Abstract
Objectives. We compared the effects of 2 interventions on alcohol use, use of a new syringe at last injection, and condom use at last sexual encounter in a community sample of injection drug users.
Methods. Between 2003 and 2006, 851 out-of-treatment injection drug users were recruited in Raleigh, NC, and Durham, NC, through street outreach and were randomly assigned to either a 6-session educational intervention or a 6-session motivational intervention. Intervention effects were examined at 6 and 12 months after enrollment.
Results. In multiple logistic regression analyses adjusted for baseline alcohol use and HCV status, participants assigned to the motivational intervention were significantly less likely than were participants in the educational intervention to be drinking at the 6-month follow-up (odds ratio = 0.67; 95% confidence interval = 0.46, 0.97). There were no significant between-group differences in use of a new syringe at last injection or condom use at last sexual encounter at either follow-up.
Conclusions. Reducing alcohol use among persons with HCV may slow disease progression and provide important health benefits. Additional strategies are needed for slowing HCV disease progression until more effective HCV treatments are available.
HCV is the most prevalent bloodborne infection in the United States; an estimated 3.2 to 3.5 million persons are chronically infected.1,2 Although the incidence of HCV has decreased since the early 1990s, prevalence remains high because of the slow progression of the disease.3 HCV currently accounts for an estimated 8000 to 12 000 deaths annually, and that number is predicted to grow substantially as persons are infected longer.4 HCV-related liver disease is projected to cause 165 900 deaths in the United States from 2010 to 2019. An additional 27 200 deaths caused by HCV-related hepatocellular carcinoma are projected during the same period.2
HCV is a particular problem among injection drug users (IDUs). The prevalence in this group ranges from 50% to 90%,5,6 and incidence rates range from 13% to 47% per year.7–9 Although HCV incidence among IDUs has declined, 50% of new HCV infections still occur in this risk group.10 Clearly, more-effective interventions are needed to reduce incidence among HCV-negative IDUs, but simultaneously, increased efforts are also needed to identify and treat HCV-positive IDUs.5,11,12 Current treatment options, however, have several drawbacks: they produce a sustained response in only 50% of patients, have severe side effects, and are expensive.13,14 Furthermore, many persons infected with HCV may not even need treatment, and those who do may be either unwilling or unable to access it.15–18 In light of the limitations of current treatment options, it seems clear that a priority for public health workers should be the development of behavioral interventions to slow disease progression among infected individuals.
One obvious behavioral target for these interventions is reducing or eliminating alcohol use. Heavy alcohol use is one of the most important behavioral predictors of HCV progression.19–21 Alcohol use is also common among IDUs. In one study of young HCV-positive IDUs, 37% met the criteria for harmful drinking on the Alcohol Use Disorders Identification Test.22 About one quarter of heroin users entering treatment in 2002 reported alcohol as a secondary substance,23 and Hillebrand et al.24 found that 41% of their methadone maintenance patients met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for past-year alcohol dependence.25 These statistics are particularly disturbing in light of recent evidence showing deleterious effects on HCV progression and liver disease of even moderate alcohol use.26,27
One approach that has shown promise in reducing alcohol use is motivational interviewing. Motivational interviewing is a client-centered intervention approach that has been used to reduce alcohol use and other problem behaviors in a variety of settings.28 A meta-analysis of brief motivational interviewing interventions found that it was more effective in reducing alcohol use than were a variety of other brief interventions, with an aggregate effect size of 0.43.29 Motivational interviewing has also been shown to reduce alcohol use among IDUs who were needle exchange clients.30 However, the effectiveness of motivational interviewing in reducing sexual and injection-related HIV risk behaviors has been mixed.31–33
We present the results of a randomized trial of 2 HCV risk-reduction interventions in IDUs. We compared a motivational intervention with an educational intervention, both of which emphasized reductions in alcohol use as well as use of a new syringe for injection and condom use during sexual intercourse. Our primary objective was to compare the effects of the motivational intervention with those of an educational intervention on eliminating alcohol use among out-of-treatment IDUs, most of whom were infected with HCV. Secondary objectives of the study were to compare the effects of the motivational intervention with those of the educational intervention on use of a new syringe at last injection and condom use at last sexual encounter among out-of-treatment IDUs.
METHODS
Eight hundred fifty-one out-of-treatment IDUs were enrolled in the study between July 2003 and January 2006 in the Raleigh, NC, and Durham, NC, areas. We recruited participants by using a targeted sampling approach34,35 that included street outreach, in which recovering drug users went into communities to recruit active drug users and to distribute risk-reduction materials (e.g., bleach, condoms).36 After field screening, the prospective participants were referred to a project office where they received a detailed description of the study and provided informed consent. To minimize underreporting of sensitive behaviors and social desirability, we collected the data by using audio computer-assisted self-interview technology. After the participants completed the initial interview, they were randomly assigned to either an educational or a motivational intervention. We collected baseline data across 2 visits that were completed about 1 week apart. Follow-up interviews were scheduled for 6 and 12 months after enrollment. Eligibility criteria for the study included the following: a minimum age of 18 years; self-reported injection drug use in the previous 30 days; visible tracks (injection marks) or a urine specimen positive for heroin (morphine), cocaine, or methamphetamine; no formal substance abuse treatment in the previous 30 days; and current residence in 1 of the 2 counties in which the study was conducted. This study was approved by RTI International's Office of Research Protection.
Screening and Recruitment
Outreach workers completed 1786 brief screening interviews in the field; of those, 1236 met the preliminary eligibility criteria for the study and were referred to a field site. A total of 861 persons were screened at the field sites, and 855 were enrolled in the study. Four of these were later identified as duplicates and were dropped from the study. Because we did not collect and retain identifying information on the participants until they were screened in the office and provided written informed consent, it was not possible to calculate refusal rates or to assess differences between potentially eligible persons who enrolled in the study and those who did not from the screening data.
Attrition and Intervention Participation
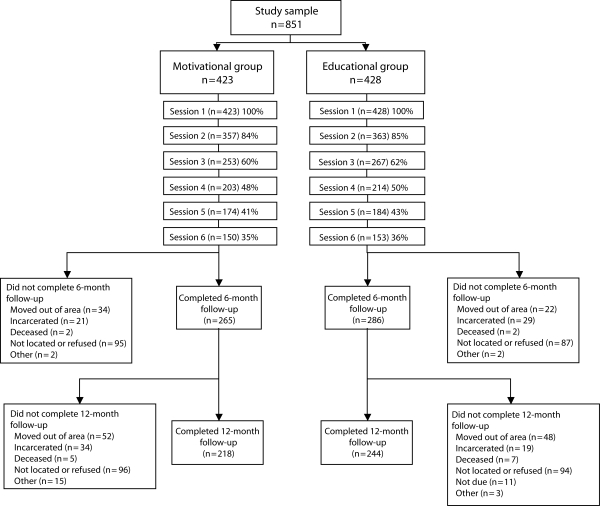
Of the 847 participants enrolled in the study who were still alive on the date that their 6-month follow-up was due, 74% completed at least 1 follow-up interview (n = 625). At the 6-month follow-up, excluding participants who had died, moved out of the area, or were incarcerated, 75% completed an interview. At the 12-month follow-up, excluding participants who had died, moved out of area, were incarcerated, or were not yet due for a follow-up interview when the study ended, 72% completed an interview. Participants who did not complete a follow-up were significantly less likely to be female (19% vs 27%; P = .049) and African American (49% vs 66%; P < .001) and were more likely to be younger (mean ±SD age of 38.3 ±9.9 years versus 41.2 ±9.3 years; P = .001). Additionally, they were less likely to test positive for HCV (45% vs 55%; P = .05). Additional details regarding attrition and intervention participation are displayed in the study flow diagram in Figure 1.
FIGURE 1.
Participant flow diagram: Chronic Hepatitis Intervention Study, Raleigh, NC, and Durham, NC, 2003–2006.
Interventions
Participants in both intervention groups were offered testing for HIV, HCV, and hepatitis B virus antibodies at the end of their first intervention session. Those who were tested received their test results and appropriate referrals during their second session. A description of each intervention follows, and additional details are provided in Table 1.
TABLE 1.
Comparison of the Length, Format, and Content of the 6-Session Educational and Motivational Interventions Presented to Injection Drug Users: Raleigh–Durham, North Carolina, July 2003 Through January 2006
| Educational Intervention |
Motivational Intervention |
|||||
| Session number | No. of Minutes, Mean (SD) | Format | Content | No. of Minutes, Mean (SD) | Format | Content |
| 1 | 23 (11) | 23 computer slides; demonstration and rehearsal of syringe cleaning and condom application; testing for HIV/HBV/HCV; passive referrals to substance abuse treatment and other services | 1. HIV/HBV/HCV disease, infection, transmission | 24 (13) | 19 computer slides; demonstration and rehearsal of syringe cleaning and condom application by interventionist; testing for HIV/HBV/HCV; passive referrals to substance abuse treatment and other services | 1. HIV/HCV/HBV disease, infection, transmission |
| 2. Modes of transmission | 2. Modes of transmission | |||||
| 3. Injection risk behaviors | 3. Injection risk behaviors | |||||
| 4. Syringe cleaning | 4. Syringe cleaning | |||||
| 5. Sexual risk behaviors | 5. Sexual risk behaviors | |||||
| 6. Male and female condom use | 6. Male and female condom use | |||||
| 7. Benefits of drug treatment | 7. Benefits of drug treatment | |||||
| 8. Description of HIV, HBV, and HCV antibody tests | 8. Description of HIV, HBV, and HCV antibody tests | |||||
| 2 | 20 (10) | 6 computer slides; HIV/HCV/HBV test results; educational materials; active referrals to substance abuse treatment, HAV/HBV vaccinations, HIV care, HCV follow-up | 1. Description of the meaning of positive and negative HIV, HBV, HCV test results | 19 (9) | 24 computer slides; HIV/HCV/HBV test results; educational materials; active referrals to substance abuse treatment, HAV/HBV vaccinations, HIV care, HCV follow-up | 1. Description of the meaning of HIV, HBV, HCV positive and negative test results |
| 2. Information on slowing or preventing onset of serious liver disease for people with chronic HCV or HBV infection including recommendations not to drink | 2. Disease-specific prevention measures for negative participants | |||||
| 3. Disease-specific control measures for positive participants | ||||||
| 4. Treatment information for each disease | ||||||
| 3. Referrals to HIV treatment and HCV, HBV evaluation for positive participants and HBV vaccination for HBV-negative participants | 5. Information on probable outcomes of HCV infection and on slowing HCV disease progression | |||||
| 3 | 52 (14) | Video | Video on HAV, HBV, and HCV | 41 (14) | Counseling | Focused on beginning motivation to change |
| 4 | 78 (15) | Video | HIV informational video regarding HIV transmission, natural history, and treatment and prevention | 37 (14) | Counseling | Focused on developing a plan for change |
| 5 | 39 (10) | Video | Video on drug preparation and sharing, demonstrating how a drug mixture becomes contaminated at different points in the process; video on HCV | 35 (12) | Counseling | Reviewed progress and worked on overcoming obstacles to change |
| 6 | 50 (9) | Video | In this video, recovering drug users describe consequences of addiction and programs that have helped them conquer it | 35 (9) | Counseling | Reviewed progress, developed strategies for overcoming obstacles, and reaffirmed commitment to change |
Note. HAV = hepatitis A virus; HBV = hepatitis B virus.
Educational intervention.
The educational intervention consisted of 6 sessions. The first 2 sessions were based on the cue cards from the National Institute on Drug Abuse standard intervention that was revised in 2000 to include information on hepatitis B virus and HCV in addition to HIV.32 These sessions were followed by 4 additional sessions based on videos that lasted approximately 1 hour each. Topics covered during these sessions included hepatitis A, hepatitis B, hepatitis C, HIV, indirect sharing practices, and addiction. Participants were given a $10 food coupon at the end of sessions 3 through 6.
Motivational intervention.
The motivational intervention also consisted of 6 sessions. Participants assigned to the motivational intervention received 2 cue-card sessions presented as PowerPoint (Microsoft Corporation, Redmond, Washington) slides followed by 4 additional motivational sessions. Session 1 included 20 slides that were adapted from the revised National Institute on Drug Abuse standard intervention37 and lasted approximately 45 minutes. Session 2 included 24 slides; however, the number of slides a participant was shown was determined by his or her test results. The 4 additional motivational sessions lasted approximately 30 minutes each and focused on increasing motivation to change, developing a plan for change, reviewing progress, and reaffirming commitment to change. These sessions were conducted by lay persons from the community who were trained in motivational interviewing techniques. Although the explicit emphasis of the sessions was on increasing motivation to change, the activities were also designed to build self-efficacy and self-regulation skills. Participants received a $10 food coupon at the end of each session.
Measures
Participants were screened for HIV antibodies by using the Orasure HIV-1 enzyme-linked immunosorbent assay test (Orasure Technologies, Bethlehem, Pennsylvania). Specimens that were reactive on 2 assays were confirmed by Orasure Western blot. Participants were screened for HCV antibodies by using the HCV EIA 3.0 test (Orthoclinical Diagnostics, Rochester, New York) with a signal to cutoff ratio of 8 or more. Specimens with a signal to cutoff ratio that was less than 8 were confirmed by using qualitative polymerase chain reaction. Specimens were initially tested for hepatitis B virus core antibodies. Those specimens testing positive were then tested for the hepatitis B virus surface antigen to distinguish between resolved and active infection. Five participants refused the HIV antibody test, and 15 refused HCV antibody testing, which required a blood draw.
We examined intervention outcomes at the 6-month and 12-month follow-ups separately. The primary outcome of interest was any alcohol use in the previous 30 days. We chose no alcohol use, rather than reductions in alcohol use, as the primary outcome because safe levels of alcohol use for persons with HCV infection are poorly defined and abstinence is recommended.20,36,38 Use of a new syringe at last injection and condom use at last sexual encounter were selected to assess secondary outcomes for injection and sexual risk reduction, respectively. Because we were comparing 2 potentially effective interventions, we assessed the effects of each intervention separately on each outcome as well as compared the relative effectiveness of the 2 interventions.
Analysis
We used McNemar's test to assess the significance of within-group changes from baseline to each follow-up separately for each intervention condition. Multiple logistic regression analyses were performed to assess intervention effects on alcohol use during the previous 30 days, use of a new syringe at last injection, and unprotected intercourse at last sexual encounter, while adjusting for baseline behavior. In addition, the alcohol use models included HCV status because of the effect of alcohol on HCV progression. For each of the outcomes, we ran models to look at intervention effects at the 6-month and the 12-month follow-up separately.
RESULTS
Sample Characteristics
The baseline characteristics of the 625 participants who completed one or more follow-up interviews are shown by intervention group in Table 2. The sample was predominantly male (73%) and African American (66%), and the participants’ mean age was 41 years (SD = 9 years). Sixty-nine percent had completed high school, 70% were unemployed, 35% were homeless, and 20% were married or living as married. Sixty-seven percent had been in substance abuse treatment, and 57% had been in prison. HIV prevalence was 9%, and HCV prevalence was 55%. Drug use and risk behavior data are shown in Table 2 by intervention group. With the exception of alcohol use, which was significantly higher in the motivational group, the groups were not significantly different in any of the variables for which we compared them.
TABLE 2.
Baseline Characteristics of the Injection Drug Users, by Intervention Group: Raleigh–Durham, North Carolina, July 2003 Through January 2006
| Background characteristics | Total (N = 625) | EducationalIntervention | MotivationalIntervention | P |
| Age, y, mean (SD) | 41.2 (9.3) | 41.1 (8.9) | 41.3 (9.7) | .78 |
| Race/ethnicity, % | .97 | |||
| African American | 66.4 | 66.6 | 66.2 | |
| Non-Hispanic White | 26.8 | 26.4 | 27.2 | |
| Other | 6.8 | 7.0 | 6.6 | |
| Men, % | 72.9 | 70.2 | 76.0 | .11 |
| High school graduate, % | 69.2 | 71.1 | 66.9 | .26 |
| Unemployed, % | 69.9 | 67.3 | 72.8 | .14 |
| Married or living as married, % | 20.3 | 21.0 | 19.5 | .66 |
| Currently homeless, % | 34.8 | 33.9 | 35.8 | .63 |
| Ever in substance abuse treatment (lifetime), % | 67.1 | 65.0 | 69.5 | .24 |
| Ever in prison (lifetime), % | 57.0 | 58.4 | 55.5 | .47 |
| HIV positive, % | 8.9 | 7.6 | 10.3 | .24 |
| HCV positive, % | 54.8 | 55.3 | 54.2 | .78 |
| Alcohol and drug use (previous 30 days) | ||||
| Used alcohol, % | 70.4 | 66.9 | 74.5 | .04 |
| No. of days drank alcohol, mean (SD) | 11.9 (11.8) | 11.0 (11.9) | 13.0 (11.7) | .04 |
| No. of drinks per day when drinking, mean (SD) | 4.1 (4.9) | 3.6 (4.4) | 4.7 (5.4) | .004 |
| Used crack, % | 72.5 | 73.0 | 72.0 | .79 |
| Used powder cocaine, % | 64.8 | 63.3 | 66.4 | .42 |
| Heroin, % | 69.6 | 68.5 | 70.9 | .53 |
| Speedball (heroin and cocaine in combination), % | 58.4 | |||
| Methamphetamine, % | 12.1 | 10.5 | 140 | .18 |
| Injection risk behaviors | ||||
| Ever shared a syringe (lifetime), % | 47.5 | 45.8 | 49.3 | .39 |
| Shared a syringe, previous 30 days, % | 17.0 | 16.0 | 18.2 | .47 |
| Shared cooker, cotton, or rinse water, previous 30 days, % | 23.2 | 22.5 | 24.1 | .63 |
| Used a new syringe at last injection, % | 87.1 | 86.2 | 88.1 | .47 |
| Sexual behavior previous 30 days | ||||
| >1 sexual partner, % | 26.5 | 27.0 | 26.0 | .77 |
| Gave money or drugs for sex previous 30 days, % | 24.0 | 23.5 | 24.6 | .73 |
| Traded sex for money or drugs previous 30 days, % | 15.8 | 16.7 | 14.8 | .52 |
| Unprotected at last sexual intercourse, % | 57.4 | 56.1 | 58.8 | .52 |
Intervention Outcomes
Within-group changes from baseline to follow-up.
The percentage of participants who reported drinking alcohol in the previous 30 days decreased significantly in each intervention group at both the 6-month and the 12-month follow-up interviews. In the educational condition, the percentage of participants who reported alcohol use in the previous 30 days decreased from 67% to 54% between baseline and the 6-month follow-up (P < .001) and from 67% to 48% between baseline and the 12-month follow-up (P < .001). In the motivational group, the percentage of participants who reported alcohol use decreased from 74% to 48% between baseline and the 6-month follow-up (P < .001) and from 74% to 52% between baseline and the 12-month follow-up (P < .001).
The percentage of participants in the educational group who reported using a new syringe for their last injection was 87% at baseline, 88% at the 6-month follow-up, and 82% at the 12-month follow-up. The percentage of participants in the motivational group who reported using a new syringe for their last injection was 88% at baseline, 91% at the 6-month follow-up, and 89% at the 12-month follow-up. None of these within-group differences in use of a new syringe at last injection were statistically significant at P < .05. In the educational group, the percentage of participants who reported engaging in unprotected intercourse during their last sexual encounter decreased from 54% to 35% between baseline and the 6-month follow-up (P < .001) and to 37% at the 12-month follow-up (P < .001). In the motivational group, the percentage of participants who reported engaging in unprotected intercourse during their last sexual encounter decreased from 59% to 39% between baseline and the 6-month follow-up (P < .001) and to 38% at the 12-month follow-up (P < .001).
Between-group intervention effects.
As shown in Table 3, in multiple logistic regression analyses adjusted for baseline alcohol use and HCV status, participants in the motivational group were significantly less likely to be drinking at the 6-month follow-up than were participants in the educational group (odds ratio [OR] = 0.67; 95% confidence interval [CI] = 0.46, 0.97; P = .035). Being in the intervention group was not a significant predictor of alcohol use at the 12-month follow-up. In multiple logistic regression analyses adjusted for use of a new syringe at last injection at baseline, being in the intervention group was not significantly associated with use of a new syringe at last injection at either the 6-month or the 12-month follow-up (Table 3). In logistic regression models that adjusted for unprotected intercourse at last sexual encounter at baseline and partner type (i.e., main or casual), being in the intervention group was not a significant predictor of unprotected intercourse during last sexual encounter at either the 6-month or the 12-month follow-up (Table 3).
TABLE 3.
Intervention Outcomes of the 6-Session Educational and Motivational Interventions Presented to Injection Drug Users: Raleigh–Durham, North Carolina, July 2003 Through January 2006
| Outcome and Predictors | b | OR (95% CI) |
| Alcohol use previous 30 days, 6-month follow-up (n = 495) | ||
| Motivational group | −0.40 | 0.67* (0.46, 0.97) |
| Baseline alcohol use | 1.38 | 3.97*** (2.58, 6.11) |
| HCV positive | −0.44 | 0.65* (0.44, 0.94) |
| Alcohol use previous 30 days, 12-month follow-up (n = 447) | ||
| Motivational condition | 0.01 | 1.01 (0.69, 1.49) |
| Baseline alcohol use | 1.20 | 3.32*** (2.13, 5.20) |
| HCV positive | −0.06 | 0.94 (0.64, 1.38) |
| Used a new syringe last injection, 6-month follow-up (n = 503) | ||
| Motivational group | 0.36 | 1.43 (0.79, 2.60) |
| Used a new syringe last injection at baseline | 1.01 | 2.75** (1.37, 5.52) |
| Used a new syringe last injection, 12-month follow-up (n = 454) | ||
| Motivational group | 0.51 | 1.67 (0.97, 2.87) |
| Used a new syringe last injection at baseline | 0.64 | 1.89 (0.97, 3.69) |
| Unprotected vaginal or anal intercourse at last sexual encounter, 6-month follow-up (n = 436) | ||
| Motivational group | 0.07 | 1.08 (0.69, 1.67) |
| Unprotected intercourse at baseline | 1.81 | 6.09*** (3.76, 9.84) |
| Primary partner | 1.30 | 3.68*** (2.29, 5.92) |
| Unprotected vaginal or anal intercourse at last sexual encounter, 12-month follow-up (n = 397) | ||
| Motivational group | 0.00 | 1.00 (0.65, 1.56) |
| Unprotected intercourse at baseline | 1.40 | 4.04*** (2.55, 6.39) |
| Primary partner | 0.90 | 2.46*** (1.54, 3.91) |
Note. OR = odds ratio; CI = confidence interval.
*P < .05; **P < .01; ***P < .001.
DISCUSSION
The findings of this randomized trial indicated that a brief 6-session motivational intervention was more effective than was a 6-session educational intervention in reducing alcohol use at the 6-month follow-up. There were no significant between-group differences in alcohol use at the 12-month follow-up, although alcohol use in both groups was still substantially lower than at baseline. Although these results are encouraging, approximately 50% of participants in both intervention groups continued to drink, so more-effective interventions are still needed. Injection risk was low at baseline; almost 90% of the participants in each intervention group reported using new syringes at baseline. Participants in both intervention groups were significantly less likely to report unprotected intercourse at the 6-month and 12-month follow-up interviews than they were at baseline, but we found no significant between-group differences.
The finding that 35% to 40% of participants in both groups reported engaging in unprotected intercourse at their last sexual encounter is disappointing but not surprising. A common finding of intervention studies with IDUs is that interventions tend to be more successful in reducing injection-related risk behaviors than in reducing sexual risk behaviors.39 The emphasis of this study on HCV, which is transmitted more efficiently through injection than through sexual practices, may have inadvertently further diminished the effects of the motivational intervention on reducing sexual risk.
Limitations
As with most studies of drug users and other high-risk populations, this study suffered from several limitations. Most notably, our study had high rates of attrition caused by incarceration, death, and moving out of the area, which is a typical problem with community-based studies of IDUs. Although audio computer-assisted self-interview technology was used to minimize socially desirable responses, the accuracy of self-reports of drug use and sexual behavior is difficult to verify. The reliability of self-reports may suffer from inaccurate recall, whereas validity may suffer from intentional misreporting in addition to faulty memory. We also cannot rule out the possibility that the association between the intervention and the behavioral risk reductions reflected self-selection, with participants who were more motivated to change attending more sessions.
A targeted sampling approach was used to recruit a geographically diverse and racially mixed sample. Despite these efforts, the sample was predominantly African American from the inner cities of Raleigh and Durham. Because outreach workers tend to reach drug users who are accessible on the street in high drug use areas, IDUs that spend large amounts of time in these areas are likely to be overrepresented. Conversely, suburban drug users, mid- to upper-level drug dealers, many working professionals, and other employed drug users may be underrepresented in this sample. Therefore, caution should be used in generalizing findings in this study to IDU populations of other races and to suburban IDUs.
Conclusions
Few HCV or HIV risk-reduction interventions designed to reduce risky injection and sexual practices among high-risk groups have focused on reductions in alcohol use.40 Yet, alcohol use has been shown to be associated with HCV in several important ways. Not only has alcohol use been shown to exacerbate many of the risky injection and sexual practices that lead to acquisition of these diseases, but it has also been shown to affect health-related quality of life41 and to promote HCV progression and liver disease among those already infected. The results of our study showed that a relatively brief and inexpensive motivational intervention was effective at significantly reducing alcohol use as well as reducing sexual risk and maintaining lower injection risk in a population of out-of-treatment IDUs.
The high prevalence of HCV infection among IDUs, the barriers to HCV treatment, and the severe side effects and limited effectiveness of current treatments make this a population in particular need of new strategies for slowing HCV disease progression. In addition, given the millions of persons already chronically infected with HCV and the fact that current treatments for this disease are expensive and have significant side effects, it is clear that new strategies are needed to slow HCV progression among all of those already infected. Although this intervention was conducted among a population of out-of-treatment IDUs, we expect that it could be easily adapted and be equally, if not more, effective among other groups with high rates of alcohol use and HCV infection, such as former injectors, methadone patients, and US military veterans. Indeed, the fact that this intervention was effective among a population that is particularly challenging to recruit and retain in studies makes the findings of positive behavioral changes in this intervention all the more impressive. The motivational intervention in this study may be one such important alternative to treatment that not only has no side effects but also is less expensive and time consuming to administer and more accessible to those without insurance.
Acknowledgments
This study was funded by the National Institute on Drug Abuse (grant R01DA13763). Funding for the development of the motivational intervention was provided by the University of North Carolina Center for AIDS Research program of the National Institutes of Health (grant 9P30 AI050410).
We thank the entire field staff of the Chronic Hepatitis Intervention Project for Drug Users and all of the participants in the study.
Human Participant Protection
This study was approved by RTI International's Office of Research Protection and Ethics Institutional Review Board.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714 [DOI] [PubMed] [Google Scholar]
- 2.McHutchison JG, Bacon BR. Chronic hepatitis C: an age wave of disease burden. Am J Manag Care. 2005;11(suppl 10):S286–S295; quiz S307–S311 [PubMed] [Google Scholar]
- 3.Chen CM, Yoon YH, Yi HY, Lucas DL. Alcohol and hepatitis C mortality among males and females in the United States: a life table analysis. Alcohol Clin Exp Res. 2007;31:285–292 [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health Consensus Development Conference Statement: Management of Hepatitis C. Bethesda, MD: National Institutes of Health; 2002 [Google Scholar]
- 5.Edlin BR, Carden MR. Injection drug users: the overlooked core of the hepatitis C epidemic. Clin Infect Dis. 2006;42:673–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulkowski MS, Thomas DL. Epidemiology and natural history of hepatitis C virus infection in injection drug users: implications for treatment. Clin Infect Dis. 2005;40(Suppl 5):S263–S269 [DOI] [PubMed] [Google Scholar]
- 7.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15:543–549 [DOI] [PubMed] [Google Scholar]
- 8.Maher L, Li JO, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure? Aust N Z J Public Health. 2007;31:30–35 [DOI] [PubMed] [Google Scholar]
- 9.Miller CL, Johnston C, Spittal PM, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36:737–742 [DOI] [PubMed] [Google Scholar]
- 10.Wasley A, Miller JT, Finelli L. Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ. 2007;56:1–24 [PubMed] [Google Scholar]
- 11.Backmund M, Reimer J, Meyer K, Gerlach JT, Zachoval R. Hepatitis C virus infection and injection drug users: prevention, risk factors, and treatment. Clin Infect Dis. 2005;40(suppl 5):S330–S335 [DOI] [PubMed] [Google Scholar]
- 12.Page-Shafer K, Hahn JA, Lum PJ. Preventing hepatitis C virus infection in injection drug users: risk reduction is not enough. AIDS. 2007;21:1967–1969 [DOI] [PubMed] [Google Scholar]
- 13.Firpi RJ, Nelson DR. Current and future hepatitis C therapies. Arch Med Res. 2007;38:678–690 [DOI] [PubMed] [Google Scholar]
- 14.Rajendra A, Wong JB. Economics of chronic hepatitis B and hepatitis C. J Hepatol. 2007;47:608–617 [DOI] [PubMed] [Google Scholar]
- 15.Evon DM, Verma A, Dougherty KA, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007;52:3251–3258 [DOI] [PubMed] [Google Scholar]
- 16.Kim AI, Saab S. Treatment of hepatitis C. Am J Med. 2005;118:808–815 [DOI] [PubMed] [Google Scholar]
- 17.Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes among patients with psychiatric illness. Psychiatr Serv. 2006;57:570–572 [DOI] [PubMed] [Google Scholar]
- 18.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398, vi [DOI] [PubMed] [Google Scholar]
- 19.Delarocque-Astagneau E, Roudot-Thoraval F, Campese C, Desenclos JC, the Hepatitis C Surveillance System Steering Committee Past excessive alcohol consumption: a major determinant of severe liver disease among newly referred hepatitis C virus infected patients in hepatology reference centers, France, 2001. Ann Epidemiol. 2005;15:551–557 [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1150–1159 [DOI] [PubMed] [Google Scholar]
- 21.Kayali Z, Tan S, Shinkunas L, et al. Risk factors for hepatitis C fibrosis: a prospective study of United States veterans compared with nonveterans. J Viral Hepat. 2007;14:11–21 [DOI] [PubMed] [Google Scholar]
- 22.Campbell JV, Hagan H, Latka MH, et al. High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug Alcohol Depend. 2006;81(3):259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Office of Applied Studies, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services Treatment Episode Data Set (TEDS), 2005 [computer file]. Prepared by Synectics for Management Decisions I. Treatment Episode Data Set (TEDS), 2005 [Computer file]. In: ICPSR04626-v1 Ann Arbor, MI: Inter-university Consortium for Political and Social Research [producer and distributor]; 2007 [Google Scholar]
- 24.Hillebrand J, Marsden J, Finch E, Strang J. Excessive alcohol consumption and drinking expectations among clients in methadone maintenance. J Subst Abuse Treat. 2001;21:155–160 [DOI] [PubMed] [Google Scholar]
- 25.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 26.Monto A, Patel K, Bostrom A, et al. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004;39:826–834 [DOI] [PubMed] [Google Scholar]
- 27.Szabo G. Moderate drinking, inflammation, and liver disease. Ann Epidemiol 2007;17(5 Suppl):S49–S54 [Google Scholar]
- 28.Rubak S, Sandboek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305–312 [PMC free article] [PubMed] [Google Scholar]
- 29.Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41:328–335 [DOI] [PubMed] [Google Scholar]
- 30.Stein MD, Charuvastra A, Maksad J, Anderson BJ. A randomized trial of a brief alcohol intervention for needle exchangers (BRAINE). Addiction. 2002;97:691–700 [DOI] [PubMed] [Google Scholar]
- 31.Baker A, Kochan N. Controlled evaluation of a brief intervention for HIV prevention among injecting drug users not in treatment. AIDS Care. 1994;6:559–570 [DOI] [PubMed] [Google Scholar]
- 32.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71:843–861 [DOI] [PubMed] [Google Scholar]
- 33.Stein MD, Anderson B, Charuvastra A, Maksad J, Friedmann PD. A brief intervention for hazardous drinkers in a needle exchange program. J Subst Abuse Treat. 2002;22:23–31 [DOI] [PubMed] [Google Scholar]
- 34.Carlson RG, Wang J, Siegal HA, Falck RS, Guo J. An ethnographic approach to targeted sampling: problems and solutions in AIDS prevention research among injection drug and crack-cocaine users. Hum Organ. 1994;53:279–286 [Google Scholar]
- 35.Watters JK, Biernacki P. Targeted sampling: options for the study of hidden populations. Soc Probl. 1989;36:416–430 [Google Scholar]
- 36.Jamal MM, Morgan TR. Liver disease in alcohol and hepatitis C. Best Pract Res Clin Gastroenterol. 2003;17:649–662 [DOI] [PubMed] [Google Scholar]
- 37.National Institute on Drug Abuse The NIDA Community-Based Outreach Model: A Manual to Reduce the Risk of HIV and Other Blood-Borne Infections in Drug Users. Bethesda, MD: National Institute on Drug Abuse; 2000 [Google Scholar]
- 38.Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761–772 [DOI] [PubMed] [Google Scholar]
- 39.Williams M, McCoy HV, Bowen A, Saunders L, Freeman R, Chen D. An evaluation of a brief HIV risk reduction intervention using empirically derived drug use and sexual risk indices. AIDS Behav. 2001;5:31–43 [Google Scholar]
- 40.Stein MD, Anderson B, Charuvastra A, Maksad J, Friedmann PD. A brief intervention for hazardous drinkers in a needle exchange program. J Subst Abuse Treat. 2002;22:23–31 [DOI] [PubMed] [Google Scholar]
- 41.Costenbader EC, Zule WA, Coomes CM. The impact of illicit drug use and harmful drinking on quality of life among injection drug users at high risk for hepatitis C infection. Drug Alcohol Depend. 2007;89:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]