Abstract
Electrocorticographic (ECoG) spectral patterns obtained during language tasks from 12 epilepsy patients (age: 12–44 years) were analysed in order to identify and characterize cortical language areas. ECoG from 63 subdural electrodes (500 Hz/channel) chronically implanted over frontal, parietal and temporal lobes were examined. Two language tasks were performed. During the first language task, patients listened to a series of 50 words preceded by warning tones, and were asked to repeat each word. During a second memory task, subjects heard the 50 words from the first task randomly mixed with 50 new words and were asked to repeat the word only if it was a new word. Increases in ECoG gamma power (70–100 Hz) were observed in response to hearing tones (primary auditory cortex), hearing words (posterior temporal and parietal cortex) and repeating words (lateral frontal and anterior parietal cortex). These findings were compared to direct electrical stimulation and separate analysis of ECoG gamma changes during spontaneous inter-personal conversations. The results indicate that high-frequency ECoG reliably differentiates cortical areas associated with receptive and expressive speech processes for individual patients. Compared to listening to words, greater frontal lobe and decreased temporal lobe gamma activity was observed while speaking. The data support the concept of distributed functionally specific language modules interacting to serve receptive and expressive speech, with frontal lobe ‘corollary discharges’ suppressing low-level receptive cortical language areas in the temporal lobe during speaking.
Keywords: language mapping, cortical mapping, direct cortical stimulation, functional mapping, epilepsy surgery, electrocorticography, ECoG power
Introduction
Changes in the spectral composition of the electroencephalogram (EEG) as a function of cognition were noted in the initial description of EEG (Berger, 1929), with its potential for use for functional mapping of cortex suggested a short time later (Kornmüller, 1932). Studies during the interim years examined EEG correlates of cognition and laid the groundwork for utilizing spectral measures during language tasks to map cortical areas associated with speech and other cognitive functions in clinical settings (Chatrian et al., 1959; Ojemann et al., 1989a). Interest in higher frequency oscillatory activity originated with Freeman's observation that olfactory events were associated with localized gamma activity (∼40 Hz) in the rabbit olfactory bulb (Freeman, 1975). Task-related gamma band dynamics have been studied at the level of single-unit activity (Gray and McCormick, 1996), the ECoG (Murthy and Fetz, 1992; Edwards et al., 2005; Miller et al., 2007) and scalp recordings (Pfurtscheller and Aranibar, 1977). The degree to which high-frequency activity is associated with language processes has been increasingly studied (Crone et al., 1998; Aoki et al., 1999; Crone et al., 2001a, b; Xiang et al., 2001; Trautner et al., 2006; Crone et al., 2006).
Although non-invasive functional imaging has seen dramatic advances, direct electrical stimulation of cortex during language tasks remains the ‘gold standard’ for determining the location of eloquent cortex in surgical settings (Penfield and Boldrey, 1937; Penfield and Roberts, 1959; Ojemann et al., 1989b; Lesser et al., 1994). Although haemodynamic techniques have been used to locate cortical and subcortical language areas (Peterson et al., 1988; Mummery et al., 1999; Belin et al., 2000; Binder et al., 2000), several problems have precluded its utilization in the surgical suite: the lack of clear indexes of reliability and validity, difficulty testing children, discordance with other neurophysiological findings, and issues regarding accurate registration of the functional findings to the operative setting (Bookheimer et al., 1997; Sinai et al., 2005; Powell and Duncan, 2006). However, direct electrical stimulation of cortex has limitations as well: proper application is time-consuming, it is difficult to perform on children, direct stimulation may cause seizures and mapping areas related to higher cognitive function, such as receptive speech and the various forms of memory, is difficult (Boatman et al., 1995).
We report here the analysis of spectral changes in electrocorticographic (ECoG) patterns from adult and paediatric epileptic patients performing a simple language task. ECoG findings reveal that perisylvian frontal, parietal and temporal cortical areas differentially and dynamically express localized high-frequency gamma activity (70–100 Hz) while hearing, recognizing and speaking words. The present findings suggest that ECoG gamma measures may be helpful in identifying eloquent language areas.
Methods
Subjects
Twelve epilepsy patients were studied during invasive work-up for medically intractable seizures. Patient demographics, diagnoses, grid placement and outcome are listed in Table 1. One 10-year-old child was excluded because she could not perform the task. Patients were tested during stable interictal periods, after seizures had been recorded and characterized using ECoG video monitoring from 52 to 157 subdural electrodes chronically implanted over frontal, parietal and temporal lobes (10 or 7.5 mm spacing). Electrode arrays were placed according to the clinical needs of the patient. A gap between the frontal and parietal arrays occasionally precluded coverage of the motor-mouth region. Two patients received bilateral implants. The location of the arrays relative to cortical gyral anatomy was photographed both at implant and explant. None of the patients experienced a post-operative language deficit. Written consent was obtained from each patient or their parent, according to the direction of the institutional review board at the University of Chicago.
Table 1.
Patient demographics, diagnoses, electrode locations and surgical outcomes
| Patient | Age | Gender | Onset | Freq | Grids | Seizures | Dx | Path | Rx | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | F | Infancy | 3/day | Lf, Lt, Lp | t-C w/G | TS | Dysplasia | L f, L p, topect, VNS | Class III |
| 2 | 13 | F | 2 month | 4/day | Rf, Rt, Rp | CP | Stroke | Necrosis | R TLE, R Occ topect | Class I |
| 3 | 14 | F | 20 month | 3/month | Lf, Lp, Lt | CP w/G | L TLE | NF1 | L SAH | Class I |
| 4 | 14 | F | 6 years | 3/week | Lf, Lp, Lt | CP w/G | L TLE | Hip scleros | L TL | Class I |
| 5 | 15 | M | 10 years | 3/week | Lf, Lp, Lt | CP | L FTLE | Infarct | L f topect | Class IV |
| 6 | 16 | M | 6 years | 1/week | Bf, Lt, Bt | CP w/G | L TLE | Dysplasia | None | Class IV |
| 7 | 18 | M | 14 years | 2/year | Lf, Lp, Lt | CP w/G | L TLE | Gliosis | L t topect | Class I |
| 8 | 20 | F | 11 years | 2/day | Lf, Lp, Lt | CP w/G | L FLE | Heterotop | L f, L p topect, VNS | Class III |
| 9 | 21 | M | 1 years | 10/month | Lf, Lp, Lt | CP w/G | L FLE | None | VNS | Class III |
| 10 | 22 | F | 8 years | 2/month | Bf, Rp, Bt | CP | R TLE | Hip Scleros | R SAH | Class I |
| 11 | 30 | F | 9 years | 4/year | Lf, Lt | t-C w/G | L TLE | Atrophy | L SAH | Class I |
| 12 | 43 | F | 8 years | 4/day | Lf, Lp, Lt | CP | L FTLE | None | L SAH | Class II |
L = left; R = right; B = bilateral; f = frontal; p = parietal; t = temporal; CP = complex partial seizures; G = generalization; TLE = temporal lobe epilepsy; NF = neurofibromatosis; TS = tuberous sclerosis; SAH = selective amigdalohippocampectomy; topect = topectomy; TL = temporal lobectomy; VNS = vagal nerve stimulator.
Direct cortical stimulation
Eloquent cortex was identified using direct electrical stimulation at the bedside after an adequate number of seizures had been recorded. Trains of pulses lasting 2–10 s (biphasic pulses, 0.3 ms duration, 50 Hz, 2–10 mA) were systematically applied to pairs of electrodes in an attempt to elicit positive or negative functional signs or symptoms, without afterdischarges. Depending on the cortical location, behavioural or language tasks were employed to identify motor, sensory or language-related cortical areas (Lüders et al., 1988; Ojemann et al., 1989b).
Evoked potentials
Somatosensory evoked potentials (N/P20) were recorded from 64 channels (1 Hz–1 kHz bandpass) in response to contralateral median nerve stimulation (0.2 ms duration pulses at motor threshold, 5.7 Hz) to confirm the location of the primary somatosensory hand area within the central sulcus (Wood et al., 1988). Similarly, auditory evoked potentials (N/P100) (1–100 Hz bandpass) elicited by 800 Hz tones (250 ms duration) were used to locate primary auditory cortex within the Sylvian fissure (Celesia, 1976; Lee et al., 1984).
Language tasks
Two language tasks were performed during a 30 min recording session. For the first language task, patients listened to a series of 50 words preceded by warning tones, and were simply asked to repeat each word aloud. During the second language task, subjects heard the 50 words from the first task randomly mixed with 50 new words. They were asked to repeat only the new words. The warning tones (800 Hz) were followed 2 s later by presentation of one- or two-syllable common words that would be familiar to children (e.g. ‘rain’, ‘sweater’, ‘tighten’) spoken by a male voice presented through open-field speakers adjusted to a comfortable hearing level. They were common nouns, verbs or adjectives that were easy to pronounce. The Kucera–Francis word frequency count per 100 000 words for the word lists was 72 (145 SD) and the number of phonemes per word was 3.7 (0.9 SD). A practice trial of different words was rehearsed to familiarize the patient with the task. Each trial lasted 7 s, including 5 s to repeat the word. The trials were presented in the same voice and timing in both tasks. The computer stimuli and patient's voice were recorded with a microphone and processed along with the ECoG recordings. Stimuli were presented using the GENTASK function of the Compumedics STIM 2 system (Compumedics, Inc., El Paso, TX).
ECoG recordings
ECoG was recorded from 52 to 63 subdural electrodes (500 Hz/channel, 1–100 Hz bandpass, 12 dB/octave roll-off) located over the traditional expressive and receptive peri-Sylvian language areas of the lateral convexity of the frontal and parietal lobes, and temporal lobe language areas. A 64th channel recorded the acoustic impression of the tone and word stimuli and the patient's voice. This channel usually prevented recording the ECoG from the proximal end of one of the 1 × 8 strips. All ECoG channels were recorded referenced to Pz (Fpz ground), and then digitally converted to a grand mean reference. TTL triggers marked tone onset (t), word onset (w) and voice-onset time (vot), the last of which was placed manually by visual review of the microphone channel using the EDIT function of SYNAMPS (Compumedics, Inc., El Paso, TX).
Calculations of power
ECoG recordings were analysed by computing averaged power spectra for each of the 63 channels, after parsing the files into 512-point non-overlapping epochs. The frequency specificity of the event-related changes in ECoG power was assessed by subtracting the power during the 1 s epoch immediately preceding the trigger from the epoch immediately after the trigger. Visual inspection of these normally distributed samples revealed no effect at lower frequency bands. Power spectra were obtained at the midpoint of conventional EEG frequency bands [1.95 (delta), 5.86 (theta), 9.77 (alpha), 20.51 (beta), 40.04 (low gamma) and 84.96 Hz (high gamma)]. Changes in high gamma band power were computed for all 63 channels using averaged event-related band-pass filtering (85 Hz centre frequency, 15 Hz roll-off, 100-point smoothing, non-phase locked), computed across an 8 s interval (Thatcher et al., 1994; Andrew and Pfurtscheller, 1996).
Gamma activation during normal conversations
Over 100 h of video recordings of five patients were examined to identify times patients were conversing with family or staff. ECoG power spectra were calculated (0–200 Hz) while patients were engaged in conversation. Gamma power during non-talking periods was subtracted from talking periods to isolate the effect of conversing. These epochs were 1–2 min in duration. No reference was made to whether the patient was speaking or listening during the conversation.
Image visualization and co-registration
Radiological findings for the 12 patients are summarized in Table 2. Precise location of the implanted electrodes was determined from post-operative CT scans using pbrain, an open source application developed in our laboratory (http://neuroimaging.scipy.org/) (Hunter et al., 2005). Cortex segmentation was achieved with the open-source ITK-SNAP segmenting software (http://www.itksnap.org/) (Yushkevich et al., 2006). Co-registration of MRI and CT coordinate data were performed by selecting between 5 and 12 fiducial points common to the respective scans and solving for a transformation matrix using a local software implementation of the generalized solution to the least-squares ‘Procrustes’ problem (Schonemann and Carroll, 1970). Fiducial points included the tip of the nose, nasion, the centre of the globes, the pre-auricular fossae, the tympanic membranes, the centre of the foramen magnum, the optic chiasm, the cella and the inion. Co-registration allowed simultaneous visualization of the cortex, subdural electrodes and neurophysiologic recordings (Fig. 1). The final location of the ECoG electrodes on the cortex was verified by comparing their location with photographs taken during surgery. To visualize the spatial distribution of each patient's electrodes with respect to a single cortex, each subject's data were co-registered to a single subject's CT scan.
Table 2.
Radiological findings for the 12 patients
| Patient | Hand | MRI | Wada | PET |
|---|---|---|---|---|
| 1 | R | Tuberous scler. | No | No |
| 2 | R | R POT cyst | No | Reduced R PTO |
| 3 | R | MTS (L > R) | B language (L > R) | Reduced B T (L > R) |
| 4 | R | Normal | No | Reduced B T (L > R), LP |
| 5 | R | LT incr. signal | L language, L memory | Reduced L T |
| 6 | R | Normal | L language, R memory | Reduced L T |
| 7 | L | L F cyst | R language, B memory | Reduced L FTP |
| 8 | R | L F schizenceph. | No | Normal |
| 9 | R | Normal | No | Reduced B T |
| 10 | R | R T incr. signal | L language, B memory | Normal |
| 11 | R | Normal | L language, B memory | No |
| 12 | R | Normal | No | No |
L = left; R = right; B = bilateral; F = frontal; O = occipital; P = parietal; T = temporal; MTS = mesial temporal sclerosis.
Fig. 1.
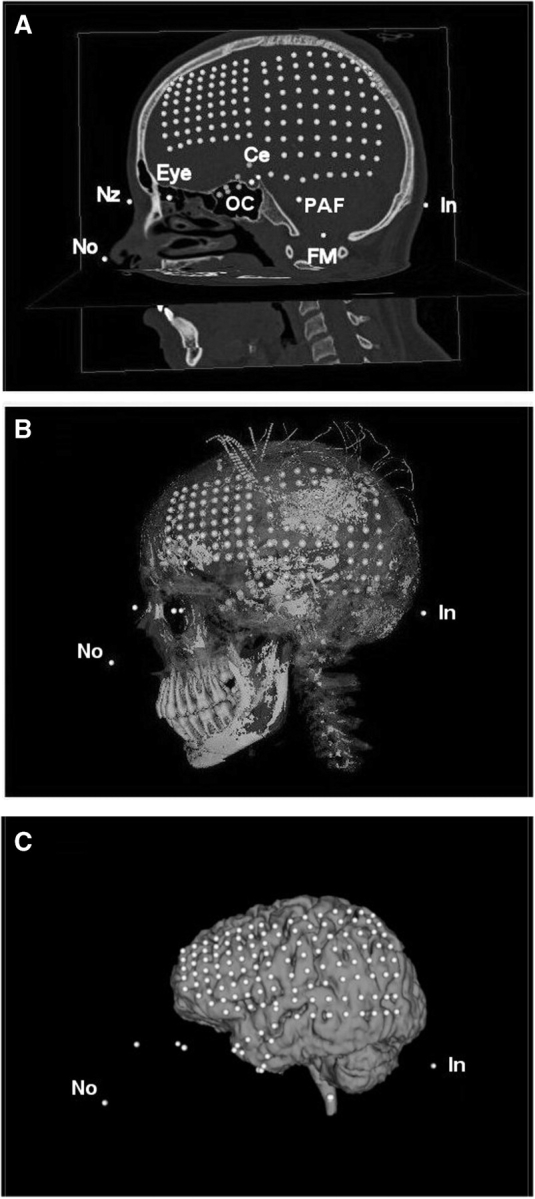
The identification and registration of electrodes and fiducial points on CT and MRI scans. (A) Electrodes as identified in CT image of head. Up to 12 fiducial points were identified and marked with a cursor: tip of nose (No), nasion (Nz), optic chiasm (OC), cella (Ce), pre-auricular fossae (PAF), centre of foramen magnum (FM), inion (In). (B) The same locations displayed in a 3D rendered image of the skull as imaged by CT. (C) The electrodes and fiducial points brought into registration with the 3D rendered brain from MRI using a Procrustes algorithm.
Statistical analyses
Mean power in each band was determined to be greater than zero using single-sample t-tests (all P < 0.05) (SPSS V. 14.0, SPSS, Chicago, IL). Comparison of talking and non-talking event-related gamma power was evaluated using conventional two-sample t-tests. The accuracy of the patient's verbal response during the memory task was assessed using ROC curves and signal detection analyses (Swets, 1979).
Results
Voice-onset-time
The latency of the patient's verbal response (voice-onset time) was determined by visual inspection of the microphone channel and calculated relative to the onset of the word stimuli. Typical reaction-time distributions were obtained (Fig. 2). The mean delay increased by 0.44 s for the memory task compared to the repetition task (1.29 ± 0.35 s versus 1.74 ± 0.60 s). During the repetition task, patients repeated almost all words correctly. For the memory task, in addition to having a delayed response, subject's mean correct response rate decreased from 99% for the simple repetition task, to 69% (57–88%). Subjects were able to correctly discriminate new words from old words with an average sensitivity of 0.78 and specificity of 0.56, with the accuracy of responses ranging from d′ = 1.5 to d′ = 0.26. Response bias for the memory task ranged widely from β = 0.01 to β = 5.9. Variations in the patient's performance were not related to the patient's age or clinical assessment of their memory abilities.
Fig. 2.
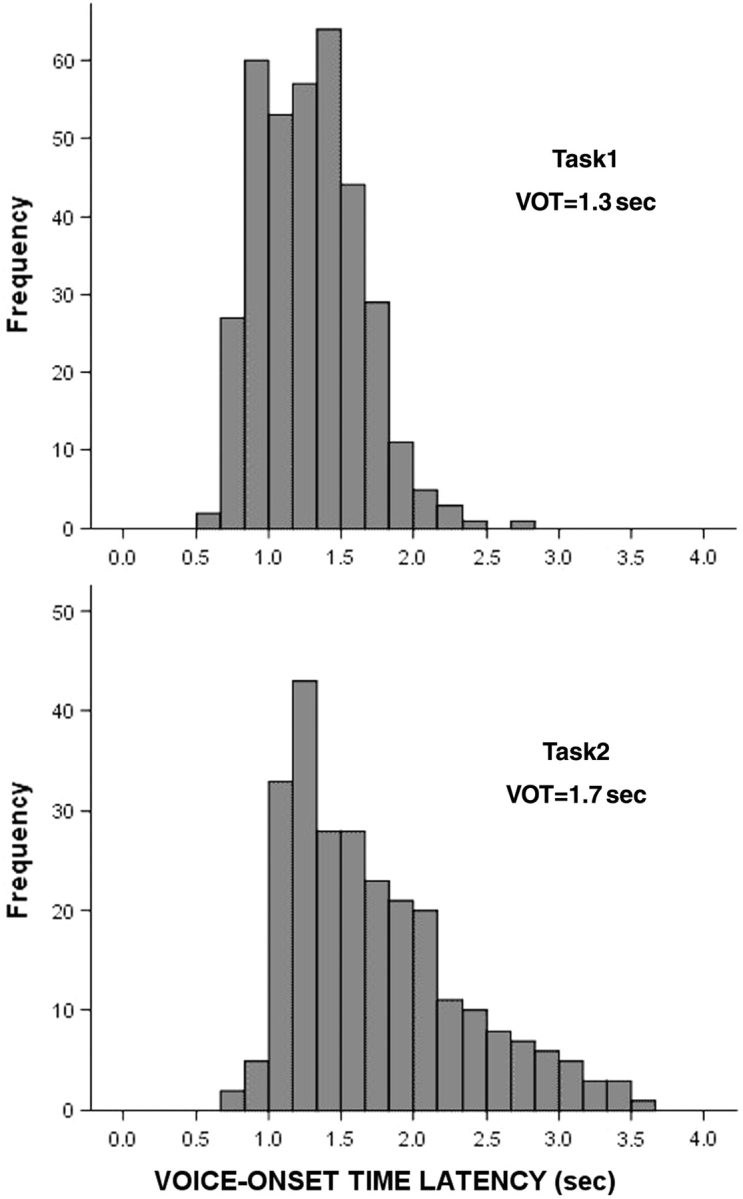
The distribution of voice-onset-time latency (vot) for all 12 patients for the simple repetition task (top) and the recognition memory task (bottom). Voice-onset-time was delayed and skewed to the right for the memory task. Time is calculated from the onset of the computer-presented word.
Sensory evoked potentials
The first step in obtaining functional maps of the patient's cortex was to establish the location of primary sensory and motor areas. The primary sensory hand area was identified by recording the average evoked response to contralateral median nerve stimulation. In all subjects, a high-amplitude N/P20 component was observed over the lateral convexity of the central region. The inversion identified the Rolandic Fissure. Localization of primary somatosensory cortex was confirmed via direct electrical stimulation to elicit paraesthesias or involuntary movements of the hand and face. Similarly, auditory cortex was located by analysis of the distribution of the early cortical auditory evoked potentials elicited by tones. For all patients with electrodes over the parietal–temporal junction, the inversion of polarity of the primary cortical response (N/P100) was located in the region of the posterior Sylvian fissure, consistent with the putative location of primary auditory cortex. The auditory 100 ms component peaked at a mean of 90 (±16) ms, with latencies not significantly different for tones or words. Location of the electrodes with the earliest auditory evoked response is shown in Fig. 3. Early auditory evoked potentials with separate cortical distributions were also recorded over frontal (8/10 patients) and parietal cortex (6/12 patients).
Fig. 3.
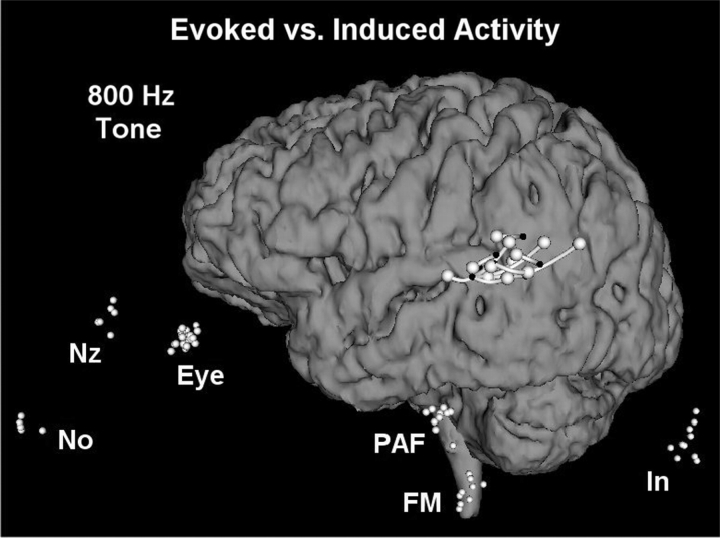
The location of the inversion of the initial component of the left hemisphere auditory evoked potential to tone stimulation for eight patients determined the location of primary auditory cortex (small black dots), compared to the location of tone-induced gamma activity (large white spheres). Pipes link data from individual patients. Small white dots indicate the location of each subject's fiducial points after registration with the fiducial points within the MRI from Patient 5.
ECoG power
Compared to baseline, power spectra from electrodes placed near the primary auditory cortex, computed 1 s after the triggers, were significantly enhanced in the high gamma band (70–100 Hz) for all 12 patients. Increases in ECoG gamma (power ratios greater than 1) were observed in response to hearing tones (primary auditory cortex) (t = 2.7, df = 11, P < 0.05), hearing words (usually posterior temporal cortex) (t = 1.2, df = 11, P < 0.001) and repeating words (usually lateral frontal cortex) (t = 1.2, df = 11, P < 0.001). Similar pre- to post-stimulus changes were not reliably observed in the lower frequency bands (Fig. 4).
Fig. 4.
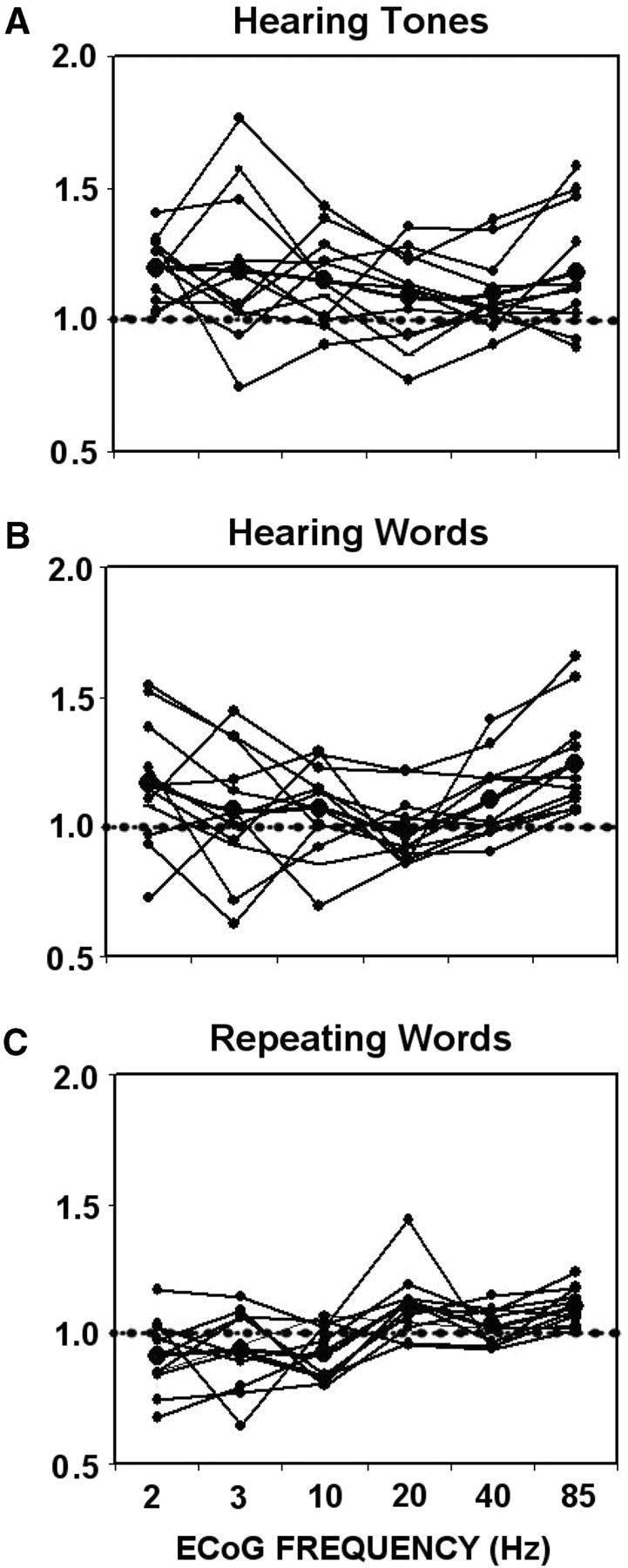
Ratios (post-stim/pre-stim) indicating changes in ECoG power in all six frequency bands caused by (A) hearing tones, (B) hearing words and (C) repeating words. The greatest enhancement was observed at 85 Hz for hearing and repeating words.
Gamma activity
As only gamma band changes were reliably observed, subsequent analyses were restricted to high gamma activity (70–100 Hz). Gamma activity was examined using the average event-related band power function of the Neuroscan EDIT module. Non-phase-locked gamma activity emitted during the event was calculated. The few channels that contained inter-ictal spiking, which contributed unpredictable artefacts into high-frequency bands, were removed. Event-related activation was clearly observed in approximately one-fifth of the electrodes (145/721). A comparison of the first 25 trials to the second 25 trials showed the distribution of gamma activity to be highly reliable (Fig. 5). For 8/12 patients with electrodes placed over the Sylvian fissure, 21/721 electrodes (3%) revealed activation to all three event types (Fig. 6, top functions). The majority of the 145 responding electrodes, however, showed selective activation. No electrodes sites were observed that responded only to tones. Increased gamma activity was observed over the posterior superior temporal gyrus for all 12 patients. Gamma responses to the tones were located immediately posterior to the early auditory evoked potential for 8/12 patients. Gamma responses were also activated by hearing words. Interestingly, 39/721 (5%) of electrodes with a widespread temporal lobe distribution exhibited gamma activation while hearing and speaking the words. These superior temporal gyrus areas were the same areas in which the early tone auditory evoked potentials were recorded for 8/12 patients. For two patients, hearing words caused increased gamma activity in the premotor hand area.
Fig. 5.
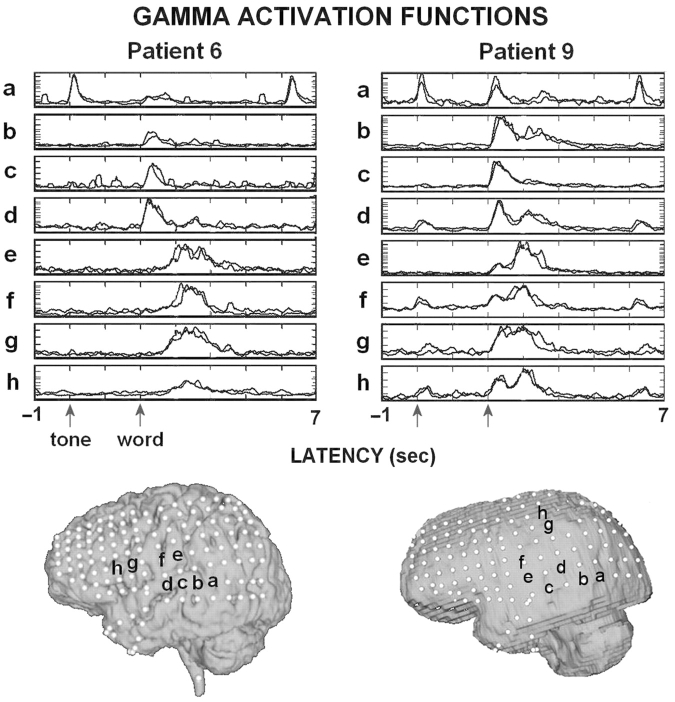
Gamma activations for eight selected channels for Patients 6 and 9 showing differential activation to hearing the tone, hearing the word and speaking the word. Two replications of 25 averaged trials are superimposed and normalized to reveal the reliability of the activations during Task 1. The location of the functions is indicated by a–h below. Similar reproducibility was observed for Task 2.
Fig. 6.
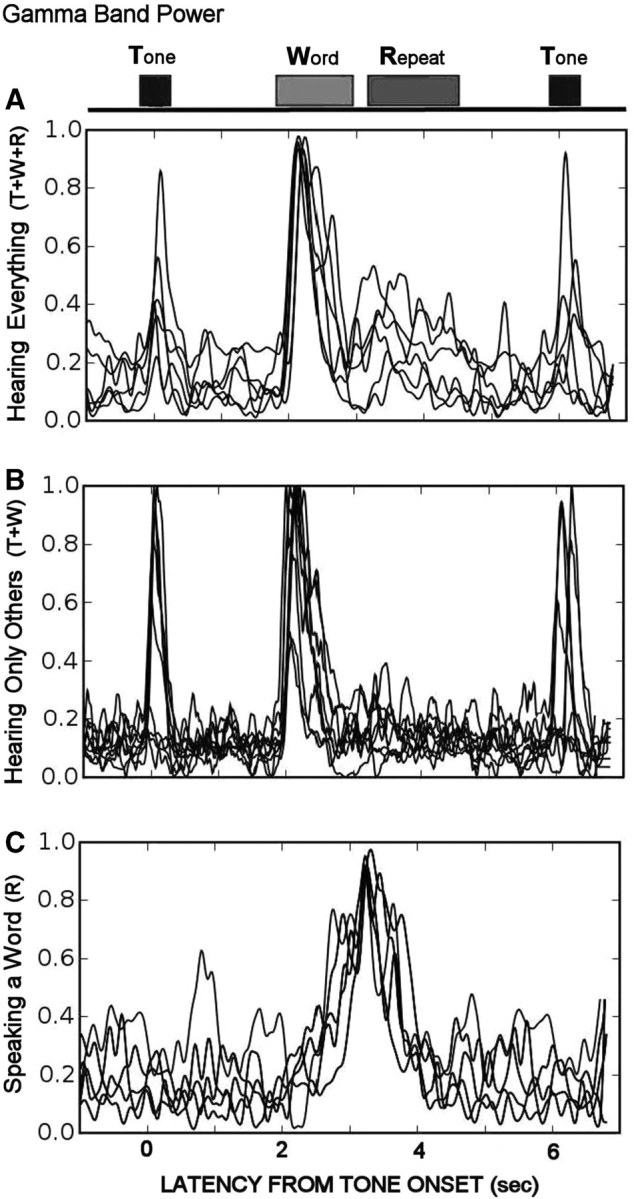
Changes in ECoG power in the high gamma band (70–100 Hz) for each event. Within each box, each tracing represents a different subject. (A) ECoG channels close to the primary auditory cortex showed increased gamma activity for all events. (B) ECoG channels located over primary auditory areas of the temporal lobe responded to all external events except the subject's own voice. (C) Frontal/parietal ECoG channels showed increased gamma activity only when the subjects said the words.
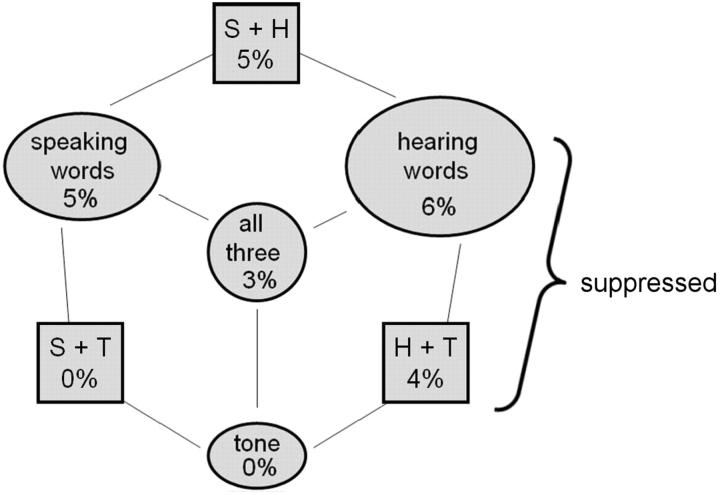
Forty-three of the 721 electrodes (6%) were activated only when the patients (11/12) repeated words (Fig. 6, bottom functions). These changes were located over frontal or parietal cortex, as well as the temporal lobe. In contrast, 42 of the electrodes located over the temporal lobe showed no activity while words were spoken by the patient, even though these sites showed increased activity after computer presentation of the words, apparently being suppressed during speaking (Fig. 6, middle functions). Seven patients showed a brief reduction in gamma activity over the most anterior dorsal lateral frontal cortex immediately before the word was presented, which was usually associated with an increase in power in the lower frequency bands, suggestive of transient ECoG slowing. Examples of the anterior shift in the distribution of gamma activity when the patients heard the word compared to when they spoke the word for three patients is shown in Fig. 7. Informal analyses regarding whether differences in gamma activation were related to the patient's age, or memory performance, or whether a specific word was remembered correctly in Task 2 or 1 (Dm) were inconclusive.
Fig. 7.
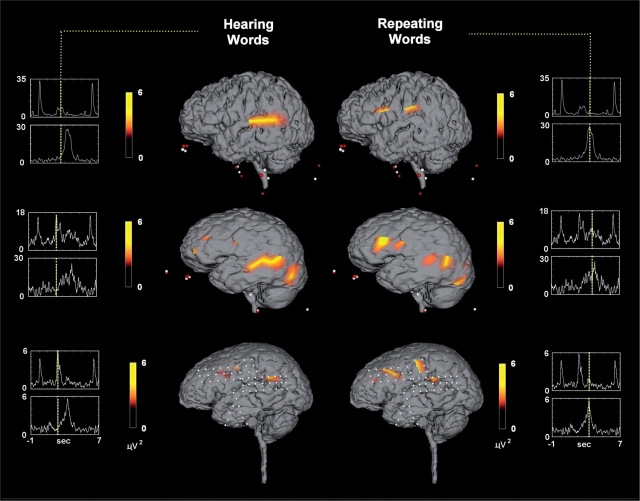
Distribution of gamma activity for Patients 3, 5 and 8 during the presentation of the words (left) and while the patient repeated the word (right). Greatest activation to hearing the words was over the posterior superior temporal gyrus. Activation over the frontal lobe was observed when the patient repeated the words. White dots = fiducial points.
Grouped data
Electrodes exhibiting task-specific gamma activations for all 12 subjects were registered and superimposed onto Patient 5's brain (Fig. 8). The accuracy with which the location of the electrodes identified in CT scans could be registered with the subject's MRI scans depends on the consistency with which the same fiducial points could be identified in the MRI and CT images. The discrepancy between the two scans is detailed in Table 3, including within-subject and between-subject registration accuracy. Because some fiducial points were difficult to identify in both scans, the two most discrepant fiducial points were omitted. Fiducial points behind the optic chiasm were always included to ensure that the points were widely spread throughout the field-of-view. This process yielded a registration accuracy of about 3 mm. Within-patient registration was further improved by comparing registered electrodes with photographs taken during surgery.
Fig. 8.
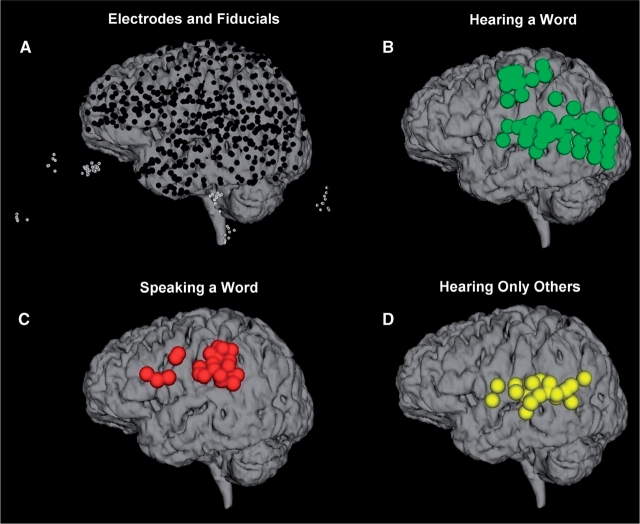
Findings from all patients are superimposed on the brain from Patient 5. (A) The locations of all of the electrodes visible over the left hemisphere (black dots) after registering them based on their fiducial points (white circles). (B) Widespread gamma activity was observed during the presentation of words (green). (C) When the patients repeated the words, activation was primarily observed over frontal and parietal cortex (red). (D) Electrodes that responded to hearing the words when presented by the computer, but not when the patient repeated the word (yellow).
Table 3.
Breakdown of the CT/MRI fiducial point registration discrepancy (mm)
| Within-subject |
Between-subjects |
|||
|---|---|---|---|---|
| mean | (max) | mean | (max) | |
| All possible fiducial points | 4.2 | (7.5) | 5.9 | (10.7) |
| Individual fiducial points | ||||
| Nose tip | 2.8 | (2.8) | 3.3 | (3.9) |
| Nasion | 3.7 (7.6) | 4.7 | (7.0) | |
| Globes | 3.0 | (5.9) | 7.0 | (12.2) |
| Optic chiasm | 2.5 | (4.4) | 3.4 | (4.4) |
| Cella | 3.8 (6.1) | 4.8 | (9.2) | |
| Tympanic membranes | ||||
| Pre-auricular fossae | 4.3 (6.0) | 7.0 | (11.2) | |
| Foramen magnum | 6.4 (8.3) | 8.1 | (12.1) | |
| Inion | 5.4 (9.3) | 8.2 | (10.8) | |
| Subset of best fiducial points | 3.1 | (5.0) | 3.2 | (4.8) |
Electrodes that exhibited gamma activation in response to tones were located over the superior temporal cortex, immediately below or posterior to the motor strip, and over posterior regions of the temporal lobe (the more posterior tone-related activations were also distributed more inferiorly). A greater number of electrodes exhibited gamma activation in response to words. These electrodes had a distribution similar to those responding to tones, but gamma activity was additionally observed over primary motor hand and face areas. Gamma activation while speaking the word was observed over sensory and motor face areas, as well as anteriorally over Broca's area. Recordings of 92 electrodes located over the right hemisphere of two subjects revealed similar findings.
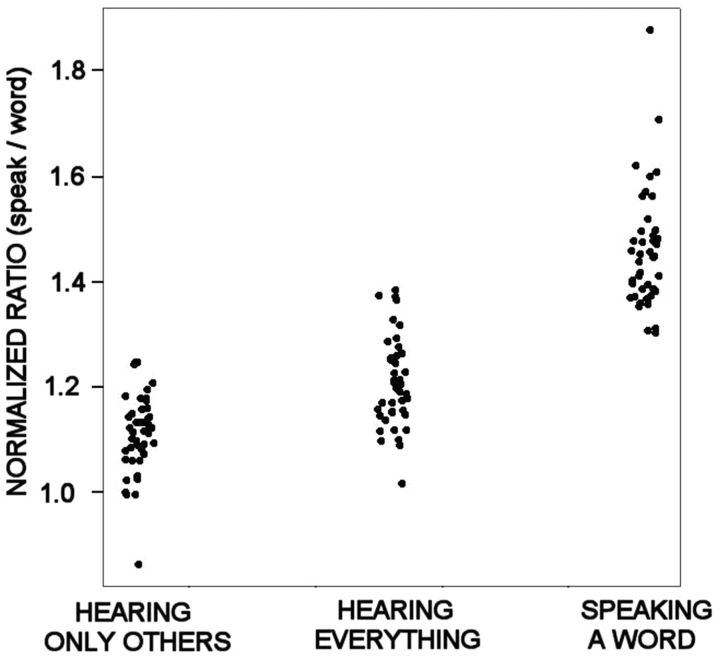
Gamma suppression
A quantification of the above categorizations for separating the activation functions into three categories is illustrated in Fig. 9. High gamma band power was measured while subjects listened to the word and while repeating the word. A comparison of the ratios of these measurements (power while repeating the word/power while hearing the word) is shown for each of the categorizations (a square-root transformation was utilized to create homogeneity of variance). A one-way analysis of variance with post hoc comparisons revealed that all three categorizations were significantly different from each other (F = 166, df = 2, 128, P < 0.001). The finding that the power ratio of the ‘hearing only-others’ category was significantly lower than the ‘hearing everything’ category supports the idea that the processing of one's own voice is suppressed.
Fig. 9.
Three primary patterns of activation were observed: Increased gamma activity only when the patient heard the word presented by the computer (superior temporal cortex), increased gamma activity to both the heard and spoken word (posterior temporal/parietal cortex), and increased gamma activity only during speaking the word (frontal/parietal cortex). These categories were confirmed by calculating a ratio of maximum power during speaking/power during hearing the word presented by the computer.
A comparison of peak gamma power emitted while hearing each voice for the 87 recordings containing responses to these events revealed that the mean amplitude ratio of the activation to one's own voice compared to that of others was 0.63, indicating a 37% attenuation of high gamma activity when hearing one's own voice (t = 9.2, df = 86, P < 0.001). The suppressed receptive areas were distributed more anterior than unsuppressed temporal lobe language areas (Fig. 8).
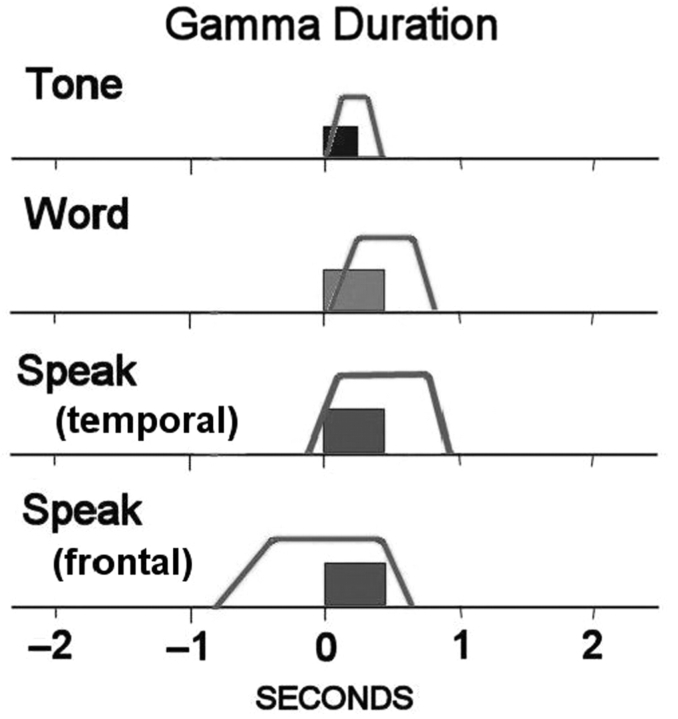
Gamma onset/offset
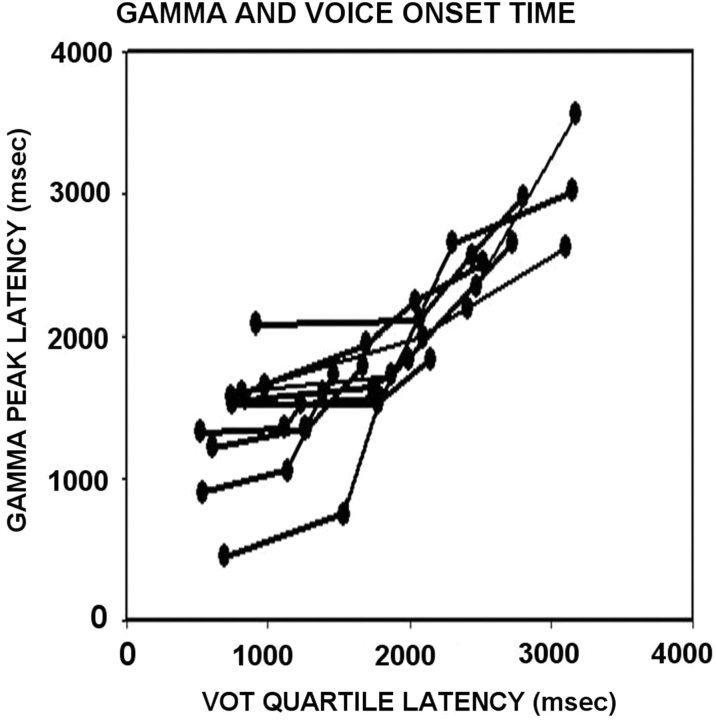
Although gamma activation was observed within a few milliseconds of the onset of the tone and word, on average, gamma activity lasted 211 ms longer than the tone stimulus, and 394 ms longer than words. Over the temporal lobe, gamma activity associated with speaking the word was similar, beginning near voice-onset-time and ending 537 ms after the word. However, over the frontal lobe, a different pattern was observed. Frontal gamma activity started 805 ms before voice-onset time, but like the tone, only extended 203 ms beyond the end of the spoken word (Fig. 10). If our own voice was unusually loud, one would expect that the temporal lobe gamma activity associated with hearing one's own voice to last longer than the acoustic event itself (Table 4). This was not the case. This suggests that gamma activity in response to one's voice is attenuated to be perceptually comparable to external auditory events. Frontal gamma activity not only preceded the spoken word, it covaried with it in a linear fashion. The temporal relationship between voice-onset time (VOT) and gamma activity associated with speech production is illustrated in Fig. 11. Trials were divided into quartiles and averaged in order to make the measurements. The peak of the gamma activity covaried with VOT for each subject (r2 = 0.56).
Fig. 10.
A schematic diagram of the mean onset and offset times of gamma activity relative to the task events. During the presentation of the tones and words, the onset of gamma activation was close to the onset of the stimulus, but lasted longer than the auditory event. When the patient repeated the word, the onset of gamma activation from electrodes over the temporal lob was similar to hearing the tone and word. Over the frontal lobe the onset of gamma activation associated with repeating the words started before voice-onset time, suggesting preparation to speak.
Table 4.
The duration of high-frequency ECoG activation relative to the acoustical event (ms)
| Acoustic event |
Gamma activity |
Total gamma duration |
|||
|---|---|---|---|---|---|
| Onset | Offset | Onset | Offset | ||
| External tone | 0 | 250 | 18 | 461 | 443 |
| External word | 0 | 450 | 52 | 844 | 792 |
| Spoken word (Temporal lobe) | 0 | 450 | −138 | 936 | 1074 |
| Spoken word (Frontal lobe) | 0 | 450 | −805 | 653 | 1451 |
Fig. 11.
Scatterplot of the temporal relationship between gamma activity and voice-onset time over the frontal lobe during the memory task for each patient. On trials with delayed voice-onset times the onset of the gamma activity was also delayed, relative to those trials with early voice-onset times. Activity was grouped into VOT quartiles (N = 25) in order to reliably measure the gamma parameters.
Clinical confirmation
Independent confirmation of these electrophysiologic findings was obtained. First, the gamma activations were compared to the results of direct electrical stimulation of cortex and the evoked potential maps. Of the sixty-four positive and negative signs and symptoms elicited by direct electrical stimulation and routing mapping studies, forty-four of them were associated with increased gamma activity, yielding a sensitivity of 63%. Specificity was 57%. A common type of confirmation was that an increase in gamma power during speaking the word was observed for electrodes in which electrical stimulation caused a disruption of speech (5/12 patients), often accompanied by motor signs or paraesthesias in the mouth. However, unambiguous increases in gamma activity were observed from 145 electrodes, many of which did not respond to electrical stimulation. The most frequent confirmation was that the early averaged auditory evoked potential was recorded from electrodes that exhibited increased gamma in response to hearing the tone (10/12 patients). For one patient, a left anterior temporal area was resected under 1/11 electrodes that exhibited a small increase in gamma activity in response to hearing the word. None of our patients had any documented post-operative language deficits. Overall, the discriminability of the gamma activations in terms of predicting the clinical maps was modest (d′ = 0.63), with a bias toward false alarms (C = –0.15). Our impression is that these numbers are deflated because of the limitations of our direct electrical stimulation protocol, in which bipolar stimulation is administered through adjacent pairs of electrodes, sometimes leaving uncertainty about which electrode was causing the symptoms. Also, the clinical language mapping tasks were not identical to the experimental task.
Gamma power during conversations
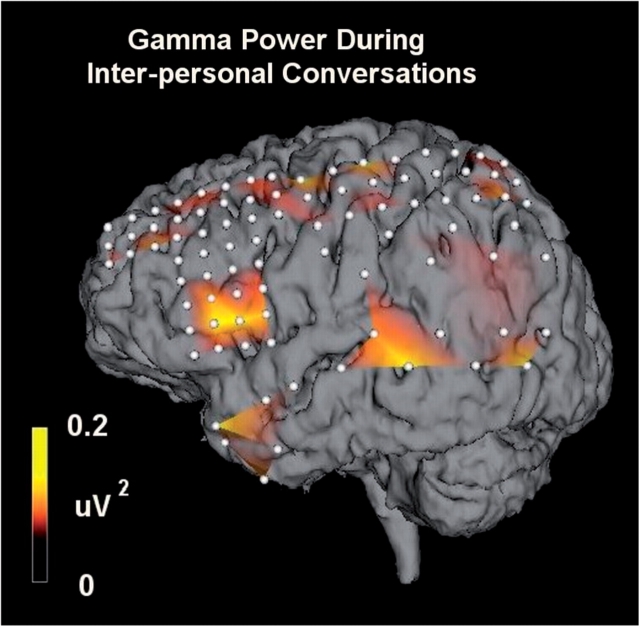
Similar language-related gamma changes in ECoG power spectra were observed when patients engaged in spontaneous conversation. Increased power in the high gamma band was maximal over the perisylvian frontal lobe and the posterior superior temporal gyrus, reminiscent of activation observed during the formal language tasks (depicted for Patient 5 in Fig. 12, and compare with Patient 5, Fig. 7). This was true for two of the five patients studied.
Fig. 12.
The distribution of increased high gamma activity during inter-personal conversations. Increased gamma activity was observed over the perisylvian frontal lobe and posterior superior temporal gyrus during periods when Patient 5 was involved in interpersonal conversations.
Discussion
Mapping ECoG activation in the high gamma band between 70 and 100 Hz reliably identified cortical areas associated with receptive and expressive speech processes in individual patients. Activation associated with hearing tones was primarily restricted to the posterior superior temporal gyrus, indicating activation of the primary auditory cortex as well as more posterior auditory areas. Activation associated with hearing words was more widespread, and included posterior superior temporal gyrus and lateral parietal regions, suggestive of activation in Wernicke's and surrounding areas (Wernicke, 1874). This activation included and extended beyond areas showing the primary auditory evoked response, and these areas and direct electrical stimulation of these areas usually produced no positive or negative language signs (Hashimoto et al., 2000). Whereas hearing words did not activate Broca's area, activation associated with repeating words was located in the perisylvian frontal and parietal cortex, consistent with activation of the sensory/motor mouth area and Broca's language area (Broca, 1865). Analysis of recordings obtained during spontaneous interpersonal conversations confirmed many of these findings, and revealed additional widespread areas of activation over motor cortex and over the anterior frontal lobe. The widespread activation likely reflects the fact that self-directed conversations involve more executive processes than the simple language tasks employed in the word-repetition task.
Present results confirm findings reported by Crone et al. (2001a, b) and others reporting increases in gamma band activity during language tasks. The gamma increases are broad-band, with activity observed between 40 Hz and our highest recorded frequency (100 Hz). Others, digitizing at rates higher than us, have reported activation reaching above 200 Hz (Hart et al., 1998). As increased gamma band power was reliably observed for all 12 patients, this technique may have value in assessing and planning resections for individual patients. These increases in power at high frequencies were also associated with reductions in power in lower frequency bands (Towle et al., 1995; Miller et al., 2007). The overall pattern of activation is consistent with the concept that language functions are mediated by a distributed network of modules that contribute to different aspects of expressive and receptive speech, located in the perisylvian frontal, parietal and temporal lobes (Goldman-Rakic, 1988; Mesulam, 1990; Bressler, 1995). When viewed over time in individual patients, the pattern of activity was often spatially non-contiguous, reminiscent of the mosaic pattern described by Ojemann et al. (1989b). Areas of activation much larger than a square centimeter were also observed. Consistent with Berger's original observations, Miller et al. (2007) report that increases in gamma activity are associated with reductions in power at lower frequencies. We have previously reported a suppression of frontal lobe alpha activity related to performing difficult language tasks (Towle et al., 1995), but did not analyse a possible increase in high-frequency activity in that study. In the present study we observed reductions in gamma power preceding voice-onset time, which in this word repetition task may reflect a brief relaxation of receptive speech-related processes and an initiation of expressive processes. Observed in the most anterior frontal electrodes, this reduction was associated with an increase in delta and theta power. Jokisch and Jensen (2007) interpreted similar task-related increases of alpha activity during a visual memory task as indicating inhibition of information processing. Freeman-like cortical gamma activity has been elicited in response to stimulation of the posterior intralaminar region of the ventral acoustic thalamus in the rat (Brett and Barth, 1997). Event-related gamma activity has also been reported in other cortical regions, such as in rat and monkey motor and somatosensory area (Murthy and Fetz, 1992; Jones and Barth, 1995; MacDonald and Barth, 1995), suggesting it is a general correlate of cortical information processing.
Investigations of the primate auditory system reveal multiple parallel pathways (Rauschecker, 1998a), each with different functional specialization. For example, primate vocalizations activate A1 superior temporal gyrus, whereas less complex sounds activate the caudomedial area (Rauschecker, 1998b). Similar functional specificity has been described in humans (Jasper and Penfield, 1949; Wise et al., 2001). It is clear from human PET and functional MRI studies that widely distributed cortical areas are involved while listening to words (Peterson et al., 1988; Bookheimer et al., 1997). Six distinct mirror-image tonotopically organized regions have been identified in human auditory cortex using functional MRI (Talavage et al., 2004). Casual observation might give the impression that superior temporal gyrus activation was posterior to primary auditory cortex. Cytoarchitectonic and morphometric investigations, however, reveal that the auditory cortex includes, in addition to Heschl's Gyrus and planum temporale (Brodmann, 1909), area A1 centred about 4 cm from the anterior tip of the temporal lobe, and STA extending almost 6 cm from the temporal pole (Rivier and Clarke, 1997). Auditory areas extend to the most posterior extent of the Sylvian Fissure (Zetzsche et al., 2001), to include the ascending posterior planum (Merlo et al., 1998) and even rostral into the parietal lobe (Galaburda and Sanides, 1980). Recent multimodal architectonic mapping reveals that auditory cortex (Te3) includes the lateral portion of the posterior temporal gyrus, and extends rostrally and caudally (Morosan et al., 2005). The auditory areas for which one's own voice was suppressed were at or immediately posterior to the early tone evoked potential, and anterior through most of the receptive speech processing areas. The suppression of one's own voice is likely due to frontal areas suppressing activity in cortical auditory areas such as area Te3.
The previous findings indicate that the human language system involves widespread areas of the cortex, and includes language modules that serve a variety of linguistic needs. In the clinical setting it is important to discriminate between areas essential and less essential for language. In the present study, a one-to-one association between areas showing increased gamma activity and language areas identified via cortical stimulation was not observed. These findings suggest that many of the areas where activity was observed are likely ‘boutique’ language processing centres, adding to the richness of the language experience, but in some circumstances, potentially resectable. Such areas have also been identified in functional MRI and PET studies of language. Disrupting these areas with electrical stimulation or even resecting these areas might not cause a deficit evident on casual clinical observation (although likely demonstrable with more detailed testing). It therefore seems reasonable to consider the location of these areas when planning how much of the anterior temporal lobe or peri-Sylvian areas to resect. Unfortunately, given that it was not possible to deviate from our standard clinical evaluation, the research language task and cortical stimulation findings could not be directly compared. Future studies using a common task are needed to better assess associations between gamma activity and essential and non-essential language areas.
An interesting problem of speech perception is the question of how one's own voice is processed. Do we perceive our own speech in the same way we monitor the speech of others? Why does not our own voice, generated so close to our ears, resonate louder than other voices and cause auditory desensitization? One mechanism to accomplish perceptual normalization is peripheral sensory gating. In humans, the stapedius reflex mechanically reduces sound transmission through the middle ear by 20–25 dB, and is activated just before one speaks. Similar reflexes exist in many sound-generating animals, including non-human primates (Müller-Preuss and Ploog, 1981; Eliades and Wang, 2003), birds (Coleman et al., 2007), bats (Suga and Schlegel, 1972), electric fish (Bell, 2001) and crickets (Poulet and Hedwig, 2003). Distal sensory gating tends to attenuate all auditory input. In addition to the previous mechanism, a second mechanism of ‘corollary discharge’ has been proposed, in which frontal areas regulate activity in superior temporal gyrus auditory regions (Creutzfeldt et al., 1989b; Houde et al., 2002; Heinks-Maldonado et al., 2005). For example, it has been hypothesized that a lack of frontal modulation of superior temporal gyrus activity underlies the failure in individuals with schizophrenia to recognize inner speech as self-generated, and may partially account for auditory hallucinations (Ford et al., 2002). Similar frontal lobe modulation of primary/secondary sensory areas is thought to account for the visual phenomenon of saccadic suppression (Ross et al., 2001) and gaze stabilization during movement (Cullen and Roy, 2004), to explain why one cannot tickle oneself (Blakemore et al., 2000), and to explain why ‘biting the bullet’ suppresses pain (Le Pera et al., 2007). Our findings support a similar mechanism during speaking.
Although perception of our own voice is seemingly desirable, allowing us to monitor and modulate its intensity and prosody, portions of the receptive language system in the superior temporal lobe are deactivated when we speak, a process that may help us to differentiate our voice from others, as well as to speak and listen at the same time. Suppression during speaking was observed for 10% of the implanted electrodes (Fig. 13). Creutzfeldt et al. (1989b) reported activation and suppression of single-unit spiking in response to hearing one's own voice. Their recordings from the superior temporal gyrus mainly revealed activation of spiking activity. However, in the middle temporal gyrus they encountered roughly equal amounts of activation and suppression of single unit spiking during speaking, implying the activation of selective gating or inhibitory processes (Trautner et al., 2006). The finding that processing of one's own voice tended to be suppressed in more anterior voice processing regions near primary auditory cortex suggests that the suppression, likely coming from frontal cortex, acts on relatively low level cortical auditory areas. Similarly, input from other sensory modalities is thought to be integrated with visual processing at low cortical levels (Schroeder and Foxe, 2005).
Fig. 13.
The proportion of electrodes that exhibited each of the seven possible different patterns of activation (S = speaking, H = hearing, T = tone). Gamma activity was suppressed for 10% of the electrodes during speaking.
We were unable to reliably demonstrate increased lateral coherence or phase synchrony between activated areas in the gamma band (Gray et al., 1989; Shen et al., 1999; Weiss and Mueller, 2003). We also did not see a tendency for greater gamma activity related to words that contained coarse fricative phonemes (Creutzfeldt et al., 1989a). We did not observe reliable activation of Broca's area while hearing words (Xiang et al., 2001; Matsumoto et al., 2004). We were unable to support the role of the medial temporal lobe in distinguishing new versus old words (Daselaar et al., 2006), perhaps to limited electrode coverage in that region.
A major limitation of this study is the constraint on the number of electrodes from which we could record. Recordings were only obtained from about half of the implanted electrodes. This somewhat sparse spatial sampling of the cortex probably explains why not all phenomena were observed for all patients. Cortical areas specifically related to recognition memory during the second task were not evident in these recordings, perhaps due to the sparse coverage of medial temporal regions. However, a similar failure to observe changes in high gamma activity in the basal temporal region during an auditory new word/old word discrimination task was reported by Tanji et al. (2005), even though they found clear gamma activations in this region for visual character reading tasks. Our similar findings reinforce the concept that the superior temporal gyrus is devoted to auditory processing, but more inferior regions are dedicated to visual processing. Although we only had a limited number of electrodes placed over the right hemisphere, we did not detect any hemispheric difference. Similarly, we did not observe any systematic differences related to gender or patient age.
The functional analyses described in this study do not require that the patient undergo an MRI or CT scan. This is an advantage for younger patients, who may not be able to tolerate awake scanning, and reduces the complexity and cost of this type of work-up. Although many of the electrophysiological phenomena were observed to arise under a few adjacent electrodes, some patterns followed the course of a gyrus or were unique to a single electrode, suggesting that volume conduction was not a major issue. Because the width of cortical gyri is about 1 cm, a more desirable inter-electrode spacing would be on the order of 5 mm. Although closer spacing of electrodes is possible, the need for greater spatial resolution must be balanced with practical constraints of chronic wires safely exiting the scalp.
Because our findings indicate that expressive and receptive speech areas may be identified from the analysis of passive ECoG recordings, direct electrical stimulation of cortex may potentially be relegated to a more abbreviated confirmatory role in functional mapping. The finding that similar patterns of activation were observed during language tasks and during spontaneous conversation increases our confidence in the external validity of these findings. More extensive analysis of spontaneous conversations might ultimately eliminate the need of formal language tasks. Such innovations, if validated, could shorten and reduce the risk, discomfort and staffing necessary to manage the invasive epilepsy work-up.
Our capacity to simultaneously record from only about half (721/1486) of the total number of implanted electrodes suggests that our findings may underestimate the total area of superficial cortex activated by speech. We preferentially chose to record from electrodes near traditional speech areas and also excluded 24 electrodes with frequent inter-ictal spiking, which tended to contaminate the gamma band. In spite of these limitations, the data support the concept of widely distributed and functionally specific language modules dynamically interacting to serve receptive and expressive speech. The findings also indicate that widespread areas of the temporal lobe are suppressed when a person speaks. As increased gamma band power was reliably observed for all 12 subjects, this technique may have clinical value in assessing and planning surgical resections for individual patients. Recent advances in our understanding of the anatomical basis of speech challenge the classical description of language processes, in which Broca's Area in the left frontal lobe exclusively serves expressive speech (Broca, 1865), and Wernicke's Area in the left posterior temporal lobe mediates receptive speech (Wernicke, 1874; Wise et al., 2001). Using non-invasive haemodynamic functional imaging techniques, activity in additional brain regions is observed when performing various speech processes (Peterson et al., 1988; Price et al., 1996; Bookheimer et al., 1997; Belin et al., 2000). These brain regions extend beyond those identified via the classical technique of applying direct electrical stimulation to the cortex to disrupt normal language function (Penfield and Roberts, 1959; Ojemann et al., 1989a; Lesser et al., 1994; Boatman et al., 1995). Although multiple language areas have been identified using haemodynamic imaging techniques, the slow time course of the haemodynamic response makes it difficult to determine how brain regions differently respond to constantly changing linguistic demands. Neural activity within cortical regions occurs on a millisecond time scale, making some brain processes associated with cognition better explored by examining the electrophysiological signals associated with neural activity. The origin of the alleged corollary discharges escapes the present analysis, but may become evident from analysis of cross-correlations, coherence or phase synchrony relationships between recorded channels.
Acknowledgements
This study was supported in part by NIH 5 R01 NS40514, The Brain Research Foundation and the Susman and Asher Foundation. We are also grateful to Daniel Choi, Simone Davion, Diana Hanan, Gioconda Mojica, Jeff Wang, Brent Parris and Anna Poon, who worked on various aspects of this study.
References
- Andrew C, Pfurtscheller G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol. 1996;98:144–8. doi: 10.1016/0013-4694(95)00228-6. [DOI] [PubMed] [Google Scholar]
- Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA. Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor tasks. Clin Neurophysiol. 1999;110:524–37. doi: 10.1016/s1388-2457(98)00064-9. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RF, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–12. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Bell CC. Memory-based expectations in electrosensory systems. Curr Opin Neurobiol. 2001;11:481–7. doi: 10.1016/s0959-4388(00)00238-5. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektroencephelogram des Menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–28. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert D, Frith C. Why can't you tickle yourself? NeuroReport. 2000;11:R11–6. doi: 10.1097/00001756-200008030-00002. [DOI] [PubMed] [Google Scholar]
- Boatman D, Lesser RP, Gordon B. Auditory speech processing in the left temporal lobe: an electrical interference study. Brain Lang. 1995;51:269–90. doi: 10.1006/brln.1995.1061. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Malow BA, Gaillard WD, Sato S, et al. A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology. 1997;48:1056–65. doi: 10.1212/wnl.48.4.1056. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Brett B, Barth DS. Subcortical modulation of high-frequency (gamma-band) oscillating potentials in auditory cortex. J Neurophysiol. 1997;78:573–81. doi: 10.1152/jn.1997.78.2.573. [DOI] [PubMed] [Google Scholar]
- Broca P. Sur le siège de la faculté du langage articulé. Bull Soc Anthropol. 1865;6:377–93. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig: 1909. [Google Scholar]
- Celesia GG. Organization of auditory cortical areas in man. Brain. 1976;99:403–14. doi: 10.1093/brain/99.3.403. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Petersen MC, Lazarte JA. The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalogr Clin Neurophysiol. 1959;11:497–510. doi: 10.1016/0013-4694(59)90048-3. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci. 2007;27:10024–36. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, Lettich E. Neuronal activity in the human lateral temporal lobe. I. Response to speech. Exp Brain Res. 1989a;77:451–75. doi: 10.1007/BF00249600. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, Lettich E. Neuronal activity in the human lateral temporal lobe. II. Responses to the subjects own voice. Exp Brain Res. 1989b;77:476–89. doi: 10.1007/BF00249601. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol. 2001a;112:565–82. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Hao L, Hart J, Jr, Boatman D, Lesser RP, Irizarry R, et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001b;57:2045–53. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–95. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol. 2004;91:1919–33. doi: 10.1152/jn.00988.2003. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Prince SE, Cabeza R. The medial temporal lobe distinguishes old from new independently of consciousness. J Neurosci. 2006;26:5835–9. doi: 10.1523/JNEUROSCI.0258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–80. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol. 2003;89:2194–207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–92. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Mass action in the nervous system. New York: Academic Press; 1975. [Google Scholar]
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Topography of cognition: Parallel distributed networks in primate association cortex. Ann Rev Neurosci. 1988;11:137–56. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–7. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–13. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Crone NE, Lesser RP, Sieracki J, Miglioretti DL, Hall C, et al. Temporal dynamics of verbal object comprehension. Proc Natl Acad Sci. 1998;95:6498–503. doi: 10.1073/pnas.95.11.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Homae F, Nakajima K, Miyashita Y, Sakai KL. Functional differentiation in the human auditory and language areas revealed by a dichotic listening task. NeuroImage. 2000;12:147–58. doi: 10.1006/nimg.2000.0603. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM. Fine-tuning of auditory cortex during speech production. Psychophysiol. 2005;42:180–90. doi: 10.1111/j.1469-8986.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: an MEG study. J Cogn Neurosci. 2002;14:1125–38. doi: 10.1162/089892902760807140. [DOI] [PubMed] [Google Scholar]
- Hunter JD, Hanan DM, Singer BF, Shaikh S, Brubaker KA, Hecox KE, et al. Locating chronically implanted electrodes using surface reconstruction. Clin Neurophysiol. 2005;116:1984–7. doi: 10.1016/j.clinph.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Jasper H, Penfield W. Electrocorticograms in man: effect of voluntary movement upon the electrical activity of the precentral gyrus. Psychiat Zeitschrift Neurol. 1949;183:163–74. [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–51. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Sensory-evoked high-frequency (gamma-band) oscillating potentials in somatosensory cortex of the unanesthetized rat. Brain Res. 1997;768:167–76. doi: 10.1016/s0006-8993(97)00639-2. [DOI] [PubMed] [Google Scholar]
- Kornmüller AE. Architektonische lokalisation bioelektrischer erscheinungen auf der grosshirnrinde: Untersuchungen am Kaninchen bei Augenbelichtung. Psychiatneurol Wschr. 1932;44:447–59. [Google Scholar]
- Lee YS, Lueders H, Dinner DS, Lesser RP, Hahn J, Klem G. Recording of auditory evoked potentials in man using chronic subdural electrodes. Brain. 1984;107:115–31. doi: 10.1093/brain/107.1.115. [DOI] [PubMed] [Google Scholar]
- Le Pera D, Brancucci A, De Armas L, Del Percio C, Miliucci R, Babiloni C, et al. Inhibitory effect of voluntary movement preparation on cutaneous heat pain and laser-evoked potentials. Eur J Neurosci. 2007;25:1900–7. doi: 10.1111/j.1460-9568.2007.05389.x. [DOI] [PubMed] [Google Scholar]
- Lesser R, Gordon B, Uematsu S. Electrical stimulation and language. J Clin Neurophysiol. 1994;11:191–204. doi: 10.1097/00004691-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J, Lüders H. Localization of cortical function—new information from extraoperative monitoring of patients with epilepsy. Epilepsia. 1988;29:S56–65. doi: 10.1111/j.1528-1157.1988.tb05799.x. [DOI] [PubMed] [Google Scholar]
- MacDonald KD, Barth DS. High frequency (gamma-band) oscillating potentials in rat somatosensory and auditory cortex. Brain Res. 1995;694:1–12. doi: 10.1016/0006-8993(95)00662-a. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Merlo A, Albanese E, Gomez E, Mir J, Mascitti T, Albanese A. Correlation between cortical surfaces and their asymmetries in postmortem human brains. Revista Chilena de Anatom. 1998;16:169–76. [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RPN, Anderson NR, Moran DW, et al. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27:2424–32. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosan P, Schleicher A, Amunts K, Zilles K. Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol. 2005;210:401–6. doi: 10.1007/s00429-005-0029-1. [DOI] [PubMed] [Google Scholar]
- Müller-Preuss P, Ploog D. Inhibition of auditory cortical neurons during phonation. Brain Res. 1981;215:61–76. doi: 10.1016/0006-8993(81)90491-1. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Scott SK, Ashburner J, Wise RJS. Functional neuroimaging of speech perception in six normal and two aphasic patients. J Acoust Soc Am. 1999;106:449–57. doi: 10.1121/1.427068. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89:5670–4. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Fried I, Lettich E. Electrocorticographic (ECoG) correlates of language. I. Desynchronization in temporal language cortex during object naming. Electroencephalogr Clin Neurophysiol. 1989a;73:453–63. doi: 10.1016/0013-4694(89)90095-3. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989b;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield W, Roberts L. Speech and brain-mechanisms. Princeton: Princeton University Press; 1959. [Google Scholar]
- Peterson SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–9. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;42:817–26. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Poulet JFA, Hedwig B. A corollary discharge mechanism modulates central auditory processing in singing crickets. J Neurophysiol (Bethesda) 2003;89:1528–40. doi: 10.1152/jn.0846.2002. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Duncan JS. Functional magnetic resonance imaging for assessment of language and memory in clinical practice. Curr Opin Neurol. 2005;18:161–6. doi: 10.1097/01.wco.0000162858.60144.ca. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Warburton EA, Moore CJ, Howard D, Patterson K, et al. A functional neuroimaging description of two deep dyslexic patients. Brain. 1996;119:919–31. [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neuro-Otol. 1998a;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998b;8:516–21. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Rivier F, Clarke S. Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: evidence for multiple auditory areas. NeuroImage. 1997;6:288–304. doi: 10.1006/nimg.1997.0304. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci. 2001;24:113–21. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- Schönemann PH, Carroll RM. Fitting one matrix to another under choice of a central dilation and a rigid motion. Psychometrika. 1970;35:245–55. [Google Scholar]
- Schroeder CE, Foxe J. Multisensory contributions to low-level, ‘unisensory' processing. Curr Opin Neurobiol. 2005;15:454–8. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Shen B, Nadkarni M, Zappulla RA. Spectral modulation of cortical connections measured by EEG coherence in humans. Clin Neurophysiol. 1999;110:115–25. doi: 10.1016/s0013-4694(98)00104-7. [DOI] [PubMed] [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–70. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- Suga N, Schlegel P. Neural attenuation of responses to emitted sounds in echo locating bats. Science. 1972;177:82–4. doi: 10.1126/science.177.4043.82. [DOI] [PubMed] [Google Scholar]
- Swets JA. ROC analysis applied to the evaluation of medical imaging techniques. Invest Radiol. 1979;14:109–21. doi: 10.1097/00004424-197903000-00002. [DOI] [PubMed] [Google Scholar]
- Talavage TM, Sereno MI, Melcher JR, Ledden PJ, Rosen BR, Dale AM. Tonotopic organization in human auditory cortex revealed by progressions of frequency sensitivity. J Neurophysiol. 2004;91:1282–96. doi: 10.1152/jn.01125.2002. [DOI] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency γ-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–93. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW, Toro C, Pflieger ME, Hallett M. Human network dynamics using multimodal registration of EEG, PET, and MRI. In: Thatcher RW, Hallett M, Zeffiro T, John ER, Juerta M, editors. Functional neuroimaging: Technical foundations. San Diego, New York: Academic Press; 1994. pp. 269–78. [Google Scholar]
- Towle VL, Cohen S, Alperin N, Hoffmann K, Cogen P, Milton J, et al. Displaying electrocorticographic findings on gyral anatomy. Electroencephalogr Clin Neurophysiol. 1995;94:221–8. doi: 10.1016/0013-4694(95)98474-m. [DOI] [PubMed] [Google Scholar]
- Trautner P, Rosburg T, Dietl T, Fell J, Korzyukov OA, Kurthen M, et al. Sensory gating of auditory evoked and induced gamma band activity in intracranial recordings. NeuroImage. 2006;32:790–8. doi: 10.1016/j.neuroimage.2006.04.203. [DOI] [PubMed] [Google Scholar]
- Weiss S, Mueller HM. The contribution of EEG coherence to the investigation of language. Brain Lang. 2003;85:325–43. doi: 10.1016/s0093-934x(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der Aphasische Symtmenkomplex. Eine Psychologische Studie auf Anatomischer Basis. Breslau: M Cohn und Weigart; 1874. [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke's area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Wood CC, Spencer DD, Allison T, McCarthy G, Williamson PD, Goff WR. Localization of human sensorimotor cortex during surgery by cortical surface recording of somatosensory evoked potentials. J Neurosurg. 1988;68:99–111. doi: 10.3171/jns.1988.68.1.0099. [DOI] [PubMed] [Google Scholar]
- Xiang J, Wilson D, Otsubo H, Ishii R, Chuang S. Neuromagnetic spectral distribution of implicit processing of words. NeuroReport. 2001;12:1–5. doi: 10.1097/00001756-200112210-00014. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Meisenzahl EM, Preuss UW, Holder JJ, Kathmann N, Leinsinger G, et al. In-vivo analysis of the human planum temporale (PT): does the definition of PT borders influence the results with regard to cerebral asymmetry and correlation with handedness? Psychiat Res: Neuroimag Sect. 2001;107:99–115. doi: 10.1016/s0925-4927(01)00087-7. [DOI] [PubMed] [Google Scholar]