Abstract
We examined the efficacy of herpes simplex virus vector-mediated gene transfer of erythropoietin in preventing neuropathy in mouse model of streptozotocin-diabetes. A replication-incompetent herpes simplex virus vector with erythropoietin under the control of the human cytomegalovirus promoter (vector DHEPO) was constructed. DHEPO expressed and released erythropoietin from primary dorsal root ganglion neurons in vitro, and following subcutaneous inoculation in the foot, expressed erythropoietin in dorsal root ganglion neurons in vivo. At 2 weeks after induction of diabetes, subcutaneous inoculation of erythropoietin prevented the reduction in sensory nerve amplitude characteristic of diabetic neuropathy measured 4 weeks later, preserved autonomic function measured by pilocarpine-induced sweating, and prevented the loss of nerve fibres in the skin and reduction of neuropeptide calcitonin gene-related peptide in the dorsal horn of spinal cord of the diabetic mice. We further investigated whether vector-mediated local expression of erythropoietin in dorsal root ganglion neurons can protect in vivo as well as in vitro hyperglycemia-induced axonal degeneration. Our findings show that the AKT/GSK-3β dependent pathway plays an important role in mediating the protection of erythropoietin against diabetic neuropathy. Herpes simplex virus-mediated transfer of erythropoietin to dorsal root ganglia may prove useful in treatment of diabetic neuropathy.
Keywords: diabetes, gene therapy, herpes simplex virus, erythropoietin
Introduction
Sensory polyneuropathy is a common complication of diabetes, for which there are no available effective treatments. Neurotrophic peptides have been shown to be effective in preventing or reversing diabetic neuropathy in animal models of the disease (Apfel et al., 1994; Ishii and Lupien, 1995; Tomlinson et al., 1997), but the clinical application of these potent pleiotropic peptides to treat human disease is limited by off-target effects that result from systemic administration (Apfel, 2002). We and others have found that replication defective herpes simplex virus (HSV)-based vectors can be used to transfer genes from skin application to dorsal root ganglia (DRG) to achieve focal expression and local release of the gene product from primary sensory neurons (Chattopadhyay et al., 2002, 2003, 2004; Sasaki et al., 2004). Using this approach, we have previously demonstrated that HSV-mediated gene transfer of nerve growth factor, neurotrophin-3 or vascular endothelial growth factor can be used to prevent the development of sensory neuropathy in streptozotocin (STZ)-diabetic mice (Goss et al., 2002; Chattopadhyay et al., 2005, 2007).
Erythropoietin (EPO) was originally characterized for its haematopoietic effects, but in recent years has been shown to have important functions in the nervous system. Non-erythropoietic actions of EPO include a critical role in the development and maintenance of neural structures (Juul et al., 1998; Marti et al., 2000), and protection from or enhanced repair after injury to the nervous system (Brines et al., 2000; Celik et al., 2002; Campana and Myers, 2003; Toth et al., 2008). Erythropoietin confers protection against development or improvement of peripheral neuropathies associated with diabetes (Bianchi et al., 2004; Schmidt et al., 2008), HIV (Keswani et al., 2004), cisplatinum (Bianchi et al., 2006), uremia (Khoshdel et al., 2008) and ageing (Lauretani et al., 2008). However, many clinical situations require multiple doses of recombinant-EPO, which leads to potentially harmful increases in the red cell mass (Stohlawetz et al., 2000). Leist et al. (2004) found that carbamylated EPO is neuroprotective and triggers EPO-mediated tissue-protective pathways without cross-talk with the haematopoietic system. Therefore, we tested a single administration of HSV-mediated EPO gene therapy by subcutaneous inoculation in the footpad which showed not only increase in functional EPO expression locally in the DRG, but also showed no significant changes in the haematocrit level, 1 month after the vector treatment. In this study, we further investigated whether HSV-mediated transfer of EPO to DRG in vivo could prevent neuropathy without the easily detectable off-target effect of increase in haematocrit in STZ-diabetic mice. We found that subcutaneous inoculation of a non-replicating HSV vector expressing EPO prevented the electrophysiologic, anatomic and behavioural manifestations of diabetic neuropathy in STZ-diabetic mice. Vector-mediated EPO led to increased phosphorylation of AKT and GSK-3β in DRG neurons in diabetic animals in vivo and in primary DRG neurons exposed to hyperglycemic conditions in vitro. These studies suggest that the AKT/GSK-3β dependent pathway plays an important role in mediating the protection of EPO against diabetic neuropathy. The neuroprotective effects of EPO offer an important alternative to the use of traditional neurotrophic factors by gene transfer in the treatment of this significant clinical disease.
Materials and methods
Vector constructs
Vector DHEPO was constructed by insertion of a cassette consisting of the human cytomegalovirus immediate early promoter (HCMV IEp) and the human EPO coding sequence into the UL41 locus of a non-replicating HSV vector defective in expression of the immediate early genes ICP4, ICP27 and UL41 (Fig. 1A). Control vector DHZ.5 was identical except that it contained the reporter gene lacZ in place of EPO (Fig. 1B).
Figure 1.
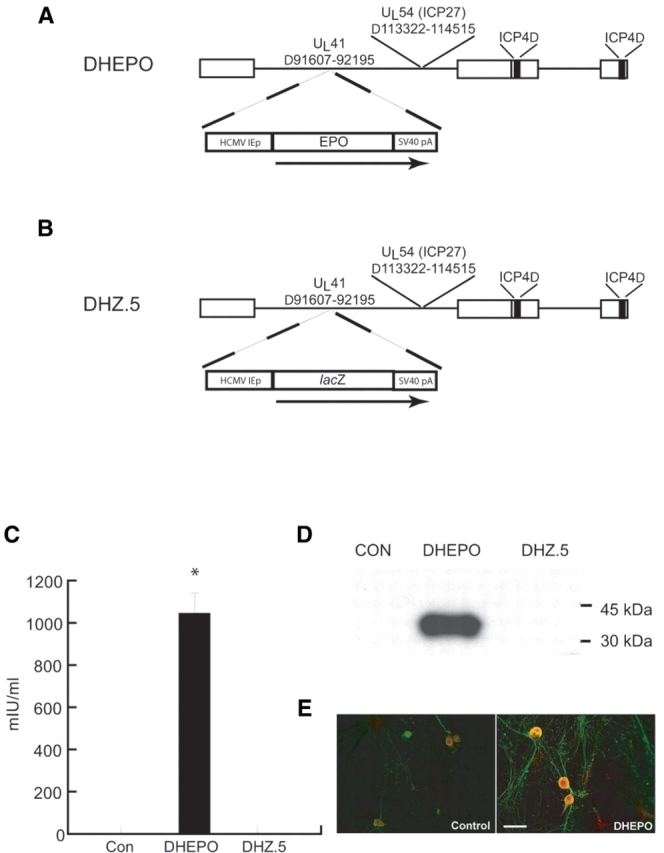
Schematic representation of the replication-incompetent HSV vector DHEPO and its expression in vitro. (A) Transgene EPO expression is driven by the HCMV promoter; (B) control vector DHZ.5 contained the reporter gene lacZ in place of EPO. (C) DHEPO produces EPO in vitro. Primary DRG neurons in culture infected at a multiplicity of infection of 1 with DHEPO released substantial amounts of EPO into supernatant over 24 h measured by ELISA. (D) EPO expression was found in cell lysate (Western blot), (E) by immunocytochemistry. Bar = 20 μm. *P < 0.0001.
Experimental protocol
Experiments were performed on Swiss Webster mice weighing 25–30 g (Charles River, Wilmington, MA, USA) and in compliance with approved institutional animal care and use protocols. Forty mice were rendered diabetic by injection of STZ (100 mg/kg twice in 48 h; Sigma-Aldrich, St Louis, MO, USA). Two weeks after STZ blood glucose was 29 ± 0.72 mmol/l for the diabetic animals and 8.3 ± 0.5 mmol/l for the controls. Two weeks after STZ administration, subsets of 10 diabetic mice received 10 µl containing 1 × 107 plaque forming units (pfu) of HSV-based therapeutic vector DHEPO or control vector DHZ.5 subcutaneously into each footpad. Four weeks after the vector inoculation and 6 weeks after STZ-induction, neuropathy was measured by electrophysiological and behavioural parameters. After completion of the study, DRG from all the animals were collected for Western blot analysis and immunocytochemical studies. For vector expression study, 10 Swiss Webster were injected with either 10 µl containing 1 × 107 pfu of DHEPO or phosphate buffered saline (PBS) and 7 days later DRG were collected for further analysis.
Cell culture
Dorsal root ganglia from 17-day rat embryos were dissociated with 2.5 g/l trypsin, 1mmol/l EDTA for 30 min at 37°C with constant shaking and then plated on poly-d-lysine-coated coverslips at 105 cells per well in a 24-well plate in 500 μl of defined Neurobasal media containing B27, Glutamax I, Albumax II, and penicillin/streptomycin (Gibco-BRL, Carlsbad, CA, USA), supplemented with 100 ng/ml of 7.0S NGF per ml (Sigma, St Louis, MO, USA). At 17 days in vitro, for the expression study, DHEPO and DHZ vector were added. Cells were collected 24 h after the experiments (three wells were pooled to one sample). For experiments involving exposure to hyperglycemia, the cells were infected with DHEPO or DHZ and 24 h later 20 mM glucose added in the medium in addition to the basal 25 mM glucose essential for DRG neuron culture (total 45 mM glucose) and NGF was maintained at 100 ng/ml but in the absence of the B-27 additives. Cells from DRG neuron culture and L4-6 DRG from rats were homogenized with lysis buffer (20 mmol/l Tris pH 7, 20 ml/l SDS, 100 ml/l glycerol and 1:100 dilution of Protease Inhibitor cocktail, Phosphatase Inhibitor cocktail; Sigma, St Louis, MO, USA). The homogenized cells and tissues were then centrifuged at 10 000g for 10 min at 4°C, the supernatant was stored at −80°C. An aliquot of supernatant was taken for protein estimation using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). For PI3K inhibitor study, cells were infected with DHEPO and 24 h later 20 mM glucose and 10 μM LY294002 were added to the medium in the absence of the B-27 overnight. Ten micromolar LY294002 was added again 1 h before collection. For vector expression studies, cells were infected at a multiplicity of infection of 1 for 1 h, after which the medium was changed. The amount of EPO released in the medium was determined using a commercial EPO ELISA kit (R&D Systems Inc, Minneapolis, MN, USA).
Electrophysiologic measurement
Nerve conduction recordings were performed on the right hind foot using a Nicolet Viking II (Nicolet Biomedical, Madison, WI, USA). Mice were anaesthetized with ketamine/xylazine (80/10 mg/kg i.p.), the hind limbs were secured at an angle of 30–45° relative to the long axis of the body, and motor nerve conduction velocity and amplitude in the sciatic nerve was determined. The recording electrode was inserted into the gastrocnemius muscle and the stimulating electrode pair was placed at the sciatic notch or the knee; the reference electrode was inserted subcutaneously into the fifth digit of the hind limb. A ground electrode was inserted into the tail, and subcutaneous temperature was maintained at 36–37°C. Both latency and baseline-to-peak amplitudes were determined. For foot sensory nerve recordings, the electrode placed in the sciatic notch was used as the recording electrode, with the stimulating electrode placed at the ankle and the reference electrode placed at the first digit.
Statistical analysis
The statistical significance of the difference between groups was determined by analysis of variance (ANOVA) using the Bonferroni correction for multiple post hoc analyses (Systat 9.0 Software, SPSS, Inc., Chicago, IL, USA). Results are expressed as means ± SEM.
Sudomotor test
Autonomic function, reflecting innervation of sweat glands in the paw, was evaluated by measuring the number of sweat droplets induced by injection of pilocarpine (Sigma-Aldrich Co., St Louis, MO, USA) using the silicone imprint technique. Sweating was stimulated by subcutaneous injection of pilocarpine nitrate (0.3 mg/kg). At 10 min after the injection, silicone (Silaplast and Silasoft, Detax GmbH & Co., Ettlingen, Germany) was spread over the plantar surface of the hind paw and left in place for 10 min. The amount of sweating was determined by counting the number of sweat droplet impressions made in the silicone mold.
Hotplate test
Small fibre (thermal pain) sensory function was assessed by the hotplate test. Animals were placed on a metal plate heated to 47°C, and the temperature increased at 2°C per minute. The withdrawal latency from the plate was measured in seconds.
Haematocrit
A total of 20 Swiss Webster mice were used for this study. Ten animals were inoculated with DHEPO (10 µl containing 1 × 107 pfu) subcutaneously into each footpad. One month later, 10 control and 10 vector inoculated animals were euthanized; blood was collected and haematocrit determined by the animal core laboratory. The study was repeated twice.
Immunocytochemistry
Mouse hearts were perfused with 9 g/l NaCl followed by Zamboni's fixative. The spinal cord, DRG, and footpads were removed, post-fixed with Zamboni's fixative for 2 h, then cryo-protected with 300 g/l sucrose in PBS. In all, 10 µm cryostat sections were collected on gelatin-coated slides and fixed with 20 g/l paraformaldehyde for 15 min, washed with PBS, and incubated with blocking solution (PBS with 10 ml/l normal goat serum and 3 ml/l Triton X-100) for 1 h, then washed once. The DRG and spinal cord were incubated with the primary antibody, rabbit anti-calcitonin gene-related peptide (CGRP; Peninsula Laboratories, Inc., San Carlos, CA, USA) or anti-EPO, 1:1000 dilutions (Sigma), for 2 h at room temperature. The footpads were incubated with anti-protein gene product 9.5, 1:1000 dilutions (Chemicon International, Inc., Temecula, CA, USA) overnight at 4°C, and washed three times. After incubation in the secondary fluorescent antibody, Alexa Fluor 594 goat anti-rabbit IgG, 1:500 dilution (Molecular Probes, Eugene, OR, USA), for 2 h at room temperature, the specimens were washed three times and mounted in water-based Fluoromount G (Electron Microscopy Sciences, Fort Washington, PA, USA).
Morphometric analysis
Digitized images of immunostained sections were captured with a Nikon E1000 microscope, and analysed using the MetaMorph computer-based image analysis system (Universal Imaging Corporation, Downingtown, PA, USA). The area occupied by immunostained central sensory nerve terminals in the dorsal horn of the spinal cord, or immunostained peripheral nerve terminals in the subcutaneous tissue of the footpad, was determined as a percentage of the total area. Three cross-sections of the tissues from each animal were evaluated by an investigator blinded to the treatment group. Data are expressed as mean ± SEM (n = 5 mice per group of animals).
Western blot analysis
Total cell extract (20 μg protein per lane) was separated by polyacrylamide gel electrophoresis (PAGE), transferred to an Immobilon-P membrane (0.45 μm; Millipore, Bedford, MA, USA), blocked with 50 g/l non-fat milk, then incubated with the primary antibody (anti-EPO; anti-pAKT, anti-AKT; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; anti-pGSK3β, anti-GSK3β; Cell Signaling Technology, Beverly, MA, USA) followed by horseradish peroxidase-conjugated anti-rabbit IgG (1:10 000; Sigma) and visualized with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA). The lower half of the membrane was probed with anti-β-actin (1:2000; Sigma, St Louis, MO, USA) as a loading control. The intensity of each band was determined by quantitative chemiluminescence using a PC-based image analysis system (ChemiDoc XRS System; Bio-Rad).
Results
DHEPO produces erythropoietin in vitro and in vivo
Primary DRG neurons in culture infected at a multiplicity of infection of one with DHEPO released substantial amounts of EPO into the supernatant over 24 h measured by ELISA (*P < 0.0001 DHEPO compared with control or DHZ). Erythropoietin was found in cell lysate measured by Western blot and by immunocytochemistry (Fig. 1C–E). In vivo, 1 week after subcutaneous inoculation of DHEPO (1 × 107 pfu) in the footpad of the animals EPO expression in DRG, quantified by ELISA, was substantially higher in the DHEPO animals compared with controls (102.8 ± 4.4 mIU/ml; control 81.6 ± 7.2 mIU/ml, *P < 0.05, Fig. 2A) and confirmed by Western blot (Fig. 2B).
Figure 2.
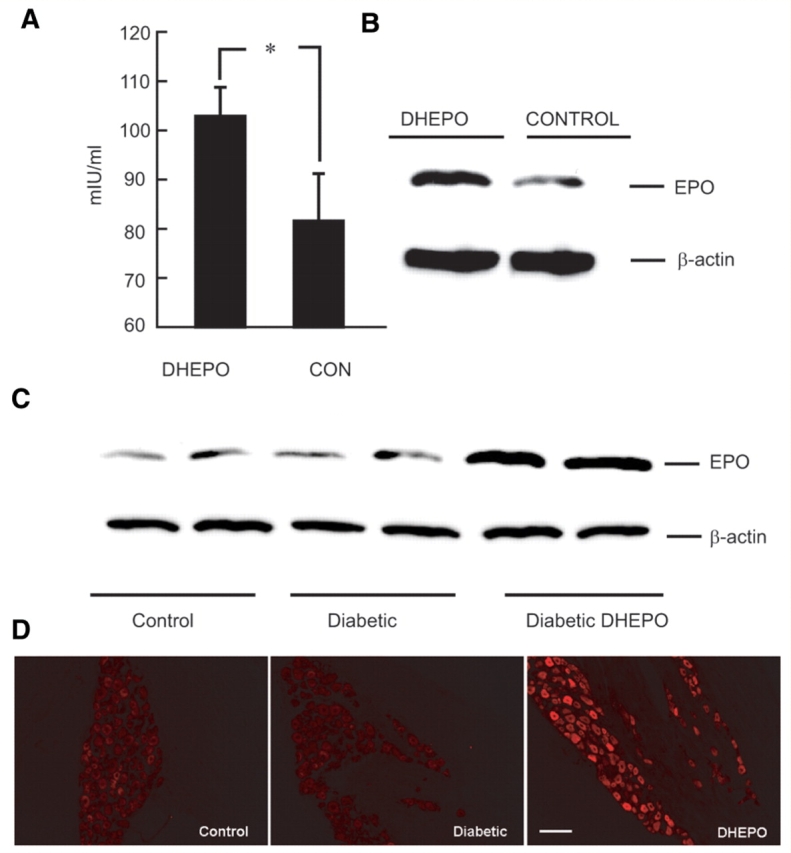
DHEPO produces EPO in DRG in vivo. Transgene expression of EPO was detected in DRG of rats by ELISA (A) and by Western blot (B) 7 days post-inoculation. (C) STZ-diabetic mice inoculated with DHEPO demonstrated increased expression of EPO by Western blot compared with control or STZ-diabetic animals; (D) DRG of DHEPO-inoculated animals showed intense EPO immunoreactivity in large and small neurons (4 weeks after vector inoculation, n = 5 per group). Bar = 20 μm.
DHEPO produces erythropoietin in dorsal root ganglia of diabetic animals
In STZ-diabetic mice inoculated with DHEPO subcutaneously 2 weeks after diabetes in both hind feet, an intense EPO immunoreactivity was seen in both large and small neurons of the lumbar dorsal root ganlgia 4 weeks after vector inoculation. The amount of EPO determined by Western blot was increased in DHEPO compared with STZ-diabetic or control animals 4 weeks of inoculation (Fig. 2C and D).
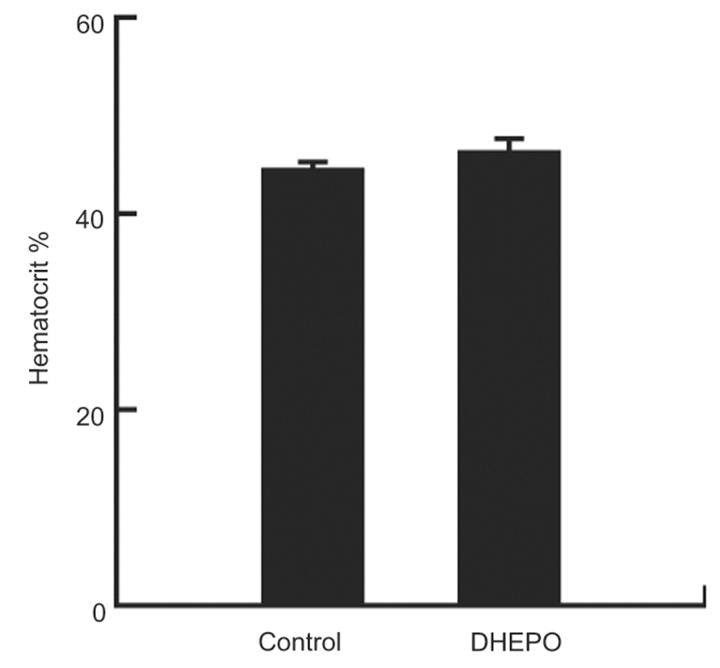
Inoculation of DHEPO does not increase blood haematocrit level in vector-treated animals
A single administration of DHEPO by subcutaneous inoculation in both footpads showed increased EPO expression locally in the DRG (data not shown), but showed no significant changes in the blood haematocrit level compared with the control animals, 1 month after the vector treatment (Fig. 3).
Figure 3.
Effect of DHEPO treatment on changes of blood haematocrit level. Four weeks after treatment, the haematocrit level was unchanged in animals treated with DHEPO, compared with the naïve control (n = 10 per group).
Inoculation of DHEPO preserves sensory nerve function in diabetic animals
Diabetic animals showed sensory neuropathy by a significant reduction in the foot sensory nerve amplitude (control = 25.0 ± 1.8 µV, STZ = 12.5 ± 1.3 µV; **P < 0.001 control compared with diabetic). Transduction of DRG in vivo by subcutaneous inoculation of DHEPO protected against the decrease in foot sensory amplitude measured 4 weeks later (DHEPO 21.9 ± 1.7 µV, DHZ 10.1 ± 1.5 µV; **P < 0.001 DHEPO compared with DHZ.5; Fig. 4A).
Figure 4.
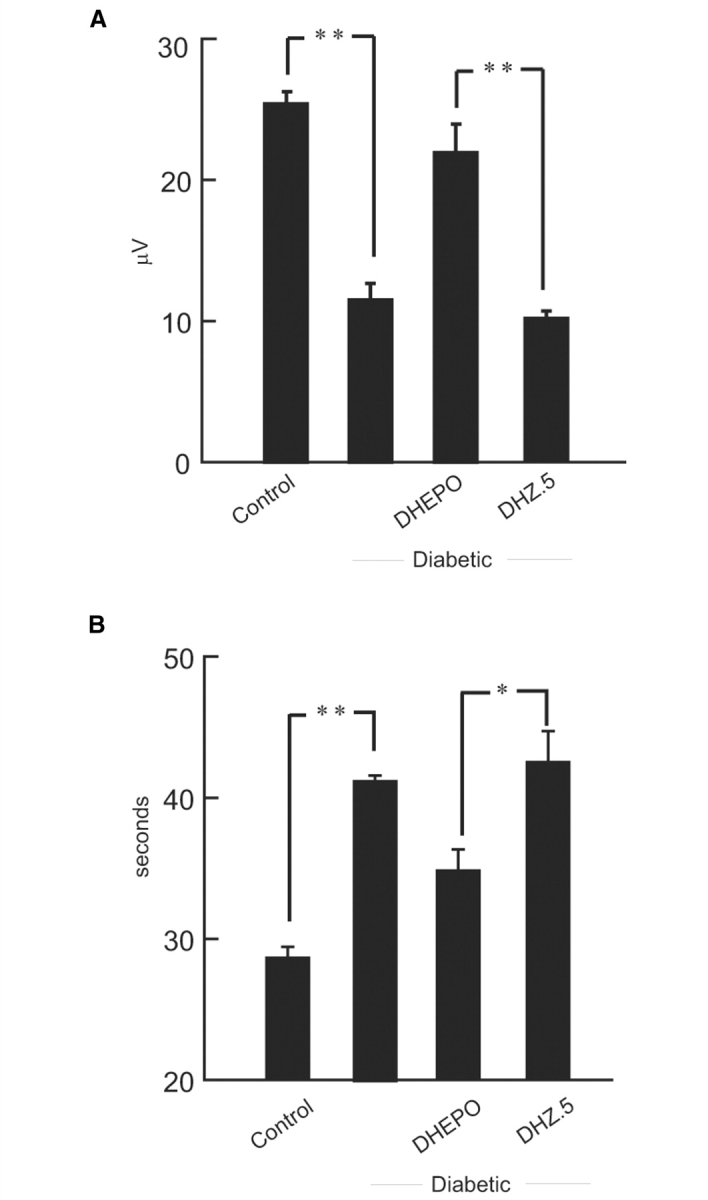
Inoculation of DHEPO preserves sensory behaviour in diabetic animals. (A) The amplitude of the evoked sensory response was reduced substantially in animals with diabetes. Inoculation of DHEPO preserves sensory nerve amplitude compared with DHZ. (B) Diabetic animals showed sensory nerve impairment manifested by an increased latency to withdraw foot from heat. Diabetic mice inoculated with DHEPO showed near normal thermal pain sensation (n = 10 per group).
Inoculation of DHEPO preserves thermal pain sensation
Streptozotocin diabetic mice showed a reduced thermal sensitivity in the hot plate test, manifested by an increased latency to withdrawal from a thermal stimulus (control 28.6 ± 0.6 s, STZ 41.2 ± 0.3 s; **P < 0.001 control compared with diabetic). Thermal pain sensation was relatively preserved in animals inoculated with DHEPO (DHEPO 34.8 ± 1.3 s, DHZ 43.1 ± 1.8 s, *P < 0.005 DHEPO compared with DHZ.5; Fig. 4B) indicating protection of small unmyelinated (C) fibres by vector-mediated EPO release.
Inoculation with DHEPO preserves skin innervation
Nerve terminals in the epidermis of the plantar surface of the foot, determined by protein gene product 9.5 immunostaining and quantified as the proportional area of the skin and subcutaneous tissue occupied by nerve branches, was substantially reduced in diabetic animals (control 2.5 ± 2.2 μm2, diabetic 0.8 ± 0.5 μm2, **P < 0.01; control compared with diabetic). Animals inoculated with DHEPO showed substantial preservation of skin innervation (DHEPO 1.9 ± 2.8 μm2, DHZ 1.1 ± 0.7 μm2; *P < 0.05 DHEPO compared with DHZ; Fig. 5A and B).
Figure 5.
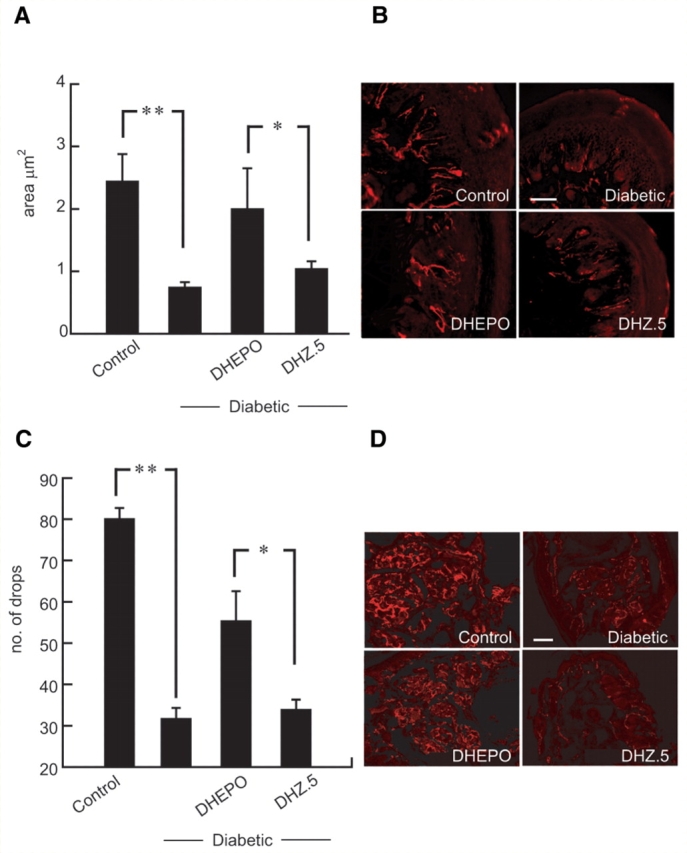
Inoculation of DHEPO prevents epidermal loss of fibres and autonomic neuropathy. (A) The area, quantified as proportional area occupied by the nerve in the footpad, was substantially reduced in diabetic animals but preserved in diabetic animals inoculated with DHEPO. (B) Nerve fibres in the footpad visualized by protein gene product 9.5 immunoreactivity. (C) Sudomotor function after pilocarpine injection was evaluated by silicone imprint. The number of sweat droplets per sweat gland was substantially reduced in diabetic compared with control animals. Diabetic animals inoculated with DHEPO showed substantial preservation of sweating compared with diabetic alone or DHZ-inoculated diabetic mice. (D) Immunohistochemistry with protein gene product 9.5 showed substantial preservation of nerve fibres surrounding the sweat glands in the footpad of diabetic animals inoculated with DHEPO compared with diabetic alone or DHZ-inoculated diabetic mice. (Bar = 20 μm; n = 5 per group).
Inoculation of DHEPO prevents autonomic neuropathy
Sudomotor function after pilocarpine injection was evaluated by silicone imprint. Innervation of sweat glands in the paw was evaluated by measuring the number of sweat droplets after injection of pilocarpine. The number of sweat droplets per sweat gland was substantially reduced in diabetic compared with control animals. This reduction in sweat droplets in diabetic mice (control 82.6 ± 4.6, STZ 30.3 ± 1.2, drops **P < 0.001; control compared with diabetic) characteristic of autonomic neuropathy was prevented in animals inoculated with DHEPO (DHEPO 55.3 ± 5.4, DHZ 32.5 ± 1.5 drops *P < 0.005; DHEPO compared with DHZ). The reduction in pilocarpine-induced sweat production correlated with a marked decrease in protein gene product 9.5-immunoreactive fibres surrounding the sweat glands in diabetic animals whereas diabetic animals inoculated with DHEPO showed significant preservation of nerve terminals in the sweat gland of the footpad while control vector-treated animals showed no preservation of innervation (Fig. 5C and D).
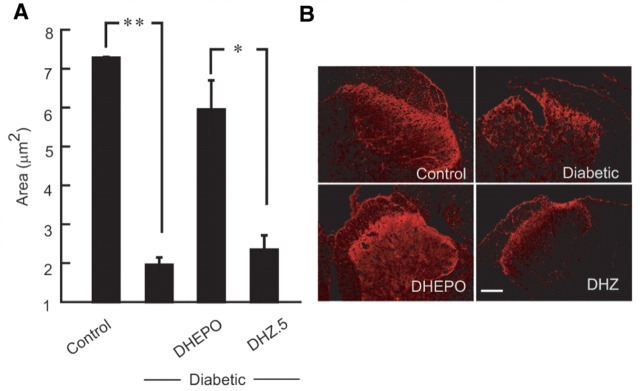
Inoculation of DHEPO preserves CGRP expression in diabetic mice
Diabetic neuropathy is characterized by reductions in neuropeptide content in sensory nerve terminals of primary afferents in the dorsal horn of spinal cord. Calcitonin gene-related peptide (CGRP) immunoreactivity was quantitated by measuring the area of occupied by the CGRP positive nerve terminals in the dorsal horn of the spinal cord. The area of CGRP positive fibres in the dorsal of diabetic mice were reduced compared with control animals, (control 7.3 ± 0.2 μm2, diabetic 2.0 ± 0.2 μm2; **P < 0.001). Diabetic animals inoculated with DHEPO showed preservation of CGRP immunoreactivity in the dorsal horn of the diabetic animals whereas control vector-treated animals were no different than the diabetic alone group (DHEPO-inoculated diabetic 5.9 ± 2.2 μm2; DHZ-inoculated diabetic 2.4 ± 1.4 μm2; *P < 0.005, Fig. 6).
Figure 6.
Inoculation of DHEPO preserves CGRP expression in diabetic mice. (A) The area, quantified as proportional area occupied by the CGRP positive nerve terminals in the dorsal horn of spinal cord, was substantially reduced in diabetic animals but preserved in diabetic animals inoculated with DHEPO compared with DHZ. (B) Immunohistochemical illustrations of CGRP positive nerve terminals in dorsal horn of spinal cord in all four groups. (Bar = 20 μm; n = 5 per group).
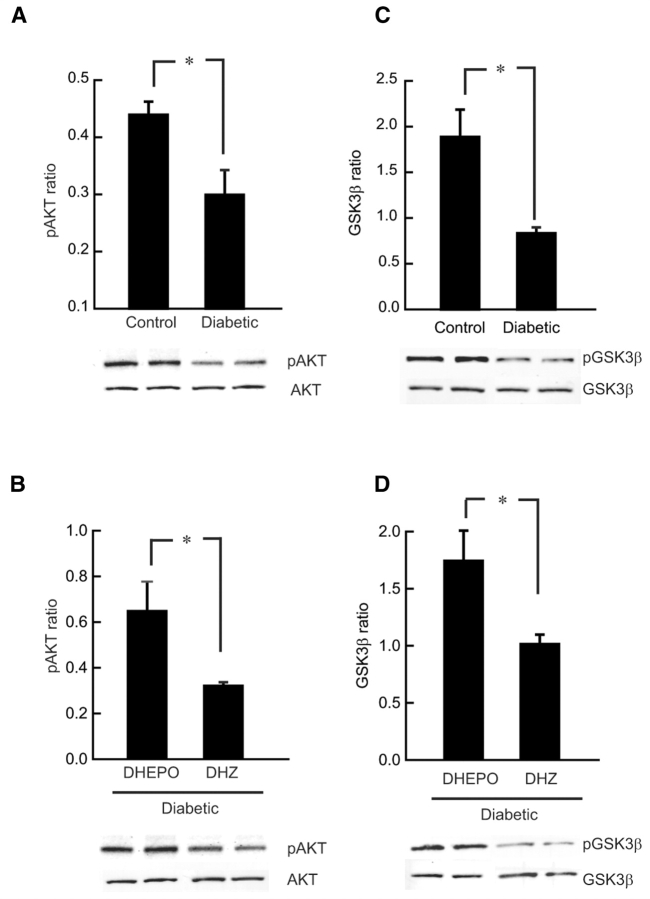
Erythropoietin activates the PI3K/AKT pathway
It has been demonstrated in other systems that the PI3K/AKT pathway contributes to EPO-mediated neuroprotection (Siren et al., 2001a). In vivo, diabetic DRG showed reduced AKT phosphorylation detected by western blot analysis (*P < 0.01 compared with control). This reduction of AKT phosphorylation was reversed in STZ-diabetic animals treated with DHEPO where EPO-mediated increase in AKT phosphorylation was observed (*P < 0.01 compared with DHZ; Fig. 7A and B).
Figure 7.
Erythropoietin stimulates AKT and GSK-3β phosphorylation in diabetic DRG in vivo. (A) DRG of diabetic animals showed reduced AKT phosphorylation compared with control animal. (B) AKT phosphorylation was increased in DRG of DHEPO-treated diabetic animals compared with control vector-treated animals. (C) DRG of the diabetic animals showed decreased phosphorylation of GSK-3β at Ser9 compared with the DRG of the control animals. (D) Inoculation of DHEPO in diabetic animals induced phosphorylation of GSK-3β, a representative western blot of pGSK-3β showed increased phosphorylation in DHEPO cells compared with control vector DHZ (n = 3 per group).
Erythropoietin phosphorylates GSK-3β in dorsal root ganglia of diabetic-DHEPO treated animals
In diabetic animals phosphorylation of GSK-3β at Ser9 in DRG was inhibited after 6 weeks of diabetes compared with controls (*P < 0.01; Fig. 7C). This decrease in phosphorylation of GSK-3β occurred in parallel with decreased phosphorylation of AKT (Fig. 7A and C). Animals treated with EPO expressing vector (DHEPO) 2 weeks after diabetes, showed increase in phosphorylation of GSK-3β in DRG (**P < 0.05 compared with DHZ) which is also analogous to the AKT phosphorylation (Fig. 7B and D).
DHEPO blocks the hyperglycemia-related decrease in phospho-AKT through activation of PI3K
Exposure of primary DRG neurons in culture to 45 mM glucose overnight resulted in decreased phospho-AKT (Fig. 8A) (*P < 0.05 control cells compared with glucose groups) similar to the diabetic DRG in vivo. DRG neurons transfected for 24 hr with DHEPO (glucose + DHEPO; G + E) at multiplicity of infection of 1, prior to exposure to 45 mM glucose showed preservation of phospho-AKT levels (*P < 0.01 DHEPO with 45 mM of glucose compared with 45 mM glucose only group) similar to the DHEPO-diabetic DRG in vivo (Fig. 8A and B).
Figure 8.
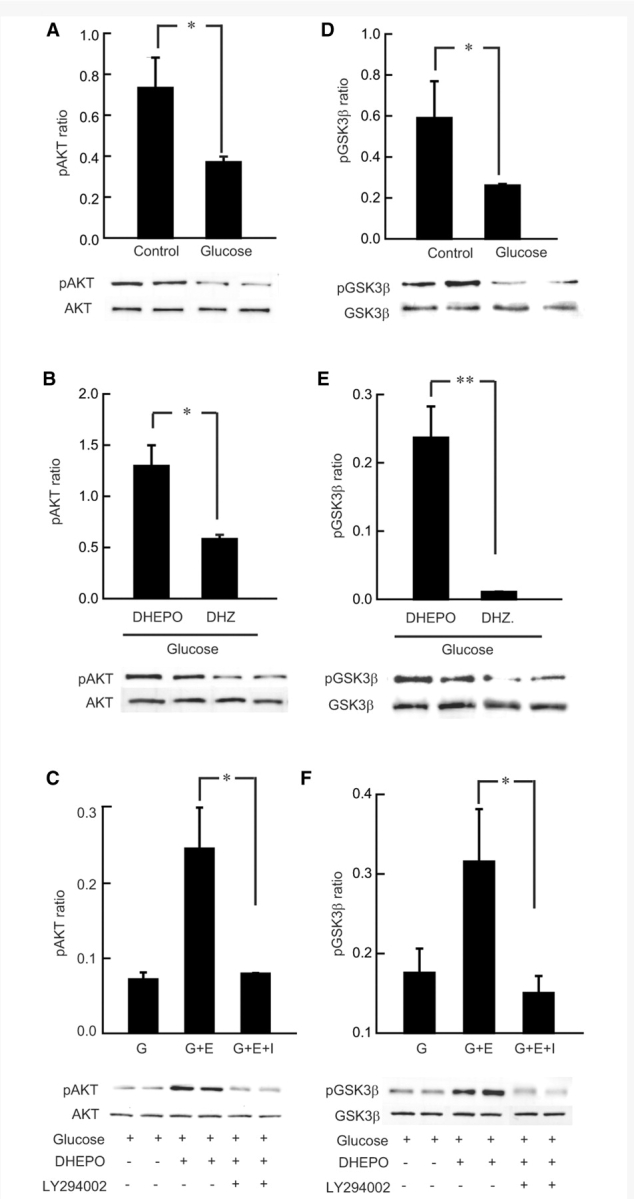
DHEPO treatment increases AKT and GSK-3β phosphorylation in hyperglycemic DRG neurons. (A) In vitro administration of 45 mM glucose (G) showed reduced AKT phosphorylation 18 h after hyperglycemia in primary DRG neurons. (B) A single infection of DHEPO in vitro at multiplicity of infection of 1, 24 h before the glucose administration induced AKT phosphorylation in the DHEPO-treated hyperglycemic cells (G + E) compared with control vector-treated cells. (C) AKT phosphorylation was inhibited by cotreatment with PI3K inhibitor LY294002 along with glucose (G + E + I), 24 h after DHEPO administration. (D) Primary DRG neurons under hyperglycemia showed decreased phosphorylation of GSK-3β at Ser9 compared with control cells in normoglycemic conditions. (E) Inoculation of DHEPO in primary DRG cultured neurons 24 h prior to hyperglycemia-induced phosphorylation of GSK-3β, western blot of pGSK-3β showed increased phosphorylation in DHEPO cells compared with control vector-treated cells. (F) GSK-3β phosphorylation was inhibited by cotreatment with PI3K inhibitor LY294002 along with glucose, 24 h after DHEPO administration.
To test whether EPO stimulated an increase in phosphorylation of AKT in the PI3K/AKT pathway, PI3K was blocked by LY294002. The effects of DHEPO was blocked by addition of 10 μM PI3K inhibitor (*P < 0.01; DHEPO compared with DHEPO with PI3K inhibitor, glucose + E + I; Fig. 8C). PI3K inhibition reduced EPO-stimulated increase in phosphorylation of AKT.
Erythropoietin increases the GSK-3β phosphorylation in glucose-treated primary dorsal root ganglia neurons
When AKT is activated by phosphorylation, it in turn serves to phosphorylate and thereby inactivate GSK-3β. With respect to GSK-3β, a reduction of p-AKT reduces the phosphorylation of GSK-3β. Therefore, GSK-3β phosphorylation was also evaluated 18 h after 45 mM glucose treatment. Phosphorylation of GSK-3β at Ser9 was inhibited following glucose-treatment in primary DRG neurons (*P < 0.05; Fig. 8D). After 18 h of hyperglycemia, decreased phosphorylation of GSK-3β occurred in parallel with decreased phosphorylation of AKT (Fig. 8A and D). A concurrent increase in AKT and GSK-3β phosphorylation was seen in neurons treated with EPO (**P < 0.001, *P < 0.01; Fig. 8B and E). LY294002 pretreatment abolished phosphorylation of both AKT and GSK-3β by EPO (Fig. 8C and F). These data confirm that GSK-3β is a substrate of AKT contributing to the EPO-mediated neuroprotection against neurotoxicity of glucose.
Discussion
The results of our study indicate that transduction of DRG neurons by subcutaneous inoculation of an HSV vector expressing EPO can prevent the progression of diabetic neuropathy in a mouse model. A wide variety of experimental studies have shown that EPO and its receptor are expressed in the nervous system and exerts remarkable neuroprotection in animal and in vitro models of nervous system disorders including traumatic brain injury and spinal cord injury (Morishita et al., 1997; Juul et al., 1998; Marti et al., 2000; Sinor and Greenberg, 2000). Alterations of vascular integrity have been described in diabetes. In clinical studies of Diabetes Mellitus, plasma EPO is often low in diabetic patients with anaemia or without anaemia (Symeonidis et al., 2006). Furthermore, the failure of these individuals to produce EPO in response to a declining haemoglobin level suggests an impaired EPO response in diabetic patients (Thomas et al., 2005). While in vitro studies with vascular endothelial cells, exposed to elevated glucose, have elucidated a strong cytoprotective effect of EPO. Administration of EPO can significantly improve endothelial cell survival in a very low dose (Chong et al., 2007).
HSV-mediated gene transfer to the DRG provides for local release of neurotrophic substances that may act in an autocrine or paracrine manner to protect peripheral sensory neurons from axonal degeneration. We have previously demonstrated the effect of HSV-mediated gene transfer in models of neuropathy induced by overdose of pyridoxine in the rat, STZ diabetes in the mouse, and neuropathy induced by the chemotherapeutic drug cisplatin in the rat (Chattopadhyay et al., 2002, 2004, 2005, 2007; Goss et al., 2002). Our study extends those observations to demonstrate that the local release of EPO achieved by transduction of DRG neurons with an HSV-based vector can prevent the progression of diabetic neuropathy in the mouse, and suggests an alternative approach to the use of EPO in the treatment of this disease.
Despite substantial evidence in animal models demonstrating that neurotrophic factors are beneficial in the treatment or prevention of peripheral neuropathy, the treatment of human neuropathies has been limited, in part because of the short life of these peptide factors and the side effects engendered by systemic administration. Erythropoietin has a wide range of neuroprotective actions in animal models of central and peripheral nervous system disorders. However, the prolonged and repetitive administration of EPO needed for neuroprotection has the potential to cause side effects. In animals and humans, EPO may cause hypertension (Lim, 1991), thrombosis (Bokemeyer et al., 2007), and haematopoiesis as demonstrated by increased blood haematocrit levels. Besides these potential side effects after prolonged treatment, Bianchi et al. (2007) found that EPO does not cause unwanted tumour growth in an experimental model of peripheral neuropathy induced by cisplatinum. The observation in these studies that restricted expression of EPO in DRG neurons achieved by HSV-mediated gene transfer in mice did not result in an increase in haematocrit suggests that such potential systemic complications may be avoided by this method. It seems likely that the ability to protect nerves by autocrine or paracrine local effects of restricted EPO release would similarly allow one to avoid systemic complications in larger animals, though this remains to be formally tested.
Our study has shown that EPO has a beneficiary effect on altered thermal nociception, and electrophysiology in diabetic animals similar to that has been reported earlier by others (Bianchi et al., 2004; Roesler et al., 2004). Campana and colleagues also showed that EPO receptor-dependent cell signalling might reduce axonal degeneration and facilitate recovery from chronic pain states (Campana, 2007). The vector DHEPO has demonstrated neuroprotective effect in electrophysiologic, behavioural as well as restoration of CGRP positive nerve terminals in dorsal horn and protein gene product 9.5 positive nerve fibres in the footpad of the mice with diabetic sensory neuropathy. Decreased levels of CGRP mRNA in L4 and L5 DRG of STZ-diabetic rats have previously been shown (Diemel et al., 1994). Brewster et al. (1994) also found reductions in substance P and CGRP-like immunoreactivity in sciatic nerve and DRG. We have shown a decrease in CGRP expression in dorsal horn by immunocytochemistry, which is preserved in animals treated with DHEPO. Recently, Toth et al. (2008) showed that EPO substantially increased the density and intensity of CGRP expression within outgrowing axons and also improved the behavioural measures of sensorimotor function in rats. These results suggest that EPO induces neuropeptide gene expression, and the effect of EPO on preserving nerve function may be due, in part, to the preservation of the neuropeptide phenotype. Diabetic neuropathy commonly affects the longest nerve fibres first, so that quantification of axon number demonstrates the loss of cutaneous nerve fibres in diabetic patients (Kennedy et al., 1996; Hirai et al., 2000). In STZ-diabetic mice, there is also a significant loss in cutaneous innervation in the hind limb. We found a decrease in total cutaneous innervation in the footpad of mice by protein gene product 9.5 immunostaining, which was prevented by transgene expression of EPO in the DRG, suggesting that EPO acts as a neurotrophic factor and plays an important role in the preservation of peripheral nerve structure and function. Toth et al. (2008) reported similar results showing that local EPO signalling enhances regenerating peripheral nervous system axons and similar preservation of the cutaneous innervation has been demonstrated by treatment with neurotrophic factors such as VEGF, GDNF and neurterin (Christianson et al., 2003).
Sudomotor dysfunction is a common feature of diabetic autonomic neuropathy, and correlates with a reduction in neuropeptide concentration and sweat gland activity (Navarro et al., 1997). In our study, EPO expression preserved autonomic function, measured by pilocarpine-induced sweating. We believe that this results from retrograde transport of the vector from the skin to the autonomic ganglia, although this has not been established conclusively. Another possibility is that EPO released from sensory nerve terminals in the skin interacts with receptors on the autonomic fibres peripherally. Sympathetic neurons express EPO receptor, as detected by immunohistochemistry, and treatment with EPO and carbamylated EPO (which is neuroprotective but not haematopoietic) of STZ-diabetic NOD-SCID mice prevented the development of neuritic dystrophy. Neither EPO nor carbamylated EPO had a demonstrable effect on the metabolic severity of diabetes (Schmidt et al., 2008).
The biological relevance of EPO in neuroprotection has been demonstrated by the presence of the EPO receptor, a cytokine receptor type I superfamily, in the nervous system. EPO binding to EPO receptor results in activation by dimerization and autosphosphorylation of Janus-tyrosine kinase-2 (JAK-2) leading to activation of several downstream signalling pathways, including Ras-mitogen-activated protein-kinases (MAPKs), phosphatidylinositol-3-kinase (PI3K), and signal transducers and activators of transcription-5 (STAT-5) (Siren et al., 2001a; Ruscher et al., 2002; Chong et al., 2003; Ghezzi and Brines, 2004). These pathways seem to be crucial for the neuroprotective effect of EPO, because specific inhibitors of MAPK and PI3K pathways largely abolished the EPO-increased neuronal survival in a model of hypoxia, oxidative stress and excitotoxic injury (Siren et al., 2001b). Activation of EPO receptors on neurons and Schwann cells triggers neuroprotective pathways involving the PI3K/AKT cascade (Siren et al., 2001a; Lipton, 2004). Erythropoietin has been shown to enhance the activation of the PI3K/AKT pathway, which is diminished in DRG and nerves of rats with STZ-diabetes (Cai and Helke, 2003; Huang et al., 2005). Interestingly, in our studies we established that reduction in phosphorylation of AKT in diabetic DRG and hyperglycemic primary DRG neurons could be preserved by DHEPO vector administration.
Erythropoietin—EPO receptor signalling also involves GSK3β, a serine/threonine protein kinase, which is one of the downstream targets of AKT/PKB pathway (Li et al., 2004; Maiese et al., 2004; Shang et al., 2007). Although GSK3β was initially described as an inhibitor of glycogen synthesis through phosphorylation of glycogen synthase, it has been implicated in multiple additional cellular processes including cell maturation, metabolism, gene transcription, cell-cycle regulation and apoptosis (Elyaman et al., 2002; Smith et al., 2002; Jope and Johnson, 2004). GSK3β is a key negative regulator of multiple intracellular signalling pathways. We investigated the effect of EPO on AKT activation and GSK3β phosphorylation in primary DRG cells under hyperglycemia and in STZ-diabetic mice. This elevated phosphorylation of GSK3β may also contribute to the increased neuroprotection in the DRG cells; therefore, AKT/GSK-3β dependent pathway plays an important role in mediating the protection of EPO against diabetic neuropathy.
These observations strongly suggest that HSV-mediated EPO expression prevents axonal degeneration in STZ-diabetic model of neuropathy and hyperglycemia-induced degeneration of primary DRG culture cells. In this study, a single administration of DHEPO was sufficient to obtain a protective effect over a period of 4 weeks without any adverse side effects. In a study of spinal cord regeneration, a bell-shaped response to EPO treatment was observed with very high doses proving ineffective (Toth et al., 2008). Whether such a dose–response relationship is also true in peripheral nerve has not been established. The dosing provided in these experiments by vector-mediated gene transfer proved effective, but it is likely that for the neuroprotective effects of EPO in diabetic neuropathy to be used as a treatment of patients, it will be necessary to develop a vector with a regulatable gene expression system so as to allow for control of the level and duration of gene expression.
Funding
Diabetes Action Research and Education Foundation (to M.C.); National Institutes of Health and the Department of Veterans Affairs (to D.J.F. and M.M.).
Acknowledgements
We acknowledge the technical assistance of Vikram Singh Thakur in vector propagation, Shue Liu in tissue culture, and Carrissia Holloway in animal care.
Glossary
Abbreviations
- CGRP
Calcitonin gene-related peptide
- DRG
dorsal root ganglia
- EPO
erythropoietin
- GSK-3β
glycogen synthase kinase-3β
- HSV
herpes simplex virus
- PGP 9.5
protein gene product 9.5
- PI3K
phosphoinositide 3-kinase
- STZ
streptozotocin
References
- Apfel SC. Is the therapeutic application of neurotrophic factors dead? Ann Neurol. 2002;51:8–11. doi: 10.1002/ana.10099. [DOI] [PubMed] [Google Scholar]
- Apfel SC, Arezzo JC, Brownlee M, Federoff H, Kessler JA. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994;634:7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Brines M, Lauria G, Savino C, Gilardini A, Nicolini G, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental Cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006;12:2607–12. doi: 10.1158/1078-0432.CCR-05-2177. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA. 2004;101:823–8. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Gilardini A, Rodriguez-Menendez V, Oggioni N, Canta A, Colombo T, et al. Cisplatin-induced peripheral neuropathy: neuroprotection by erythropoietin without affecting tumour growth. Eur J Cancer. 2007;43:710–7. doi: 10.1016/j.ejca.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43:258–70. doi: 10.1016/j.ejca.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Brewster WJ, Fernyhough P, Diemel LT, Mohiuddin L, Tomlinson DR. Diabetic neuropathy, nerve growth factor and other neurotrophic factors. Trends Neurosci. 1994;8:321–5. doi: 10.1016/0166-2236(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai F, Helke CJ. Abnormal PI3 kinase/Akt signal pathway in vagal afferent neurons and vagus nerve of streptozotocin-diabetic rats. Brain Res Mol Brain Res. 2003;110:234–44. doi: 10.1016/s0169-328x(02)00652-6. [DOI] [PubMed] [Google Scholar]
- Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–7. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci. 2003;18:1497–506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002;99:2258–63. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Goss J, Lacomis D, Goins W, Glorioso JC, Mata M, et al. Protective effect of HSV-mediated gene transfer of nerve growth factor in pyridoxine neuropathy demonstrates functional activity of trkA receptors in large sensory neurons of adult animals. Eur J Neurosci. 2003;17:732–40. doi: 10.1046/j.1460-9568.2003.02500.x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Goss J, Wolfe D, Goins WC, Huang S, Glorioso JC, et al. Protective effect of herpes simplex virus-mediated neurotrophin gene transfer in cisplatin neuropathy. Brain. 2004;127:929–39. doi: 10.1093/brain/awh103. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Krisky D, Wolfe D, Glorioso JC, Mata M, Fink DJ. HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy. Gene Ther. 2005;12:1377–84. doi: 10.1038/sj.gt.3302533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M, Goss J, Wolfe D, Huang S, Glorioso JC, et al. Prolonged preservation of nerve function in diabetic neuropathy in mice by herpes simplex virus-mediated gene transfer. Diabetologia. 2007;50:1550–8. doi: 10.1007/s00125-007-0702-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Wolfe D, Huang S, Goss J, Glorioso JC, Mata M, et al. In vivo gene therapy for pyridoxine-induced neuropathy by herpes simplex virus-mediated gene transfer of neurotrophin-3. Ann Neurol. 2002;51:19–27. doi: 10.1002/ana.10061. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–18. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179:188–99. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Diemel LT, Brewster WJ, Fernyhough P, Tomlinson DR. Expression of neuropeptides in experimental diabetes: effects of treatment with nerve growth factor or brain-derived neurotrophic factor. Brain Res. 1994;21:171–5. doi: 10.1016/0169-328x(94)90391-3. [DOI] [PubMed] [Google Scholar]
- Elyaman W, Terro F, Wong NS, Hugon J. In vivo activation and nuclear translocation of phosphorylated glycogen synthase kinase-3beta in neuronal apoptosis: links to tau phosphorylation. Eur J Neurosci. 2002;15:651–60. doi: 10.1046/j.1460-9568.2002.01899.x. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11(Suppl 1):S37–44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- Goss JR, Goins WF, Lacomis D, Mata M, Glorioso JC, Fink DJ. Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neruropathy in streptozotocin-induced diabetes in the mouse. Diabetes. 2002;51:2227–32. doi: 10.2337/diabetes.51.7.2227. [DOI] [PubMed] [Google Scholar]
- Hirai A, Yasuda H, Joko M, Maeda T, Kikkawa R. Evaluation of diabetic neuropathy through the quantitation of cutaneous nerves. J Neurol Sci. 2000;172:55–62. doi: 10.1016/s0022-510x(99)00290-7. [DOI] [PubMed] [Google Scholar]
- Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194:279–83. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Ishii DN, Lupien SB. Insulin-like growth factors protect against diabetic neuropathy: effects on sensory nerve regeneration in rats. J Neurosci Res. 1995;40:138–44. doi: 10.1002/jnr.490400116. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–49. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–8. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Leitz GJ, Hoke A. Erythropoietin is neuroprotective in models of HIV sensory neuropathy. Neurosci Lett. 2004;371:102–5. doi: 10.1016/j.neulet.2004.08.080. [DOI] [PubMed] [Google Scholar]
- Khoshdel A, Carney S, Gillies A, Mourad A, Jones B, Nanra R, et al. Potential roles of erythropoietin in the management of anaemia and other complications diabetes. Diabetes Obes Metab. 2008;10:1–9. doi: 10.1111/j.1463-1326.2007.00711.x. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Strotmeyer ES, Corsi AM, Di Iorio A, Guralnik JM, et al. Erythropoietin and polyneuropathy in older persons. Mech Ageing Dev. 2008;129:299–303. doi: 10.1016/j.mad.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Erythropoietin on a tightrope: balancing neuronal and vascular protection between intrinsic and extrinsic pathways. Neurosignals. 2004;13:265–89. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- Lim VS. Recombinant human erythropoietin in predialysis patients. Am J Kidney Dis. 1991;18:34–7. [PubMed] [Google Scholar]
- Lipton SA. Erythropoietin for neurologic protection and diabetic neuropathy. N Engl J Med. 2004;350:2516–7. doi: 10.1056/NEJMcibr041121. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–83. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Marti HH, Bernaudin M, Petit E, Bauer C. Neuroprotection and angiogenesis: dual role of erythropoietin in brain ischemia. News Physiol Sci. 2000;15:225–9. doi: 10.1152/physiologyonline.2000.15.5.225. [DOI] [PubMed] [Google Scholar]
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–16. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- Navarro X, Verdu E, Wendelschafer-Crabb G, Kennedy WR. Immunohistochemical study of skin reinnervation by regenerative axons. J Comp Neurol. 1997;380:164–74. doi: 10.1002/(sici)1096-9861(19970407)380:2<164::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Roesler R, Quevedo J, Schroder N. Erythropoietin, glutamate, and neuroprotection. N Engl J Med. 2004;351:1465–6. doi: 10.1056/NEJM200409303511423. author reply 1465–6. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723–30. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Green KG, Feng D, Dorsey DA, Parvin CA, Lee JM, et al. Erythropoietin and its carbamylated derivative prevent the development of experimental diabetic autonomic neuropathy in STZ-induced diabetic NOD-SCID mice. Exp Neurol. 2008;209:161–70. doi: 10.1016/j.expneurol.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Wu Y, Yao S, Wang X, Feng D, Yang W. Protective effect of erythropoietin against ketamine-induced apoptosis in cultured rat cortical neurons: involvement of PI3K/Akt and GSK-3 beta pathway. Apoptosis. 2007;12:2187–95. doi: 10.1007/s10495-007-0141-1. [DOI] [PubMed] [Google Scholar]
- Sinor AD, Greenberg DA. Erythropoietin protects cultured cortical neurons, but not astroglia, from hypoxia and AMPA toxicity. Neurosci Lett. 2000;290:213–5. doi: 10.1016/s0304-3940(00)01361-6. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001a;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001b;101:271–6. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- Smith E, Coetzee GA, Frenkel B. Glucocorticoids inhibit cell cycle progression in differentiating osteoblasts via glycogen synthase kinase-3beta. J Biol Chem. 2002;277:18191–7. doi: 10.1074/jbc.M109708200. [DOI] [PubMed] [Google Scholar]
- Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95:2983–9. [PubMed] [Google Scholar]
- Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, Leotsinidis M, Kyriazopoulou V, Vassilakos P, et al. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006;85:79–85. doi: 10.1007/s00277-005-1102-9. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Cooper ME, Tsalamandris C, MacIsaac R, Jerums G. Anemia with impaired erythropoietin response in diabetic patients. Arch Intern Med. 2005;165:466–9. doi: 10.1001/archinte.165.4.466. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46(Suppl 2):S43–9. doi: 10.2337/diab.46.2.s43. [DOI] [PubMed] [Google Scholar]
- Toth C, Martinez JA, Liu WQ, Diggle J, Guo GF, Ramji N, et al. Local erythropoietin signaling enhances regeneration in peripheral axons. Neuroscience. 2008;154:767–83. doi: 10.1016/j.neuroscience.2008.03.052. [DOI] [PubMed] [Google Scholar]