Abstract
Objective
Vascular oxidative stress and inflammation are contributing factors in atherosclerosis. We recently found that the iron chelator, desferrioxamine (DFO), suppresses NADPH oxidase-mediated oxidative stress and expression of cellular adhesion molecules in mice treated with lipopolysaccharide (LPS). The objective of the present study was to investigate whether and how LPS and iron enhance, and DFO inhibits, NADPH oxidase activity in human aortic endothelial cells (HAEC).
Methods and Results
Incubation of HAEC for 24 hrs with 5 μg/mL LPS led to a four-fold increase in NADPH oxidase activity, which was strongly suppressed by pretreatment of the cells for 24 hrs with 100 μmol/L DFO. Incubating HAEC with LPS also significantly increased cellular iron and heme levels and mRNA and protein levels of p22phox, a heme-containing, catalytic subunit of NADPH oxidase. All of these effects of LPS on HAEC were strongly inhibited by DFO. Exposing HAEC to 100 mol/L iron (ferric citrate) for 48 hrs exerted similar effects as LPS, and these effects were strongly inhibited by co-incubation with DFO. Furthermore, neither LPS nor DFO affected mRNA and protein levels of p47phox, a non-heme containing, regulatory subunit of NADPH oxidase, or the mRNA level of NOX4, an isoform of the principal catalytic subunit of NADPH oxidase in endothelial cells. In contrast, heme oxygenase-1 was strongly suppressed by DFO, both in the absence and presence of LPS or iron.
Conclusions
Our data indicate that prolonged exposure to LPS or iron increases endothelial NADPH oxidase activity by increasing p22phox gene transcription and cellular levels of iron, heme, and p22phox protein. Iron chelation by DFO effectively suppresses endothelial NADPH oxidase activity, which may be helpful as an adjunct in reducing vascular oxidative stress and inflammation in atherosclerosis.
Keywords: desferrioxamine, iron, lipopolysaccharide, NADPH oxidase, NOX4, p22phox, p47phox
Vascular inflammation and oxidative stress play prominent roles in the pathogenesis of atherosclerosis and cardiovascular diseases.1–3 Lipopolysaccharide (LPS), a common inflammatory agonist, is the biologically active constituent of endotoxin derived from the outer membrane of Gram-negative bacteria. Bacterial endotoxin has been recently recognized as a potential mediator of inflammatory responses in atherosclerosis.4
NADPH oxidase appears to be the principal enzymatic source of superoxide radicals (O2.−) in the vascular wall.5–7 NADPH oxidase-mediated oxidative stress has been implicated in the activation of redox-sensitive transcription factors and vascular expression of inflammatory genes, including cytokines, chemokines, and cellular adhesion molecules, e.g., vascular cell adhesion molecule-1 (VCAM-1).8–9 Increased surface expression of VCAM-1 is an early marker of endothelial activation, which contributes to monocyte infiltration and vascular remodeling in the initial stages of atherosclerosis.10–11
The catalytic subunit of NADPH oxidase is a membrane-bound protein called cytochrome b558, which is comprised of two subunits, NOX and p22phox. These two catalytic subunits associate with several cytoplasmic regulatory subunits, viz., Rac1, p40phox, p47phox, p67phox, p41nox, and/or p51nox. Several homologues of NOX, called NOX1-5, have been identified in different cell types.12–15 NOX2, which is also known as gp91phox, is found in phagocytic cells, such as neutrophils and monocytes. Vascular endothelial cells contain NOX1, 2, and 4, and vascular smooth muscle cells NOX1, 4, and 5.14,15 While it is possible that additional, as of yet unidentified NADPH oxidase subunits exist, it is well established that all NOX-containing enzymes require p22phox for catalytic activity, because p22phox serves as the docking protein for the other subunits and stabilizes the NOX subunit.16 Furthermore, it has been shown that each molecule of cytochrome b558 contains two molecules of heme.17 The two heme-bound iron ions are essential for electron transfer from NADPH to oxygen and, hence, O2.− generation by the enzyme.17,18 Removing iron from heme by heme oxygenase-1 (HO-1), or blocking heme synthesis, lowers NADPH oxidase activity due to destabilization and degradation of cytochrome b558.19,20
Therefore, there is a close relationship between cellular iron status and NADPH oxidase activity. Accordingly, the activity of NADPH oxidase was found to be significantly lower in patients with iron-deficient anemia, and iron supplementation increased–and eventually normalized–NADPH oxidase activity.21 In addition, several studies have observed an increased iron level in human atherosclerotic plaque.22–24 Exactly how iron regulates NADPH oxidase and its subunits in arterial inflammation and atherosclerosis has not been established, and the role of iron in atherosclerosis remains controversial.3,25
We have recently reported that the iron chelator, desferrioxamine (DFO), suppresses NADPH oxidase-mediated oxidative stress and VCAM-1 expression in an in vivo model of LPS-induced inflammation.26 In the present study, we sought to investigate the mechanisms by which iron enhances, and DFO suppresses, NADPH oxidase activity in human aortic endothelial cells (HAEC).
Methods
Endothelial Cell Culture
Human aortic endothelial cells obtained from Cambrex Bio Science were cultured in endothelial cell growth medium at 37°C in a humidified 95% air-5% CO2 atmosphere. Cells were harvested at confluence with 0.05% trypsin-0.02% EDTA (Cambrex Bio Science) and plated at a split ratio of 1:3. For experiments, passage five to eight cells were grown to confluence in 96-well plates or 100-mm Petri dishes, using an endothelial culture medium consisting of M199 medium (Sigma) supplemented with 20% fetal bovine serum (FBS, Life Technologies), 100 ng/mL streptomycin, 100 IU/mL penicillin, 250 ng/mL fungizone, 1 mmol/L glutamine (Sigma), and 1 ng/mL human recombinant basic fibroblast growth factor (Roche).27 Cell viability was assessed by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) cell proliferation and viability assay kit (R&D Systems).
NADPH Oxidase Activity
Cells were washed with ice-cold Hanks’ balanced salt solution (HBSS) and transferred to lysis buffer (Cell Signaling). The cell lysates were centrifuged for 10 min at 12,000g and 4°C, and 20 μL of the supernatant was subjected to protein analysis (BCA kit, Bio-Rad). NADPH oxidase activity was assessed by measuring O2.− production in the presence of the substrate, NADPH (100 μmol/L, Sigma) as lucigenin-enhanced chemiluminescence (5 μmol/L lucigenin, Sigma).9,26,28 No enzymatic activity could be detected in the absence of NADPH. Reactions were initiated by the addition of 10–20 μL cell lysate containing 25–50 μg extracted protein. NADPH oxidase activity was expressed as relative light units (RLU)/min/mg protein.
Western Blot Analysis of p22phox, p47phox, and Heme Oxygenase-1
Equal amounts of protein (30 μg in 20 μL lysis buffer) were electrophoresed on 12% SDS polyacrylamide gels (Invitrogen), electro-transferred to a PVDF membrane (Invitrogen), and blotted with primary antibodies against p22phox (1:1000, a kind gift from Dr. Frans B. Wientjes, University College London, UK; or 1:200, Santa Cruz), p47phox (1:200, Santa Cruz), or HO-1 (1:250, Stressgen). The secondary antibodies used were goat anti-rabbit IgG (1:2000) for p22phox and p47phox, and goat anti-mouse IgG (1:2000) for HO-1. Blots were developed using ECL plus reagent (Amersham Biosciences). Prestained protein markers (Bio-Rad Laboratories) were used for molecular mass determination. To confirm equal protein loading, PVDF membranes were stripped and blotted with an antibody against actin. Molecular band intensity was determined by densitometry using NIH Scion image software.8,9,26
Total Cellular Iron Content
Cells were transferred to lysis buffer (Cell Signaling), the lysates were centrifuged for 10 min at 12,000g and 4°C, and 20 μL of the supernatant was subjected to protein analysis (BCA kit, Bio-Rad). The remaining sample was digested in 69% nitric acid overnight at room temperature. Subsequently, the samples were diluted 1:100 in 1% nitric acid and subjected to a total iron assay using inductively coupled plasma-optical emission spectroscopy (ICP-OES).24 ICP-OES was also used to measure the total iron content in M199 containing 20% FBS. The standard curve was prepared using a standardized iron solution (ICP-026, Ultra Scientific).
Cellular Heme Level
Cells were transferred to lysis buffer (Cell Signaling), the lysates were centrifuged for 10 min at 12,000g and 4°C, and 20 μL of the supernatant was subjected to protein analysis (BCA kit, Bio-Rad). The remaining sample was transferred to a glass tube and boiled in 2 M oxalic acid (Sigma) for 30 min to release iron from heme, generating protoporphyrin IX, which was measured using a fluorescence microplate reader (Spectra MAX Gemini XS, Molecular Devices, excitation wavelength 410 nm, emission wavelength 600 nm).29,30
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Quantification of human p22phox, p47phox, NOX4, and GAPDH was performed by amplification of cellular cDNA, using a DNA Engine Opticon® 2 System real-time thermocycler (Bio-Rad).14 Optimized amplification conditions were: 300 nmol/L primers (Invitrogen) for p22phox, p47phox, NOX4, and GAPDH; 3 mmol/L MgCl2; and annealing at 58°C. Copy numbers were calculated by the instrument software from standard curves generated from human p22phox, p47phox, NOX4, and GAPDH templates. The primer sequences used were as follows:
-
p22phox
F: 5′CGCTGGCGTCCGGCCTGATCCTCA 3′.
R: 5′ACGCACAGCCGCCAGTAGGTAGAT 3′
-
p47phox
F: 5′TTGAGAAGCGCTTCGTACCC 3′
R: 5′CGTCAAACCACTTGGGAGCT 3′
-
NOX4
F: 5′CAGA AGGT TCCAAGCA GGAG 3′
R: 5′GTTAAGGG CATTCACC AGAT 3′
-
GAPDH
F: 5′GAAGGTGAAGGTCGGAGTC 3′
R: 5′GAAGATGGTGATGGGATTTC 3′
Statistical Analysis
Data are expressed as mean ± SEM. Student’s unpaired t-test or factorial analysis of variance (ANOVA) was used for statistical analysis of the original data. Significance was accepted at P<0.05.
Results
DFO inhibits the LPS-induced increase in NADPH oxidase activity in human aortic endothelial cells
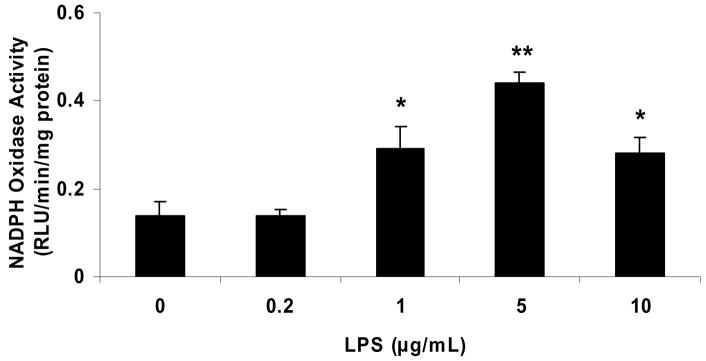
Incubation of HAEC for 18 hrs with ≥1 μg/mL LPS (Escherichia coli 055:B5, detoxified) significantly increased NADPH oxidase activity, whereas no increase was observed with 0.2 μg/mL LPS (Fig. 1a). None of the LPS concentrations used (0.2–10 μg/mL) caused a significant decrease in cell viability after 24 hrs of incubation, as assessed by the MTT assay (data not shown).
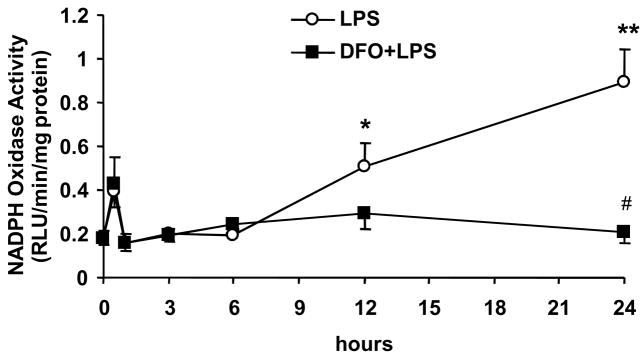
Figure 1. DFO inhibits the LPS-induced increase in NADPH oxidase activity in human aortic endothelial cells.
HAEC were incubated for 18 hrs with the indicated concentrations of LPS (panel a) or up to 24 hrs with 5 μg/mL LPS, without or with 24-hr pretreatment with 100 μmol/L DFO (panel b). After incubation, the cells were lysed and NADPH oxidase activity was measured as described in Methods. Panel a: *P<0.05 and **P<0.01 vs. 0 μg/mL LPS; n=4–6. Panel b: *P<0.05 and **P<0.01 vs. 0 hr LPS; #P<0.05 vs. 24 hrs LPS; n=4.
Exposure of HAEC to 5 μg/mL LPS significantly increased NADPH oxidase activity after 12 and 24 hrs of incubation (Fig. 1b). This LPS-induced increase in NADPH oxidase activity was eliminated by pretreatment of the cells for 24 hrs with 100 μmol/L of the iron chelator, DFO (Fig. 1b); or for 1 hr with 100 μmol/L of the NADPH oxidase inhibitor, apocynin (data not shown). Thus, LPS increases endothelial NADPH oxidase activity in a time- and dose-dependent manner, and iron appears to play a critical role in this process.
Treatment of HAEC for 24 hrs with 100 units/mL tumor necrosis factor α (TNFα) also significantly increased NADPH oxidase activity about two-fold compared to untreated control cells, and this effect was abolished by DFO. In contrast, incubation of HAEC for 24 hrs with 10 ng/mL interleukin-1β did not affect NADPH oxidase activity, either in the absence or presence of DFO (data not shown).
DFO inhibits the LPS or iron-induced increase in cellular iron and heme levels
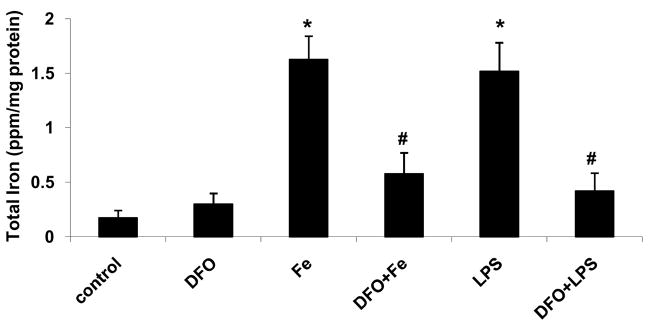
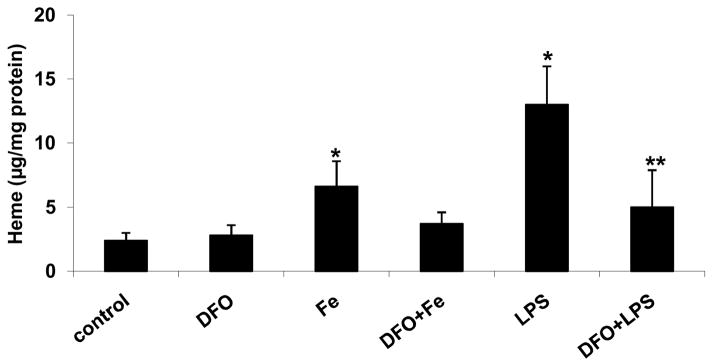
Incubation of HAEC with 5 μg/mL LPS for 24 hrs significantly increased cellular iron and heme levels (Figs. 2a and b, respectively). These effects of LPS were strongly suppressed by 24-hr pretreatment with 100 μmol/L DFO. Similarly, incubation of HAEC with 100 μmol/L ferric citrate for 48 hrs significantly increased cellular iron and heme levels, which was inhibited by 45-hr co-incubation with DFO (Figs. 2a and b).
Figure 2. DFO inhibits the LPS or iron-induced increase in cellular iron and heme levels.
HAEC were incubated for 24 hrs without (control) or with 5 μg/mL lipopolysaccharide (LPS), or treated for 24 hrs with 100 μmol/L DFO and then incubated for 24 hrs with LPS (DFO+LPS). In addition, HAEC were incubated with 100 μmol/L ferric citrate for 48 hrs (Fe) or for 3 hrs with 100 μmol/L ferric citrate followed by addition of 100 μmol/L DFO and incubation for another 45 hrs (DFO+Fe). After incubation, the cells were lysed and assayed for total iron (panel a) or heme (panel b) as described in Methods. Panel a: *P<0.05 vs. control; #P<0.05 vs. Fe or LPS; n=4. Panel b: *P<0.05 vs. control; **P<0.01 vs. LPS; n=4.
DFO inhibits the LPS or iron-induced increase in p22phox protein and NADPH oxidase activity
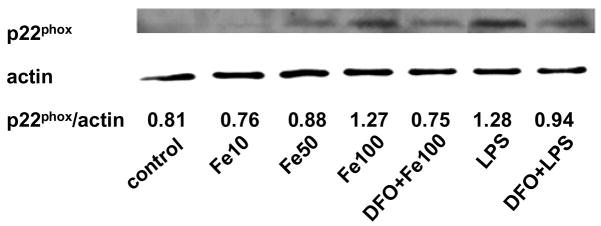
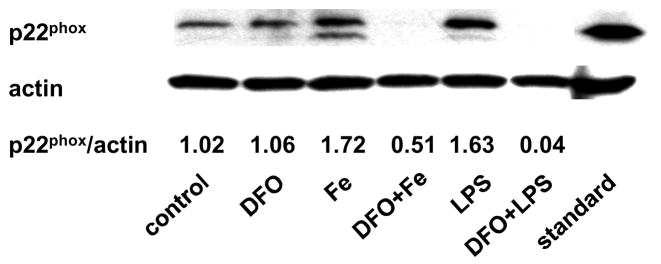
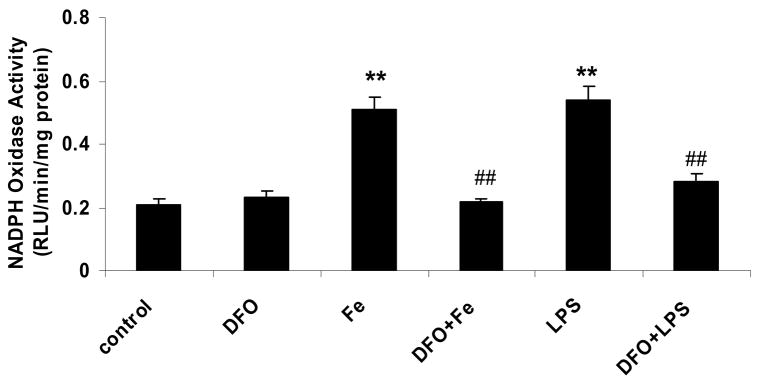
Incubation of HAEC with 5 μg/mL LPS for 24 hrs increased the protein level of p22phox, a heme-containing, catalytic subunit of NADPH oxidase (Fig. 3a). The same observation was made in the human monocytic cell line, THP-1 (Fig. 3b), which was used to confirm results obtained with HAEC. Incubation of HAEC or THP-1 cells with 100 μmol/L ferric citrate for 48 hrs also increased p22phox (Figs. 3a and b). These effects of LPS and iron on the p22phox protein level were strongly inhibited by DFO (Figs. 3a and b). Furthermore, LPS and iron increased NADPH oxidase activity in HAEC after 24 or 48 hrs of incubation, respectively, which was blocked by DFO (Fig. 3c, see also Fig. 1b). Hence, the changes observed in NADPH oxidase activity in HAEC (Fig. 3c) paralleled the corresponding changes in p22phox in the same cells (Fig. 3a).
Figure 3. DFO inhibits the LPS or iron-induced increase in p22phox protein and NADPH oxidase activity.
HAEC (panels a and c) or THP-1 cells (panel b) were incubated as described in the legend of Fig. 2, using 5 μg/mL LPS, 100 μmol/L ferric citrate, or 100 μmol/L DFO. For panel a, HAEC were also incubated for 48 hrs with 10 or 50 μmol/L ferric citrate (Fe10 and Fe50, respectively). After incubation, the cells were lysed and assayed for p22phox by Western blot analysis (panels a and b) or NADPH oxidase activity (panel c) as described in Methods. Each Western blot is representative of three independent experiments. Actin was used as loading control, and the numbers below the individual bands in panels a and b indicate the mean ratio of p22phox to actin determined by densitometry (n=3). In panel b, “standard” indicates the THP-1 lysate from Santa Cruz Biotechnology, used as positive control. Panel c: **P<0.01 vs. control; ##P<0.01 vs. Fe or LPS; n=4.
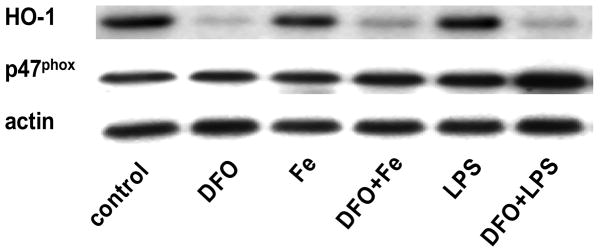
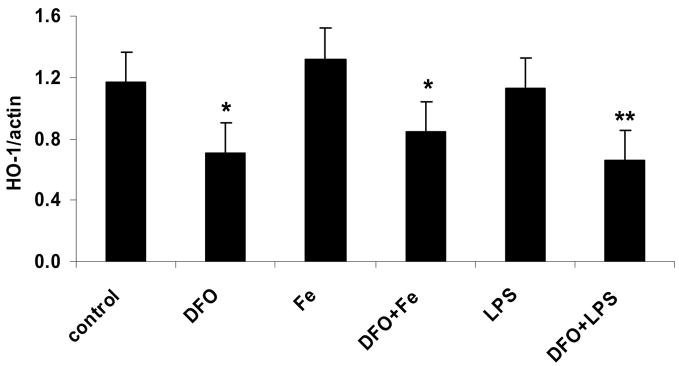
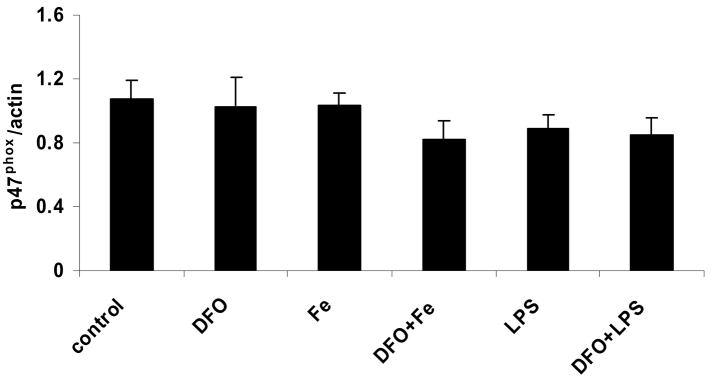
LPS and iron do not increase, but DFO decreases, heme oxygenase-1 protein, whereas p47phox protein is unaffected
Incubation of HAEC with iron or LPS did not significantly increase the protein level of HO-1 (Figs. 4a and b). In contrast, treatment with DFO strongly suppressed HO-1, both in the absence and presence of iron or LPS (Figs. 4a and b). Furthermore, the protein level of p47phox, a non heme-containing, regulatory subunit of NADPH oxidase, was not significantly affected by any of the treatments of HAEC, i.e., LPS or iron without or with DFO (Figs. 4a and c). These results are in striking contrast to the changes observed in the p22phox protein level (Figs. 3a and b).
Figure 4. LPS and iron do not increase, but DFO decreases, heme oxygenase-1 protein, whereas p47phox protein is unaffected.
HAEC were incubated as described in the legend of Fig. 2. After incubation, the cells were lysed and assayed for HO-1 and p47phox by Western blot analysis (panel a) as described in Methods. Each Western blot is representative of three (HO-1) or four (p47phox) independent experiments. Actin was used as loading control, and protein levels of HO-1 and p47phox were quantified by densitometry and expressed as the mean ratio of HO-1 to actin (panel b) or p47phox to actin (panel c). Panel b: *P<0.05 and **P<0.01 vs. control; n=3.
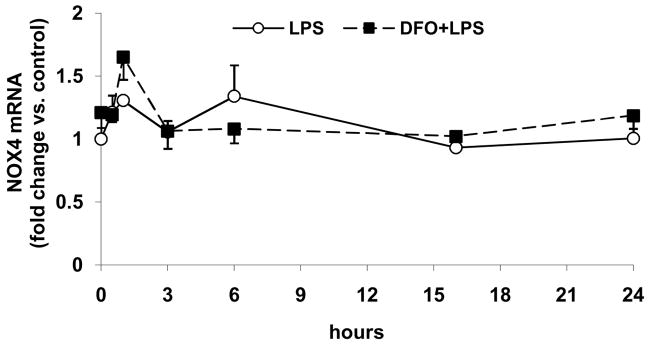
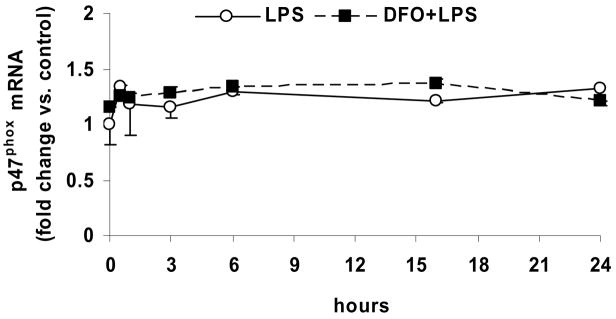
LPS time-dependently increases gene expression of p22phox, which is inhibited by DFO, but has no effect on p47phox and NOX4 gene expression
Incubation of HAEC with LPS for up to 24 hrs, without or with 24-hr pretreatment with DFO, had no significant effect on the mRNA level of NOX4 (Fig. 5a), which is the principal catalytic subunit of endothelial NADPH oxidase. The message level of p47phox also was not affected by LPS or DFO (Fig. 5b), in agreement with the unchanged p47phox protein level (Figs. 4a and c).
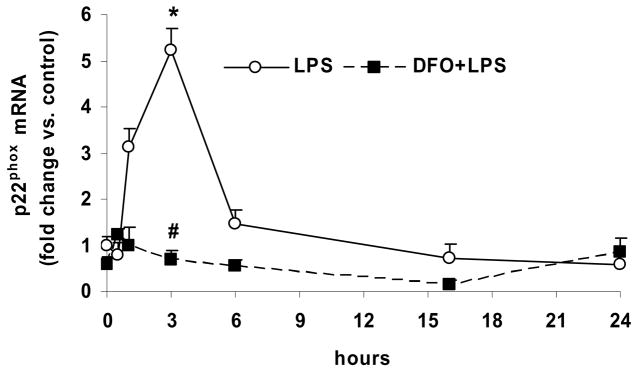
Figure 5. LPS time-dependently increases gene expression of p22phox, which is inhibited by DFO, but has no effect on p47phox and NOX4 gene expression.
HAEC were incubated for up to 24 hrs with 5 μg/mL LPS, without or with 24-hr pretreatment with 100 μmol/L DFO. After incubations, the cells were lysed and total RNA was extracted and subjected to real-time RT-qPCR for NOX4 (panel a), p47phox (panel b) or p22phox (panel c) as described in Methods. Results are expressed as fold change vs. control (0 hr LPS). Panel c: *P<0.05 vs. 0 hr LPS; #P<0.05 vs. 3 hrs LPS; n=3, each determined in duplicate.
However, LPS treatment time-dependently increased the mRNA level of p22phox, which reached a maximum after 3 hrs of incubation and subsequently declined rapidly (Fig. 5c). The LPS-induced increase in the p22phox mRNA level was eliminated by pretreatment of the cells with DFO (Figs. 5c). These data support the notion that LPS increases NADPH oxidase activity in HAEC by upregulating gene transcription of p22phox, but not the enzyme’s other subunits, NOX4 and p47phox.
LPS increases cellular iron and heme levels only after 24 hrs of incubation, which is inhibited by DFO
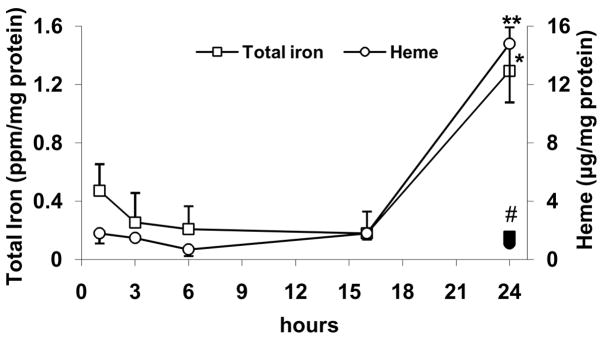
Finally, the time-course of LPS-induced changes in cellular iron and heme levels was assessed. As shown in Fig. 6, exposing HAEC to LPS did not affect iron and heme levels for up to 16 hrs of incubation. However, significant increases were observed at 24 hrs, which were blocked by pretreatment of the cells with DFO (Fig. 6).
Figure 6. LPS increases cellular iron and heme levels only after 24 hrs of incubation, which is inhibited by DFO.
HAEC were incubated as described in the legend of Fig. 5. After incubations, the cells were lysed and assayed for total iron or heme as described in Methods. *P<0.05 vs. 0 hr LPS (iron); **P<0.01 vs. 0 hr LPS (heme); #P<0.05 vs. 24 hrs LPS (iron or heme); n=4, each determined in triplicate.
Discussion
Lipopolysaccharide-induced oxidative stress is causally related to increased NADPH oxidase activity; however, the underlying mechanisms are incompletely understood.4,26,31,32 The major findings of this study are that prolonged exposure of human aortic endothelial cells to LPS or iron increases cellular levels of iron, heme, and p22phox, a heme-containing, catalytic subunit of NADPH oxidase; and treatment of the cells with the iron chelator, desferroxamine, inhibits these effects and prevents the LPS or iron-induced increase in NADPH oxidase activity.
We found that LPS increased NADPH oxidase activity in HAEC in a time- and dose-dependent manner, which is consistent with the observation that LPS dose-dependently increased O2.− production in human blood vessels.33 Interestingly, our time-course studies showed that LPS caused a small, transient increase in NADPH oxidase activity within the first 30 minutes of incubation (see Fig. 1b), likely due to activation of pre-existing NADPH oxidase. However, this increase was not statistically significant and did not appear to be inhibited by DFO. A significant, sustained increase in NADPH oxidase activity was observed between 12 and 24 hours of incubation with LPS, which was abrogated by DFO. These findings are in agreement with published data that increased O2.− production by NADPH oxidase required prolonged exposure to LPS, both in vitro and in vivo.26,31,32
Prolonged exposure to LPS also increased cellular iron, heme, and p22phox protein levels in HAEC. As a possible explanation for these observations, LPS has been shown to upregulate the divalent metal transporter 1 (DMT1), an iron importer, in bronchial epithelial cells.34 Increased iron uptake supplies cellular iron for heme biosynthesis, which in turn may help stabilize the heme protein, p22phox.19,20 We also observed that TNFα increased NADPH oxidase activity in HAEC in a DFO-sensitive manner, and TNFα, like LPS, is known to upregulate DMT1 in bronchial epithelial cells.34 However, although DMT1 is abundant in HAEC, its level was not affected by incubation with LPS (data not shown). Therefore, the mechanism by which LPS stimulates iron uptake into HAEC remains to be fully elucidated.
The above long-term effect of LPS on cellular iron and heme levels may explain why p22phox protein and NADPH oxidase activity were increased in HAEC after 24 hours of incubation. An additional, major role of iron in NADPH oxidase activity is indicated by the observation that DFO abrogated LPS-induced p22phox gene transcription, which peaked at around 3 hours of incubation with LPS. It is conceivable that cellular labile (“free”) iron, e.g., by increasing oxidative stress, plays a critical in LPS or TNFα-induced activation of the redox-sensitive transcription factors, NFκB and AP-1, and subsequent p22phox gene expression.35–38 In contrast, LPS and DFO had no effect on p47phox and NOX4 gene expression, suggesting a different mechanism of transcriptional regulation independent of iron.
Incubating HAEC with excess iron, in the form of ferric citrate, mimicked the effects of LPS on cellular iron, heme, and p22phox protein levels. Ferric citrate was used because the majority of labile iron in humans is found as a complex of ferric iron with citrate.3,39 As discussed above, DFO inhibited the iron or LPS-induced changes in cellular iron, heme, p22phox, and NADPH oxidase. DFO is transported into cells via endocytosis and remains associated with endosomes,40 from which labile iron is transported to mitochondria for heme biosynthesis.41 Thus, DFO chelates free iron and blocks heme synthesis, which may explain why it affected the cellular level of the heme protein, p22phox, but not the non-heme protein, p47phox.
Neither iron nor LPS affected heme oxygenase-1 in HAEC. Induction of HO-1 has been shown to lower NADPH oxidase activity due to decreased heme availability and destabilization and degradation of p22phox and cytochrome b558.19,20 Hence, our findings presented here and elsewhere26 indicate that LPS and iron increase NADPH oxidase activity independently of HO-1, most likely by upregulating p22phox gene expression and stabilizing the protein by increasing cellular iron uptake and de novo synthesis of heme.
Interestingly, DFO blocked HO-1 expression irrespective of the addition of LPS or iron. These data suggest that there is a negative feedback loop between iron and HO-1: chelation of iron with DFO reduces the iron supply for synthesis of heme and stabilization of p22phox, and thus NADPH oxidase activity declines. In turn, this mechanism may negatively regulate HO-1 in order to prevent further degradation of heme and, hence, decreased NADPH oxidase activity. The iron content in HAEC growth media (M199 containing 20% FBS) is about 10 μmol/L, whereas up to 100 μmol/L ferric citrate was added in our experiments to induce an effect on p22phox and NADPH oxidase activity. Nevertheless, the low iron content in the media seems enough to strongly induce HO-1 expression in HAEC, because neither added iron nor LPS further increased HO-1.
In summary, our data show that prolonged exposure to LPS or iron increases endothelial NADPH oxidase activity, in parallel with increased p22phox gene transcription and increased cellular levels of iron, heme, and p22phox protein. All of these effects of LPS and iron were strongly inhibited by the iron chelator, desferrioxamine. Therefore, chelation of excess iron may help attenuate vascular oxidative stress and inflammation and inhibit the development of atherosclerotic vascular diseases.
Acknowledgments
This publication was made possible by grant number P01 AT002034 from the National Center for Complementary and Alternative Medicine (NCCAM), P30 ES000210 from the National Institute of Environmental Health Sciences (NIEHS), and Beginning Grant-in-Aid number 0760018Z from the American Heart Association (AHA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM, NIEHS, NIH, or AHA.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 3.Kruszewski M. The role of labile iron pool in cardiovascular diseases. Acta Biochim Pol. 2004;51:471–480. [PubMed] [Google Scholar]
- 4.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Crockett E, Wang DH, Galligan JJ, Fink GD, Chen AF. Gene transfer of endothelial NO synthase and manganese superoxide dismutase on arterial vascular cell adhesion molecule-1 expression and superoxide production in deoxycorticosterone acetate-salt hypertension. Arterioscler Thromb Vasc Biol. 2002;22:249–255. doi: 10.1161/hq0202.104124. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Chu Y, Fink GD, Engelhardt JF, Heistad DD, Chen AF. Endothelin-1 stimulates arterial VCAM-1 expression via NADPH oxidase-derived superoxide in mineralocorticoid hypertension. Hypertension. 2003;42:997–1003. doi: 10.1161/01.HYP.0000095980.43859.59. [DOI] [PubMed] [Google Scholar]
- 10.Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. 2001;14:44S–54S. doi: 10.1016/s0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
- 11.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107:1209–1210. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 13.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 14.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 15.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 16.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 17.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leuko Biol. 2004:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 18.Knopfel M, Solioz M. Characterization of a cytochrome b(558) ferric/cupric reductase from rabbit duodenal brush border membranes. Biochem Biophys Res Commun. 2002;291:220–225. doi: 10.1006/bbrc.2002.6423. [DOI] [PubMed] [Google Scholar]
- 19.Taille C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Zhen L, Dinauer MC. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J Biol Chem. 1997;272:27288–27294. doi: 10.1074/jbc.272.43.27288. [DOI] [PubMed] [Google Scholar]
- 21.Kurtoglu E, Ugur A, Baltaci AK, Mogolkoc R, Undar L. Activity of neutrophil NADPH oxidase in iron-deficient anemia. Biol Trace Elem Res. 2003;96:109–115. doi: 10.1385/BTER:96:1-3:109. [DOI] [PubMed] [Google Scholar]
- 22.Evans PJ, Smith C, Mitchinson MJ, Halliwell B. Metal ion release from mechanically-disrupted human arterial wall. Implications for the development of atherosclerosis. Free Radic Res. 1995;23:465–469. doi: 10.3109/10715769509065267. [DOI] [PubMed] [Google Scholar]
- 23.Gackowski D, Kruszewski M, Jawien A, Ciecierski M, Olinski R. Further evidence that oxidative stress may be a risk factor responsible for the development of atherosclerosis. Free Radic Biol Med. 2001;31:542–547. doi: 10.1016/s0891-5849(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 24.Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol. 2004;24:949–954. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan JL. Stored iron and vascular reactivity. Arterioscler Thromb Vasc Biol. 2005;25:1532–1535. doi: 10.1161/01.ATV.0000174124.20147.22. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Frei B. Iron chelation inhibits NF B-mediated adhesion molecule expression by inhibiting p22phox protein expression and NADPH oxidase activity. Arterioscler Thromb Vasc Biol. 2006;26:2638–2643. doi: 10.1161/01.ATV.0000245820.34238.da. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W-J, Frei B. α-Lipoic acid inhibits TNF-α-induced NF-κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–2432. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension. 2003;42:316–321. doi: 10.1161/01.HYP.0000084853.47326.F2. [DOI] [PubMed] [Google Scholar]
- 29.Ward JH, Jordan I, Kushner JP, Kaplan J. Heme regulation of HeLa cell transferrin receptor number. J Biol Chem. 1984;259:13235–13240. [PubMed] [Google Scholar]
- 30.Morrison GR. Fluorometric microdetermination of heme protein. Anal Chem. 1965;37:1124–1126. doi: 10.1021/ac60228a014. [DOI] [PubMed] [Google Scholar]
- 31.Kawahara T, Kohjima M, Kuwano Y, Mino H, Teshima-Kondo S, Takeya R, Tsunawaki S, Wada A, Sumimoto H, Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–C457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 32.Brandes RP, Koddenberg G, Gwinner W, Kim D, Kruse HJ, Busse R, Mugge A. Role of increased production of superoxide anions by NAD(P)H oxidase and xanthine oxidase in prolonged endotoxemia. Hypertension. 1999;33:1243–1249. doi: 10.1161/01.hyp.33.5.1243. [DOI] [PubMed] [Google Scholar]
- 33.Rice JB, Stoll LL, Li WG, Denning GM, Weydert J, Charipar E, Richenbacher WE, Miller FJ, Jr, Weintraub NL. Low-level endotoxin induces potent inflammatory activation of human blood vessels: inhibition by statins. Arterioscler Thromb Vasc Biol. 2003;23:1576–1582. doi: 10.1161/01.ATV.0000081741.38087.F9. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Garrick MD, Yang F, Dailey LA, Piantadosi CA, Ghio AJ. TNF, IFN-gamma, and endotoxin increase expression of DMT1 in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L24–L33. doi: 10.1152/ajplung.00428.2003. [DOI] [PubMed] [Google Scholar]
- 35.Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22phox by NF-κB in human aortic smooth muscle cells. Arch Physiol Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 37.Manea A, Manea SA, Gafencu AV, Raicu M, Simionescu M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler Thromb Vasc Biol. 2008;28:878–885. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- 38.Zhang WJ, Frei B. Intracellular metal ion chelators inhibit TNFalpha-induced SP-1 activation and adhesion molecule expression in human aortic endothelial cells. Free Radic Biol Med. 2003;34:674–682. doi: 10.1016/s0891-5849(02)01375-8. [DOI] [PubMed] [Google Scholar]
- 39.Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non–transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989;264:4417–4422. [PubMed] [Google Scholar]
- 40.Doulias PT, Christoforidis S, Brunk UT, Galaris D. Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radic Biol Med. 2003;35:719–728. doi: 10.1016/s0891-5849(03)00396-4. [DOI] [PubMed] [Google Scholar]
- 41.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]