Abstract
Ventricular enlargement may be an objective and sensitive measure of neuropathological change associated with mild cognitive impairment (MCI) and Alzheimer's disease (AD), suitable to assess disease progression for multi-centre studies. This study compared (i) ventricular enlargement after six months in subjects with MCI, AD and normal elderly controls (NEC) in a multi-centre study, (ii) volumetric and cognitive changes between Apolipoprotein E genotypes, (iii) ventricular enlargement in subjects who progressed from MCI to AD, and (iv) sample sizes for multi-centre MCI and AD studies based on measures of ventricular enlargement. Three dimensional T1-weighted MRI and cognitive measures were acquired from 504 subjects (NEC n = 152, MCI n = 247 and AD n = 105) participating in the multi-centre Alzheimer's Disease Neuroimaging Initiative. Cerebral ventricular volume was quantified at baseline and after six months using semi-automated software. For the primary analysis of ventricle and neurocognitive measures, between group differences were evaluated using an analysis of covariance, and repeated measures t-tests were used for within group comparisons. For secondary analyses, all groups were dichotomized for Apolipoprotein E genotype based on the presence of an ε4 polymorphism. In addition, the MCI group was dichotomized into those individuals who progressed to a clinical diagnosis of AD, and those subjects that remained stable with MCI after six months. Group differences on neurocognitive and ventricle measures were evaluated by independent t-tests. General sample size calculations were computed for all groups derived from ventricle measurements and neurocognitive scores. The AD group had greater ventricular enlargement compared to both subjects with MCI (P = 0.0004) and NEC (P < 0.0001), and subjects with MCI had a greater rate of ventricular enlargement compared to NEC (P = 0.0001). MCI subjects that progressed to clinical AD after six months had greater ventricular enlargement than stable MCI subjects (P = 0.0270). Ventricular enlargement was different between Apolipoprotein E genotypes within the AD group (P = 0.010). The number of subjects required to demonstrate a 20% change in ventricular enlargement was substantially lower than that required to demonstrate a 20% change in cognitive scores. Ventricular enlargement represents a feasible short-term marker of disease progression in subjects with MCI and subjects with AD for multi-centre studies.
Introduction
Brain tissue atrophy rates measured on serial magnetic resonance imaging (MRI) scans may provide an objective and quantitative method to examine neuropathological changes associated with mild cognitive impairment (MCI) and Alzheimer's disease (AD) (Fox et al., 2000; Bradley et al., 2002; Wang et al., 2002; Jack et al., 2004; Schott et al., 2005). Serial MRI techniques that measure neurodegeneration principally focus on volumetric analysis of the hippocampus (Jack et al., 1997; Leinsinger et al., 2003; Jack et al., 2004; Devanand et al., 2007), whole brain (Fox and Freeborough, 1997; Fox et al., 2000; Smith et al., 2002; Jack et al., 2004; Schott et al., 2005), and ventricles (Bradley et al., 2002; Wang et al., 2002; Silbert et al., 2003; Jack et al., 2004; Thompson et al., 2004; Schott et al., 2005; Giesel et al., 2006; Carmichael et al., 2007; Fleisher et al., 2008). Hippocampal volumetric analysis typically involves manual or semi-manual tracing techniques (Giesel et al., 2006) that require a significant amount of time and interaction from experienced operators, increasing costs and decreasing reproducibility. Conversely, measurement of cerebral ventricular volume is amenable to robust automatic segmentation due to the sharp contrast between the signal intensity of cerebral spinal fluid (CSF) in the ventricles and surrounding tissue in T1-weighted MRI images. Moreover, the position of the ventricles near the centre of the brain places this structure near the magnet isocentre. As a result, geometric distortions across the ventricle due to gradient non-linearities are minimized.
The use of cerebral ventricular volume as a measure of AD progression is supported by several studies. Hemispheric atrophy rates, measured by ventricular enlargement, correlate more strongly with changes on cognitive tests than medial temporal lobe (MTL) atrophy rates (Jack et al., 2004), and capture significant variation between NEC and subjects with MCI, and AD (Bradley et al., 2002; Jack et al., 2005; Schott et al., 2005). This sensitivity occurs, in part, because portions of the lateral ventricles are adjacent to MTL structures that atrophy notably in the preclinical stages of dementia (Ferrarini et al., 2006; Giesel et al., 2006). The rate of ventricular volume change is also highly correlated with an increase in senile plaques and neurofibrillary tangles (Silbert et al., 2003). Previously reported sample sizes required to detect meaningful reductions from the expected rate of annual change were markedly lower when using lateral ventricular volumes compared to psychometric, MTL and whole brain MR measurements (Fox et al., 2000; Jack et al., 2004; Schott et al., 2005). Anatomical measurements such as ventricle volume are likely to provide complementary information to neurocognitive testing, and provide insight into the mechanisms of disease modifying therapies. It may be possible to use such measures to select subjects likely to respond to specific disease-modifying therapies and subsequently assess the biological efficacy of these treatments.
The allele ε4 of the apolipoprotien ε (APOE) gene has been well established as a primary risk factor for AD, and APOE has previously been associated with increased neurofibrillary tangles, increased plaque burden (Schmechel et al., 1993), and cognitive decline (Bizzarro et al., 2005; Blesa et al., 2006). In addition, previous retrospective studies (Farlow et al., 2004) and a few prospective studies (Bizzarro et al., 2005; Frankfort et al., 2007) of cholinesterase inhibitor treatment of AD have noted differential therapeutic responses between APOE genotypes. However, associations between APOE genotype and measures of structural rates of change are not well characterized in subjects with MCI and AD. Further, the majority of studies evaluating genotype and brain tissue atrophy are cross-sectional (Jack et al., 1998; Bigler et al., 2000; den Heijer et al., 2002); although one previous longitudinal study examined the interaction between ventricular enlargement and genotype in a small sample of AD subjects using manual quantification methods (Wahlund et al., 1999). Thus, there is a strong rationale for the characterization of differences in ventricular enlargement between genotypes, and to determine the number of subjects required to detect a change in the expected natural history of ventricular enlargement.
Despite the evidence supporting the use of ventricular volume as a measure of disease progression in AD and MCI, there are only two studies of ventricular volume change over short time intervals (less than 1 year) (Bradley et al., 2002; Schott et al., 2005). However, these studies were from single centres, were limited by the small sample sizes used, did not compare structural measures to neurocognitive scores, did not include an MCI group and did not examine differences in APOE genotype (Bradley et al., 2002; Schott et al., 2005). In the current study, a large image dataset compiled from over forty-eight centres was obtained for NEC, MCI (including a subset of MCI subjects that converted to AD after six months), and AD subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI). The primary goal of ADNI is to test whether serial MRI, positron emission tomography, other biological markers and clinical and neurocognitive assessment can, alone or in combination, measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.
The primary purpose of the current study was to examine the cross-sectional and longitudinal ventricular volume differences between and within NEC, MCI and AD subjects after only six months in a multi-centre study. The secondary objectives were to determine (i) whether ventricular dilatation in AD is sensitive to disease progression after six months, (ii) whether there is a difference in the rate of ventricular enlargement between APOE genotypes, (iii) the number of subjects necessary to detect a meaningful change from the natural history of ventricular enlargement with respect to genotype, and (iv) whether the rate of ventricular enlargement over six months correlates with the cognitive measures usually used in AD clinical trials including the Mini Mental State Exam (MMSE) (Folstein et al., 1975), and the Alzheimer's Disease Assessment Scale-cognitive (ADAS-cog) test scores (Rosen et al., 1984). We hypothesized that ventricular dilatation after six months would discriminate NEC, MCI, MCI to AD progressors, and AD patients, and be a more sensitive measure of disease progression than cognitive scores.
Materials and Methods
Subjects
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu\ADNI). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), pharmaceutical companies and non-profit organizations, as a $63 million, 5-year public–private partnership. The principal investigator of this initiative is Michael W. Weiner, MD, VA Medical Centre and University of California–San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the US and Canada. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research; approximately 200 cognitively normal older subjects to be followed for three years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years. Written informed consent was obtained from patients or their families. Data acquisition was approved by the local ethics review board at each participating site.
The current study included 504 subjects from the ADNI database that had both baseline and six-month follow-up data available at the time of analysis (August–September 2007), including 105 AD, 247 MCI and 152 NEC subjects. The subject selection protocol and clinical evaluation has been previously reported (Alzheimer's Disease Neuroimaging Initiative, 2008). To summarize, at baseline, classification of the diagnostic group was based on clinical judgment assimilating medical history, clinical evaluation and several neurocognitive tests. At six-month, follow-up subjects were evaluated in a multiple-step procedure, to determine whether MCI and NEC remained appropriate diagnoses or whether the patient had progressed to possible or probable AD according to established NINCDS/ADRDA criteria.
All images selected from the ADNI database were acquired on 1.5 Tesla General Electric (GE) Medical Systems (N = 262), Philips (N = 11), or Siemens (N = 207), MR clinical scanners in accordance with the standard ADNI MR imaging protocol (Jack et al., 2008). In addition, a subset of individuals had scans on a GE system at baseline and then a Siemens system at six months (N = 24). Measurements of ventricular volume were made from 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE) images acquired in the sagittal plane (for detailed pulse sequence parameters see Jack et al., 2008). Raw images uncorrected by ADNI site-specific phantom calibration results were used. For the scans completed on the GE and Siemens systems there was no N3 correction, B1 correction, gradient warping correction, or phantom-based scaling. The scans completed on the Philips systems were automatically B1 corrected on the scanner.
Psychometric assessments
Psychometric assessments were acquired at both baseline and six months for all subjects in each of the three groups. Although there were several tests administered to subjects in the ADNI protocol, two of these tests were chosen for this study based on their use in multicentre studies and previously demonstrated correlation with structural MR measures at intervals greater than or equal to one year (Jack et al., 2004; Thompson et al., 2004; Duarte et al., 2006). The Alzheimer's Disease Assessment Scale-cognitive subscale scores (ADAS-cog) (Rosen et al., 1984) were acquired to test for associations with volumetric measures, as these cognitive scores are the primary endpoints for dementia trials (Fox et al., 2005). The Mini-Mental State Examination (MMSE) (Folstein et al., 1975) was used because of its ubiquitous application in clinical settings.
Ventricular volume measurement
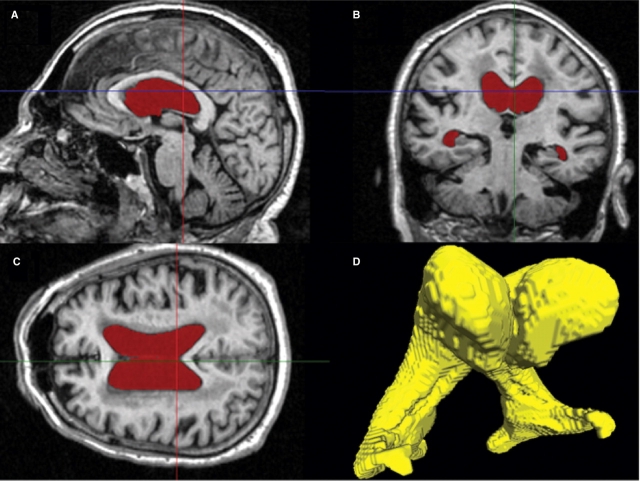
All volumetric analysis was performed on a Windows XP workstation using the semi-automated software Brain Ventricle Quantification (BVQ) (Accomazzi et al., 2005) developed by Cedara Software and refined collaboratively by Cedara Software and Robarts Research Institute. A single researcher (S.M.N.), who was blinded to the age, gender, all clinical information, diagnostic group and chronological ordering within each scan pair, performed all volumetric analyses of the ADNI data. Operator-selected seed points were placed in each lateral ventricle and a region-growing algorithm automatically expanded the seed points within the 3D space of the image to the margin of the periventricular tissue. The region-growing procedure combined image intensity and shape analysis (using morphological operators) and was specifically optimized for the segmentation of the lateral ventricles (Accomazzi et al., 2005; Saha et al., 2000). The lateral ventricles were then automatically rendered in three dimensions and in the coronal, sagittal and axial planes for inspection (Fig. 1). In certain cases extraneous anatomical volumes (usually third and fourth ventricular volumes) were removed by identifying the tissue connecting the ventricle proper and the extraneous volumes. BVQ then automatically removed the extraneous tissue to the border of the lateral ventricles. This type of minimal manual interaction was required in approximately one-third of all subjects. Each 3D volume took approximately 1 min to segment. An additional minute was needed for analyses that require semi-automated editing, usually to remove volume that had been attributed to the third ventricle.
Fig. 1.
Sagittal (A), coronal (B) and transverse (C) T1-weighted MRI images of one subject with the pixels assigned to the lateral ventricles by the Brain Ventricle Quantification software coloured in red. A 3D rendered view of the ventricle from this subject (D) is used for quality control purpose.
Statistical analysis
Automatic volumetric measurement stability was assessed through a repeatability analysis. Intra-rater and inter-rater correlation coefficients (ICC) were determined from a set of 27 subjects, which consisted of 3 groups (AD, MCI, NEC) of 9 subjects randomly selected from the ADNI database. This sample size was chosen to calculate ICC with a significance level of 0.05 and 80% power using two time points (intra-rater) and two operators (inter-rater) (Walter et al., 1998). Two operators (S.M.N., R.R.) performed volume measurements at baseline and again one week later on all 27 datasets. All ICC calculations were performed using a 1-way ANOVA model in SAS 9.1 (SAS Institute Inc., North Carolina). A subset of data were also examined to determine whether the incorporation of the available image corrections (gradient warp correction, B1 correction, N3 correction and phantom scaling correction) had an appreciable effect on ventricle volume measurement. To perform the analysis, ventricle volumes were segmented on 28 randomly chosen subjects (NEC = 10, MCI = 11, AD = 7) using phantom-scaled/optimized images (including gradient warp correction, B1 correction and N3 correction) and the raw images, both at baseline and at six-month follow-up.
The rate of ventricular enlargement was computed in each subject by taking the absolute difference between six-month and baseline volumetric measurements, as well as by taking the percent change from baseline ventricle volume. Normalization to whole-brain volume was not necessary because each subject served as their own control (Jack et al., 2004). Further, normalization of volumes to other brain structures was not performed prior to analysis of cross-sectional ventricular volumes, as Carmichael et al. have previously demonstrated that this normalization does not significantly affect results (Carmichael et al., 2007).
Statistical analysis was performed using SPSS 15 (SPSS Incorporated, Chicago Illinois). The primary analysis consisted of comparisons between all groups (NEC, MCI and AD) on baseline and longitudinal measures, and within groups for longitudinal measures. An analysis of covariance was computed by the general linear model, and Bonferroni tests to adjust for multiple comparisons were conducted for all between group post-hoc investigations. Age, education and scan interval were included as covariates where appropriate based on an ANCOVA. A repeated measures t-test was applied for each within group analysis (changes in MMSE, ADAS-cog and ventricular volume) for all groups and these nine tests were Bonferroni corrected for multiple comparisons.
For secondary analyses, Levene's test was used to analyse homogeneity of variance between ventricular volumes and rates of enlargement between all groups. All statistical tests were two sided, with significance set at the 0.05 level. No secondary analyses were corrected for multiple comparisons. Associations between baseline cognitive scores, rate of cognitive change, baseline ventricular volumes and ventricular enlargement were tested using linear Pearson correlations for each subgroup.
The MCI group was dichotomized by grouping subjects who progressed to a clinical diagnosis of AD after six months and subjects that remained stable. Differences between groups were assessed by an independent sample t-test and longitudinal change was assessed with a repeated measures t-test. In addition, each group (NEC, MCI, AD) was dichotomized into ε4− (ε2/ε3 heterozygote or ε2/ε3 homozygote) and ε4+ (ε4 homozygote or ε4 heterozygote) subjects for consistency with previous studies (Bigler et al., 2000; Farlow et al., 2004; Blesa et al., 2006). The ventricular and cognitive change measures were compared between strata within each group (NEC, MCI and AD) using an independent sample t-test. All t-tests were two sided.
Sample size calculations were performed using a conventional protocol employed by Fox et al. (Equation 1 in (Fox et al., 2000)) for cerebral atrophy. This calculation assumed that there were no differences in standard deviations between groups and that detection of a 20% change is derived with 90% power at a 5% level of significance, for two-sided significance tests.
Results
Ventricular volume measurements using the BVQ software (Fig. 1) were highly reproducible (Table 1). The intra-operator and inter-operator correlation coefficients were greater than 0.98 (Table 1). The ICC was also high for both baseline (0.998) and six-month (0.999) ventricle volumes when comparing volumes derived from raw images and those derived from the scaled and corrected images. A chi-squared test showed that the different types of scanners were equally distributed in the three primary study groups. There was no significant main effect of site for ventricular rates of change. In addition, there was no statistically significant interaction between group and site for measures of six-month ventricular change.
Table 1.
ICC Reliability results for semi-automated (Ventricular volume) Brain Ventricle Quantification measurements
| Reliability measure | ICC | [95% CI] |
|---|---|---|
| Intra-operator 1 | 0.99997 | [0.99994–0.99999] |
| Intra-operator 2 | 0.98098 | [0.95935–0.99131] |
| Inter-operator at baseline | 0.99977 | [0.99950–0.99989] |
| Inter-operator at follow-up | 0.98100 | [0.95939–0.99132] |
ICC = Inter/Intra rater correlation coefficient; CI = Confidence interval.
Demographic information is provided in Table 2. No subjects had a lumbar puncture before their MR scan. Scan interval was not significantly different between groups and did not influence the outcome of the group-wise comparisons. Specifically, the average scan interval for NEC ± SD = 7.0 ± 0.1 months, MCI group = 7.1 ± 0.1 months, and for subjects with AD = 6.8 ± 0.1 months. There was a gender difference found within groups. Specifically, there were significantly more male subjects within both MCI (P < 0.0001) and AD (P = 0.0248) groups. However, there were no significant differences in gender between groups. There was no significant difference in age or education between groups. More than half of the patients with MCI were on dementia medication. There were 98 subjects in the AD group on cholinesterase inhibitor therapy, and there were 61 subjects on Memantine.
Table 2.
Demographic data and cognitive scores
| Group | NEC |
MCI |
AD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ε4− | ε4+ | All subjects | Stable | Converter | ε4− | ε4+ | All subjects | ε4− | ε4+ | All subjects | |
| Sample size | 109 | 43 | 152 | 228 | 18 | 110 | 136 | 246 | 30 | 74 | 104 |
| Age (years) (mean ± SD) | 76.5 ± 5.2 | 76.1 ± 5.2 | 76.4 ± 5.2 | 74.7 ± 7.7 | 75.0 ± 7.3 | 75.7 ± 8.4 | 74.0 ± 6.9 | 74.7 ± 7.3 | 77.7 ± 8.6 | 73.8 ± 6.5 | 74.9 ± 15.0 |
| Sex (male) | 56 | 25 | 81 | 148 | 3 | 77 | 85 | 165 | 13 | 42 | 64 |
| Education (years) (mean ± SD) | 16 ± 3 | 16 ± 3 | 16 ± 3 | 16 ± 3 | 16 ± 4 | 16 ± 3 | 16 ± 3 | 16 ± 3 | 16 ± 3 | 15 ± 3 | 15 ± 3 |
| MMSE at baseline (mean ± SD)a | 29.1 ± 1.0 | 29.2 ± 0.8 | 29.1 ± 0·9 | 27.0 ± 1.8 | 25.9 ± 1.6 | 27.1 ± 1.8 | 26.7 ± 1.8 | 26.9 ± 1.8 | 23.5 ± 1.9 | 23.3 ± 1.9 | 23.3 ± 1.9 |
| ΔMMSE (mean ± SD) | −0.1 ± 1.2 | −0.09 ± 1.3 | −0.1 ± 1.2 | −0.5 ± 2.2 | −1.7 ± 2.2 | −0.3 ± 2.2 | −0.9 ± 2.4 | −0.6 ± 2.3 | 0.2 ± 3.0 | −1.3 ± 3.3 | −0.9 ± 3.3 |
| ADAS-cog at baseline (mean ± SD)b | 5.9 ± 3.0 | 6.8 ± 3.5 | 6.2 ± 3.2 | 11.5 ± 4.3 | 13.2 ± 5.2 | 10.8 ± 4.3 | 12.4 ± 4.3 | 11·7 ± 4·4 | 15.6 ± 5.2 | 18.3 ± 5.9 | 17.5 ± 5.8 |
| ΔADAS-cog (mean ± SD) | 0.0 ± 2.9 | 0.1 ± 3.8 | 0.0 ± 3.2 | 0.6 ± 4.1 | 1.2 ± 4.7 | 0.8 ± 4.1 | 0.4 ± 4.1 | 0.6 ± 4·2 | 2.0 ± 4.0 | 2.6 ± 4.2 | 2.4 ± 4.2 |
aMini mental state exam (MMSE): 2 missing at 6 months in MCI group. bAlzheimer's disease assessment scale-cognitive subscale (ADAS-cog): 2 missing at 6 months in MCI group.
ε4+ = at least one ε4 allele; ε4− = no ε4 allele; NEC = Normal elderly control; MCI = Mild cognitive impairment; AD = alzheimer's disease.
There were no early terminations among the subjects examined in this study at six months. However, two subjects did not have an MRI scan at six-month follow-up (MCI = 1, AD = 1) and were not included in the longitudinal analysis; two subjects did not have an MMSE administered at six months (MCI = 2); two subjects did not have the ADAS-cog administered at six months (MCI = 2).
Longitudinal and cross-sectional ventricular volume measurements
Total ventricular volume and the rate of change over six months are reported in Table 3. At baseline, ventricular volume was significantly larger in both AD subjects (P < 0.0001) and MCI subjects (P = 0.0001) compared to the NEC group. All groups, including NEC, showed a significant increase in absolute and percent ventricular volume after six months (Table 3). The AD group had a significantly greater absolute ventricular enlargement than both subjects with MCI (P = 0.0004) and NEC (P < 0.0001). Patients with MCI also had a significantly greater rate of enlargement than NEC (P = 0.0001). Similarly, when analysing ventricular change as a percentage of baseline ventricular volume, the AD group had a significantly greater rate than the NEC group (P < 0.0001) and MCI group (P = 0.0004), and the MCI group had a significantly greater rate than controls (P = 0.0034). The cross-sectional variance of ventricular volumes in the AD group at baseline was significantly greater than the NEC group (P = 0.0015). The MCI group was not significantly different from NEC and AD for cross-sectional variance. The variance for the rate of ventricular enlargement was significantly greater in both the MCI and the AD groups compared to NEC (P < 0.0001).
Table 3.
Ventricular cross-sectional and longitudinal data
| Baseline ventricular volume (cm3) | Ventricular enlargement after six months (cm3) | Percent ventricular enlargement after six months from baseline | ||
|---|---|---|---|---|
| (mean ± SD) | (mean ± SD) | (mean ± SD) | p-value* | |
| NEC (all subjects) | 38.3 ± 19.1 | 0.6 ± 1.4 | 1.5 ± 4.3 | <0.0001 |
| MCI (all subjects) | 45.8 ± 21.4 | 1.6 ± 2.4 | 3.4 ± 6.1 | <0.0001 |
| AD (all subjects) | 49.9 ± 25.3 | 2.6 ± 2.0 | 5.7 ± 4.9 | <0.0001 |
| MCI stable | 45.6 ± 20.7 | 1.5 ± 2.3 | 3.2 ± 6.0 | = 0.0031 |
| MCI to AD progressors | 48.2 ± 29.2 | 2.8 ± 3.4 | 5.5 ± 6.1 | <0.0001 |
| NEC ε4− | 37.9 ± 18.0 | 0.6 ± 1.3 | 1.5 ± 4.0 | = 0.0102 |
| NEC ε4+ | 39.1 ± 21.8 | 0.7 ± 1.7 | 1.7 ± 5.1 | <0.0001 |
| MCI ε4− | 47.4 ± 22.6 | 1.4 ± 2.7 | 2.7 ± 6.3 | <0.0001 |
| MCI ε4+ | 44.6 ± 20.4 | 1.7 ± 2.1 | 3.9 ± 5.9 | <0.0001 |
| AD ε4− | 47.3 ± 28.0 | 1.8 ± 1.7 | 4.6 ± 4.5 | <0.0001 |
| AD ε4+ | 50.9 ± 24.2 | 3.0 ± 2.1 | 6.2 ± 5.0 | <0.0001 |
*Indicates P-values for repeated measures t-tests for two time-points (baseline and six-months).
NEC = Normal elderly control; MCI = Mild cognitive impairment; AD = Alzheimer's disease; ε4− = Subjects with no APOE ε4 allele; ε4+ = Subjects with at least one APOE ε4 allele.
Longitudinal and cross-sectional cognitive measurements
The cognitive test results for all patient groups at baseline and six months are summarized in Table 2. A significant positive correlation between baseline ventricular volume and age was found within each subgroup (NEC: r = 0.174, P = 0.033, MCI: r = 0.315, P < 0.0001, AD: r = 0.311, P < 0.0001). The MCI group displayed a significant decline in MMSE scores (P < 0.0001) after six months. Only the ADAS-cog scores increased in the AD group after six months (P < 0.0001). Within the MCI group, ventricular enlargement was significantly correlated with decline in MMSE score (r = −0.216, P = 0.0007). Further, change in ADAS-cog scores were significantly correlated with ventricular enlargement in the MCI group (r = 0.128, P = 0.046).
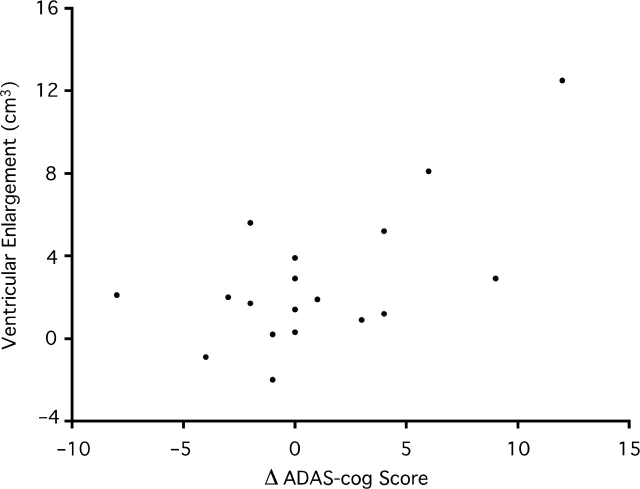
Eighteen subjects diagnosed with MCI at baseline, clinically progressed to AD after six months. Two-hundred-twenty-nine remained stable with MCI. Both groups had significant ventricular enlargement (Table 3). Progressors demonstrated significantly greater ventricular enlargement than subjects who remained stable (P = 0.027). There was no statistical difference in baseline ventricular volumes between MCI strata. Subjects with MCI that progressed to AD had a significantly greater rate of cognitive decline than stable subjects (P = 0.020), and progressors had significantly greater cognitive deficit measured at baseline on the MMSE (P = 0.012). There was no significant decline in MCI progressors on the ADAS-cog after six months or significant difference in rate of decline on the ADAS-cog between MCI strata after six months. However, there was a significant positive association in MCI progressors between ventricular enlargement and cognitive decline measured on the ADAS-cog (r = 0.627, P = 0.0051) (Fig. 2).
Fig. 2.
A Scatter plot of the association between absolute ventricular enlargement (cm3) and change in score on the Alzheimer's Disease Assessment–Cognitive Subscale (ADAS-cog) in subjects with mild cognitive impairment that progressed to Alzheimer's disease. An increase in ADAS-Cog score is taken as evidence of cognitive decline.
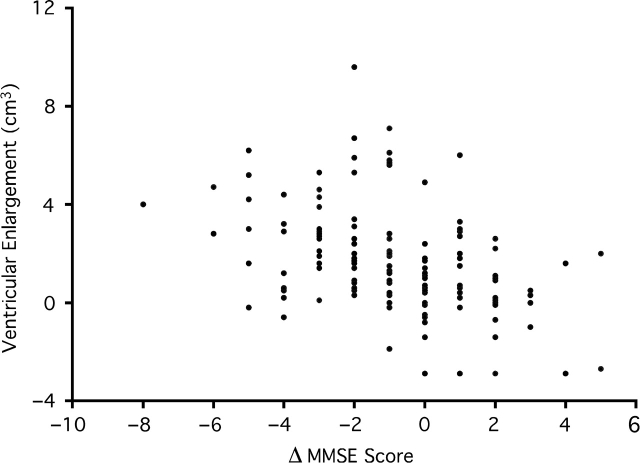
Ventricular enlargement was significantly greater in the AD ε4+ group compared to AD ε4− subjects (P = 0.010) (Table 3). However, there were no significant differences for ventricular measures realized in either the MCI or NEC genotypic groups. However, ε4+ MCI subjects had greater cognitive decline on the MMSE (P = 0.0357) compared to ε4- subjects. In addition, rate of change on the MMSE was significantly associated with ventricular enlargement for ε4+ subjects with MCI (r = −0.420, P < 0.0001). No other significant correlations were observed when dichotomizing for APOE genotype (Fig. 3).
Fig. 3.
A scatter plot of the association between absolute ventricular enlargement (cm3) and change in score on the Mini Mental State Exam (MMSE) for Apolipoprotein ε4+ subjects with mild cognitive impairment. A decline in MMSE score suggests a decline in cognition.
The estimated sample sizes required to detect a 20% change in ventricle volume, MMSE and ADAS-cog change based on the six month rate of ventricular enlargement are presented in Table 4. Since there were no significant differences in ventricular enlargement between MCI genotypes, sample sizes were not derived for these strata.
Table 4.
Six-month estimated sample sizes required to detect a 20% change from the expected absolute rate of change in ventricular volumes, ADAS-cog scores, and MMSE scores
| Lateral ventricular enlargement | Δ MMSE score | Δ ADAS- Cog score | ||
|---|---|---|---|---|
| AD | Six-month | 342 | 7056 | 1607 |
| AD (ε4−) | Six-month | 468 | ≫20 000 | 2100 |
| AD (ε4+) | Six-month | 257 | 3382 | 1370 |
| MCI | Six-month | 1180 | 7712 | ≫20 000 |
MMSE = Mini Mental State Exam; ADAS-cog = Alzheimer's disease assessment Scale–cognitive subscale; AD = Alzheimer's disease; MCI = Mild cognitive impairment.
Discussion
This study examined both total ventricular volume at baseline and ventricular enlargement over six months using a large ADNI subset of NEC, subjects with MCI and subjects with AD. Both AD and MCI subjects had significantly greater mean baseline ventricle volumes compared to controls. However, considerable overlap between individuals existed between all groups. Statistically significant ventricular enlargement was observed in all groups after six months. Subjects with AD had a 60% greater ventricular enlargement compared to subjects with MCI and a 4-fold greater enlargement compared to NEC measured over a six-month interval. In the MCI group, ventricular volume and ventricular enlargement were associated with baseline cognitive scores and cognitive decline, while in the AD group ventricular enlargement was associated with baseline cognitive score. After dichotomizing the MCI group based on clinical status at six months, those individuals who progressed to AD had greater ventricular enlargement and lower MMSE scores on average after six months.
Raw T1-weighted images (without gradient warp correction, B1 correction, N3 correction, or phantom scaling correction) were used for the analyses. Ventricle volume measurements from these images were highly reproducible within and between raters. Additionally, comparison to scaled and corrected images using a subset of data produced very high inter-class correlation coefficients suggesting that the raw images provided comparable measurements to the phantom-scaled and corrected images–with respect to the measurement of ventricular volume. These data suggest that our ventricular volume marker is robust to scanner inhomogeneities and supports the use of either raw images or the corrected images for this metric. The robust nature of the measurement is in part due to the geographical position of the ventricles near the centre of the brain which places this structure near the magnetic isocentre where gradient non-linearities are minimized.
The primary outcome in this study is absolute ventricular change. A previous study has concluded that absolute rates of change are more statistically efficient measures than normalized change (Vickers, 2001) and demonstrated that fractional change or percent change from baseline does not correct for imbalance between groups at baseline. Percent change measures may also create a non-normally distributed statistic from normally distributed data (Vickers, 2001). In the current study, normalized ventricular change was found to be a less efficient metric, as there was more variation relative to the mean for the normalized ventricular change data in comparison to the absolute ventricular change measures.
The finding that subjects with MCI have similar total ventricular volumes to subjects with AD, suggests significant levels of atrophy may occur in the brain prior to a clinical diagnosis of dementia. Nevertheless, there was large overlap in volumes between both pathological groups and controls, which corroborates other cross-sectional volumetric studies (Table 5). However, only one other cross-sectional study in Table 5 reported baseline ventricular volume in MCI subjects (Giesel et al., 2006). A gender difference did exist within both the MCI and AD study groups, however, there were no gender differences between groups; thus, it is unlikely that skewed gender ratios affected the volumetric results.
Table 5.
Absolute baseline ventricular volumes and annual or annualized rate of lateral ventricular enlargement reported in the literature
| Study | Sample size |
Absolute ventricular volume (mean ± SD) |
Annual ventricular enlargement (mean ± SD) |
Units | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NEC | MCI | AD | NEC | MCI | AD | NEC | MCI | AD | ||
| Giesel et al., 2006 | 21 | 21 | 10 | 27.7 ± 12.5 | 24.2 ± 10.1 | 48.4± 24.3 | NA | NA | NA | ml |
| Schott et al., 2005 | 38 | 19 | 31.7 ± 22.1 | NA | 52.7 ± 25.5 | 0.8 ± 1.4 | NA | 4.1 ± 2.3 | ml | |
| Ridha et al., 2008 | 52a | NA | NA | 41.1 ± 17.8 | NA | NA | 4.58 ± 3.75b | ml | ||
| Silbert et al., 2003 | 15 | 24 | NA | NA | NA | 3.3 ± 3.5 | NA | 5.5 ± 3.2 | cm3 | |
| Jack et al., 2004 | Stable = 40 Converter = 15 | Stable = 15 Converter = 26 | Slow Progressor = 32 | NA | NA | NA | Stable: 1.7 (0.9)c | Stable: 2.6 (1.3)c | Slow Progressor: 4.3 (3.3)c | % |
| Fast Progressor = 33 | Converter: 3.4 (1.6)c | Converter: 3.4 (2.8)c | Fast Progressor: 6.4 (3.7)c | |||||||
| Wang et al., 2002 | 14 | 14 | ∼30 ± NAd | ∼60 ± NAd | 0.8 ± NA | NA | 8.2 ± NA | cm3 | ||
| Current Study | 152 | 247 | 105 | 38.3 ± 19.1 | 45.8 ± 21.4 | 49.9 ± 25.3 | 1.1 ± 2.4b | 2.7 ± 4.0b | 4.6 ± 3.7b | cm3 |
NEC = Normal elderly control; MCI = Mild cognitive impairment; AD = Alzheimer's disease.
aAD placebo group. bAnnualized Value (absolute ventricular change/scan interval in years). cMedian percent change/year (Interquartile range). dEstimated ventricular volume and standard deviations.
The mean rates of ventricular enlargement for the NEC and AD group in this multi-centre study are consistent with previously published single-centre measures (Table 5). The MCI group had a rate of enlargement intermediate to the difference between the NEC and AD groups. The large intra-group variance in both cross-sectional and six-month longitudinal data may reflect biological differences within and between subgroups, which has been characterized in other studies (Wang et al., 2002; Giesel et al., 2006). Specifically, cross-sectional measures in the current study reveal relatively large variations across all groups, which suggest large morphological differences among individuals. The AD group had particularly large ventricular variation compared to NEC subjects at baseline. This result suggests that the pathology of dementia and rate of atrophy varies widely within AD subjects. In addition, there is a large variation in ventricular enlargement within pathological groups in comparison to control subjects, which is likely attributable to differential rates of disease progression (slow and fast progressors) and disease severity.
The subset of subjects with MCI at baseline who progressed to AD after six months demonstrated nearly twice the rate of ventricular enlargement compared to stable MCI subjects. Progressors presented a similar rate of enlargement to that of subjects with AD. Mild cognitively impaired subjects that progressed to AD also demonstrated cognitive decline measured by the MMSE. Thus, absolute ventricular enlargement is demonstrably sensitive to clinically measured disease progression over short intervals in a multi-centre study. This result supports the notion that longitudinal absolute measures of structural change measured over a set interval may provide more predictive value of progression from MCI to AD than cross-sectional volumes. However, a previous single centre study by Jack et al. with relatively small sample sizes did not show a significant difference between the percent ventricular enlargement of subjects that converted from MCI to AD and subjects with stable MCI (Jack et al., 2004). They did, however, see a significant percent change difference between these groups for whole brain atrophy (Jack et al., 2004). In the current study, the MCI progressor group did not have a significantly different change on the ADAS-cog when compared to the MCI stable group. However, decline as measured by an increase in ADAS-cog score, was moderately associated with ventricular enlargement. This suggests that as cognition worsens on global cognitive measures, there is associated macroscopic loss of brain tissue.
The current study demonstrates that AD carriers with at least one ε4 allele have a pronounced increase in ventricular enlargement, in the absence of detectable cognitive differences, in comparison to ε4- subjects. There were no differences in ventricular change between MCI genotypes; however, the rate of cognitive decline was greater for ε4+ MCI subjects. These results suggest a pronounced effect of the APOE ε4 gene on cerebral atrophy for mild AD. A recent comprehensive qualitative review lists only four previous cross-sectional studies and one longitudinal study examining the association between ventricular volume and APOE genotype (Cherbuin et al., 2007). The only reported longitudinal study found no difference between APOE genotypes within an AD group, although it found a greater rate of enlargement in ε4 carriers with other dementias in comparison to non-carriers (Wahlund et al., 1999); however, this study used manual methods, was based on a small sample of AD subjects and examined a younger AD group with greater cognitive deficit measured on the MMSE than the current study. The majority of studies that incorporate an AD group are cross sectional and thus fail to capture the association of APOE and dynamic structural changes in subjects with AD. Measures of change are important when considering the heterogeneity of ventricular volumes among all AD subjects at baseline. A longer follow-up interval may also demonstrate more appreciable difference in structural brain changes between MCI and NEC APOE groups.
The temporal horns of the lateral ventricles are adjacent to paralimbic tissue and demonstrably capture changes in these regions, which are pathologically susceptible during the prodromal stages of dementia (Chetelat and Baron, 2003). A previous study of surface map changes in the temporal horns of controls and subjects with AD found regional enlargement correlates to disease progression (Thompson et al., 2004). A recent ventricular subfield analysis of subjects with AD, however, postulates there are several other hemispherical brain structures contributing to ventricular dilatation in conjunction with MTL structures (Ferrarini et al., 2006). This result is congruous with the topographical staging of cerebral neurodegeneration delineated by Braak and Braak in subjects with AD (Braak and Braak, 1994). Hence, the total lateral ventricular measures may capture hemispherical atrophy in conjunction with MTL atrophy, which analysis of strictly the temporal horns would exclude. These more global lateral ventricular enlargement measures, may explain the significantly greater rates of enlargement in subjects with AD compared to patients with MCI. Furthermore, one study found that a robust measure of temporal horn volume incorporated total lateral ventricular volume (Giesel et al., 2006). Thus, total lateral ventricular volume may be the most sensitive single measure to discriminate enlargement between NEC, subjects with MCI and subjects with AD over short durations.
An important application of volumetric MRI measurements of disease progression is towards evaluating drug therapy in AD multi-centre clinical trials, and during prodromal stages of dementia, notably in subjects with MCI. In addition, measures at short intervals, for example six months, expedite the process of drug innovation. Currently, cognitive scores are used as endpoints in clinical trials. Neuroimaging is increasingly used to evaluate structural changes in response to therapeutic intervention. In the current study, the AD group had a stable mean MMSE score after six months, which may be ascribed to the efficacy of therapeutic interventions to ameliorate cognitive symptoms over short durations, as the majority of AD subjects were administered cholinesterase inhibitor therapy. However, the same group did have an increase in mean ADAS-cog score, which is a more sensitive cognitive measure. The MCI group demonstrated both a modest average increase on the ADAS-cog and decrease on the MMSE over the same time interval. Ventricular volume changes were also detected in these groups during this period. Sample sizes needed to detect ventricular enlargement for MCI subjects and AD subjects were lower than the sample sizes required when using psychometric measures to detect changes from the natural history of cognitive or functional decline (Table 4). The smaller sample size derived from structural measures is due to the lower variability in measures of ventricular volume compared to the change in neurocognitive scores. Moreover, high education levels and the prevalent use of cholinesterase inhibitor therapy in conjunction with the use of Memantine, may partially explain the relatively large samples required to detect a 20% reduction in the rate of decline as measured by the MMSE and ADAS-cog (Table 4). Thus, ventricular volume can provide complementary insight into insidious disease progression in the absence of cognitive decline. Moreover, there is recent evidence to suggest ventricular volume may provide additive diagnostic utility to other neuroimaging measures (Jack et al., 2008).
There are several threats to the internal validity of neurocognitive tests that short testing intervals may exacerbate (van Belle et al., 1990). Furthermore, certain individuals may develop greater cognitive reserves in response to longer durations of education and/or cognitively demanding occupations (Sanchez et al., 2002), which may generate high cognitive scores despite underlying disease progression. In summary, neurocognitive measures require greater samples to detect significant cognitive decline in patients, particularly in subjects with mild AD over short intervals, whereas measurements of ventricular dilatation may provide insight into AD progression, particularly for multi-centre studies.
Furthermore, pharmacogenetic interactions may mediate the efficacy of certain therapeutic agents (Farlow et al., 2004; Bizzarro et al., 2005; Frankfort et al., 2007). There are several retrospective studies (Farlow et al., 2004) and a few prospective studies (Bizzarro et al., 2005; Frankfort et al., 2007) that have examined the differential cognitive response to cholinesterase inhibitors between APOE genotypes; however, the results are equivocal with varying methodologies. In addition, there is some evidence to suggest ε4+ subjects with MCI have greater cognitive response to Donepezil (Petersen et al., 2005). Nevertheless, there are few studies examining structural brain changes between APOE genotypes in response to treatment (Bigler et al., 2000; Wilcock et al., 2000; Bizzarro et al., 2005; Visser et al., 2005; Blesa et al., 2006; Frankfort et al., 2007). The current study demonstrates greater ventricular enlargement in AD subjects with an ε4+ genotype and supports the notion that dichotomizing subjects based on genotype may provide the greatest sensitivity to detect changes in the natural history of disease progression (Table 4). Although it is possible that temporal effects (time since diagnosis), age of sample and disease severity may alter APOE and therapeutic interactions, fewer subjects are required when examining ventricular differences, particularly for ε4+ genotypic groups. In addition, there was a significant association between ventricular enlargement and cognitive decline observed in ε4+ subjects with MCI. This association was not demonstrated in ε4− subjects, and suggests that the ε4+ subjects are driving the significant association between ventricular enlargement and cognitive decline demonstrated when pooling all subjects with MCI.
In summary, absolute ventricular volumes and ventricular enlargement measured over a six-month interval were greater in subjects with AD and MCI compared to age-matched controls. Ventricular enlargement also demonstrated sensitivity to disease progression by way of discriminating between subjects with stable MCI and those that progressed to AD. Further, ventricular enlargement demonstrated effects of genotype on pathological phenotype in AD. As a potential measure of disease progression for multi-centre studies of both AD and MCI subjects, ventricular enlargement measures would significantly reduce the number of subjects required to demonstrate a change from the natural history of Alzheimer's disease progression.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; Principal Investigator: Michael Weiner; NIH grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer's Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the US Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles. Funding for this study was provided by The Division of Geriatric Medicine, University of Western Ontario, The Ivey-BMO Financial Group Scientist in Brain Disorders Imaging Award. The authors thank Larry W. Stitt, Assistant Director at the Biostatistical Support Unit, Department of Epidemiology & Biostatistics at the University of Western Ontario for consultation regarding statistical analysis and calculation of reliability coefficients. The original version of this manuscript was approved by the ADNI data and publication committee (DPC).
References
- Accomazzi V, Lazarowich R, Barlow CJ, Davey B inventors; Cedara Software Corp., assignee. 2005. Feb 10, Image region segmentation system and method. US Patent Application 20050031202. [Google Scholar]
- Bigler ED, Lowry CM, Anderson CV, Johnson SC, Terry J, Steed M. Dementia, quantitative neuroimaging, and apolipoprotein E genotype. American Journal of Neuroradiology. 2000;21:1857–68. [PMC free article] [PubMed] [Google Scholar]
- Bizzarro A, Marra C, Acciarri A, Valenza A, Tiziano FD, Brahe C, et al. Apolipoprotein E ε4 allele differentiates the clinical response to donepezil in Alzheimer's disease. Dementia & Geriatric Cognitive Disorders. 2005;20:254–61. doi: 10.1159/000087371. [DOI] [PubMed] [Google Scholar]
- Blesa R, Aguilar M, Casanova JP, Boada M, Martinez S, Alom J, et al. Relationship between the efficacy of rivastigmine and apolipoprotein E (ε4) in patients with mild to moderately severe Alzheimer disease. Alzheimer Disease & Associated Disorders. 2006;20:248–54. doi: 10.1097/01.wad.0000213880.93665.c7. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Morphological criteria for the recognition of Alzheimer's disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15:355–6. doi: 10.1016/0197-4580(94)90032-9. 379–80. [DOI] [PubMed] [Google Scholar]
- Bradley KM, Bydder GM, Budge MM, Hajnal JV, White SJ, Ripley BD, et al. Serial brain MRI at 3-6 month intervals as a surrogate marker for Alzheimer's disease. Br J Radiol. 2002;75:506–13. doi: 10.1259/bjr.75.894.750506. [DOI] [PubMed] [Google Scholar]
- Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, et al. Ventricular volume and dementia progression in the cardiovascular health study. Neurobiol Aging. 2007;28:389–97. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Leach LS, Christensen H, Anstey KJ. Neuroimaging and APOE genotype: A systematic qualitative review. Dement Geriatr Cogn Disord. 2007;24:348–62. doi: 10.1159/000109150. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Baron JC. Early diagnosis of Alzheimer's disease: Contribution of structural neuroimaging. Neuroimage. 2003;18:525–41. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- ADNI: Alzheimer's Disease Neuroimaging Initiative. Research [Internet] 2008 [cited 2008 June]. Available from: http://www.loni.ucla.edu/ADNI/Research/
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–8. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: Prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Duarte A, Hayasaka S, Du A, Schuff N, Jahng GH, Kramer J, et al. Volumetric correlates of memory and executive function in normal elderly, mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2006;406:60–5. doi: 10.1016/j.neulet.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M, Lane R, Kudaravalli S, He Y. Differential qualitative responses to rivastigmine in APOE ε4 carriers and noncarriers. Pharmacogenomics Journal. 2004;4:332–5. doi: 10.1038/sj.tpj.6500267. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, Palm WM, Olofsen H, van Buchem MA, Reiber JH, Admiraal-Behloul F. Shape differences of the brain ventricles in Alzheimer's disease. Neuroimage. 2006;32:1060–9. doi: 10.1016/j.neuroimage.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sun S, Taylor C, Ward CP, Gamst AC, Petersen RC, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70:191–9. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: Validation and application to Alzheimer's disease. Journal of Magnetic Resonance Imaging. 1997;7:1069–75. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: Power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000;57:339–44. doi: 10.1001/archneur.57.3.339. [DOI] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. AN1792(QS-21)-201 Study. Effects of abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Frankfort SV, Appels BA, de Boer A, Tulner LR, van Campen JP, Koks CH, et al. Identification of responders and reactive domains to rivastigmine in Alzheimer's disease. Pharmacoepidemiology & Drug Safety. 2007;16:545–51. doi: 10.1002/pds.1345. [DOI] [PubMed] [Google Scholar]
- Giesel FL, Hahn HK, Thomann PA, Widjaja E, Wignall E, von Tengg-Kobligk H, et al. Temporal horn index and volume of medial temporal lobe atrophy using a new semiautomated method for rapid and precise assessment. Am J Neuroradiol. 2006;27:1454–8. [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–94. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, O’Brien PC, Waring SC, Tangalos EG, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer's disease. Ann Neurol. 1998;43:303–10. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Weigand SD, O’Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–31. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. C11 PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinsinger G, Teipel S, Wismuller A, Born C, Meindl T, Flatz W, et al. Volumetric MRI for evaluation of regional pattern and progressin of neocortical degeneration in Alzheimer's disease. Radiologe. 2003;43:537–42. doi: 10.1007/s00117-003-0928-1. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Ridha BH, Anderson VM, Barnes J, Boyes RG, Price SL, Rossor MN, et al. Volumetric MRI and cognitive measures in Alzheimer disease. J Neurol. 2008;255:567–74. doi: 10.1007/s00415-008-0750-9. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Saha PK, Udupa JK, Odhner D. Scale-based fuzzy connected image segmentation: Theory, algorithms, and validation. CVIU. 2000;77:145–74. [Google Scholar]
- Sanchez JL, Rodriguez M, Carro J. Influence of cognitive reserve on neuropsychologic functioning in Alzheimer's disease type sporadic in subjects of spanish nationality. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 2002;15:113–22. [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci. 1993;90:9649–53. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: A serial MRI study over 6 and 12 months. Neurology. 2005;65:119–24. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–92. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–66. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- van Belle G, Uhlmann RF, Hughes JP, Larson EB. Reliability of estimates of changes in mental status test performance in senile dementia of the Alzheimer type. J Clin Epidemiol. 1990;43:589–95. doi: 10.1016/0895-4356(90)90163-j. [DOI] [PubMed] [Google Scholar]
- Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: A simulation study. BMC Medical Research Methodology. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Pelgrim E, Verhey FR. Dutch ENA-NL-01 Study G. Medial temporal lobe atrophy and APOE genotype do not predict cognitive improvement upon treatment with rivastigmine in Alzheimer's disease patients. Dementia & Geriatric Cognitive Disorders. 2005;19:126–33. doi: 10.1159/000082883. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Julin P, Lannfelt L, Lindqvist J, Svensson L. Inheritance of the ApoE ε4 allele increases the rate of brain atrophy in dementia patients. Dementia & Geriatric Cognitive Disorders. 1999;10:262–8. doi: 10.1159/000017130. [DOI] [PubMed] [Google Scholar]
- Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101–10. doi: 10.1002/(sici)1097-0258(19980115)17:1<101::aid-sim727>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Wang D, Chalk JB, Rose SE, de Zubicaray G, Cowin G, Galloway GJ, et al. MR image-based measurement of rates of change in volumes of brain structures. part II: Application to a study of Alzheimer's disease and normal aging. Magn Reson Imaging. 2002;20:41–8. doi: 10.1016/s0730-725x(02)00472-1. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: Multicentre randomised controlled trial. galantamine international-1 study group. BMJ. 2000;321:1445–9. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]