.1061402. [DOI] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–6. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kim K, Lerou P, Yabuuchi A, Lengerke C, Ng K, West J, et al. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007a;315:482–6. doi: 10.1126/science.1133542. [DOI] [PubMed] [Google Scholar]
- Kim K, Ng K, Rugg-Gunn PJ, Shieh J-H, Kirak O, Jaenisch R, et al. Recombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transfer. Cell Stem Cell. 2007b;1:346–52. doi: 10.1016/j.stem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kittappa R, Chang WW, Awatramani RB, McKay RD. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007;5:e325. doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Park CH, Koh HC, Cho YH, Kyhm JH, Kim YS, et al. Human embryonic stem cell-derived neural precursors as a continuous, stable, and on-demand source for human dopamine neurons. J Neurochem. 2007;103:1417–29. doi: 10.1111/j.1471-4159.2007.04898.x. [DOI] [PubMed] [Google Scholar]
- Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, et al. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–4. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]
- Kuwagata M, Ogawa T, Nagata T, Shioda S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2'-deoxyuridine in mice. Neurotoxicology. 2007;28:780–9. doi: 10.1016/j.neuro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–75. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–9. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P. The time of genesis, embryonic origin and differentiation of the brain stem monoamine neurons in the rhesus monkey. Brain Res. 1982;256:35–57. doi: 10.1016/0165-3806(82)90095-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Mertineit C, Clarke HJ, Bestor TH, Trasler JM. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–8. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Ungerstedt U. Striatal efferent fibers play a role in maintaining rotational behavior in the rat. Science. 1977;198:62–4. doi: 10.1126/science.897683. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells—hype and hope. Nature. 2000;406:361–4. doi: 10.1038/35019186. [DOI] [PubMed] [Google Scholar]
- Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson's disease. J Neurochem. 2007;101:483–97. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipov S, Clepper L, Sritanaudomchai H, Fujimoto A, Wolf D. Methylation status of imprinting centers for H19/IGF2 and SNURF/SNRPN in primate embryonic stem cells. Stem Cells. 2007;25:581–8. doi: 10.1634/stemcells.2006-0120. [DOI] [PubMed] [Google Scholar]
- Murase S, McKay RD. A specific survival response in dopamine neurons at most risk in Parkinson's disease. J Neurosci. 2006;26:9750–60. doi: 10.1523/JNEUROSCI.2745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JE, Johnston TH, Collingridge GL, Garner CC, Brotchie JM. Subcellular redistribution of the synapse-associated proteins PSD-95 and SAP97 in animal models of Parkinson's disease and L-DOPA-induced dyskinesia. FASEB J. 2005;19:583–5. doi: 10.1096/fj.04-1854fje. [DOI] [PubMed] [Google Scholar]
- Nikkhah G, Duan WM, Knappe U, Jodicke A, Bjorklund A. Restoration of complex sensorimotor behavior and skilled forelimb use by a modified nigral cell suspension transplantation approach in the rat Parkinson model. Neuroscience. 1993;56:33–43. doi: 10.1016/0306-4522(93)90559-x. [DOI] [PubMed] [Google Scholar]
- Ohmachi S, Watanabe Y, Mikami T, Kusu N, Ibi T, Akaike A, et al. FGF-20, a novel neurotrophic factor, preferentially expressed in the substantia nigra pars compacta of rat brain. Biochem Biophys Res Commun. 2000;277:355–60. doi: 10.1006/bbrc.2000.3675. [DOI] [PubMed] [Google Scholar]
- Parish CL, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen AT, Salto C, et al. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest. 2008;118:149–60. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–8. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, et al. Patient-Specific Stem Cell Lines Derived from Human Parthenogenetic Blastocysts. Cloning Stem Cells. 2007;9:432–49. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–28. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–68. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J Neurosci Res. 2001;65:284–8. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Studer L, Ferrari D, Perrier A, Lee H, Vinuela A, et al. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–22. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz SC, Wittlinger J, Schober R, Storch A, Schwarz J. Transplantation of human neural precursor cells in the 6-OHDA lesioned rats: effect of immunosuppression with cyclosporine A. Parkinsonism Relat Disord. 2006;12:302–8. doi: 10.1016/j.parkreldis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Sgado P, Alberi L, Gherbassi D, Galasso SL, Ramakers GM, Alavian KN, et al. Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice. Proc Natl Acad Sci USA. 2006;103:15242–7. doi: 10.1073/pnas.0602116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottman H, Dilber MS, Hovatta O. The derivation of clinical-grade human embryonic stem cell lines. FEBS Lett. 2006;580:2875–8. doi: 10.1016/j.febslet.2006.03.083. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–8. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Simantov R, Bjorklund L, Cooper O, Pruszak J, Kowalke F, et al. Context-dependent neuronal differentiation and germ layer induction of Smad4-/- and Cripto-/- embryonic stem cells. Mol Cell Neurosci. 2005;28:417–29. doi: 10.1016/j.mcn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol. 2005;23:601–6. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–9. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer M, Cesnulevicius K, Winkler C, Kolb J, Lipokatic-Takacs E, Jungnickel J, et al. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci. 2007;27:459–71. doi: 10.1523/JNEUROSCI.4493-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8:832–43. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Bockers T, Witter MP. Synaptic contacts between identified neurons visualized in the confocal laser scanning microscope. Neuroanatomical tracing combined with immunofluorescence detection of post-synaptic density proteins and target neuron-markers. J Neurosci Meth. 2003;128:129–42. doi: 10.1016/s0165-0270(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Wu H, Xu J, Pang ZP, Ge W, Kim KJ, Blanchi B, et al. Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc Natl Acad Sci USA. 2007;104:13821–6. doi: 10.1073/pnas.0706199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–38. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.