Abstract
Inappropriate social behaviours are early and distinctive symptoms of the temporal and frontal variants of frontotemporal lobar degeneration (FTLD). Knowledge of social behaviour is essential for appropriate social conduct. It is unknown, however, in what way this knowledge is degraded in FTLD. In a recent functional MRI study, we have identified a right-lateralized superior anterior temporal lobe (aTL) region showing selective activation for ‘social concepts’ (i.e. concepts describing social behaviour: e.g. ‘polite’, ‘stingy’) as compared with concepts describing less socially relevant animal behaviour (‘animal function concepts’: e.g. ‘trainable’, ‘nutritious’). In a further fMRI study, superior aTL activation was independent of the context of actions and feelings associated with these social concepts. Here, we investigated whether the right superior sector of the aTL is necessary for context-independent knowledge of social concepts. We assessed neuronal glucose uptake using 18-fluoro-deoxy-glucose-positron emission tomography (FDG-PET) and a novel semantic discrimination task which probed knowledge of social and animal function concepts in patients with FTLD (n = 29) and corticobasal syndrome (n = 18). FTLD and corticobasal syndrome groups performed equally poorly on animal function concepts but FTLD patients showed more pronounced impairments on social concepts than corticobasal syndrome patients. FTLD patients with right superior aTL hypometabolism, as determined on individual ROI analyses, were significantly more impaired on social concepts than on animal function concepts. FTLD patients with selective impairments for social concepts, as determined on individual neuropsychological profiles, showed higher levels of inappropriate social behaviours (‘disinhibition’) and demonstrated more pronounced hypometabolism in the right superior aTL, the left temporal pole and the right lateral orbitofrontal and dorsomedial prefrontal cortex as compared with FTLD patients showing selective impairments of animal function concepts. Combining both FTLD subgroup analyses, based on anatomical and neuropsychological criteria, by using inclusive masks, revealed the right superior aTL as associated with selective impairments of social concepts in both analyses. These results corroborate the hypothesis that the right aTL is necessary for representing conceptual social knowledge. Further, we provide first evidence for the potential importance of conceptual social knowledge impairments as contributing to behavioural symptoms of FTLD.
Keywords: frontotemporal dementia, semantics, social cognition, anterior temporal lobe, social behaviour
Introduction
Socially appropriate behaviour requires knowledge of adequate social actions within a given sequential context [e.g. ‘to appropriately kiss a romantic partner on her/his lips after a romantic date but not the waiter/waitress after dinner in a restaurant’ (Wood and Grafman, 2003)], but also knowledge of the abstract conceptual quality of a given social action within a given context [e.g. enabling us to flexibly interpret not being greeted by a colleague who is passing by in a corridor at work as a sign of ‘disrespect’, ‘impoliteness’, ‘shyness’ or ‘absent-mindedness’ (Zahn et al., 2008)]. Recently, we have demonstrated that this abstract conceptual social knowledge, which is independent of the context of actions (Zahn et al., 2008) and emotions (Zahn et al., 2007, 2008), is represented in the superior anterior temporal lobe (aTL). A superior sector within the aTL with lateralization to the right hemisphere (BA38/22) was identified which represents the richness of detail with which social concepts (e.g. ‘ambitious’, ‘polite’, ‘tactless’, ‘stingy’, ‘honourable’) describe social behaviour (Zahn et al., 2007). Contrary to concepts that are expressed by names for living things (e.g. ‘dog’), social concepts (e.g. ‘loyal’) are expressed by names for social behaviour or properties of living things [e.g. ‘acting in a loyal way’ or ‘being loyal’ (Hampson et al., 1986)]. Therefore, as the most suitable class of concepts for comparison with social concepts, we chose concepts that describe animal behaviour or properties [e.g. ‘being nutritious’, ‘useful’, ‘trainable’ and ‘healthy’ (McRae et al., 2005)].
The superior aTL region was selectively activated for social as compared with animal function concepts (Zahn et al., 2007). This result was obtained while carefully controlling for psycholinguistic differences between concepts, such as imageability and confirmed by independent parametric regression analyses. Functional activation studies in healthy subjects, however, cannot demonstrate whether a region is necessary to perform a cognitive function and whether damage to that region produces impairments (Price et al., 1999). In this study, we investigated whether the right superior aTL is necessary for abstract conceptual social knowledge.
Whether and how knowledge of social concepts in patients with brain lesions is affected is unknown. To our knowledge, social concepts have disappeared from modern semantic test batteries. Case reports from the beginning of the 20th century, however, point to putative dissociations of the neural basis of social as opposed to other types of concepts. Kleist, for example, referred to social concepts, such as ‘bravery’ or ‘honesty’ as ‘concepts of subjective nature’ and reported a case of selective impairment of the ability to define subjective concepts with intact knowledge of concepts denoting objects (Kleist, 1922).
Knowledge of concepts denoting objects has been extensively studied in semantic dementia, a subtype of frontotemporal lobar degeneration (FTLD) (Neary et al., 1998). Patients with semantic dementia show marked atrophy of the aTLs and prominent impairment of conceptual knowledge for concrete objects in all modalities [e.g. verbal and visual (Patterson et al., 2007)]. Further, patients with semantic dementia show early behavioural changes such as ‘disinhibition’ (Bozeat et al., 2000; Rosen et al., 2002) which were traditionally attributed to frontal lobe damage and occur as a prominent symptom in behavioural variants of FTLD. In semantic dementia, however, there is ventromedial prefrontal, in addition to marked aTL, atrophy (Mummery et al., 2000; Rosen et al., 2002; Liu et al., 2004) and ‘disinhibited’ behavioural variants of FTLD often show both anterior temporal and ventral frontal abnormalities (Snowden et al., 2001) which would be in keeping with attributing behavioural changes in semantic dementia to frontal lobe damage. Recent studies have provided striking evidence counter to this interpretation. For example, it has been demonstrated that atrophy of the right aTL itself is associated with behavioural changes, such as lack of empathy (Rankin et al., 2006) and ‘disinhibition’ (Liu et al., 2004).
Changes in social behaviour after aTL lesions in primates and humans have mostly been interpreted as evidence for ‘emotional or social’ functions of the aTL (Franzen and Myers, 1973; Streitfeld, 1980; Liu et al., 2004; Olson et al., 2007). These social functions of the aTL have been discussed independently from the evidence on amodal conceptual knowledge representations within the aTL (Bozeat et al., 2000; McClelland and Rogers, 2003; Garrard and Carroll, 2006; Jefferies and Lambon Ralph, 2006; Patterson et al., 2007). One possible explanation for the shared role of at least parts of the aTL for amodal conceptual knowledge and social cognition is that it hosts abstract conceptual representations of social behaviour which are critical for differentiated social evaluations and therefore relevant to everyday social behaviour (Moll et al., 2005; Zahn et al., 2007). Semantic knowledge representations in the aTL have also been proposed as a possible explanation for its frequent activation in functional neuroimaging studies that require the interpretation of other people's mental states [‘Theory of Mind’ (Frith and Frith, 2003)].
Despite this plausible link between loss of semantic knowledge and inappropriate social behaviour, to our knowledge, the only study so far which has investigated the topic did not show a relationship between semantic impairment for object concepts and behavioural symptoms in FTLD (Williams et al., 2005). Furthermore, this study demonstrated separable rather than common regional pathology underlying semantic and behavioural impairments. Semantic impairment was associated with left aTL in accordance with studies correlating semantic tests with neuroimaging measures in FTLD (Grossman et al., 2004), semantic dementia (Mummery et al., 2000), Alzheimer's disease (Zahn et al., 2004, 2006) and aphasia due to stroke (DeLeon et al., 2007). In contrast, behavioural impairment correlated with medial prefrontal volumes (Williams et al., 2005) which have been associated with mental state attribution impairments in FTLD [‘Theory of Mind’ (Gregory et al., 1998)]. One possible explanation for these negative findings is that social conceptual knowledge was not tested and that knowledge of social concepts may dissociate from knowledge of the object concepts tested by Williams et al. (2005).
Here, we combined information from 18-fluoro-deoxy-glucose-positron emission tomography (FDG-PET) with performance on an experimental semantic task requiring knowledge of social concepts or animal function concepts, respectively. The aim of this study was to probe whether damage to the right superior aTL in patients with a clinical syndrome of FTLD leads to selective impairments of social conceptual knowledge. As a control group we chose patients with corticobasal syndrome (CBS). CBS is a progressive neurodegenerative disorder characterized by apraxia and progressive asymmetric rigidity (Litvan et al., 2003; Boeve, 2005). Patients with CBS show less pronounced aTL pathology despite considerable naming impairment (Garraux et al., 2000; Grossman et al., 2004; Eckert et al., 2005; Josephs et al., 2008) and only infrequently exhibit inappropriate social behaviour (Geda et al., 2007).
We employed three analyses in order to probe the role of the right superior aTL in the representation of social conceptual knowledge. The first analysis compared FTLD with CBS patients. We hypothesized that FTLD patients would show: (i) greater impairment of social concepts than CBS patients; (ii) more pronounced hypometabolism in the right superior aTL; and (iii) more severe abnormalities in social behaviour than patients with CBS. The second analysis specifically compared subgroups of FTLD, based on presence or absence of hypometabolism in the right superior aTL on individual FDG-PET region of interest (ROI) analyses. We hypothesized that the FTLD subgroup with hypometabolism in the right superior aTL (compared with the FTLD subgroup showing normal metabolism in this region) should perform worse on social concepts in comparison with animal function concepts. The third and supporting analysis employed subgroup comparisons within FTLD based on dissociations of performance on social concepts and animal function concepts as defined by the individual neuropsychological profile. We hypothesized that patients with ‘Social concept selective impairment’ would display greater hypometabolism of the right superior aTL than patients with ‘Animal function concept selective impairment’.
Methods
Patients and controls
Patients were referred by specialists to participate in a larger observational study at the clinical centre of the National Institutes of Health (NIH) intramural program in Bethesda, MD, USA. Patients stayed at NIH for 1 week and were accompanied by their main caregiver. They were clinically assessed by a senior neurologist and a senior neuropsychologist and underwent extensive neuropsychological test examination as well as MRI and FDG-PET. General inclusion criteria for this study were strong right-handedness and English as first language. Diagnosis of FTLD (including behavioural variant, progressive non-fluent aphasia, semantic dementia) and CBS was based on clinical, neuropsychological criteria as well as visual inspection of MRI and FDG-PET and followed established consensus criteria [FTLD: Lund-Manchester criteria (Neary et al., 1998); CBS: (Boeve, 2005)]. FTD-Motorneuron-Disease (FTD-MND) was diagnosed when there were symptoms of motor neuron disease (significant muscle atrophy, bulbar signs, prominent fasciculations or typical electromyographic signs) in addition to symptoms of behavioural variant FTD.
Cortico-basal syndrome was diagnosed when the lead symptom was progressive asymmetric rigidity and ideomotor apraxia and when there was evidence of other cortical neurological signs (e.g. alien limb phenomena, cortical sensory loss, myoclonus, mirror movements) and basal ganglia dysfunction (e.g. bradykinesia, dystonia, tremor). Progressive non-fluent aphasia and semantic dementia patients had a history of at least 2 years of prominent impairment of language or speech without further clinically relevant symptoms. Progressive non-fluent aphasia patients had non-fluent speech and phonological errors during spontaneous speech as required by the Lund-Manchester consensus criteria. Semantic dementia patients had fluent speech with comprehension and naming impairments as lead symptom. The lead symptoms in behavioural variant FTD patients were progressive dysexecutive and behavioural abnormalities with an early loss of insight as noted by caregivers. Early amnesia and visuo-spatial deficits were absent in the history of all FTLD patients. All FTLD patients showed abnormalities within fronto-temporal areas upon visual inspection of PET and/or MRI. Substance abuse during the study could be ruled out by close monitoring over the 1-week period of the study. None of the patients had a history of substance abuse that could account for their behavioural, language, cognitive or motor symptoms in accordance with the Lund-Manchester consensus criteria.
Thirty-one patients with a clinical diagnosis of FTLD were consecutively enrolled. Two FTLD patients had to be excluded prior to the statistical analysis (no MRI: n = 1, poor PET image quality: n = 1) leading to a final FTLD sample of n = 29 (19 males, age: mean = 61.3 ± 8.9 years, education: mean = 15.4 ± 3.0 years; ± refers to standard deviation throughout the text). FTLD patients had been clinically classified into progressive non-fluent aphasia (n = 4), semantic dementia (n = 2), behavioural variant FTD (n = 21) and behavioural variant FTD-Motor Neuron Disease (n = 2). Nineteen patients with a clinical diagnosis of CBS were consecutively enrolled and one CBS patient had to be excluded prior to the statistical analysis (no MRI). The final CBS sample consisted of n = 18 (10 males, age: mean = 65.3 ± 9.6 years, education: mean = 14.5 ± 3.1 years).
Thirty age- and education-matched (to FTLD group) healthy subjects took part in the neuropsychological part of the study to gain normative data on the social concept discrimination task (15 males, age: mean = 61.5 ± 8.5 years; education: mean = 17.0 ± 2.6 years).
A smaller number of 12 age- and education-matched healthy subjects took part in the FDG-PET part of the study to minimize the number of subjects exposed to radiation (eight males, age: mean = 61.3 ± 6.6 years; education: mean = 17.1 ± 3.2 years).
Demographic variables were compared for all groups using one-way analyses of variance (ANOVA) and follow-up with pairwise comparisons (Bonferroni-corrected P = 0.05). Gender (Contingency coefficient = 0.14, P = 0.48) and age [F(2,74) = 1.31, P = 0.28] were matched between CBS, FTLD and neuropsychological control groups. FTLD patients were matched in education with CBS [mean difference: 0.91, P = 0.88, 95% Confidence Interval (CI): −1.19 to 3.02] and neuropsychological controls (mean difference: −1.55, P = 0.12, 95% CI: −3.38 to 0.28). Education level was higher in normal controls than in CBS patients (mean difference: 2.47, P = 0.02, 95% CI: 0.37–4.56). The FDG-PET normal control group was matched for gender (Contingency coefficient = 0.10, P = 0.75), age [F(2,56) = 1.28, P = 0.29] and education [F(2,56) = 2.56, P = 0.09] to both patient groups.
All healthy volunteers had a normal neurological examination and no history of psychiatric or neurological disorders. Informed written consent was obtained according to procedures approved by the NINDS Institutional Review Board. Normal control subjects were compensated for their participation according to NIH standards.
Clinical and neuropsychological assessment
Social concept discrimination task design
This novel task (which can be obtained from the authors) was designed as a self-paced computerized test (Experimental Run Time System, Berisoft Cooperation, Germany, http://www.erts.de) in analogy to the Pyramids and Palm Trees Test [three word version (Howard and Patterson, 1992)] in which a prime word (e.g. ‘adventurous’) was presented at the top of a screen and participants had to decide which of two concepts at the bottom was more related in meaning to the prime concept at the top. Target (e.g. ‘courage’) and distracter (e.g. ‘controlled’) concepts were chosen from the same category as the prime concepts (e.g. animal function concept prime: ‘trainable’, target: ‘ridden’, distracter: ‘bites’) and had been used in our previous fMRI studies (Zahn et al., 2007, 2008). Ninety items were presented randomly [30 positive social concept triads (pSOC), 30 negative social concept triads (nSOC), 30 animal function concept triads (ANI)].
Based on the performance of our aged normal control group, we excluded items from the final analysis in which there was <80% response agreement leaving a total of 73 items (pSOC: 25, nSOC: 24, ANI: 24). We compared the conditions (only using the final 73 items) for differences on 28 variables using non-parametric one-way ANOVAs (Kruskal-Wallis tests) and an approximate Bonferroni-corrected P < 0.10 (uncorrected P < 0.004) to detect potentially confounding differences between task conditions [Kucera Francis word frequency, word familiarity, imageability and concreteness obtained from the MRC psycholinguistic database (Coltheart, 1981)]. Forward word associativity for word pairs (i.e. the percentage of subjects naming a word, used in the task as a distracter or target, as the first word that comes to their mind when prompted with the prime word), semantic relatedness (i.e. meaning relatedness of concept pairs), category-breadth (i.e. ‘how many different kinds of behaviours the word can apply to’), descriptiveness of behaviour [i.e. ‘how well each word described a detailed specific set of social behaviours of persons (SOC) or of biological behaviours of animals (ANI)] were obtained from a normative study [(Zahn et al., 2007), supporting materials: http://www.pnas.org/cgi/content/full/0607061104/DC1]. The conditions were matched on:
response time
mean percentage of correct responses
prime, target and distracter word familiarity
prime, target and distracter word frequency
target and distracter descriptiveness
SOC: prime minus target difference in social desirability (Hampson et al., 1987)
prime–target and prime–distracter semantic relatedness
prime–target and prime–distracter pair forward word associativity (%)
prime minus target and prime minus distracter differences in category breadth Z-values
The conditions showed significant differences on:
Prime, target and distracter number of syllables
Prime, target and distracter concreteness and imageability
Prime descriptiveness
Pairwise post hoc comparisons showed significantly higher numbers of syllables for pSOC and nSOC compared with ANI and lower values on all the other differing variables, except that prime descriptiveness was only lower in pSOC compared with ANI but not comparing nSOC and ANI. There were no significant differences between pSOC and nSOC conditions except for lower prime descriptiveness in the pSOC condition. Imageability and concreteness were highly correlated (Spearman Rho ranges from 0.77 to 0.84 for primes, distracters and targets), therefore we only corrected for imageability in our analyses. Number of syllables and descriptiveness (mean of prime, target and distracter) had no effect on performance (percentage correct per stimulus, mean over FTLD group and CBS group separately, simple linear regression analyses over all 73 stimuli; there were no outliers outside of mean ± 2.5 SDs) and could therefore be disregarded as confounders.
With regard to word class, verbs and nouns were significantly more frequent in the ANI condition than in the SOC conditions, whereas adjectives were significantly more frequent in SOC compared with ANI conditions. Word class of targets and distracters significantly influenced performance (one-way ANOVAs on percentage correct per stimulus, mean over FTLD and CBS groups separately). Pairwise post hoc comparisons demonstrated that presence of verbs versus nouns/adjectives had no significant influence on performance, but that the overall word class effect was solely due to better performance for adjectives compared with nouns/verbs. Therefore the potential effect of adjectives, more frequent in the SOC conditions, compared with nouns/verbs, more frequent in the ANI condition, needed to be controlled for.
The only potential remaining variable to be controlled for in our final analyses, was therefore imageability (=concreteness), which had a significantly negative influence on performance in FTLD. This was achieved by computing individual binary logistic regression models (SPSS 15.0) for each subject in the FTLD group in which a significant dissociation between SOC and ANI conditions had occurred. Imageability per stimulus was a predictor covariate and correct/incorrect response the categorical outcome (no constant modelled).
Behavioural data analysis
Neuropsychological data were analysed using SPSS15 (www.spss.com), we report two-tailed significances for all analyses with no directed hypothesis and one-tailed significances otherwise (lower performance on social concepts relative to animal function concepts in patients with right superior aTL damage compared with patients with no damage to this region). We used one-way ANOVA and pairwise post hoc t-tests for comparisons in which the sample size was either at least n = 30 or data were normally distributed. In all other analyses we used non-parametric one-way ANOVAs (Kruskal Wallis test) and Mann–Whitney U-tests (asymptotic P-values and Mann–Whitney U-statistic were reported) for pairwise comparisons of FTLD subgroups based on neuropsychological dissociations. Also, when there were unequal variances and sample sizes we used non-parametric statistics. The alpha-level was set to P = 0.05 unless otherwise stated. For student t-tests, in case the Levene's test for equality of variances was significant at P = 0.05, P-values under the assumption of unequal variances were reported.
Image acquisition
MRI
Structural MR imaging was performed on a General Electric 1.5 T scanner (3D-T1-SPGR sequence, 120 contiguous slices, slice thickness = 1.5 mm, in-plane resolution = 0.9375 mm × 0.9375 mm, flip angle = 20°).
FDG-PET
All FDG-PET scans were acquired using a GE Advance PET Scanner. Subjects were fasting since midnight before the scan and had no caffeine, alcohol or nicotine for 24 h before the scan. An arterial line was inserted for arterial blood sampling. The subject was given an intravenous injection of 5 millicuries of FDG with eyes covered with patches and the ears plugged. Starting with the time of injection and continuing through the cerebral uptake period and subsequent scan, 25 arterial blood specimens were taken at fixed intervals for assay of plasma radioactivity and glucose content. A transmission scan was used to correct the emission data. At the end of the 45-min uptake period, the emission (PET) scan was performed (15 min for one set of 35 brain slices).
Image analysis
Imaging data were analysed using statistical parametric mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm/software/spm5). The following pre-processing steps were applied: normalization with 3 × 3 × 3 mm3 voxel size and smoothing (FWHM = 12 mm). Normalization was performed by first normalizing the 3D MRI using the SPM5 T1-template and then applying the same transformation on the FDG-PET images. For the group whole brain analyses, we created an explicit cerebrum mask by using the Talairach Daemon (Lancaster et al., 2000) hemisphere templates implemented into the WFU Pickatlas (Maldjian et al., 2003) to increase power by excluding extracerebral and white matter voxels. No implicit masks were used as this leads to drop out of regions with severe atrophy. We used a multiple regression model using patient group (FTD, CBS, Normal Control) or subgroup (FTD subgroups based on neuropsychological or anatomical criteria) as a categorical covariate. Global normalization was performed using proportional scaling.
Localization of areas was determined by using anatomical landmarks (Nieuwenhuys et al., 1978; Mai et al., 2004) and by looking at activations in original Montreal Neurological Imaging (MNI) space projected onto a standard MNI template. In addition, Talairach transformed coordinates (using Matthew Brett's formula, http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml) were used to identify corresponding Brodmann Areas on the Talairach atlas (Talairach and Tournoux, 1988) using Talairach Daemon software version 2 [http://ric.uthscsa.edu/projects/talairachdaemon.html; (Lancaster et al., 2000)].
Single case ROI analysis
To determine whether hypometabolism was present in the right or left anterior superior or middle/inferior temporal lobe, we used an apriori defined bilateral aTL ROI. The aTL ROI was identical to the one used in our previous studies (Zahn et al., 2007, 2008). The ROI was created using bilateral Brodmann (38, 22, 21) maps from the WFU Pickatlas (Maldjian et al., 2003) integrated in SPM5. The original maps were cropped to exclude tissue posterior to the MNI y-coordinate = −10. As in our previous paper, for the individual case analysis, the superior aTL included the superior temporal sulcus and cortex dorsal to this. The middle/inferior aTL included aTL inferior to the superior temporal sulcus. Hypometabolism maps comparing each patient with the normal controls were projected onto the normalized individual patient 3D MRI (Fig. 1A) and inspected by RZ. Significant hypometabolism on an individual level was assumed if clusters survived uncorrected P = 0.05 and false discovery rate-corrected P = 0.05 over the bilateral ROI. In cases of severe aTL atrophy, hypometabolism which fell into the enlarged sulci was identified as aTL by adjacency to residual parts left intact. The aTL ROI which had been defined by using a standardized MNI template aligned well with normalized patient MRIs.
Figure 1.
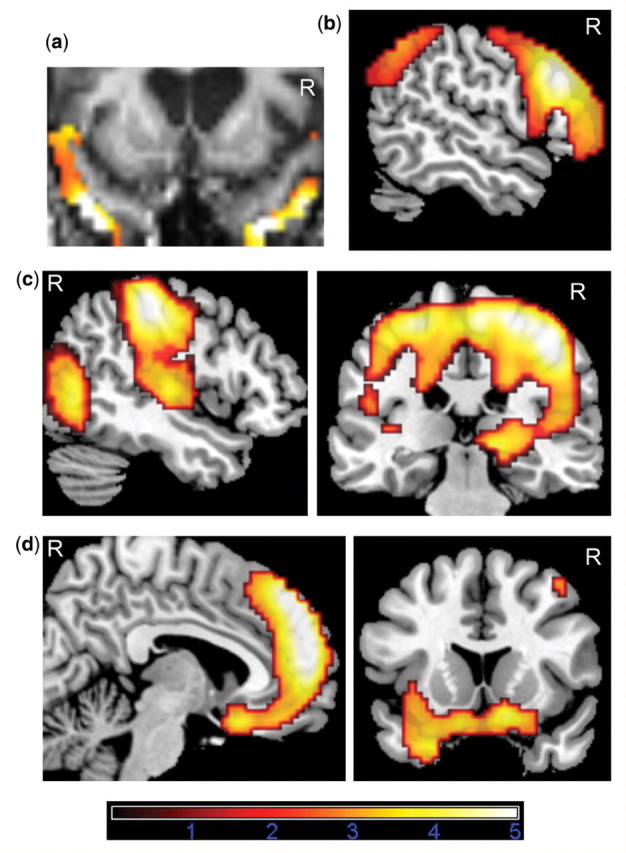
FDG-PET analysis using SPM5. (A) shows an example of a single case analysis in which hypometabolic clusters in an FTLD patient surviving FDR-corrected P = 0.05 over the volume of the bilateral apriori aTL ROI were overlaid onto his normalized MRI to determine whether anterior temporal cortex inferior or within or superior to the superior temporal sulcus showed hypometabolism. (B) Common areas of hypometabolism in FTLD versus Normal controls and CBS versus Normal controls (inclusive mask at P = 0.005). (C) Hypometabolism in CBS versus FTLD. (D) Hypometabolism in FTLD versus CBS. Areas exceeding a voxel level threshold of P = 0.005 uncorrected, five voxels are displayed. Only regions which additionally survive FWE- or cluster-corrected P = 0.05 over the whole brain or bilateral apriori aTL ROI are reported in Table 1 and discussed in text.
To correct for multiple comparisons in all reported analyses, we used the same bilateral apriori aTL ROI as in our previous fMRI study (Zahn et al., 2007) which was predicted to be relevant for context-independent conceptual social knowledge. Only regions surviving FWE-corrected P = 0.05 over the bilateral predefined ROI volume were reported. Activations outside of this apriori ROI were reported when they survived a whole brain FWE-corrected threshold of P = 0.05. All reported coordinates are in Montreal Neurological Institute Standard Space. MRIcron [http://www.sph.sc.edu/comd/rorden/mricron/(Rorden and Brett, 2000)] was used to display saved statistical masks overlaid on a standard template.
Results
Comparison of FTLD and CBS groups
Patients with FTLD showed more behavioural symptoms (Neurobehavioural Rating Scale) and performed worse on global cognitive functioning (Mattis Dementia Rating Scale), verbal comprehension (Token Test), category fluency and set shifting (Card Sorting) as compared to patients with CBS (Table 1).
Table 1.
Neuropsychological standard test results
| CBS total (n = 18) | FTLD total (n = 29) | P-value | FTLD no R sup aTL lesion (n = 19) | FTLD w. R sup aTL lesion (n = 10) | P-value | Effect of Social Concept Selective Impairment: P-value (chi-square) | ||
|---|---|---|---|---|---|---|---|---|
| Neurobehavioural Rating Scale Total | Mean ± SD | 19.3 ± 12.6 | 27.5 ± 12.0 | 0.04* | 28.4 ± 13.2 | 26.0 ± 10.2 | 0.62 | 0.04*,# (6.4) |
| Mattis Dementia Rating Scale Scaled Score (Jurica et al., 2001) | Mean ± SD | 5.8 ± 4.1 | 3.5 ± 2.0 | 0.04* | 3.0 ± 1.5 | 4.3 ± 2.6 | 0.17 | 0.08 (5.0) |
| Impaired | 10/16 | 24/28 | 17/18 | 7/10 | ||||
| DELIS Trail Making Test Number/Letter Scaled Score | Mean ± SD | 8.5 ± 4.7 | 6.7 ± 4.3 | 0.28 | 5.5 ± 4.7 | 8.8 ± 2.7 | 0.06 | 0.34 (2.2) |
| Impaired | 3/12 | 9/21 | 8/13 | 1/8 | ||||
| Boston Naming Correct (Zec et al., 2007) | Mean ± SD | 45.1 ± 10.3 | 38.8 ± 16.3 | 0.12 | 41.2 ± 13.2 | 34.5 ± 20.8 | 0.37 | 0.96 (0.09) |
| Impaired | 12/17 | 22/28 | 14/18 | 8/10 | ||||
| Token Test Correct (Steinberg et al., 2005) | Mean ± SD | 90.4 ± 12.5 | 77.3 ± 18.9 | 0.01* | 73.8 ± 19.6 | 83.6 ± 16.7 | 0.20 | 0.13 (4.1) |
| Impaired | 0/16 | 1/28 | 1/18 | 0/10 | ||||
| DELIS Category Fluency Scaled Score | Mean ± SD | 5.8 ± 3.3 | 3.3 ± 3.2 | 0.02* | 2.7 ± 2.4 | 4.4 ± 4.3 | 0.18 | 0.05* (5.9) |
| Impaired | 8/17 | 24/28 | 16/18 | 8/10 | ||||
| DELIS Letter Fluency Scaled Score | Mean ± SD | 5.5 ± 2.9 | 4.3 ± 3.6 | 0.26 | 4.0 ± 3.5 | 4.9 ± 4.0 | 0.56 | 0.25 (2.8) |
| Impaired | 13/17 | 22/27 | 13/17 | 9/10 | ||||
| DELIS Free Sorting Description | Mean ± SD | 7.4 ± 2.9 | 4.9 ± 3.5 | 0.02* | 6.4 ± 3.6 | 0.09 | 0.01* (8.6) | |
| Impaired | 8/16 | 17/25 | 12/16 | 5/9 | ||||
| Matrix reasoning WAIS III raw score | Mean ± SD | – | 7.5 ± 4.5 | – | 6.8 ± 4.4 | 8.7 ± 4.6 | 0.30 | 0.06 (5.6) |
| Impaired | – | 9/26 | 6/16 | 3/10 | ||||
| Auditory Delayed Memory WMSIII Scaled Score | Mean ± SD | 16.7 ± 5.8 | 13.9 ± 7.5 | 0.24 | 14.3 ± 7.6 | 13.3 ± 7.9 | 0.77 | 0.02*,# (7.9) |
| Impaired | 6/15 | 13/23 | 8/15 | 5/8 | ||||
| Visual Delayed Memory WMSIII Scaled Score | Mean ± SD | 15.4 ± 5.3 | 12.1 ± 5.5 | 0.07 | 11.4 ± 5.1 | 13.6 ± 6.4 | 0.36 | 0.13 (4.1) |
| Impaired | 10/16 | 16/26 | 11/18 | 5/8 |
The number of patients within each group with impairments (performance equivalent to or below percentile rank 10 on age-corrected norm scores or mean—1.25 SD). FTLD subgroups did not differ on gender distribution (P > 0.20), age or education (P > 0.10).
*Significant group difference. Neurobehavioural Rating Scale (McCauley et al., 2001) scores were coded from 0 to 6 (with 27 items and 0 equivalent to no symptom). DELIS = DELIS Kaplan Executive Function System (Delis et al., 2001) and Scaled Scores are age-corrected with a mean of 10 and SD of 3. Effects of subgroups based on individual case dissociations (SOC < ANI, SOC=ANI, ANI<SOC) were tested using a non-parametric ANOVA (df = 2). Only two follow-up pairwise post hoc comparisons for SOC < ANI versus the ANI < SOC group were significant and marked with #. In both cases the SOC < ANI group performed worse than the other group. WAISIII = Wechsler Adult Intelligence Scale, WMSIII = Wechsler Memory Scale (Wechsler, 1997).
There was a significant main effect of group (one-way ANOVA including normal controls with post hoc pairwise Bonferroni-corrected comparisons) on both types of concepts [SOC: F(2,74) = 16.84, P < 0.0001, ANI: F(2,74) = 25.26, P < 0.0001]. Both FTLD and CBS showed significantly lower performance on both types of concepts compared with controls (P < 0.0001). However, only on social concepts were patients with FTLD performing worse than CBS patients (P = 0.04), whereas both groups performed equally poorly on animal function concepts (P = 0.19). These results did not change when we excluded the four PNFA patients from our FTLD group when comparing with CBS (SOC: P = 0.05, ANI: P = 0.16). Overall, performance on animal function concepts was worse than on social concepts in both groups [FTLD: t(28) = −3.15, P = 0.004; CBS: t(17) = −4.13, P = 0.001]. There was no significant difference in normal controls [t(29) = −1.70, P = 0.10].
We computed Z-scores for each type of concept and patient group and normal controls separately to standardize performance on a comparable scale in both conditions. Single case dissociations in the form of Social concept selective impairments and Animal function concept selective impairments were defined as differences in Z-scores outside the normal mean difference range (mean ± 1.5 SD). Selective impairments on social concepts occurred in 6 of 29 FTLD and in only 1 of 18 CBS patients. Animal function concept selective impairments were observed in 13 of 29 FTLD and 10 of 18 CBS patients (n = 47, Contingency coefficient = 0.20, P = 0.36).
On FDG-PET, both the FTLD and the CBS groups demonstrated dorsolateral and posterior lateral prefrontal hypometabolism compared with controls (Fig. 1B and Table 2). CBS showed greater perirolandic, superior parietal and precuneus hypometabolism compared with FTLD (Fig. 1C and Table 2). FTLD exhibited greater medial and ventral prefrontal as well as left inferior anterior temporal hypometabolism (Fig. 1D and Table 2).
Table 2.
FDG-PET—comparisons of FTLD and CBS groups
| Hemisphere | Area | x | y | z | Brodmann Area | Cluster size | T-score |
|---|---|---|---|---|---|---|---|
| FTD < NC | |||||||
| R | Dorsolateral prefrontal cortex | 54 | 27 | 30 | 46 | 7869 | 7.42 |
| L&R | Dorsomedial prefrontal cortex | −6 | 48 | 42 | 8/10 | – | 6.85 |
| R | Posterior parietal cortex | 54 | −57 | 48 | 40 | 417 | 4.67 |
| CBS < NC | |||||||
| R | Superior Parietal Lobule | 36 | −57 | 60 | 7 | 4891 | 5.57 |
| R&L | Posterior cingulate gyrus | 9 | −15 | 27 | 23 | 945 | 4.09 |
| R | Thalamus | 0 | −15 | 3 | – | – | 3.67 |
| L | Anterior superior/middle frontal gyrus | −33 | 66 | 0 | 10/9 | 1097 | 4.01 |
| FTD < CBS | |||||||
| L&R | Dorsomedial prefrontal cortex | −9 | 45 | 42 | 8/9 | 5647 | 5.29 |
| L | Anterior inferior temporal cortex | −51 | −9 | −30 | 20 | 21 | 3.09 |
| CBS < FTLD | |||||||
| R&L | Postcentral/superior parietal/precuneus | 30 | −45 | 60 | 5/7 | 10171 | 6.59 |
FDG-PET analysis using SPM5. Only areas exceeding a voxel level threshold of P = 0.005 uncorrected, five voxels and FWE-corrected or cluster-corrected P = 0.05 over the whole brain or the bilateral apriori aTL ROI are reported. Side maxima in larger clusters are marked in italics.
Anatomical subgroups of FTLD based on right superior aTL involvement
The individual aTL ROI analyses revealed 10 of 29 FTLD patients with significant hypometabolism of the right superior aTL and 19 of 29 without abnormalities in this region. These subgroups of FTLD showed no demographic differences or differences on standard neuropsychological tests, global cognitive functioning or behavioural scales (Table 1).
FTLD patients with hypometabolism of the right superior aTL (n = 10) showed significantly inferior performance on social compared with animal function concepts [t(9) = −1.8, P = 0.05, based on the difference of Z-scores SOC minus ANI for each individual, results for individual cases in Supplementary Table 4]. In contrast, patients with no abnormalities in this region (n = 19) showed no differences in performance between social and animal function concepts and the comparison of both groups of patients revealed significantly higher selectivity of impairment for social concepts in patients with right superior aTL damage [t(27) = 1.8, P = 0.04, Supplementary Fig. 4]. This group difference remains significant when excluding the four PNFA patients from the analysis [t(23) = 1.98, P = 0.03, see also Supplementary Table 4].
A group comparison of the two subgroups of FTLD patients based on the individual ROI analyses of (i) patients with right superior aTL hypometabolism versus (ii) patients with no right superior aTL hypometabolism showed maximal hypometabolism in group 1 within the right superior temporal sulcus which confirmed the individual ROI analyses (Fig. 2A and Table 3). This hypometabolism further extended into more right inferior temporal and inferior frontal regions.
Figure 2.
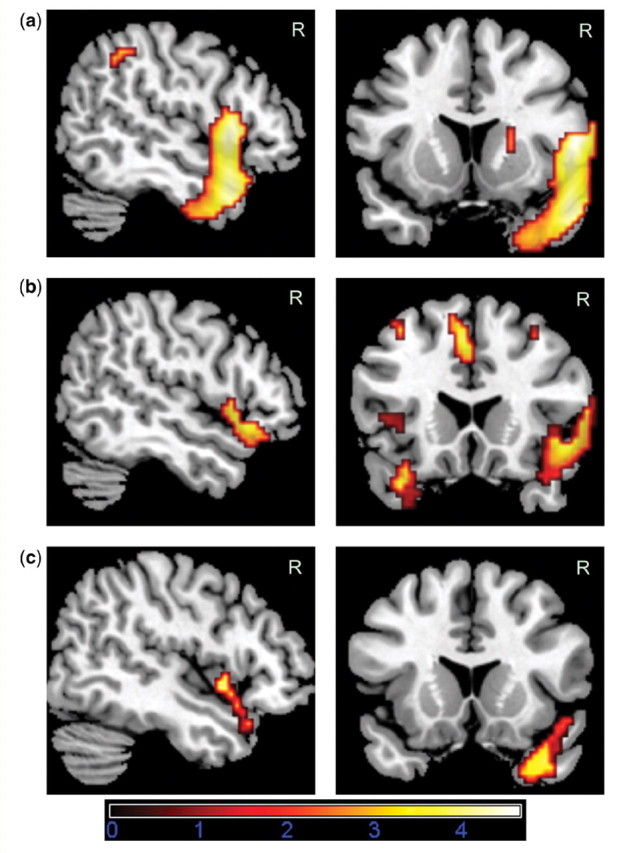
FDG-PET analyses using SPM5. Areas exceeding a voxel level threshold of P = 0.005 uncorrected, five voxels are displayed. Only regions additionally surviving FWE- or cluster-corrected P = 0.05 over bilateral apriori aTL ROI or the whole brain are reported in Table 2 and the text. (A) Analysis 1: FTLD patients with R sup aTL lesion < Normal controls, only regions shown that also show more hypometabolism in FTLD patients with R sup aTL lesion versus FTLD patients without R sup aTL lesion (inclusive mask at P = 0.005) and do not show significant hypometabolism in FTLD patients with no R sup aTL lesion versus Normal controls (exclusive mask at P = 0.005). (B) Analysis 2: FTLD Pat. with social concept selective impairment < Normal controls, only regions shown which are also more hypometabolic in FTLD patients with social concept selective impairments than in those with the reverse dissociation (i.e. animal function concept selective impairments, inclusive mask at P = 0.05) and in which there was no significant hypometabolism for the animal function concept selective impairment group versus Normal controls (exclusive mask at P=.005). (C) Areas common to both analyses displayed at P = 0.005 (Analysis 1 at P =.005 inclusively masked by Analysis 2 at P = 0.05).
Table 3.
FDG-PET—comparisons of FTLD subgroups
| Hemisphere | Area | x | y | z | Brodmann Area | Cluster size | T-score |
|---|---|---|---|---|---|---|---|
| Analysis 1: FTLD Pat. with R sup aTL lesion < NCa | |||||||
| R | Anterior superior temporal sulcus | 54 | 9 | −24 | 21/22 | 1358 | 5.19 |
| L | Anterior middle/inferior temporal gyrus | −51 | 3 | −36 | 21 | 20 | 4.19 |
| Analysis 2: FTLD Pat. with social concept selective impairment < NCb | |||||||
| L&R | Dorsomedial prefrontal cortex | −12 | 39 | 45 | 8/9 | 409 | 5.16 |
| R | Lateral orbitofrontal/anterior superior temporal cortex | 48 | 21 | −9 | 47/38 | 246 | 4.26 |
| L | Anterior superior temporal gyrus | −30 | 18 | −36 | 38 | 37 | 4.03 |
| Analysis 3: Analysis 1 inclusively masked by Analysis 2 | |||||||
| R | Anterior superior temporal gyrus | 51 | 9 | −3 | 22/38 | 94 | 4.67 |
FDG-PET analyses using SPM5. Only areas exceeding a voxel level threshold of P = 0.005 uncorrected, five voxels and FWE- or cluster-corrected P = 0.05 over the bilateral apriori aTL ROI or the whole brain are reported.
aOnly regions reported that also show more hypometabolism in FTLD Pat with R sup aTL lesion versus FTLD Pat. without R sup aTL lesion (inclusive mask at P = 0.005) and do not show significant hypometabolism in FTLD Pat. with no R sup aTL lesion versus Normal controls (exclusive mask at P = 0.005). bOnly regions reported which are also more hypometabolic in FTLD patients with social concept selective impairments than in those with the reverse dissociation (i.e. animal function concept selective impairments, inclusive mask at P = 0.05) and in which there was no significant hypometabolism for the animal function concept selective impairment group versus Normal controls (exclusive mask at P = 0.005). Analysis 3 (P = 0.005) was conducted by inclusively masking Analysis 1 at P = 0.005 for all component masks with Analysis 2 at P = 0.05 for all component masks. Inclusive masking was performed by using the Wake Forest Pickatlas ROI tool (Maldjian et al., 2003).
Neuropsychological subgroups of FTLD based on social concept selective impairment
When testing the effect of presence of Social concept selective impairments versus presence of Animal function concept selective impairments versus absence of both, there were no differences on standard neuropsychological tests and global cognitive functioning, except auditory delayed memory (WMSIII) being poorer in patients with Social concept selective impairments (Table 1). Also demographic variables did not differ between groups (Table 1). In contrast, as predicted there were significantly more behavioural symptoms (in particular ‘disinhibition’ and ‘emotional withdrawal’) in patients with Social concept selective impairments (Fig. 3).
Figure 3.
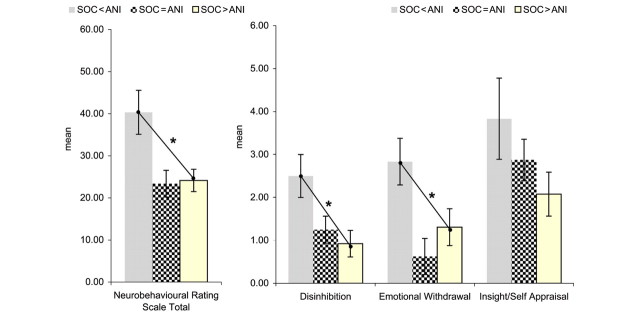
Mean scores on the Neurobehavioural Rating Scale in FTLD patients show significant differences between three subgroups (statistics see Table 1) (i) social concept selective impairment group (n = 6), (ii) social concept equal to animal function concept performance group (n = 8), (iii) animal function concept selective impairment group (n = 13). Group 1 (i.e. the social concept selective impairment group) compared with group 3 showed significantly higher total scores (Mann–Whitney U = 13.0, P = 0.02), ‘Disinhibition’ (U = 14.5, P = 0.02) as well as ‘Emotional Withdrawal’ (U = 17.0, P = 0.05) scores. There was no significant difference on ‘Insight/Self-appraisal’ scores (U = 19.0, P = 0.09). *Significant at P = 0.05 for pairwise comparisons
Effects of imageability which was the only variable, apart from word class, that differed between social and animal function concepts and affected performance (see Methods section) could be ruled out. In FTLD patients with Social concept selective impairment, there was only one patient with a significant effect of imageability with higher imageability associated with decreased performance, which therefore decreased the likelihood of Social concept selective impairments and cannot account for it. In FTLD patients with Animal function concept selective Impairment there were 7 out of 13 with significant imageability effects; in all cases higher imageability decreased performance. In order to test whether Animal function concept selective impairments were due to imageability effects, we correlated the difference of Z-scores for social minus animal function concepts with the individual regression coefficients for the effect of imageability on performance. No significant correlation emerged (n = 19, ρ = −0.29, P = 0.23). Further, when comparing the regression coefficients between patients with Social concept selective impairments with patients with Animal function concept selective impairments there was a strong group difference for the difference of Z-scores for correct responses on social concept minus animal function concept condition (U < 0.0001, P = 0.001) in the expected direction, but no difference on regression coefficients for imageability effects (U = 24.5, P = 0.14). This demonstrates that social concept selective impairments or animal function concept selective impairments were not due to lower imageability/concreteness of social concepts.
In the FTLD group, performance was higher when targets or distracters were adjectives, more frequent in the social concept condition, than when nouns and verbs were presented [t(71) > 2.5, P < 0.02]. In contrast, whether prime, target or distracter words were verbs, more frequent in the animal function concept condition, did not influence performance in the FTLD group [t(71) < 0.94, P > 0.35].
On FDG-PET, patients with Social concept selective impairments displayed the predicted right superior aTL hypometabolism, but also right orbitofrontal and bilateral dorsomedial prefrontal abnormalities when compared with patients showing animal function concept selective impairments (Fig. 2B and Table 3). In addition, there was significantly stronger left temporal pole hypometabolism in patients with social concept selective impairments (Fig. 2B and Table 3).
Combination of anatomical and neuropsychological subgroup analyses
When combining the results of both FTLD subgroup analyses, the one based on anatomical criteria and the other based on neuropsychological dissociations by using inclusive masking, the only common region of hypometabolism was the right superior aTL (Fig. 2C and Table 3). The location of hypometabolism extended into the lateral sulcus which was widened in FTLD patients due to atrophy (for an example, see Fig. 1A). Importantly, hypometabolism did not reach into adjacent orbitofrontal or insular regions (Fig. 2C, coronal section), which allowed clear localization of hypometabolism to the aTL.
Discussion
In this study, we probed knowledge of social concepts in 29 patients with FTLD and 18 patients with CBS and used FDG-PET to infer differences in the distribution of regional dysfunction.
Our comparison of patient groups with FTLD and CBS confirms the prediction that social concepts were affected more strongly in FTLD than in CBS. This finding in itself, however, cannot be unequivocally interpreted as FTLD patients showed higher levels of general cognitive impairment compared with CBS patients. The distribution of metabolic changes in CBS with stronger perirolandic, thalamic and superior parietal hypometabolism is consistent with earlier reports (Garraux et al., 2000; Eckert et al., 2005). Consistent dorsolateral, medial frontal and orbitofrontal hypometabolism in our FTLD group are also in keeping with findings in behavioural variant FTD (Salmon et al., 2006; Diehl-Schmid et al., 2007). Consistent left inferior aTL hypometabolism had been reported earlier in semantic dementia (Diehl et al., 2004; Desgranges et al., 2007) as well as progressive non-fluent aphasia (Zahn et al., 2005). The lack of consistent involvement of the superior aTL in the FTLD group as a whole is due to heterogeneity with respect to right superior aTL involvement in FTLD. This is in accordance with the reported heterogeneity in group studies of semantic dementia, in which the right superior aTL is affected in some studies (Boxer et al., 2003; Gorno-Tempini et al., 2004a) whereas other studies reported relative sparing of this region [(Good et al., 2002; Rosen et al., 2002; Diehl et al., 2004; Desgranges et al., 2007), for a review of voxel-based morphometry studies in the different subgroups of FTLD see also: (Whitwell and Jack, 2005)]. Comparable with our study sample, it was reported that only around one-third of FTLD patients show right-lateralized hypometabolism (Jeong et al., 2005).
The central analysis corroborates our main hypothesis that right superior aTL dysfunction leads to impairments of social concepts. Patients with hypometabolism in this region, but not patients without such hypometabolism, were selectively impaired on social concepts as compared with animal function concepts. Relative sparing of animal function concepts in the group with right superior aTL damage may be explained by sparing of bilateral middle aTL which was less consistently involved in this group. Bilateral middle aTL activations were found to be activated for both social and animal function concepts in a previous fMRI study conducted by our group (Zahn et al., 2007).
To further support this interpretation, we probed whether comparing FTLD patients selectively impaired on social concepts versus patients with animal function concept selective impairment, as defined individually for each patient, lead to converging evidence for involvement of the right superior aTL in the representation of conceptual social knowledge. In agreement with this hypothesis, there was right superior aTL hypometabolism sparing the middle aTL in patients with social concept selective impairment compared with the group with the reverse dissociation. This was not due to differences on other standard neuropsychological tests. Further, we carefully ruled out that dissociations between both conditions were either related to task difficulty or psycholinguistic differences including imageability/concreteness.
In addition, we were able to rule out that dissociations were due to higher frequency of verbs in the animal function concept condition. Dissociations for verbs and nouns in neurodegenerative disorders have been repeatedly reported with verb-selective impairments after premotor and inferior frontal pathology in motor neuron disease-associated dementia (Bak et al., 2001), progressive non-fluent aphasia (Hillis et al., 2004) and corticobasal degeneration (Cotelli et al., 2006; Silveri and Ciccarelli, 2007), more severe impairment of action naming than object naming in FTLD when compared with Alzheimer's disease (Cappa et al., 1998) and noun-selective naming impairments in progressive fluent aphasia (Hillis et al., 2004). However, in patients with semantic dementia in which neurodegeneration of the aTL is most prominent, conceptual impairment equally affects different word classes (Bird et al., 2000; Cotelli et al., 2006). Thus, the better performance for adjectives was most likely due to better performance on social concepts. Selective impairments for social concepts cannot be explained by relatively higher frequency of adjectives, because even if one assumed a word class effect, this should have led to underestimation of social concept selective impairments.
Interestingly, patients with social concept selective impairments showed significantly higher levels of behavioural symptoms. This was found when using the total score on the neurobehavioural rating scale and two of three symptoms which had been associated with temporal lobe pathology in FTLD before: ‘Disinhibition’ (Rosen et al., 2005) [also related to frivolous behaviour (Mendez et al., 2006)] and ‘Emotional Withdrawal’ which is related to the demonstrated lack of empathy as associated with superior aTL atrophy in FTLD (Rankin et al., 2006). There was no difference on ‘Insight/Self Appraisal’ as may have been expected from the literature on personality changes after right temporal atrophy (Gorno-Tempini et al., 2004b). A recent study showed bilateral temporal pole hypometabolism to be related to insight into one's own changes of behaviour in behavioural variant FTD (Ruby et al., 2007) using a comparison of caregiver and self-ratings, which may have been more accurate to measure this symptom than the observer-based rating employed here.
Patients with social concept selective impairment, however, did not only show pronounced hypometabolism in the right superior aTL but also in the right lateral orbitofrontal and dorsomedial prefrontal cortex. Therefore, the significantly greater number of behavioural abnormalities cannot be solely attributed to right aTL involvement. The association of orbitofrontal and temporal pole pathology in FTLD patients with ‘disinhibition’ has been well described (Snowden et al., 2001). Further studies will need to establish what the exact contribution of the right superior aTL to these symptoms is. This is because the comparison of patients with and without right superior aTL damage revealed no significant difference in observed behavioural symptoms in this study.
In close agreement with the hypothesis generated from our previous functional MRI studies (Zahn et al., 2007, 2008), the right superior aTL was associated with social concept selective impairments in both types of analyses, based on subgroups split by neuropsychological or anatomical criteria. These results are in agreement with the central role of the aTLs for representing abstract conceptual knowledge (Bozeat et al., 2000; McClelland and Rogers, 2003; Garrard and Carroll, 2006; Jefferies and Lambon Ralph, 2006; Spitsyna et al., 2006; Patterson et al., 2007; Pobric et al., 2007), and the importance of the right temporal lobe for social cognitive impairments in FTLD (EdwardsLee et al., 1997; Liu et al., 2004).
On a more cautionary note, our supporting analyses should be interpreted with care because of the rather small number of patients with social concept selective impairment identified when using a case series approach. To mitigate this concern, we based our main conclusions on the comparison of FTLD patients with right superior aTL hypometabolism versus FTLD patients with no such hypometabolism. This comparison showed a large area of right aTL hypometabolism with a peak in the superior temporal sulcus region and selective impairments for social concepts on a group basis. This analysis in itself strongly pointed to cortex around the right anterior superior temporal sulcus as necessary for the representation of conceptual social knowledge. In conjunction with the available evidence on the lack of correlation between social conceptual detail and activation of adjacent more inferior/middle aTL and lateral inferior frontal regions using fMRI in healthy controls (Zahn et al., 2007), we conclude that hypometabolism of these latter regions in our FTLD group with right superior aTL hypometabolism were less likely to have caused selective impairments of social concepts than the right superior temporal part of the hypometabolic area.
The main limitations of our study are that in principle we cannot rule out the contribution of additional regions other than the right aTL to the representation of context-independent conceptual social knowledge, because our analysis was mainly based on a prior hypothesis derived from fMRI in healthy populations. Further, this study did not establish an independent contribution of the right aTL to abnormalities of social behaviour. Therefore, the positive association between changes in social behaviour and impairments of conceptual social knowledge could have arisen by a joint effect of right aTL hypometabolism in addition to ventral and medial frontal hypometabolism. This finding is in accordance with previous studies demonstrating behavioural changes such as inappropriate social behaviour (‘disinhibition’) to be associated with both right aTL and ventromedial frontal abnormalities in FTLD (Liu et al., 2004). In our study, the group of patients with right superior aTL hypometabolism had equal levels of ventral and medial frontal hypometabolism compared with the group with no right superior aTL hypometabolism which could explain equally high levels of behavioural abnormalities in both groups. Further studies are needed to disentangle the difference in behavioural manifestations arising from right aTL and ventral frontal damage. This would be possible by using novel measures of behavioural symptoms that are able to distinguish behavioural abnormalities due to a lack of understanding of the context-independent meaning of social behaviour, depending on the aTL (Zahn et al., 2007) and a lack of understanding of the sequential context of social behaviour and the motivation to act accordingly which depends on fronto-limbic circuits (Zahn et al., 2008).
Conclusions
Taken together, our findings support the hypothesis that the right aTL, in particular its superior sector, is necessary for the representation of context-independent conceptual social knowledge. This study in itself did not rule out the additional contribution of adjacent middle aTL and inferior frontal regions, but functional imaging data in healthy populations demonstrated that these regions do not represent context-independent conceptual social knowledge (Zahn et al., 2007). Future studies need to address how loss of conceptual social knowledge representations in the aTL contributes to abnormal social behaviour in FTLD and whether this can be dissociated from the well-known contribution of ventral frontal regions to guiding social behaviour.
Supplementary material
Supplementary material is available at Brain online.
Funding
This study was supported by NINDS intramural funding to J.G. and a German Academy of Natural Scientists Leopoldina Fellowship funded by the Federal Ministry of Education and Research (BMBF-LPD 9901/8-122) to R.Z. Further R.Z. was supported in part by a Stepping Stones Fellowship award (Faculty of Medical and Human Sciences, The University of Manchester). J.M. was supported in part by the LABS-D’Or Hospital Network, Rio de Janeiro, Brazil.
Supplementary Material
Acknowledgements
We thank Eric Wassermann for neurological exams, Kris Knutson for imaging analysis advice, Alyson Cavanagh and Karen DeTucci for testing patients and Elizabeth Garber for help with normative data.
Glossary
Abbreviations:
- aTL
anterior temporal lobe
- CBS
corticobasal syndrome
- FDG-PET
18-fluoro-deoxy-glucose-positron emission tomography
- FTLD
frontotemporal lobar degeneration
- MNI
Montreal Neurological Institute
- ROI
region of interest
References
- Bak TH, O’Donovan DG, Xuereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain. 2001;124:103–20. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- Bird H, Ralph MAL, Patterson K, Hodges JR. The rise and fall of frequency and imageability: noun and verb production in semantic dementia. Brain Lang. 2000;73:17–49. doi: 10.1006/brln.2000.2293. [DOI] [PubMed] [Google Scholar]
- Boeve BF. Corticobasal degeneration: the syndrome and the disease. In: Litvan I, editor. Atypical parkinsonian disorders: clinical and research aspects. Totowa, NJ: Humana Press Inc.; 2005. pp. 309–34. [Google Scholar]
- Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner M, Gorno-Tempini ML, et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol. 2003;60:949–56. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Binetti G, Pezzini A, Padovani A, Rozzini L, Trabucchi M. Object and action naming in Alzheimer's disease and frontotemporal dementia. Neurology. 1998;50:351–5. doi: 10.1212/wnl.50.2.351. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Q J Exp Psychol A Hum Exp Psychol. 1981;33:497–505. [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Alberici A, Calabria M, Agosti C, et al. Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology. 2006;20:558–65. doi: 10.1037/0894-4105.20.5.558. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–22. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis Kaplan executive function system. San Antonio, USA: The Psychological Corporation; 2001. [Google Scholar]
- Desgranges B, Matuszewski V, Piolino P, Chetelat G, Mezenge F, Landeau B, et al. Anatomical and functional alterations in semantic dementia: a voxel-based MRI and PET study. Neurobiol Ag. 2007;28:1904–13. doi: 10.1016/j.neurobiolaging.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Forstl H, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Ag. 2007;28:42–50. doi: 10.1016/j.neurobiolaging.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Diehl J, Grimmer T, Drzezga A, Riemenschneider M, Forstl H, Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Ag. 2004;25:1051–6. doi: 10.1016/j.neurobiolaging.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26:912–21. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- EdwardsLee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–40. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Franzen EA, Myers RE. Neural control of social-behavior—prefrontal and anterior temporal cortex. Neuropsychologia. 1973;11:141–57. doi: 10.1016/0028-3932(73)90002-x. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Phil Trans R Soc London Ser B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard P, Carroll E. Lost in semantic space: a multi-modal, non-verbal assessment of feature knowledge in semantic dementia. Brain. 2006;129:1152–63. doi: 10.1093/brain/awl069. [DOI] [PubMed] [Google Scholar]
- Garraux G, Salmon E, Peigneux P, Kreisler A, Degueldre C, Lemaire C, et al. Voxel-based distribution of metabolic impairment in corticobasal degeneration. Mov Disord. 2000;15:894–904. doi: 10.1002/1531-8257(200009)15:5<894::aid-mds1021>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Geda YE, Boeve BF, Negash S, Graff-Radford NR, Knopman DS, Parisi JE, et al. Neuropsychiatric features in 36 pathologically confirmed cases of corticobasal degeneration. J Neuropsychiatry Clin Neurosci. 2007;19:77–80. doi: 10.1176/jnp.2007.19.1.77. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004a;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004b;40:631–44. doi: 10.1016/s0010-9452(08)70159-x. [DOI] [PubMed] [Google Scholar]
- Gregory CA, McKenna PJ, Hodges JR. Dementia of frontal type and simple schizophrenia: two sides of the same coin? Neurocase. 1998;4:1–6. [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Hampson S, Goldberg L, John O. Category-breadth and social-desirability values for 573 personality terms. Eur J Personal. 1987;1:241–58. [Google Scholar]
- Hampson S, John O, Goldberg L. Category breadth and hierarchical structure in personality: studies of asymmetries in judgments of trait implications. J Pers Soc Psychol. 1986;51:37–54. doi: 10.1037/0022-3514.51.1.37. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Ann Neurol. 2004;55:268–75. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The pyramids and palm trees test. Flempton: Thames Valley Test Company; 1992. [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, Kang E, et al. F-18-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nuclear Med. 2005;46:233–9. [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Ag. 2008;29:280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2. Lutz, USA: Psychological Assessment Resources; 2001. [Google Scholar]
- Kleist K. Kriegsverletzungen des Gehirns in ihrer Bedeutung für die Hirnlokalisation und Hirnpathologie. In: Bonhoeffer K, editor. Geistes- und Nervenkrankheiten. Leipzig: Verlag von Johann Ambrosius Barth; 1922. pp. 754–5. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas ES, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Movement Disorders. 2003;18:467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–8. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Assheuer J, Paxinos G. Atlas of the human brain. Amsterdam/Boston: Elsevier Academic Press; 2004. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Levin HS, Vanier M, Mazaux JM, Boake C, Goldfader PR, et al. The neurobehavioural rating scale-revised: sensitivity and validity in closed head injury assessment. J Neurol Neurosurg Psychiatry. 2001;71:643–51. doi: 10.1136/jnnp.71.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003;4:310–22. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McRae K, Cree GS, Seidenberg MS, McNorgan C. Semantic feature production norms for a large set of living and nonliving things. Behav Res Methods. 2005;37:547–59. doi: 10.3758/bf03192726. [DOI] [PubMed] [Google Scholar]
- Mendez MF, McMurtray A, Chen AK, Shapira JS, Mishkin F, Miller BL. Functional neuroimaging and presenting psychiatric features in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2006;77:4–7. doi: 10.1136/jnnp.2005.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Van Huijzen C. The human central nervous system. Berlin, Heidelberg, New York: Springer; 1978. [Google Scholar]
- Olson IR, Ploaker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Ralph MAL. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci USA. 2007;104:20137–41. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frackowiak RSJ, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci. 1999;11:371–82. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–56. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Ruby P, Schmidt C, Hogge M, D’Argembeau A, Collette F, Salmon E. Social mind representation: where does it fail in frontotemporal dementia? J Cogn Neurosci. 2007;19:671–83. doi: 10.1162/jocn.2007.19.4.671. [DOI] [PubMed] [Google Scholar]
- Salmon E, Kerrouche N, Herholz K, Perani D, Holthoff V, Beuthien-Baumann B, et al. Decomposition of metabolic brain clusters in the frontal variant of frontotemporal dementia. Neuroimage. 2006;30:871–8. doi: 10.1016/j.neuroimage.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Ciccarelli N. The deficit for the word-class "verb" in corticobasal degeneration: linguistic expression of the movement disorder? Neuropsychologia. 2007;45:2570–9. doi: 10.1016/j.neuropsychologia.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJ. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–36. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BA, Bieliauskas LA, Smith GE, Langellotti C, Ivnik RJ. Mayo's older Americans normative studies: age- and IQ-adjusted norms for the Boston Naming Test, the Mae Token Test, and the Judgment of Line Orientation Test. Clin Neuropsychol. 2005;19:280–328. doi: 10.1080/13854040590945229. [DOI] [PubMed] [Google Scholar]
- Streitfeld BD. The fiber-connections of the temporal-lobe with emphasis on the Rhesus-monkey. Int J Neurosci. 1980;11:51–71. doi: 10.3109/00207458009147579. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Wechsler D. WAISIII and WMSIII—Wechsler Adult Intelligence Scale and Wechsler Adult Memory Scale,. 3rd. San Antonio, USA: The Psychological Corporation; 1997. [Google Scholar]
- Whitwell JL, Jack CR., Jr Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Top Magn Reson Imaging. 2005;16:409–25. doi: 10.1097/01.rmr.0000245457.98029.e1. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–47. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Zahn R, Buechert M, Overmans J, Talazko J, Specht K, Ko CW, et al. Mapping of temporal and parietal cortex in progressive nonfluent aphasia and Alzheimer's disease using chemical shift imaging, voxel-based morphometry and positron emission tomography. Psychiatry Res. 2005;140:115–31. doi: 10.1016/j.pscychresns.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zahn R, Garrard P, Talazko J, Gondan M, Bubrowski P, Juengling F, et al. Patterns of regional brain hypometabolism associated with knowledge of semantic features and categories in Alzheimer's disease. J Cogn Neurosci. 2006;18:2138–51. doi: 10.1162/jocn.2006.18.12.2138. [DOI] [PubMed] [Google Scholar]
- Zahn R, Juengling FD, Bubrowski P, Jost E, Dykierek P, Talazko J, et al. Hemispheric asymmetries of hypometabolism associated with semantic memory impairment in Alzheimer's disease: a study using positron emission tomography with fluorodeoxyglucose-F18. Psychiatr Res Neuroimaging. 2004;132:159–72. doi: 10.1016/j.pscychresns.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Garrido G, Krueger F, Huey ED, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–5. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, et al. The neural basis of human social values: evidence from functional MRI. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn080. doi: 10.1093/cer cor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zec RF, Burkett NR, Markwell SJ, Larsen DL. Normative data stratified for age, education, and gender on the Boston naming test. Clin Neuropsychol. 2007;21:617–37. doi: 10.1080/13854040701339356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


