Abstract
The superior temporal gyrus, which contains the auditory cortex, including the planum temporale, is the most consistently altered neocortical structure in schizophrenia (Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res 2001; 49: 1–52). Auditory hallucinations are associated with abnormalities in this region and activation in Heschl's gyrus. Our review of 34 MRI and 5 post-mortem studies of planum temporale reveals that half of those measuring region size reported a change in schizophrenia, usually consistent with a reduction in the left hemisphere and a relative increase in the right hemisphere. Furthermore, female subjects are under-represented in the literature and insight from sex differences may be lost. Here we present evidence from post-mortem brain (N = 21 patients, compared with 17 previously reported controls) that normal age-associated changes in planum temporale are not found in schizophrenia. These age-associated differences are reported in an adult population (age range 29–90 years) and were not found in the primary auditory cortex of Heschl's gyrus, indicating that they are selective to the more plastic regions of association cortex involved in cognition. Areas and volumes of Heschl's gyrus and planum temporale and the separation of the minicolumns that are held to be the structural units of the cerebral cortex were assessed in patients. Minicolumn distribution in planum temporale and Heschl's gyrus was assessed on Nissl-stained sections by semi-automated microscope image analysis. The cortical surface area of planum temporale in the left hemisphere (usually asymmetrically larger) was positively correlated with its constituent minicolumn spacing in patients and controls. Surface area asymmetry of planum temporale was reduced in patients with schizophrenia by a reduction in the left hemisphere (F = 7.7, df 1,32, P < 0.01). The relationship between cortical asymmetry and the connecting, interhemispheric callosal white matter was also investigated; minicolumn asymmetry of both Heschl's gyrus and planum temporale was correlated with axon number in the wrong subregions of the corpus callosum in patients. The spacing of minicolumns was altered in a sex-dependent manner due to the absence of age-related minicolumn thinning in schizophrenia. This is interpreted as a failure of adult neuroplasticity that maintains neuropil space. The arrested capacity to absorb anomalous events and cognitive demands may confer vulnerability to schizophrenic symptoms when adult neuroplastic demands are not met.
Keywords: auditory processing, neuroplasticity, cerebral asymmetry, corpus callosum, language processing, schizophrenia
Introduction
The planum temporale (particularly on the left) has been found to be smaller and even to reduce over time in schizophrenia (Kasai et al., 2003) and its reduced size has been correlated with the degree of thought disorder in patients (Shenton et al., 1992). Another common symptom, the perception of auditory hallucinations, is accompanied by increased blood flow (functional MRI—Shergill et al., 2000) and neural activity (magnetoencephalographzy—Ropohl et al., 2004) in the planum temporale and the primary auditory cortex in Heschl's gyrus. Enlargement of the ventricles has also been found correlated with reduced superior temporal gyrus volume (Chance et al., 2003).
Reductions or reversals of asymmetry of the planum temporale have been reported in schizophrenia (Rossi et al., 1992; Petty et al., 1995; Barta et al., 1997). Although it has not been found in a number of subsequent studies (Kulynych et al., 1993, 1995; Kleinschmidt et al., 1994; Rossi et al., 1994; O’Leary et al., 1995; Ward et al., 1995; Frangou et al., 1997), meta-analyses (Shapleske et al., 1999; Sommer et al., 2001) confirm a loss of asymmetry in patients relative to controls in the literature as a whole.
The developmental expansion of the cortical surface depends on the proliferation of minicolumnar units of cells according to the radial unit hypothesis (Rakic, 1995). During embryogenesis, the columns are formed as cells migrate radially towards the brain's surface. Consequently, the nature of the pathophysiology underlying altered region size in schizophrenia may be reflected in minicolumn organization. Cortical minicolumnar structure is visible in axonal and dendritic bundles and, most commonly, cell body distribution. In adult cortex, these usually have a periodicity of 20–90 µm, depending on region and method.
We previously reported region and minicolumn size asymmetries in the superior temporal lobe in normal subjects as a putative substrate of language processing (Chance et al., 2006a). Following the principle that language lateralization involves an interaction between the auditory cortices of the two hemispheres mediated by the corpus callosum, we identified asymmetries in minicolumn number in the normal auditory cortex that related to variation in the number of axons passing through the connecting regions of the corpus callosum; the posterior midbody contains the connections between the primary auditory regions of Heschl's gyri in both hemispheres and the isthmus contains the connections between the plana temporale (Chance et al., 2006a). It has been suggested that cortical misconnections underlie the symptoms of schizophrenia (Friston and Frith, 1995) and that the corpus callosum may be particularly vulnerable (Crow et al., 1998).
Minicolumn organization, little investigated in schizophrenia, therefore offers an approach to cytoarchitectural anomalies relating to abnormal region size and deficits in speech perception in schizophrenia. For example, reduced electrophysiological auditory mismatch responses have been associated with altered lateralization in the planum temporale in schizophrenia (Kircher et al., 2004). Sex-dependent, asymmetric alteration in the evoked activation of auditory cortex has also been reported (Rojas et al., 1997).
Given previous reports of grey matter reduction and reduced asymmetry, we hypothesized that schizophrenia patients would have reduced asymmetry of auditory cortex including PT, with smaller cortical surface area associated with smaller minicolumn spacing. Furthermore, based on the misconnectiviy hypotheses of Crow, Friston and Frith, we predicted that patients would have lost correlations between minicolumn asymmetry and axon numbers (measured previously in these brains) in the posterior midbody and isthmus of the corpus callosum.
Material and Methods
Subjects
Formalin-fixed brain tissue was sampled from 21 patients with schizophrenia (11 female, 10 male) conforming to DSM IV criteria, for comparison with a group of 17 control subjects (10 female, 7 male) for which minicolumn and callosal data have been reported previously using the same methods (Chance et al., 2006a). Although control data were analysed separately to address independent scientific questions of cerebral lateralization in normal humans (Chance et al., 2006a), the data for both patients and controls were originally gathered by the same raters at the same time while blind to diagnosis. Tissue was collected with consent in accordance with standard neuropathological practice and is registered with UK national investigations on organ retention. Cases were selected to yield comparable group mean fixation times and ages at death as far as possible, although a close match was not possible. Causes of death are listed in Table 1. Patients were included on the basis of the assessment of clinical notes by a consultant psychiatrist (T.J.C. or Dr S.J. Cooper, Belfast). Assessment of tissue sample pathology was carried out by a consultant neuropathologist (M.M. Esiri or B. McDonald, Oxford) and cases with significant pathology, such as Alzheimer's disease or cerebrovascular disease, were excluded using CERAD criteria. Control subjects had no history of neuropsychiatric illness. Demographic details and potentially confounding variables, including age at death, post-mortem interval and fixation time, were subjected to statistical analysis (see below).
Table 1.
Causes of death
| Diagnosis | Sex | Age | Cause of death |
|---|---|---|---|
| C | F | 53 | Carcinomatosis due to carcinoma of kidney |
| C | F | 59 | Multi-organ failure in a patient with myelodysplastic syndrome |
| C | F | 63 | Acute pulmonary oedema due to myocardial infarction due to coronary artery atheroma |
| C | F | 71 | Ruptured abdominal aortic aneurysm due to atherosclerosis |
| C | F | 72 | Pulmonary embolus, carcinomatosis (cancer of left lower lobe) |
| C | F | 73 | Haemothorax |
| C | F | 80 | Carcinomatosis (primary tumour probably lung), ulceration and haemorrhage of oesophagus |
| C | F | 82 | Pulmonary oedema due to brown atrophy to heart due to coronary artery atherosclerosis |
| C | F | 89 | Cardiac failure |
| C | F | 90 | Acute myocardial ischaemia due to coronary artery atheroma |
| C | M | 40 | Pulmonary oedema due to myocardial infarction and fibrosis due to coronary artery atherosclerosis with myocardial hypertrophy |
| C | M | 53 | Acute coronary insufficiency, coronary atherosclerosis |
| C | M | 54 | Ischaemic heart disease due to coronary artery atheroma |
| C | M | 62 | Congestive cardiac failure due to acute myocardial ischaemia due to coronary artery atheroma (operated) |
| C | M | 66 | Myocardial infarction due to coronary artery occlusion |
| C | M | 68 | Congestive cardiac failure due to coronary artery atheroma |
| C | M | 76 | Retroperitoneal haemorrhage due to ruptured abdominal aortic aneurysm due to aortic atherosclerosis |
| S | F | 44 | Sudden, other |
| S | F | 48 | Acute pulmonary oedema, hypertensive heart disease, Hodgkin's disease |
| S | F | 66 | Probably from bleeding duodenal ulcer and dehydration |
| S | F | 70 | Other, unknown |
| S | F | 71 | Suppurative bronchopneumonia, aspiration |
| S | F | 73 | Coronary thrombosis; septicaemia |
| S | F | 79 | Bilateral bronchopneumonia |
| S | F | 80 | Perforated duodenal ulcer and peritonitis |
| S | F | 83 | Pulmonary embolism |
| S | F | 84 | Other, unknown |
| S | F | 90 | Pulmonary embolus |
| S | M | 29 | Chest injuries (suicide) |
| S | M | 41 | Pulmonary oedema due to left ventricular failure and renal failure |
| S | M | 58 | Pulmonary oedema, ischaemic heart disease |
| S | M | 59 | Myocardial ischaemia, coronary occlusion, atherosclerosis, bronchopneumonia |
| S | M | 60 | Chest infection following carcinoma |
| S | M | 65 | Bronchopneumonia |
| S | M | 66 | Haemopericardium, cardiac infarction, coronary atherosclerosis |
| S | M | 67 | Respiratory infection |
| S | M | 76 | Acute myocardial ischaemia due to coronary artery atheroma |
| S | M | 87 | Other, non-acute |
F = female; M = male; C = control; S = schizophrenia. Age at death is given in years (in ascending order within diagnosis × sex groups).
No comorbidity of alcohol or illicit drug misuse was detected in our sample's records. Patients had received long-term anti-psychotic medication. Unfortunately, insufficient detail on lifetime medication was available for subsequent statistical analysis; however, we note that Benes et al. (2001) found no structural changes in cortical areas when comparing patients who had been exposed to neuroleptics with drug-naive patients.
Tissue samples
The brains had been supported by the basilar artery in 10% formalin for fixation and assigned a randomized code by a third party, so that measurements could be made by persons blind to sex, diagnosis and age. Five millimeter thick blocks of temporal lobe were cut orthogonal to the long axis of the lobe, systematically random with respect to the anterior boundary of Heschl's gyrus, sampling exhaustively through HG and PT, as defined below. Blocks were cut by hand using a calibrated metal guide. All blocks were used for the assessment of gross volume and area measurements. For the analysis of minicolumns, two 25 μm thick paraffin sections were cut from separate blocks within each region of interest (ROI), spaced to preserve the systematic random nature of the sample so that the entire ROI had a chance of being sampled. This was done in each hemisphere and the sections were Cresyl violet Nissl stained. Each ROI from each hemisphere was therefore analysed on two slides. Cortical tissue shrinkage due to embedding in these brains has been estimated with a mean of 23.7% (measurements on the 5 mm thick blocks were taken before embedding and afterwards and a measure of shrinkage was calculated) and no systematic difference was found between groups. The corpus callosum was not sampled in this study and the data used here were drawn from a previous study on the brains of the same subjects reported in Highley et al. (1999a). The material used in the present study was removed from formalin and tissue blocks were placed in embedding medium at the same time as in the previous studies that have been reported for these brains. Consequently, the comparison between recent minicolumn measures in the cortex and axon measures previously reported is not confounded by the time between studies.
Anatomical measurements
Gross anatomical measurements
HG was defined as Heschl's gyrus, bounded by Heschl's sulcus posteriorly, the First Transverse sulcus anteriorly (Kim et al., 2000) and laterally by the superolateral margin of the STG (Zetzsche et al., 2001) containing cytoarchitectural regions TC and TBC following the definitions of von Economo and Koskinas (1925). The lower bank of the Sylvian fissure, posterior to HG was measured as PT. This consisted of the planum temporale bounded anteriorly by Heschl's sulcus, including regions TB and TA1, excluding the posterior ascending ramus. The PT was painted while still intact to clearly identify the beginning of the ascending ramus as the posterior border of the PT.
The callosal subregion boundaries, as reported in Highley et al. (1999a), were defined as proportions of the total length of the corpus callosum. Cortical volume was estimated by point counting within the grey matter of each region. Surface area was estimated by counting intersections between the cortical surface and cycloidal test lines (with changing orientation through 180° and known dimensions). Images of tissue slices were superimposed over the probe grids. A parallel slice design was used, as reported by Pakkenberg and Gundersen (1997). It should be noted that the present study was not strictly stereological since the identification of STG anatomy and minicolumnar organization requires a non-random orientation of tissue. Although probes were used that reduce the bias otherwise incurred by subjective outlining of structures, the parallel slice design (coronal slicing) does not satisfy the random orientation criteria to be strictly unbiased as discussed in Pakkenberg and Gundersen (1997).
Each structure was sampled twice by replacement of the point grid or test lines on each count of every slice, random with respect to the boundaries of the ROI, to generate a mean estimate. Estimation of PT and HG surface area and cortical volume was repeated for 10 hemispheres to determine reliability—intraclass correlation coefficient for all measures was good (≥0.9). Across the entire final dataset, strong correlations were found between volume and surface area (Pearson correlations 0.87–0.94 for the four ROIs), indicating good agreement between the methods. To validate the surface area measured by cycloids, a comparison was made on a subgroup (10 plana) with an area measure based on manual surface outlining using the CortexTrace software (S.A.C., University of Oxford). Although surface outlining constitutes a more biased method as it depends on entirely user-guided tracing and was therefore undesirable for the main study, the agreement between methods was high (correlation 0.96, P < 0.01), indicating that the cycloid sampling was in good agreement with outlining methods such as those employed by many MRI analyses.
Columnar measurements
Minicolumn width and peripheral neuropil width were quantified using semi-automated computerized image analysis so that user bias is minimal. The method is model based and has been reported in detail with stereological validation and discussion of assumptions by Casanova and Switala (2005). The tissue sections were a systematically spaced subset that preserved randomization with respect to the anterior and posterior boundaries of the ROIs and therefore maintained systematic random sampling of the full extent of the regions while retaining coronal orientation. Minicolumns are clearest in lamina III, so minicolumn detection was optimized for lamina III. In summary, photographs of cortical lamina III were taken at 100× magnification, in coronal view, and the photographs were digitized at 0.48 µm resolution and tessellated. Two resulting photomicrographs (each about 1.5 mm2 in area) were generated from each slide. Fields were selected systematically, randomly from the section, although regions of high cortical curvature such as the fundi of sulci or the apices of gyri were excluded since, while minicolumns are still clearly visible, high curvature affects cell distribution (Chance et al., 2004). A mean of 261.6 minicolumns were sampled per brain (65.4 minicolumns per region, per hemisphere).
The image was automatically segmented to select neurons and nearest neighbour measurements of clustering were applied to determine the periodicity of columnar distribution. Segmentation was based on grey level intensity of the digitized image, with automated shape and size thresholds for cell identification, as validated previously (Casanova and Switala, 2005). The software is able to recognize minicolumns despite variations in stain, as long as cell object size and background contrast pass a threshold of acceptability for inclusion. Every image was studied for quality control by the user and artefacts were manually selected for exclusion from the analysis. A minicolumn is composed of the cell dense core and the cell sparse periphery of the cell column where local circuits, synapses and dendritic branches predominate. Centre-to-centre minicolumn spacing is calculated from the combination of the core and peripheral space.
The potential confounds of over-projection and lost caps are prevented by the thickness of sections—25 µm—being approximately matched to the spacing of minicolumns (taking into account z-axis shrinkage); therefore, a single plane of minicolumns is in view—for detailed discussion see Casanova and Switala (2005).
Tissue quality from one hemisphere did not pass the confidence threshold for automatic minicolumn segmentation in three cases for HG (1 male left, 1 female left and 1 female right) and once for PT (1 female right), so minicolumn measures were obtained only from the remaining hemisphere in these cases. To relate minicolumn spacing to regional surface area for the calculation of regional minicolumn number, the surface area per column was estimated based on a hexagonal distribution [as indicated by other researchers (Gabbott, 2003; Favorov and Kelly, 1994a, b)]. Asymmetry coefficients of minicolumn spacing were calculated as the magnitude of difference between hemispheres, expressed as a percentage of the bihemispheric mean ((left−right)/((left+right)/2)) × 100.
Statistical analysis
The four key measures, surface area, cortical volume, minicolumn spacing and minicolumn number, were analysed by repeated measures analysis of variance (rmANOVA). Statistical analyses were conducted using SPSS software (version 12.0) to apply rmANOVAs with diagnosis and gender as between-subject factors and either one level (hemisphere) or two levels (hemisphere and region) of within-subject factors. The influence of potential confounding factors in the rmANOVAs, including age at death, post-mortem interval and fixation time, was accounted for—t-tests were used to identify differences between groups.
The influence of age on columnar organization (Chance et al., 2006b) was controlled by including age at death as a covariate in all rmANOVAs of columnar variables. It was also retained in rmANOVAs of region size variables, if it was a significant covariate. t-tests of post-mortem interval showed no differences between groups (and six cases had missing values, see Table 2, where the time since death was uncertain and not recorded in hours), so it was not included as a covariate in rmANOVAs. Fixation time was found to differ between groups and so was subjected to covariate analysis. However, fixation time data for one case was found to be an approximation (inaccurate by up to 6 months), so this covariate was only retained in rmANOVAs if found to be a significant covariate. All groups passed Kolmogorov–Smirnov tests, indicating a normal distribution, on all measured parameters. All repeated measures analyses also passed Box's M-test for equality of variance except Heschl's gyrus volume, which was corrected as reported below.
Table 2.
Demographic variables and covariates
| Group | Age at death (years) | PMI (h)a | Fixation time (months)b | Age of illness onset (years)b |
|---|---|---|---|---|
| Male controls | 59.9 ± 11.9 | 36.4 ± 15.9 | 20.9 ± 9.8 | NA |
| Female controls | 73.2 ± 12.4 | 36.9 ± 19.0 | 26.9 ± 13.3 | NA |
| Male schizophrenia | 59.5 ± 15.8 | 35.4 ± 21.2 | 32.8 ± 20.1 | 24.4 ± 6.4 |
| Female schizophrenia | 71.6 ± 14.5 | 35.0 ± 23.6 | 58.2 ± 20.2 | 38.4 ± 15.5 |
aInaccurate information for six cases (see text for details).
bInaccurate information for one case (fixation time uncertain by up to 6 months in one case, age of onset not recorded for one case).
NA = Not applicable.
Three Pearson correlation analyses were performed—the first examined the relationship between minicolumn spacing and region size (surface area) for each region and the second considered the relationship between minicolumn number asymmetry and callosal axon number. A correlation analysis of age and minicolumn spacing was also performed.
Several unique tests were performed in this analysis. Due to the undesirability of multiple testing, data were compressed into single tests (i.e. rmANOVAs) wherever possible. The rmANOVA tests for inter-subject differences while also modelling an additional level of contrasts between intra-subject repeated measures (i.e. left and right hemispheres, or HG and PT). Mean values are reported for diagnosis, sex and hemisphere in Tables 3 and 4. In the main text, only statistically significant results are reported. Lateralized hemisphere differences are described only when there is an explicit statistical interaction depending on hemisphere. Where a main result did not involve a difference between hemispheres, a mean of left and right has been reported in the text.
Table 3.
Cortical volume and surface area
| Group | Volume (mm3) |
Area (mm2) |
||||||
|---|---|---|---|---|---|---|---|---|
| Planum |
Heschl's |
Planum |
Heschl's |
|||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Male | 2170.4 ± 553.5 | 1590.3 ± 259.9 | 2593.6 ± 600.4 | 2180.6 ± 316.8 | 902.5 ± 280. 8 | 609.7 ± 95.7 | 1052.2 ± 209.5 | 908.2 ± 123.9 |
| controls | (0.13) | (0.06) | (0.08) | (0.06) | (0.1) | (0.07) | (0.09) | (0.06) |
| Female | 1910.4 ± 421.1 | 1486.5 ± 584.9 | 2041.4 ± 600.8 | 1648.5 ± 333.8 | 709.5 ± 173.2 | 544.5 ± 191.2 | 946.9 ± 257.8 | 748.4 ± 196.5 |
| controls | (0.08) | (0.11) | (0.09) | (0.08) | (0.07) | (0.12) | (0.09) | (0.06) |
| Male | 2186.5 ± 600.4 | 1536.4 ± 756.0 | 2279.5 ± 849.2 | 2014.8 ± 694.6 | 840.9 ± 310.9 | 615.1 ± 293.9 | 1007.8 ± 350.4 | 890.9 ± 239.2 |
| patients | (0.12) | (0.15) | (0.11) | (0.08) | (0.09) | (0.16) | (0.12) | (0.11) |
| Female | 1715 ± 610.5 | 1517.5 ± 526.7 | 1707.5 ± 406.3 | 1783.4 ± 355.4 | 636 ± 240.3 | 606.2 ± 212.5 | 758.3 ± 220.0 | 847. 4 ± 196.4 |
| patients | (0.11) | (0.11) | (0.09) | (0.07) | (0.11) | (0.1) | (0.07) | (0.06) |
Means, SD and co-efficients of error of measurements.
Table 4.
Minicolumn spacing and number
| Group | Heschl's minicolumn spacing (μm) |
Planum minicolumn spacing (μm) |
Heschl's minicolumn number |
Planum minicolumn number |
||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Male control | 79.4 ± 11.3 | 77.7 ± 10.17 | 88.5 ± 12.6 | 83.8 ± 8.5 | 184 024 ± 59 505 | 177 980 ± 48 627 | 122 397 ± 29 949 | 100 926 ± 25 454 |
| Female control | 74.0 ± 7.52 | 73.2 ± 13.2 | 80.8 ± 7.6 | 79.3 ± 10.2 | 210 923 ± 51 557 | 183 143 ± 68 440 | 129 785 ± 51 483 | 108 530 ± 51 759 |
| Male schizophrenia | 75.0 ± 17.6 | 72.0 ± 8.1 | 85.7 ± 10.6 | 77.4 ± 14.0 | 162 520 ± 37 750 | 219 999 ± 66 735 | 137 530 ± 56 826 | 134 214 ± 74 075 |
| Female schizophrenia | 79.7 ± 13.9 | 80.5 ± 10.5 | 86.3 ± 12.8 | 88.1 ± 11.7 | 152 654 ± 46 574 | 164 490 ± 29 945 | 96 498 ± 20 811 | 90 440 ± 37 137 |
Means and SD of measurements.
Fewer cases [N = 10 schizophrenia (5M, 5F), 16 controls (7M, 9F)] were sampled for Heschl's gyrus due to tissue exclusion, for example by damage during post-mortem brain extraction. (CEs are not included since minicolumn measures were by non-stereological automated method, previously validated).
Tests refer to specific separable elements of the study: (i) altered minicolumn configuration (Casanova et al., 2005), (ii) altered gross regional morphometry (area and volume) (Kawasaki et al., 2007), (iii) the relationship between region size and minicolumn spacing (Chance et al., 2006a), (iv) the relationship between minicolumn asymmetry and corpus callosum (compared with Chance et al., 2006a) and (v) different effects of age on minicolumn spacing (Chance et al., 2006b). F-statistics, t-statistics or Pearson's r coefficents have been reported, as appropriate.
Results
Minicolumn spacing
In the planum temporale, centre-to-centre minicolumn spacing was altered in schizophrenia in a sex-dependent manner, with a decrease in males (control mean = 86.2 µm, patient mean = 81.6 µm) and an increase in females (control mean = 80.1 µm, patient mean = 87.2 µm) (sex × diagnosis F = 4.5, df 1,29, P = 0.04). No other main effects of diagnosis, sex or hemisphere were observed. Age at death and fixation time were not significant covariates.
As there was a difference in the number of cases for which successful measurements were possible in each region, minicolumn data for Heschl's gyrus were treated separately from the planum temporale (Table 4). In Heschl's gyrus, there was no effect of diagnosis, sex, hemisphere or their interactions on minicolumn spacing. Age was not a significant covariate although it was included in the ANOVA, as described in the methods (above) and fixation was also included due to a significant interaction with hemisphere (F = 5.5, df 1,17, P = 0.03).
The cell sparse, peripheral region of minicolumns was found to constitute a mean 29% of minicolumn spacing in all sex × diagnosis groups and was not subjected to separate tests.
Minicolumn number
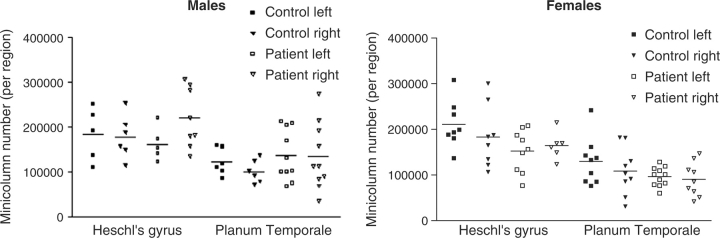
A decrease in total minicolumn number per region was found in the PT in female patients (control mean = 119 158, patient mean = 93 469 [although note that values for the female right hemisphere fall within the normal control range)] and an increase in male patients compared with controls (control mean = 111 662, patient mean = 135 872) (sex × diagnosis F = 5.9, df 1,28, P = 0.02) (Fig. 1). No other significant effects were observed and neither fixation nor age was a significant covariate.
Fig. 1.
Minicolumn number in HG and PT is increased in males and decreased in females with schizophrenia. (Males are shown in the graph on the left, females are shown in the graph on the right. Control subjects are indicated by solid shapes, patients by unfilled shapes. HG is represented by the four columns of data on the left of each graph and PT by the four columns on the right. The left hemisphere is represented by squares, the right hemisphere by triangles.)
Tested separately in HG, minicolumn number was not changed, although there was a non-significant trend for a sex × diagnosis interaction that echoed the finding in planum temporale of a decrease in female patients and an increase in male patients (F = 3.9, df 1,17, P = 0.065). This result was not significant partly because the left hemisphere in males did not conform to this pattern. Neither fixation nor age was a significant covariate.
Region size
Reduced surface areas of both PT and HG were found in patients relative to controls in the left hemisphere (two-level analysis, diagnosis × hemisphere F = 7.7, df 1,32, P < 0.01) (Table 3). (This hemisphere effect is also emphasized by a small increase in the right hemisphere, particularly in female patients compared with controls.) Furthermore, surface areas were less in females than males (two-level analysis, sex effect F = 7.2, df 1,32, P = 0.01), particularly in the left hemisphere in both patients and controls, resulting in overall less asymmetry in females (two-level analysis, sex × hemisphere F = 6.6, df 1,32, P = 0.02). Fixation time, with a strong trend for an interaction, was included as a covariate (hemisphere × fixation, F = 3.8, df 1,32, P = 0.06). Age was not a significant covariate and therefore not included.
For the analysis of cortical volume, a two-level analysis including region as a factor failed Box's M-test for homogeneity of variance and so HG was analysed independently from PT since both structures passed Box's M-test independently. For HG no change was found in schizophrenia, although the gyrus was larger on the left (hemisphere effect F = 7.1, df 1,33, P = 0.01) and larger in males than females (sex effect F = 8.4, df 1,33, P < 0.01). Fixation and age were not significant covariates and therefore not included in the ANOVA. In the separate test of PT, volume was also unchanged in schizophrenia (F = 0.1, df 1,33, P = 0.75), while the usual left > right volume asymmetry persisted (hemisphere effect, F = 16.1, df 1,33, P < 0.01), with no differences dependent on sex. Age and fixation were not significant covariates and therefore not included in ANOVA.
Correlations
Region size and minicolumn spacing
In HG, minicolumn spacing was independent of surface area (correlations between surface area and minicolumn spacing were r = 0.2, P = 0.31 for the left hemisphere and r = 0.3, P = 0.12 for the right hemisphere). However, in PT, minicolumn spacing in the left hemisphere was positively correlated with surface area (r = 0.37, P = 0.03). The right hemisphere, which has smaller minicolumn spacing, did not show this correlation (r = 0.04, P = 0.82).
Mean minicolumn spacing asymmetry of PT is more than that of HG in normal control subjects (Chance et al., 2006a). In schizophrenia, by contrast, the asymmetry was less in PT (mean asymmetry coefficient = 3.4% of bihemispheric mean) than in HG (mean asymmetry coefficient = 6.7% of bihemispheric mean).
Minicolumn asymmetry and callosal axons
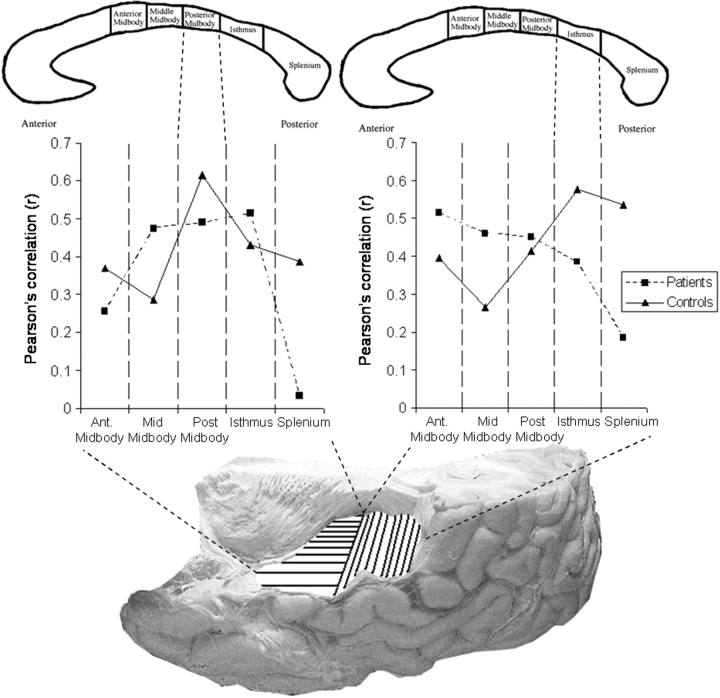
The relationship of minicolumn number asymmetry in HG and PT to axonal fibre number in the five middle and posterior subregions of the corpus callosum was tested. In patients, the correlation values did not have clear peaks selective to the appropriate regions of the corpus callosum. Instead, the data showed poor selectivity for the expected callosal subregions, with similar correlations for several different subregions (Fig. 2).
Fig. 2.
Bottom: Image of the superior surface of the left temporal lobe. HG (horizontal shading) and PT (vertical shading) project through different subregions of the corpus callosum. Top: Two images of the corpus callosum. For HG the key subregion (upper left) is identified, by dotted lines linking it to the graph on the left, as the posterior midbody, for PT the key subregion (upper right) is the isthmus, linked by dotted lines to the graph on the right. Middle: The middle graphs show correlations between minicolumn number asymmetry and axonal fibre number for subregions of the corpus callosum. Middle left: Pearson r-values are plotted for HG primary auditory cortex against the five subregions. Middle right: Pearson r-values are plotted for PT association cortex against the five subregions. For both graphs, control subject data are represented by triangles (solid line) and patient data by squares (dashed line). [r-Values are directionless (√r2) to ease comparison, indicating a correlation between increasing axon number and either increasing leftwards asymmetry (for HG) or decreasing leftwards asymmetry (for PT).] In controls, a clear peak correlation is seen for the subregions through which the respective cortical regions (HG and PT) are known to project. However, this peak correlation is not clear and is not selective to the appropriate subregion in patients.
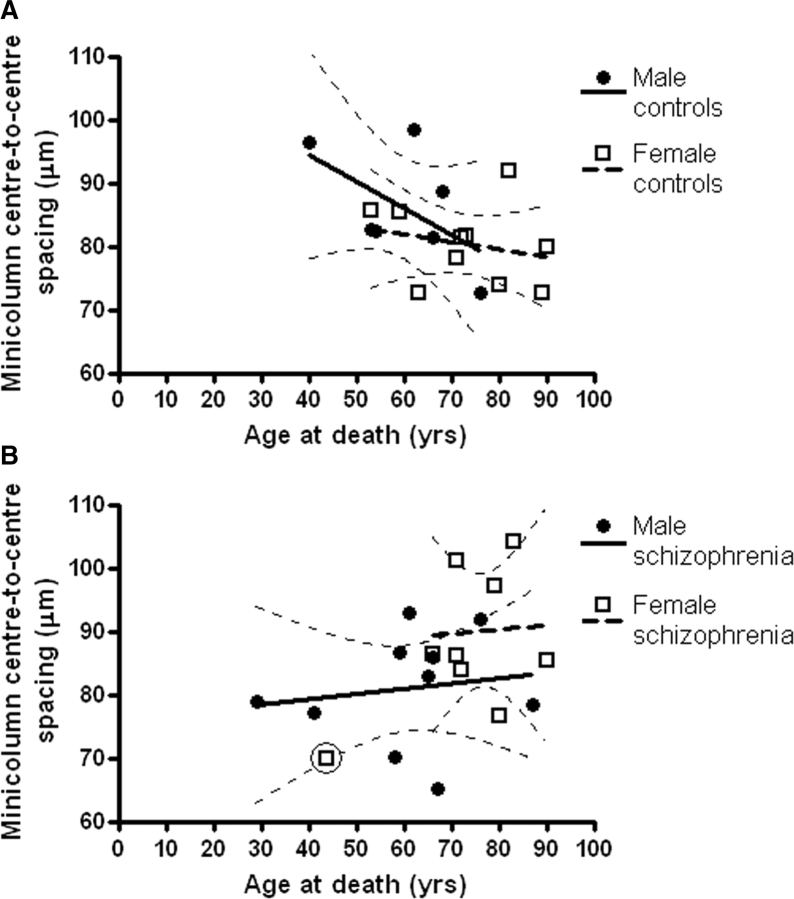
Minicolumn spacing and age
Due to normal age-associated thinning, Pearson's correlation analysis found a negative correlation between minicolumn spacing and age for the PT in Controls (r = −0.51, P = 0.04). As seen in Fig. 3A, both males and females show a negative correlation. In contrast, patients did not show a negative ageing effect (r = 0.4, P = 0.09). This trend for a positive correlation depended on the combination of males and females, including a single female outlier. More rigorous statistical consideration suggested that the sexes should be separated and the outlier excluded. Following this, the regression lines were horizontal, still showing no effect of age (Fig. 3B).
Fig. 3.
Age-associated minicolumn shrinkage is found in the Planum Temporale in controls but this is absent in patients. Graphs show the bihemispheric mean minicolumn spacing in the planum temporale for male and female groups, with linear regression lines and 95% CIs. Overall, the negative correlation for control subjects (A) is significant (r = −0.51, P = 0.04) but there was no negative relationship for patients (B). (One outlier in the female patient group has been ringed and was excluded from linear regression due to its disproportionate effect on the result, see Results section).
HG did not show an effect of age in controls (as reported previously, r = −0.21, P = 0.46) or patients (r = 0.05, P = 0.89).
Discussion
Literature review
Our review of previous imaging studies (Table 5) identified several features of the literature. Overall, half of the studies report a change in PT in schizophrenia, 13 report a decrease, 9 of which are selective to the left hemisphere, and 3 report an increase, 2 of which are selective to the right hemisphere. Most studies investigated Heschl's gyrus as well and since changes occur in both HG and PT, their interaction deserves attention. Structural changes in these areas are among the most frequent to be associated with functional deficits. Change in Heschl's gyrus has been associated with hallucinations, semantics, mismatch negativity, illness duration and auditory sensory memory. Asymmetry or left hemispheric size of PT has been correlated with delusions, positive symptoms, phonetic mismatch strength, hallucinatory behaviour, social withdrawal, stereotyped thinking, memory deficits, P300 amplitude, suspiciousness, left ear advantage, phonology, psychosis duration and thought disorder. Of this wide range, the most consistent relationship is that of PT size with auditory mismatch responses (McCarley et al., 1993, 2002; Yamasue et al., 2004; Salisbury et al., 2007). From the 34 studies reviewed, the male–female ratio among patients was approximately 5:2. For the majority of studies, the numbers of female subjects were too small to analyse sex differences (only 41% of studies had more than five females in the patient group of which three studies found sex differences) that should be addressed in future work. Although there are far fewer post-mortem studies, some sex differences are also reported (Table 6).
Table 5.
Summary table of MRI studies examining the Planum Temporale (and Heschl's gyrus) in schizophrenia
| Focus of study | References | Sample size |
Finding | Asymmetry | Sex difference | Functional correlates | Notes | |
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | |||||||
| Volume | Kawasaki et al. (2007) | 60 (30 M, 30 F) | 60 (30 M, 30 F) | PT reduced asymmetry due to left PT ↓ | PT L > R in controls and patients | No | Clinical variables did not correlate | |
| Cortical thickness | Qiu et al. (2007) | 20 (10M, 10F) | 20 (10M, 10F) | Anterior left PT thinner, posterior thicker | Not quantified | Not tested | No | |
| Volume | Salisbury et al. (2007) | 32 (22M, 10F) | 20 (17M, 3F) | Left HG grey matter ↓ | Grey matter L > R all subjects. Asymmetric HG reduction | Not tested | Asymmetric HG reduction correlated with MMN reduction | First episode, longitudinal |
| Volume correlates | Weinstein et al. (2007) | 0 | 12 (7M, 5F) | No comparison with controls | Only left hemisphere measured | No | PT grey matter correlated with thought disorder and fMRI activation | |
| Volume correlates | Takahashi et al. (2007) | 0 | 38 (20M, 18F) | No comparison with controls | PT and HG L > R but not statistically significant | Not tested | Correlation between untreated psychosis duration and left PT volume | |
| Volume | Yamasaki et al. (2007) | 17 | 22 | HG no change PT ↓ bilateral | PT and HG L > R but not statistically significant | All males | PT reduction correlated with delusional behaviour | |
| Volume correlates | (2007) Walder et al. (related to Goldstein et al. (2002)) | 15 (6M, 9F) | 19 (11M, 8F) | (See Goldstein et al. (2002)) | (See Goldstein et al. (2002)) | PT: L > R in males | All: left PT associated with phonology. Females: right HG associated with semantics and phonology | |
| Volume | Takahashi et al. (2006) | 72 (38M, 34F) | 65 (35M, 30F) | STG↓, HG↓, PT↓ | Controls: L > R in STG, HG, PT, Scz: PT left sided reduction | No | No | |
| Volume | Sumich et al. (2005) | 0 (no control group) | 25 | – | See functional correlates | All males | Left HG vol associated with hallucinations, Left PT associated with delusions | |
| Volume | Crespo-Facorro et al. (2004) | 30 | 30 | Right HG↓ | All groups: L > R PT and HG. Scz: Right HG↓(volume and surface area) | All males | HG↓: Longer illness duration, PT↓: positive symptoms | |
| Spatial probability | Park et al. (2004) | 21 (up to 5 female) | 17 (up to 4 female) | Greater structural variation of HG and PT in scz | No | Not tested | No | First episode |
| Volume | Yamasue et al. (2004) | 19 (13M, 6F) | 13 (7M, 6F) | Left PT↓ | Controls: no clear asymmetry, Scz: left PT↓ | Not tested | Left PT reduction associated with phonetic mismatch strength | |
| Volume | Kasai et al. (2003) | 22 (20M, 2F) | 13 (10M, 3F) | Left HG↓, left PT↓ | All groups: L > R HG, Scz: left HG↓, left PT↓ | Not tested | No | First episode, longitudinal change over time |
| Volume | Sallet et al. (2003) | 20 (12M, 8F) | 40 (24M, 16F) | No difference | No clear asymmetry | None reported | Left PT vol associated: hallucinatory behaviour, social withdrawal and stereotyped thinking. Reversed asymmetry: memory deficits and judgement of NSRS | |
| Volume | Sumich et al. (2002) | 16 | 25 | Left PT↓ | Controls: no clear asymmetry, Scz: left PT↓ | All males | No | |
| Volume | Meisenzahl et al. (2002) | 30 | 30 | No difference | All groups: L > R PT | All males | No | First episode |
| Volume | McCarley et al. (2002) | 18 (15M, 3F) | 15 (12M, 3F) | Bilateral HG↓, left PT↓ | Controls: L > R in HG, PT, Scz: PT left sided reduction | Not tested | Left post. STG↓: left temporal P300 amplitude↓ | First episode |
| Volume | Goldstein et al. (2002) | 48 (27M, 21F) | 40 (27M, 13F) | HG↓ in males, right PT↑ in females | Scz: right PT↑ in females | Controls: L > R PT. Scz: HG↓ in males, right PT↑ in females, left PT↓ in males | No | |
| Volume | Shapleske et al. (2001) | 32 | 74 | No difference | All groups: L > R PT | All males | No correlations with symptom clusters | |
| Volume | Hirayasu et al. (2000) | 22 (20M, 2F) | 20 (16M, 4F) | Bilateral HG↓, left PT↓ | All groups: L > R HG, Scz: left PT↓ | Not tested | No | First episode |
| Volume | Kwon et al. (1999) | 16 | 16 | Left PT↓ | All groups: HG no clear asymmetry, Controls: L > R PT, Scz: left PT↓ | All males | Suspiciousness/persecution correlated with left PT volume | |
| Volume | Frangou et al. (1997) | 39 (19M, 20F) | 32 (21M, 11F) | No difference | All groups: L > R PT | No | No | |
| Volume/surface area | Barta et al. (1997) (also Pearlson et al.1996) | 32 | 28 | Scz: right PT thickness↓ | No clear asymmetry | Reversed PT surface area in male and female patients | No | |
| Surface area | Kulynych et al. (1995) | 12 | 12 | No difference | All groups: L > R PT, no asymmetry HG | All males | No | |
| Surface area | Mosnik et al. (1995) | 10 | 10 | Scz: PT surface smaller | All groups: L > R PT | All males | PT asym↑: left ear advantage↑ | |
| Volume | O'Leary et al. (1995) | 10 | 10 | No difference | All groups L > R PT | PT rCBF greater individual variability during language tests | ||
| Surface area | Ward et al. (1995) | 30 | 30 | PT no difference, HG↓ | All groups: L > R | None reported | HG↓: auditory sensory memory↓ | |
| Surface area | Petty et al. (1995) | 14 (9M, 5F) | 14 (9M, 5F) | PT reversed asymmetry | Controls: PT L > R, Scz: PT R > L | No | Greater thought disorder: greater reversal of asymmetry | |
| Surface area | Shenton et al. (1995) | 15 | 15 | PT surface↑ (L > R) | All groups: PT L > R | All males | No | |
| Surface area | Rossi et al. (1994) | 23 (13M, 10F) | 22 (13M, 9F), | No difference | All groups: L > R | Not tested | PT asymmetry↓: thought disorder↑ | |
| Surface area | Kleinschmidt et al. (1994) | 26 (13M, 13F) | 26 (13M, 13F), | No difference | All groups: L > R | No | No | First episode |
| Surface area | DeLisi et al. (1994) | 40 (24M, 16F) | 85 (50M, 24F), | No difference | Diagnosis difference dependent on antero–posterior boundary | Female PT asymmetry reduced | No relationship to thought disorder or hallucinations | First episode |
| Surface area | Vladar et al. (1993) | 8 (discordant twins) | 8 (discordant twins) | No difference | All groups: L > R | Not tested | No | |
| Surface area | Rossi et al. (1992) | 12 | 20 | Scz: left PT↓, right PT↑ | Controls: PT L > R, Scz: PT R > L | No | ||
| Approximately 60% studies report volume as primary measure | Summary: | 772 (F : M ratio ∼2 : 5) | 907 (F : M ratio ∼2 : 5) | 13/29 studies report decrease of PT, 9 selective to left | 21/30 studies report L > R in controls | 3/11 studies report sex difference, most underpowered | Correlations replicated with auditory mismatch and thought disorder | First episode N = 226. 5/8 studies positive |
Studies since 1992 were identified on the basis of a PubMed search in October 2007 using the search terms ‘Planum Temporale’ and ‘schizophrenia’ or inclusion in the previous meta-analyses of Sommer et al. (2001) or Shapleske et al. (1999). 32 of 34 studies report region size; ↓ = reduction, ↑ = increase.
Table 6.
Post mortem studies are few compared to MRI
| Focus of study | References | Sample size |
Finding | Asymmetry | Sex difference | Functional correlates | Notes | |
|---|---|---|---|---|---|---|---|---|
| Control | Patients | |||||||
| Cell density | Chance et al. (2005) | 12 (5M, 7F) | 12 (7M, 5F) | Calbindin interneuron density↓ | No | Scz: Male calbindin interneuron size↑, Female size↓ | No | PM cell density |
| Cell distribution | Beasley et al. (2005) | 15 (9M, 6F) | 15 (9M, 6F) | Neuron clustering↓, no change in density | Both hemispheres not tested | No | PM cell distribution | |
| Cell size | Sweet et al. (2003) | 18 (10M, 8F) | 18 (10M, 8F) | Pyramidal neuron size↓ | Only left hemisphere tested | No | No | PM cell size |
| 5-HT receptors | Pralong et al. (2000) | 20 (3F, 17M) | 20 (3F, 17M) | 5-HT2a receptor binding density reduced | Only left hemisphere tested | Not tested | PM receptors | |
| Cortical volume | Falkai et al. (1995) | 24 | 24 | Left PT↓ | Controls: L > R, Scz: R > L | Scz: Male right PT size↑, Female right PT size↓ | No | PM cortical volume |
Overall neuron density is not clearly altered but more subtle reductions of interneuron subpopulations, neuron size and clustering appear to contribute to the altered cortical volume reported in MRI. There is some evidence for sex differences but in general asymmetries and sex differences have not been adequately investigated.
Neuropathological study
Region size
The surface areas of both Heschl's gyrus and the planum temporale in the left hemisphere were reduced in schizophrenia. The change in cortical volume was less. Consistent with a meta-analysis of MRI studies, the volume of planum temporale cortex on the left was not reduced significantly. Thus, loss of asymmetry of the planum temporale reflects a greater change in surface area than volume.
The findings relate to a problem outstanding in the imaging literature. Some authors (e.g. Kulynych et al., 1995; Frangou et al., 1997; Meisenzahl et al., 2002) have failed to find the losses of asymmetry of the planum temporale reported by others [for meta-analyses see Shapleske et al. (1999) and Sommer et al. (2001)]. Barta et al. (1997) suggested that measurements of the surface area of the planum temporale were more important than those of volume and reported reversal of surface area asymmetry but an absence of asymmetry of volume. Our findings are in agreement with this suggestion. The significance of surface area as a measurement is brought into focus by the findings of Harasty et al. (2003) that asymmetry of the planum temporale in normal individuals is due to expansion (‘ballooning’) of the cortex on the left side relative to the right. A change in surface area implies a change in minicolumn number and spacing. We have shown that this is the case not only in the planum temporale, which shows the greatest change, but also in Heschl's gyrus, which may relate to early auditory perceptual abnormalities in patients.
Differences between HG and PT
Given that there are surface area differences of HG but no accompanying minicolumn spacing differences and that HG minicolumn spacing was not correlated with surface area, variation in HG size appears to be more dependent on the early established proliferation in number rather than spacing of minicolumns. In PT, in contrast, both surface area and minicolumn spacing differences were found in schizophrenia and minicolumn spacing in the left hemisphere was positively correlated with surface area, consistent with Harasty et al. (2003), indicating that the size and asymmetry of this region is linked more closely to the spacing of its minicolumns. Surface area, therefore, depends partly on proliferation of minicolumns, but also, particularly in association cortex, on later expansion of minicolumn spacing. These are the variables (proliferation and spacing) that are altered in schizophrenia and most of all in the latest maturing, most asymmetric cortex (thus alteration of the size of the left PT was correlated most closely to the spacing of minicolumns).
Regional differences may relate to the hierarchical relationship between PT and HG in which PT is the recipient of feed-forward projections from the primary auditory area of HG and plays a role in more integrative, associative processing than HG. The two regions differ in maturation (Guillery, 2005; Chance, 2006; Toga et al., 2006), dendritic arborization (Elston et al., 1999), asymmetry and neuroplasticity (Arendt, 2004).
For minicolumn spacing in HG, there was a statistical interaction between fixation and hemisphere. Effects of fixation time are of greater concern for methods such as immunohistochemistry for which antigen retrieval techniques may be necessary but the Nissl stains used here are relatively robust and unlikely to be systematically affected by fixation. Given that shrinkage due to formalin fixation stabilizes after a few weeks and all of the brains in the present study were fixed for longer than that and furthermore both hemispheres were in formalin for identical periods, the interaction between fixation time and hemisphere seems unlikely to be causal. Any suggestion that the neuropil is more vulnerable to shrinkage in one hemisphere is contradicted by the absence of an equivalent, asymmetric fixation effect in PT. Similarly, the longer fixation time in female patients did not result in a sex-dependent fixation effect.
Callosal interaction
In schizophrenia, among the middle and posterior callosal subregions, the strongest correlation for area PT was with fibre number in the anterior midbody, forward of the appropriate subregion, the isthmus, through which posterior auditory association cortex projects (Pandya and Seltzer, 1996). However, the direction of the relationship was the same as for controls (Chance et al., 2006a) indicating that a larger number of minicolumns in the right PT (reduced leftwards asymmetry) was associated with increased axon number. For HG, the strongest correlation was with fibre number in the isthmus, which is posterior to the expected subregion—the posterior midbody—through which primary auditory cortex is known to project. In this case, increasing number of minicolumns in the left HG (increased leftwards asymmetry) was associated with increased axon number (Fig. 2), also similar to controls.
The results indicate different relationships between minicolumn asymmetry and callosal axon number for HG and PT. The PT data show that less leftwards asymmetry is correlated with increasing axon number, consistent with predictions that greater leftwards, typical cortical asymmetry is associated with fewer interhemispheric connections. The relationship for HG is the reverse. A further interpretation is that an increased number of interhemispheric axons is associated with a more rightward bias for PT and a leftward bias for HG. This is of interest given that auditory processing that varies in the temporal domain is processed preferentially by HG in the left hemisphere whereas variation in the spectral domain is preferentially processed by the auditory belt areas in the right hemisphere (Jamison et al., 2006). Therefore, it is possible that the domain-sensitive processing bias for each region depends on callosal interaction.
Sex differences and minicolumn changes
There were sex differences in the present study. In summary, surface area (and volume in HG) was generally larger and more asymmetrical in males—a common sex difference. For minicolumn measures (number and spacing) in PT, the values for patients resembled those of the control group of the opposite sex. A similar effect was not significant in HG.
The peculiar reversal of the effect of schizophrenia in each sex in the minicolumn measures may imply a different pathology in each sex. However, the age difference between sexes here raised the possibility of a more parsimonious interpretation that the direction of change in schizophrenia was dependent on age. Further investigation supported this unifying view, although it revealed that there are slight differences between the sexes in the normal trajectory of age-related changes that should be taken into account. Therefore, the apparent reversal of the effect of schizophrenia in PT may be understood in terms of normal sex differences in the proliferation and neuropil basis of minicolumn spacing, over which is superimposed a failure of age-associated, neuroplastic processes in schizophrenia.
The normally faster maturing female brain (Kretschmann et al., 1979) is associated with more narrow minicolumns relative to the male brain. It appears that the prolonged development in males contributes to wider minicolumns, larger region size and greater asymmetries in the mature brain compared with that of females.
Neuroplasticity and disease course
Normal dendritic remodelling and age-associated changes in minicolumn spacing are associated with neuroplasticity that persists into adult life (Arendt, 2004; Chance et al., 2006b). In schizophrenia, there is an absence of ageing changes in minicolumn spacing, as reported here, that is consistent with loss of plasticity. The greater dependence in males on a longer period of maturation confers vulnerability to a greater deficit in schizophrenia. In males the greater deficit results in greater loss of Minicolumn spacing. In females the deficit is less so that in old age, and in the absence of the normal age-associated thinning, minicolumn spacing in patients is actually greater than that of controls.
Other sex differences have been found in this series of brains in the asymmetrical volumes of the superior temporal (Highley et al., 1999b), fusiform and parahippocampal (McDonald et al., 2000) gyri. These differences are potentially relevant to a sex difference in the manifestation of the disease: onset is earlier in males than females (Penrose, 1991; Hafner, 2003) and in general earlier onset is a predictor of poor outcome (Eaton et al., 1992), particularly in males. Therefore, it is paradoxical that onsets of psychosis are earlier in males although the female brain usually matures faster than that of the male (Kretschmann et al., 1979).
We draw attention to the fact that the corpus callosum goes on developing in size later in females than in males (Cowell et al., 1992; Pujol et al., 1993) continuing through the third and fourth decades of life, providing a close correlate of age of onset. The sex-dependent findings in the planum temporale here parallel those of the corpus callosum reported previously (Highley et al., 1999a): the densities of minicolumns in PT and axons in corpus callosum are decreased in females with schizophrenia and increased in males.
There may be an association between the arrest of axodendritic plasticity seen in the minicolumn data and the peak of callosal maturation (i.e. myelination). In male patients an early arrest means that neuropil expansion is low compared with controls, leading to more dense minicolumns. In females with a relatively late arrest in schizophrenia, the deficit is smaller.
Conclusions
The effect of illness on auditory cortex region size and asymmetry can be understood in terms of the lifetime trajectory of neuropil change in the underlying cytoarchitecture. The absence of ageing effects in schizophrenia supports the concept of failure of axo-dendritic plasticity, which is most acute in those areas of the brain that go on developing longest (asymmetric association cortex). Schizophrenia can be conceived to involve transcallosal misconnection with timing of onset that reflects the sex difference in maturation of the corpus callosum. We propose that altered auditory perception in schizophrenia is related to differences in maturation and asymmetry of language cortex and its interhemispheric connections.
Funding
Alzheimer Scotland, UK; the Medical Research Council, UK; the TJ Crow Psychosis Trust, UK; the SANE charity, UK; National Institute of Mental Health (RO1 MH62654 to M.F.C., RO1 MH61606 to A.E.S.).
Acknowledgements
Thanks to Mary Walker (University of Oxford, UK) for technical support and to Prof. Margaret Esiri (University of Oxford, UK) and Dr Brendan McDonald (John Radcliffe Hospital, Oxford, UK).
References
- Arendt T. Neurodegeneration and plasticity. Int J Dev Neurosci. 2004;22:507–14. doi: 10.1016/j.ijdevneu.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Brill LB 2nd, Royall R, McGilchrist IK, Pulver AE, et al. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry. 1997;154:661–7. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Chana G, Honavar M, Landau S, Everall IP, Cotter D. Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophr Res. 2005;73:69–78. doi: 10.1016/j.schres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Casanova MF, de Zeeuw L, Switala A, Kreczmanski P, Korr H, Ulfig N, et al. Mean cell spacing abnormalities in the neocortex of patients with schizophrenia. Psychiatry Res. 2005;133:1–12. doi: 10.1016/j.psychres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Switala AE. Minicolumnar morphometry: computerized image analysis. In: Casanova MF, editor. Neocortical modularity and the cell minicolumn. New York: Nova Biomedical; 2005. pp. 161–80. [Google Scholar]
- Chance SA. Subtle changes in the ageing human brain. Nutr Health. 2006;18:217–24. doi: 10.1177/026010600601800303. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ. Minicolumnar structure in Heschl's gyrus and Planum temporale: asymmetries in relation to sex and callosal fibre number. Neuroscience. 2006a;143:1041–50. doi: 10.1016/j.neuroscience.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Chance SA, Casanova MF, Switala AE, Crow TJ, Esiri MM. Minicolumn thinning in temporal lobe association cortex but not primary auditory cortex in normal human ageing. Acta Neuropathol (Berl) 2006b;111:459–64. doi: 10.1007/s00401-005-0014-z. [DOI] [PubMed] [Google Scholar]
- Chance SA, Esiri MM, Crow TJ. Ventricular enlargement in schizophrenia: a primary change in the temporal lobe? Schizophrenia Res. 2003;62:123–31. doi: 10.1016/s0920-9964(02)00344-4. [DOI] [PubMed] [Google Scholar]
- Chance SA, Tzotzoli PM, Vitelli A, Esiri MM, Crow TJ. The cytoarchitecture of sulcal folding in Heschl's sulcus and the temporal cortex in the normal brain and schizophrenia: lamina thickness and cell density. Neurosci Lett. 2004;367:384–8. doi: 10.1016/j.neulet.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Chance SA, Walker M, Crow TJ. Reduced density of calbindin immunoreactive interneurons in the planum temporale in schizophrenia. Brain Res. 2005;1046:32–7. doi: 10.1016/j.brainres.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Allen LS, Zalatimo NS, Denenberg VH. A developmental study of sex and age interactions in the human corpus callosum. Brain Res Dev Brain Res. 1992;66:187–92. doi: 10.1016/0165-3806(92)90079-c. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim JJ, Chemerinski E, Magnotta V, Andreasen NC, Nopoulos P. Morphometry of the superior temporal plane in schizophrenia: relationship to clinical correlates. J Neuropsychiatry Clin Neurosci. 2004;16:284–94. doi: 10.1176/jnp.16.3.284. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Crow LR, Done DJ, Leask S, Crow TJ. Schizophrenia as a transcallosal misconnection syndrome. Schizophr Res. 1998;30:111–4. doi: 10.1016/s0920-9964(97)00139-4. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Hoff AL, Neale C, Kushner M. Asymmetry in the superior temporal lobe in men and women first schizophrenic patients. Measure of the planum temporale and superior temporal gyrus in MRI. Schizophr. Res. 1994;12:19–28. doi: 10.1016/0920-9964(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Bilker W, Haro JM, Herrman H, Mortensen PB, Freeman H, et al. Long-term course of hospitalization for schizophrenia: part II. Change with passage of time. Schizophr Bull. 1992;18:229–41. doi: 10.1093/schbul/18.2.229. [DOI] [PubMed] [Google Scholar]
- Elston GN, Tweedale R, Rosa MG. Cellular heterogeneity in cerebral cortex: a study of the morphology of pyramidal neurones in visual areas of the marmoset monkey. J Comp Neurol. 1999;415:33–51. doi: 10.1002/(sici)1096-9861(19991206)415:1<33::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, et al. Disturbed planum temporale asymmetry in schizophrenia. A quantitative post-mortem study. Schizophr Res. 1995;14:161–76. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Kelly G. Minicolumnar organization within somatosensory cortical segregates I: development of afferent connections. Cereb Cortex. 1994a;4:408–27. doi: 10.1093/cercor/4.4.408. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Kelly G. Minicolumnar organization within somatosensory cortical segregates II: emergent functional properties. Cereb Cortex. 1994b;4:428–42. doi: 10.1093/cercor/4.4.428. [DOI] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Sigmundsson T, Barta P, Pearlson GE, Murray RM. The Maudsley family study: IV. Normal planum temporale asymmetry in familial schizophrenia: a volumetric MRI study. Br J Psychiatry. 1997;170:328–33. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Gabbott PL. Radial organisation of neurons and dendrites in human cortical areas 25, 32, and 32'. Brain Res. 2003;992:298–304. doi: 10.1016/j.brainres.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:154–64. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Is postnatal neocortical maturation hierarchical? Trends Neurosci. 2005;28:512–7. doi: 10.1016/j.tins.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Harasty J, Seldon HL, Chan P, Halliday G, Harding A. The left human speech-processing cortex is thinner but longer than the right. Laterality. 2003;8:247–60. doi: 10.1080/13576500244000175. [DOI] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999a;122:99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- Highley JR, McDonald B, Walker MA, Esiri MM, Crow TJ. Schizophrenia and temporal lobe asymmetry. A post-mortem stereological study of tissue volume. Br J Psychiatry. 1999b;175:127–34. doi: 10.1192/bjp.175.2.127. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–9. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison HL, Watkins KE, Bishop DVM, Matthews PM. Hemispheric specialization for processing auditory nonspeech stimuli. Cereb Cortex. 2006;16:1266–75. doi: 10.1093/cercor/bhj068. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–75. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Takahashi T, Nohara S, McGuire PK, Seto H, et al. Biol Psychiatry. 2007. Anomalous cerebral asymmetry in patients with schizophrenia demonstrated by voxel-based morphometry. October 13 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kim JJ, Crespo-Facorro B, Andreasen NC, O'Leary DS, Zhang B, Harris G, et al. An MRI-based parcellation method for the temporal lobe. Neuroimage. 2000;11:271–88. doi: 10.1006/nimg.2000.0543. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Rapp A, Grodd W, Buchkremer G, Weiskopf N, Lutzenberger W, et al. Mismatch negativity responses in schizophrenia: a combined fMRI and whole-head MEG study. Am J Psychiatry. 2004;161:294–304. doi: 10.1176/appi.ajp.161.2.294. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Falkai P, Huang Y, Schneider T, Furst G, Steinmetz H. In vivo morphometry of planum temporale asymmetry in first episode schizophrenics. Schizophr Res. 1994;12:9–18. doi: 10.1016/0920-9964(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ, Schleicher A, Wingert F, Zilles K, Loblich HJ. Human brain growth in the 19th and 20th century. J Neurol Sci. 1979;40:169–88. doi: 10.1016/0022-510x(79)90202-8. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Fantie BD, Jones DW, Weinberger DR. Normal asymmetry of the planum temporale in patients with schizophrenia: three dimensional cortical morphometry with MRI. Br J Psychiatry. 1995;166:742–9. doi: 10.1192/bjp.166.6.742. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. A 3D surface rendering in MRI morphometry: a study of the planum temporale. J Comput Assisted Tomogr. 1993;17:529–35. doi: 10.1097/00004728-199307000-00003. [DOI] [PubMed] [Google Scholar]
- Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, et al. Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry. 1999;56:142–8. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, et al. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–31. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50:190–7. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, et al. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a postmortem study. Am J Psychiatry. 2000;157:40–7. doi: 10.1176/ajp.157.1.40. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Zetzsche T, Preuss U, Frodl T, Leinsinger G, Moller HJ. Does the definition of borders of the planum temporale influence the results in schizophrenia? Am J Psychiatry. 2002;159:1198–200. doi: 10.1176/appi.ajp.159.7.1198. [DOI] [PubMed] [Google Scholar]
- Mosnik MD, O’Leary DS, Tranel AP, Andreasen NC. Left hemisphere language dominance in schizophrenic patients and normal controls: a study of functional and anatomical asymmetries. Schizophr Res. 1995;2:30–31. [Google Scholar]
- O’Leary DS, Kesler M, Mosnik DM, Tronel AP, Hurtig R, Hichwa R, et al. Regional cerebral blood flow within Heschl's gyrus and the planum temporale in schizophrenic patients and controls during language tasks: an MRI and PET study. Schizophr Res. 1995;2:94. [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–20. [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissural fibers. In: Lepore E, Ptito M, Jasper HH, editors. Neurology and neurobiology. Vol. 17. New York: Liss; 1986. pp. 47–73. Two hemispheres-one brain. [Google Scholar]
- Park HJ, Levitt J, Shenton ME, Salisbury DF, Kubicki M, Kikinis R, et al. An MRI study of spatial probability brain map differences between first-episode schizophrenia and normal controls. Neuroimage. 2004;22:1231–46. doi: 10.1016/j.neuroimage.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, et al. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Penrose LS. Survey of cases of familial mental illness. L. S. Penrose, July 1945. Eur Arch Psychiatry Clin Neurosci. 1991;240:315–24. doi: 10.1007/BF02279760. discussion 314–5. [DOI] [PubMed] [Google Scholar]
- Petty RG, Barta PE, Pearlson GD, Mcgilchrist IK, Lewis RW, Tein AY, et al. Reversal of asymmetry of planum temporale in schizophrenia. Am J Psychiatry. 1995;152:715–21. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- Pralong D, Tomaskovic-Crook E, Opeskin K, Copolov D, Dean B. Serotonin(2A) receptors are reduced in the planum temporale from subjects with schizophrenia. Schizophr Res. 2000;44:35–45. doi: 10.1016/s0920-9964(99)00150-4. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–5. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- Qiu A, Vaillant M, Barta P, Ratnanather JT, Miller MI. Hum Brain Mapp. 2007. Region-of-interest-based analysis with application of cortical thickness variation of left planum temporale in schizophrenia and psychotic bipolar disorder. August 17 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–8. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale P, Sheeder J, Simon J, Reite M. Sex-specific expression of Heschl's gyrus functional and structural abnormalities in paranoid schizophrenia. Am J Psychiatry. 1997;154:1655–62. doi: 10.1176/ajp.154.12.1655. [DOI] [PubMed] [Google Scholar]
- Ropohl A, Sperling W, Elstner S, Tomandl B, Reulbach U, Kaltenhauser M, et al. Cortical activity associated with auditory hallucinations. Neuroreport. 2004;15:523–6. doi: 10.1097/00001756-200403010-00028. [DOI] [PubMed] [Google Scholar]
- Rossi A, Serio A, Stratta P, Petruzzi C, Schiazza G, Mancini F, et al. Planum temporale asymmetry and thought disorder in schizophrenia. Schizophr Res. 1994;12:1–7. doi: 10.1016/0920-9964(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Rossi A, Stratta P, Mattei P, Cupillari M, Bozzao A, Gullucci M, et al. The planum temporale in schizophrenia: a MRI study. Schizophr Res. 1992;7:19–22. doi: 10.1016/0920-9964(92)90069-h. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–9. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet PC, Elkis H, Alves TM, Oliveira JR, Sassi E, de Castro CC, et al. Rightward cerebral asymmetry in subtypes of schizophrenia according to Leonhard's classification and to DSM-IV: a structural MRI study. Psychiatry Res. 2003;123:65–79. doi: 10.1016/s0925-4927(03)00020-9. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Simmons A, David AS, Woodruff PW. Are auditory hallucinations the consequence of abnormal cerebral lateralization? A morphometric MRI study of the sylvian fissure and planum temporale. Biol Psychiatry. 2001;49:685–93. doi: 10.1016/s0006-3223(00)01006-4. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Hokama HH, Kikinis R, Dickey C, Lornsen WE, Ballard M, et al. A 3D MRI study of the planum temporale in schizophrenia. Schizophr Res. 1995;15:98. [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–12. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–8. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344–51. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Sumich A, Chitnis XA, Fannon DG, O’Ceallaigh S, Doku VC, Falrowicz A, et al. Temporal lobe abnormalities in first-episode psychosis. Am J Psychiatry. 2002;159:1232–5. doi: 10.1176/appi.ajp.159.7.1232. [DOI] [PubMed] [Google Scholar]
- Sumich A, Chitnis XA, Fannon DG, O’Ceallaigh S, Doku VC, Faldrowicz A, et al. Unreality symptoms and volumetric measures of Heschl's gyrus and planum temporale in first-episode psychosis. Biol Psychiatry. 2005;57:947–50. doi: 10.1016/j.biopsych.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Tanino R, Zhou SY, Hagino H, Niu L, et al. Volume reduction of the left planum temporale gray matter associated with long duration of untreated psychosis in schizophrenia: a preliminary report. Psychiatry Res. 2007;154:209–19. doi: 10.1016/j.pscychresns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou SY, Tanino R, Hagino H, Kawasaki Y, et al. Morphologic alterations of the parcellated superior temporal gyrus in schizophrenia spectrum. Schizophr Res. 2006;83:131–43. doi: 10.1016/j.schres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–59. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar K, Kulynych JJ, Jones DW, Torrey EF, Weinberger DR. An MRI study of the planum temporale in monozygotic twins discordant for schizophrenia. Biol. Psychiatry. 1993;33:123a. [Google Scholar]
- von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Wien und Berlin: Verlag von Julius Springer; 1925. [Google Scholar]
- Walder DJ, Seidman LJ, Makris N, Tsuang MT, Kennedy DN, Goldstein JM. Neuroanatomic substrates of sex differences in language dysfunction in schizophrenia: a pilot study. Schizophr Res. 2007;90:295–301. doi: 10.1016/j.schres.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PB, Loneragon C, Liebert B, Calts SV, Chaturvedi S, Pearson M, et al. MRI measures of auditory cortex area correlate with an ERP index of auditory sensory memory in schizophrenia. Schizophr Res. 1995;15:102. [Google Scholar]
- Weinstein S, Woodward TS, Ngan ET. Brain activation mediates the association between structural abnormality and symptom severity in schizophrenia. Neuroimage. 2007;36:188–93. doi: 10.1016/j.neuroimage.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Yamasue H, Abe O, Yamada H, Iwanami A, Hirayasu Y, et al. Reduced planum temporale volume and delusional behaviour in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:318–24. doi: 10.1007/s00406-007-0723-5. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Yamada H, Yumoto M, Kamio S, Kudo N, Uetsuki M, et al. Abnormal association between reduced magnetic mismatch field to speech sounds and smaller left planum temporale volume in schizophrenia. Neuroimage. 2004;22:720–7. doi: 10.1016/j.neuroimage.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Meisenzahl EM, Preuss UW, Holder JJ, Kathmann N, Leinsinger G, et al. In-vivo analysis of the human planum temporale (PT): does the definition of PT borders influence the results with regard to cerebral asymmetry and correlation with handedness? Psychiatry Res. 2001;107:99–115. doi: 10.1016/s0925-4927(01)00087-7. [DOI] [PubMed] [Google Scholar]