Abstract
Estrogen metabolism may play an important role in mammary carcinogenesis in postmenopausal women. We evaluated the effects of prior oral contraceptive (OC) treatment and current soy isoflavone consumption on endogenous estrogen metabolite concentration and biomarkers of tissue estrogen exposure in a monkey model. One hundred eighty-one female cynomolgus macaques were randomized to receive OC or placebo for 26 months premenopausally, then ovariectomized and randomized to one of three diets for 36 months: an isoflavone-depleted soy protein isolate (Soy−) diet, a diet containing soy protein isolate with a human equivalent of 129 mg isoflavone/d (Soy+), or a Soy− diet supplemented with conjugated equine estrogens (CEE+) at a human equivalent dose of 0.625 mg/d. Reverse-phase high-performance liquid chromatography directly coupled with tandem mass spectrometry was used to measure the concentrations of estrogen species in urine samples. Generally, prior OC treatment was associated with significantly reduced urinary estrogen metabolites (25–55% reduction; P < 0.05 for each versus OC−). Animals that consumed isoflavones postmenopausally had increased urinary 2-hydroxyestrone and 16α-hydroxyestrone (50% and 56% increases, respectively), but reduced levels of 2-hydroxyestradiol, 2-methoxyestradiol, and 17-epiestriol (92%, 63%, and 66%, respectively), compared with animals fed a Soy− diet. Isoflavones did not have widespread effects on uterine or mammary proliferation biomarkers, whereas prior OC significantly reduced two of three proliferation end points in the endometrium. Premenopausal OCs may have long-term systemic effects on response to estrogen and its metabolism whereas postmenopausal dietary isoflavones may alter endogenous estrogen metabolism in a modest but selective manner.
Introduction
According to the American Cancer Society, cancers originating in the breast and uterus are estimated to be the first and fourth most common sites of cancer incidence among women, respectively, accounting for a projected 32% of new cancer cases in 2007 (1). Given the functions of these tissues, estrogen responsiveness plays a critical role in their normal and pathologic character. Lifetime overexposure to estrogens has long been linked to the etiology of breast and endometrial cancer through various life characteristics such as age at menarche and menopause, parity status, exogenous hormone use, obesity, and diet (2, 3).
Most hypotheses implicating estrogen in the cause or promotion of hormone-responsive cancers identify a mitogenic role possibly promoting carcinogenesis by stimulating growth of neoplastic cells. Over the past two decades, however, more investigations have focused on the role of certain estrogen metabolites [i.e., hydroxy estrogens (OHE) and their quinone species] as genotoxins and mitogens in promoting cancer (4). For example, 16α-hydroxyestrone is proposed to be an inducer of proliferation (5, 6), and 4-hydroxyestradiol and 4-hydroxyestrone are postulated to be direct (through DNA adduct formation) and indirect (through generation of reactive oxygen species) producers of DNA damage in carcinogenesis (7). Estrogen metabolites modified at the C2 position, however, may act as inhibitors of carcinogenesis and angiogenesis (8). Based, in part, on these findings, a number of studies have sought to link an increased ratio of 2-hydroxyestrogen to 16-hydroxyestrogen formation to decreased risk for breast cancer, with mixed results (9).
The relationship between estrogen and breast cancer risk has driven many recent studies to investigate how dietary and lifestyle factors may interact with estrogen exposure. Several studies suggest that dietary soy may decrease serum levels of E1 (10–12) and 4-hydroxyestrone (13) and increase the ratio of 2-hydroxyestrone/16-hydroxyestrone (13–16), although the latter effect may be dependent on gut flora conversion of the soy isoflavone daidzein to equol (17).
We investigated how the use of OCs premenopausally and/or dietary isoflavone consumption postmenopausally might alter the estrogen metabolite profile using a cynomolgus monkey model. This model allows a degree of control over dietary and hormonal treatments not possible in human primate subjects while also providing primate-specific reproductive physiology. We examined whether these two factors affected tissue biomarkers of estrogen exposure and proliferation and the relationship between those biomarkers and concentrations of selected urinary estrogen metabolites. We hypothesized that the isoflavone-rich diet would produce an estrogen metabolite profile with lower levels of genotoxic or mitogenic estrogen metabolites and higher amounts of benign or beneficial metabolites, whereas the premenopausal OCs would have no significant effects on postmenopausal estrogen metabolism.
Materials and Methods
Animals and Study Design
One hundred eighty-one premenopausal cynomolgus monkeys (Macaca fascicularis) were obtained from the Institut Bogor, Indonesia. These monkeys were used in a multifaceted, randomized, controlled study designed to investigate the effects of premenopausal OCs and postmenopausal soy isoflavones or CEE on cardiovascular, bone, neurobiology, and cancer-risk marker end points. Data on many of these end points as well as diet composition and study design have been reported previously (12, 16, 18–22).
In brief, half of the premenopausal monkeys were given a triphasic oral contraceptive (OC+) over a 26-mo period (Triphasil, Wyeth Pharmaceuticals). Initial randomization before the premenopausal treatment phase was based on body weight and plasma total cholesterol/high-density lipoprotein cholesterol ratios and stratified by social group status. At the end of this premenopausal phase of the study, the animals were ovariectomized to make them surgically menopausal.
The postmenopausal phase of this study was a three-group, parallel-arm design, with treatments lasting 36 mo. An equivalent number of social groups were sampled from each premenopausal condition and randomized to the postmenopausal dietary interventions. The treatment diet included soy protein isolate containing isoflavone (Soy+) at a dose approximately equivalent to 129 mg/d for women, expressed as aglycone units. The control diet contained isolated soy protein that had been alcohol washed to remove the isoflavone (Soy−), so that the protein source remained consistent between the groups. The third diet group was fed isoflavone-depleted soy protein supplemented with CEE (Premarin, Wyeth Pharmaceuticals) at a human equivalent dose of 0.625 mg/d as a positive estrogen control. The isolated soy proteins used for this study were generously provided by The Solae Company. The diets were formulated to be isocaloric for protein, carbohydrate, and fat and comparable for cholesterol, calcium, and phosphorus. The monkeys were fed ∼ 120 kcal/kg body weight split into two feedings (one third in the morning, two thirds in the afternoon). Urine samples were collected from each monkey housed singly in cages overnight during month 35 of the 36-mo postmenopausal phase of the study and frozen for subsequent analysis of metabolite concentrations. Mammary and uterine tissues were collected at necropsy, with samples from each animal flash frozen in liquid nitrogen and stored at −80°C for mRNA analyses and formalin fixed and paraffin embedded for histomorphometric analysis. All procedures involving animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee.
Estrogen Profiling of Macaque Urine Using High-Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry Analysis
Female cynomolgus monkey urine was centrifuged to remove debris, aliquoted, and frozen at −20°C until analysis. Urine samples for 166 animals and complete treatment information for 163 animals were obtained. Endogenous estrogens and estrogen metabolites were quantified using reverse-phase high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in selected reaction monitoring mode as previously described (23). The estrogens quantified were estrone (E1), estradiol (E2), and estriol (E3). The estrogen metabolites measured were 16-epiestriol (16epiE3), 17-epiestriol (17epiE3), 16-ketoestradiol (16KE2), 16α-hydroxyestrone (16αOHE1), 2-methoxyestrone (2MeOE1), 4-methoxyestrone (4MeOE1), 2-hydroxyestrone-3-methyl ether (3MeOE1), 2-methoxyestradiol (2MeOE2), 4-methoxyestradiol (4MeOE2), 2-hydroxyestrone (2OHE1), 4-hydroxyestrone (4OHE1), and 2-hydroxyestradiol (2OHE2).
Briefly, calibration standards were prepared in charcoal-stripped estrogen-free human urine by adding 20 µL of the deuterium-labeled endogenous estrogens and estrogen metabolites working internal standard solution (1.6 ng of deuterium-labeled estrogen metabolites) and various volumes of estrogen metabolite working standard solution, which typically contained between 0.02 and 19.2 ng of estrogen metabolites. Quality control samples were prepared in estrogen-free urine at three levels (0.12, 0.96, and 6.4 ng of estrogen metabolites/mL). Animal urine (0.5 mL) was used for each analysis and samples from each treatment groups were randomized in each run of the assay. Because endogenous estrogens and their metabolites in urine are mostly present as glucuronide and sulfate conjugates, a hydrolysis step was included using β-glucuronidase/sulfatase from Helix pomatia (type H-2). After hydrolysis, estrogen metabolites were extracted with dichloromethane. The organic phase was isolated, evaporated to dryness under nitrogen gas, and reconstituted with a sodium bicarbonate buffer containing dansyl chloride for subsequent derivatization of the estrogen metabolites. Calibration standards and quality control samples were hydrolyzed, extracted, and derivatized following the same procedure as that of unknown urine samples. After derivatization, all samples were analyzed by reverse-phase high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in selected reaction monitoring mode using a Finnigan TSQ Quantum-AM triple quadrupole mass spectrometer coupled to a Surveyor high-performance liquid chromatography system (ThermoFinnigan). Both the high-performance liquid chromatography and mass spectrometer were controlled by Xcalibur software (ThermoFinnigan). The lower limit of quantitation for each estrogen metabolite is 0.02 ng/0.5 mL urine sample and the intrabatch precision (percent coefficient of variation) for each estrogen metabolite is provided in Table 1. Two animals were excluded due to uncertainty about the urine samples based on their mass spectrometry profiles, leaving data from 161 animals for subsequent analyses. Animal urine creatinine was measured using an ACE ALERA automated analyzer (Alfa Wassermann, Inc.).
Table 1.
Precision (percent coefficient of variation) of quality control sample estrogen measurement
| Estrogen metabolite |
0.12 ng EM/mL |
0.96 ng EM/mL |
6.4 ng EM/mL |
|---|---|---|---|
| E3 | 3.16 | 0.09 | 0.47 |
| 16-ketoE2 | 2.18 | 0.32 | 0.54 |
| 16a-OHE1 | 2.08 | 0.44 | 2.61 |
| 16-epiE3 | 1 | 0.71 | 1.02 |
| 17-epiE3 | 1.12 | 0.68 | 1.23 |
| 3-MeOE1 | 1.4 | 0.53 | 1.82 |
| 2-MeOE1 | 0.46 | 1.36 | 0.2 |
| 4-MeOE1 | 0.86 | 0.13 | 0.51 |
| 2-MeOE2 | 2.46 | 1.64 | 1.35 |
| E1 | 2.4 | 0.44 | 0.55 |
| 4-MeOE2 | 0.67 | 0.06 | 0.45 |
| E2 | 0.87 | 0.2 | 0.17 |
| 2-OHE1 | 0.4 | 0.66 | 1.72 |
| 2-OHE2 | 1.38 | 1.4 | 0.66 |
| 4-OHE1 | 0.88 | 0.98 | 0.7 |
Abbreviation: EM, estrogen metabolite.
Histomorphometry
Breast and uterine epithelial area and thickness were quantified by histomorphometry, as described previously (24). Briefly, H&E-stained slides were digitized using a Hitachi VK-C370 camera and video capture board (Scion LG-3, Scion, Inc.). Area and thickness measurements were taken with public domain software (NIH Image version 1.60).4 Three microscopic fields were randomly selected and examined at higher magnification. Percent glandular and epithelial areas were determined by manual tracing of total epithelial units within the endometrium and expressed as a percentage of the total area examined. Percent lobular area in the mammary tissue sections was determined in the same manner. Endometrial thickness was measured at the point of greatest perpendicular depth. H&E-stained mammary glands and uteri were also evaluated qualitatively for histologic changes by two board-certified veterinary pathologists (J.M.C. and N.D.K.). All measurements were made blinded to treatment group. Adjacent tissue sections from each animal were used for histomorphometry and immunostaining.
Mammary mRNA Expression Analysis by Reverse Transcription-PCR
To evaluate changes in estrogen exposure in the mammary gland, Ki67, pS2, and progesterone receptor mRNA levels were measured. Primer-probe set assays were used for human Ki67 and progesterone receptor from the premade inventory of gene expression assays from Applied Biosystems (ABI; assay ID nos. Hs00606991_m1 and Hs00172183_m1, respectively). Design of a macaque-specific primer-probe set for pS2 and reverse transcription-PCR protocol specifics have previously been reported (16). Relative expression amounts (R0) of mRNA were calculated using a DART analysis worksheet (25) with the change in raw fluorescence (ΔRn) data generated by the ABI Prism software. The R0 values were normalized to an internal control (glyceraldehyde-3-phosphate dehydrogenase) and an external plate control (mammary tumor sample) to account for interplate differences in reaction efficiency because the entire sample set could not be run on a single plate.
Statistical Analysis
All data were log-transformed before statistical analysis to establish homoscedasticity. Statistical significance was determined using factorial two-way ANOVA by treatment for log-transformed variables in which estrogen metabolite concentrations could be detected. Due to a large proportion (range, 25–70%) of measurements for several estrogen metabolite concentrations being undetectable (urinary levels of 17epiE3, 2MeOE1, 2MeOE2, 3MeOE1, 4MeOE1, 4MeOE2, 2OHE2, and 4OHE1), a two-part regression model was used to analyze differences in probability of detection by treatment group and levels of amount present in those monkeys with detectable levels (26). The number of animals in each treatment group with detectable levels of each metabolite and the total percentage of detectable measurements of the 161 urine samples are provided in Table 2. The overall mean amount of the hormone present in the sample was estimated by multiplying the probability of detection by the amount detected in those with detectable hormone amounts, and the variance was estimated using both components of the model as well (27). Data represented in all figures are from analyses including all treatment groups unless otherwise indicated. We did not expect any significant effects of past OCs on any end points examined, so to rule out potential confounding effects of OCs on the more recent postmenopausal treatment, we tested for interactions. Full factorial (OC treatment × postmenopausal diet) interaction effects were examined for all estrogen metabolites, as is common practice when evaluating main effects with ANOVA/general linear models. The interaction was only statistically significant for urinary 2MeOE1 levels, and therefore only significant main effects are presented unless otherwise stated. OC treatment effect compares OC treated (OC+; n = 78) versus not treated (OC−; n = 83) regardless of postmenopausal treatment, and postmenopausal diet effect examines CEE+ (n = 54) or Soy+ (n = 55) diet versus Soy− diet (n = 52) regardless of prior OC treatment. Analyses were done using the SAS statistical analysis software (version 9.1.3, SAS Institute).
Table 2.
Estrogen metabolite detection proportions by treatment group combination
| n | 17epiE3 | 3MeOE1 | 2MeOE1 | 4MeOE1 | 2MeOE2 | 4MeOE2 | 2OHE2 | 4OHE1 | |
|---|---|---|---|---|---|---|---|---|---|
| OC−Soy− | 26 | 6 | 0 | 19 | 0 | 4 | 0 | 5 | 1 |
| OC−Soy+ | 28 | 3 | 2 | 15 | 0 | 2 | 0 | 1 | 0 |
| OC−CEE+ | 29 | 28 | 20 | 28 | 24 | 27 | 24 | 27 | 28 |
| OC+Soy− | 26 | 6 | 1 | 19 | 0 | 3 | 0 | 5 | 3 |
| OC+Soy+ | 27 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| OC+CEE+ | 25 | 25 | 18 | 25 | 18 | 25 | 25 | 24 | 23 |
| totals | 161 | 69 | 41 | 112 | 42 | 61 | 49 | 62 | 55 |
| % detected | 43 | 25 | 70 | 26 | 38 | 30 | 39 | 34 |
Results
Premenopausal Usage of OC Results in Significantly Lower Levels of Most Estrogen Metabolites 3 Years after Surgical Menopause
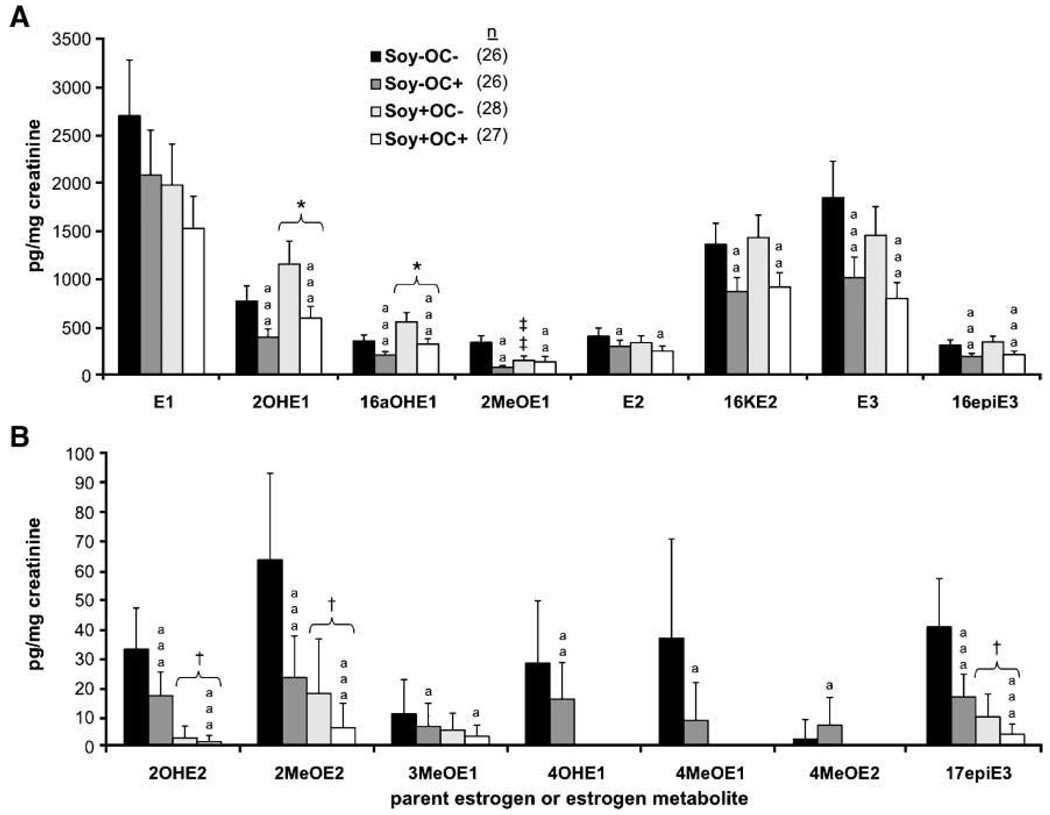
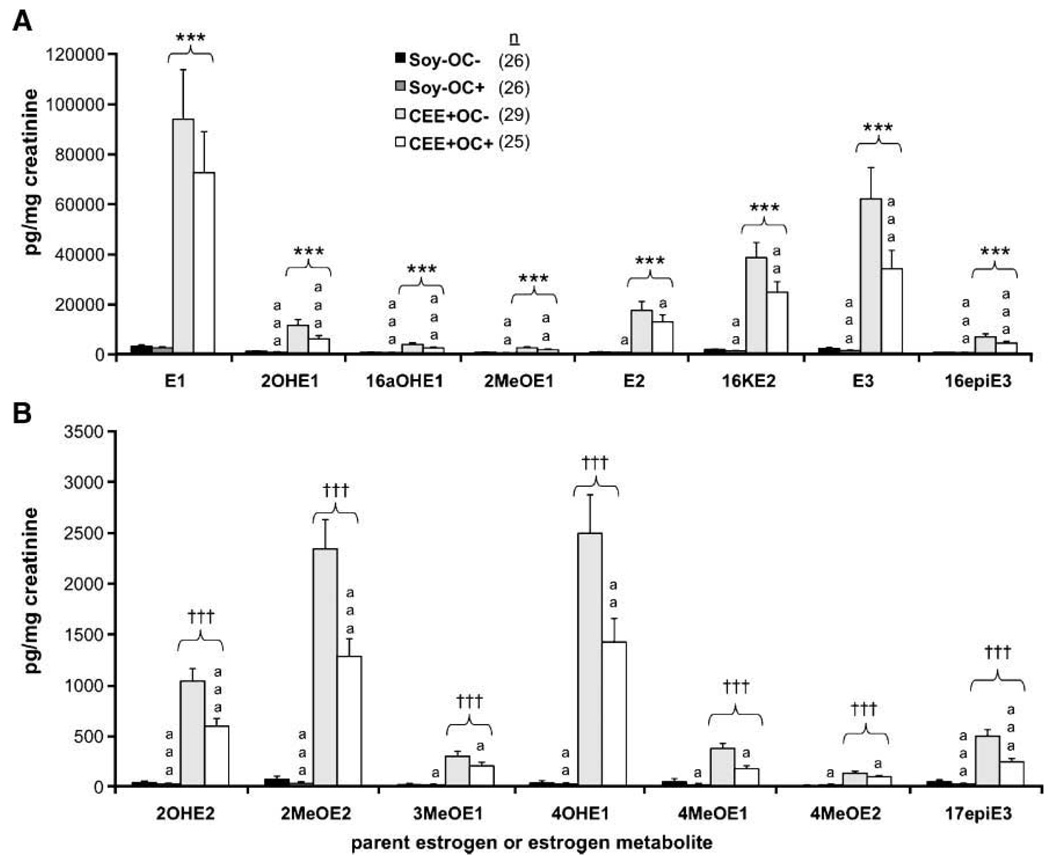
As shown in Fig. 1 and Fig. 2, OC treatment (regardless of postmenopausal diet) resulted in significant decreases in the levels of all metabolites measured except for E1 and 4MeOE2. Among the most abundant metabolites (Fig. 1A and Fig. 2A), overall percent changes ranged from a 25% reduction in E1 to a 50% reduction in 2OHE1 with OC administration. Percent reductions were comparable among the less abundant metabolites (Fig. 1B and Fig. 2B), ranging from 25% to 57%.
Figure 1.
Postmenopausal urinary estrogen metabolites from control, OC, and soy isoflavone–treated monkeys. Columns, geometric mean urinary content (corrected to creatinine) of the most abundant (A) and least abundant (B) estrogen metabolites; bars, SE. *, P < 0.05, versus Soy− group (two-way ANOVA); ***, P < 0.0001, versus Soy− group (two-way ANOVA); †, P < 0.05, versus Soy− group (two-part regression); † † † , P < 0.0001, versus Soy− group (two-part regression); ‡ ‡ ‡, P < 0.0001, interactive effect of OC+ and Soy+ (versus OC−Soy−); a, P < 0.05, versus OC−; aa, P < 0.01, versus OC−; aaa, P < 0.0001, versus OC−.
Figure 2.
Postmenopausal urinary estrogen metabolites from control, OC-treated, and CEE-treated monkeys. Columns, geometric mean urinary content (corrected to creatinine) of the most abundant (A) and least abundant (B) estrogen metabolites; bars, SE. *, P < 0.05, versus Soy− group (two-way ANOVA); ***, P < 0.0001, versus Soy− group (two-way ANOVA); † , P < 0.05, versus Soy− group (two-part logistic regression); † † †, P < 0.0001, versus Soy− group (two-part regression); a, P < 0.05, versus OC−; aa, P < 0.01, versus OC−; aaa, P < 0.0001, versus OC−.
Postmenopausal Dietary Isoflavones Induced Few Significant Effects on Postmenopausal Estrogen Metabolite Concentrations
Monkeys fed an isoflavone-rich diet (Soy+) postmenopausally had increased levels of urinary 2OHE1 and 16αOHE1 excretion (50% and 56% increases, respectively) and decreased levels of 2OHE2, 2MeOE2, and 17epiE3 (92%, 72%, and 76% reductions, respectively) compared with those monkeys fed the Soy− diet (Fig. 1A and B). These changes in urinary estrogen metabolite excretion were significant regardless of premenopausal OC administration status. Urinary excretion of 2MeOE1 was differentially affected by the interaction of isoflavone and OC treatment. There was a 57% decrease in 2MeOE1 levels associated with the OC treatment among the monkeys not fed an isoflavone-rich diet. Alternatively, the addition of Soy+ treatment resulted in no significant difference in 2MeOE1 excretion between the control and OC-treated monkeys. The visually apparent difference in mean urinary 4OHE1 levels between Soy− and Soy+ treated monkeys was not statistically significant because the difference in frequency of detectable 4OHE1 between Soy+ and Soy− fed animals did not reach statistical significance (P = 0.052, two-tailed Fisher’s exact test). Although none of the Soy+ monkeys had detectable levels of 4OHE1 in their urine, only four of the Soy− monkeys had detectable urinary concentrations of 4OHE1.
Mammary and Endometrial Biomarkers of Estrogen Exposure and Proliferation Were Most Significantly Changed in CEE-Treated Monkeys, with Some Modest Changes Associated with Isoflavone or OC Treatments
CEE treatment resulted in at least a doubling of average endometrial thickness, percent glandular and epithelial areas in the endometrium, and percent lobular area in the mammary tissues compared with monkeys fed the control diet alone (Table 3). Compared with monkeys on the control Soy− diet, those treated with CEE also experienced a 2-fold increase in Ki67, a 6-fold increase in progesterone receptor, and a 12-fold induction in pS2 mRNA expression within mammary tissues (Table 3). Overall, premenopausal OC administration (regardless of postmenopausal treatment) modestly reduced average thickness and percent epithelial area of the endometrium by 23% and 17% respectively (Table 3). Its effect on mammary Ki67 mRNA expression was much more robust: increasing levels by 110% compared with placebo-treated control animals (Table 3). Postmenopausal isoflavones were associated with a 48% reduction in mammary expression of Ki67mRNA compared with Soy− animals (Table 3), whereas it had no significant effect on any other tissue biomarker of estrogen exposure or proliferation. All of these effects, however, are in stark contrast to the wide-ranging and large increases in all proliferation and estrogen exposure end points stimulated by CEE treatment.
Table 3.
Premenopausal and postmenopausal treatment effects on proliferation and estrogen exposure biomarkers
| Treatment group |
Endometrial | Mammary | |||||
|---|---|---|---|---|---|---|---|
| Avg thickness (mm) |
Epithelial area (%) |
Glandular area (%) |
Lobular area (%) |
PGR mRNA | Ki67 mRNA | pS2 mRNA | |
| OC− | 1.4 (1.2–1.5) | 10.1 (9.2–11.1) | 14.0 (12.6–15.5) | 0.78 (0.63–0.97) | 16.8 (14.0–20.0) | 0.014 (0.011–0.018) | 0.50 (0.38–0.67) |
| OC+ | 1.1 (1.0–1.3)* | 8.6 (7.8–9.5)* | 12.4 (11.2–13.8) | 0.90 (0.72–1.13) | 17.1 (14.2–20.8) | 0.029 (0.023–0.038)* | 0.58 (0.43–0.79) |
| Soy− | 0.89 (0.78–1.0) | 7.3 (6.5–8.2) | 9.3 (8.1–10.5) | 0.64 (0.49–0.84) | 9.7 (7.7–12.1) | 0.019 (0.014–0.026) | 0.25 (0.17–0.35) |
| Soy+ | 0.83 (0.72–0.94) | 6.9 (6.1–7.7) | 8.7 (7.7–9.9) | 0.56 (0.43–0.73) | 8.1 (6.4–10.2) | 0.011 (0.008–0.015)† | 0.20 (0.14–0.29) |
| CEE+ | 2.7 (2.4–3.1)‡ | 16.1 (14.3–18.0)‡ | 28.4 (15.0–32.2)‡ | 1.64 (1.26–2.14)‡ | 62.2 (49.6–78.0)‡ | 0.041 (0.030–0.055)‡ | 3.16 (2.21–4.52)‡ |
NOTE: Values represent geometric means with 95% confidence intervals. Treatment group n values are the same as stated in Materials and Methods.
Abbreviation: PGR, progesterone receptor.
P < 0.01, versus OC− group.
P < 0.01, versus Soy− group.
P < 0.0001, versus Soy− group.
Premenopausal and Postmenopausal Treatment Effects on Mammary Gland and Endometrial Proliferation End Points May Occur through Changes in Estrogen Metabolism
To further explore significant treatment effects on biomarkers of estrogen-induced proliferation, we used a simple mediation analysis based on the concepts of Baron and Kenny (28). Using linear regression modeling, we explored whether changes in estrogen and estrogen metabolite levels mediated significant premenopausal or postmenopausal treatment effects on mammary and endometrial measures of proliferation. Table 3 illustrates the significant relationships between the dietary and hormonal treatments and proliferation biomarkers. Table 4 summarizes those relationships and lists the estrogens and/or estrogen metabolites that cause the treatment-outcome effect to drop to nonsignificance once added to the regression model. Inclusion of each mediation variable reduced the significant percent change in treatment group means by at least 9%, and each of these variables alone was significantly correlated with the indicated proliferation end point (data not shown). E1 and E2 levels seemed to be the primary mediators for CEE-associated increases in endometrial percent epithelial area. The percent change was reduced from a 120% increase in percent epithelial area without any mediating variables to nonsignificant 26% and 56% increases after inclusion of E1 or E2, respectively, in the regression model. Similarly, percent increase in mammary percent lobular area was reduced by 18% (from 154% to 136%) with E2 incorporation. In contrast, 2OHE1 and 16αOHE1 variability most strongly attenuated OC-associated reductions in endometrial thickness (23% decrease without mediating variables reduced to a 3% or 4% decrease with 2OHE1 or 16αOHE1 included, respectively) and percent epithelial area (percent decrease also reduced to 3% or 4% with 2OHE1 or 16αOHE1). Mediation by 16αOHE1 also produced a 13% reduction of percent decrease in mammary Ki67 mRNA expression associated with Soy+ diet (45% decrease in mammary Ki67 expression without mediators lessened to a nonsignificant 31% decrease after 16αOHE1 inclusion).
Table 4.
Mediation effects of selected estrogens and their metabolites on treatment-induced changes in mammary gland and endometrial proliferation biomarkers
| Change in means (%)* | P† | Significant covariate‡ estrogens/EM | |
|---|---|---|---|
| Soy+ vs Soy−§ | |||
| Mammary Ki67 mRNA expression | −44.7 | 0.006 | 16αOHE1‖ |
| OC+ vs OC− | |||
| Mammary Ki67 mRNA expression | 101.7 | 0.0004 | None |
| Endometrial thickness | −23.1 | 0.027 | E1,‖ E2,¶ 16αOHE1,** 2OHE1,** 2MeOE1¶ |
| Endometrial epithelial area (%) | −17.1 | 0.042 | E1,‖ E2,¶ 16αOHE1,** 2OHE1,** 2MeOE1¶ |
| CEE+ vs Soy− | |||
| Mammary lobular area (%) | 153.9 | 1.26e–05 | E2¶ |
| Endometrial thickness | 211.2 | 4.70e–19 | None |
| Endometrial epithelial area (%) | 120.3 | 6.08e–15 | E1¶, E2¶ |
| Endometrial glandular area (%) | 206.7 | 2.36e–20 | None |
Percentage change in unadjusted least square means from untreated to treated for designated biomarker.
P value associated with change in unadjusted least square means.
Estrogen or estrogen metabolite which, on inclusion in regression model, abolished significant treatment effect.
Treatment comparisons for the indicated proliferation biomarker(s).
Inclusion increases P value to >0.05.
Inclusion increases P value to >0.1.
Inclusion increases P value to >0.5.
Discussion
In this study, we investigated the long-term effects of prior OCs and/or dietary soy isoflavones on markers of endogenous estrogen metabolism and proliferation in the breast and uterus. Premenopausal OC administration resulted in a significant reduction of most endogenous urinary estrogen metabolites measured during postmenopausal years almost 3 years after OC treatment. The results suggest that premenopausal OC use may alter subsequent exposure to estrogen even after OC use is ceased. This effect could be through a reduction of endogenous biosynthesis or an increase in catabolism or excretion of estrogens, but elucidation of the exact mechanism is still necessary. Recently, Chan and colleagues (29) also reported a reduction in urinary estrogen concentrations in postmenopausal women who were previous users of OCs, supporting our current findings.
Varied long-term effects of past OC use have also been noted in other studies of other estrogen-sensitive organ systems, such as cardiovascular, bone, and brain (30–34). This evidence suggests that OCs affect not only immediate ovarian hormone production but also long-term production or catabolism of steroid hormones within target tissues. Some studies indicate that steroid hormones, both from endogenous and exogenous sources, can affect the expression of genes through epigenetic modification within various target tissues (35–37). This process is thought to occur mainly through alterations in methylation and demethylation patterns of promoter elements of estrogen-producing enzymes. In subsequent studies, it would be highly informative to investigate differences in methylation patterns of OC-treated and nontreated subjects to determine if long-term usage of OCs may affect target systems through tissue-specific gene imprinting.
Results from this study related to dietary isoflavone effects on urinary estrogen metabolite excretion are generally consistent with previous results from our lab (16), which used commercial immunoassays and was limited to the non–OC-treated subset of animals. Dietary isoflavones decreased urinary excretion of E1 and increased urinary 2OHE1 although the magnitudes of percent difference somewhat vary (−27% versus −54% for E1 and +50% versus +72% for 2OHE1). However, there was variability in the direction and magnitude of change in urinary 16αOHE1 levels between the previous publication and the current analysis. We report a 56% increase in 16αOHE1 in isoflavone-fed monkeys whereas the previous findings indicate a 26% decrease. This led to a significant increase in the ratio of 2αOHE1/16αOHE1 reported previously, where this study did not make a similar finding (data not shown). Whereas the prior study involving these monkeys did not include the OC+ treated animals, these differences are most likely attributable to the modification in methods used to quantitate the metabolites; we used high-performance liquid chromatography-tandem mass spectrometry whereas an ELISA, which exhibits a great deal of variability in sensitivity and precision, was used previously.
In contrast to premenopausal OC exposure, dietary soy isoflavones tended to have varied effects on different metabolite concentrations. A possible mechanism for this observation may relate to modulation of cytochrome P450 isoforms. There are few published studies, however, investigating isoflavone effects on cytochrome P450 activity. Khan and colleagues (38) have reported soy isoflavone inhibition of cytochrome P450 activation of benzo[a]pyrene into a genotoxic carcinogen in Swiss albino mice through decreasing benzo[a]pyrene–induced expression and activity of cytochrome P450 1A1 and 1A2. Other investigators have reported similar findings using in vitro models, but these studies found the significant effects to arise only at very high and supraphysiologic doses of isoflavones (39–41). The relevance of these effects to dietary isoflavone exposure is thus unclear.
The lack of OC effect on Soy+ treated animals’ urinary 2MeOE1 excretion suggests that these two interventions may balance each other along this pathway of E1 metabolism. 2MeOE1 is produced by COMT with 2OHE1 as its substrate. We have shown that prior OC use is associated with reduced 2OHE1 levels, thereby reducing the substrate for COMT. Others have published that soy isoflavones inhibit COMT expression and activity at physiologically relevant concentrations (42, 43). This suggests that the effects of the Soy+ treatment not only counteract the OC effects on 2OHE1 but also hinders its phase II O-methylation, resulting in no net difference in 2MeOE1 excretion between OC−Soy+ and OC+Soy+ monkeys. The ability for soy isoflavones to partially reverse potentially negative outcomes of previous OC exposure warrants further investigation. Future studies will focus on cytochrome P450 1A1, 1B1, and 3A4 activities as these are three isoforms expressed in the breast that are involved in estrogen metabolism (44). The mechanism behind isoflavones increasing 2OHE1 while decreasing 4OHE1 or increasing hydroxylated estrogens while decreasing methoxylated estrogens is a matter for further investigation.
There was no statistical difference in the levels of 4OHE1 between Soy+ and Soy− animals because none was detected in the urine of the isoflavone-fed animals, yet there was no statistically significant difference in the probability of its detection between these groups either. The tendency for isoflavone-fed animals to excrete no 4OHE1 suggests that dietary soy isoflavones may suppress the activity and/or expression of cytochrome P450 1B1, the primary enzyme responsible for hydroxylation of E1 at the C4 position (45). The validity of this potential effect should be examined in the context of subjects with higher endogenous estrogen levels.
Long-term CEE treatment of these macaques resulted in significantly higher ratios of both 2αOHE1/16αOHE1 and 2OHE1/4OHE1 (data not shown), as well as drastically increased levels of all estrogen metabolites compared with animals fed the control diet. Most likely, this is a reflection of the clearance of exogenous estrogens. However, the uniquely heterogeneous mix of highly estrogenic compounds present within CEE may be stimulating 2-hydroxylase enzyme expression or activity, creating potential for altered ratios of E1 metabolites. This phenomenon would require additional investigation comparing treatment with CEE versus 17β-estradiol. CEE alone was used in this study, however, because it is the most commonly prescribed estrogen replacement therapy in the United States, and one major aim of this investigation was to model the female experience with exogenous hormone exposure in non-human primates.
Apart from OCs, several other lifestyle factors have been associated with alterations in circulating estrogen levels. Increased body mass index, fat intake, and alcohol consumption have been strongly correlated with increased estrogen levels, whereas smoking, increased parity, soy food intake, and exercise have been associated with modest decreases in estrogen concentrations in postmenopausal women (46). Various mechanisms have been proposed for these effects, including increased local aromatization of androgens with increased body mass index (47), isoflavone-induced changes in hepatic conjugation of estrogens (48), and alterations in hepatic production of SHBG and/or enzymes responsible for catabolism of circulating estrogens with smoking (49).
Prior OC treatment may affect subsequent target tissue response to estrogens. Although prior OC use produced many significant changes in estrogen metabolite concentrations, these changes did not translate to widespread robust effects on markers of estrogen exposure and cell proliferation in macaque mammary gland and endometrium. Prior OC administration decreased markers of proliferation in the uterus while increasing proliferation markers in the mammary gland, which corroborates the findings of early epidemiologic studies reporting marked long-lasting reductions in endometrial cancer risk but a slight increase in breast cancer risk associated with recent use of OC (50, 51). Mechanisms for long-term regulation of mRNA expression effects by prior OC use are unknown but may include induction of terminal differentiation of epithelial progenitor cells and possibly modulation of DNA methylation patterns.
In conclusion, these data provide evidence that prior OC therapy and dietary soy isoflavones may exert effects on estrogen exposure. Moreover, our current findings support previous epidemiologic studies about OC effects on uterine and breast proliferation. Analyses included in this study support the hypothesis that hormonal and dietary effects on estrogen-responsive tissues occur, in part, by alterations in estrogen metabolism. Direct effects of these treatments on mammary gland and endometrium cannot and should not be ruled out based on these statistical descriptions of mediation. However, these results suggest that OC and isoflavone influences on estrogen metabolite production are significant components of their physiologic actions.
Because this was not a longitudinal prospective investigation of hormonal and dietary treatment effects on endogenous estrogen metabolism, we cannot account for any baseline differences in urinary estrogen excretion that may have existed between treatment groups. However, these animals were randomized on characteristics that are highly correlated with endogenous estrogen concentrations (body weight and total plasma cholesterol/high-density lipoprotein cholesterol ratio). The groups of animals were all of the same mean approximate age and multiparous, so it is reasonable to assume that interindividual variations in baseline estrogen excretion were not major factors in these results. More work is necessary to elucidate mechanisms behind these effects and to determine if these effects are specific to certain contraceptive formulations or attributable to specific isoflavones. These findings indicate that there may be additional and novel effects of prolonged exposures to exogenous hormones and phytochemicals.
Acknowledgments
We thank Dr. Thomas B. Clarkson and colleagues for designing and implementing this study, and Gerald Perry, Maryanne Post, and Jean Gardin for their technical contributions.
Grant support: National Cancer Institute, NIH, under contract NO1-CO-12400 (X. Xu and T.D. Veenstra), National Center for Complementary and Alternative Medicine, NIH RO1 AT00639 (J.M. Cline). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the United States Government.
Footnotes
Disclosure of Potential Conflicts of Interest
C.E. Wood and J.M. Cline were previous members of an advisory board for Wyeth-Ayerst Laboratories, Inc. J.M. Cline is a co-inventor of “Treatment of Endometriosis Using Soy Phytoestrogens.” The other authors disclosed no potential conflicts of interest.
Available from: http://rsb.info.nih.gov/nih-image/.
References
- 1.American Cancer Society. Cancer facts and figures 2007. Atlanta: American Cancer Society; 2007. pp. 1–56. [Google Scholar]
- 2.Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas. 2001;38:103–116. doi: 10.1016/s0378-5122(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 3.Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:341–354. doi: 10.1053/beog.2000.0180. [DOI] [PubMed] [Google Scholar]
- 4.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 5.Swaneck GE, Fishman J. Covalent binding of the endogenous estrogen 16α-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci U S A. 1988;85:7831–7835. doi: 10.1073/pnas.85.21.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis JS, Thomas TJ, Klinge CM, Gallo MA, Thomas T. Regulation of cell cycle and cyclins by 16α-hydroxyestrone in MCF-7 breast cancer cells. J Mol Endocrinol. 2001;27:293–307. doi: 10.1677/jme.0.0270293. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 8.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-Hydroxyestrone: the “good” estrogen. J Endocrinol. 1996;150:S259–S265. [PubMed] [Google Scholar]
- 9.Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7:112–129. [PubMed] [Google Scholar]
- 10.Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE. The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer. 2002;94:1166–1174. doi: 10.1002/cncr.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu AH, Stanczyk FZ, Seow A, Lee HP, Yu MC. Soy intake and other lifestyle determinants of serum estrogen levels among postmenopausal Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2002;11:844–851. [PubMed] [Google Scholar]
- 12.Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab. 2004;89:3462–3468. doi: 10.1210/jc.2003-032067. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:781–786. [PubMed] [Google Scholar]
- 14.Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:1101–1108. [PubMed] [Google Scholar]
- 15.Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16α-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000;60:1299–1305. [PubMed] [Google Scholar]
- 16.Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2007;28:801–808. doi: 10.1093/carcin/bgl163. [DOI] [PubMed] [Google Scholar]
- 17.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;135:603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 19.Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88:4362–4370. doi: 10.1210/jc.2003-030493. [DOI] [PubMed] [Google Scholar]
- 20.Register TC, Cann JA, Kaplan JR, et al. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90:1734–1740. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 22.Henderson JA, Shively CA. Triphasic oral contraceptive treatment alters the behavior and neurobiology of female cynomolgus monkeys. Psychoneuroendocrinology. 2004;29:21–34. doi: 10.1016/s0306-4530(02)00132-4. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Veenstra TD, Fox SD, et al. Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 24.Foth D, Cline JM. Effects of mammalian and plant estrogens on mammary glands and uteri of macaques. Am J Clin Nutr. 1998;68 Suppl 6:S1413–S1417. doi: 10.1093/ajcn/68.6.1413S. [DOI] [PubMed] [Google Scholar]
- 25.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31:e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan N, Manning WG, Morris CN, Newhouse JP. A comparison of alternative models for the demand for medical care. J Bus Econ Stat. 1983;1:115–126. [Google Scholar]
- 27.Aitchison J. On the distribution of a positive random variable having a discrete probability mass at the origin. J Am Stat Assoc. 1955;50:901–908. [Google Scholar]
- 28.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 29.Chan MF, Dowsett M, Folkerd E, et al. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15:332–339. doi: 10.1097/gme.0b013e31806458d9. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DC, Martin H, Tsai-Morris CH. The in vitro and in vivo effect of estradiol upon the 17α-hydroxylase and C17,20-lyase activity in the ovaries of immature hypophysectomized rats. Mol Cell Endocrinol. 1984;35:199–204. doi: 10.1016/0303-7207(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 31.Brady WA, Kritz-Silverstein D, Barrett-Connor E, Morales AJ. Prior oral contraceptive use is associated with higher blood pressure in older women. J Womens Health. 1998;7:221–228. doi: 10.1089/jwh.1998.7.221. [DOI] [PubMed] [Google Scholar]
- 32.Kleerekoper M, Brienza RS, Schultz LR, Johnson CC Henry Ford Hospital Osteoporosis Cooperative Research Group. Oral contraceptive use may protect against low bone mass. Arch Intern Med. 1991;151:1971–1976. [PubMed] [Google Scholar]
- 33.Michaelsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral-contraceptive use and risk of hip fracture: a case-control study. Lancet. 1999;353:1481–1484. doi: 10.1016/S0140-6736(98)09044-8. [DOI] [PubMed] [Google Scholar]
- 34.Shively CA. Behavioral and neurobiological effects of estrogen replacement therapy and a history of triphasic oral contraceptive exposure. Psychoneuroendocrinology. 1998;23:713–732. doi: 10.1016/s0306-4530(98)00039-0. [DOI] [PubMed] [Google Scholar]
- 35.Wilks A, Seldran M, Jost JP. An estrogen-dependent demethylation at the 5′ end of the chicken vitellogenin gene is independent of DNA synthesis. Nucleic Acids Res. 1984;12:1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Washburn KA, Moore R, et al. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–4359. [PubMed] [Google Scholar]
- 37.Contractor RG, Foran CM, Li S, Willett KL. Evidence of gender-and tissue-specific promoter methylation and the potential for ethinylestradiol-induced changes in Japanese medaka (Oryzias latipes) estrogen receptor and aromatase genes. J Toxicol Environ Health A. 2004;67:1–22. doi: 10.1080/15287390490253633. [DOI] [PubMed] [Google Scholar]
- 38.Khan TH, Prasad L, Anuradha, Sultana S. Soy isoflavones inhibits the genotoxicity of benzo(a)pyrene in Swiss albino mice. Hum Exp Toxicol. 2005;24:149–155. doi: 10.1191/0960327105ht504oa. [DOI] [PubMed] [Google Scholar]
- 39.Shertzer HG, Puga A, Chang C, et al. Inhibition of CYP1A1 enzyme activity in mouse hepatoma cell culture by soybean isoflavones. Chem Biol Interact. 1999;123:31–49. doi: 10.1016/s0009-2797(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 40.Chan HY, Leung LK. A potential protective mechanism of soya isoflavones against 7,12-dimethylbenz[a]anthracene tumour initiation. Br J Nutr. 2003;90:457–465. doi: 10.1079/bjn2003913. [DOI] [PubMed] [Google Scholar]
- 41.Shon YH, Park SD, Nam KS. Effective chemopreventive activity of genistein against human breast cancer cells. J Biochem Mol Biol. 2006;39:448–451. doi: 10.5483/bmbrep.2006.39.4.448. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann L, Jiang L, Wagner J. Soy isoflavones decrease the catechol-O-methyltransferase-mediated inactivation of 4-hydroxyestradiol in cultured MCF-7 cells. Carcinogenesis. 2008;29:363–370. doi: 10.1093/carcin/bgm235. [DOI] [PubMed] [Google Scholar]
- 43.Wagner J, Jiang L, Lehmann L. Phytoestrogens modulate the expression of 17α-estradiol metabolizing enzymes in cultured MCF-7 cells. Adv Exp Med Biol. 2008;617:625–632. doi: 10.1007/978-0-387-69080-3_65. [DOI] [PubMed] [Google Scholar]
- 44.Modugno F, Knoll C, Kanbour-Shakir A, Romkes M. A potential role for the estrogen-metabolizing cytochrome P450 enzymes in human breast carcinogenesis. Breast Cancer Res Treat. 2003;82:191–197. doi: 10.1023/B:BREA.0000004376.21491.44. [DOI] [PubMed] [Google Scholar]
- 45.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 β-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kendall A, Folkerd EJ, Dowsett M. Influences on circulating oestrogens in postmenopausal women: relationship with breast cancer. J Steroid Biochem Mol Biol. 2007;103:99–109. doi: 10.1016/j.jsbmb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Azziz R. Reproductive endocrinologic alterations in female asymptomatic obesity. Fertil Steril. 1989;52:703–725. doi: 10.1016/s0015-0282(16)61020-8. [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffer E, Treiling CR, Hoehle SI, Metzler M. Isoflavones modulate the glucuronidation of estradiol in human liver microsomes. Carcinogenesis. 2005;26:2172–2178. doi: 10.1093/carcin/bgi197. [DOI] [PubMed] [Google Scholar]
- 49.Cassidenti DL, Vijod AG, Vijod MA, Stanczyk FZ, Lobo RA. Shortterm effects of smoking on the pharmacokinetic profiles of micronized estradiol in postmenopausal women. Am J Obstet Gynecol. 1990;163:1953–1960. doi: 10.1016/0002-9378(90)90780-b. [DOI] [PubMed] [Google Scholar]
- 50.Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner’s guide to metaanalysis. Hum Reprod. 1997;12:1851–1863. doi: 10.1093/humrep/12.9.1851. [DOI] [PubMed] [Google Scholar]
- 51.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: further results. Contraception. 1996;54 Suppl 3:S1–S106. doi: 10.1016/s0010-7824(15)30002-0. [DOI] [PubMed] [Google Scholar]